Abstract
PHOSPHO1 is one of principal proteins involved in initiating bone matrix mineralisation. Recent studies have found that Phospho1 KO mice (Phospho1-R74X) display multiple skeletal abnormalities with spontaneous fractures, bowed long bones, osteomalacia and scoliosis. These analyses have however been limited to young mice and it remains unclear whether the role of PHOSPHO1 is conserved in the mature murine skeleton where bone turnover is limited. In this study, we have used ex-vivo computerised tomography to examine the effect of Phospho1 deletion on tibial bone architecture in mice at a range of ages (5, 7, 16 and 34 weeks of age) to establish whether its role is conserved during skeletal growth and maturation. Matrix mineralisation has also been reported to influence terminal osteoblast differentiation into osteocytes and we have also explored whether hypomineralised bones in Phospho1 KO mice exhibit modified osteocyte lacunar and vascular porosity. Our data reveal that Phospho1 deficiency generates age-related defects in trabecular architecture and compromised cortical microarchitecture with greater porosity accompanied by marked alterations in osteocyte shape, significant increases in osteocytic lacuna and vessel number. Our in vitro studies examining the behaviour of osteoblast derived from Phospho1 KO and wild-type mice reveal reduced levels of matrix mineralisation and modified osteocytogenic programming in cells deficient in PHOSPHO1. Together our data suggest that deficiency in PHOSPHO1 exerts modifications in bone architecture that are transient and depend upon age, yet produces consistent modification in lacunar and vascular porosity. It is possible that the inhibitory role of PHOSPHO1 on osteocyte differentiation leads to these age-related changes in bone architecture. It is also intriguing to note that this apparent acceleration in osteocyte differentiation evident in the hypomineralised bones of Phospho1 KO mice suggests an uncoupling of the interplay between osteocytogenesis and biomineralisation. Further studies are required to dissect the molecular processes underlying the regulatory influences exerted by PHOSPHO1 on the skeleton with ageing.
Keywords: PHOSPHO1, Mineralisation, Osteoblast, Osteocyte, Vascular porosity, MicroCT
Highlights
-
•
Our data suggest that PHOSPHO1 deficiency exerts modifications in bone architecture that are transient and depend upon age.
-
•
Phospho1 deficiency leads to increased osteocytic lacunar and vascular porosity.
-
•
Phospho1 driven mineralisation is not coupled with osteocyte differentiation.
1. Introduction
Bone formation involves a cascade of events leading to the deposition of mineral (biomineralisation), critical to skeletal maintenance throughout life. Mineralisation occurs by a series a complex physico-chemical and biochemical processes that facilitate the deposition of a solid hydroxyapatite (HA) phase [1]. Biomineralisation can be considered a two-step process, which involves de novo induction of mineral formation within the protective enclave of the lumen of osteoblast and chondrocyte matrix vesicles (MVs) followed by propagation of induced mineral into the extravesicular matrix [2], [3]. These formation and propagation steps of HA deposition are carefully regulated by a balance of mineralisation promoters and inhibitors.
The recognised local inhibitors include inorganic pyrophosphate (PPi) and organic non-collagenous proteins or peptides of the extracellular matrix (ECM) such as osteopontin [4], [5], [6]. Bone mineralisation is also dependent on a tight local balance between extracellular (e) levels of Pi and PPi and when ePPi is deficient or in excess, the skeleton is either over- or under-mineralised, respectively [7], [8]. The complex interplay between PPi formation, transport and degradation directly controls the ePi/PPi balance and thereby the propagation of HA out with the confines of the MV.
Current evidence suggests that there are several principal proteins involved in regulating bone mineralisation, which include tissue-nonspecific alkaline phosphatase (TNAP), an ectoenzyme expressed on the surface of chondrocytes, osteoblasts and their shed MVs [9]. TNAP hydrolysis maintains ePPi levels at physiological concentrations which also yields Pi for HA formation within the ECM [10]. Nucleotide pyrophosphatase phosphodiesterase 1 (NPP1) also regulates mineralisation by generating PPi ectoplasmically from nucleoside triphosphate substrates [11], [12] whereas the multiple-pass transmembrane protein ANK achieves this by mediating intracellular to extracellular channelling of PPi [13], [14]. Mouse models with NPP1 or ANK mutations show decreased levels of PPi and bone hypermineralisation [14], [15]. PHOSPHO1 (phosphatase, orphan 1) which directly regulates PPi availability, has also now been identified.
TNAP deficiency in humans results in hypophosphatasia (HPP) and is linked to increased plasma PPi levels due to impaired pyrophosphatase function. Similarly, mice deficient in TNAP function (Alpl−/−) are born with normally calcified skeletons but by postnatal day 6 skeletal hypomineralisation becomes apparent and worsens with age until their early demise by postnatal day 20 [7], [16]. The failure of bones to calcify after birth appears to result from a block in HA propagation in the ECM, beyond the confines of the MV membrane [17], [18], as a consequence of accumulated ePPi levels due to lack of TNAP's pyrophosphatase activity [19], [20], [21] and concomitant pyrophosphate-induced increase in osteoblast production of osteopontin [22], [23]. Importantly, electron microscopy has revealed that MVs from Alpl−/− mice and from patients with hypophosphatasia possess the ability to initiate HA formation within the sheltered interior of the MV [24], [25]. These findings suggest that alternative mechanisms may regulate the intravesicular initiation of mineral formation. One candidate is PHOSPHO1, a soluble cytosolic phosphatase and a member of the haloacid dehalogenase (HAD) superfamily of hydrolases [26].
PHOSPHO1 was first identified in the chick where it is expressed at 120-fold higher levels in mineralising than non-mineralising tissues [27]. It is active in osteoblast and chondrocyte MVs and has specificity for phosphoethanolamine (PEA) and phosphocholine (PCho) [28], [29]. The reduced ability of the chick wing and leg long bones to mineralize in the presence of the PHOSPHO1 inhibitor, lansoprazole, provided initial confirmation of the pivotal functional role of PHOSPHO1 in skeletal mineralisation [30]. More recently, PHOSPHO1 deficient mice, Phospho1-R74X (Phospho1 KO) were found to show elevated ePPi levels and to display multiple skeletal abnormalities, including spontaneous fractures, bowed long bones, osteomalacia and scoliosis in early life [31]. These pathological changes were clearly evident at 1 month of age in Phospho1 KO mice, and this effect is thought to become progressively worse with age. Furthermore, tibiae from Phospho1 KO mice are more ductile and did not fracture during 3-point bending but deformed plastically [32], [33], likely due to a reduced elastic modulus and hardness [32], [33].
Previous micro-computed tomography (μCT) analysis of 1-month-old Phospho1 KO showed increased trabecular number and decreased trabecular space but no significant difference in BV/TV ratio compared to WT mice, along with a significant reduction in cortical mineral density in both femur and tibia [31]. Together, these findings suggest that PHOSPHO1 serves a critical role in bone mineralisation during development and growth. This, we have hypothesised, is related to its capacity to scavenge Pi from both PEA and PCho in order to generate the Pi concentration needed to establish a Pi/PPi ratio permissive for the initial formation of HA crystal inside the MVs [3], [29].
To date, analyses of the Phospho1 KO phenotype have been limited to young mice which are characterised by active modelling of the skeleton and as PHOSPHO1 has been implicated in the initiation of bone mineralisation, it is unclear whether this role is conserved in later life in the mature murine skeleton where bone turnover is limited [31], [32]. Furthermore, the level of mineralisation and the properties of the bone matrix are associated with bone strength and stiffness [34], [35], [36]. We therefore sought to determine how Phospho1 contributes to tibial surface strain and stiffness using digital image correlation as reported previously [37]. Since matrix mineralisation has also been reported to influence terminal osteoblast differentiation into osteocytes [38], [39] and angiogenesis [40], [41], [42], [43], [44], [45], [46], it is also important to determine whether the osteoblast-to-osteocyte transition and vascular porosity [47], [48], [49], [50], [51] are impaired in PHOSPHO1 deficient mice as this may have profound effects on skeletal architecture and biomechanical properties due to an impaired ability of the skeleton to respond appropriately to mechanical loading [52], [53]. In this study, we have therefore used high resolution CT to examine the effect of Phospho1 deletion on tibial bone architecture in mice at a range of ages to establish whether its role is conserved during growth and maturation of the skeleton. Furthermore, we have also explored whether the hypomineralised bones in these mice exhibit modified osteocytic and vascular content.
2. Materials and methods
2.1. Animal model
Phospho1-R74X-null mutant (Phospho1 KO) mice were generated by N-ethyl-N-nitrosourea mutagenesis (ENU) as described previously [31]. Mice were housed up to 4 per cage in polypropylene cages with wood chip and paper bedding and provided standard mouse chow and water ad libitum throughout the study. Weaners up to 8 weeks of age were fed a standard rodent breeding diet and thereafter a standard rodent maintenance diet (Special Diet Services, South Witham, UK). All procedures complied with the UK Animals (Scientific Procedures) Act 1986 and were reviewed and approved by the ethics committee of The Roslin Institute, University of Edinburgh and the Royal Veterinary College (London, UK).
We chose to study the role of Phospho1 in the maintenance of bone architecture at various life stages and therefore used male mice at 5 (young, early stage of growth), 7 (growing, later stage of growth), 16 (skeletally mature) and 34 (post-maturation) weeks of age.
2.2. Load-related tibial bone surface strains using digital image correlation
Digital image correlation (DIC) was used to describe strain distribution engendered by load application through points of articulation [37], [54]. Accordingly, male wild-type (WT) and Phospho1 KO mice (n = 4/strain) at 5, 7 and 16 weeks old were euthanized, and right tibiae were exposed and covered with a thin layer of matt, water-based, white paint. Bones were subsequently speckled with matt, acrylic, black ink using a high precision air brush [54]. Legs were inserted in custom built loading cups attached to a material testing machine (Instron 5800, High Wycombe, UK) and loaded at a rate of 8 N/min up to 12 N/min. These cups ensured the bone to be loaded axially across the knee and ankle joints [55]. Two CCD cameras (100 mm lenses, GOM GmbH, Germany) mounted on a tripod at a reciprocal distance of 148 cm were positioned horizontally in front of the loading cups, at a distance of 42 cm, to provide a 15 × 12 mm field of view with 1.2 mm depth of focus. The two cameras were rotated towards each other meeting at 25° angle on the bone surface. A high-precision panel 15 mm × 12 mm was used to calibrate the system (GOM GmbH, Germany). The bone was illuminated by two diode lamps with polarised filters. During the loading, images of the medial side of the tibiae surface were recorded at 1 N interval using the ARAMIS 5M System (GOM GmbH, Germany), with a resolution of 7.5 × 10.9 μm. Post processing of the images was done using 19 × 19 pixel square facets, with 15 pixel step facet. Strains on the bone surface were computed with a computation size of 5 and a validity quote of 65%. Accuracy was determined at zero loading (zero strains) by taking three images in the un-deformed state during the experiments. Maximum and average strains on the medial surface were calculated at 12 N for all samples. The noise was consistent throughout all the tests and of approximately 0.03%.
2.3. High-resolution micro and nano-computed tomography
2.3.1. μCT
Tibiae from 5-, 7- and 16- (n = 6/age group) as well as 34-week old (n = 5) Phospho1 KO mice and WT were fixed in 70% EtOH and stored until scanning using the Skyscan 1172 (Skyscan, Kontich, Belgium), with X-ray tube operated at 50 kV and 200 μA, 1600 ms exposure time with a 0.5 mm aluminium filter and a voxel size of 5 μm. The scanning time for each sample was approximately 2 h. The slices were then reconstructed using NRecon 1.6.9.4 (Skyscan, Kontich, Belgium). 2D/3D analyses were performed using CTAn 1.13.5.1 + version software (Skyscan, Kontich, Belgium). CTVol 2.6.0 r98 version (Skyscan, Kontich, Belgium) was used for 3D visualisation. Phantom calibrated μCT was used to assess cortical tissue mineral density (TMD) on a stack of 100 slices for cortical region at 37% of total tibial length using two Skyscan-supplied bone phantoms with known mineral density values of 0.25 and 0.75 g/cm3 calcium hydroxyapatite. The phantoms were scanned and reconstructed with the same settings used to scan tibiae from WT and Phospho1 KO mice.
