Abstract
Octopamine is a biogenic amine first identified in octopus. It has been well studied in arthropods and a few gastropods, serving as a neurotransmitter and hormone. The presence of octopamine has rarely been reported in bivalves and has not been reported in Crassostrea virginica. We utilized HPLC to identify and measure octopamine in cerebral ganglia, visceral ganglia, gill, palps, mantle, heart and hemolymph of C. virginica. Endogenous octopamine levels increased when animals were treated with tyramine, an octopamine precursor. A preliminary study in our lab found that octopamine has a cardio-excitatory action on C. virginica heart. In the present study we also used immunoblotting techniques to identify an octopamine-like receptor (Pan TAAR, trace amine-associated receptor) in oyster heart. The study confirms the presence of octopamine in the nervous system, innervated organs and hemolymph of C. virginica and identifies the presence of an octopamine-like receptor in heart, strengthening the contention that octopamine is important in the physiology of C. virginica as it is in other invertebrates.
Introduction
Studies of the nervous system of bivalve molluscs show the biogenic amines serotonin, norepinephrine and dopamine to be present and serve as neurotransmitters and neurohormones1–8. Many of these studies focus on the physiology of the heart and gill. Hearts of different bivalve species tend to have different responses to various neurotransmitters. Bivalve heart tends to be inhibited by acetylcholine and in most species excited by serotonin9,10. Biogenic amines are present and have neurophysiological functions in Crassostrea virginica11–14. Dopamine and norepinephrine are involved in stress responses of C. virginica and endogenous dopamine and norepinephrine levels increased in response to mechanical stress15,16. Temperature or salinity changes also increase dopamine and norepinephrine levels in bivalves17,18.
Octopamine is a biogenic amine that was first identified in salivary glands of octopus19. It has been well studied in gastropods and insects where it serves as a neurotransmitter and hormone20–24. It has been less well studied in bivalve molluscs25. In the clam Tapes watlingi octopamine was found to have an excitatory action on the isolated, spontaneously beating heart26 and octopamine receptors were identified in the animal’s accessory ventricle27. A preliminary study in our lab found that octopamine has a cardio-excitatory action on C. virginica hearts28. The present study sought to determine if octopamine and an octopamine receptor were present in the heart and other tissues of C. virginica.
Materials and Methods
Adult C. virginica of approximately 80 mm shell length were obtained from Frank M. Flower and Sons Oyster Farm in Oyster Bay, NY, USA. They were maintained in the lab for up to two weeks in temperature-regulated aquaria in Instant Ocean artificial sea water (ASW) at 16 – 18°C, specific gravity of 1.024 ± 0.001, salinity of 31.9 ppt, and pH of 7.8 ± 0.2. Each animal was tested for health prior to experimentation by the resistance it offered to being opened. Animals that fully closed in response to tactile stimulation and required at least moderate hand pressure to being opened were used for the experiments. Octopamine hydrochloride, tyramine, and 1-octanesulfonic acid (sodium salt, Sigma Ultra) were obtained from Sigma-Aldrich (St. Louis, MO, USA. For HPLC analysis. Gemini 5µ C18 reverse phase HPLC columns were obtained from Phenomenex (Torrance, CA). For immunoblotting analysis, NP-40 lysis buffer, Bradford reagent, Laemmli 2X loading buffer containing βME, Bio-Rad Mini-Protean TGX gels, Bio-Rad Precision Plus Protein WesternC Standards, Tris/glycine SDS buffer and Bio-Rad Precision Protein StrepTactin-HRP conjugate were obtained from Bio-Rad. Western Blot Signal Enhancer was obtained from Pierce. Pan TAAR (trace amine-associated receptor) 1° antibodies (goat polyclonal, sc-54398), and polyclonal HRP-conjugated 2° antibodies (chicken anti-goat, sc2953) were obtained from Santa Cruz Biotechnology. CN/DAB Substrate and all other reagents were obtained from Fisher Scientific (Pittsburgh, PA, USA).
HPLC Analysis of Tissues
Oyster tissues (cerebral and visceral ganglia, gill, palps, mantle and heart) were excised, blotted and weighed. Approximately 1 gram of each tissue was homogenized in 2 ml of 0.4 M HCl with a Brinkman Polytron homogenizer with Omni International disposable probe tips. One ml of hemolymph was drawn from adductor muscle with a syringe and mixed with 1 ml of 0.4 M HCl. The samples were centrifuged (15,000 × g, 20 minutes) and resulting supernatant vacuum filtered through 0.24 micron filters. Tissue filtrates were analyzed for endogenous octopamine levels using HPLC with fluorescence detection12. Samples (20 µl) were injected into a Beckman System Gold 126/168 HPLC system fitted with a Phenomenex-Gemini 5µ C18 reverse phase, ion pairing column with a guard column. All reagents were HPLC grade. The acetate/methanol (85:15 v/v) mobile phase (50 mM acetate buffer, pH 4.7 containing 1.1 mM of 1-octanesulfonic acid and 0.11 mM EDTA) with a flow rate of 2 ml/min in isocratic mode. A Jasco FP 2020 Plus Spectrofluorometer fitted with a 16 µl flow cell was used for detection of native fluorescence (280 nm excitation, 320 nm emission). Octopamine levels were quantified by comparing the peak areas of samples to those of standards and are reported as ng/g wet weight for tissues (gill, palps, heart and mantle), ng/ml for hemolymph and ng/ganglion for both cerebral and visceral ganglia. Statistical analysis was determined by a t Test.
