Abstract
Bacillus anthracis, the causative agent of anthrax, requires surface (S)-layer proteins for the pathogenesis of infection. Previous work characterized S-layer protein binding via the surface layer homology domain to a pyruvylated carbohydrate in the envelope of vegetative forms. The molecular identity of this carbohydrate and the mechanism of its display in the bacterial envelope are still unknown. Analyzing acid-solubilized, purified carbohydrates by mass spectrometry and NMR spectroscopy, we identify secondary cell wall polysaccharide (SCWP) as the ligand of S-layer proteins. In agreement with the model that surface layer homology domains bind to pyruvylated carbohydrate, SCWP was observed to be linked to pyruvate in a manner requiring csaB, the only structural gene known to be required for S-layer assembly. B. anthracis does not elaborate wall teichoic acids; however, its genome harbors tagO and tagA, genes responsible for the synthesis of the linkage unit that tethers teichoic acids to the peptidoglycan layer. The tagO gene appears essential for B. anthracis growth and complements the tagO mutant phenotypes of staphylococci. Tunicamycin-mediated inhibition of TagO resulted in deformed, S-layer-deficient bacilli. Together, these results suggest that tagO-mediated assembly of linkage units tethers pyruvylated SCWP to the B. anthracis envelope, thereby enabling S-layer assembly and providing for the pathogenesis of anthrax infections.
Keywords: Bacillus anthracis, secondary cell wall polysaccharide, surface-layer, pyruvylation, tagO
Introduction
Ingestion, inoculation, or inhalation of Bacillus anthracis spores can lead to anthrax disease in mammalian hosts.1 Typically, anthrax is a disease of herbivorous mammals, capable of producing infection in humans exposed to spores via contaminated animal products or, more recently, by acts of bioterrorism.2 The envelope of B. anthracis is a dynamic organelle composed of peptidoglycan, lipoteichoic acid, polysaccharides, proteins, and poly-γ-D-glutamic acid capsule.3 These surface molecules must interact directly with host cells and tissues during all stages of infection. Within the envelope, anthrax bacilli synthesize and assemble a surface (S)-layer, a paracrystalline lattice of protein that covers the entire cell surface.3 Although many microbes elaborate an S-layer, B. anthracis provides a model system for the study of S-layer assembly in Gram-positive bacteria, laying groundwork for understanding how these molecules are immobilized within the envelope.
S-layers in B. anthracis comprise two secreted proteins: Sap and EA1, hypothesized to bind the murein sacculus non-covalently by virtue of three conserved tandem repeats of a surface layer homology (SLH) domain,4,5 which specifically engages a pyruvylated cell wall polysaccharide.6 An N-terminal signal peptide and three tandem copies of the SLH domain are sufficient for the secretion and immobilization of proteins onto the surface of B. anthracis via this mechanism.7 In addition to the Sap and EA1 proteins, 22 other B. anthracis genes (bslA–bslU) encode both a signal peptide and three SLH domains and are therefore potentially surface-displayed by a similar mechanism.8
Directly adjacent to sap and eag, the structural genes for the Sap and EA1 S-layer proteins, is positioned a two-gene operon encoding csaAB (cell surface attachment). csaB is essential for S-layer assembly, as csaB mutants secrete both Sap and EA1 but fail to retain either on the cell surface.6 csaB-dependent binding of SLH domains to the cell wall has also been observed in Thermus thermophilus, another microbe that elaborates S-layers.9 Presumably, bacilli deficient in csaB are incapable of properly retaining any SLH protein and, consequently, secrete these molecules into the extracellular environment. The predicted primary translation product of the csaB open reading frame displays homology to exopolysaccharide pyruvyl transferases of other bacteria, suggesting a role for csaB in synthesizing the pyruvylated polysaccharides.6 Purified polysaccharides from B. anthracis Sterne bind the SLH domains of Sap and EA1, whereas analogous polysaccharides purified from ΔcsaB cells do not.6 When analyzed by NMR spectroscopy, the only differences between the two polymers were methyl proton chemical shifts correlated with ketal carbon and carboxylic carbon chemical shifts.6 Similar shifts are documented in spectra of polysaccharides purified from other S-layer-containing organisms, suggesting a conserved structure.10,11 These chemical shifts are diagnostic of ketal pyruvate modification of these polysaccharides and, by extension, assign a role for CsaB in this process.
To date, no further structural information regarding the pyruvylation of B. anthracis polysaccharides has been presented. However, the structure of the major secondary cell wall polysaccharide (SCWP) has been determined for various B. anthracis and Bacillus cereus strains via linkage analysis and NMR spectroscopy.12,13 Atomic-level resolution of the SCWP revealed structural differences, which confer the exquisite specificity observed between the B. anthracis and B. cereus SCWP-binding phage lysins.14 Nevertheless, the published structure did not provide evidence of pyruvate modifications to these polysaccharides, leaving the identity of the polymeric SLH ligand unknown.
Given the published reports, placing ketal pyruvate on polysaccharides of various S-layer-containing bacteria6,9 and the similarity between the proposed structures of the SCWP from Geobacillus stearothermophilus PV72/p2 (including a ketal pyruvate) and B. anthracis,11,12 we sought to purify SLH-binding polysaccharides to test the hypothesis that these molecules contain pyruvate. Towards this end, we developed a fluorescence-based measurement of SLH protein deposition onto the cell walls of anthrax bacilli and used this system to assay purified components of the cell wall for their ability to bind SLH domains. This approach makes no assumption as to the composition of the SLH ligand, but rather examines a variety of constituent cell wall molecules for their ability to bind SLH domains. Hydrofluoric-acid-extractable polysaccharides (HF-PS) bind the SLH domain, which is consistent with previous studies. These SLH ligand polysaccharides exhibit mass spectra identical with the published structural models for the B. anthracis SCWP.12 High-resolution mass measurement and tandem mass spectrometry (MS) of these carbohydrates unequivocally confirmed their identity as the SCWP and also provided evidence for greater structural homogeneity than previously appreciated. Importantly, the mass spectra of SCWP, purified from wild-type Sterne cells, contain multiple additional ions not observed in ΔcsaB strains. These ions arise from pyruvylated forms of the SCWP, providing the first direct evidence linking ketal pyruvate to the SCWP structure and unifying studies of S-layer assembly and SCWP structure. These data expand the roles of SCWP to include SLH protein retention and S-layer assembly in B. anthracis.
Many Gram-positive organisms are known to elaborate an SCWP and we sought to identify the genetic pathway responsible for SCWP production. TagA and TagO are glycosyl transferases that synthesize the cell wall linkage unit of wall teichoic acid (WTA).15,16 Genetic linkage between tagA and csaB homologs in multiple organisms and the lack of WTA in the B. anthracis envelope prompted the hypothesis that TagO and TagA play essential roles in the synthesis of SCWP. We demonstrate that B. anthracis cells grown in the presence of the TagO-specific inhibitor tunicamycin display aberrant forms and no longer bind SLH domains. This implies a novel function of the tag machinery in B. anthracis: synthesis of the SCWP. Taken together, these data promote understanding of S-layer assembly, SCWP function, and TagO activity in the assembly of SCWP molecules.
Results
ΔcsaB mutants no longer retain SLH proteins
Previous studies reported that B. anthracis strains deficient in csaB are incapable of retaining the Sap and EA1 S-layer proteins.6 To examine the contribution of csaB to S-layer assembly, we constructed an in-frame deletion of the entire csaB open reading frame in B. anthracis Sterne. As expected, strains lacking the csaB gene grew aberrantly, producing large masses of cells that rapidly settled out of liquid culture (data not shown).6 To determine if this retention-deficient phenotype includes all SLH proteins, we examined the entire protein profile of SDS-extracted culture sediments by SDS-PAGE and Coomassie blue staining and by immunoblotting for BslA, an SLH adhesin.8 Whereas extracts of Sterne culture sediments contain abundant species corresponding to Sap, BslA, and presumably other SLH proteins, these species no longer sediment with bacilli in ΔcsaB cultures (Fig. 1a). Proteins precipitated from the supernatants of these cultures appeared similar (data not shown). Transformation of the ΔcsaB strain with pcsaB, a plasmid containing the csaB open reading frame, complements this defect in trans, restoring the profile of sedimented, SDS-extracted proteins whereas transformation with the empty vector, pLM5, does not (Fig. 1a). Immunoblots using BslA-specific antisera to probe proteins extracted from culture sediments and supernatants confirmed that these strains still produce and export SLH proteins. As expected, immunoreactive species are detected in the supernatant of all cultures (all SLH proteins are shed from the cell surface into the media supernatant over time) but only cells harboring the csaB gene contain immunoreactive species in the sediment (Fig. 1a).
Fig. 1.
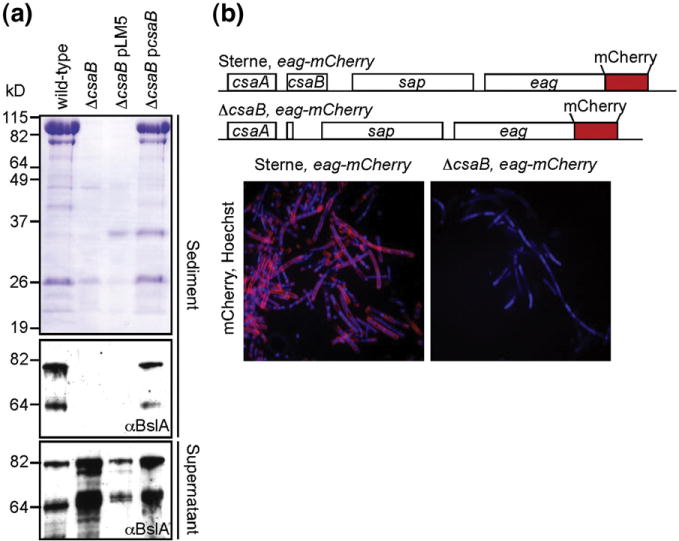
csaB is required for SLH protein retention on the B. anthracis cell wall. (a) Coomassie-blue-stained SDS-PAGE of protein extracts from culture sediments (upper panel). Strains harboring csaB contain numerous protein species that no longer sediment when the csaB gene is deleted. Immunoblots of these samples (middle panel) using antiserum against the SLH protein BslA suggest that SLH proteins are no longer retained even though all strains produce BslA, as total precipitated proteins from culture filtrate contain specific signals (bottom panel). (b) Genetic organization of B. anthracis Sterne eag-mCherry and ΔcsaB eag-mCherry strains. (c) Fluorescent micrographs of these cells harvested in late-exponential phase stained with Hoechst (blue). B. anthracis Sterne eag-mCherry assemble the EA1-mCherry (red) hybrid into the S-layer, validating the use of fluorescent reporters in measuring SLH protein assembly.
As all previous measurements of SLH retention have relied on SDS-PAGE and immunoblot analysis of whole cultures,6 we engineered a reporter protein whose in vitro assembly onto the cell surface could be visualized microscopically and fluorometrically. To verify that fluorescent reporter hybrids to SLH proteins are tolerated for cell wall binding, we inserted the mCherry open reading frame directly before the TAA stop of eag on the B. anthracis chromosome, generating Sterne eag-mCherry. Fluorescent micrographs of late-exponential and stationary phase cultures of this strain stained with the double-stranded-DNA-specific dye Hoechst reveal abundant red fluorescence surrounding most cells (Fig. 1b). In contrast, ΔcsaB strains carrying this hybrid do not exhibit any red fluorescence on the cell surface (Fig. 1b). These microscopy data not only corroborate observations reported for whole culture extracts but also enable us to follow the trafficking of specific substrates to the cell envelope. More importantly, these data provide proof of principle for the use of fluorescence molecules as reporters of SLH–cell wall binding.
Recombinant SapSLH–mCherry binds cell walls from strains harboring csaB
Given that S-layer protein–mCherry hybrids are assembled on the cell surface in vivo, we sought to develop an in vitro assay to measure the deposition of fluorescent SLH proteins on the B. anthracis cell wall. Such an assay would allow screening of cell wall extracts for their ability to compete for SLH–cell wall binding and, consequently, identify fractions containing the SLH ligand. To accomplish this, we purified decahistidyl-tagged mCherry or SapSLH–mCherry, a translational hybrid between the SLH domains of the B. anthracis S-layer protein Sap and mCherry from Escherichia coli. Nickel affinity purification yields abundant quantities of both recombinant proteins with comparable fluorescence yields (Fig. 2a). To measure binding of the SLH domains to the cell wall, we incubated equimolar concentrations of mCherry or SapSLH–mCherry with equal numbers of B. anthracis cells, which had been stripped of all endogenous non-covalently bound proteins by boiling in urea. After incubation, the suspensions were centrifuged and the sediment was washed extensively and then imaged microscopically. Cells of B. anthracis Sterne incubated with SapSLH–mCherry were abundantly stained, whereas incubation of these walls with mCherry alone displayed no staining (Fig. 2b). In contrast, cell wall material from B. anthracis ΔcsaB showed no labeling by either fluorescent protein unless transformed with the complementing pcsaB plasmid (Fig. 2b). Thus, SapSLH–mCherry binds to the cell wall of bacilli in vitro dependent on the presence of both the SLH domain and the csaB gene, recapitulating requirements known from in vivo studies and validating it as a measurement of SLH assembly in the B. anthracis envelope. Development of this assay provided a tool with which to quantify binding of the SLH domain to cell walls fluorometrically and allowing us to measure the ability of purified compounds to compete for this binding.