2.3.2. NanoCT
The tibial-fibula junction in 5-, 16-, and 34-week old (n = 4/age group) Phospho1 KO mice and WT was scanned using the Skyscan 1172 (Skyscan, Kontich, Belgium) X-ray microtomograph. The samples were placed in Orthodontic Wax (Kerr, CA, USA) at 50 kV and 200 μA, 9800 ms exposure time with a 0.25 mm aluminium filter (99.999% purity, Goodfellow, Huntington, UK), voxel size of 0.6 μm, 360° at a rotation step of 0.25°. Two-frame averaging was used to improve the signal-to-noise ratio. The scan time for each sample was approximately 7 h. Prior to reconstruction, thermal shift in projection images was corrected in NRecon 1.6.9.4 (Skyscan, Kontich, Belgium). The slices were then reconstructed in NRecon using a ring correction factor of 15, smoothing of 1 and 35% beam hardening correction.
2.4. Morphometrical analysis
2.4.1. Trabecular and positional cortical analysis
Prior to analysis, μ-CT images were re-oriented in DataViewer 1.5.0 (Skyscan, Kontich, Belgium), such that the cross-section within the transverse plane was perpendicular to the long axis of the bone. Tibial length was measured in CTAn 1.13.5.1 + software using a straight line measuring tool and the appearance of the trabecular ‘bridge’ connecting the two primary spongiosa bone ‘islands’ was set as reference point for analysis of the metaphyseal trabecular bone adjacent to the epiphyseal growth plate. 5% of the total bone length from this point (towards the diaphysis) was utilised for trabecular analysis of the proximal tibia. Cortical bone was analysed at one point along the bone shaft, at 37% of the total length (proximal-middle) from the reference starting slice (first appearance of medial tibial condyles). These areas were chosen in reference to previously published data on cancellous and cortical tibial bone [52], [56], [57]. As bones from different mice varied in length it was more useful to define a percentage of bone length for analysis of these regions in order to reduce undersampling/oversampling effects. The selected trabecular and cortical regions of interests were analysed using CTAn BatMan software (Skyscan, Kontich, Belgium) and morphometric parameters were recorded.
2.4.2. Whole bone cortical analysis
Whole bone analysis was performed on datasets derived from whole CT scans using BoneJ [58] (version 1.13.14) a plugin for ImageJ [59]. Following segmentation, alignment and removal of fibula from the dataset, a minimum bone threshold was selected for each bone to separate higher density bone from soft tissues and air. The most proximal and the most distal 10% portions of tibial length were excluded from analysis, as these regions include the trabecular bone. This threshold was used in “Slice Geometry” within BoneJ plugin to calculate cross sectional area (CSA), second moment of area around minor axis (Imin), second moment of area around major axis (Imax) and mean thickness determined by local thickness in 2D (Mean Thick).
2.4.3. NanoCT lacunae and canal analysis
300 consecutive images from the tibia–fibula junction were selected from each specimen. The images were loaded in CTAn software (Skyscan, Kontich, Belgium). Initially, foreground was segmented from background and a series of noise removal ‘despeckling’ steps were performed. Pores smaller than 13 μm3 and larger than 1500 μm3 were assumed to be noise and canals, respectively and the rest were considered to be lacunae. Osteocyte lacunar indices included average lacunar number (N.Lc), average volume (Lc.V), thickness (Lc.Th), separation (Lc.Sp), connectivity density (Lc.Con.Dnn) as well as canal indices including average canal number (Ca.N), average volume (Ca.V), thickness (Ca.Th), separation (Ca.Sp) and canal connectivity density (Ca.Con.Dnn) were calculated by measuring the 3D parameters of each discreet object within the volume of interest after segmentation. Shape analysis of the lacunae was conducted utilizing ‘Analyze Particles’ function in BoneJ. Shape parameters were then computed for each ellipsoid based upon the resulting three radii. The best-fit ellipsoid provided lacuna major radius (Lc.λ1), lacuna intermediate radius (Lc.λ2) and lacuna minor radius (Lc.λ3), which correspond to the lacuna's principal axes (i.e. the eigenvalues of the inertial matrix). These values allowed calculation of the degree of lacunar equancy (Lc.Eq >= Lc.λ3 / Lc.λ1), degree of lacunar elongation [Lc.El = 1 − (Lc.λ2 / Lc.λ1)] and degree of lacunar flatness [Lc.Fl >= 1 − (Lc.λ3 / Lc.λ2)] [60], [61]. The composition of the structure was then plotted using a Flinn diagram [62] showing major:intermediate axis ratio on the y-axis and the intermediate:minor axis ratio on the x-axis.
2.5. Primary osteoblast cultures
Primary calvarial osteoblast cells were isolated from 3 day-old WT and Phospho1 KO mice [39]. Briefly, excised calvaria underwent sequential enzyme digestion [1 mg/ml collagenase type II (10 min); 1 mg/ml collagenase (30 min); 4 mM EDTA (10 min); 1 mg/ml collagenase (30 min)]. The cells were collected from each digest, re-suspended in α-MEM supplemented with 10% FBS and 50 μg/ml gentamicin and cultured at 37 °C with 5% CO2 until confluent. For experiments, cells were seeded at a density of 1.5 × 104 cells/cm2. At confluency (day 0), growth medium was supplemented with 50 μg/ml ascorbic acid (Sigma) and 6 mM calcium chloride for up to 28 days to induce extracellular matrix mineralisation. The medium was changed every second/third day and samples collected at days 0, 7, 14, 21 and 28 of culture.
2.6. Assessment and quantification of mineralisation
Cell monolayers were fixed with 4% paraformaldehyde (PFA) for 5 min at 4 °C. After several washes in PBS, cells were stained with aqueous 2% (w/v) Alizarin red solution (Sigma) at pH 4.2, for 5 min at room temperature, before washing with water, to remove any unbound stain. The Alizarin red stain was subsequently solubilised in 10% cetylpyridinium chloride (Sigma) and the optical density of the resultant solution determined at 570 nm by spectrophotometry (Thermo Multiskan Ascent).
2.7. Real-time quantitative PCR (RT-qPCR)
RNA was extracted from primary osteoblast cell cultures using an RNeasy mini kit (Invitrogen) according to the manufacturer's instructions. For each sample, total RNA content was assessed by absorbance at 260 nm and purity by A260/A280 ratios, and then reverse-transcribed. RT-qPCR was performed using the SYBR green detection method on a Stratagene Mx3000P real-time qPCR system (Stratagene, CA, USA), or a LC480 instrument (Roche) as previously described. Pdpn primers were purchased from PrimerDesign Ltd, Southampton, UK (forward AAC AAG TCA CCC CAA TAG AGA TAA T, reverse CTA ACA AGA CGC CAA CTA TGA TTC). Sost primers were purchased from Qiagen (sequences not disclosed). Reactions were run in triplicate and routinely normalised against Gapdh (PrimerDesign Ltd. Sequences not disclosed).
2.8. Western blotting
The tibial diaphysis from 3 week old WT and Phospho1 KO mice was snap-frozen in liquid nitrogen and stored at − 80 °C. Bones were subsequently ground in liquid nitrogen and then homogenised in 500 μl RIPA buffer (150 mM NaCl, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0) (Sigma) containing protease inhibitors (Roche). Lysates were frozen at − 20 °C. Protein concentrations were determined by a DC assay (Bio-Rad, Hemel Hempsted, UK) and 10 μg of protein was separated using a 10% bis-tris gel and then transferred to a nitrocellulose membrane and probed with goat anti-mouse E11 (1:1000, R&D Systems) and goat anti-mouse Sclerostin (1:500, R&D systems), followed by HRP-linked rabbit anti-goat secondary antibody (1:3000, Dako, Cambridge, UK), diluted in 5% non-fat milk (Marvel, Lincs UK). Membranes were washed in TBST and the immune complexes visualised by chemiluminescence using the ECL detection kit and an ECL film-based technique (GE Healthcare, Amersham, UK). Equal loading of protein was confirmed by stripping the blot in Restore Western stripping buffer (Pierce, Rockford, USA) for 30 min at 37 °C and subsequent re-probing with HRP-conjugated anti β-actin antibody (170000, Sigma). Densitometric analysis was performed using ImageJ Software (U. S. National Institutes of Health, Maryland, USA).
2.9. Statistical analysis
Statistical analyses were performed using either GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA) or “R”, version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org). Continuous measurements were summarised as means ± SEM. Linear model (two-way analysis of variance) was used to determine the effects of age (5, 7, 16 and 34 weeks) and genotype (WT and Phospho1 KO) and their interaction on all phenotypic measurements and normality of residuals was assessed using the Shapiro–Wilk test. Bonferroni post-hoc correction was carried out for whole bone measurements, whilst no p-value adjustment was made on the post-hoc comparison for CSA, Imin, Imax and mean thickness from 10 to 90% tibial length. This was to preserve the original inferential statistics across the 10–90% tibial length and results were interpreted cautiously across tibial length. Statistical significance level was set at 5%.
3. Results
3.1. Phospho1 deficiency reduces tibial average strain and stiffness in young, growing mice and this effect diminishes with skeletal maturation
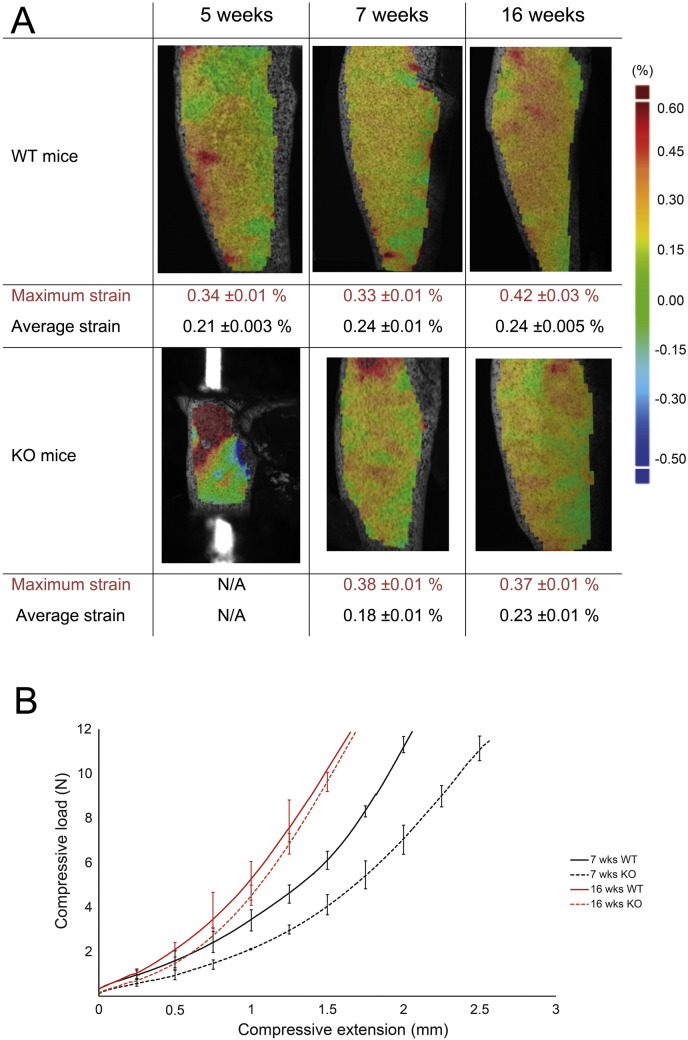
Spatial strain distribution was calculated across the medial tibia, based on the three components of displacement measured by the digital image correlation (DIC) system; however, Fig. 1 shows only strain in the axial (loading) direction, as transverse and shear strains both had relatively low magnitude in comparison. In agreement with previous studies [37], [54], axial compressive loads generated a non-uniform strain field across the surface of the tibia, with tension on the medial side because of its curved shape. It was not possible to detect strain distribution in tibiae from 5 week-old Phospho1 KO mice due to their relatively small size (the cups were too close and the bone was not visible by the cameras, Fig. 1A).