Western Blot Analysis for Heart Octopamine Receptor
Three oyster hearts were dissected, rinsed well in ASW, blotted and weighed, and placed in eppendorf tubes with 2.5 ml of ice cold NP-40 lysis buffer containing protease and phosphatase inhibitors. Each tube was sonicated on ice for 2–3, 5 sec bursts with a Brinkman Polytron and then kept on ice for 30 min before being centrifuged (10,000 × g for 20 min). The resultant lysate supernatants were pooled and aliquots were analyzed for protein concentration by Bradford assay. The remaining lysate supernatants were adjusted to a protein concentration of 4–5 mg/mL for SDS PAGE.
Preparation of Samples for Loading into SDS-PAGE Gels
Aliquots of lysate protein were denatured with Laemmli 2X loading buffer containing βME (1:1 ratio) and allowed to sit for one hour at room temperature. Laemmli-treated samples (20–40 µg total protein) were wet-loaded into wells of polyacrylamide gels (Bio-Rad Mini-Protean TGX gels), alongside pre-stained molecular weight markers (Bio-Rad Precision Plus Protein WesternC Standards). Gels underwent electrophoresis in Tris/glycine SDS buffer for 1 hour at 150 v.
Western Blot Analysis
After electrophoresis, gels were removed from their plate, rinsed in transfer buffer (25 mM Tris, 190 mM glycine, 20% methanol, pH 8.3), and sandwiched for transfer onto nitrocellulose membranes. Before immunoblotting was started, pre-stained markers were visualization to access whether proteins migrated uniformly and