Fig. 2.
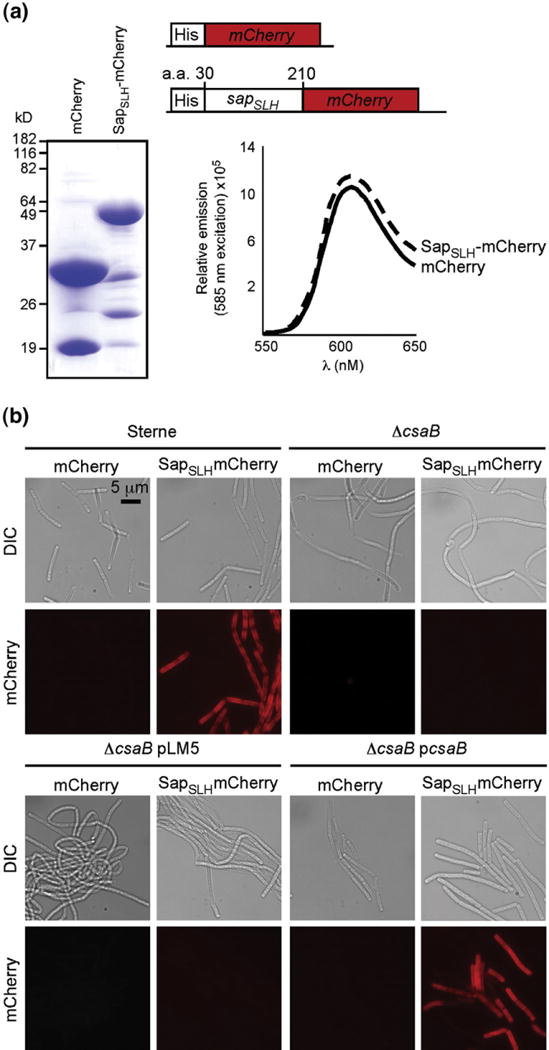
Purified, recombinant SapSLH–mCherry, but not mCherry, binds to the cell walls of B. anthracis strains harboring csaB. (a) Coomassie-stained SDS-PAGE of hexahistidyl-purified mCherry and a translational hybrid between the Sap SLH domains (amino acids 30 to 210 of the sap gene) and mCherry. Abundant, soluble forms of both proteins were purified from E. coli and, at equal concentrations, exhibited equivalent fluorescent yields with identical peak excitation (585 nm) and emission (608 nm) spectra. (b) Fluorescent micrographs of B. anthracis Sterne, ΔcsaB, ΔcsaB pLM5, and ΔcsaB pcsaB cells incubated with 1 μM mCherry or SapSLH–mCherry. Staining of the cell wall depends upon the presence of the SLH domain and the csaB gene, validating the use of this as an in vitro model of S-layer assembly.
Mutanolysin and hydrofluoric acid cell wall extracts from B. anthracis Sterne, but not ΔcsaB, cell walls inhibit SLH binding to the cell wall
We next used our in vitro fluorescence assay of SLH assembly to score various extracts of the B. anthracis cell wall for inhibitory activity, aiming to characterize a purified SLH ligand. Sterne and ΔcsaB cell walls, free from protein, lipid, and nucleic acid contaminants, were then solubilized with mutanolysin or extracted with hydrofluoric acid, the former containing all components of the murein sacculus and the latter containing only cell-wall-linked polysaccharides (HF-PS).17,18 We measured the concentration of mutanolysin-solubilized peptidoglycan and HF-PS by the Morgan–Elson and Anthrone method, respectively.19,20 These two extracts, purified from either wild-type or ΔcsaB strains, were then included in the fluorescence binding assay described above. The ability of these extracts to inhibit SLH–cell wall binding is determined by the quantity of fluorescence that would co-sediment with the cell walls after incubation. As expected, mutanolysin-solubilized peptidoglycan from B. anthracis Sterne cells inhibited the binding of SapSLH–mCherry to the cell wall in a dose-dependent manner, whereas no inhibition by soluble peptidoglycan from the ΔcsaB strain or by our normalization standard N-acetyl-glucosamine (Sigma) was observed regardless of concentration (Fig. 3a). Micrographs of stained bacilli were collected from each reaction, qualitatively verifying the fluorescence measurements plotted in Fig. 3a (Fig. 3c). Similarly, neutralized HF-PS of B. anthracis Sterne cell walls inhibited SapSLH–mCherry binding to the cell wall. This inhibitory activity is csaB-dependent, as neither ΔcsaB cell walls nor a D-glucose normalization standard (Fisher) impacted SapSLH–mCherry binding (Fig. 3b). These measurements were also confirmed by microscopy (Fig. 3d).
Fig. 3.
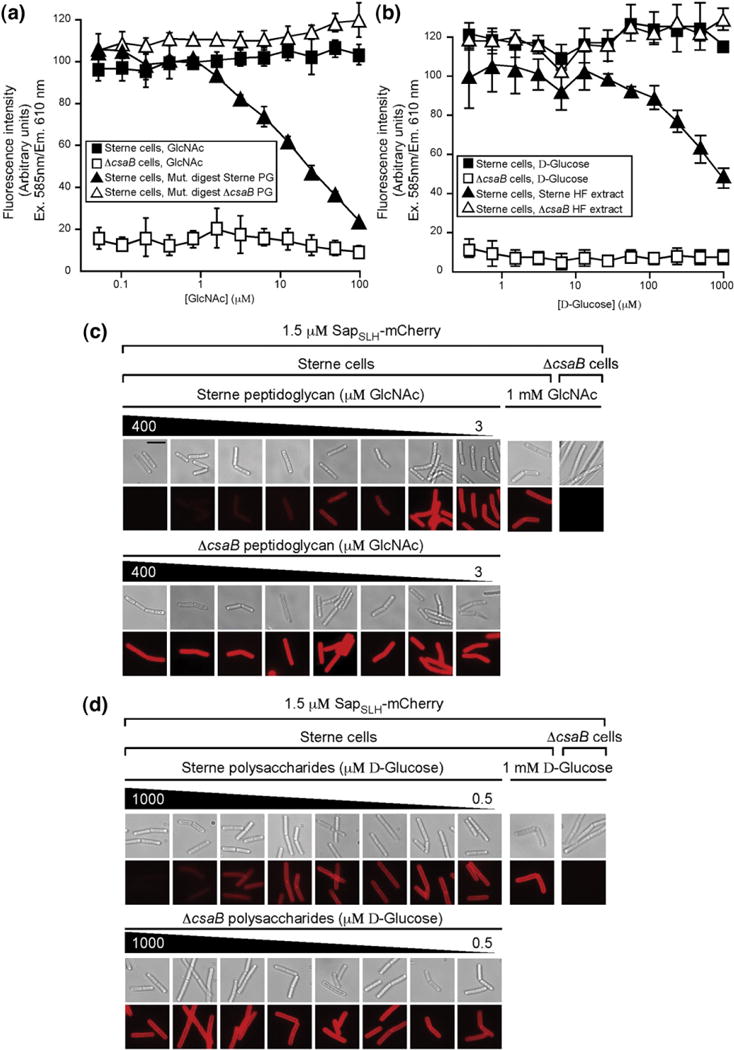
Crude, soluble peptidoglycan and HF-PS inhibit SapSLH–mCherry binding to the B. anthracis cell wall. (a) Fluorescence intensities in the reaction sediment of SapSLH–mCherry (1 μM) incubated with equal numbers of ΔcsaB cells (open squares) or with Sterne cells (filled squares) and the indicated concentration of GlcNAc (normalization control), or with SapSLH–mCherry (1 μM) incubated with Sterne cell suspensions containing the indicated concentrations of soluble peptidoglycan from Sterne (filled triangles) or ΔcsaB cell walls (open triangles). Only soluble peptidoglycan fragments from Sterne cell walls can inhibit the binding of SapSLH–mCherry to the cell wall. (b) Fluorescence intensities in the reaction sediment of SapSLH–mCherry (1 μM) incubated with equal numbers of ΔcsaB cells (open squares) or with Sterne cells (filled squares) and the indicated concentration of D-glucose (normalization control), or with SapSLH–mCherry (1 μM) incubated with Sterne cell suspensions containing the indicated concentrations of HF-PS from Sterne (filled triangles) or ΔcsaB cell walls (open triangles). Only HF-PS from Sterne cell walls can inhibit the binding of SapSLH–mCherry to the cell wall. (c) Fluorescence micrographs of cells from representative reactions plotted in (a) validate the measurements in the upper panel. The scale bar represents 5 μm. (d) Fluorescence micrographs of cells from representative reactions plotted in (b) validate the measurements in the upper panel.
Extracts were then subjected to rpHPLC in order to obtain chromatographically pure compounds for structural analysis. Crude, soluble peptidoglycan from B. anthracis Sterne eluted as many peaks over a 100-min gradient, most of which exhibited a low level of inhibitory activity (Fig. S1). These data suggested that the SLH ligand cannot be purified to homogeneity from mutanolysin-solubilized peptidoglycan. In contrast, HF-PS from both strains displayed similar chromatograms (A206) with a few discrete peaks eluting with similar profiles (Fig. 4a). Each fraction was collected, neutralized, and scored for inhibitory activity in the in vitro SapSLH–mCherry cell wall binding assay as before. Fluorescence intensities were recorded in duplicate in 96- well format. Strikingly, all fractions containing significant inhibitory activity corresponded to a single, large elution peak (Fig. 4b). Although this peak is present in both the Sterne and ΔcsaB strains, only the eluant fraction from Sterne cell walls displayed inhibitory activity. This fraction represents a chromatographically pure HF-PS amenable to structural analyses.
Fig. 4.
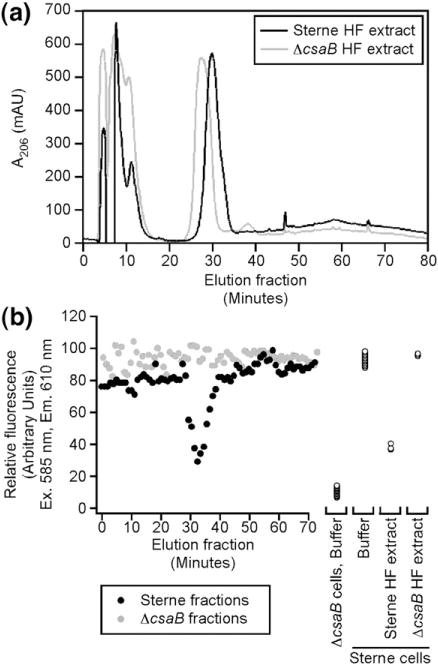
rpHPLC purification of the HF-PS from Sterne and ΔcsaB strains reveals a single peak containing the SLH ligand. (a) rpHPLC chromatographs (A206) of HF-PS extracts from B. anthracis Sterne (black trace) and ΔcsaB (gray trace) both contain a single, predominant peak. (b) All fractions from the purifications in (a) were dried, dissolved in neutral buffer, and incubated with SapSLH–mCherry (1 μM) and Sterne cell walls. Fluorescence intensity in the sediment is plotted. The large, predominant peak from the Sterne strain was inhibitory in this reaction and was subjected to further analysis.
HF-PS are pyruvylated in a csaB-dependant manner
Our rpHPLC purification of HF-PS from B. anthracis Sterne and ΔcsaB yielded approximately equal quantities of polysaccharides (as measured by A206) with dramatically different inhibitory activity (Fig. 4). We reasoned, therefore, that these polysaccharides must contain important differences in structure. To test this hypothesis, we subjected the material purified by rpHPLC to matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS and to 1H nuclear magnetic resonance spectroscopy.
The spectra of ions we obtained by MALDI-TOF were remarkable for two reasons. First, both spectra contain the same pattern of major ions and repeating units as the SCWP of B. anthracis, whose structure has been determined (Fig. 5a).12 This result identifies the major SCWP as the SLH ligand. The most abundant ion in these spectra occurred at m/z ~1136, which corresponds to a sodium adduct of the repeating SCWP oligosaccharide (Table 1). This oligosaccharide has a →6)-α-GlcNAc-(1→4)-β-ManNAc-(1→4)-β-GlcNAc-(1→ backbone, substituted with β-Gal via 1→3 and 1→4 linkages to the α-GlcNAc and with a β-Gal via 1→3 linkage to the β-GlcNAc.12 In addition to the conservation of major peaks between these and other mass spectra,12 we detected numerous additional ions, corresponding to structural permutation of the SCWP, indicating that this molecule displays significant heterogeneity in glycosyl and acetyl composition after extraction and purification (Table 1). Second, the wild-type spectrum contains a series of ions absent from the ΔcsaB spectrum whose mass-to-charge ratios differ from ions present in both samples by 70, the increase that can be expected from ketal pyruvate linked to a pyranose sugar (Fig. 5b, bold). The composition of these pyruvylated ions is summarized in Table 2.
Fig. 5.

