Abstract
Synapses are highly plastic and are modified by changes in patterns of neural activity or sensory experience. Plasticity of cortical excitatory synapses is thought to be important for learning and memory, leading to alterations in sensory representations and cognitive maps. However, these changes must be coordinated across other synapses within local circuits to preserve neural coding schemes and the organization of excitatory and inhibitory inputs, i.e., excitatory-inhibitory balance. Recent studies indicate that inhibitory synapses are also plastic and are controlled directly by a large number of neuromodulators, particularly during episodes of learning. Many modulators transiently alter excitatory-inhibitory balance by decreasing inhibition, and thus disinhibition has emerged as a major mechanism by which neuromodulation might enable long-term synaptic modifications naturally. This review examines the relationships between neuromodulation and synaptic plasticity, focusing on the induction of long-term changes that collectively enhance cortical excitatory-inhibitory balance for improving perception and behavior.
Keywords: cortex, inhibition, neuromodulation, synaptic plasticity
INTRODUCTION
Synapses are plastic, meaning their relative strengths, structures, and functions can be modified by changes in experience and activity. This confers neural circuits with a remarkable degree of flexibility, allowing the nervous system to adapt to changes in statistics and patterns of sensory input and to alterations of reward contingencies and learn from other forms of experience (Buonomano & Merzenich 1998, Carcea & Froemke 2013, Katz & Shatz 1996). Most studies of long-term synaptic plasticity have focused on long-term potentiation (LTP) and long-term depression (LTD) of specific excitatory synapses onto excitatory neurons in the hippocampus and neocortex (Bliss & Collingridge 1993). Much is now known about the induction of excitatory plasticity, in terms of the spike rates and precise spike timing required for LTP or LTD (Bi & Poo 1998, Bienenstock et al. 1982, Froemke et al. 2006, Kirkwood et al. 1993, Sjöström et al. 2001), as well as the mechanisms by which these long-term changes are expressed (Feldman 2009, Malenka & Nicoll 1999). Despite these advances, we are still far from understanding how cellular phenomena like LTP and LTD relate to learning, memory, and other cognitive processes (Cahill et al. 2001, Martin et al. 2000, Thompson 2005).
In part, this is because changes to specific excitatory synapses must be coordinated within existing networks of numerous other excitatory and inhibitory synapses. This may require modifications to excitatory synapses that were not activated during episodes of learning, conditioning, or synaptic plasticity induction procedures (i.e., forms of heterosynaptic plasticity). Additionally, connections onto or made by various inhibitory neuron subtypes may also be plastic (Vogels et al. 2013). However, much less is known about the rules and mechanisms of heterosynaptic and inhibitory plasticity, especially in the context of complex circuitry in vivo, in which multiple forms of plasticity with differential dynamics may be induced.
Similarly, it is unclear how stimulation procedures used for inducing synaptic modifications (e.g., spike pairing or high-frequency stimulation) relate to natural patterns of activity observed in vivo during episodes of learning or training. This includes areas beyond local sensory processing circuits, such as neuromodulatory systems that provide contextual or feedback signals (Marder 2012, Yu & Dayan 2005). Neuromodulation is critical for the induction (or expression) of many forms of cortical plasticity in vivo (Carcea & Froemke 2013, Gu 2002, Shulz et al. 2000, Weinberger 2007). Although many neuromodulators exist, recent progress has advanced our understanding of how neuromodulation enables plasticity in vitro and in vivo. We are also beginning to understand how synaptic modifications, at least those initiated within specific circuits, affect cognition and behavior, especially in the auditory system (Carcea & Froemke 2013, Shamma & Fritz 2014).
This review focuses on the relations between cortical neuromodulation and long-term synaptic plasticity. Initially, I provide an overview of excitatory plasticity induced by changes in local circuit activity and discuss inhibitory plasticity in the context of regulating overall cortical excitatory-inhibitory balance. I describe how cortical modulation enables synaptic modifications in vivo, comparing several neuromodulators that seem to be important for attentional control in distinct ways: acetylcholine, noradrenalin, and oxytocin. Although these and other neuromodulators can have diverse mechanisms of action, one consistent observation is that many modulators transiently reduce inhibitory transmission. Rapid and reversible disinhibition might improve signal-to-noise ratios for processing sensory input, effectively gating excitatory LTP and further mechanisms for reorganizing cortical circuits. Together, these recent findings demonstrate how cortical neuro-modulation can modify synaptic strength, improving excitatory-inhibitory balance and in some cases enhancing sensory perception and behavioral performance.
LONG-TERM SYNAPTIC PLASTICITY
Excitatory Plasticity
Most of what we know about long-term synaptic plasticity comes from studies of LTP and LTD of excitatory connections onto excitatory cells in the neocortex and hippocampus. Classically, LTP and LTD are induced by changes in presynaptic input firing rate, with brief (approximately 1-s) trains of high-frequency stimulation above 20 Hz generally inducing LTP, whereas sustained low-frequency stimulation (1 Hz for several minutes) induces LTD (Bliss & Collingridge 1993, Bliss & Lømo 1973, Kirkwood et al. 1993). This rate dependence of long-term synaptic plasticity is referred to as the BCM model, after the authors who codified it with the caveat that the specific transition point from LTD to LTP as a function of frequency is under homeostatic regulation (Abraham & Bear 1996, Bienenstock et al. 1982). Considerable evidence supports the application of this model across species and systems, and high-frequency stimulation is now conventionally used to probe the function and integrity of synaptic transmission throughout the nervous system (Kirkwood et al. 1993, Malenka & Nicoll 1999).
More recently, repetitive pairing of presynaptic stimulation with postsynaptic depolarization or postsynaptic spike firing has also been found to induce excitatory LTP and LTD. Changes induced by repetitive spike pairing are referred to as spike timing–dependent plasticity (STDP). The hallmark of STDP is a relatively brief time window on the order of tens of milliseconds by which paired pre- and postsynaptic spiking determines the sign and magnitude of long-term synaptic modification (Figure 1a). Specifically, at most excitatory connections, spike timing– dependent LTP is induced when postsynaptic spikes reliably follow presynaptic activation within 10 ms, whereas timing-dependent LTD is induced when postsynaptic spikes occur first before presynaptic spiking by 10–100 ms (Bell et al. 1997, Bi & Poo 1998, D’amour & Froemke 2015, Debanne et al. 1994, Feldman 2000, Froemke and Dan 2002, Markram et al. 1997, Sjöström et al. 2001, Song et al. 2000). These timing requirements for STDP induced by repetitive spike pairing are similar for many different types of excitatory synapses in vitro and in vivo (Dan & Poo 2006, Meliza & Dan 2006, Pawlak et al. 2013, Wittenberg & Wang 2006, Zhang et al. 1998) and are likely due to the activation kinetics of glutamate receptors and Ca2+ channels as well as interactions between them (Froemke et al. 2005, Larsen et al. 2010, Urakubo et al. 2008). This has made STDP useful for theoretical studies of synaptic plasticity in neural networks (Clopath et al. 2010, Gjorgjieva et al. 2011, Gütig & Sompolinsky 2006, Pfister & Gerstner 2006, Song et al. 2000, Vogels et al. 2011, Yao et al. 2004), as pre- and postsynaptic spike pairs can be considered fundamental units of synaptic modification.
Figure 1.
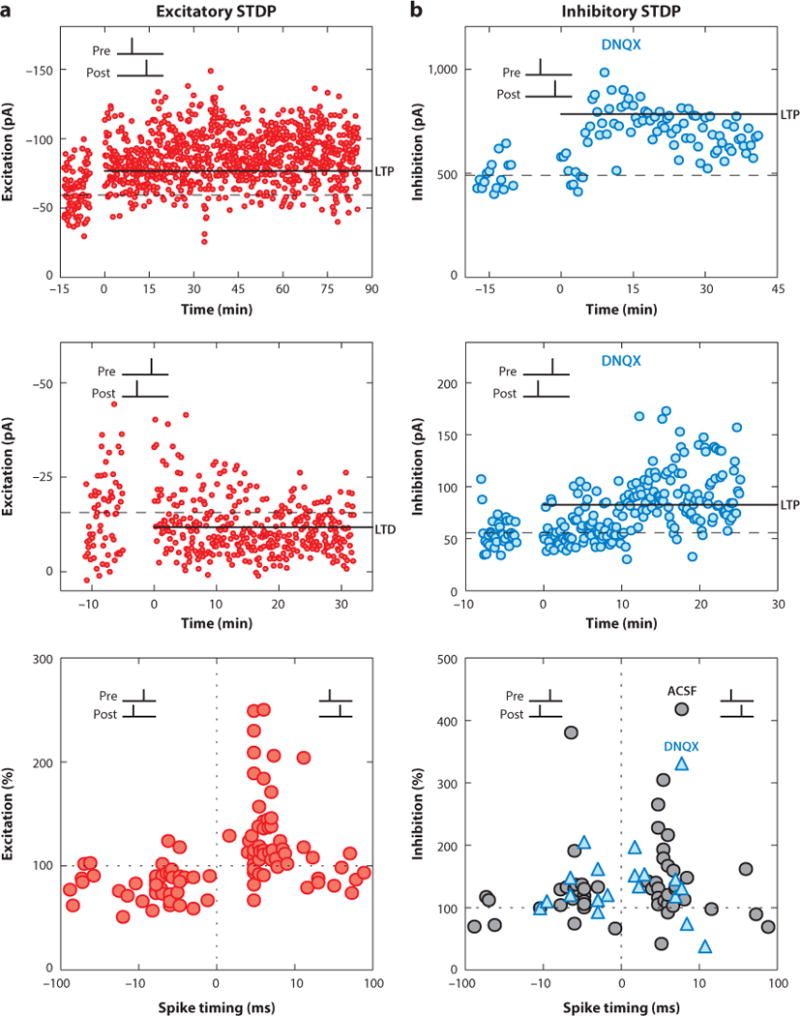
STDP of excitatory (a) and inhibitory (b) synaptic responses recorded in the same cells from slices of young mouse auditory cortex. (Top and middle) Examples of LTP and LTD induced by spike pairings of various timing intervals. Blue symbols in panel b indicate experiments performed with excitation blocked via DNQX. (Bottom) Summary of all cells, showing that spike pairing differentially affects excitation versus inhibition. Excitatory STDP has a Hebbian asymmetric window [short-interval pre-before-postsynaptic pairing (pre→post pairing) induced LTP, and post→pre pairing induced LTD], whereas inhibitory STDP is symmetric (LTP is induced by both pre→post and post→pre pairings). Adapted from D’amour & Froemke (2015). Abbreviations: ACSF, artificial cerebrospinal fluid; DNQX, 6,7-dinitroquinoxaline-2,3-dione; LTD, long-term depression; LTP, long-term potentiation; pA, picoamperes; STDP, spike timing–dependent plasticity.