Fig. 1.
A) Longitudinal strain map on the medial side of the bone surface of WT and Phospho1 KO tibia at 5, 7 and 16 weeks of age with maximum and average values obtained following 12 N compressive load. B) Loading displacement for 7 and 16 week old Phospho1 KO and WT mice.
Consistent with previous findings [32], [63], patterns of tissue strain showed that tibiae in Phospho1 KO mice were more compliant at 5 and less so at 7 weeks of age than corresponding bones in WT group. However, average patterns of strain appeared to converge with advancing age, and were very similar in tibiae of WT and Phospho1 KO at 16 weeks of age (Fig. 1A). Measurement of compressive displacement at varying load magnitudes supported these findings by showing that tibia of Phospho1 KO mice were more compressible (25%) than WT mice at 7 weeks, under the same load (Fig. 1B). In contrast, compressive extension in Phospho1 KO tibiae did not differ from WT at 16 weeks (Fig. 1B); both WT and Phospho1 KO mice exhibited a maturation-related reduction in compressive displacement between 7 and 16 weeks of age and this modification was more marked in Phospho1 KO mice (Fig. 1B). Thus, in line with previous studies [32], [63] we found that the bone of Phospho1 KO mice is less stiff during growth and we also show that these differences in the degree of stiffness appear to be corrected upon attainment of skeletal maturity.
3.2. Phospho1 deficiency generates age-related defects in trabecular bone, but produces compromised cortical bone architecture at all ages
To explore whether these genotype-related differences in load-strain relationships are reflected in bone organisation, we have chosen specific ‘landmark’ locations along the tibia to analyse both trabecular and cortical bone architectures [52], [56], [57]. We find that Phospho1 deficiency results in reduced tibial length in mice at all ages examined (Fig. 2A; p < 0.05), which was 17, 14, 10, and 11% shorter than WT tibia at 5, 7, 16 and 34 weeks respectively. These data are also consistent with greater age-related lengthening of tibiae in Phospho1 KO compared to WT mice. Both age (p < 0.001) and genotype (p < 0.001) affect tibial length and an interaction between age and genotype is detected (Table 1; p < 0.05).
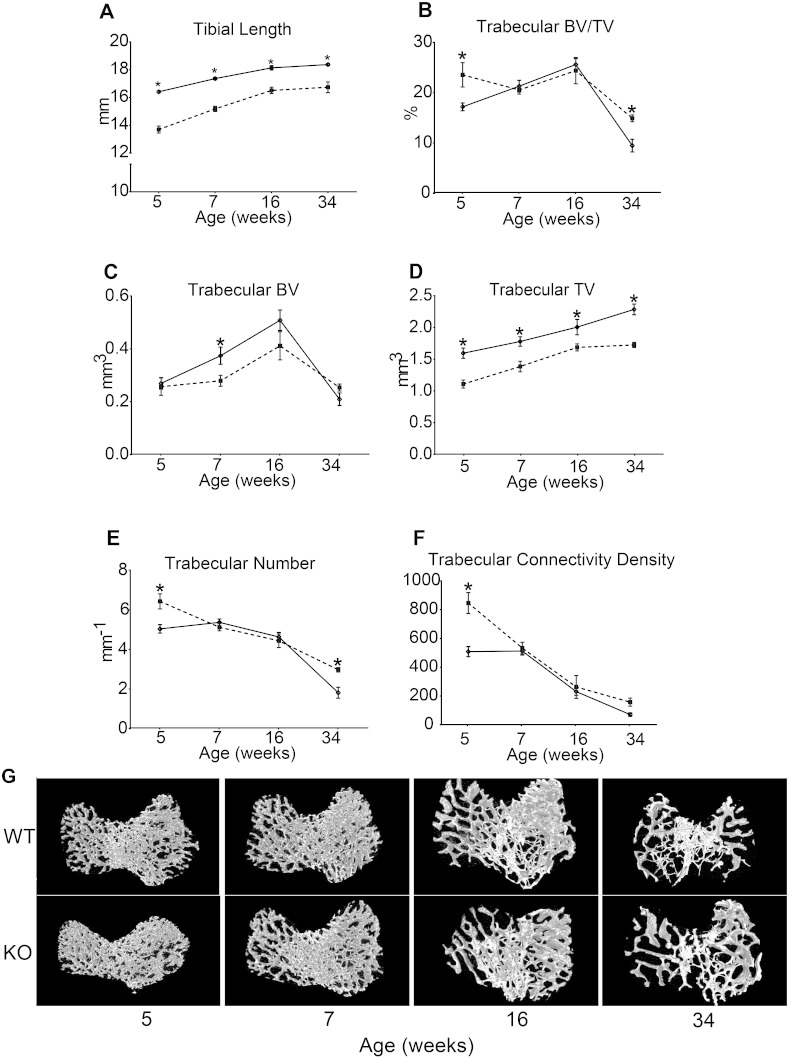
Fig. 2.
Trabecular bone phenotype of WT (solid) and Phospho1 KO (dashed) tibia at 5, 7, 16 and 34 weeks of age. (A) Tibial length. Ex vivo high-resolution analyses of distal proximal metaphysical tibia to determine (B) trabecular bone volume/total volume (BV/TV), (C) trabecular bone volume (BV), (D) trabecular total volume (TV), (E) trabecular number, (F) trabecular connectivity density and (G) representative 3D μCT images of tibial trabecular bone in WT and KO mice. Linear graphs represent means ± SEM. Group sizes were n = 6 for 5-, 7- and 16- as well as n = 5 for 34-week old WT and KO mice. Statistical comparisons: p < 0.05 WT and KO of same age.
Table 1.
Results from the ANOVA, testing the significance of the main effects of genotype and age and their interactions between WT and Phospho1 KO in metaphyseal trabecular and cortical bone.
| Parameters | Genotype | Age | Genotype ∗ age |
|---|---|---|---|
| Tibial length | < 0.001 | < 0.001 | < 0.05 |
| Trabecular bone | |||
| BV/TV (%) | NS | < 0.001 | NS |
| BV (mm3) | NS | < 0.001 | NS |
| TV | < 0.001 | < 0.001 | NS |
| Tb.N | < 0.05 | < 0.001 | < 0.01 |
| Conn.Dn (mm) | < 0.01 | < 0.001 | < 0.01 |
| Cortical bone | |||
| BV/TV (%) | < 0.001 | < 0.001 | NS |
| BV (mm3) | NS | NS | < 0.001 |
| Ct.Th | < 0.001 | < 0.001 | NS |
| Tot.Po % | < 0.001 | < 0.001 | NS |
| B.Ar | NS | NS | < 0.001 |
| MMI | NS | < 0.001 | NS |
| TMD | < 0.001 | < 0.001 | NS |
μCT based comparison of the tibial trabecular bone compartment revealed significantly higher BV/TV and trabecular number in Phospho1 KO mice at 5 and 34 weeks of age (Fig. 2B; p < 0.05), but no significant differences in these parameters at either 7 or 16 weeks of age. This, however, would appear to mask the significantly lower bone volume in 7 week-old Phospho1 KO mice (p < 0.05) which was not apparent at either 5 or 34 weeks of age and corresponds with lower total volume of the metaphyseal trabecular compartment in Phospho1 KO than in WT mice at all ages (p < 0.05). There were no significant differences in trabecular thickness between WT and KO bones but, as expected, this increased with age (p < 0.001, Appendix 1). Trabecular connectivity density (Fig. 2F) was significantly greater in Phospho1 KO mouse tibiae at 5 (p < 0.05) and somewhat elevated at 34 weeks, but no differences were apparent between KO and WT bones at 7 or 16 weeks of age.
Multiple comparisons across all groups revealed significant effects of Phospho1 deficiency on trabecular total volume, number and connectivity density (p < 0.001; < 0.05 and < 0.01 respectively, Table 1) and significant interaction between genotype and age in trabecular number, connectivity density (p < 0.01; Table 1), trabecular separation and eccentricity (p < 0.05; Appendix 1). Together these analyses reveal that Phospho1 deficiency leads to the elaboration of a smaller metaphyseal trabecular area at all ages, reduces BV at only some ages, and results in greater trabecular number at other ages, compared with WT mice. These data indicate that Phospho1 deficiency leads to a defect in whole bone structure below the metaphyseal growth plate where age-related trends in trabecular architecture differ between the WT and Phospho1 deficient bones.
A previous study reported reduced cortical mineral density and osteoid accumulation in the cortical bone of Phospho1 KO mice compared with WT [32]. Our examination of the cortical bone at 37% of the total tibia length in Phospho1 KO and WT mice shows that cortical bone volume was not modified by Phospho1 deficiency at any of the ages studied; post-hoc analysis did, however, reveal expected age-related trends (p < 0.001; Fig. 3A). Age-related trends were also evident in TV (p < 0.001; Appendix 1) but this did not appear to be modified in Phospho1 KO mice. In contrast, bone volume/total volume was significantly lower in bones of Phospho1 KO mice older than 5 weeks (7, 16 and 34 weeks; Fig. 3B; p < 0.05). Thus, genotype (p < 0.001; Table 1) and age (p < 0.001; Table 1) both affected BV/TV but these did not exhibit interaction, supporting an age-independent effect of Phospho1 deficiency.
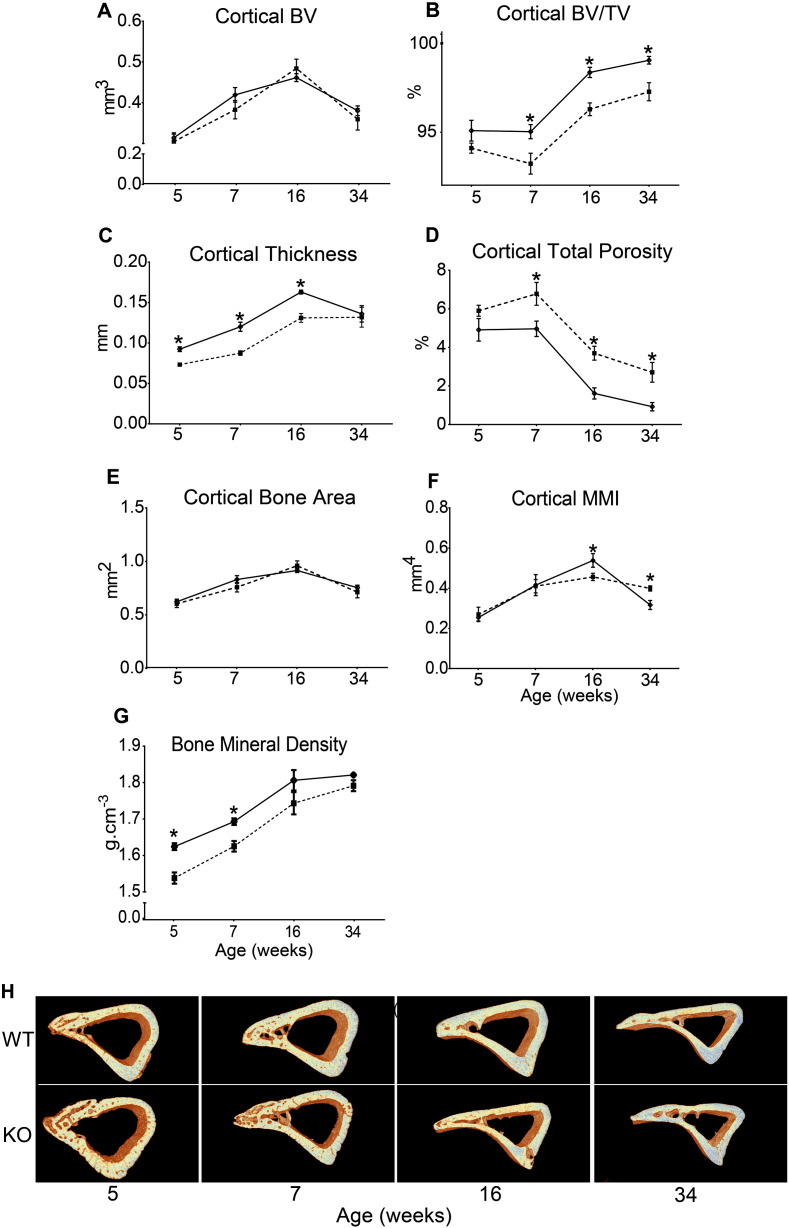
Fig. 3.