MALDI-TOF mass spectra of rpHPLC-purified HF-PS from Sterne and ΔcsaB. (a) MALDI-TOF spectra were collected from rpHPLC-purified HF-PS. Both Sterne and ΔcsaB spectra contain the same series of ions, which is identical with those ions described in the identification of the SCWP structure. Ions in bold represent multimers of the basic Hex3HexNAc3 oligosaccharide unit of the SCWP. These spectra identify the SLH ligand polysaccharide as the SCWP of B. anthracis. (b) A more detailed view of the mass spectra from (a), displaying ions from m/z=1200 to m/z=1800. A series of ions (bold) were discovered in the Sterne HF-PS that were absent from ΔcsaB HF-PS and differed in mass ratio from ions present in both by m/z=70, the mass ratio difference expected from a ketal pyruvate modification.
Table 1.
Ions present in MALDI-TOF spectra from HF-PS extracted from both Sterne and ΔcsaB
| Observed m/z (Sterne PS) a | Observed m/z (ΔcsaB PS) b | Proposed structure | Theoretical m/z (monoisotopic) c | Sterne Δm/z c–a | ΔcsaB Δm/z c–b |
|---|---|---|---|---|---|
| 1118.3309 | 1118.3593 | [GlcNAc2ManNAc1Gal3−H2O+Na]+ | 1118.3843 | 0.0534 | 0.0250 |
| 1136.3836 | 1136.3666 | [GlcNAc2ManNAc1Gal3 GlcNAc2ManNAc1Gal3+Na]+ | 1136.3948 | 0.0112 | 0.0282 |
| 1152.3512 | 1152.3607 | [GlcNAc2ManNAc1Gal3+O+Na]+ | 1152.3897 | 0.0385 | 0.0290 |
| 1178.3952 | 1178.3367 | [GlcNAc2ManNAc1GalOAcGal2+Na]+ | 1178.4053 | 0.0101 | 0.0686 |
| 1321.3619 | 1321.4404 | [GlcNAc3ManNAc1Gal3−H2O+Na]+ | 1321.4633 | 0.1014 | 0.0229 |
| 1339.4456 | 1339.4075 | [GlcNAc3ManNAc1Gal3+Na]+ | 1339.4738 | 0.0282 | 0.0663 |
| 1362.4947 | 1362.4520 | [GlcNAc3ManNAc2Gal2−H2O+Na]+ | 1362.4898 | −0.0049 | 0.0378 |
| 1380.5203 | 1380.4245 | [GlcNAc3ManNAc2Gal2+Na]+ | 1380.5003 | −0.0200 | 0.0758 |
| 1403.4946 | 1403.4623 | [GlcNAc3ManNAc2Gal2+2Na]+ | 1403.4901 | −0.0045 | 0.0278 |
| 1421.5325 | 1421.4854 | [GlcNAc4ManNAc2Gal1+Na]+ | 1421.5268 | −0.0057 | 0.0414 |
| 1483.4787 | 1483.4963 | [GlcNManNGlcNAc3ManNAc1Gal2−O+Na]+ | 1483.5634 | 0.0847 | 0.0671 |
| 1499.5223 | 1499.5162 | [GlcNManNGlcNAc3ManNAc1Gal2+Na]+ | 1499.5583 | 0.0360 | 0.0421 |
| 1524.5672 | 1524.5162 | [GlcNAc3ManNAc1Gal4+2Na]+ | 1524.5161 | −0.0511 | −0.0001 |
| 1542.6977 | 1542.5310 | [GlcNAc3ManNAc2Gal3+Na]+ | 1542.5528 | −0.1449 | 0.0218 |
| 1565.5451 | 1565.5510 | [GlcNAc3ManNAc2Gal3+2Na]+ | 1565.5426 | −0.0025 | −0.0084 |
| 1583.6014 | 1583.5364 | [GlcNAc4ManNAc2Gal2+Na]+ | 1583.5793 | −0.0221 | 0.0429 |
| 1625.6340 | 1625.5233 | [GlcNAc4ManNAc2GalOAcGal+Na]+ | 1625.5898 | −0.0442 | 0.0665 |
| 1661.6583 | 1661.5688 | [GlcNManNGlcNAc3ManNAc1Gal3+Na]+ | 1661.6108 | −0.0475 | 0.0420 |
| 1686.5755 | 1686.5566 | [GlcNAc3ManNAc1Gal5+2Na]+ | 1686.5686 | −0.0069 | 0.0120 |
| 1703.7262 | 1703.6128 | [GlcNGlcNAc3ManNAc2Gal3+Na]+ | 1703.6213 | −0.1049 | 0.0085 |
| 1727.6852 | 1727.6150 | [GlcNAc3ManNAc2Gal4+2Na]+ | 1727.5951 | −0.0901 | −0.0199 |
| 1745.7051 | 1745.5923 | [GlcNAc4ManNAc2Gal3+Na]+ | 1745.6318 | −0.0733 | 0.0395 |
| 1848.6754 | 1848.6306 | [GlcNAc3ManNAc1Gal6+2Na]+ | 1848.6211 | −0.0543 | −0.0095 |
| 1865.7980 | 1865.6704 | [GlcNGlcNAc3ManNAc2Gal4+2Na]+ | 1865.6738 | −0.1242 | 0.0034 |
| 1889.6858 | 1889.6458 | [GlcNAc3ManNAc2Gal5+2Na]+ | 1889.6476 | −0.0382 | 0.0018 |
| 1907.7384 | 1907.6608 | [GlcNAc4ManNAc2Gal6+Na]+ | 1907.6843 | −0.0541 | 0.0235 |
| 1931.7430 | 1931.6667 | [GlcNGlcNAc2ManNAc2Gal6+2Na]+ | 1931.6581 | −0.0849 | −0.0086 |
| 2009.7289 | 2009.7438 | [GlcNGlcNAc2ManNAc2Gal5+2Na]+ | 2009.6896 | −0.0393 | −0.0542 |
| 2027.6312 | 2027.7323 | [GlcNGlcNAc3ManNAc2Gal5+Na]+ | 2027.7263 | 0.0951 | −0.0060 |
| 2051.7870 | 2051.7031 | [GlcNAc3ManNAc2Gal5+2Na]+ | 2051.7001 | −0.0869 | −0.0030 |
| 2069.8491 | 2069.7141 | [GlcNAc4ManNAc2Gal5+Na]+ | 2069.7368 | −0.1123 | 0.0227 |
| 2093.8138 | 2093.7082 | [GlcNAc3ManNAc2GalOAcGal5+2Na]+ | 2093.7106 | −0.1032 | 0.0024 |
| 2111.8258 | 2111.7463 | [GlcNAc4ManNAc2GalOAcGal6+Na]+ | 2111.7473 | −0.0785 | 0.0010 |
| 2189.8865 | 2189.7595 | [GlcNGlcNAc3ManNAc2Gal6+Na]+ | 2189.7788 | −0.1077 | 0.0193 |
| 2213.8951 | 2213.7842 | [GlcNAc4ManNAc2Gal6−H2O+Na]+ | 2213.7788 | −0.1163 | −0.0054 |
| 2231.8272 | 2231.7766 | [GlcNAc4ManNAc2Gal6+Na]+ | 2231.7893 | −0.0379 | 0.0127 |
| 2254.8276 | 2254.7766 | [GlcNAc4ManNAc2Gal6+2Na]+ | 2254.7791 | −0.0485 | 0.0025 |
| 2274.8674 | 2274.7607 | [GlcNAc3ManNAc2GalOAc2Gal5+Na]+ | 2274.7838 | −0.0836 | 0.0231 |
| 2294.8876 | 2294.7683 | [GlcNAc3ManNAc2GalOAc3Gal4+H]+ | 2294.8123 | −0.0753 | 0.0440 |
| 2312.9262 | 2312.8281 | [GlcNGlcNAc5ManNAc3Gal3+Na]+ | 2312.8583 | −0.0679 | 0.0302 |
| 2354.9798 | 2354.8459 | [GlcNAc6ManNAc3Gal3+Na]+ | 2354.8688 | −0.1110 | 0.0229 |
| 2432.9039 | 2432.8970 | [GlcN2GlcNAc4ManNAc3Gal4+Na]+ | 2432.9003 | −0.0036 | 0.0033 |
| 2456.9717 | 2456.8613 | [GlcNGlcNAc4ManNAc3Gal5+2Na]+ | 2456.8741 | −0.0976 | 0.0128 |
| 2499.1209 | 2498.8989 | [GlcNAc5ManNAc3Gal5+Na]+ | 2498.8846 | −0.2363 | −0.0143 |
| 2517.0349 | 2516.9009 | [GlcNAc6ManNAc3Gal4+Na]+ | 2516.9213 | −0.1136 | 0.0204 |
| 2577.0763 | 2576.8870 | [GlcN2GlcNAc2ManNAc2Gal5+2Na]+ | 2576.9161 | −0.1602 | 0.0291 |
| 2595.0364 | 2594.9209 | [GlcN2GlcNAc4ManNAc2Gal5+Na]+ | 2594.9528 | −0.0836 | 0.0319 |
| 2620.1232 | 2619.8814 | [GlcNAc5ManNAc2Gal7+2Na]+ | 2619.9106 | −0.2126 | 0.0292 |
| 2637.0117 | 2636.9578 | [GlcNGlcNAc5ManNAc3Gal5+Na]+ | 2636.9633 | −0.0484 | 0.0055 |
| 2660.9568 | 2660.9664 | [GlcNAc5ManNAc3Gal6+2Na]+ | 2660.9371 | −0.0197 | −0.0293 |
| 2800.2063 | 2800.0208 | [GlcNAc5ManNAc3Gal7+Na]+ | 2799.9998 | −0.2065 | −0.0210 |
| 2823.1167 | 2823.0181 | [GlcNAc5ManNAc3Gal7+2Na]+ | 2822.9896 | −0.1271 | −0.0285 |
| 2841.2496 | 2841.0042 | [GlcNAc6ManNAc3Gal6+Na]+ | 2841.0263 | −0.2233 | 0.0221 |
| 2862.1650 | 2862.1125 | [GlcNAc5ManNAc3GalOAc2Gal5+H]+ | 2862.0388 | −0.1262 | −0.0737 |
| 2964.3171 | 2964.0210 | [GlcNAc4ManNAc2GalOAc2Gal8+Na]+ | 2964.0203 | −0.2968 | −0.0007 |
| 2987.2883 | 2987.0659 | [GlcNAc5ManNAc2GalOAc2Gal7−H2O+Na]+ | 2987.0363 | −0.2520 | −0.0296 |
| 3003.1480 | 3003.0693 | [GlcNAc6ManNAc3Gal7+Na]+ | 3003.0788 | −0.0692 | 0.0095 |
| 3045.2293 | 3045.0647 | [GlcNAc6ManNAc3GalOAcGal6+Na]+ | 3045.0893 | −0.1400 | 0.0246 |
| 3165.3916 | 3165.1147 | [GlcNAc6ManNAc3Gal8+Na]+ | 3165.1313 | −0.2603 | 0.0166 |
| 3309.3999 | 3309.1826 | [GlcNAc6ManNAc3Gal9−H2O+Na]+ | 3309.1733 | −0.2266 | −0.0093 |
| 3327.3966 | 3327.2034 | [GlcNAc6ManNAc3Gal9+Na]+ | 3327.1838 | −0.2128 | −0.0196 |
| 3367.4485 | 3367.2002 | [GlcNGlcNAc6ManNAc4Gal7+Na]+ | 3367.2263 | −0.2222 | 0.0261 |
| 3732.6504 | 3732.3103 | [GlcNGlcNAc7ManNAc4Gal8+Na]+ | 3732.3578 | −0.2926 | 0.0475 |
| 4098.6257 | 4098.4512 | [GlcNAc8ManNAc4Gal10+Na]+ | 4098.4733 | −0.1524 | 0.0221 |
| 4221.7969 | 4221.5103 | [GlcNAc10ManNAc5Gal7+Na]+ | 4221.5528 | −0.2441 | 0.0425 |
| 4260.5003 | 4260.5073 | [GlcNAc8ManNAc4Gal11+Na]+ | 4260.5258 | 0.0255 | 0.0185 |
| 4422.4986 | 4422.6673 | [GlcNAc8ManNAc4Gal12+Na]+ | 4422.5783 | 0.0797 | −0.0890 |
Table 2.
Ions present only in MALDI-TOF spectra of HF-PS extracted from Sterne and predicted to contain a ketal pyruvate
| Observed m/z (Sterne PS) a | Proposed structure | Theoretical m/z (monoisotopic) b | Sterne Δm/z b–a |
|---|---|---|---|
| 1247.3360 | [GlcNAc3ManNAc1Gal2kPyr+Na]+ | 1247.4267 | 0.0907 |
| 1451.6573 | [GlcNAc3ManNAc1GalOAcGal2kPyr+Na]+ | 1451.4897 | −0.1676 |
| 1409.5652 | [GlcNAc3ManNAc1Gal3kPyr+Na]+ | 1409.4792 | −0.0860 |
| 1612.6528 | [GlcNAc3ManNAc2Gal3kPyr+Na]+ | 1612.5582 | −0.0946 |
| 1653.5932 | [GlcNAc4ManNAc2Gal2kPyr+Na]+ | 1653.5847 | −0.0085 |
These data led to the hypothesis that the SCWP is pyruvylated and that it serves as the SLH ligand polysaccharide. To test this, and to confirm the structural assignment of the polysaccharides, we subjected the SCWP and its pyruvylated forms to high-resolution MS to generate elemental compositional data and therefore provide a test of the hypothesis. Polysaccharides were purified from B. anthracis Sterne as before, and fractions were analyzed off-line by electrospray ionization interfaced with a Fourier transform mass spectrometer (ESI-FTMS). The 115 most intense monoisotopic species in the spectra (Fig. 6a) were within 4 mDa of the masses calculated for the corresponding predicted structures (Table 3); without exception, all of these masses could be explained as structural permutations of the SCWP.