evenly during the electrophoresis process. The wet-transfer was done in a Mini Trans-BlotR electrophoretic transfer cell (Bio-Rad) under constant current (20 v) for 150 min in the presence of a cooling module to prevent excess heating. After transfer the membranes were rinsed with ddH2O, treated with a Western Blot Signal Enhancer, rinsed 5× with ddH2O, and then blocked with 5% non-fat dry milk in TBS-T for one hour at room temperature. After blocking, membranes were incubated at 4°C with 1° antibody (Pan TAAR at 1:400 dilution). Pan TAAR is reactive with octopamine, beta-phenylethylamine, p-tyramine (p-TYR) and tryptamine receptors, but unresponsive to classical biogenic amines (serotonin, dopamine, norepinephrine and epinephrine) and histamine receptors. The Pan TAAR 1° antibodies were diluted in TBS-T and 2% blocker for 24 hours, then the membranes were washed extensively with TBS-T, followed by incubation at room temperature for 60 min with HRP-conjugated 2° antibody (1:4000 dilution) in TBS-T and 2% blocker, and Bio-Rad Precision Protein StrepTactin-HRP conjugate (1:5000 dilution) was used to resolve protein standards. After incubation the membranes were washed extensively with PBS-T and chromogenic detection of HRP-conjugated standards and lysate proteins were resolved using CN/DAB Substrate. After the protein blots were chromogenically developed, the images were captured with a Carestream Gl212 Pro Molecular Imaging System.
Results and Discussion