Cortical bone phenotype of WT (solid) and Phospho1 KO (dashed) tibia at 5, 7, 16 and 34 weeks of age. Ex vivo high-resolution analyses of cortical bone at 37% of total tibial length showing (A) cortical total volume (TV), (B) cortical bone volume/total volume (BV/TV), (C) cortical cross sectional thickness, (D) cortical total porosity, (E) cortical degree of anisotropy, (F) cortical mean polar moment of inertia, (G) cortical tissue mineral density and (H) representative 3D μCT images of tibial cortical bone at 37% tibial length in WT and KO mice. Linear graphs represent means ± SEM. Group sizes were n = 6 for 5-, 7- and 16- as well as n = 5 for 34-week old WT and KO mice. Statistical comparisons: p < 0.05 WT and KO of same age.
Cortical cross sectional thickness was significantly lower (p < 0.01; Fig. 3C) in Phospho1 KO mice at all ages, except at 34 weeks. Furthermore, both genotype and age affected cortical thickness (p < 0.001) but no interaction was found. Cortical total porosity was significantly higher in Phospho1 KO bones at 7, 16 and 34 weeks (p < 0.001; Fig. 3D) and no interaction between genotype and age was detected. Moreover, our data show that Phospho1 deficiency did not affect cortical bone area at any age (Fig. 3E). No differences in mean polar moment of inertia were observed in Phospho1 KO mice at 5 or 7 weeks but levels were higher compared to WT at 16 weeks and lower at 34 weeks (p < 0.05; Fig. 3F). Thus, cortical analysis at this particular location along the tibial shaft suggested that Phospho1 deficiency compromised cortical microarchitecture and leads to greater cortical porosity at all ages. Furthermore, our data show that TMD was significantly lower at 5 and 7 weeks in Phospho1 deficient mice (p < 0.05; Fig. 3G) but no differences were observed at 16 and 34 weeks of age.
3.3. Phospho1 deficiency produces age-related architectural changes in gross tibial anatomy and generates marked structural anomalies not observed by conventional analysis
To determine whether these cortical bone deficiencies at this particular location in Phospho1 KO mice were generalised along the tibial shaft, we undertook whole-bone cortical analysis. We excluded the first and last 10% of total length, where there was significant trabecular bone volume, and removed the fibula by manual segmentation. Medians of the residuals normality test p-values along the % tibial length were 0.477, 0.157, 0.104 and 0.111 for CSA, Imin, Imax and Ct.Th, respectively.
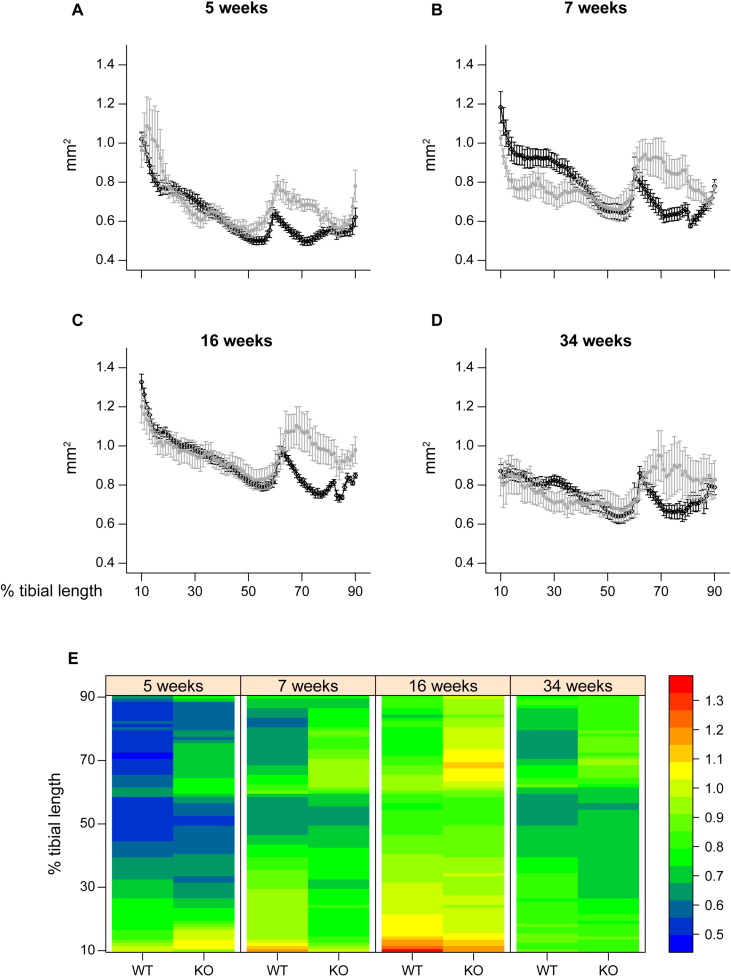
Our examination found that cross-sectional area (CSA) was significantly higher in KO compared to WT tibiae near the distal tibia at all ages (Fig. 4; 60–90%). In contrast, CSA was lower in proximal regions (Fig. 4; ~ 20–35%) in 7 and 34 week old Phospho1 KO mice. Despite this, no significant interaction between age and genotype was detected.
Fig. 4.
Cross sectional area (CSA) of WT (black) and Phospho1 KO (grey) tibia at 5, 7, 16 and 34 weeks of age. Whole bone analyses of cortical bone between 10–90% of total tibial length, excluding proximal and distal metaphyseal bone showing cross sectional area at (A) 5 weeks, (B) 7 weeks, (C) 16 weeks and (D) 34 weeks. Line graphs represent means ± SEM. Group sizes were n = 6 for 5-, 7- and 16- as well as n = 5 for 34-week old WT and KO mice. (E) Graphical heat map representation of average tibial cross sectional area.
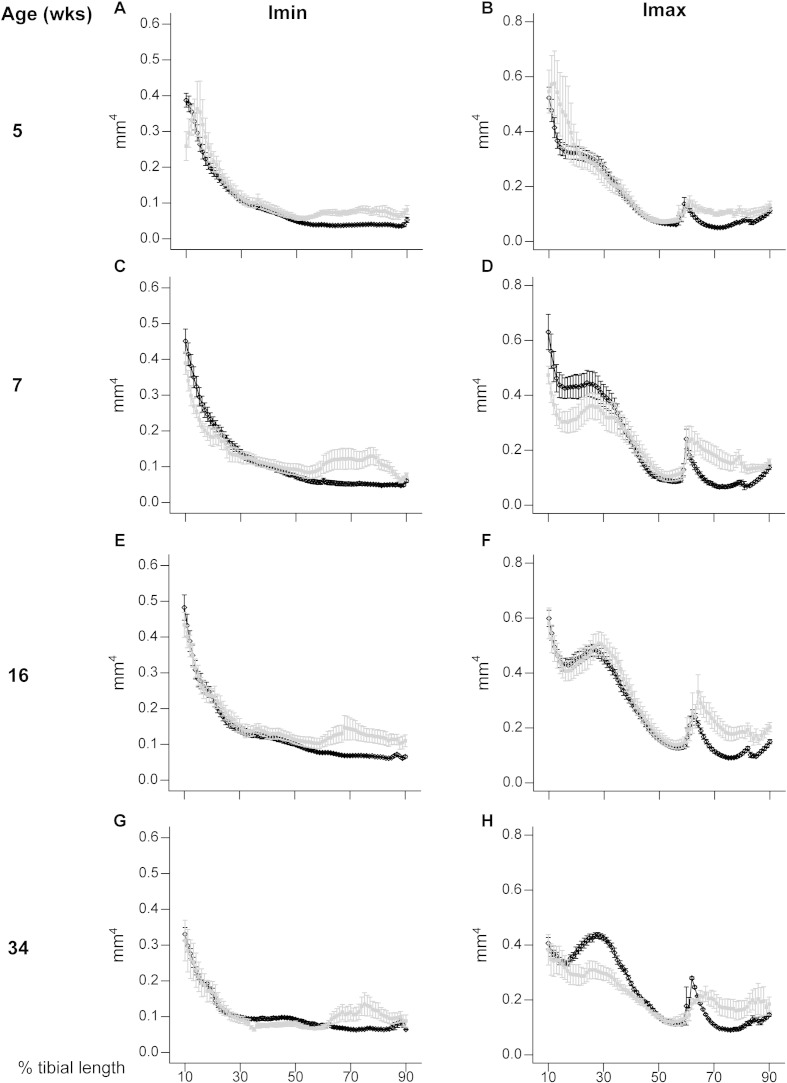
To provide an estimate of tibial resistance to bending forces, we also calculated the second moment of area around minor (Imin) and major axes (Imax). These data show that Imin and Imax in Phospho1 KO tibiae deviate from WT patterns and are significantly greater toward the distal tibia at all ages; no genotype: age interaction was detected (Fig. 5). In contrast, Imax is lower in proximal regions of the tibia of Phospho1 KO mice. Together, CSA, Imin and Imax indicate that Phospho1 deficiency produces tibial architecture likely reflecting regionalised changes in bending resistance at all ages, which is lower in proximal regions in 7 and 34 week-old mice and greater in the distal third at all ages (Fig. 7).
Fig. 5.
Minimum and maximum second moments of area (Imin and I max respectively) of WT (black) and Phospho1 KO (grey) tibia at 5, 7, 16 and 34 weeks of age. Whole bone analyses of cortical bone between 10–90% of total tibial length, excluding proximal and distal metaphyseal bone showing Imin and Imax at (A, B) 5 weeks, (C, D) 7 weeks, (E, F) 16 weeks and (G, H) 34 weeks. Line graphs represent means ± SEM. Group sizes were n = 6 for 5-, 7- and 16- as well as n = 5 for 34-week old WT and KO mice.