Fig. 6.
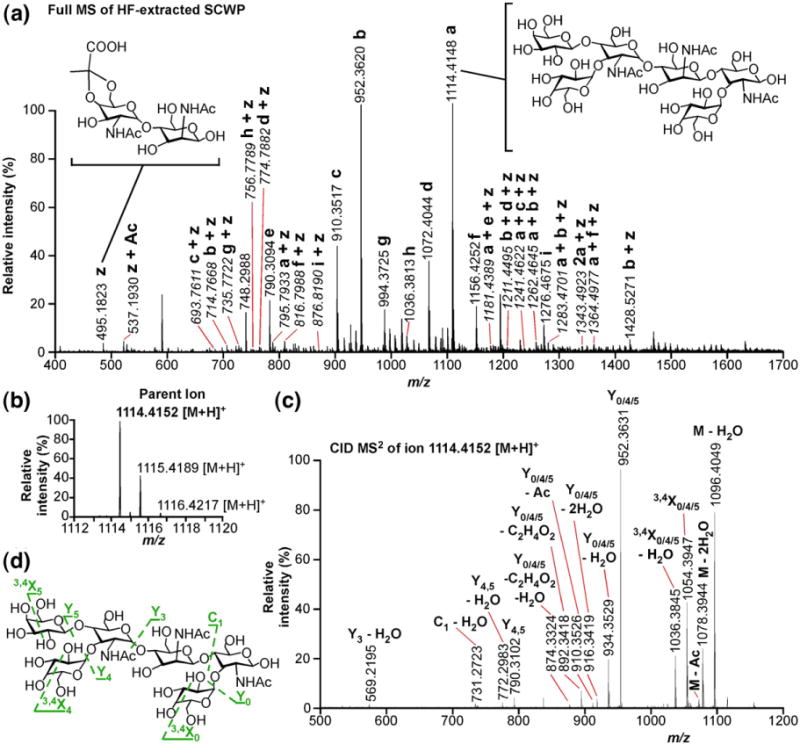
High-resolution FTMS of the HF-PS from Sterne. (a) Full mass spectrum of HF-extracted, SLH ligand polysaccharide from B. anthracis Sterne. The monoisotopic masses and structures of 115 species are given in Table 3. Ion a is the most abundant and represents the repeating SCWP hexasaccharide (right inset structure); ions b–i arise from structural perturbation of (a). Notably, many less abundant ions have structures composed of a–i and z, which comprise two N-acetylated amino hexose sugars and a ketal pyruvate (right inset structure). Ions whose m/z ratios are italicized are doubly charged (z=2). (b) Isolated parent ion m/z=1114.4152. (c) Collision-induced disassociation (CID) tandem MS of m/z=1114.4152 ion generates 15 major daughter ions. (d) Structure of m/z=1114.4152 parent ion and CID events that yield the daughter ions labeled in (b).
Table 3.
Summary of monoisotopic ions present in FTMS of HF-PS extracted from B. anthracis Sterne
| Observed (monoisotopic) m/z a | Proposed structure | Theoretical (monoisotopic) m/z b | Dm/z b–a |
|---|---|---|---|
| 495.1823 | [GlcNAc-ManNAc-kPyr+H]+ | 495.1817 | −0.0006 |
| 537.1930 | [GlcNAc-ManOAcNAc-kPyr+H]+ | 537.1922 | −0.0008 |
| 586.2456 | [GlcN-GlcNAc-ManNAc+H]+ | 586.2448 | −0.0008 |
| 628.2561 | [GlcNAc2-ManNAc+H]+ | 628.2553 | −0.0008 |
| 693.7611 | [GlcN-GlcNAc2-ManNAc2-Gal2-kPyr+2H]2+ | 693.7605 | −0.0006 |
| 706.2882 | [GlcNAc2-ManNAc-Gal+H]+ | 706.2868 | −0.0014 |
| 714.7668 | [GlcNAc3-ManNAc2-Gal2-kPyr+2H]2+ | 714.7658 | −0.0010 |
| 730.2882 | [GlcN-GlcNAc-ManNAc-Gal−H2O+H]+ | 730.2868 | −0.0014 |
| 735.7722 | [GlcNAc3-ManNAc2-GalOAc-Gal-kPyr+2H]2+ | 735.7710 | −0.0012 |
| 739.2940 | [GlcN2-GlcNAc2-ManNAc2-Gal2+2H]2+ | 739.2921 | −0.0019 |
| 748.2988 | [GlcN-GlcNAc-ManNAc-Gal+H]+ | 748.2973 | −0.0015 |
| 756.7789 | [GlcNAc3-ManNAc2-GalOAc2-kPyr+2H]2+ | 756.7763 | −0.0026 |
| 760.2993 | [GlcN-GlcNAc3-ManNAc2-Gal2+2H]2+ | 760.2973 | −0.0020 |
| 772.2995 | [GlcNAc2-ManNAc-Gal−H2O+H]+ | 772.2973 | −0.0022 |
| 774.7882 | [GlcN-GlcNAc3-ManNAc1-Gal3-kPyr+2H]2+ | 774.7868 | −0.0014 |
| 790.3094 | [GlcNAc2-ManNAc-Gal+H]+ | 790.3078 | −0.0016 |
| 795.7933 | [GlcNAc3-ManNAc2-Gal3-kPyr+2H]2+ | 795.7920 | −0.0013 |
| 816.7988 | [GlcNAc3-ManNAc2-GalOAc-Gal2-kPyr+2H]2+ | 816.7973 | −0.0015 |
| 820.3196 | [GlcN2-GlcNAc2-ManNAc2-Gal3+2H]2+ | 820.3183 | −0.0013 |
| 829.3262 | [GlcN2-GlcNAc2-ManNAc2-Gal3+H2O+2H]2+ | 829.3236 | −0.0026 |
| 832.3202 | [GlcNAc2-ManNAc1-GalOAc+H]+ | 832.3183 | −0.0019 |
| 837.8029 | [GlcNAc3-ManNAc2-GalOAc2-Gal-kPyr+2H]2+ | 837.8025 | −0.0004 |
| 841.3248 | [GlcN-GlcNAc3-ManNAc2-Gal3+2H]2+ | 841.3236 | −0.0012 |
| 850.3314 | [GlcN-GlcNAc3-ManNAc2-Gal3+H2O+2H]2+ | 850.3288 | −0.0026 |
| 862.3310 | [GlcNAc4-ManNAc2-Gal3+2H]2+ | 862.3288 | −0.0022 |
| 876.8190 | [GlcNAc3-ManNAc2-Gal4-kPyr+2H]2+ | 876.8183 | −0.0007 |
| 892.3413 | [GlcN-GlcNAc-ManNAc-Gal2−H2O+H]2+ | 892.3393 | −0.0020 |
| 897.8234 | [GlcNAc3-ManNAc2-GalOAc-Gal3-kPyr+2H]2+ | 897.8235 | 0.0001 |
| 901.3466 | [GlcN2-GlcNAc2-ManNAc2-Gal4-kPyr+2H]2+ | 901.3446 | −0.0020 |
| 910.3517 | [GlcN-GlcNAc-ManNAc-Gal2+H]+ | 910.3498 | −0.0019 |
| 922.3520 | [GlcN-GlcNAc3-ManNAc2-Gal4+2H]2+ | 922.3498 | −0.0022 |
| 931.3559 | [GlcN-GlcNAc3-ManNAc2-Gal4+H2O+2H]2+ | 931.3551 | −0.0009 |
| 934.3518 | [GlcNAc2-ManNAc-Gal2−H2O+H]+ | 934.3498 | −0.0020 |
| 943.3567 | [GlcNAc4-ManNAc2-Gal4+2H]2+ | 943.3551 | −0.0017 |
| 952.3620 | [GlcNAc2-ManNAc-Gal2+H]+ | 952.3603 | −0.0017 |
| 964.3620 | [GlcNAc4-ManNAc2-GalOAc-Gal3+2H]2+ | 964.3603 | −0.0017 |
| 973.3656 | [GlcN-GlcNAc3-ManNAc2-Gal5−C2H4O2+2H]2+ | 973.3656 | 0.0000 |
| 976.3637 | [GlcNAc2-ManNAc-GalOAc-Gal−H2O+H]+ | 976.3603 | −0.0034 |
| 982.3722 | [GlcN-GlcNAc3-ManNAc2-Gal4+2H]2+ | 982.3708 | −0.0014 |
| 991.3777 | [GlcN-GlcNAc3-ManNAc2-Gal4+H2O+2H]2+ | 991.3761 | −0.0017 |
| 994.3725 | [GlcNAc2-ManNAc-GalOAc-Gal+H]+ | 994.3708 | −0.0017 |
| 1003.3776 | [GlcN-GlcNAc2-ManNAc2-Gal5+2H]2+ | 1003.3761 | −0.0016 |
| 1012.3820 | [GlcN-GlcNAc2-ManNAc2-Gal5+H2O+2H]2+ | 1012.3813 | −0.0007 |
| 1015.3763 | [GlcNAc4-ManNAc2-Gal5−H2O+2H]2+ | 1015.3761 | −0.0003 |
| 1024.3831 | [GlcNAc4-ManNAc2-Gal5+2H]2+ | 1024.3813 | −0.0018 |
| 1032.8964 | [GlcN-GlcNAc4-ManNAc2-Gal4+H2O+2H]2+ | 1032.8946 | −0.0019 |
| 1036.3817 | [GlcNAc2-ManNAc-GalOAc2+H]+ | 1036.3813 | −0.0004 |
| 1045.3883 | [GlcN-GlcNAc4-ManNAc2-Gal4+H2O+2H]2+ | 1045.3866 | −0.0018 |
| 1054.3920 | [GlcN2-GlcNAc2-ManNAc2-Gal6−H2O+2H]2+ | 1054.3918 | −0.0002 |
| 1063.3987 | [GlcN2-GlcNAc2-ManNAc2-Gal6+2H]2+ | 1063.3971 | −0.0016 |
| 1066.3956 | [GlcNAc4-ManNAc2-GalOAc2-Gal3+2H]2+ | 1066.3918 | −0.0038 |
| 1072.4044 | [GlcN-GlcNAc-ManNAc-Gal2+H]+ | 1072.4023 | −0.0021 |
| 1084.4040 | [GlcN2-GlcNAc2-ManNAc2-Gal6+2H]2+ | 1084.4023 | −0.0017 |
| 1092.9188 | [GlcN2-GlcNAc3-ManNAc2-Gal7+H2O+2H]2+ | 1092.9156 | −0.0032 |
| 1096.4043 | [GlcNAc3-ManNAc-Gal3−H2O+H]+ | 1096.4023 | −0.0020 |
| 1100.4114 | [GlcNAc5-ManNAc3-Gal3-kPyr+2H]2+ | 1100.4105 | −0.0009 |
| 1105.4093 | [GlcNAc4-ManNAc2-Gal6+2H]2+ | 1105.4076 | −0.0018 |
| 1114.4148 | [GlcNAc2-ManNAc1-Gal3+H]+ | 1114.4128 | −0.0020 |
| 1121.4158 | [GlcNAc5-ManNAc3-GalOAc-Gal2-kPyr + 2H]2+ | 1121.4158 | −0.0001 |
| 1126.4155 | [GlcNAc4-ManNAc2-GalOAc-Gal5+2H]2+ | 1126.4128 | −0.0027 |
| 1131.4420 | [GlcN-GlcNAc2-ManNAc-Gal2+H2O+H]+ | 1131.4393 | −0.0027 |
| 1134.9285 | [GlcNAc5-ManNAc2-Gal5+H2O+2H]2+ | 1134.9261 | −0.0024 |
| 1138.4152 | [GlcNAc2-ManNAc1-GalOAc-Gal2−H2O+H]+ | 1138.4128 | −0.0024 |
| 1147.4144 | [GlcNAc4-ManNAc2-GalOAc2-Gal4+2H]2+ | 1147.4181 | 0.0037 |
| 1156.4252 | [GlcNAc2-ManNAc1-GalOAc-Gal2+H]+ | 1156.4233 | −0.0019 |
| 1165.4297 | [GlcN-GlcNAc3-ManNAc2-Gal5+2H]2+ | 1165.4286 | −0.0011 |
| 1169.4402 | [GlcN-GlcNAc4-ManNAc3-Gal4-kPyr+H2O+2H]2+ | 1169.4368 | −0.0034 |
| 1174.4331 | [GlcN-GlcNAc3-ManNAc2-Gal7+H2O+2H]2+ | 1174.4338 | 0.0007 |
| 1181.4389 | [GlcNAc5-ManNAc3-Gal4-kPyr+2H]2+ | 1181.4368 | −0.0022 |
| 1186.4353 | [GlcNAc4-ManNAc2-Gal7+2H]2+ | 1186.4338 | −0.0015 |
| 1190.4426 | [GlcNAc5-ManNAc3-Gal4-kPyr+H2O + 2H]2+ | 1190.4420 | −0.0006 |
| 1194.9508 | [GlcN-GlcNAc4-ManNAc2-Gal6+H2O+2H]2+ | 1194.9471 | −0.0038 |
| 1198.4353 | [GlcNAc2-ManNAc1-GalOAc2-Gal+H]+ | 1198.4338 | −0.0015 |
| 1202.4457 | [GlcNAc5-ManNAc3-GalOAc-Gal3-kPyr+2H]2+ | 1202.4420 | −0.0037 |
| 1211.4495 | [GlcNAc5-ManNAc3-GalOAc-Gal3-kPyr+H2O+2H]2+ | 1211.4473 | −0.0023 |
| 1214.9717 | [GlcN2-GlcNAc4-ManNAc3-Gal4+H2O+2H]2+ | 1214.9683 | −0.0034 |
| 1234.4578 | [GlcN-GlcNAc-ManNAc1-Gal4+H]+ | 1234.4548 | −0.0030 |
| 1241.4622 | [GlcN-GlcNAc4-ManNAc3-Gal5-kPyr+2H]2+ | 1241.4578 | −0.0044 |
| 1250.4643 | [GlcN-GlcNAc4-ManNAc3-Gal5-kPyr+H2O+2H]2+ | 1250.4630 | −0.0013 |
| 1262.4645 | [GlcNAc5-ManNAc3-Gal5-kPyr+2H]2+ | 1262.4630 | −0.0015 |
| 1266.4742 | [GlcNAc3-ManNAc2-Gal1-kPyr+H]+ | 1266.4712 | −0.0030 |
| 1271.4719 | [GlcNAc5-ManNAc3-Gal5-kPyr+H2O+2H]2+ | 1271.4683 | −0.0037 |
| 1276.4675 | [GlcNAc2-ManNAc1-Gal4+H]+ | 1276.4653 | −0.0022 |
| 1283.4701 | [GlcNAc5-ManNAc3-GalOAc-Gal4-kPyr+2H]2+ | 1283.4683 | −0.0019 |
| 1292.4777 | [GlcNAc5-ManNAc3-GalOAc-Gal4-kPyr+H2O+2H]2+ | 1292.4735 | −0.0042 |
| 1308.4826 | [GlcNAc5-ManNAc3-Gal6+2H]2+ | 1308.4866 | 0.0040 |
| 1304.4719 | [GlcNAc5-ManNAc3-GalOAc2-Gal3-kPyr+2H]2+ | 1304.4735 | 0.0016 |
| 1313.4803 | [GlcNAc5-ManNAc3-GalOAc2-Gal3-kPyr+H2O+2H]2+ | 1313.4788 | −0.0015 |
| 1322.4853 | [GlcN2-GlcNAc3-ManNAc3-Gal6-kPyr+2H]2+ | 1322.4840 | −0.0013 |
| 1329.0025 | [GlcNAc6-ManNAc3-Gal5+2H]2+ | 1328.9998 | −0.0027 |
| 1331.4937 | [GlcN2-GlcNAc3-ManNAc3-Gal6-kPyr+H2O+2H]2+ | 1331.4893 | −0.0044 |
| 1343.4923 | [GlcNAc5-ManNAc3-Gal6-kPyr+2H]2+ | 1343.4893 | −0.0030 |
| 1352.5004 | [GlcNAc5-ManNAc3-Gal6-kPyr+H2O+2H]2+ | 1352.4945 | −0.0059 |
| 1364.4977 | [GlcNAc5-ManNAc3-GalOAc-Gal5-kPyr+2H]2+ | 1364.4945 | −0.0032 |
| 1373.0083 | [GlcNAc6-ManNAc3-Gal5-kPyr+H2O+2H]2+ | 1373.0078 | −0.0005 |
| 1377.0226 | [GlcN2-GlcNAc4-ManNAc3-Gal6+H2O+2H]2+ | 1377.0208 | −0.0018 |
| 1385.4979 | [GlcNAc5-ManNAc3-GalOAc2-Gal4-kPyr+2H]2+ | 1385.4998 | 0.0018 |
| 1389.0226 | [GlcN-GlcNAc5-ManNAc3-Gal6+2H]2+ | 1389.0208 | −0.0018 |