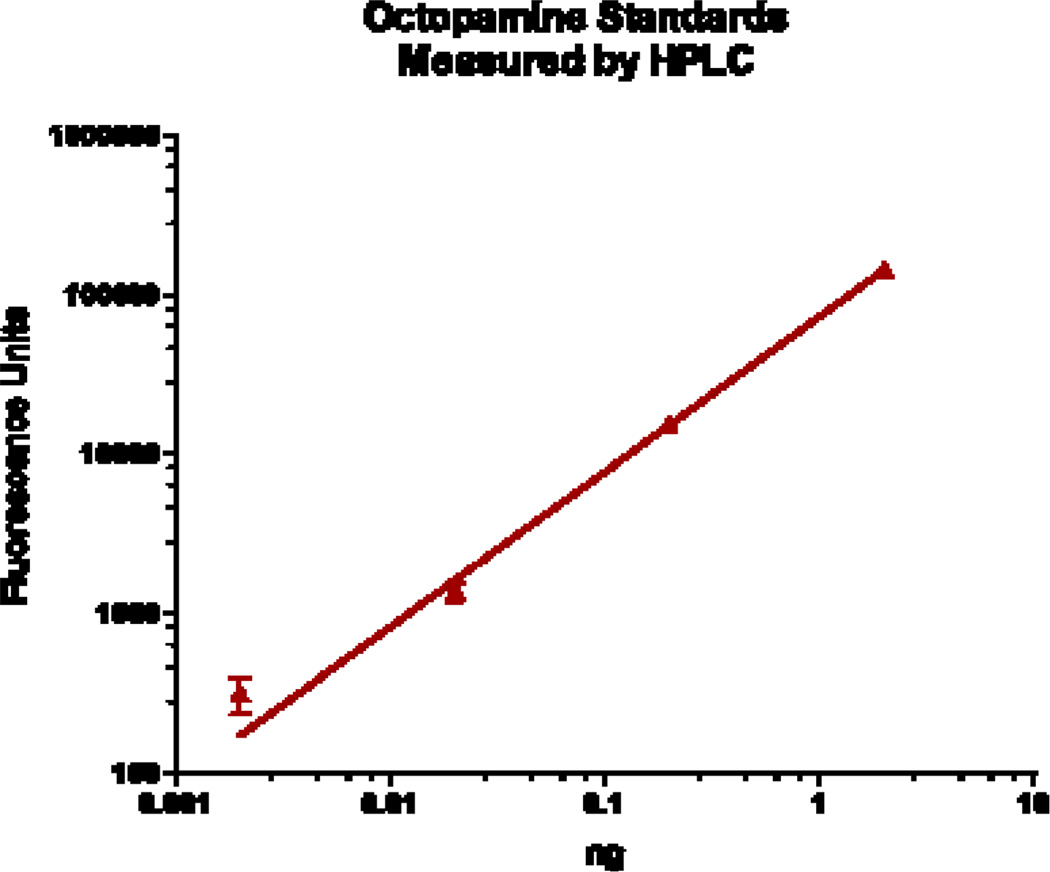
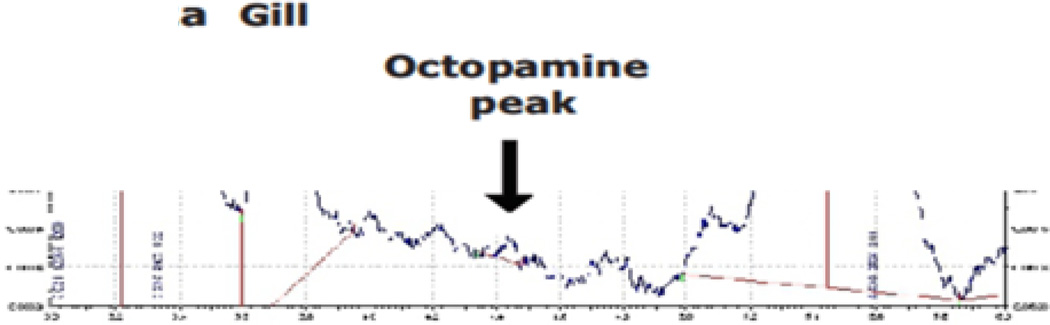
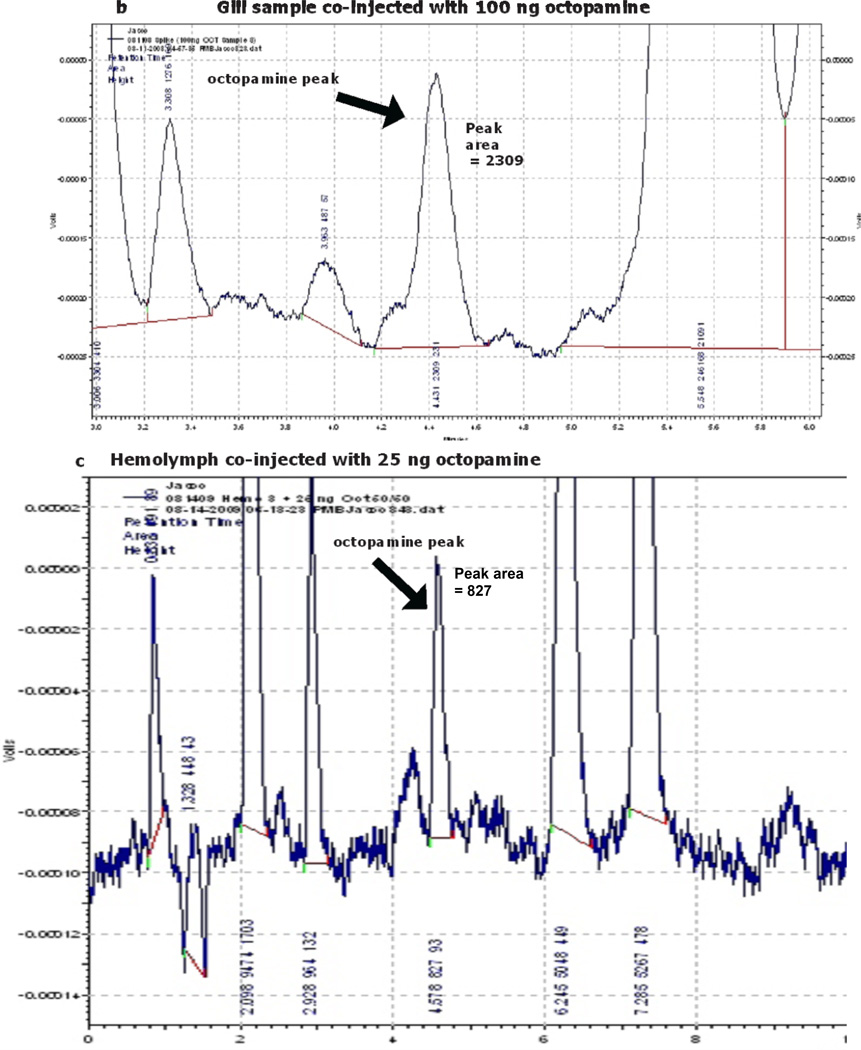
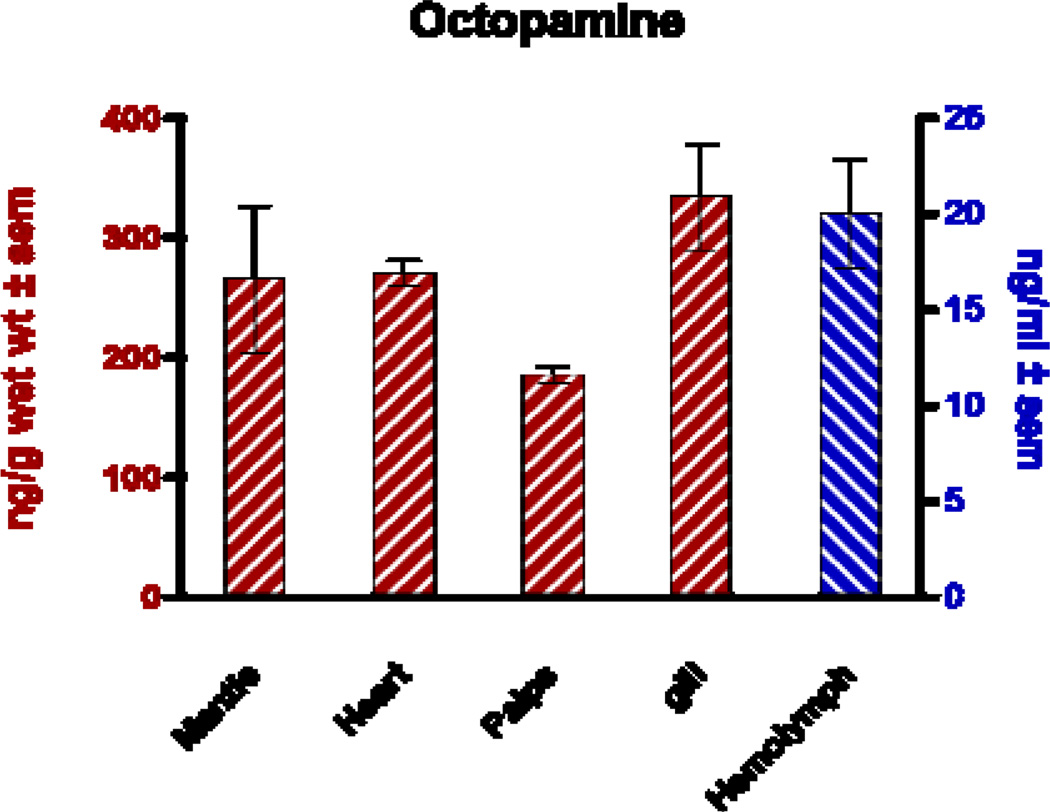
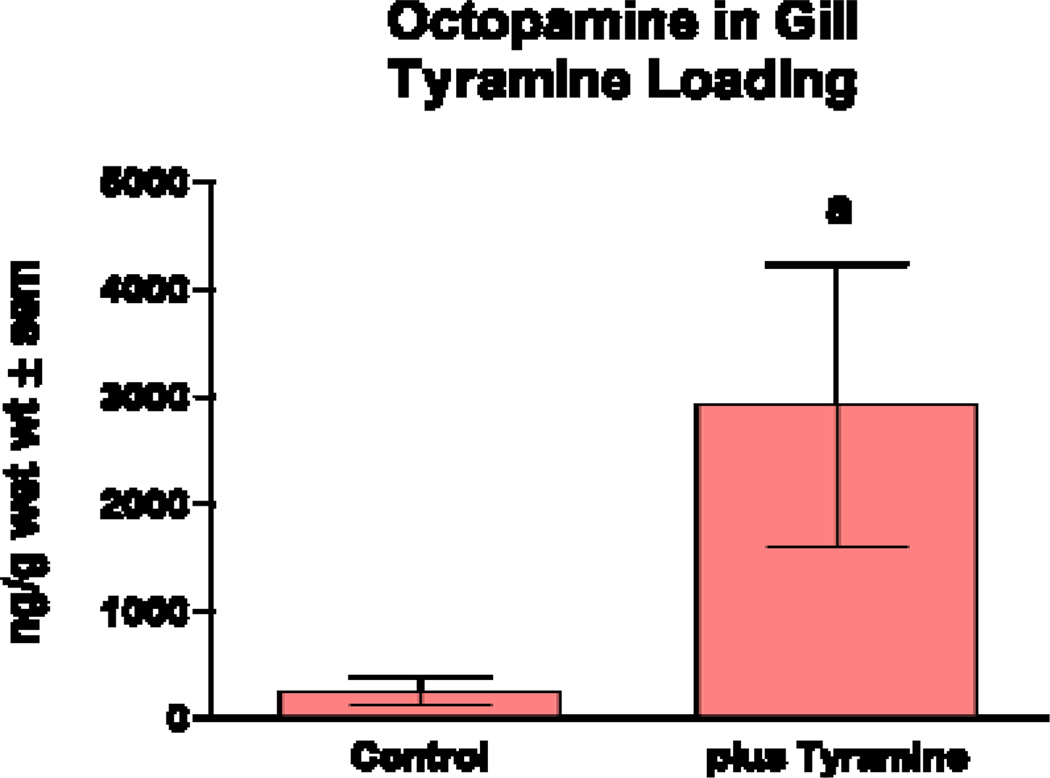
Reports of octopamine presence in peripheral tissues of bivalves or gastropods are scarce, beyond the few reports showing it to be present in heart and hemolymph23,29,30. In this study we looked for octopamine using HPLC and found octopamine to be present in hemolymph and other peripheral tissues of the oyster C. virginica. This HPLC procedure with fluorescence detection showed an octopamine retention time of 4.1 minutes and was able to detect octopamine standards at amounts lower than 10 pg. The standard curve was fairly linear up to about 2 ng (Fig. 1). The identification of octopamine from tissue samples was confirmed by identical retention time and spectral characteristics compared to octopamine standards (Fig. 2a). Co-injecting octopamine standards with gill tissue samples (Fig. 2b) or hemolymph samples (Fig, 2c) revealed a single octopamine peak with the same 4.1 minute retention time. Octopamine was present in all C. virginia tissues samples tested (Fig. 3). Tissue amounts were in the 300 ng/g range for gill, palps, heart and mantle, while hemolymph had about 20 ng/ml. Our results with C. virginica compares well with other reports of octopamine, which was present in the hearts of the snail, Helix and the clam, Tapes, at about 4X and 1/4 as much, respectively30,31. Since