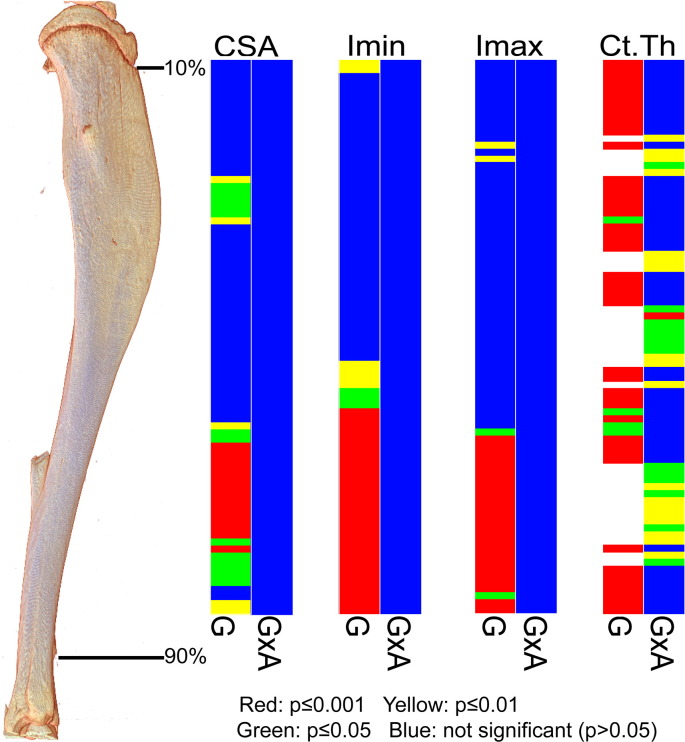
Fig. 7.
Graphical heat map representation of statistical significance of the effect of genotype (Phospho1 deficiency) (G) and its interaction with age (GxA) on CSA, Imin, Imax and Ct.Th of tibia between 10 and 90% of length. Red p ≤ 0.000–0.001, yellow p ≤ 0.001–0.01, green p ≤ 0.01–0.05 and blue p > 0.05.
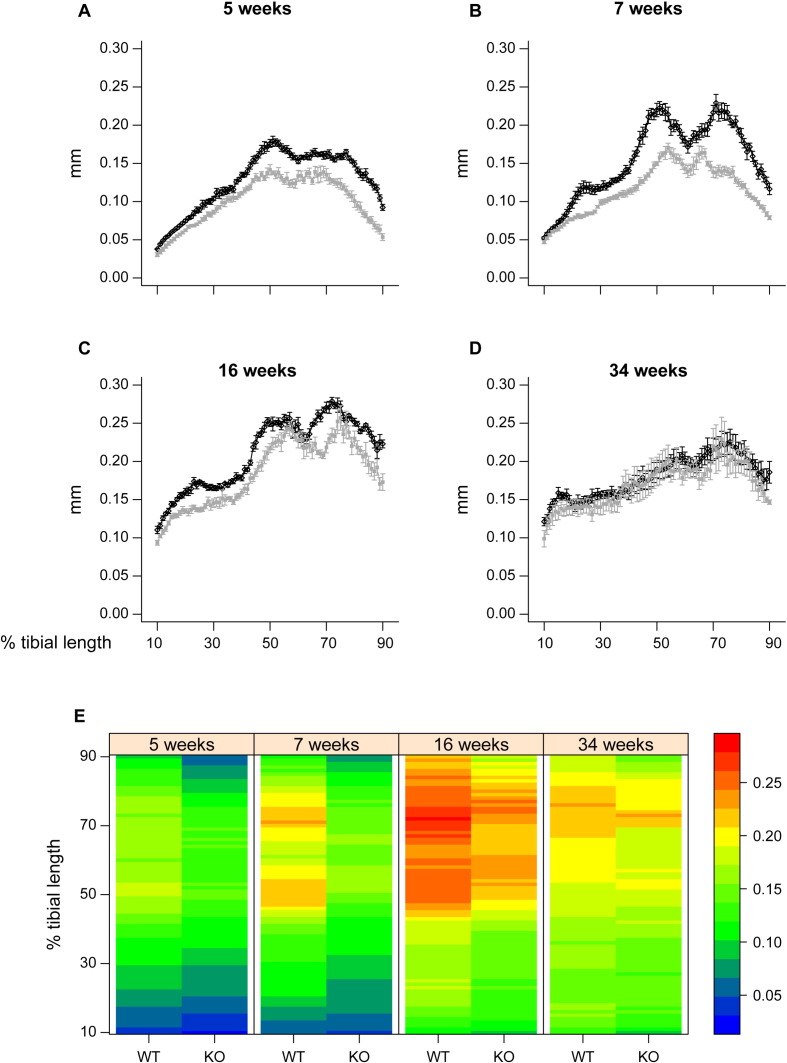
Phospho1 KO mice also exhibited lower cross sectional thickness in almost all tibial regions at 5, 7 and 16 weeks, but did not differ from WT at 34 weeks of age (Fig. 6). Comparison across ages reveals that this lack of difference at 34 weeks is primarily due to greater decline in thickness with maturation/ageing in WT than in KO tibiae. These divergent age-related changes in cortical thickness in Phospho1 KO tibiae contribute to significant age:genotype interactions (at locations along almost all the tibial length; Fig. 7). In addition to revealing greater utility of such whole bone analyses (that measurement at one location, 37%, may not necessarily always be representative), these data demonstrate that Phospho1 deficiency produces proximodistally, regionalised modifications in indices of bending strength and cortical architecture, and leads to age-related changes in cross sectional thickness along the tibial length which diverge from WT.
Fig. 6.
Mean cortical thickness of WT (black) and Phospho1 KO (grey) tibia at 5, 7, 16 and 34 weeks of age. Whole bone analyses of cortical bone between 10–90% of total tibial length, excluding proximal and distal metaphyseal bone showing mean cortical thickness at (A) 5 weeks, (B) 7 weeks, (C) 16 weeks and (D) 34 weeks. Line graphs represent means ± SEM. Group sizes were n = 6 for 5-, 7- and 16- as well as n = 5 for 34-week old WT and KO mice. (E) Graphical heat map representation of average tibial mean cortical thickness.
3.4. Phospho1 deficiency increases lacunar and vascular bone porosity
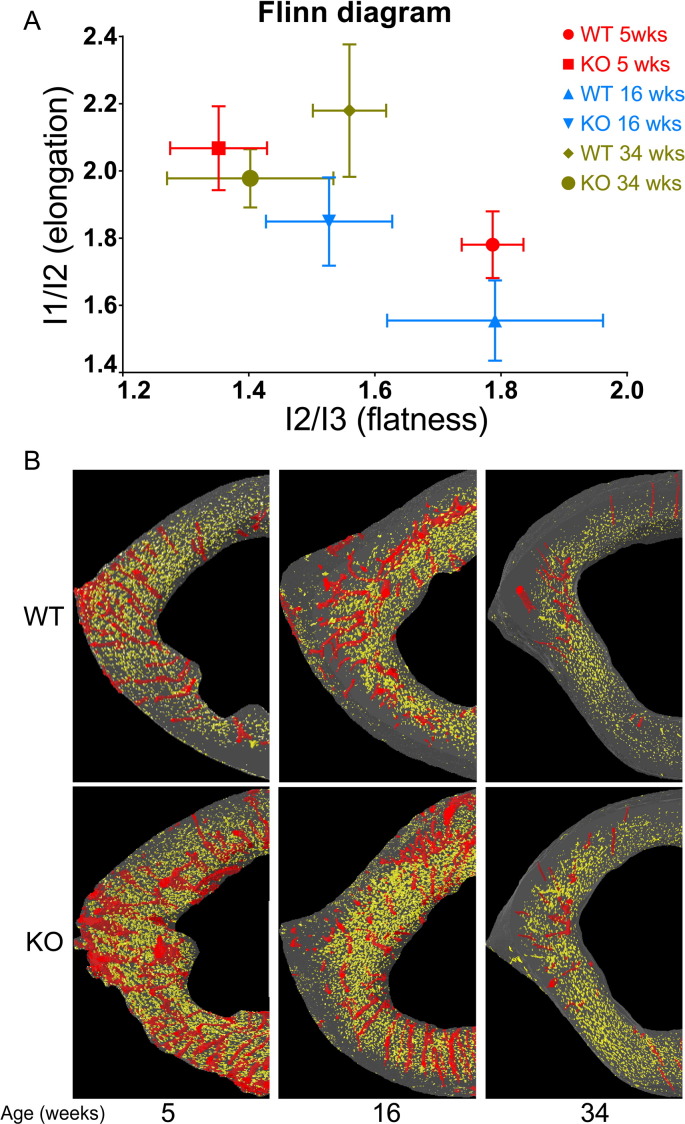
Regional control of bone architecture is thought to involve integration with the mechanical milieu by osteocytes and the process of mineralisation has been linked to osteocytogenesis [38], [39]. We therefore sought to determine whether Phospho1 deficiency modifies osteocyte organisation, as well as vascular porosity, by measuring 3D morphometric parameters in high resolution images. Consistent with earlier analyses, we confirmed both lower TV and cortical thickness in Phospho1 KO tibiae at all ages (Table 2). Our morphometric evaluation of the cortical bone at the tibia–fibula junction shows that porosity (or lacunar space) is greater in Phospho1 KO than in WT bones, containing significantly greater numbers of osteocyte lacunae (N.Lc; p < 0.001; Table 2) with significantly greater volume (Lc.V; p < 0.001; Table 2). Whilst age also significantly impacts upon osteocyte lacunar number and volume, no interaction with genotype was observed. Intriguingly, analysis of lacunar shape showed that Phospho1 KO bone contained osteocytes occupying somewhat elongated lacuna with significantly lower levels of lacunar flatness (Lc.El; p < 0.01). Based on these data, we have constructed a Flinn diagram [62] (Fig. 8) which shows that lacunar elongation and flatness were also both significantly affected post-maturation, but no age-related interaction with Phospho1 deficiency was evident. Whilst Phospho1 deficiency did not significantly alter lacunar equancy (Lc.Eq) in any age, relationships between lacunar elongation and flatness are plotted (Fig. 8) and support divergence in osteocyte shape in Phospho1 deficient bone.
Table 2.
Porosity parameters representing lacuna and vascular porosity of male WT and Phospho1 KO mice at 5, 7, 16 and 34 weeks of age, detailing post-hoc comparisons for significant age:genotype interactions. Data represent means ± SEM with group sizes of n = 4 for WT and Phospho1 KO mice from different ages.
| Morphometric index | WT 5 weeks n = 4 |
KO 5 weeks n = 4 |
WT 16 weeks n = 4 |
KO 16 weeks n = 4 |
WT 34 weeks n = 4 |
KO 34 weeks n = 4 |
Effect of genotype | Effect of age | Interaction age ∗ genotype |
|---|---|---|---|---|---|---|---|---|---|
| Bone parameters | |||||||||
| Ct.TV (mm− 3) | 0.085 ± 0.005 | 0.157 ± 0.002 | 0.140 ± 0.005 | 0.180 ± 0.012 | 0.117 ± 0.009 | 0.132 ± 0.022 | < 0.01 | < 0.01 | NS |
| Ct.Th (mm) | 0.089 ± 0.000 | 0.063 ± 0.002 | 0.119 ± 0.007 | 0.114 ± 0.011 | 0.144 ± 0.007 | 0.124 ± 0.003 | < 0.01 | < 0.001 | NS |
| Canal parameters | |||||||||
| N.Ca | 74 ± 8.495 | 282 ± 54.580 | 62 ± 13.444 | 136 ± 44.434 | 20 ± 4.366 | 63 ± 22.595 | < 0.001 | < 0.01 | < 0.05 |
| N.Ca/Ct.TV (mm− 3) | 895.7 ± 137.35 | 1928.8 ± 458.01 | 509 ± 93.76 | 715 ± 185.91 | 164 ± 34.26 | 430.6 ± 92.88 | < 0.05 | < 0.001 | NS |
| Ca.V/Ct.TV (%) | 1.266 ± 0.233 | 2.375 ± 0.558 | 1.033 ± 0.302 | 1.128 ± 0.228 | 0.142 ± 0.045 | 0.546 ± 0.141 | < 0.05 | < 0.001 | NS |
| Lacunae parameters | |||||||||
| N.Lc | 1707 ± 234.6 | 5900 ± 768.9 | 2930 ± 574.3 | 5367 ± 740 | 789 ± 373 | 2197 ± 573 | < 0.001 | < 0.001 | NS |
| N.Lc/Ct.TV (mm− 3) | 20,483 ± 3327 | 38,761 ± 5951 | 25,026 ± 1519 | 29,336 ± 2282 | 6276 ± 2830 | 15,917 ± 1629 | < 0.01 | < 0.001 | NS |
| Lc.V/Ct.TV (%) | 0.571 ± 0.103 | 0.995 ± 0.137 | 0.665 ± 0.033 | 0.675 ± 0.142 | 0.157 ± 0.060 | 0.404 ± 0.060 | < 0.05 | < 0.001 | NS |
| < Lc.Eq > | 0.316 ± 0.011 | 0.363 ± 0.023 | 0.367 ± 0.014 | 0.548 ± 0.196 | 0.394 ± 0.074 | 0.368 ± 0.023 | NS | NS | NS |
| < Lc.El > | 0.433 ± 0.031 | 0.511 ± 0.026 | 0.346 ± 0.050 | 0.450 ± 0.043 | 0.527 ± 0.049 | 0.491 ± 0.023 | NS | < 0.05 | NS |
| < Lc.Fl > | 0.439 ± 0.014 | 0.253 ± 0.041 | 0.426 ± 0.060 | 0.337 ± 0.038 | 0.356 ± 0.024 | 0.267 ± 0.070 | < 0.01 | NS | NS |
Fig. 8.