However, STDP learning rules appear to be much more heterogeneous across different synapses and systems when three or more spikes occur in a short period of time or when synapses are physically located further in the dendrites from the site of axosomatic action potential generation. This heterogeneity in STDP is presumably due to the differences in postsynaptic responses depending on the dynamics of short-term plasticity (affecting the response to trains of presynaptic spikes) and the electrical properties of dendrites, including the change in shape of backpropagating action potentials (Froemke et al. 2010a,b). Thus, the details of short-term synaptic plasticity and dendritic integration are both major factors in determining the net change in synaptic strength in response to complex pre- and postsynaptic spike trains.
Both frequency-dependent and spike timing–dependent synaptic modifications are likely due to similar or the same underlying biological processes (Feldman 2009, Sjöström & Nelson 2002). In terms of mechanism, these stimulus patterns depolarize postsynaptic neurons, allowing for N-methyl-D-aspartate (NMDA) receptor activation and postsynaptic Ca2+ increases, which are sufficient to induce either LTP or LTD in a manner that depends on the duration and amplitude of Ca2+ influx (Froemke et al. 2005, Nishiyama et al. 2000, Yang et al. 1999). Postsynaptic spikes are not strictly necessary for induction of long-term synaptic plasticity (Lisman & Spruston 2005). In particular, recent in vivo studies in the visual, auditory, and barrel cortex have shown that repetitive or patterned sensory stimulation can enhance synaptic transmission and receptive field organization, even without the postsynaptic cell firing action potentials (Dorrn et al. 2010, Froemke et al. 2007, Li et al. 2008), as long as cortical NMDA receptors are functional (Froemke et al. 2013, Gambino et al. 2014).
Ultimately, the biophysical and biochemical properties of postsynaptic NMDA receptor signaling may underlie the induction of most (but not all) forms of long-term synaptic plasticity (Feldman 2012, Malenka & Nicoll 1999, Urakubo et al. 2008). GABAergic inhibition keeps NMDA receptors nominally in check, and transient reduction of inhibition is an effective means for gating plasticity in response to electrical or sensory stimulation (Artola et al. 1990, Chun et al. 2013, Froemke et al. 2007, Kuhlman et al. 2013). Growing evidence indicates that many neuromodulators, changes in sensory experience in vivo, or both lead to enduring modifications of neural circuits initially via transient disinhibition. In the sections below, I examine this hypothesis in detail.
Heterosynaptic Plasticity
Most studies examine excitatory modifications independently from inhibition. In part, this is because LTP and LTD can apparently be induced by BCM-type or STDP protocols in vitro regardless of whether inhibitory synapses are functional or blocked (Feldman 2000, Froemke & Dan 2002). However, excitatory LTP is a destabilizing positive feedback process, increasing the excitability of the circuit as a whole unless other compensatory changes occur within affected neurons or elsewhere in the local network (Song et al. 2000, Turrigiano 2008, Vogels et al. 2013).
Some of these compensatory changes could occur on other excitatory synapses that were not activated during pairing. For example, forms of homeostatic or heterosynaptic excitatory plasticity (e.g., heterosynaptic LTD) might act to decrease the overall level of excitation. Changes in activity can lead to homeostatic adjustments in spontaneous excitatory activity. Specifically, Turrigiano et al. (1998) showed that increases of activity in cultured cortical neurons by prolonged disinhibition led to a decrease in miniature excitatory postsynaptic current (mEPSC) amplitude and a normalization of firing rates after 48 h. This type of synaptic scaling has been observed in many neural systems (Turrigiano 2008), although in many cases the wide-scale nature and prolonged time course of these changes (hours to days) may not be sufficient to correct for the immediate increase in excitability after LTP induction, depending on the extent of synaptic modifications throughout the nervous system.
Heterosynaptic plasticity provides a complementary process for rapidly normalizing synaptic strength over individual cells and multicellular networks. After LTP induction at one set of excitatory inputs, heterosynaptic LTD can be observed within several minutes at other excitatory synapses onto the same cell (Christie et al. 1994, Lynch et al. 1977). Combined with homosynaptic LTD at activated inputs, heterosynaptic LTD helps control network dynamics and excitability within neural circuits (Stent 1973, von der Malsburg 1973).
Other types of modifications to cortical synapses in vivo and in vitro also occur, including heterosynaptic LTP (Dorrn et al. 2010, Meliza & Dan 2006, Royer & Paré 2003) and sensitization of neighboring inputs, lowering the threshold for STDP induction (Harvey & Svoboda 2007). Not all unpaired synapses undergo heterosynaptic modifications (Froemke et al. 2013, Royer & Paré 2003), and some inputs are conventionally used as a control pathway to ensure that changes to cellular excitability are minimal (Bliss & Lømo 1973, Kirkwood et al. 1993). The flexibility and input specificity of heterosynaptic modifications, combined with the speed at which these changes can be expressed, potentially enable this form of plasticity to regulate receptive field organization and neuronal computations with high resolution.
Which inputs are sensitive to heterosynaptic regulation? The mechanistic details of heterosynaptic modifications likely impose spatial and temporal constraints on the extent of heterosynaptic LTP or LTD, helping to determine which inputs are comodified during and after pairing or other plasticity-inducing episodes. Heterosynaptic LTD requires NMDA receptor activation (presumably at paired inputs) and depends on the release of Ca2+ from internal stores (Nishiyama et al. 2000, Royer & Paré 2003). In some cases, heterosynaptic interactions may involve other intracellular and intercellular diffusible factors (Scanziani et al. 1996), including small molecules like Ras (Harvey et al. 2008). Intracellular signaling systems like Ca2+ release from endoplasmic reticulum may enable coordination and induction of heterosynaptic plasticity at a much larger scale—possibly throughout the entire neuron (Dudman et al. 2007, Nakamura et al. 1999). The timescale for the induction of heterosynaptic modifications is also rather brief, occurring within approximately 10 min after induction of changes to paired inputs (Froemke et al. 2013, Harvey & Svoboda 2007).
Heterosynaptic modifications may play important roles in retuning cortical circuits in vivo. In general, reorganization of cortical receptive fields or shifts in tuning curves involve increased responses to paired or overrepresented stimuli, in parallel with decreases in responses to the original best stimulus or a deprived stimulus (Feldman 2009, Hensch & Fagiolini 2005, Weinberger 2007). Increases in responses to sensory stimuli are now generally accepted to be due to LTP at paired or reinforced inputs (Buonomano & Merzenich 1998, Froemke & Martins 2011). Behaviorally, strengthening auditory thalamocortical inputs to the amygdala via BCM-type stimulation procedures is sufficient to enhance auditory fear conditioning in trained animals (Nabavi et al. 2014). In contrast, heterosynaptic LTD may underlie the reduction in tuning curves at the original best stimuli. Recently, we showed in both the developing and adult auditory cortex (Dorrn et al. 2010, Froemke et al. 2013) that LTP induced at one tone frequency was matched by a form of heterosynaptic LTD at the original best frequency. Heterosynaptic LTD at the original best stimulus was activity dependent, occurring during the 10 min that followed induction of synaptic modifications. Surprisingly, if the original best stimulus was not presented during that period, the next largest stimulus was decreased instead (Froemke et al. 2013). This suggests that the inputs undergoing heterosynaptic modifications are not fixed or predetermined but are dynamically specified and computed from ongoing input statistics immediately following LTP induction. These coordinated changes act to preserve the relative tuning shape and width of cortical receptive fields, which may be important for sensory processing or other neural computations (Montgomery & Wehr 2010, Pouget et al. 1999). Furthermore, heterosynaptic LTD can act to control excitability by selectively decreasing the largest set of inputs reliably engaged in a brief period after synaptic plasticity induction and NMDA receptor activation.
Homosynaptic and heterosynaptic modifications of excitatory inputs onto inhibitory cells can also be induced by BCM-type and STDP protocols, possibly serving to control excitability in cortical and hippocampal networks (Lamsa et al. 2010, Lu et al. 2007). In their remarkable study of intercalated GABAergic neurons of the amygdala, Royer & Paré (2003) simultaneously monitored several excitatory inputs onto these cells before and after inducing long-term synaptic modifications. They found that high-frequency stimulation induced LTP at tetanized inputs but produced heterosynaptic LTD at specific neighboring unpaired inputs. Conversely, after inducing LTD with low-frequency stimulation, heterosynaptic LTP was induced at neighboring inputs. In each case, these bidirectional heterosynaptic changes acted to normalize the total amount of excitation received by these inhibitory neurons.