tyramine is a direct precursor to octopamine, in other experiments gills were incubated for 24 hours with 5 mM tyramine to determine if the treatment would result in an increase in endogenous octopamine levels. HPLC analysis of tyramine treated gills found an octopamine peak representing more than a 10 fold increase in endogenous octopamine concentration compared to controls (Fig. 4).
Fig. 1.
Standard curve of octopamine standards analyzed by HPLC HPLC with native fluorescence detection (280 nm excitation, 320 nm emission). X and Y axis are log scale.
Fig. 2.
a. Record of HPLC analysis of octopamine from gill.
c.d. Record of HPLC analysis of octopamine from gill (c) and hemolymph (d) that was co-injected with octopamine standard.
Fig. 3.
Octopamine levels in peripheral tissues and hemolymph determined by HPLC analysis.
Fig. 4.
Octopamine levels in gill incubated with 5 mM of tyramine for 24 hours. Statistical analysis was determined by a t-Test. ap < 0.05 compared to controls.
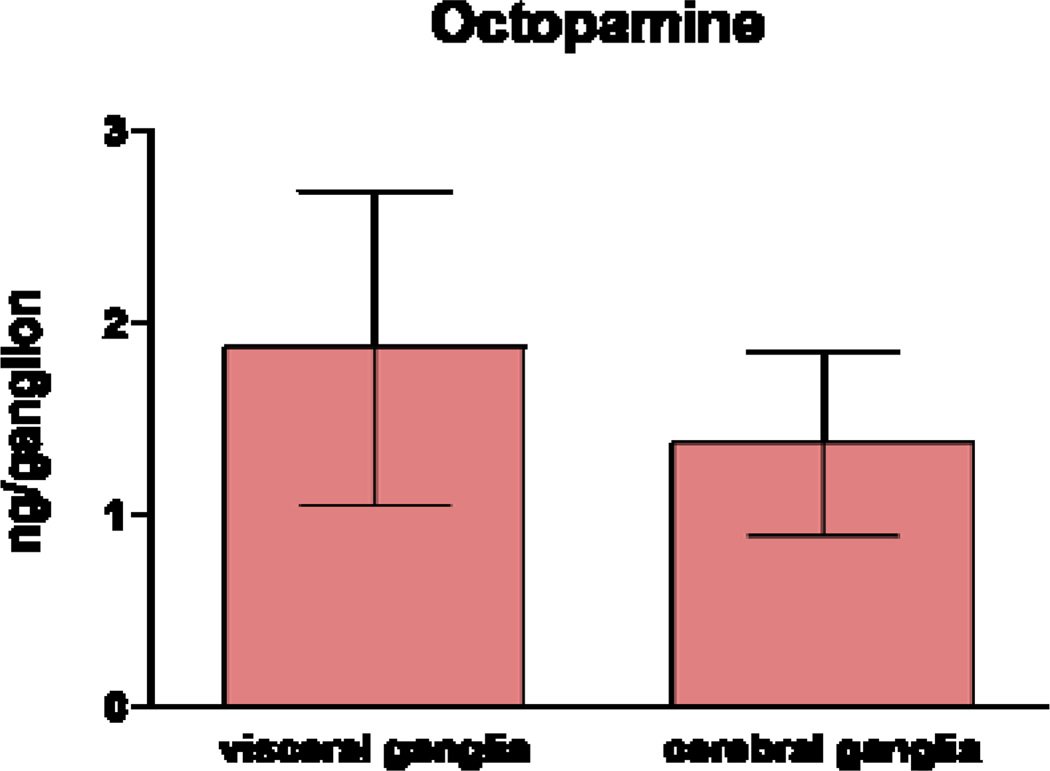
Compared to peripheral tissues, there are numerous reports of octopamine in nerves and ganglia of gastropods23, and even a few in nerves and ganglia of bivalves25. Our HPLC results found that the cerebral and visceral ganglia of C. virginica had approximately 1 ng/ganglion each (Fig 5). Octopamine amounts in ganglia of snails and nudibranchs, which are much larger than the ganglia of C. virginica, were reported to be about 10 to 100 times greater29.