| 1398.0293 | [GlcN-GlcNAc5-ManNAc3-Gal6+H2O+2H]2+ | 1398.0261 | −0.0032 |
| 1410.0305 | [GlcNAc6-ManNAc3-Gal6+2H]2+ | 1410.0261 | −0.0045 |
| 1419.0344 | [GlcNAc6-ManNAc3-Gal6+H2O+2H]2+ | 1419.0313 | −0.0031 |
| 1428.5271 | [GlcNAc3-ManNAc2-Gal2-kPyr+H]+ | 1428.5237 | −0.0034 |
| 1470.0510 | [GlcN-GlcNAc5-ManNAc3-Gal7+2H]2+ | 1470.0471 | −0.0040 |
| 1479.0597 | [GlcN-GlcNAc5-ManNAc3-Gal7+H2O+2H]2+ | 1479.0523 | −0.0074 |
| 1491.0571 | [GlcNAc6-ManNAc3-Gal7+2H]2+ | 1491.0523 | −0.0048 |
| 1500.0636 | [GlcNAc6-ManNAc3-Gal7+H2O+2H]2+ | 1500.0576 | −0.0060 |
| 1512.5538 | [GlcNAc3-ManNAc2-GalOAc2-kPyr+H]+ | 1512.5447 | −0.0091 |
| 1551.0746 | [GlcN-GlcNAc5-ManNAc3-Gal8+2H]2+ | 1551.0733 | −0.0013 |
| 1560.0836 | [GlcN-GlcNAc5-ManNAc3-Gal8+H2O+2H]2+ | 1560.0786 | −0.0050 |
| 1572.0835 | [GlcNAc6-ManNAc3-Gal8+2H]2+ | 1572.0786 | −0.0050 |
| 1581.0867 | [GlcNAc6-ManNAc3-Gal8+H2O+2H]2+ | 1581.0838 | −0.0029 |
| 1590.5824 | [GlcN2-GlcNAc3-ManNAc3-Gal10+2H]2+ | 1590.5811 | −0.0014 |
| 1632.5934 | [GlcNAc5-ManNAc3-Gal10+2H]2+ | 1632.5916 | −0.0018 |
| 1653.1103 | [GlcNAc6-ManNAc3-Gal9+2H]2+ | 1653.1048 | −0.0055 |
| 1662.1193 | [GlcNAc6-ManNAc3-Gal9+H2O+2H]2+ | 1662.1101 | −0.0093 |
Ions in bold contain a ketal pyruvate.
The most abundant ion in the FTMS data set (m/z=1114.4148) corresponds to the repeating unit of the SCWP, a singly charged, protonated hexasaccharide (Fig. 6a, a); structural variants of this oligosaccharide comprise the remainder of the most abundant ions (b, c, d, e, f, g, h, and i). Interestingly, the ESI-FTMS spectra revealed an ion (Fig. 6a, z), the proposed structure of which includes two acetylated amino sugar residues and a ketal pyruvate. In total, 37 of the 115 ions identified in the ESI-FTMS spectra are proposed to contain a ketal pyruvate and two HexNAc residues in addition to the SCWP (Table 3, bold). These high-resolution data further support the hypothesis that pyruvylated SCWP is the SLH ligand. A representative structure for z is given as an inset in Fig. 6a. Although the pyruvylated SCWP ions were insufficiently intense for tandem mass spectrometric analysis, for every major variant of the SCWP, we observed the same ion modified by the addition of a ketal pyruvate and two acetylated, amino sugars (Fig. 6a, a+z, b+z, c+z, d+z, e+z, f+z, g+z, h+z, i+z).
Collision-induced disassociation tandem mass spectra (CID-MS2) of ion a (m/z = 1114.4152, Fig. 6b) generated 15 major fragment ions (Fig. 6c), all of which could be plausibly assigned as arising from simple or known cleavages: loss of an acetyl residue, dehydration, cleavage across a glycosidic bond (Y, C ions), or cross-ring cleavages (X ions) (Fig. 6d). The most abundant fragment ion (b, m/z=952.3631), postulated to arise from loss of any one of three galactosyl residues, is also present in the full FTMS spectrum. This ion was isolated and analyzed by CID-MS2 as well (Fig. S2), further supporting our assignment of the SLH ligand polysaccharide as the SCWP.
Finally, because the pyruvylated forms of the SCWP generated less intense signals than their unmodified counterparts, we performed a narrow select ion monitoring (nSIM) experiment to accurately measure and document the mass of these compounds. Four of the most abundant ions (m/z = 693.7611, 714.7668, 735.7722, and 816.7988) whose predicted structures included a ketal pyruvate were selected and analyzed. The nSIM spectra of these species produced an accurate and robust mass measurement, confirming their hypothesized structures (Fig. 7).
Fig. 7.
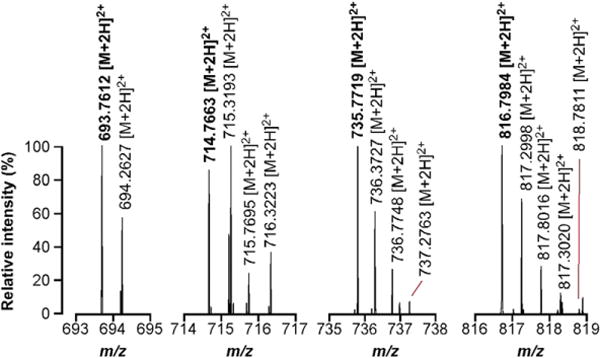
nSIM measurement of pyruvylated forms of the SCWP. Four independent nSIM experiments were performed to accurately measure the mass of four pyruvylated forms of the SCWP and, therefore, to confirm our structural model. Monoisotopic signals are displayed in bold; peaks with m/z values increasing by 0.5 amu represent isotopic variants of the same compound.
Initial work on SLH assembly concluded that a cell wall polysaccharide containing a ketal pyruvate served as the SLH ligand.6,9 This conclusion was based on the presence of chemical shifts in one- and two-dimensional NMR spectra diagnostic of a ketal pyruvate.6,9–11 These studies did not, however, identify the larger structure of this polysaccharide. 1H NMR spectra from both B. anthracis Sterne and ΔcsaB polysaccharides were used to compare the polysaccharides we purified to those previously published (Fig. S3). The spectrum obtained from the Sterne strain differs from its isogenic ΔcsaB strain by a proton shift, δ 1.54 ppm, which arises from the methyl group attached to the ketal carbon. Our spectra bear striking resemblance to those of SCWP, further supporting the model that pyruvylated SCWP is the SLH ligand and confirming that the NMR spectra generated in these earlier studies arose from samples containing the SCWP.6,12
Although several structural studies on the SCWP have been published, none of these studies demonstrated pyruvyl moieties in this polymer; neither have these studies identified other gene products required for its synthesis. Our mass spectra unequivocally identify this material as the SCWP, whose structure has been solved by NMR and linkage analysis, and provide the first direct evidence linking the SCWP structure and csaB-mediated pyruvylation. We observed a perfect correlation between pyruvylation of the SCWP and the addition of two acetylated amino sugars, demonstrating that the SCWP is modified by the addition of a unit structure in order to become an SLH ligand. Moreover, our purification strategy correlates structure (ketal pyruvate) with function (SLH binding) and advances testable hypotheses as to the attachment location of the ketal pyruvate to the polysaccharide (Fig. S4).
Tunicamycin inhibits TagO and subsequent SLH-binding, implicating tagO in the assembly of the SCWP
We aimed to identify the gene products required for the synthesis of the SLH ligand polysaccharide or S-layer assembly. With the exception of csaB, no other gene products have been assigned an essential role in the assembly of this polymer. Previous work suggested that SCWP is essential for growth of B. anthracis.13 We reasoned that because csaB modifies the SCWP, it might be genetically linked to genes required for its biosynthesis in SLH-domain-containing organisms. Members of the TagA/WecB/CpsF family of glycosyl transferases are frequently linked to csaB homologs that are themselves associated with SLH proteins in a variety of organisms, including Geobacillus thermodenitrificans NG80-2, Desulfotomaculum reducens MI-1, and Alkaliphilus metalliredigens QYMF. Members of the TagA/WecB/CpsF enzyme superfamily catalyze the transfer of nucleotide-activated sugars into growing polysaccharide chains.21–23 The enzymes with significant linkage to csaB had greatest homology to tagA of Bacillus subtilis and Staphylococcus aureus, an enzyme responsible for the addition of N-acetyl-mannosamine to undecaprenyl-pyrophosphate-N-acetyl-glucosamine, the second step in WTA synthesis.22
B. anthracis does not synthesize glycerol or ribitol phosphate (RibP) WTA.24 Moreover, it lacks homologs of tarI, tarJ, tarL, tagB, tagD, or tagF, genes whose products catalyze activation and polymerization of RibP and glycerol phosphate (GroP) monomers to teichoic acid linkage units. As such, cell walls of B. anthracis do not contain poly(RibP) or poly(GroP). However, the genome of B. anthracis does encode homologs of nearly every other tag gene, including tagO. In S. aureus and in B. subtilis, strains lacking tagB, tagD, tagE, tagF, tagG, tagH, or tagX display a synthetic lethal phenotype if they contain the tagO gene.25,26 These observations beg the question, what product, if any, do the tag genes synthesize in B. anthracis? The close linkage between tagA homologs and csaB homologs in various strains in conjunction with the similarity of the GlcNAc-ManNAc–GlcNAc backbone of the SCWP and the WTA linkage unit of GroP–ManNAc–GlcNAc led us to hypothesize that the tag genes of B. anthracis serve to synthesize and assemble the SCWP.