A) Flinn diagram displaying lacunar shapes in WT and Phospho1 KO tibia at tibia–fibula junction from various ages. The x axis represents lacunar flatness which was calculated by dividing lacunar intermediate radius (l2: length of best-fit ellipsoid's intermediate radius) with lacunar minor radius (l3: length of best-fit ellipsoid's minor radius). The y axis represents lacunar elongation which was calculated by dividing lacunar major radius (l1: length of best-fit ellipsoid's major radius) with lacunar intermediate radius (l2: length of best-fit ellipsoid's intermediate radius). Data represent means with group sizes of n = 4 for WT and KO mice from different ages. B) Surface representation of the lacunar (yellow) and red (vascular porosity) segmented from 300 consecutive images from tibia–fibula junction from both genotypes and each age.
Greater porosity in Phospho1 KO bone was also consistent with measures of vascular porosity, in which significantly higher canal number (N.Ca; p < 0.001 Table 2), density (N.Ca/Ct.TV; p < 0.05; Table 2) and volume (normalised by cortical tissue volume; Ca.V/Ct.TV; p < 0.05; Table 2) were evident in Phospho1 KO bones. There was significant age-related decline in each of these parameters, and canal number showed significantly (p < 0.05) greater decline in Phospho1 KO bone. These data indicate that the lower tissue volume and reduced cortical thickness at the tibia–fibula junction of Phospho1 deficient mice are closely associated with increased porosity characterised by significant increases in the number of osteocytic lacuna and vessels.
3.5. Phospho1 KO osteoblasts produce a hypomineralised matrix and show distinct osteocyte differentiation kinetics
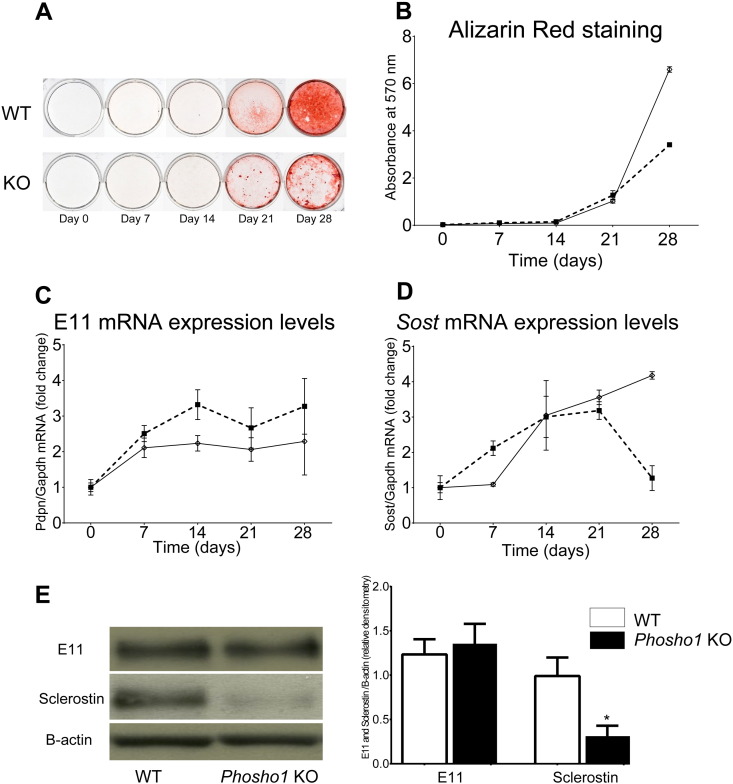
To explore whether these structural modifications and greater osteocyte lacunar numbers might reflect inherent characteristics of osteoblasts undergoing mineralisation in the absence of Phospho1, we examined the behaviour of primary osteoblasts from WT and Phospho1 KO mice. Initial phenotypic characterisation of primary osteoblasts demonstrated significantly lower levels of matrix mineralisation in Phospho1-deficient primary osteoblast cultures than WT by days 21 and 28 of culture (p < 0.001; Fig. 9A/B). Despite reduced matrix mineralisation of Phospho1 KO osteoblasts, RT-qPCR analysis showed that mRNA levels of E11/Pdpn, the early osteocyte marker, were significantly increased (vs WT) at time points beyond day 7 (p < 0.05; Fig. 9C), suggesting that osteoblast-to-osteocyte transition is nonetheless accelerated in the absence of PHOSPHO1. Phospho1 KO osteoblast cultures also exhibited increased mRNA expression for Sost, a late osteocyte marker from day 7, and faster decline in these levels at day 28, compared to WT (p < 0.001; Fig. 9D). Western blot analysis of protein extracted from the tibia of 3 week old mice confirmed decreased sclerostin protein expression in Phospho1 KO tibia in comparison to WT bones (p < 0.05; Fig. 9E). These data suggest that Phospho1 exerts inherent effects on osteoblast characteristics and influences the osteocytogenic programme.
Fig. 9.
Characterisation of primary osteoblast-like cells isolated from WT and Phospho1 KO mice to determine (A) mineralisation of cells, (B) E11/Pdpn mRNA expression levels, (D) Sost mRNA expression levels and (E) Western blots of E11 and sclerostin. For in vitro culture studies (A, B, C and D) results are the means ± SEM of three independent experiments (n = 4 per study). For Western blots (E) data represent means ± SEM with group sizes of n = 4 for WT and KO mice.
4. Discussion
Our data indicate that tibiae of Phospho1 KO mice are less stiff during growth but rectified by skeletal maturation. Whole bone analyses show that Phospho1 deficiency lowers proximal indices of bending resistance at only some ages, but that levels are consistently higher in distal regions at all ages. This is supported by data showing that Phospho1 deficiency generates different age-related trends in whole bone structure below the growth plate in WT and Phospho1 deficient bones, and compromised cortical microarchitecture with greater porosity at all ages. Our data reveal that modified Phospho1 deficient bone morphology also encompasses marked alterations in osteocyte shape and significant increases in osteocytic lacuna and vessel number. The prospect that this is due to inherent deficits in osteoblast behaviour is bolstered by our data showing that primary Phospho1 deficient osteoblast-like cells exhibit lower levels of matrix mineralisation and modified osteocytogenic programming in vitro.
Bone mineralisation is an essential and carefully controlled process for skeletal function and is regulated by both promoters and inhibitors of matrix mineralisation [64], [65]. Biomineralisation is dependent on PPi/Pi homeostasis which is regulated by the actions of TNAP [10], [20], [21], [66], [67], NPP1 [11], [12], ANK [13], [14] and PHOSPHO1. Our knowledge of the involvement of these proteins in the regulation of biomineralisation and skeletal maintenance with maturation/ageing is incomplete. Furthermore, earlier studies did not extensively investigate the role of Phospho1 in age-related maintenance of bone architecture and mechanical properties post-maturation.
A previous study reported that Phospho1 deficiency resulted in increased trabecular number and decreased trabecular space in tibia [31]. This reported gain in trabecular bone mass was, in further studies, ascribed to a structural support role rectifying for the observed weaker (thinner and more porous) cortex [32]. However, this apparent compensation does not match with mechanical properties and greater incidence of greenstick fracture observed in Phospho1 KO mice, nor does it provide an explanation for why the effects of Phospho1-deficiency differ in the trabecular and cortical compartments. Herein, we extensively investigate the role of Phospho1 deletion on bone architecture with ageing.
In agreement with a previous study [32], [54] our DIC and load-deformation data showed that at younger age, Phospho1 KO tibia was less stiff and was more compressible (25%) than WT bones; this effect was however diminished in 16-week-old skeletally mature mice, suggesting an age-dependent role on mechanical properties of Phospho1. As described previously [31], [32], [33], Phospho1 deficiency results in a lower accumulation of mineral in bones, that consequently, lowers stiffness resulting in a more deformable bone. This more ductile bone is consistent with the protection of long bones from Phospho1 KO mice against fracture during 3-point bending (36). This greater deformability in Phospho1 KO mice is supported by the bowing of long bones from an early juvenile age and by fractures exhibiting predominantly green-stick presentation [31]. Although we have not tested bending properties of mice at 34 weeks of age, our data suggest that PHOSPHO1 serves a relatively limited role in the biomechanics of the mature skeleton and that biomechanical deficiencies due to the absence of PHOSPHO1 during growth and development are eventually corrected (in later life) by alternative mechanisms. Whether differences in cortical porosity and polar moment of inertia, evident at 34 weeks, will be retained when mice become even older remains to be elucidated in future studies. The mechanism(s) for these age-related corrections in average strain and stiffness are unclear but alternative mineralisation mechanisms clearly exist as the complete ablation of PHOSPHO1 function only leads to a decrease in the calcification ability of MVs but not to a complete lack of calcification [31], [32]. It is feasible that whilst the generation of Pi within MVs through the actions of PHOSPHO1 is optimum for rapid and timely ECM mineralisation in early growth of the skeleton, this process can be mimicked, through time, by the influx of TNAP generated ePi into MVs via the phosphate transporter, PiT1 [3] in later life.
Our previous studies have also disclosed that the impaired ECM mineralisation noted in Phospho1 KO mice may be in part explained by elevated osteopontin but not PPi levels. Indeed, the ablation of osteopontin improves the skeletal phenotype of Phospho1 KO mice [68]. Whether differences in osteopontin expression between young and older PHOSPHO1 deficient mice can explain the correction in bone phenotype with age is unknown and requires further examination.
Our positional μCT analysis indicates, in agreement with a previous study [31], that Phospho1 deficiency resulted in increased trabecular number, despite relative reductions in trabecular separation not reaching statistical significance. This observation without considering other structural parameters, such as BV and TV, suggests that Phospho1 acts as a negative regulator of trabecular bone and hence in its absence, more trabeculae are formed. Alternatively, it is possible that greater bone formation occurs in order to compensate for the bone's relatively hypomineralised status. In this study, we report that despite smaller proximal metaphyseal volume in KO group in all ages, BV was similar to WT mice at 5 and 34 weeks of age and therefore increased BV/TV at these ages is a reflection of smaller trabecular total volume. Moreover, in agreement with Yadav et al. [9] cortical thickness was lower at 5 weeks and here we report that this effect is maintained through growth and adulthood but disappears in post-maturation group. In contrast, total cortical porosity was not different at 5 weeks but consistently higher in KO genotype in post-maturation groups. This type of positional analysis of both trabecular and cortical compartments suggests a transitory role of PHOSPHO1 in the maintenance of the trabecular bone and a more consistent role in cortical compartment.