Inhibitory Plasticity
If excitatory synapses are modified (e.g., via STDP, changes in sensory experience, or episodes of learning), inhibitory synapses will also likely need to be rapidly adjusted as well. Over the past decade, numerous studies have shown that inhibitory synapses themselves are also plastic. Some initial work on inhibitory plasticity focused on the observation that early in development, GABAergic inputs are initially depolarizing and become hyperpolarizing over the first week or two of postnatal life in rodents because of changes in chloride transporters (Ben-Ari et al. 2012, Luhmann & Prince 1991, Owens et al. 1996). Woodin et al. (2003) showed that this process was activity dependent. They observed a form of STDP at hippocampal GABAergic synapses in vitro in which repetitive pre- and postsynaptic spike pairing decreased GABA reversal potentials, weakening GABAergic synaptic strength depending on membrane potential. Other studies have shown that specific GABAergic synapses can be also modified by changes to receptor expression or single-channel conductance (Haas et al. 2006, Holmgren & Zilberter 2001, Komatsu 1994, Kullmann et al. 2012, Lamsa et al. 2010, Nusser et al. 1998, Wang & Maffei 2014).
Theoretical studies have begun to examine the significance of inhibitory plasticity. One form of inhibitory STDP was postulated by Vogels et al. (2011) to account for changes in synaptic tuning curves observed in primary auditory cortex (Froemke et al. 2007). In their model, inhibitory STDP was symmetric in that both pre-before-post and post-before-pre pairing induced LTP at short time intervals, whereas longer intervals between pre- and postsynaptic spiking induced LTD. This time window is similar in shape to that described initially by Woodin et al. (2003), although the effective sign of modification is opposite. Recently, we have examined inhibitory and excitatory STDP together in slices of mouse auditory cortex (D’amour & Froemke 2015). The shape of the experimentally determined inhibitory STDP window (Figure 1b) is similar but not identical to that used by Vogels et al. (2011), although several different formulations of inhibitory STDP learning rules can have similar functional consequences, in terms of calibrating or balancing inhibition with excitation and controlling overall network excitability (Luz & Shamir 2012).
EXCITATORY-INHIBITORY BALANCE
Excitatory-Inhibitory Balance in Cortical Receptive Fields
Inhibitory plasticity could be useful for calibrating the global and fine-scale levels of activity throughout the nervous system. Inhibitory synapses are thought to be largely involved in regulating excitation throughout neural networks, controlling the rate and precise timing of spike generation at the single-cell level and neuronal ensemble organization and brain state at more macroscopic network levels (Fishell & Rudy 2011, Isaacson & Scanziani 2011). Thus, inhibition and inhibitory plasticity should be studied together with excitation—specifically, by measuring changes to coactivated excitatory and inhibitory inputs onto the same postsynaptic neurons to ask if changes to excitatory and inhibitory synapses might be coordinated.
This is a critical issue, as the organization of inhibitory synapses is thought to be carefully regulated over development and by experience to precisely match the organization, function, and strengths of excitatory synapses at different levels. The coregulation of excitation and inhibition is generally referred to as excitatory-inhibitory balance, often in terms of either overall global balance or, at higher resolutions, fine-scale balance. Global balance indicates that some bulk measure of synaptic input is normative, usually in relation to a pathological or dysfunctional state such as epilepsy. Studies of global balance often examine spontaneous or ongoing excitatory and inhibitory input [mEPSCs and miniature inhibitory postsynaptic currents (mIPSCs)] and field potentials as a physiological proxy for the relative timing and magnitude of inhibition versus excitation; they may also measure the numbers of excitatory and inhibitory neurons or synapses as an anatomical readout of cortical organization (Southwell et al. 2010, Yizhar et al. 2011).
In contrast, fine-scale balance indicates that the relative magnitudes of excitatory and inhibitory inputs are matched in a point-to-point manner across one or more dimensions. Intracellular (usually whole-cell) recordings in vivo can assess the synaptic currents and conductances that contribute to cortical receptive fields at this level, as a lack of spiking to some sensory stimulus could be due to a lack of excitation, too much inhibition, a change in spike threshold, or a combination of several different mechanisms difficult to determine with extracellular recordings alone. In adult auditory cortex, in vivo whole-cell voltage-clamp recordings have demonstrated that synaptic tuning curves for excitation and inhibition are similar (Figure 2a). Excitatory and inhibitory best stimuli for both frequency and intensity tend to be the same, and the overall profiles of excitation and inhibition are highly correlated (Dorrn et al. 2010; Froemke et al. 2007, 2013; Tan & Wehr 2009; Volkov & Galazjuk 1991; Wehr & Zador 2003). A short delay (usually a few milliseconds) occurs between the onset of excitatory and inhibitory conductances, allowing for a high degree of temporal precision for action potential generation in response to sensory stimuli (Wehr & Zador 2003). Higley and Contreras (2006) observed similar cotuning of excitatory and inhibitory conductances in barrel cortex.
Figure 2.
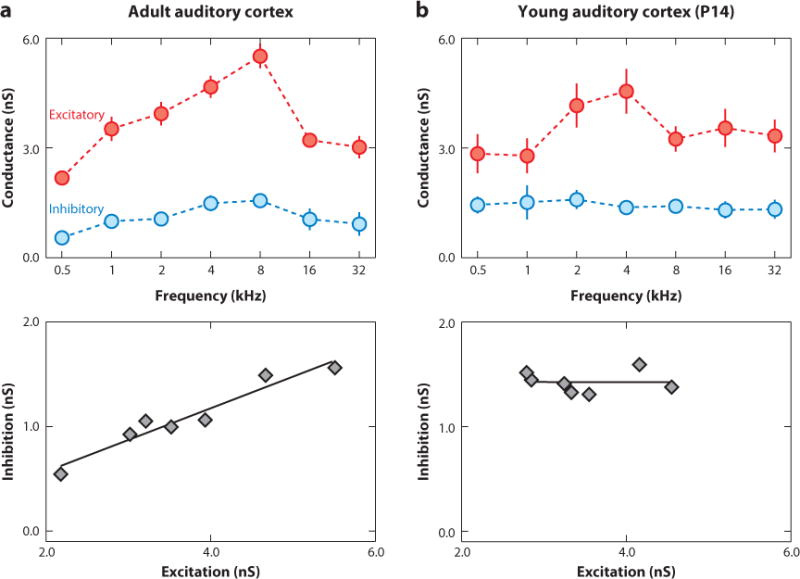
Development of cortical excitatory-inhibitory balance. (a) Balanced tone-evoked excitation and inhibition in adult rat primary auditory cortex. (Top) Frequency tuning curves for excitatory and inhibitory conductances. (Bottom) Excitation and inhibition were highly correlated (linear correlation coefficient r = 0.87). (b) Imbalanced excitatory and inhibitory frequency tuning in auditory cortex early in development. Whole-cell recording from a young [postnatal day 14 (P14)] rat (r = −0.01). Adapted from Dorrn et al. (2010). Abbreviation: nS, sanosiemens.
Thus, at least some degree of fine-scale balance appears to be a common feature of cortical organization in the adult nervous system. Excitatory and inhibitory spatial receptive fields, orientation tuning, and direction selectivity are matched and cotuned in most neurons of adult visual cortex (Ferster 1986, Hirsch et al. 1998, Liu et al. 2011, Mariño et al. 2005, Priebe & Ferster 2005), although some evidence supports cross-orientation suppression via imbalanced inhibition in some cells (Monier et al. 2003). Likewise, excitation and inhibition can be mismatched in the auditory cortex to support a range of preferred intensity tuning curves (Tan et al. 2007), perhaps increasing the sensitivity of cortical networks to lower-intensity sounds. Global and fine-scale balance of excitation and inhibition may therefore enable cortical computations by enhancing the dynamic range for sensitivity to sensory input (Priebe & Ferster 2005, Shadlen & Newsome 1998, van Vreeswijk & Sompolinsky 1996, Vogels et al. 2011). Moreover, moderate deviations from fine-scale balance may be important in some cases, as long as these imbalances are not large or long enough to pathologically disrupt cortical dynamics (Rubenstein & Merzenich 2003, Vogels et al. 2013, Yizhar et al. 2011).
Development of Cortical Excitatory-Inhibitory Balance
What generates cortical excitatory-inhibitory balance? The anatomical arrangement of inputs can constrain the types of interactions between excitation and inhibition as well as spatially define a functional domain for inhibitory control. Liu (2004) observed a conserved ratio of the numbers of excitatory and inhibitory synapses throughout the dendrites of cultured hippocampal neurons. On individual branches, the numbers of inhibitory puncta scaled with the numbers of excitatory puncta at several different developmental stages in vitro; additionally, inhibition could attenuate excitation effectively if coactivated at approximately the same time and place (on the order of approximately 20 ms and 10 μm). More recently, Xue et al. (2014) recorded responses of layer 2/3 pyramidal neurons in slices of young mouse visual cortex in response to photostimulation of layer 4. The ratio of evoked inhibition scaled with the magnitude of excitation, but interestingly, this form of equalized excitatory-inhibitory balance was due to recruitment of parvalbumin-positive but not somatostatin-positive interneurons. Thus, inhibition may be balanced with excitation in a cell-type and branch-specific manner, indicating that an anatomical basis for excitatory-inhibitory balance can be present.
However, although these measures indicate that excitation and inhibition are globally balanced even early in life, fine-scale balance emerges later and requires sensory experience (Froemke & Jones 2011). In particular, we have learned much about experience-dependent cortical plasticity from studies of the effects of sensory deprivation and exposure (Buonomano & Merzenich 1998, Feldman 2009, Katz & Shatz 1996). The auditory system has been especially useful for studies examining how cortical circuits are modified by changes in environmental acoustics. The visual system has also been important for describing how excitatory and inhibitory inputs are affected by sensory deprivation and injury (Hensch & Fagiolini 2005, Kuhlman et al. 2013), but the large literature on ocular dominance plasticity is beyond the scope of this review.