Fig. 5.
Octopamine levels in visceral and cerebral ganglia determined by HPLC analysis.
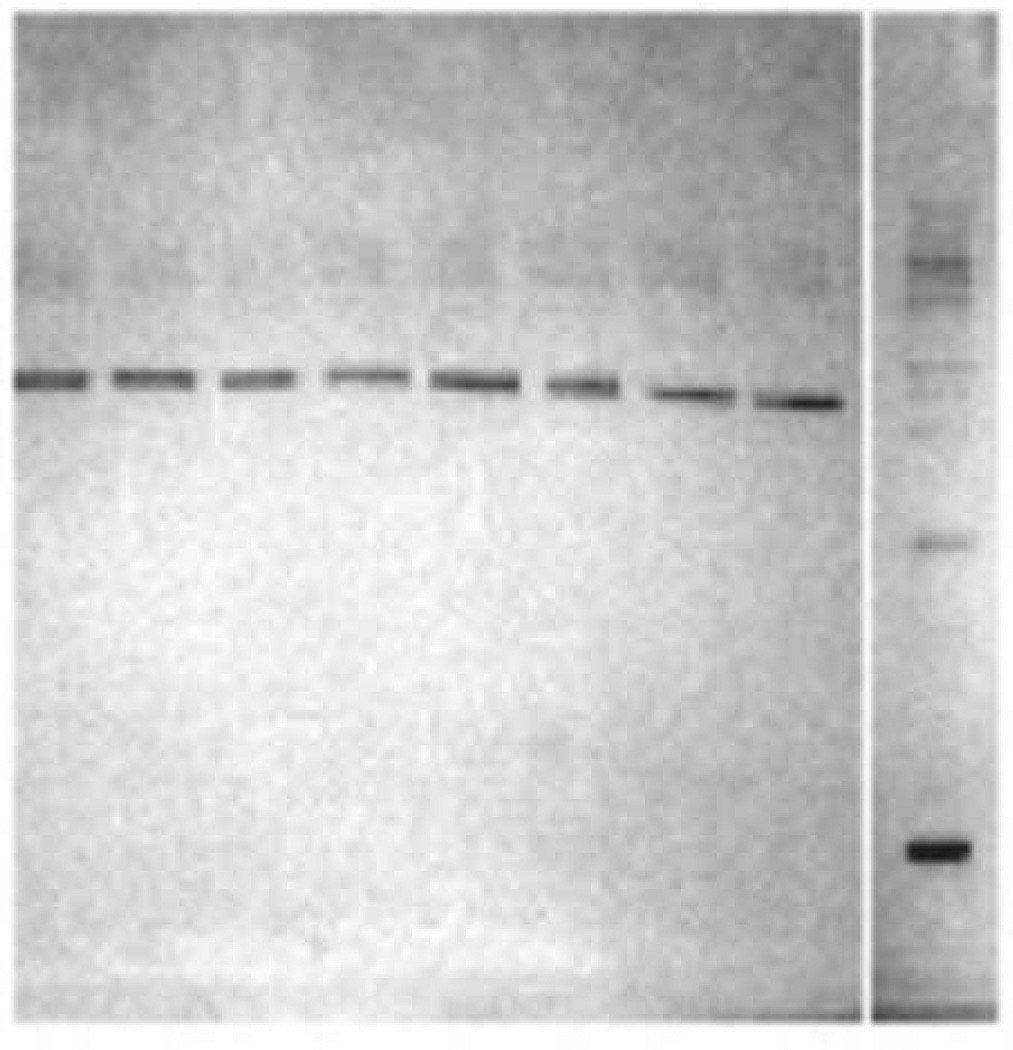
Earlier preliminary experiments in our lab showed octopamine has a physiological effect on oyster heart, and that could be blocked by the octopamine antagonists phentolamine and metoclopramide. In view of the fact that the present HPLC results found that oyster heart contains a significant amount of octopamine (270 ng/g wet weight), we used Western Blotting to look for the presence of an octopamine-like receptor in heart tissue. Using the Pan TAAR antibody, which binds to octopamine receptors, our Western Blot revealed a single strong protein band at approximately 80 kD (Fig. 6) in agreement with Farooqui et al32 who found an octopamine receptor band from honeybee brain at 78 kD. Octopamine receptors also have been identified in Tapes clam heart27.