The deficiencies observed using conventional analysis provided information about the effect of Phospho1 deficiency in a short segment of the cortical bone. Non-biased whole-bone analysis revealed that the changes described at 37% of the tibial length were not necessarily representative of changes elsewhere in the bone. Several parameters of bone strength were measured. The estimated strength and rigidity of the bone can be determined through the study of the bone's cross sectional geometry which includes cortical cross sectional area (CSA) and second moments of area. CSA is directly related to a bone's strength against compressive forces applied equally throughout the bone; factors such as bone shape and the effects of muscle contraction however result in long bones experiencing bending and torsional forces. Herein indices of rigidity, including maximum and minimum resistance against bending forces in the cross section, second moment of area around minor axis (Imin) and second moment of area around major axis (Imax) [69], were also measured in WT and Phospho1 KO bones at all ages. Our data from this approach reveal greater CSA, Imin and Imax throughout the distal third (~ 60–90%) of the tibia from Phospho1 KO mice. In contrast, Phospho1 deficiency lowered CSA in regions of the cortex closer to the proximal end of the tibia. These contrasting differences between the effect of Phospho1 deficiency on the proximal and distal compartments of the tibia may indicate differential role for Phospho1 during bone development, growth and skeletal maturity or that responses in these regions differ due to their relative undermineralisation.
Previous studies have described the relationship between osteocytes and their lacunae in humans and non-humans with mineralisation status; however, the quantification of 3D osteocyte density and morphology has always been problematic due to the relatively small number of osteocyte lacunae which cannot be visualised readily using traditional techniques including confocal microscopy. Large-scale analyses of osteocyte lacunar parameters in 3D will provide clarification on the relationship between mineralisation and osteocyte density in vivo. A number of previous studies [70], [71], [72] found no correlation between lacunar density and bone formation, architecture or resorption. In contrast, many other studies suggested that age [73], mechanical environment [51], [74], diet [75], [76] and glucocorticoid treatment [77] significantly affect lacunar morphology and density. It has also been reported that modification of loading environment leads to significant reduction in lacunar density [78]. Furthermore, Vashishth et al. [79] found positive correlations between both cortical and cancellous bone mass accrual and lacunar density. These inconsistent correlations between lacunar density and structural parameters indicate that variation in lacunar density may not be the major determinant of bone quality, although further in depth studies are needed to confirm these observations. Herein, we report that Phospho1 deficiency significantly increases lacunar density and this effect remains after maturation. Furthermore, a similar observation was made with regard to vasculature content. These data suggest that osteoblast-to-osteocyte transition may be accelerated in the absence of PHOSPHO1. This acceleration is consistent with our in vitro observations in which osteoblasts from Phospho1-deficient mice exhibit elevated levels of E11/Pdpn mRNA (the early osteocyte marker) at initial stages, yet reduced levels of Sost mRNA (a mature osteocyte marker) at later stages of culture. The increase in E11/Pdpn mRNA levels in vitro was not observed at protein levels ex-vivo, presumably due to its transitory elevation, however, more stable reduction in Sost mRNA expression in vitro was confirmed at protein level in bones from Phospho1 KO and WT mice. These data suggest that PHOSPHO1 negatively regulates osteocytogenesis and vascular porosity and in its absence both of these processes are upregulated.
Moreover, we analysed lacunar morphology to dissect the possible role of Phospho1 in regulation of lacunar shape. Using confocal microscopy, McCreadie et al. reported that lacunar shape or size was not different between older women, with and without osteoporotic fracture [80]. In contrast, using high-resolution nanoCT, van Hove et al. suggested that osteocyte morphology in the subchondral cortical bone of the lateral articular surface of the proximal tibia obtained from osteoarthritic, osteopenic, and osteopetrotic patients was significantly different, which the authors attributed to their disease state [81]. Herein, we report that Phospho1 deficiency alters lacunar shape with lacunae from KO mice exhibiting a more elongated shape compared to flat lacunae from the WT group. The shape of these osteocyte lacunae in WT mouse bone only drifted towards those shapes evident in the Phospho1 KO mice once maturation had been reached.
Together our data suggest that deficiency in PHOSPHO1 exerts modifications in bone architecture that are transient and depend upon age, yet produces consistent modification in osteocyte differentiation and vascular porosity. It is possible that the inhibitory role of PHOSPHO1 on osteocyte differentiation leads to these age-related changes in bone architecture. It is also intriguing to note that this apparent acceleration in osteocyte differentiation evident in the hypomineralised bones of Phospho1 KO mice suggests an uncoupling of the interplay between osteocytogenesis and biomineralisation. Further studies are required to dissect the molecular processes underlying the regulatory influences exerted by PHOSPHO1 on the skeleton with ageing.
Acknowledgement
This study was supported by funding from BBSRC BB/I014608/1 and Arthritis Research UK 18768 and 20413 and 20581.
Appendix A.
Appendix 1.
Bone phenotype parameters of male WT and Phospho1 KO mice at 5, 7, 16 and 34 weeks of age detailing post-hoc comparisons for effects of age and genotype and significant age:genotype interactions. Data shown are the means ± SEM.
| WT 5 weeks n = 7 |
KO 5 weeks n = 7 |
WT 7 weeks n = 7 |
KO 7 weeks n = 7 |
WT 16 weeks n = 7 |
KO 16 weeks n = 8 |
KO 37 weeks n = 5 |
KO 37 weeks n = 5 |
Effect of genotype | Effect of age | Interaction Age ∗ genotype | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trabecular bone | |||||||||||
| Tb.Th (mm) | 0.033 ± 0.000 | 0.035 ± 0.002 | 0.038 ± 0.001 | 0.039 ± 0.000 | 0.054 ± 0.001 | 0.053 ± 0.002 | 0.052 ± 0.001 | 0.049 ± 0.000 | NS | < 0.001 | NS |
| Tb.Sp (mm) | 0.134 ± 0.005 | 0.120 ± 0.004 | 0.122 ± 0.003 | 0.131 ± 0.003 | 0.143 ± 0.005 | 0.148 ± 0.008 | 0.279 ± 0.042 | 0.206 ± 0.008 | NS | < 0.001 | < 0.05 |
| Tb.DA (mm− 1) | 2.640 ± 0.079 | 2.454 ± 0.143 | 2.614 ± 0.090 | 2.418 ± 0.095 | 2.306 ± 0.043 | 2.290 ± 0.098 | 1.863 ± 0.094 | 1.823 ± 0.066 | NS | < 0.001 | NS |
| Tb.Ecc (mm) | 0.667 ± 0.039 | 0.760 ± 0.029 | 0.756 ± 0.012 | 0.686 ± 0.020 | 0.680 ± 0.021 | 0.658 ± 0.024 | 0.767 ± 0.036 | 0.684 ± 0.041 | NS | NS | < 0.05 |
| Cortical bone | |||||||||||
| Tissue volume (TV) | 0.328 ± 0.013 | 0.328 ± 0.321 | 0.439 ± 0.018 | 0.409 ± 0.025 | 0.468 ± 0.010 | 0.500 ± 0.022 | 0.382 ± 0.012 | 0.368 ± 0.026 | NS | NS | < 0.001 |
| Bone surface/bone volume (mm− 1) | 31.153 ± 0.991 | 40.737 ± 1.111 | 24.963 ± 1.169 | 33.753 ± 1.043 | 18.684 ± 0.234 | 23.684 ± 0.620 | 21.318 ± 1.551 | 22.573 ± 1.997 | < 0.001 | < 0.001 | < 0.01 |
| Cortical eccentricity | 0.698 ± 0.010 | 0.676 ± 0.015 | 0.487 ± 0.010 | 0.771 ± 0.015 | 0.796 ± 0.017 | 0.786 ± 0.016 | 0.843 ± 0.004 | 0.833 ± 0.005 | NS | < 0.001 | NS |
References
- 1.Kawasaki K., Buchanan A.V., Weiss K.M. Biomineralization in humans: making the hard choices in life. Annu. Rev. Genet. 2009;43:119–142. doi: 10.1146/annurev-genet-102108-134242. [DOI] [PubMed] [Google Scholar]
- 2.Anderson H.C., Garimella R., Tague S.E. The role of matrix vesicles in growth plate development and biomineralization. Front. Biosci. 2005;10:822–837. doi: 10.2741/1576. [DOI] [PubMed] [Google Scholar]
- 3.Millan J.L. The role of phosphatases in the initiation of skeletal mineralization. Calcif. Tissue Int. 2013;93:299–306. doi: 10.1007/s00223-012-9672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boskey A.L., Maresca M., Ullrich W., Doty S.B., Butler W.T., Prince C.W. Osteopontin–hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993;22:147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- 5.Gorski J.P. Biomineralization of bone: a fresh view of the roles of non-collagenous proteins. Front. Biosci. (Landmark Ed.) 2011;16:2598–2621. doi: 10.2741/3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter G.K., Kyle C.L., Goldberg H.A. Modulation of crystal formation by bone phosphoproteins: structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem. J. 1994;300(Pt 3):723–728. doi: 10.1042/bj3000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narisawa S., Frohlander N., Millan J.L. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev. Dyn. 1997;208:432–446. doi: 10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Russell R.G., Bisaz S., Donath A., Morgan D.B., Fleisch H. Inorganic pyrophosphate in plasma in normal persons and in patients with hypophosphatasia, osteogenesis imperfecta, and other disorders of bone. J. Clin. Invest. 1971;50:961–969. doi: 10.1172/JCI106589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whyte M.P. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994;15:439–461. doi: 10.1210/edrv-15-4-439. [DOI] [PubMed] [Google Scholar]
- 10.Majeska R.J., Wuthier R.E. Studies on matrix vesicles isolated from chick epiphyseal cartilage. Association of pyrophosphatase and ATPase activities with alkaline phosphatase. Biochim. Biophys. Acta. 1975;391:51–60. doi: 10.1016/0005-2744(75)90151-5. [DOI] [PubMed] [Google Scholar]
- 11.Terkeltaub R., Rosenbach M., Fong F., Goding J. Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteoblasts with plasma cell membrane glycoprotein-1. Relevance to calcium pyrophosphate dihydrate deposition disease. Arthritis Rheum. 1994;37:934–941. doi: 10.1002/art.1780370624. [DOI] [PubMed] [Google Scholar]
- 12.Terkeltaub R.A. Inorganic pyrophosphate generation and disposition in pathophysiology. Am. J. Physiol. Cell Physiol. 2001;281:C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- 13.Hakim F.T., Cranley R., Brown K.S., Eanes E.D., Harne L., Oppenheim J.J. Hereditary joint disorder in progressive ankylosis (ank/ank) mice. I. Association of calcium hydroxyapatite deposition with inflammatory arthropathy. Arthritis Rheum. 1984;27:1411–1420. doi: 10.1002/art.1780271212. [DOI] [PubMed] [Google Scholar]
- 14.Ho A.M., Johnson M.D., Kingsley D.M. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 15.Okawa A., Nakamura I., Goto S., Moriya H., Nakamura Y., Ikegawa S. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nat. Genet. 1998;19:271–273. doi: 10.1038/956. [DOI] [PubMed] [Google Scholar]