The ManNAc–GlcNAc moiety of the WTA linkage unit is synthesized by the sequential activity of TagO and TagA.15,16 To test our hypothesis that these enzymes are required for SCWP synthesis, we first sought to delete either tagO or tagA. We were unable to isolate a deletion of either tagO or tagA in the B. anthracis Sterne background, though both of these mutations are tolerated in B. subtilis and S. aureus.25,26 Our inability to mutate these genes is consistent with the nature of SCWP in B. anthracis. Because these efforts to generate conditional expression strains of tagO and tagA were unsuccessful, we applied a pharmacological approach to test our hypothesis.
Tunicamycin is a drug commonly used to inhibit the transfer of activated sugars to sites of protein glycosylation in eurkaryotes, but it is also a potent inhibitor of WTA synthesis.27,28 Nanomolar concentrations of tunicamycin terminate WTA synthesis in S. aureus by inhibiting the activity of TagO.29 To test the requirement of TagO in SCWP synthesis, and subsequent SLH protein binding to the cell wall, we sought to determine what effect tunicamycin exerts on B. anthracis cultures. We grew cultures of B. anthracis with or without the addition of 1 μg mL−1 tunicamycin for 4 h, normalized by OD600 (optical density at 600 nm) and then incubated cells with fluorescently labeled vancomycin [4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY)-vacomycin] (Invitrogen Molecular Probes) or with SapSLH–mCherry. Cultures grown in the presence of 1 μg mL−1 tunicamycin grew more slowly than untreated cultures. Further, the characteristic vegetative forms became coccoid in shape (Fig. 8a). Tunicamycin treatment did not inhibit MraY, which can be subject to inhibition by tunicamycin at high concentration,30 as both treated and untreated bacilli stained abundantly with BODIPY-vancomycin. As expected, BODPIY-vancomycin labeled untreated bacilli most intensely at the division septa, correlated to the foci of peptidoglycan synthesis. In contrast, treated cells displayed increased, delocalized staining throughout the cell wall upon BODPIY-vacomycin labeling (Fig. 8a). Further, a subpopulation of treated cells seemed to exhibit multiple division planes, suggesting that tunicamycin serves to disorganize features of both cell division and peptidoglycan synthesis (arrows Fig. 8a).
Fig. 8.
Treatment of B. anthracis with tunicamycin inhibits SapSLH–mCherry binding to the cell wall and TagO from B. anthracis. (a) Fluorescence micrographs of untreated B. anthracis cells (upper panels) or cells treated with 1 μg mL−1 tunicamycin and then stained with vancomycin-BODIPY (green) or with SapSLH–mCherry. B. anthracis cells treated with tunicamycin form aberrant, rounded shapes and appear to have increased, delocalized peptidoglycan synthesis and multiple division septa (white arrows on green micrographs). Treated cells also bound dramatically less SapSLH–mCherry, requiring much higher exposure times (indicated in the upper right of the micrographs) to visualize any SapSLH–mCherry binding. SapSLH–mCherry labeling occurred only at a few poles (white arrows on red micrographs), presumably those that existed prior to tunicamycin treatment. (b) PAGE analysis of WTA extracts from S. aureus RN4220 and its variants. RN4220 produces abundant WTA, which requires tagO for synthesis. WTA synthesis can be complemented in trans with a plasmid encoding the RN4220 tagO gene (ptagOS.aureus) or with a plasmid encoding the tagO gene from B. anthracis Sterne (ptagOB. anthracis). S. aureus RN4220 ΔtagO ptagOB. anthracis grown in the presence of the indicated concentrations of tunicamycin showed a dose-dependent abrogation of WTA synthesis, indicating that tunicamycin inhibits TagO from B. anthracis. The arrow indicates the position of the bromophenol blue dye front. Lipoteichoic acid synthesis was unaffected regardless of plasmid concentration or growth condition, demonstrating tunicamycin’s specificity for TagO inhibition.
Next, equal numbers of bacilli harvested from treated and untreated conditions were incubated with equimolar concentrations of SapSLH–mCherry as a proxy to measure SCWP production. We observed a dramatic reduction in the amount of staining seen on bacilli treated with tunicamycin (Fig. 8a). Increased exposure time of treated bacilli revealed an unusual staining pattern as compared to untreated cells. Whereas untreated bacilli when incubated with SapSLH–mCherry stain almost uniformly around the cell surface, tunicamycin-treated cells stain only at a few poles (arrows Fig. 8a). The paucity of staining on septa or lateral cell walls of bacilli treated with tunicamycin led us to speculate that envelope areas capable of binding SapSLH–mCherry represent portions of the bacilli that arose before tunicamycin treatment and therefore still contain the SCWP.
Based on these data, we hypothesized that tunicamycin specifically inhibits TagO of B. anthracis, resulting in aberrant forms and a deficiency in SLH protein binding. In order to test if the concentration of tunicamycin used to inhibit SLH binding to the cell wall is sufficient to inhibit the B. anthracis tagO gene product, we employed a heterologous system to measure TagO activity. As tagO and WTA are dispensable for growth in S. aureus, we asked if tagO from B. anthracis is capable of complementing the defect in WTA synthesis of an S. aureus RN4220 ΔtagO strain. The B. anthracis tagO homolog is encoded by BAS5050, and its predicted translation product, TagO, is 40% identical with TagO of S. aureus. WTA was extracted and visualized via PAGE analysis according to previously published protocols.29 Extracts of RN4220 wild-type strains contain abundant quantities of WTA, while the isogenic RN4220 ΔtagO strain produces no WTA (Fig. 8b). This defect is complemented in trans when the ΔtagO strain is transformed with either ptagOS.aureus, a plasmid encoding the S. aureus tagO open reading frame, or ptagOB. anthracis, a plasmid encoding tagO from B. anthracis Sterne (Fig. 8b). These data demonstrate that tagO from B. anthracis is sufficient to restore WTA synthesis in a RN4220 ΔtagO strain and must therefore also catalyze the transfer of UDP-GlcNAc to undecaprenylphosphate.
Given that RN4220 ΔtagO ptagOB. anthracis produces WTA as a function of TagO, we asked if tunicamycin inhibits WTA synthesis in this strain and therefore, by extension, TagO in B. anthracis. Cultures were grown in the presence of increasing concentrations of tunicamycin and normalized by optical density, and WTA extracts were subjected to PAGE analysis. Treatment with tunicamycin yielded a dose-dependent diminution of WTA production, with a concentration of 1 μg mL−1 completely ablating WTA synthesis (Fig. 8b). The production of lipoteichoic acid, an analogous polymer with a distinct synthetic machinery,31 was unaffected by genetic background or growth conditions, an indication of the specificity of tunicamycin for TagO (Fig. 8b). We conclude that TagO from B. anthracis is subject to inhibition by tunicamycin at 1 μg mL−1, and the defect in SLH binding to the cell wall is a direct result of this inhibition.
If, indeed, the TagO-synthesized linkage unit participates in the assembly of the SCWP of B. anthracis, then mass spectra of HF-extracted SCWP should contain ions representing portions of the SCWP attached to the ManNAc–GlcNAc linkage unit. Accordingly, our MALDI spectra contain a series of ions whose proposed structures contain repeats of the SCWP oligosaccharide with the addition of the ManNAc–GlcNAc linkage unit. These ions and their predicted composition are listed in bold in Table 3. Taken together, the data implicate a role for TagO in the synthesis of the SCWP of B. anthracis and potentially other organisms that elaborate similar envelope components. That the tag genetic machinery constitutes a multifunctional pathway, conserved in the synthesis of structurally distinct secondary wall polymers across various bacterial species, is an unexpected and exciting finding, which suggests that these molecules might play a much broader role in Gram-positive envelope biogenesis than previously appreciated.
Discussion
S-layers in Gram-positive eubacteria are often composed of proteins containing three tandem repeats of the SLH domain whose cell surface retention is predicated on an interaction with the cell wall.32 B. anthracis has served as a model system to understand SLH protein assembly onto the cell wall, leading to the identification of csaB, the first gene assigned an essential role in SLH protein retention.6,9 These studies provided evidence that strains harboring csaB pyruvylate a cell wall polysaccharide but did not identify the structure of this polysaccharide. Recently, a series of reports have elucidated the structure of the major SCWPs of the B. cereus sensu lato group, including B. anthracis.12,13 This work did report pyruvyl modifications on these molecules and therefore left unanswered the question, what, if any, pyruvylated polymer serves as the attachment site for S-layer proteins? Here, we report evidence that the SCWP of B. anthracis Sterne, but not ΔcsaB mutants, binds SLH domains and contains pyruvyl modifications. These data finally connect the activity of csaB, the structure of the SCWP, and S-layer assembly. Moreover, we have used tunicamycin for chemical genetic experiments to perturb the activity of TagO and, consequently, the retention of SLH proteins. On the basis of these results, we hypothesize that the tag pathway is required for the production of the SCWP in B. anthracis. Tunicamycin inhibition of TagO not only prevents binding of SLH domains to the cell wall but also induces aberrant, rounded forms sustaining multiple division septa and delocalized peptidoglycan synthesis. Studies in B. subtilis have shown that the WTA is a requirement for the bacillus shape and our data suggest that SCWP plays an analogous role.25,33 B. anthracis encodes homologs of the tag biosynthetic pathway with the exception of genes that are required to produce poly(RibP) or poly(GroP). This genetic information is consistent with the observation that poly(GroP) polymers have not been observed in B. anthracis cell walls. That the tag pathway may synthesize the SCWP in B. anthracis is an unexpected finding with the obvious supposition that the genetic machinery for secondary wall polymers is highly conserved even if the structure of polymers is not. Interestingly, we have not been able to construct null mutants of tagO or tagA, two genes that are dispensable for growth in B. subtilis and S. aureus. It is possible that null mutations in these genes generate synthetic lethal phenotypes analogous to those observed in downstream tag genes in B. subtilis and S. aureus. It is also possible that the SCWP in B. anthracis serves an essential function under the conditions tested and deletion of the synthetic machinery yields non-viable strains. Whether the SCWP is truly an essential molecule remains an area of active investigation.
Identification of the structural requirements for S-layer assembly in Gram-positive bacteria may provide additional avenues for the development of therapeutic countermeasures against emerging pathogens. Furthermore, the ability to target and assemble arrays of proteins in vitro, exploiting the SLH domain and synthetic pyruvylated polysaccharides, would have numerous translational applications in industrial processes. This report provides data that the SLH ligand SCWP is synthesized using the tag pathway and that this pathway may produce an essential polymer in B. anthracis and other organisms. These findings extend the reported functionality of the tag pathway and identify the tag machinery as a target for the design of small-molecule inhibitors, as any such molecule could be another weapon for the arsenal against drug-resistant B. anthracis.