Distinct and overlapping critical or sensitive periods exist for the development of primary auditory cortex (Insanally et al. 2009). In rodents, the earliest cortical critical period seems to be for tonotopic refinement of characteristic frequency maps, beginning with hearing onset on about postnatal day (P) 10. Initially, at P10, cortical tonotopic maps in rats are unrefined, smaller, and focused generally on mid-to-high frequencies (de Villers-Sidani et al. 2007), although subcortical auditory circuits are largely in place (Froemke & Jones 2011). Rat pups exposed to a form of patterned stimulation (pulsed pure tones of one frequency) for three days between P9 and P11 had overrepresentations of that exposed frequency in primary auditory cortical tonotopic maps that persisted into adulthood. Later in life, patterned stimulation failed to affect tonotopic map organization, even if occurring just a few days later at P14+ (de Villers-Sidani et al. 2007, Insanally et al. 2009). Conversely, young rats exposed chronically to tonic white noise for the first month of life had less organized maps, with more broadly tuned neurons, and the critical period for frequency map development was delayed seemingly indefinitely (Chang & Merzenich 2003). Thus, the critical period for frequency map development occurs immediately upon hearing onset, leading to refinement and organization of cortical frequency tuning, and the extent of the critical period itself can be regulated by the pattern of sensory experience. Critical periods for bandwidth and direction selectivity for frequency-modulated sweeps then emerge later over the next few weeks of life in rats (Insanally et al. 2009).
Changes to synaptic receptive fields and fine-scale excitatory-inhibitory balance underlie these developmental refinements in cortical receptive fields and characteristic frequency maps. We examined the development of inhibitory and excitatory synaptic frequency tuning and found that excitatory and inhibitory tone-evoked responses were present at hearing onset and equally strong throughout life, i.e., global balance was intact (Dorrn et al. 2010). However, inhibition was initially poorly tuned and mismatched with the tuning profiles of excitatory inputs (Figure 2b). Over the first month of postnatal life, excitatory-inhibitory fine-scale balance emerged as inhibitory inputs became cotuned with excitation (Dorrn et al. 2010).
Plasticity of cortical excitatory-inhibitory balance was controlled by experience, as even just a few minutes of patterned stimulation could remodel synaptic tuning curves in a way that mimicked the natural increase in balance over postnatal weeks three and four. In young but not adult cortex, both excitation and inhibition at the presented frequency as well as responses within one octave were rapidly enhanced. Over the next 10 min, responses at the original best frequencies decreased, leading to a new preference for the presented stimulus and increasing the correlation between excitation and inhibition across frequencies (Dorrn et al. 2010). Intriguingly, one of the first changes to tone-evoked responses that occurred upon repetitive presentation of patterned stimuli was a rapid decrease in tone-evoked inhibition. Cortical inhibitory responses generally seem more sensitive to repetitive stimulation, adapting first and recovering more slowly than excitatory responses (Kuhlman et al. 2013, Taub et al. 2013, Wehr & Zador 2005). Rapid disinhibition may then enable excitatory responses to generate postsynaptic action potentials reliably or lead to strong NMDA receptor activation, gating LTP of paired excitatory and inhibitory inputs and perhaps engaging additional mechanisms of heterosynaptic plasticity to reconfigure receptive fields and increase fine-scale excitatory-inhibitory balance (D’amour & Froemke 2015, Froemke & Martins 2011).
CORTICAL NEUROMODULATION
What factors are required for cortical plasticity in vivo? Patterned sensory stimulation is sufficient for inducing long-term modifications of tone-evoked synaptic and spiking responses in young but not adult cortical neurons. STDP can also be induced in young rat visual cortex in vivo, altering visual responses and receptive fields after oriented bars are paired with postsynaptic spiking (Meliza & Dan 2006, Pawlak et al. 2013). However, in adult rat auditory cortex, pairing tones with postsynaptic spiking does not induce STDP or lead to long-term changes in synaptic frequency tuning curves (Froemke et al. 2007). Although STDP can be induced in vitro, even in slices from adult animals (Feldman 2000, Froemke & Dan 2002), a fundamental difference seems to exist between the requirements for plasticity in young and adult animals, particularly in vivo.
At least two potential and possibly related differences could account for the lower thresholds for plasticity induction in young cortex. For one, as mentioned above, inhibitory circuits could be finely balanced and calibrated in the adult cortex, permitting sensory inputs to evoke action potentials via AMPA receptor transmission, before cotuned inhibition reduces excitability and NMDA receptors are activated. Given that this excitatory-inhibitory sequence depends on the details of long-range and local circuit organization, inhibition need not act like this in vitro, where evoked inhibitory events are not necessarily functionally related to excitatory events in the fine-scale balance sense (as fine-scale balance is shaped by experience, meaningful only in terms of specific stimuli used to drive synaptic modifications).
Neuromodulation is also known to play a major role in cortical plasticity (Froemke & Martins 2011, Gu 2002, Pawlak et al. 2010, Weinberger 2007). In slices, blockade of cholinergic or noradrenergic receptors can affect or prevent STDP induction (Seol et al. 2007). A tonic modulatory tone could thus be permissive for long-term synaptic plasticity, or stimulation in the slice could evoke release from spared neuromodulatory fibers. In vivo, however, neuromodulatory systems must be engaged either artificially via stimulation of some sort (e.g., pharmacologically, electrically, or optically) or through changes in behavioral context that might naturally evoke release.
Many neuromodulators affect synaptic plasticity within cortical circuits. Particularly of note are modulators involved predominantly in various forms of attentional control, allowing for changes in sensory responses to be measured and controlled by stimulation paradigms. Three of these neuromodulators important for forms of attention and perceptual learning are discussed below, specifically in the context of cortical processing and plasticity: acetylcholine, noradrenalin, and oxytocin. Growing evidence indicates that each acts to disinhibit cortical networks, transiently disrupting excitatory-inhibitory balance to enable long-term changes in sensory receptive fields. A few other modulators are also mentioned in the context of cortical plasticity and excitatory-inhibitory balance.
Acetylcholine
Investigators have implicated acetylcholine in attention or learning and memory processes for decades (Bartus et al. 1982, Carcea & Froemke 2013, Sarter et al. 2009, Weinberger 2007, Zaborszky 2002). Acetylcholine in the central nervous system is synthesized in several different nuclei (and some local interneurons, particularly in the striatum), but the major source of acetylcholine for the cortex is the basal forebrain. The basal forebrain contains cholinergic projection neurons distributed throughout the nucleus basalis, substantia innominata, and part of the ventral globus pallidus that project to neocortex, although it should be noted that many GABAergic and glutamatergic neurons also project to cortex as well as other divisions of the basal forebrain (e.g., the medial septum cholinergic systems for the hippocampus). Lesions of this system or blockade of cortical muscarinic receptors produces defects of attention and disrupts learning and memory (McGaughy et al. 2000, Mesulam 2013).
In the visual cortex, application of cholinergic agonists can increase firing rates or receptive field selectivity for stimuli paired with cholinergic receptor activation in a manner that mimics or enhances selective attention toward sensory stimuli (Herrero et al. 2008, Kang et al. 2014, Treue & Maunsell 1996). Electrical or optogenetic stimulation of nucleus basalis simultaneously increases and decorrelates cortical responses (Goard & Dan 2009, Pinto et al. 2013). In conjunction with training, cholinergic modulation can also enhance perceptual learning (Kang et al. 2014). This may occur via a transient reduction in intracortical transmission, allowing thalamocortical input driven by sensory experience to be represented more strongly, perhaps when the sensory stimuli are unexpected relative to the cortical internal model. With time, these thalamic inputs might then integrate into the internal model by modification of intracortical connections and long-term changes to receptive fields (Gilbert et al. 2009, Hasselmo 2006).
One major mechanism by which acetylcholine might have these effects is via cortical disinhibition. Nucleus basalis stimulation enhances excitatory events and decreases inhibitory events in the auditory cortex (Froemke et al. 2007, Letzkus et al. 2011, Metherate & Ashe 1993), transiently desynchronizing cortical EEG and breaking excitatory-inhibitory balance for seconds to minutes. In their classic study, Bakin & Weinberger (1996) repetitively paired nucleus basalis stimulation with pure tones for several minutes in anesthetized adult rats and observed a shift in frequency tuning curves toward the paired frequency, due to an increase in spikes evoked by the paired tone, and a decrease in spiking evoked by the original best frequency. We examined the synaptic basis for these changes in cortical frequency tuning curves with in vivo voltage-clamp recordings from deeper layer neurons and found that repetitive nucleus basalis pairing shifted excitatory tuning curves within several minutes, followed by a delayed change in inhibitory tuning (Froemke et al. 2007, 2013). Over 1–3 h, inhibitory tuning eventually matched the new profile of excitation, recovering the fine-scale balance of excitation and inhibition (Figure 3a–d). This process required sensory experience, as keeping animals in silence prevented inhibition from re-balancing excitation. Consistent with the hypothesized role for neuromodulation in perceptual learning described above, cortical but not thalamic inputs were changed persistently by nucleus basalis pairing (Froemke et al. 2007), and these changes depended on NMDA receptor activation (Froemke et al. 2013), suggesting that cholinergic disinhibition led to NMDA receptor–dependent LTP and heterosynaptic plasticity of excitation and inhibition after repetitive sensory stimulation.
Figure 3.