Fig. 6.
Western Blotting of heart tissue showing a strong protein band at about 80 kD indicating the presence of an octopamine receptor. Pan TAAR 1° antibodies (goat polyclonal) and HRP-conjugated 2° antibodies were used. The right lane contains the protein markers.
The study identifies the presence of octopamine as an endogenous biogenic amine in C. virginica, and shows that C. virginica has the enzymatic pathway to synthesis it from its precursor tyramine. The study also confirms our previous pharmacological work that suggested a possible physiological role of octopamine as a cardio-excitatory agent by identifying the presence of an octopamine-like receptor in C. virginica heart. Based upon these results we will conduct a full physiological study of the effects of octopamine on oyster heart as well as a comparative study of octopamine and octopamine receptors in other bivalves.
Acknowledgments
This work was supported in part by grants 2R25GM0600305 of the Bridge Program of NIGMS, 0516041071 of the CSTEP Program of the New York State Department of Education 0622197 and of the DUE Program of NSF.
References
- 1.Blaschko H, Milton A. Oxidation of 5-hydroxytryptamine and related cornpounds by Mytilus gill plates. Br J Pharmacol. 1960;15:42–46. doi: 10.1111/j.1476-5381.1960.tb01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welsh JH, Moorehead M. The quantitive distribution of serotonin in invertebrates, especially in their nervous system. J Neurochem. 1960;6:146–169. doi: 10.1111/j.1471-4159.1960.tb13460.x. [DOI] [PubMed] [Google Scholar]
- 3.Aiello E, Guideri G. Relationship between serotonin and nerve stimulation of ciliary activity. J Pharmacol Exp Therap. 1966;154:517–523. [PubMed] [Google Scholar]
- 4.Stefano GB, Aiello E. Histofluorescent localization of serotonin and dopamine in the nervous system and gill of Mytilus edulis (Bivalvia) Biol Bull. 1975;148:141–156. doi: 10.2307/1540655. [DOI] [PubMed] [Google Scholar]
- 5.Stefano GB, Catapane EJ, Aiello E. Dopaminergic agents: Influence on serotonin in the molluscan nervous system. Science. 1976;194:539–541. doi: 10.1126/science.973139. [DOI] [PubMed] [Google Scholar]
- 6.Stefano GB, Catapane EJ. The effects of temperature acclimation on monoamine metabolism. J Pharmacol Exp Therap. 1977a;203:449–456. [PubMed] [Google Scholar]
- 7.Stefano GB, Catapane EJ. Seasonal monoamine changes in the central nervous system of Mytilus edulis (Bivalvia) Experientia. 33:1341–1342. [Google Scholar]
- 8.Catapane EJ. Demonstration of the peripheral innervation of the gill of the marine mollusc by means of the aluminum formaldehyde histofluorescence technique. Cell Tissue Res. 1982;225:449–454. doi: 10.1007/BF00214696. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg MJ, Windsor DA. Action of acetylcholine on bivalve hearts. Science. 1962;137:534–535. doi: 10.1126/science.137.3529.534. [DOI] [PubMed] [Google Scholar]
- 10.Painter SD, Greenberg MJ. A survey of the responses of bivalve hearts to the molluscan neuropeptide FMRFamide and to 5-hydroxytryptamine. Biol Bull. 1982;162:311–332. [Google Scholar]
- 11.Carroll MA, Catapane EJ. The nervous system control of lateral ciliary activity of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. A. 2007;148:445–450. doi: 10.1016/j.cbpa.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King C, Myrthil M, Carroll MA, Catapane EJ. Effects of p-Aminosalicylic acid on the Neurotoxicity of Manganese and Levels of Dopamine and Serotonin in the Nervous System and Innervated Organs of Crassostrea virginica. In Vivo. 2008;29(3):29–34. [PMC free article] [PubMed] [Google Scholar]
- 13.Martin K, Huggins T, King C, Carroll MA, Catapane EJ. The neurotoxic effects of manganese on the dopaminergic innervation of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. C. 2008;148:152–159. doi: 10.1016/j.cbpc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson M, Huggins T, Licorish R, Carroll MA, Catapane EJ. Effects of p-Aminosalicylic Acid on the Neurotoxicity of Manganese on the Dopaminergic Innervation of the Cilia of the Lateral Cells of the Gill of the Bivalve Mollusc, Crassostrea virginica. Comp Biochem Physiol C. 2010;151(2):264–270. doi: 10.1016/j.cbpc.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muneoka Y, Kamura M. The multiplicity of neurotransmitters and neurohormones controlling Mytilus muscle. Comp. Biochem. Physiol. C. 1982;73:149–156. [Google Scholar]