Materials and Methods
Bacterial strains and plasmids
B. anthracis strain Sterne 34F2 or its variants were grown in brain heart infusion broth (BHI) (Difco) or lysis broth (LB) (Difco) at 37 or at 30 °C when transformed with pLM4-derived plasmids. E. coli DH5α and K1077 were grown in LB. S. aureus strain RN4220 and its variants were grown in tryptic soy broth (TSB). Media were supplemented with kanamycin (20 μg mL−1) to maintain plasmid selection in B. anthracis and S. aureus and with kanamycin (50 μg mL−1) and ampicillin (100 μg mL−1) in E. coli where appropriate. In all cases, plasmid DNA used to transform B. anthracis was purified from E. coli K1077 (dcm−/dam−). The B. anthracis strain Sterne ΔcsaB mutant was constructed by allelic replacement with pJK76. B. anthracis Sterne eag-mCherry and ΔcsaB eag-mCherry were constructed by allelic replacement with pJK96. pJK76 was generated by PCR amplifying 1- kb regions upstream (P243: 5′-TTTCCCGGGAGATTTTAAAGATAATGCAAAAAAGATACT-3′/P244: 5′-TTTGGTACCCCGCACTCTTAATCTCCTCCAA-3′) and downstream (P245: 5′-TTTGGTACCTCTTAATTTAAGAGGACATCCTCTTT-3′/P246: 5′ TTTGAATTCTGAATGGAGTGTACCATTGGC-3′) of the csaB open reading frame, restricting these PCR products with XmaI-KpnI and KpnI-EcoRI, respectively, and ligating them with pLM4 vector, which had been previously restricted with XmaI-EcoRI. Ligation products were transformed into E. coli DH5α, plated on selective media, and confirmed by PCR and sequencing analysis. pJK96 was constructed by PCR amplifying 1-kb regions upstream (P289: 5′-GGAATTCCATATGGGTAAATCATTCCCAGACGTTC-3′/P290: 5′-TTTCTCGAGTTTTTTTCCATATTTTTTATCTGTTAATG-3′) and downstream (P305: 5′-TTTGGTACCGTCGATTATAGATAAAGTGAAAAATCAGTG-3′/P306: 5′-TTTGAATTCCTGATGATAAATGGCTCCTGAAA) of the TAA eag stop, restricting these PCR products with XmaI-KpnI and KpnI-EcoRI, respectively, and ligating them with pLM4 vector that had been restricted with XmaI-EcoRI. Ligation products were transformed into DH5a and confirmed by PCR to generate pJK85. pJK85 was then restricted with KpnI and ligated with PCR-amplified, KpnI-restricted mCherry (P283: 5′-TTTGGTACCAGCAAGGGCGAGGAGG-3′/P284: 5′-TTTGGTACCTTACTTGTACAGCTCGTCCATG-3′). Ligation products were transformed into E. coli DH5α and screened by PCR to generate pJK96. B. anthracis transformants of pJK76 and pJK96 were grown at 30 °C overnight and single recombinants were selected by plating at 43 °C overnight on LB agar containing kanamycin. Single colonies were then passed at 30 °C four times serially to induce a second recombination event and loss of pJK76 or pJK96. Mutations were confirmed by PCR. Complementation studies were preformed using pcsaB, which was constructed by amplifying the csaB open reading frame from genomic DNA with P249: 5′-TTTTCTAGAGTGCGGTTAGTTTTATCAGGATATT-3′/P250: 5′-TTTGGTACCTTAAGATCCCATTCCTCTTTTTTTG-3′, restricting amplified DNA and pLM5 plasmid DNA with XbaI-KpnI, ligating restriction products, and transforming ligation products into E. coli DH5α. ptagOS.aureus (pJK196) was constructed by amplifying tagO from S. aureus RN4220 chromosomal DNA using P618: 5′-TTTTCTAGATTAAAAACAATTAATATCGATGAAGGTG-3′/P619: 5′-TTTGGTACCCTATTCCTCTTTATGAGATGACTTACG-3′, restricting amplified DNA and pJK4 plasmid DNA with XbaI-KpnI, ligating the restriction products, and transforming the ligation products into E. coli DH5α. ptagOB. anthracis (pJK156) was constructed by amplifying tagO (BAS5050) from B. anthracis Sterne genomic DNA using P470: 5′-TTTTCTAGAATGGACTCACAAGTGATTTATGC-3′/P471: 5′-TTTGGTACCTTAGTCTTCGCGTTTTTTCACTT-3′, restricting amplified DNA and pJK4 plasmid DNA with XbaI-KpnI, ligating the restriction products, and transforming the ligation products into E. coli DH5α. The mCherry expression vectors were constructed by amplifying mCherry with P287: 5′-TTTCTCGAGAGCAAGGGCGAGGAGG-3′/P288: 5′-TTTGGATCCTTACTTGTACAGCTCGTCCATG-3′ and restricted along with pET16b vector DNA with XhoI-BamHI, ligated, and transformed into DH5α to generate pJK81. pJK81 was then restricted with NdeI-XhoI and ligated with the NdeI-XhoI-restricted, PCR-amplified portion of sap encoding the SLH domains (amino acids 30–210) (P281: 5′-GGAATTCCATATGGGTAAAACATTCCCAGACGTTC- 3 ′/P282 : 5 ′ – TTTCTCGAGTTCTGTACCGAACTGCTTGTCAG-3′) to generate pJK87. These plasmids were transformed into E. coli BL21 (DE3).
Immunoblotting
Media supernatant proteins were prepared by precipitating proteins from culture filtrates of B. anthracis, grown overnight in BHI supplemented with 0.8% NaHCO3, by addition of trichloroacetic acid to a final concentration of 7.5% and incubation on ice for 20 min. Protein pellets were extracted with acetone, dried at room temperature, and dissolved in sample buffer. Sediment proteins were extracted with sample buffer from washed sediments of B. anthracis cultures. These samples were subjected to SDS-PAGE and electrotransferred to polyvinylidene fluoride membranes, and protein species were detected by incubation with specific antisera raised in rabbits against rBslA.8 S. aureus cultures grown overnight in TSB were centrifuged and bacteria were subjected to mechanical lysis and sedimented at 21,000g for 20 min. The sediment was boiled in sample buffer, subjected to SDS-PAGE, transferred to polyvinylidene fluoride, and blotted using a Hycult Clone 55 mouse monoclonal antibody α-lipoteichoic acid (Cell Sciences).
MALDI-TOF, 1H NMR, and FTMS
rpHPLC eluate fractions were dried in a vacuum desiccator and dissolved in 30% acetonitrile/70% water/0.1% trifluoroacetic acid (TFA). These samples were co-spotted with matrix (0.5 μL α-cyano-4-hydroxycinnamic acid) prepared at 10 mg mL−1 in the same solvent supplemented with 4 mM (NH4)2HPO4. MALDI-MS spectra were obtained in a reflectron TOF instrument (ABI Biosystems MALDI 4700) in positive ion, reflectron mode. 1H NMR spectra were collected by the University of Chicago Biological Sciences NMR facility. rpHPLC eluents of HF-PS were dried in a vacuum desiccator and suspended in 2H2O at 100 mg mL−1, and spectra were collected overnight at 600 mHz.
For accurate molecular weight measurements, HF-PS, eluted in 20% acetonitrile/80% water/0.1% TFA from a C18 Sep-Pak cartridge (Waters), were lyophilized and then re-dissolved to 20 mg/mL in 50% acetonitrile/50% water/0.1% TFA and analyzed on a hybrid linear ion-trap 7-T FT-ICR mass spectrometer (LTQ-FT Ultra; Thermo Fisher Corp., San Jose, CA, USA) fitted with an off-line nanospray source. Samples were individually loaded into 2-μm-internal-diameter, externally coated nanospray emitters (Proxeon, Cambridge, MA, USA) and desorbed using a spray voltage of 1.8 kV (versus the inlet of the mass spectrometer). These conditions produced a flow rate of 20–50 nL/min. Ion transmission into the linear trap and further to the ICR cell was automatically controlled, with ion count targets for both full-scan and MS2 experiments of 2×106. The ion trap was operated in the standard mass range (m/z=300–2000), and the resolving power of the FTMS was set at 100,000 (defined by m/Δm 50% at m/z= 400). For MS2 experiments, precursor ions were selected (isolation width, m/z=4–8) and fragmented (collision energy settings, 12–15 at the default activation q-value of 0.25) using helium as the collision gas in the linear ion trap, before transmission to and recording from the ICR cell.
All FT-ICR spectra were obtained by averaging between 50 and 200 transient signals. Precursor masses were calculated using Xtract version 3.0.1.1 (Thermo Scientific, Bremen, Germany) with an S/N threshold of 2, a minimum intensity of 2, a minimum fit of 30, and a remainder threshold of 3. Product ion spectra were processed using ProSight PC version 2 (Thermo Scientific) to produce monoisotopic mass lists (S/N=2; minimum RL value=0.9).
Purification of SapSLH–mCherry and mCherry
E. coli BL21 (DE3) carrying pJK81 or pJK87 were grown in 1-L cultures to an OD600 of 1 and induced with 1 mM IPTG for 3 h at 37 °C, harvested by centrifugation, and suspended in TN buffer (50 mM Tris–HCl, pH 7.5, and 150 mM NaCl). Cell pellets were disrupted by French press and separated into soluble and insoluble fractions by centrifugation. mCherry was purified by affinity chromotragraphy over Ni-NTA beads (Qiagen) from the soluble fraction of lysates from pJK81 cultures according to the manufacturer’s recommendations under native conditions. The insoluble portion of pJK87 cultures was dissolved in Buffer A (6 M guanidine HCl, 10 mM Tris–HCl, and 100 mM NaH2PO4, pH 8.0) and applied to Ni-NTA beads equilibrated with Buffer A. The column was washed with Buffer A and then washed extensively with TN buffer and protein purified according to the manufacturer’s recommendations under native conditions.
Fluorescence micrographs
B. anthracis Sterne and ΔcsaB eag-mCherry strains were grown in BHI overnight at 37 °C with shaking and then harvested by centrifugation, washed with phosphate-buffered saline (PBS), and stained with 1 μg·mL−1 Hoechst in PBS. Digital, confocal images were collected on a Nikon TE-2000 inverted scope with a 100× objective and a BD-CARV II spinning disk confocal unit. B. anthracis cells labeled with SapSLH–mCherry or mCherry were visualized with an Olympus PROVIS microscope with a 100× objective and digital images were collected. B. anthracis cells grown in the presence or absence of 1 μg·mL−1 tunicamycin were harvested by centrifugation, normalized by optical density, and then treated either with 1 μg·mL−1 vancomycin-BODIPY conjugate (Invitrogen) or with purified, recombinant SapSLH–mCherry, and images were collected digitally on an Olympus PROVIS microscope with a 100× objective.
SLH binding assays
B. anthracis Sterne or ΔcsaB cells were harvested by centrifugation and resuspended in 3 M urea and heated at 95 °C for 10 min. Cells were collected by centrifugation and then washed extensively with water, resuspended in PBS, and normalized by optical density (OD600). Equal numbers of these cells were then incubated with equimolar quantities of purified mCherry or SapSLH–mCherry for 10 min at room temperature in the dark. After incubation, cells were harvested by centrifugation, washed extensively with PBS, and either imaged or fluorescence intensity measured in 96-well format with a Tecan plate reader.
Purification of peptidoglycan, HF-PS, rpHPLC
B. anthracis Sterne peptidoglycan was purified from cells scraped from 100 mm×100 mm BHI agar plates and resuspended in water. Cells were harvested by centrifugation and resuspended in 500 mL 4% SDS and then submerged in a boiling water bath for 30 min. After cooling to room temperature, the cells were harvested by centrifugation (9000g, 10 min) and washed repeatedly with water until no trace of SDS remained. The cells were then suspended in 80 mL of water and subjected to mechanical lysis with a Bio-Spec bead beater and 0.1 mm glass beads for 10 pulses of 1 min, each pulse separated by a 5-min rest on ice. Cell walls were then harvested by centrifugation (8000g, 15 min) and washed once with water. This material was treated for 4 h at 37 °C with 10 μg mL−1 RNase and DNase (Roche) in 100 mM Tris–HCl, pH 7.5, supplemented with 20 mM MgSO4 and then for 16 h at 37 °C with 10 μg mL−1 trypsin (Sigma) in 100 mM Tris–HCl, pH 7.5, supplemented with 10 mM CaCl. Insoluble material was then harvested by centrifugation at 32,000g for 15 min and washed once with water and sedimented again. The pellet was resuspended in 1% SDS and submerged in a boiling water bath for 30 min. SDS was removed by repeated washing with water and centrifugation (15 min, 32,000g) until all traces of SDS were absent. This material was then washed once with 100 mM Tris–HCl, pH 8.0, once with water, once with 100 mM ethylenediaminetetraacetic acid, pH 8.0, once again with water, once with acetone, and then twice more with water; insoluble material was collected between each step by centrifugation for 15 min at 32,000g. After the final wash, the pellet was lyophilized and stored at −20 °C. Soluble peptidoglycan was prepared by suspending lyophilized wall material in water to an OD600=40 and treating 2 mL of this material in 50 mM Tris–HCl, pH 6.3, and 1.5 mM MgCl2 with 5000 μg mL−1 mutanolysin (Sigma). After incubation with tumbling overnight at 37 °C, the reaction was incubated at 95 °C for 30 min, and insoluble material was removed by centrifugation for 15 min at 20,000g. The supernatant was stored at −20 °C for future use or used immediately to measure GlcNAc concentration by the method of Morgan–Elson.19 HF-PS were removed by resuspending lyophilized peptidoglycan in 10 mL of 48% HF (Acros) at an OD600 of 25 and incubating with shaking for 18 h at 4 °C. After incubation, insoluble material was removed by centrifugation at 20,000g for 15 min and the supernatant was precipitated by adding 5 volumes of ethanol at −20 °C and storing at −20 °C for 1 h. Precipitated polysaccharides were harvested by centrifugation at 32,000g for 15 min and washed five times with ethanol at −20 °C. The pellet was then suspended in 5 mL water and applied to a Sep-Pak Plus C18 column (Waters) equilibrated with water containing 0.1% TFA. The column was washed twice with 10 mL water and 0.1% TFA and eluted with 20% acetonitrile/80% water with 0.1% TFA. The eluted material was neutralized with Tris–HCl, pH 8.0, buffer (its concentration measured by the Anthrone method) and stored at −20 °C. rpHPLC was used to further purify the HF-PS over a 250 mm×4.6 mm C18 ODS hypersile 3-μm-particle-size guard column (Thermos) to separate peptidoglycan or HF-PS with H2O–0.1% TFA (Buffer A) and acetonitrile-0.1% TFA (Buffer B) gradients as follows: 10 min, 0% Buffer B; 5 min, linear gradient 0–10% Buffer B; 20 min, linear gradient 10–20% Buffer B; 65 min, linear gradient 20–100% Buffer B; 10 min, 100% Buffer B at a flow rate of 0.5 mL min−1. The HF-PS eluted at ~30 min, 15–16% acetonitrile routinely.