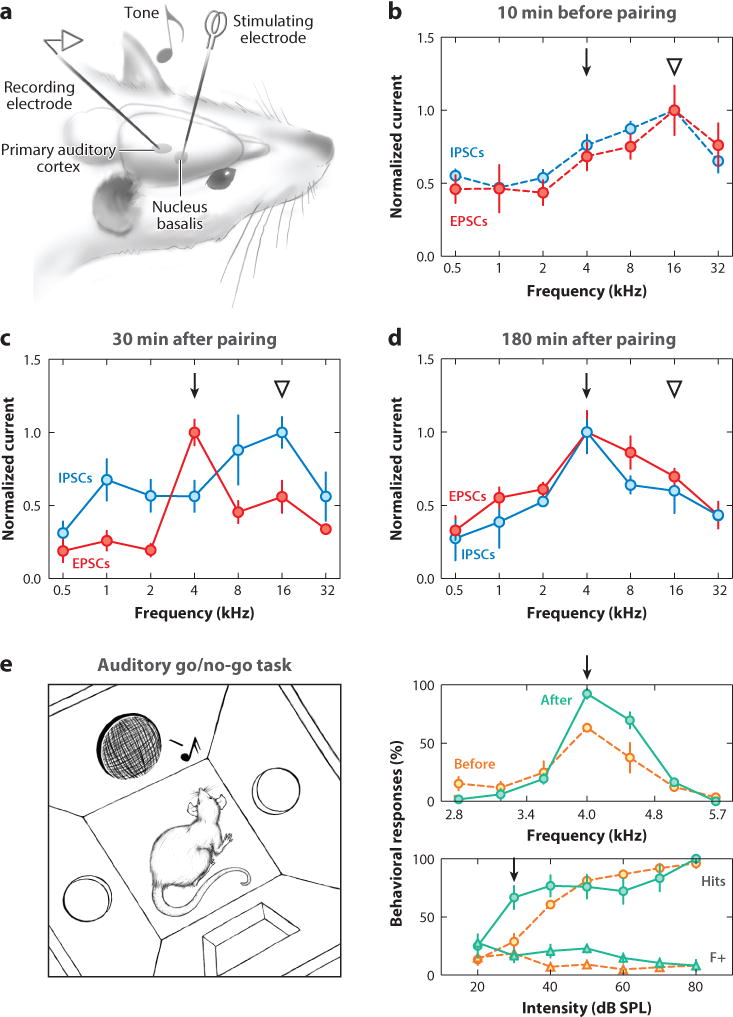
Functional consequences of nucleus basalis pairing. (a) Experimental setup for electrophysiological studies. (b) Synaptic tuning curve before pairing. Note cotuning of excitatory and inhibitory tuning curves. The triangle indicates the original best frequency, and the arrow indicates the frequency to be paired with nucleus basalis stimulation. (c) The same cells as in panel b 30 min after pairing. Inhibitory responses at the paired 4 kHz input have decreased, and the excitatory tuning curve has shifted to have a new peak at 4 kHz. (d) A different cell from the same animal as in panels b and c 180 min after pairing. Excitatory-inhibitory balance has recovered, but the tuning preference for both excitation and inhibition has shifted from the original best frequency (16 kHz) to the paired frequency (4 kHz). (e) Nucleus basalis pairing improves auditory perception. (Left) Diagram of behavioral training setup. (Right) Frequency recognition (top) and detection (bottom) of a paired 4 kHz tone before and 1–2 h after nucleus basalis pairing. Hit rates (circles) were enhanced to paired target tones, whereas false alarms (triangles) were unaffected. Adapted from Froemke et al. (2007, 2013). Abbreviations: dB SPL, decibels sound pressure level; EPSC, excitatory postsynaptic current; IPSC, inhibitory postsynaptic current.
Shifts in tuning curves and other forms of receptive field plasticity may serve as neural correlates of perceptual learning and associative memory. But how do these changes in cortical representations relate to sensory perception? Previously, Talwar et al. (2001) developed frequency-recognition and sound-detection tasks in adult rats sensitive to bilateral muscimol infusion into primary auditory cortex. They then used intracortical microstimulation to reliably produce long-lasting shifts in rat auditory cortex frequency tuning but, surprisingly, did not observe any changes in behavioral performance (Talwar & Gerstein 2001). We adopted a similar approach to investigate this issue further and determine conditions under which changes to cortical receptive fields might positively affect sensory perception (Froemke et al. 2013). We trained adult rats on a go/no-go task in which animals were rewarded for nose-poking to 4 kHz target tones of any intensity but experienced brief time-outs for nose-poking to nontarget foil tones (Figure 3e). We focused on determining liminal conditions for each animal at which detection or recognition performance was low but nonzero. We then paired those barely perceptible sounds with nucleus basalis stimulation for a few minutes and found that for several hours after pairing, behavioral performance was improved without the need for additional nucleus basalis stimulation (Figure 3e). Importantly, we could pair target tones with nucleus basalis stimulation for a few minutes when animals were anesthetized and still observed lasting gains in perceptual abilities for several hours after animals recovered (Froemke et al. 2013).
In the absence of further pairing or reinforcement, tuning curve shifts and behavioral improvements return to baseline after a few hours (Martins & Froemke 2014). Several days of pairing can lead to much longer-lasting changes in cortical receptive fields and tonotopic maps (Kilgard & Merzenich 1998), but these changes in auditory cortical map organization also appear to revert to the original configuration, despite lasting changes in behavioral performance (Reed et al. 2011). After multiple rounds of training, pairing, or both, other circuits such as frontal cortex (Fritz et al. 2010) or auditory corticostriatal projections may begin to support improved behavioral performance (Xiong et al. 2015, Znamenskiy & Zador 2013). Regardless, how cortical neurons are able to regain their original tuning preference and recover the original default prepairing or pretraining tonotopic organization remains unclear.
Nucleus basalis stimulation therefore produces a stimulus-specific decrease in tone-evoked inhibition. This disinhibition may enable selective shifts in frequency tuning without major changes in overall excitability or broadband gain (i.e., providing a form of x axis control for stimulus-response relationships, in which the peaks of tuning curves can shift toward stimuli related to episodes of heightened cholinergic tone). These physiological changes last for several hours in the absence of additional stimulation but can lead to changes elsewhere or throughout auditory sensorimotor circuits to support enduring behavioral changes in the absence of direct changes to primary sensory cortex.
Noradrenalin
Noradrenalin is a catecholamine neuromodulator also involved in attention and arousal (Aston-Jones & Cohen 2005, Eldar et al. 2013, Sara 2009), but the cholinergic and noradrenergic systems modulate cortical processing and behavior in fundamentally different ways. The functional distinctions might be reflected in or emerge from basic differences in the organization of the cholinergic versus noradrenergic nuclei and projections. The central source of noradrenalin for most of the brain is the locus coeruleus, one of several small brainstem nuclei positioned around the fourth ventricle. Whereas nucleus basalis cholinergic neurons project largely to the cortex, locus coeruleus neurons project extensively throughout the central nervous system (Berridge 2008, Fuxe et al. 2012). As locus coeruleus neurons are coupled together via gap junctions, these cells can potentially act in a coherent manner to deliver a uniform modulatory signal simultaneously to many target structures for wide-scale control of brain state (Carter et al. 2010, Constantinople & Bruno 2011, Sara 2009).
Yu and Dayan (2005) surveyed the behavioral and psychopharmacological literature and postulated that acetylcholine might encode expected uncertainty, whereas noradrenalin encodes unexpected uncertainty. In this model, cholinergic tone is related to the reliability of a specific stimulus for predicting an outcome and thus is useful for selective attention, whereas noradrenergic activity increases when a previously reliable stimulus suddenly becomes unreliable and other cues must be used. The increase in noradrenergic tone in response to surprising or possibly hazardous stimuli may be useful for updating cortical circuits via synaptic plasticity mechanisms to reflect these environmental changes or new contingencies and optimize sensorimotor transformations in a task-specific manner (Aston-Jones & Cohen 2005). However, both acetylcholine and noradrenalin are also important for cortical plasticity (Bear & Singer 1986, Carcea & Froemke 2013, Gu 2002, Seol et al. 2007), suggesting that these systems are also involved in reinforcement.
Noradrenalin modulation can produce substantially different kinds of changes in cortical receptive fields compared to acetylcholine. Across species, systems, and different anesthetics or brain states, cholinergic activation generally increases cortical excitability and shifts tuning curves toward paired stimuli in a manner that conserves overall excitability and preserves global excitatory-inhibitory balance (Froemke et al. 2007, Weinberger 2007). In contrast, repetitively pairing a pure tone with locus coeruleus stimulation often increases the responses much more dramatically, and at first, pairing that is specific to the locus coeruleus can boost responses to all stimuli, paired and unpaired, although with some preference for the paired frequency itself (Edeline et al. 2011, Martins & Froemke 2014). Long-term changes in auditory cortical tuning curves are somewhat more selective when norepinephrine iontophoresis is used instead of locus coeruleus stimulation (Manunta & Edeline 2004). These changes are incredibly enduring and persist for at least several hours or longer after a single pairing episode (Martins & Froemke 2014).
At the synaptic level, locus coeruleus pairing increases tone-evoked excitation and inhibition together. Instead of decreasing phasic, stimulus-evoked inhibition (as with nucleus basalis pairing), locus coeruleus pairing instead seems to decrease tonic, ongoing inhibition for a few minutes in the rat auditory cortex in vivo (Martins & Froemke 2014) and mouse cochlear nucleus in vitro (Kuo & Trussell 2011). The decrease in tonic inhibition leads to much larger responses (up to tenfold in amplitude) to any incoming input before excitability gradually recovers over an hour and tuning curves retain a new preference for paired inputs. Thus, noradrenalin from the locus coeruleus provides a distinct form of inhibitory control separate from that of acetylcholine, leading to overall response gain changes (i.e., shifting tuning curves along the y axis of stimulus-response relations). Such broadband enhancement of responsiveness may be a mechanism for noradrenalin to sensitize cortical circuits to incoming inputs during episodes of unexpected uncertainty.