- 16.Lacoste A, Malham SK, Cueff A, Poulet SA. Stress-Induced Catecholamine Changes in the Hemolymph of the Oyster Crassostrea gigas. Gen Comp Endocrinol. 2001;122:181–188. doi: 10.1006/gcen.2001.7629. [DOI] [PubMed] [Google Scholar]
- 17.Hiripi L, Nemcsok J, Elekes K, Salanki J. Monoamine level and periodic activity in 6-hydroxydopamine treated mussels Anodonta cygnea L. Acta Biol Acad Sci Hung. 1977;28:175–182. [PubMed] [Google Scholar]
- 18.Stefano GB, Hiripi L, Catapane EJ. The effects of short- and long term temperature stress on serotonin, dopamine and norepinephrine concentration in molluscan ganglia. J. Thermal Biol. 1978;3:79–83. [Google Scholar]
- 19.Esparmer V, Boretti G. Identification and characterization by paper chromatography of enteramine, octopamine, tyramine, histamine and allied substances in extracts of posterior salivary glands of Octopoda and in other tissue abstracts of vertebrates and invertebrates. Arch Int Pharmacodyn Ther. 1951;88:296–332. [PubMed] [Google Scholar]
- 20.Adamo SA. Norepinephrine and octopamine: linking stress and immune function across phyla. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology. 1982;73(1):149–156. [Google Scholar]
- 21.David JC, Coulon JF. Octopamine in invertebrates and vertebrates: a review. Prog Neurobiol. 1985;24(2):141–185. doi: 10.1016/0301-0082(85)90009-7. [DOI] [PubMed] [Google Scholar]
- 22.Hiripi L, Vehovszky A, Juhos S, Elekes K. An octopaminergic system in the CNS of the snails, Lymnaea stagnalis and Helix pomatia. Philos Trans R Soc London [Biol] 1998;353(1375):1621–1629. [Google Scholar]
- 23.Roeder T. Octopamine in Invertebrates. Prog Neurobiol. 1999;59:533–561. doi: 10.1016/s0301-0082(99)00016-7. [DOI] [PubMed] [Google Scholar]
- 24.Pflüger HJ, Stevenson PA. Evolutionary aspects of octopaminergic systems with emphasis on arthropods. Arthropod Structure and Development. 2005;34(3):379–396. [Google Scholar]
- 25.Karhunen T, Airaksinen MS, Tuomisto L, Panula P. Neurotransmitters in the nervous system of Macoma balthica Bivalvia. J Comp Neurol. 1993;334(3):477–488. doi: 10.1002/cne.903340311. [DOI] [PubMed] [Google Scholar]
- 26.de Rome PJ, Jamieson DD, Taylor KM, Davies LP. Ligand-binding and pharmacological studies on dopamine and octopamine receptors in the heart of the bivalve mollusc, Tapes watlingi. Comp. Biochem. Physiol. C. 1980;67(1):9–16. doi: 10.1016/0306-4492(80)90051-9. [DOI] [PubMed] [Google Scholar]
- 27.Dougan DFH, Wade DN. Octopamine receptors in the molluscan aortic bulb: effects of clozapine and chlordime-form. Comp. Biochem. Physiol. C. 1985;82:193–197. doi: 10.1016/0742-8413(85)90228-2. [DOI] [PubMed] [Google Scholar]
- 28.Pryce K, Knight J, Catapane EJ, Carroll MA. Pharmacological Study of the Effects of Octopamine on Heart Rate of Crassostrea viginica.; Annals of the 2011 Annual SICB Conference; 2011. pp. 1–20. [Google Scholar]
- 29.Saavedra JM, Brownstein MJ, Carpenter DO, Axelrod J. Octopamine: presence in single neurons of Aplysia suggests neurotransmitter function. Science. 1974;185:363–365. doi: 10.1126/science.185.4148.364. [DOI] [PubMed] [Google Scholar]
- 30.Dougan DFH, Duffield PH, Wade DN. Occurrence and synthesis of octopamine in the heart and ganglia of the mollusc Tapes watlingi. Comp. Biochem. Physiol. C. 1981;70:277–280. [Google Scholar]
- 31.Walker RJ, Kerkut GA. The first family (adrenaline, noradrenaline, dopamine, octopamine, tyramine, phenylethanolamine and phenylethylamine) Comp Biochem Physiol. 1978;61C:261–266. doi: 10.1016/0306-4492(78)90051-5. [DOI] [PubMed] [Google Scholar]
- 32.Farooqui T, Vaessin H, Smith BH. Octopamine receptors in the honeybee (Apis mellifera) brain and their disruption by RNA-mediated interference. Journal of Insect Physiology. 2004;50:701–713. doi: 10.1016/j.jinsphys.2004.04.014. [DOI] [PubMed] [Google Scholar]