Extraction of S. aureus WTA
Extraction of WTA of S. aureus strain RN4220 and its variants was carried out according to previously published protocols.29 S. aureus strains were grown as overnight cultures in 100 mL of TSB (supplemented with the appropriate antibiotic/tunicamycin concentration and 1 mM IPTG) and normalized by optical density (OD600). Overnight culture (30 mL) at OD600=6 was harvested by centrifugation (10 min, 6000g), washed once with 30 mL buffer 1 [50 mM 2-(N-morpholino)ethanesulfonic acid (Mes), pH 6.5], and suspended in 30 mL of buffer 2 (4% SDS and 50 mM Mes, pH 6.5). Cell suspensions were submerged in a boiling water bath for 1 h and collected by centrifugation (10,000g, 10 min). Insoluble material was suspended in 1 mL of buffer 2 and transferred to a 2-mL microcentrifuge tube, sedimented (21,000g, 10 min), and washed once with 1.5 mL buffer 2, once with 1.5 mL buffer 3 (2% NaCl and 50 mM Mes, pH 6.5), and finally with 1.5 mL buffer 1. After the last wash, samples were incubated with 20 μg proteinase K in 20 mM Tris–HCl, pH 8.0, and 0.5% SDS at 50 °C for ~4 h. Following digestion, wall material was collected by centrifugation (21,000g, 10 min) and washed once with 1.5 mL buffer 3 and then at least three times with distilled H2O to remove the SDS. WTA samples were then extracted with 1 mL of 0.1 M NaOH by tumbling at room temperature for 16 h. Insoluble material was removed by centrifugation (21,000g, 10 min), and the supernatant, containing hydrolyzed WTA, was neutralized by the addition of 50 μL of a 0.1-N acetic acid solution and of Tris–HCl, pH 8.5, to 100 mM.
PAGE analysis of WTA
The PAGE analysis of the WTA method was adapted from previously published protocols.29 A Bio-Rad Protean II xi electrophoresis cell (20 cm×16 cm×0.75 mm) was used for separation. A solution of 19:1 (5% C) acrylamide was supplemented with bisacrylamide to obtain a final solution of 30% T (6% C). The separating gel was 20% total acrylamide, 6% of which was the cross-linker bisacrylamide in 1 M Tris–HCl, pH 8.5. The gel was prepared by mixing 10 mL of gel buffer (3 M Tris–HCl, pH 8.5) with 20 mL of an acrylamide stock solution (30% T, 6% C). The gel was polymerized using 300 μL of 10% ammonium persulfate along with 30 μL of N,N,N′,N′-tetramethylethylenediamine. The stacking gel (4% T, 5% C) was cast using a mixture of 1 mL acrylamide stock solution (40% T, 5% C), 3 mL of gel buffer (3 M Tris–HCl, pH 8.5), and 6 mL water, polymerized with 100 μL of 10% ammonium persulfate and 10 μL of N,N, N′,N′-tetramethylethylenediamine. WTA samples were diluted 1:3 in loading buffer (50% glycerol in running buffer with a trace of bromophenol blue) and 20 μL was loaded in each well. Tris–N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl] glycine running buffer {0.1 M Tris base and 0.1 M N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine, pH 8.2} was used to run the gel for 16 h at 20 mA per gel. WTA bands were visualized using the alcian blue–silver staining protocol.34 Briefly, the gel was submerged in 1 mg/mL alcian blue in water and rotated slowly at room temperature for 30 min. The alcian blue solution was replaced after 30 min, and the gel was allowed to stain for another 30 min, washed twice with water and destained with 45% methanol and 10% acetic acid in water for 30 min, and washed twice more with water. WTA bands were developed via the Bio-Rad silver staining kit according to the manufacturer’s recommendations.
Supplementary Material
Acknowledgments
We thank Valerie Anderson for critical reading of the manuscript and members of the Schneewind laboratory for experimental advice and discussion. This work was supported by a grant from the National Institute of Allergy and Infectious Diseases, Infectious Diseases Branch (AI69227) to O.S. J.W.K. acknowledges support from the Molecular Cell Biology Training Grant (GM007183) at the University of Chicago. The authors acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (National Institute of Allergy and Infectious Diseases Award 1-U54-AI-057153).
Abbreviations
- SCWP
secondary cell wall polysaccharide
- SLH
surface layer homology
- MS
mass spectrometry
- HF-PS
hydrofluoric-acid-extractable polysaccharides
- WTA
wall teichoic acid
- MALDI-TOF
matrix-assisted laser desorption/ionization time of flight
- ESI
electrospray ionization
- FTMS
Fourier transform mass spectrometer
- BODIPY
4,4-difluoro-4-bora-3a,4a-diaza-s-indacene
- nSIM
narrow select ion monitoring
- RibP
ribitol phosphate
- GroP
glycerol phosphate
- BHI
brain heart infusion broth
- LB
lysis broth
- TSB
tryptic soy broth
- TFA
trifluoroacetic acid
- PBS
phosphate-buffered saline
- Mes
2-(N-morpholino)ethanesulfonic acid
Footnotes
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jmb.2010.06.059
References
- 1.Koch R. Die Ätiologie der Milzbrand-Krankheit, begründet auf die Entwicklungsgeschichte des Bacillus anthracis. Beitr Biol Pflanz. 1876;2:277–310. [Google Scholar]
- 2.Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 3.Fouet A. The surface of Bacillus anthracis. Mol Aspects Med. 2009;30:374–385. doi: 10.1016/j.mam.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Etienne-Toumelin I, Sirard JC, Duflot E, Mock M, Fouet A. Characterization of the Bacillus anthracis S-layer: cloning and sequencing of the structural gene. J Bacteriol. 1995;177:614–620. doi: 10.1128/jb.177.3.614-620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farchaus JW, Ribot WJ, Downs MB, Ezzell JW. Purification and characterization of the major surface array protein from a virulent Bacillus anthracis Delta Sterne-1. J Bacteriol. 1995;177:2481–2489. doi: 10.1128/jb.177.9.2481-2489.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesnage S, Fontaine T, Mignot T, Delepierre M, Mock M, Fouet A. Bacterial SLH domain proteins are non-covalently anchored to the cell surface via a conserved mechanism involving wall polysaccharide pyruvylation. EMBO J. 2000;19:4473–4484. doi: 10.1093/emboj/19.17.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesnage S, Tosi-Couture E, Fouet A. Production and cell surface anchoring of functional fusions between the SLH motifs of the Bacillus anthracis S-layer proteins and the Bacillus subtilis levansucrase. Mol Microbiol. 1999;31:927–936. doi: 10.1046/j.1365-2958.1999.01232.x. [DOI] [PubMed] [Google Scholar]
- 8.Kern JW, Schneewind O. BslA, a pXO1-encoded adhesin of Bacillus anthracis. Mol Microbiol. 2008;68:504–515. doi: 10.1111/j.1365-2958.2008.06169.x. [DOI] [PubMed] [Google Scholar]
- 9.Cava F, de Pedro MA, Schwarz H, Henne A, Berenguer J. Binding to pyruvylated compounds as an ancestral mechanism to anchor the outer envelope in primitive bacteria. Mol Microbiol. 2004;52:677–690. doi: 10.1111/j.1365-2958.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- 10.Ilk N, Kosma P, Puchberger M, Egelseer EM, Mayer HF, Sleytr UB, Sára M. Structural and functional analyses of the secondary cell wall polymer of Bacillus sphaericus CCM 2177 that serves as an S-layer-specific anchor. J Bacteriol. 1999;181:7643–7646. doi: 10.1128/jb.181.24.7643-7646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen BO, Sára M, Mader C, Mayer HF, Sleytr UB, Pabst M, et al. Structural characterization of the acid-degraded secondary cell wall polymer of Geobacillus stearothermophilus PV72/p2. Carbohydr Res. 2008;343:1346–1358. doi: 10.1016/j.carres.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Choudhury B, Leoff C, Saile E, Wilkins P, Quinn CP, Kannenberg EL, Carlson RW. The structure of the major cell wall polysaccharide of Bacillus anthracis is species-specific. J Biol Chem. 2006;281:27932–27941. doi: 10.1074/jbc.M605768200. [DOI] [PubMed] [Google Scholar]
- 13.Leoff C, Choudhury B, Saile E, Quinn CP, Carlson RW, Kannenberg EL. Structural elucidation of the nonclassical secondary cell wall polysaccharide from Bacillus cereus ATCC 10987. Comparison with the polysaccharides from Bacillus anthracis and B cereus type strain ATCC 14579 reveals both unique and common structural features. J Biol Chem. 2008;283:29812–29821. doi: 10.1074/jbc.M803234200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuch R, Fischetti VA, Nelson DC. A genetic screen to identify bacteriophage lysins. Methods Mol Biol. 2009;502:307–319. doi: 10.1007/978-1-60327-565-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown S, Zhang YH, Walker S. A revised pathway for Staphylococcus aureus wall teichoic acid biosynthesis based on in vitro reconstitution of the intracellular steps. Chem Biol. 2008;15:12–21. doi: 10.1016/j.chembiol.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia G, Kohler T, Peschel A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int J Med Microbiol. 2010;300:148–154. doi: 10.1016/j.ijmm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Ekwunife FS, Singh J, Taylor KG, Doyle RJ. Isolation and purification of cell wall polysac-charide of Bacillus anthracis. FEMS Microbiol Lett. 1991;66:257–262. doi: 10.1016/0378-1097(91)90270-k. [DOI] [PubMed] [Google Scholar]
- 18.Kojima N, Araki Y, Ito E. Structural studies on the acidic polysaccharide of Bacillus cereus AHU 1356 cell walls. Eur J Biochem. 1985;148:479–484. doi: 10.1111/j.1432-1033.1985.tb08864.x. [DOI] [PubMed] [Google Scholar]
- 19.Elson LA, Morgan WT. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27:1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris DL. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science. 1948;107:254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- 21.Alexander DC, Valvano MA. Role of rfe gene in the biosynthesis of the Escherichia coli O7-specific lipopolysaccharide and other O-specific polysaccharides containing N-acetylglucosamine. J Bacteriol. 1994;176:7079–7084. doi: 10.1128/jb.176.22.7079-7084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Elia MA, Henderson JA, Beveridge TJ, Heinrichs DE, Brown ED. The N-acetylmannosamine transferase catalyzes the first committed step of teichoic acid assembly in Bacillus subtilis and Staphylococcus aureus. J Bacteriol. 2009;191:4030–4034. doi: 10.1128/JB.00611-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haft RF, Wessels MR, Mebane MF, Conaty N, Rubens CE. Characterization of cpsF and its product CMP-N-acetylneuraminic acid synthetase, a group B streptococcal enzyme that can function in K1 capsular polysaccharide biosynthesis in Escherichia coli. Mol Microbiol. 1996;19:555–563. doi: 10.1046/j.1365-2958.1996.395931.x. [DOI] [PubMed] [Google Scholar]
- 24.Molnár J, Prágai B. Attempts to detect the presence of teichoic acid in Bacillus anthracis. Acta Microbiol Acad Sci Hung. 1971;18:105–108. [PubMed] [Google Scholar]
- 25.D’Elia MA, Millar KE, Beveridge TJ, Brown ED. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J Bacteriol. 2006;188:8313–8316. doi: 10.1128/JB.01336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Elia MA, Pereira MP, Chung YS, Zhao W, Chau A, Kenny TJ, et al. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J Bacteriol. 2006;188:4183–4189. doi: 10.1128/JB.00197-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duksin D, Bornstein P. Changes in surface properties of normal and transformed cells caused by tunicamycin, an inhibitor of protein glycosylation. Proc Natl Acad Sci USA. 1977;74:3433–3437. doi: 10.1073/pnas.74.8.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hancock IC, Wiseman G, Baddiley J. Biosynthesis of the unit that links teichoic acid to the bacterial wall: inhibition by tunicamycin. FEBS Lett. 1976;69:75–80. doi: 10.1016/0014-5793(76)80657-6. [DOI] [PubMed] [Google Scholar]
- 29.Meredith TC, Swoboda JG, Walker S. Late-stage polyribitol phosphate wall teichoic acid biosynthesis in Staphylococcus aureus. J Bacteriol. 2008;190:3046–3056. doi: 10.1128/JB.01880-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda M, Wachi M, Jung HK, Ishino F, Matsuhashi M. The Escherichia coli mraY gene encoding UDP-N-acetylmuramoyl-pentapeptide: undecaprenyl-phosphate phospho-N-acetylmuramoyl-pentapeptide transferase. J Bacteriol. 1991;173:1021–1026. doi: 10.1128/jb.173.3.1021-1026.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gründling A, Schneewind O. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc Natl Acad Sci USA. 2007;104:8478–8487. doi: 10.1073/pnas.0701821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sleytr UB, Beveridge TJ. Bacterial S-layers. Trends Microbiol. 1999;7:253–260. doi: 10.1016/s0966-842x(99)01513-9. [DOI] [PubMed] [Google Scholar]
- 33.Schäffer C, Messner P. The structure of secondary cell wall polymers: how Gram-positive bacteria stick their cell walls together. Microbiology. 2005;151:643–651. doi: 10.1099/mic.0.27749-0. [DOI] [PubMed] [Google Scholar]
- 34.Pollack JH, Neuhaus FC. Changes in wall teichoic acid during rod-sphere transition of Bacillus subtilis. J Bacteriol. 1994;176:7252–7259. doi: 10.1128/jb.176.23.7252-7259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