Locus coeruleus neurons can also respond to sensory stimuli and change their responses depending on context and experience (Sara & Segal 1991). In other words, locus coeruleus neurons are also plastic. Innocuous sounds generally do not activate locus coeruleus neurons but can lead to synaptic and spiking responses in these cells after being paired with locus coeruleus stimulation or other means of activating the circuit, such as foot shock (Martins & Froemke 2014). Subsequently, sensory input alone can activate the locus coeruleus, leading to additional noradrenergic modulation in the absence of direct stimulation of the locus. By directly reactivating the locus coeruleus and reengaging mechanisms of neuromodulation and plasticity, neural representations or memories of arousing and unexpected experiences can be maintained, perhaps indefinitely.
Oxytocin
Oxytocin is a nine amino acid peptide hormone important for parental behavior and social cognition. The peptide is synthesized in the paraventricular nucleus and supraoptic nucleus of the hypothalamus and binds to a G protein–coupled receptor with a single isoform (Gimpl & Fahrenholz 2001). Peripheral release of oxytocin via the pituitary gland is important for parturition and lactation, whereas central release has cognitive effects related to pair-bond formation (Bartz et al. 2011, Carter 1998, Dulac et al. 2014, Insel & Young 2001). Although not historically considered a major modulator of cortical processing, growing evidence suggests that oxytocin acts to enhance the salience of socially relevant sensory input by disinhibiting cortical networks, similar to acetylcholine.
Recently, Owen et al. (2013) found that the oxytocin receptor agonist [Thr4,Gly7]-oxytocin (TGOT) decreased spontaneous firing but increased stimulus-evoked action potential generation, and TGOT decreased evoked IPSCs but increased spontaneous IPSCs in CA1 pyramidal neurons from hippocampal slices. TGOT directly depolarized fast-spiking interneurons, which increased the spontaneous rate of GABA release and led to short-term depression of inhibitory connections onto excitatory cells. Similar disinhibitory effects occurred in response to cholecystokinin modulation or estrogen in hippocampal slices (Owen et al. 2013, Rudick et al. 2003), indicating that peptide and steroid hormones can act as rapid modulators of circuit activity in addition to changes to vascular tone or gene expression traditionally ascribed to these molecules (Altura & Altura 1977, Gimpl & Fahrenholz 2001).
Oxytocin can act in the mouse auditory cortex to enhance the responses to infant distress calls. When separated from the nest, mouse pups emit ultrasonic isolation calls, which mothers use to locate and retrieve them (Ehret 2005, Noirot 1972, Sewell 1970). Virgin females do not initially retrieve pups but can begin to express retrieval behavior after experience with pups. Oxytocin in the left auditory cortex, either applied pharmacologically or released via optogenetic stimulation, enables and accelerates expression of this experience-dependent retrieval behavior (Marlin et al. 2015). In recordings from auditory cortical neurons, pup distress calls evoke much stronger spiking responses in mothers than in virgins, possibly increasing the detection and discrimination of pup calls (Cohen et al. 2011, Ehret 2005, Liu & Schreiner 2007, Marlin et al. 2015). We observed imbalanced call-evoked excitation and inhibition in virgin animals, such that poorly timed synaptic inputs triggered by infant vocalizations fail to generate action potentials reliably (Marlin et al. 2015). However, with experience or after pairing oxytocin with pup call presentation, weaker virgin auditory cortical responses are transformed into more robust and temporally precise maternal-like responses. In the auditory cortex in vivo and in slices, oxytocin transiently reduces call-evoked inhibition, leading to a long-term enhancement of excitation that is then followed after 1 h by an increase and temporal restructuring of call-evoked inhibition to better match the amplitude and temporal pattern of call-evoked excitatory events (Marlin et al. 2015). These dynamics by which initially imbalanced excitation and inhibition become balanced are reminiscent of the changes to tone-evoked synaptic responses during postnatal development, when animals have had limited exposure or experience with certain sounds (Dorrn et al. 2010).
It remains a challenge to determine how excitatory and inhibitory synaptic modifications—driven by sensory experience with relatively few exemplar vocalizations—can be used to shape complex behaviors such as pup retrieval, in which animals must respond rapidly to many different specific calls in the presence of background sounds. Furthermore, little information is currently available about how hypothalamic peptides contact and communicate with cortical circuits or which elements in the auditory cortex express oxytocin receptors.
Other Neuromodulators
Many other neuromodulators disinhibit cortical circuits, providing a common mechanism for gating excitatory and inhibitory synaptic plasticity. Kruglikov & Rudy (2008) demonstrated that muscarine, serotonin, baclofen (a GABAB receptor agonist), and adenosine each reduce GABA release from fast-spiking interneurons onto excitatory cells in barrel cortex. Interestingly, noradrenalin did not function like this at these connections, suggesting that it might act on other populations of inhibitory cells or inhibitory synapses to control spontaneous activity and overall inhibitory tone.
In addition to long-range modulators originating outside of the cortex, some local retrograde messengers can also disinhibit cortical networks. Endocannabinoids mediate a form of short-term plasticity known as depolarization-induced suppression of inhibition, and endocannabinoids have been implicated as a retrograde signal important for STDP (Feldman 2009, Sjöström et al. 2003), synthesized and released from postsynaptic pyramidal neurons.
Of course, these and other neuromodulators have other modes of action besides disinhibition, depending on target neuron identity and types of receptors expressed. Dopamine in particular can bidirectionally regulate excitatory and inhibitory events, depending on which receptors are activated. D1-type receptors can enhance both NMDA receptor excitatory currents and IPSCs, whereas D2-type receptors reduce NMDA receptor responses and IPSCs (Seamans & Yang 2004). This kind of modulation potentially provides bidirectional regulation of cortical synaptic strength by directly increasing or decreasing signaling through NMDA receptors. Ventral tegmental area stimulation has been shown to regulate auditory cortical map organization bidirectionally (Bao et al. 2001), although the synaptic mechanisms by which dopamine controls receptive field plasticity remain to be determined.
CORTICAL NEUROMODULATION AND PLASTICITY: A SYNTHETIC VIEW
Taken together, these studies support the straightforward hypothesis that episodes of learning, training, and other kinds of arousing or important experiences lead to behavioral changes via modification of neural circuits within the cortex and throughout the rest of the brain. More specifically, different behavioral contexts activate distinct constellations of neuromodulatory systems, which transiently disrupt cortical excitatory-inhibitory balance to enable the induction of long-term synaptic plasticity. Sensory inputs activated during this change in modulatory tone are disinhibited, leading to more reliable spike firing, enhanced activation of NMDA receptors, and LTP of excitatory synapses. Shortly thereafter, on the timescale of minutes, heterosynaptic modifications to other excitatory synapses occur to decrease the strength of the originally strongest inputs and perhaps normalize or strengthen inputs functionally related to the paired inputs. Finally, inhibitory responses are also modified over hours to match the fine-scale profile of excitation. Once excitatory-inhibitory balance is restored, the network resumes a less plastic state of normative sensory processing.
The large number of neuromodulator systems provides a high degree of control over cortical computation and plasticity, potentially at multiple scales, owing to heterogeneity of receptor expression, anatomical projections, and other factors that control paracrine and synaptic transmission. Various modulators may act over different temporal or spatial scales, changing synaptic integration and excitatory-inhibitory balance locally or throughout the brain. For example, the cholinergic system appears to be organized topographically in terms of projections to different cortical areas (Mesulam 2013), whereas locus coeruleus neurons project extensively throughout the brain (Sara 2009). Cholinergic projections can form synapses onto target cells, and cholinesterase can act quickly to break down acetylcholine and limit diffusion away from release sites (Sarter et al. 2009, Umbriaco et al. 1994). In contrast, noradrenalin and oxytocin projections may provide forms of volume transmission (Fuxe et al. 2012). Importantly, neuromodulatory systems are interconnected extensively, so these systems are more likely to be recruited together and comodulate cortical networks and other target areas, rather than a single modulator system operating independently, e.g., during episodes of heightened attention. The locus coeruleus provides a major input to the oxytocinergic paraventricular nucleus (Sawchenko & Swanson 1982). Oxytocin may be an important modulator of nucleus basalis, as indicated by recent autoradiography and in situ hybridization data from macaques (Freeman et al. 2014). In general, then, there need not be anything particularly unique about, for example, acetylcholine for attention and perceptual learning or oxytocin for pair-bond formation. Instead, the details of the circuitry and the types of environmental stimuli that activate these circuits might impart such functional specialization.
Given the importance of neuromodulation for adult cortical plasticity, what role does postsynaptic spiking play in these plasticity processes? An increase in firing rate and STDP paradigms are effective experimental means for changing synaptic strength, but long-term synaptic modifications and changes to sensory responses can also be induced in the absence of postsynaptic spiking (Gambino et al. 2014, Lisman & Spruston 2005). However, cortical coding schemes and sensory representations ultimately rely on spikes, and in some sense, fine-scale excitatory-inhibitory balance must be defined and thus coordinated by correlated pre- and postsynaptic activity. Local dendritic and NMDA receptor spikes might also be involved in these processes during development or heightened and permissive periods of cortical neuromodulation.
CONCLUSIONS
Synaptic plasticity enables neural circuits to be dynamic, adapting to the statistics of sensory inputs and activity patterns for flexible control of perception and behavior. Much work has focused on the plasticity of excitatory synapses, but inhibitory synapses can also be modified. Populations of excitatory and inhibitory synapses must likely be adjusted in a complex and coordinated manner to preserve excitatory-inhibitory balance and prevent neural systems from remaining hypo- or hyperexcitable for prolonged periods. Although many procedures and paradigms are available for inducing plasticity in cortical neurons, the activation of subcortical modulatory systems may provide a natural mechanism for transiently disinhibiting target networks and gate subsequent modifications depending on behavioral context. Supporting this hypothesis, investigators have recently found that many neuromodulators share a common disinhibitory mode of action in the cortex. Studies in the auditory cortex in particular have proved useful for examining plasticity in vivo, to relate neuromodulation of excitatory-inhibitory balance to changes in synaptic receptive field organization and modifications of cortical receptive fields to improvements in sensory perception. Understanding these relationships will be aided by efforts to determine how various neuromodulatory systems are activated during different behavioral episodes.
Acknowledgments
D. Bliss created the artwork in Figure 3a. S.E. Ross created the artwork in Figure 3e. This work was funded by the National Institute on Deafness and Other Communication Disorders (DC009635 and DC12557), a Hirschl/Weill-Caulier Career Award, a Klingenstein Fellowship Award in the Neurosciences, a McKnight Scholarship, a Pew Scholarship, a Sloan Research Fellowship, and a Whitehead Foundation Fellowship. I thank I. Carcea and C.M. Constantinople for comments.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–30. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Altura BM, Altura BT. Vascular smooth muscle and neurohypophyseal hormones. Fed Proc. 1977;36:1853–60. [PubMed] [Google Scholar]
- Artola A, Bröcher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. The cholinergic hypothesis of geriatric memory dysfunction. PNAS. 1996;93:11219–24. [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–76. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–81. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Woodin MA, Sernagor E, Cancedda L, Vinay L, et al. Refuting the challenges of the developmental shift of polarity of GABA actions: GABA more exciting than ever! Front Cell Neurosci. 2012;6:35. doi: 10.3389/fncel.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–72. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL, Weinberger NM. The neurobiology of learning and memory: some reminders to remember. Trends Neurosci. 2001;24:578–81. doi: 10.1016/s0166-2236(00)01885-3. [DOI] [PubMed] [Google Scholar]
- Carcea I, Froemke RC. Cortical plasticity, excitatory-inhibitory balance, and sensory perception. Prog Brain Res. 2013;207:65–90. doi: 10.1016/B978-0-444-63327-9.00003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–813. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Christie BR, Kerr S, Abraham WC. Flip side of synaptic plasticity: long-term depression mechanisms in the hippocampus. Hippocampus. 1994;4:127–35. doi: 10.1002/hipo.450040203. [DOI] [PubMed] [Google Scholar]
- Chun S, Bayazitov IT, Blundon JA, Zakharenko SS. Thalamocortical long-term potentiation becomes gated after the early critical period in the auditory cortex. J Neurosci. 2013;33:7345–57. doi: 10.1523/JNEUROSCI.4500-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopath C, Busing L, Vasilaki E, Gerstner W. Connectivity reflects coding: a model of voltage-based STDP with homeostasis. Nat Neurosci. 2010;13:344–52. doi: 10.1038/nn.2479. [DOI] [PubMed] [Google Scholar]
- Cohen L, Rothschild G, Mizrahi A. Multisensory integration of natural odors and sounds in the auditory cortex. Neuron. 2011;72:357–69. doi: 10.1016/j.neuron.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–68. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’amour JA, Froemke RC. Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron. 2015 doi: 10.1016/j.neuron.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–48. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gähwiler BH, Thompson SM. Asynchronous pre- and postsynaptic activity induces associative long-term depression in area CA1 of the rat hippocampus in vitro. PNAS. 1994;91:1148–52. doi: 10.1073/pnas.91.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–89. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–36. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudman JT, Tsay D, Siegelbaum SA. A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron. 2007;56:866–79. doi: 10.1016/j.neuron.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, O’Connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345:765–70. doi: 10.1126/science.1253291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM, Manunta Y, Hennevin E. Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear Res. 2011;274:75–84. doi: 10.1016/j.heares.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Ehret G. Infant rodent ultrasounds—a gate to the understanding of sound communication. Behav Genet. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nat Neurosci. 2013;16:1146–53. doi: 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron. 2000;27:45–56. doi: 10.1016/s0896-6273(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–71. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J Neurosci. 1986;6:1284–301. doi: 10.1523/JNEUROSCI.06-05-01284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Rudy B. Mechanisms of inhibition within the telencephalon: “where the wild things are. Annu Rev Neurosci. 2011;34:535–67. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–41. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JB, David SV, Radtke-Schuller S, Yin P, Shamma SA. Adaptive, behaviorally gated, persistent encoding of task-relevant auditory information in ferret frontal cortex. Nat Neurosci. 2010;13:1011–19. doi: 10.1038/nn.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, et al. Long-term modification of cortical synapses improves sensory perception. Nat Neurosci. 2013;16:79–88. doi: 10.1038/nn.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Dan Y. Spike-timing-dependent synaptic modification induced by natural spike trains. Nature. 2002;416:433–38. doi: 10.1038/416433a. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Debanne D, Bi GQ. Temporal modulation of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010a;2:19. doi: 10.3389/fnsyn.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Jones BC. Development of auditory cortical synaptic receptive fields. Neurosci Biobehav Rev. 2011;35:2105–13. doi: 10.1016/j.neubiorev.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Letzkus JJ, Kampa BM, Hang GB, Stuart GJ. Dendritic synapse location and neocortical spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010b;2:29. doi: 10.3389/fnsyn.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Martins AR. Spectrotemporal dynamics of auditory cortical synaptic receptive field plasticity. Hear Res. 2011;279:149–61. doi: 10.1016/j.heares.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–29. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Poo MM, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–25. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Tsay IA, Raad M, Long JD, Dan Y. Contribution of individual spikes in burst-induced long-term synaptic modification. J Neurophysiol. 2006;95:1620–29. doi: 10.1152/jn.00910.2005. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Ciruela F, Manger P, et al. On the role of volume transmission and receptor–receptor interactions in social behaviour: focus on central catecholamine and oxytocin neurons. Brain Res. 2012;1476:119–31. doi: 10.1016/j.brainres.2012.01.062. [DOI] [PubMed] [Google Scholar]
- Gambino F, Pages S, Kehayas V, Baptista D, Tatti R, et al. Sensory-evoked LTP driven by dendritic plateau potentials in vivo. Nature. 2014;515:116–19. doi: 10.1038/nature13664. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Li W, Piech V. Perceptual learning and adult cortical plasticity. J Physiol. 2009;587:2743–51. doi: 10.1113/jphysiol.2009.171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gjorgjieva J, Clopath C, Audet J, Pfister JP. A triplet spike-timing–dependent plasticity model generalizes the Bienenstock–Cooper–Munro rule to higher-order spatiotemporal correlations. PNAS. 2011;108:19383–88. doi: 10.1073/pnas.1105933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–49. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–35. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Gütig R, Sompolinsky H. The tempotron: a neuron that learns spike timing–based decisions. Nat Neurosci. 2006;9:420–28. doi: 10.1038/nn1643. [DOI] [PubMed] [Google Scholar]
- Haas JS, Nowotny T, Abarbanel HD. Spike-timing-dependent plasticity of inhibitory synapses in the entorhinal cortex. J Neurophysiol. 2006;96:3305–13. doi: 10.1152/jn.00551.2006. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–40. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–15. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005;147:115–24. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–14. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J Neurosci. 2006;26:448–57. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Alonso JM, Reid RC, Martinez LM. Synaptic integration in striate cortical simple cells. J Neurosci. 1998;18:9517–28. doi: 10.1523/JNEUROSCI.18-22-09517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren CD, Zilberter Y. Coincident spiking activity induces long-term changes in inhibition of neocortical pyramidal cells. J Neurosci. 2001;21:8270–77. doi: 10.1523/JNEUROSCI.21-20-08270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insanally MN, Köver H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. J Neurosci. 2009;29:5456–62. doi: 10.1523/JNEUROSCI.5311-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–36. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–43. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JI, Huppe-Gourgués F, Vaucher E. Boosting visual cortex function and plasticity with acetylcholine to enhance visual perception. Front Syst Neurosci. 2014;8:172. doi: 10.3389/fnsys.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–38. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–48. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993;260:1518–21. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- Komatsu Y. Age-dependent long-term potentiation of inhibitory synaptic transmission in rat visual cortex. J Neurosci. 1994;14:6488–99. doi: 10.1523/JNEUROSCI.14-11-06488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–24. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501:543–46. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Moreau AW, Bakiri Y, Nicholson E. Plasticity of inhibition. Neuron. 2012;75:951–62. doi: 10.1016/j.neuron.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Kuo SP, Trussell LO. Spontaneous spiking and synaptic depression underlie noradrenergic control of feed-forward inhibition. Neuron. 2011;71:306–18. doi: 10.1016/j.neuron.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa KP, Kullmann DM, Woodin MA. Spike-timing dependent plasticity in inhibitory circuits. Front Synaptic Neurosci. 2010;2:16. doi: 10.3389/fnsyn.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RS, Rao D, Manis PB, Philpot BD. STDP in the developing sensory neocortex. Front Synaptic Neurosci. 2010;2:9. doi: 10.3389/fnsyn.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SBE, Meyer EMM, Tovote P, Courtin J, et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–35. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Li Y, Van Hooser SD, Mazurek M, White LE, Fitzpatrick D. Experience with moving visual stimuli drives the early development of cortical direction selectivity. Nature. 2008;456:952–56. doi: 10.1038/nature07417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Spruston N. Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity. Nat Neurosci. 2005;8:839–41. doi: 10.1038/nn0705-839. [DOI] [PubMed] [Google Scholar]
- Liu BH, Li YT, Ma WP, Pan CJ, Zhang LI, Tao HW. Broad inhibition sharpens orientation selectivity by expanding input dynamic range in mouse simple cells. Neuron. 2011;71:542–54. doi: 10.1016/j.neuron.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci. 2004;7:373–79. doi: 10.1038/nn1206. [DOI] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory cortical detection and discrimination correlates with communicative significance. PLOS Biol. 2007;5:e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JT, Li CY, Zhao JP, Poo MM, Zhang XH. Spike-timing-dependent plasticity of neocortical excitatory synapses on inhibitory interneurons depends on target cell type. J Neurosci. 2007;27:9711–20. doi: 10.1523/JNEUROSCI.2513-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J Neuro-physiol. 1991;65:247–63. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Luz Y, Shamir M. Balancing feed-forward excitation and inhibition via Hebbian inhibitory synaptic plasticity. PLOS Comput Biol. 2012;8:e1002334. doi: 10.1371/journal.pcbi.1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GS, Dunwiddie T, Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977;266:737–39. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation: a decade of progress? Science. 1999;285:1870–74. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J Neurophysiol. 2004;92:1445–63. doi: 10.1152/jn.00079.2004. [DOI] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño J, Schummers J, Lyon DC, Schwabe L, Beck O, et al. Invariant computations in local cortical networks with balanced excitation and inhibition. Nat Neurosci. 2005;8:194–201. doi: 10.1038/nn1391. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–15. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behavior by balancing cortical inhibition. Nature. 2015 doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Martins ARO, Froemke RC. Locus coeruleus plasticity controls cortical plasticity. Soc Neurosci Abstr. 2014;363:12. [Google Scholar]
- McGaughy J, Everitt BJ, Robbins TW, Sarter M. The role of cortical cholinergic afferent projections in cognition: impact of new selective immunotoxins. Behav Brain Res. 2000;115:251–63. doi: 10.1016/s0166-4328(00)00262-x. [DOI] [PubMed] [Google Scholar]
- Meliza CD, Dan Y. Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking. Neuron. 2006;49:183–89. doi: 10.1016/j.neuron.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J Comp Neurol. 2013;521:4124–44. doi: 10.1002/cne.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14:132–43. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Monier C, Chavane F, Baudot P, Graham LJ, Frégnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron. 2003;37:663–80. doi: 10.1016/s0896-6273(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Montgomery N, Wehr M. Auditory cortical neurons convey maximal stimulus-specific information at their best frequency. J Neurosci. 2010;30:13362–66. doi: 10.1523/JNEUROSCI.2899-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–52. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Barbara JG, Nakamura K, Ross WN. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron. 1999;24:727–37. doi: 10.1016/s0896-6273(00)81125-3. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–88. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- Noirot E. Ultrasounds and maternal behavior in small rodents. Dev Psychobiol. 1972;5:371–77. doi: 10.1002/dev.420050410. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Hájos N, Somogyi P, Mody I. Increased number of synaptic GABAA receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–77. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–62. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MBE, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–23. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V, Wickens JR, Kirkwood A, Kerr JN. Timing is not everything: Neuromodulation opens the STDP gate. Front Synap Neurosci. 2010;2:146. doi: 10.3389/fnsyn.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V, Greenberg DS, Sprekeler H, Gerstner W, Kerr JN. Changing the responses of cortical neurons from sub- to suprathreshold using single spikes in vivo. eLIFE. 2013;2:e00012. doi: 10.7554/eLife.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister JP, Gerstner W. Triplets of spikes in a model of spike timing-dependent plasticity. J Neurosci. 2006;26:9673–82. doi: 10.1523/JNEUROSCI.1425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, et al. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci. 2013;16:1857–63. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget A, Deneve S, Ducom JC, Latham PE. Narrow versus wide tuning curves: What’s best for a population code? Neural Comput. 1999;11:85–90. doi: 10.1162/089976699300016818. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Direction selectivity of excitation and inhibition in simple cells of the cat primary visual cortex. Neuron. 2005;45:133–45. doi: 10.1016/j.neuron.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, et al. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–31. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Royer S, Paré D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature. 2003;422:518–22. doi: 10.1038/nature01530. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–67. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci. 2003;23:4479–90. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–23. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Segal M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog Brain Res. 1991;88:571–85. doi: 10.1016/s0079-6123(08)63835-2. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat Rev Neurosci. 2009;10:383–90. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res Rev. 1982;4:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Malenka RC, Nicoll RA. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature. 1996;422:518–22. doi: 10.1038/nature01530. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, et al. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–29. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell GD. Ultrasonic communication in rodents. Nature. 1970;227:410. doi: 10.1038/227410a0. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci. 1998;18:3870–96. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamma S, Fritz J. Adaptive auditory computations. Curr Opin Neurobiol. 2014;25:164–68. doi: 10.1016/j.conb.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulz DE, Sosnik R, Ego V, Haidarliu S, Ahissar E. A neuronal analogue of state-dependent learning. Nature. 2000;403:549–53. doi: 10.1038/35000586. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Nelson SB. Spike timing, calcium signals and synaptic plasticity. Curr Opin Neurobiol. 2002;12:305–14. doi: 10.1016/s0959-4388(02)00325-2. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–64. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–54. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Song S, Miller KD, Abbott LF. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat Neurosci. 2000;3:919–26. doi: 10.1038/78829. [DOI] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–48. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stent G. A physiological mechanism for Hebb’s postulate of learning. PNAS. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar SK, Gerstein GL. Reorganization in awake rat auditory cortex by local microstimulation and its effect on frequency-discrimination behavior. J Neurophysiol. 2001;86:1555–72. doi: 10.1152/jn.2001.86.4.1555. [DOI] [PubMed] [Google Scholar]
- Talwar SK, Musial PG, Gerstein GL. Role of mammalian auditory cortex in the perception of elementary sound properties. J Neurophysiol. 2001;85:2350–58. doi: 10.1152/jn.2001.85.6.2350. [DOI] [PubMed] [Google Scholar]
- Tan AYY, Atencio CA, Polley DB, Merzenich MM, Schreiner CE. Unbalanced synaptic inhibition can create intensity-tuned auditory cortex neurons. Neuroscience. 2007;146:449–62. doi: 10.1016/j.neuroscience.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Tan AYY, Wehr M. Balanced tone-evoked synaptic excitation and inhibition in mouse auditory cortex. Neuroscience. 2009;163:1302–15. doi: 10.1016/j.neuroscience.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Taub AH, Katz Y, Lampl I. Cortical balance of excitation and inhibition is regulated by the rate of synaptic activity. J Neurosci. 2013;33:14359–68. doi: 10.1523/JNEUROSCI.1748-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF. In search of memory traces. Annu Rev Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JHR. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–41. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–35. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–96. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Watkins KC, Descarries L, Cozzari C, Hartman BK. Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. J Comp Neurol. 1994;348:351–73. doi: 10.1002/cne.903480304. [DOI] [PubMed] [Google Scholar]
- Urakubo H, Honda M, Froemke RC, Kuroda S. Requirement of an allosteric kinetics of NMDA receptors for spike-timing-dependent plasticity. J Neurosci. 2008;28:3310–23. doi: 10.1523/JNEUROSCI.0303-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vreeswijk C, Sompolinsky H. Chaos in neural networks with balanced excitatory and inhibitory activity. Science. 1996;274:1724–26. doi: 10.1126/science.274.5293.1724. [DOI] [PubMed] [Google Scholar]
- Vogels TP, Froemke RC, Doyon N, Gilson M, Haas JS, et al. Inhibitory synaptic plasticity: spike timing-dependence and putative network function. Front Neural Circuits. 2013;7:119. doi: 10.3389/fncir.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Sprekeler H, Zenke F, Clopath C, Gerstner W. Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science. 2011;334:1569–73. doi: 10.1126/science.1211095. [DOI] [PubMed] [Google Scholar]
- Volkov IO, Galazjuk AV. Formation of spike responses to sound tones in cat auditory cortex neurons: interaction of excitatory and inhibitory effects. Neuroscience. 1991;43:307–21. doi: 10.1016/0306-4522(91)90295-y. [DOI] [PubMed] [Google Scholar]
- von der Malsburg C. Self-organization of orientation sensitive cells in the striate cortex. Kybernetik. 1973;14:85–100. doi: 10.1007/BF00288907. [DOI] [PubMed] [Google Scholar]
- Wang L, Maffei A. Inhibitory plasticity dictates the sign of plasticity at excitatory synapses. J Neurosci. 2014;34:1083–93. doi: 10.1523/JNEUROSCI.4711-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–46. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–45. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear Res. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg GM, Wang SSH. Malleability of spike-timing-dependent plasticity at the CA3–CA1 synapse. J Neurosci. 2006;26:6610–17. doi: 10.1523/JNEUROSCI.5388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodin MA, Ganguly K, Poo MM. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl− transporter activity. Neuron. 2003;39:807–20. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
- Xiong Q, Znamenskiy P, Zador AM. Selective corticostriatal plasticity during acquisition of an auditory discrimination task. Nature. 2015 doi: 10.1038/nature14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Atallah BV, Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511:596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol. 1999;81:781–87. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- Yao H, Shen Y, Dan Y. Intracortical mechanism of stimulus-timing-dependent plasticity in visual cortical orientation tuning. PNAS. 2004;101:5081–86. doi: 10.1073/pnas.0302510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–78. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–92. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Zaborszky L. The modular organization of brain systems. Basal forebrain: the last frontier. Prog Brain Res. 2002;136:359–72. doi: 10.1016/s0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Holt CE, Harris WA, Poo MM. A critical window for cooperation and competition among developing retinotectal synapses. Nature. 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
- Znamenskiy P, Zador AM. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature. 2013;497:482–85. doi: 10.1038/nature12077. [DOI] [PMC free article] [PubMed] [Google Scholar]