- 16.Fedde K.N., Blair L., Silverstein J., Coburn S.P., Ryan L.M., Weinstein R.S., Waymire K., Narisawa S., Millan J.L., MacGregor G.R., Whyte M.P. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J. Bone Miner. Res. 1999;14:2015–2026. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedde K.N., Blair L., Silverstein J., Coburn S.P., Ryan L.M., Weinstein R.S., Waymire K., Narisawa S., Millan J.L., MacGregor G.R., Whyte M.P. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res. 1999;14:2015–2026. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson H.C., Sipe J.B., Hessle L., Dhanyamraju R., Atti E., Camacho N.P., Millan J.L. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am. J. Pathol. 2004;164:841–847. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harmey D., Hessle L., Narisawa S., Johnson K.A., Terkeltaub R., Millan J.L. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am. J. Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hessle L., Johnson K.A., Anderson H.C., Narisawa S., Sali A., Goding J.W., Terkeltaub R., Millan J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murshed M., Harmey D., Millan J.L., McKee M.D., Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Addison W.N., Azari F., Sorensen E.S., Kaartinen M.T., McKee M.D. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J. Biol. Chem. 2007;282:15872–15883. doi: 10.1074/jbc.M701116200. [DOI] [PubMed] [Google Scholar]
- 23.Harmey D., Johnson K.A., Zelken J., Camacho N.P., Hoylaerts M.F., Noda M., Terkeltaub R., Millan J.L. Elevated skeletal osteopontin levels contribute to the hypophosphatasia phenotype in Akp2(−/−) mice. J. Bone Miner. Res. 2006;21:1377–1386. doi: 10.1359/jbmr.060619. [DOI] [PubMed] [Google Scholar]
- 24.Anderson H.C., Hsu H.H., Morris D.C., Fedde K.N., Whyte M.P. Matrix vesicles in osteomalacic hypophosphatasia bone contain apatite-like mineral crystals. Am. J. Pathol. 1997;151:1555–1561. [PMC free article] [PubMed] [Google Scholar]
- 25.Ornoy A., Adomian G.E., Rimoin D.L. Histologic and ultrastructural studies on the mineralization process in hypophosphatasia. Am J Med Genet. 1985;22:743–758. doi: 10.1002/ajmg.1320220410. [DOI] [PubMed] [Google Scholar]
- 26.Stewart A.J., Schmid R., Blindauer C.A., Paisey S.J., Farquharson C. Comparative modelling of human PHOSPHO1 reveals a new group of phosphatases within the haloacid dehalogenase superfamily. Protein Eng. 2003;16:889–895. doi: 10.1093/protein/gzg126. [DOI] [PubMed] [Google Scholar]
- 27.Houston B., Seawright E., Jefferies D., Hoogland E., Lester D., Whitehead C., Farquharson C. Identification and cloning of a novel phosphatase expressed at high levels in differentiating growth plate chondrocytes. Biochim. Biophys. Acta. 1999;1448:500–506. doi: 10.1016/s0167-4889(98)00153-0. [DOI] [PubMed] [Google Scholar]
- 28.Roberts S., Narisawa S., Harmey D., Millan J.L., Farquharson C. Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization. J. Bone Miner. Res. 2007;22:617–627. doi: 10.1359/jbmr.070108. [DOI] [PubMed] [Google Scholar]
- 29.Roberts S.J., Stewart A.J., Sadler P.J., Farquharson C. Human PHOSPHO1 exhibits high specific phosphoethanolamine and phosphocholine phosphatase activities. Biochem. J. 2004;382:59–65. doi: 10.1042/BJ20040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacRae V.E., Davey M.G., McTeir L., Narisawa S., Yadav M.C., Millan J.L., Farquharson C. Inhibition of PHOSPHO1 activity results in impaired skeletal mineralization during limb development of the chick. Bone. 2010;46:1146–1155. doi: 10.1016/j.bone.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav M.C., Simao A.M., Narisawa S., Huesa C., McKee M.D., Farquharson C., Millan J.L. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification. J. Bone Miner. Res. 2011;26:286–297. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huesa C., Yadav M.C., Finnila M.A., Goodyear S.R., Robins S.P., Tanner K.E., Aspden R.M., Millan J.L., Farquharson C. PHOSPHO1 is essential for mechanically competent mineralization and the avoidance of spontaneous fractures. Bone. 2011;48:1066–1074. doi: 10.1016/j.bone.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Florez N., Garcia-Tunon E., Mukadam Q., Saiz E., Oldknow K.J., Farquharson C., Millán J.L., Boyde A., Shefelbine S.J. An investigation of the mineral in ductile and brittle cortical mouse bone. J. Bone Miner. Res. 2015:786–795. doi: 10.1002/jbmr.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Currey J.D. The mechanical consequences of variation in the mineral content of bone. J. Biomech. 1969;2:1–11. doi: 10.1016/0021-9290(69)90036-0. [DOI] [PubMed] [Google Scholar]
- 35.Currey J.D. The effect of porosity and mineral content on the Young's modulus of elasticity of compact bone. J. Biomech. 1988;21:131–139. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 36.Burr D.B. The contribution of the organic matrix to bone/'s material properties. Bone. 2002;31:8–11. doi: 10.1016/s8756-3282(02)00815-3. [DOI] [PubMed] [Google Scholar]
- 37.Sztefek P., Vanleene M., Olsson R., Collinson R., Pitsillides A.A., Shefelbine S. Using digital image correlation to determine bone surface strains during loading and after adaptation of the mouse tibia. J. Biomech. 2010;43:599–605. doi: 10.1016/j.jbiomech.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Cameron D.A., Paschall H.A., Robinson R.A. Changes in the fine structure of bone cells after the administration of parathyroid extract. J. Cell Biol. 1967;33:1–14. doi: 10.1083/jcb.33.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prideaux M., Loveridge N., Pitsillides A.A., Farquharson C. Extracellular matrix mineralization promotes E11/gp38 glycoprotein expression and drives osteocytic differentiation. PLoS One. 2012;7:e36786. doi: 10.1371/journal.pone.0036786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Wesenbeeck L., Odgren P.R., MacKay C.A., D'Angelo M., Safadi F.F., Popoff S.N., Van Hul W., Marks S.C., Jr. The osteopetrotic mutation toothless (tl) is a loss-of-function frameshift mutation in the rat Csf1 gene: evidence of a crucial role for CSF-1 in osteoclastogenesis and endochondral ossification. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14303–14308. doi: 10.1073/pnas.202332999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin. Orthop. Relat. Res. 1994:124–131. [PubMed] [Google Scholar]
- 42.Aronson J., Good B., Stewart C., Harrison B., Harp J. Preliminary studies of mineralization during distraction osteogenesis. Clin. Orthop. Relat. Res. 1990:43–49. [PubMed] [Google Scholar]
- 43.Choi I.H., Ahn J.H., Chung C.Y., Cho T.J. Vascular proliferation and blood supply during distraction osteogenesis: a scanning electron microscopic observation. J. Orthop. Res. 2000;18:698–705. doi: 10.1002/jor.1100180504. [DOI] [PubMed] [Google Scholar]
- 44.Delloye C., Delefortrie G., Coutelier L., Vincent A. Bone regenerate formation in cortical bone during distraction lengthening. An experimental study. Clin. Orthop. Relat. Res. 1990:34–42. [PubMed] [Google Scholar]
- 45.Hunter W.L., Arsenault A.L., Hodsman A.B. Rearrangement of the metaphyseal vasculature of the rat growth plate in rickets and rachitic reversal: a model of vascular arrest and angiogenesis renewed. Anat. Rec. 1991;229:453–461. doi: 10.1002/ar.1092290404. [DOI] [PubMed] [Google Scholar]
- 46.Thompson T.J., Owens P.D., Wilson D.J. Intramembranous osteogenesis and angiogenesis in the chick embryo. J. Anat. 1989;166:55–65. [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper D.M., Matyas J.R., Katzenberg M.A., Hallgrimsson B. Comparison of microcomputed tomographic and microradiographic measurements of cortical bone porosity. Calcif. Tissue Int. 2004;74:437–447. doi: 10.1007/s00223-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto T., Yoshino M., Asano T., Uesugi K., Todoh M., Tanaka M. Monochromatic synchrotron radiation muCT reveals disuse-mediated canal network rarefaction in cortical bone of growing rat tibiae. J. Appl. Physiol. 2006;100:274–280. doi: 10.1152/japplphysiol.00495.2005. [DOI] [PubMed] [Google Scholar]
- 49.Palacio-Mancheno P.E., Larriera A.I., Doty S.B., Cardoso L., Fritton S.P. 3D assessment of cortical bone porosity and tissue mineral density using high-resolution microCT: effects of resolution and threshold method. J. Bone Miner. Res. 2014;29:142–150. doi: 10.1002/jbmr.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider P., Krucker T., Meyer E., Ulmann-Schuler A., Weber B., Stampanoni M., Muller R. Simultaneous 3D visualization and quantification of murine bone and bone vasculature using micro-computed tomography and vascular replica. Microsc. Res. Tech. 2009;72:690–701. doi: 10.1002/jemt.20720. [DOI] [PubMed] [Google Scholar]
- 51.Schneider P., Meier M., Wepf R., Muller R. Towards quantitative 3D imaging of the osteocyte lacuno-canalicular network. Bone. 2010;47:848–858. doi: 10.1016/j.bone.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Moustafa A., Sugiyama T., Prasad J., Zaman G., Gross T.S., Lanyon L.E., Price J.S. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos. Int. 2012;23:1225–1234. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robling A.G., Niziolek P.J., Baldridge L.A., Condon K.W., Allen M.R., Alam I., Mantila S.M., Gluhak-Heinrich J., Bellido T.M., Harris S.E., Turner C.H. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 54.Carriero A., Abela L., Pitsillides A.A., Shefelbine S.J. Ex vivo determination of bone tissue strains for an in vivo mouse tibial loading model. J. Biomech. 2014;47:2490–2497. doi: 10.1016/j.jbiomech.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Souza R.L., Matsuura M., Eckstein F., Rawlinson S.C., Lanyon L.E., Pitsillides A.A. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37:810–818. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 56.Sugiyama T., Price J.S., Lanyon L.E. Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones. Bone. 2010;46:314–321. doi: 10.1016/j.bone.2009.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugiyama T., Saxon L.K., Zaman G., Moustafa A., Sunters A., Price J.S., Lanyon L.E. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice. Bone. 2008;43:238–248. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 58.Doube M., Klosowski M.M., Arganda-Carreras I., Cordelieres F.P., Dougherty R.P., Jackson J.S., Schmid B., Hutchinson J.R., Shefelbine S.J. BoneJ: free and extensible bone image analysis in ImageJ. Bone. 2010;47:1076–1079. doi: 10.1016/j.bone.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carter Y., Thomas C.D.L., Clement J.G., Peele A.G., Hannah K., Cooper D.M.L. Variation in osteocyte lacunar morphology and density in the human femur — a synchrotron radiation micro-CT study. Bone. 2013;52:126–132. doi: 10.1016/j.bone.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Carriero A., Doube M., Vogt M., Busse B., Zustin J., Levchuk A., Schneider P., Müller R., Shefelbine S.J. Altered lacunar and vascular porosity in osteogenesis imperfecta mouse bone as revealed by synchrotron tomography contributes to bone fragility. Bone. 2014;61:116–124. doi: 10.1016/j.bone.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 62.Flinn D. On folding during three-dimensional progressive deformation. Q. J. Geol. Soc. 1962;118:385–428. [Google Scholar]
- 63.Carriero A., Bruse J.L., Oldknow K.J., Millán J.L.L., Farquharson C., Shefelbine S.J. Reference point indentation is not indicative of whole mouse bone measures of stress intensity fracture toughness. Bone. 2014;69:174–179. doi: 10.1016/j.bone.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cecil R.N., Clarke Anderson H. Freeze-fracture studies of matrix vesicle calcification in epiphyseal growth plate. Metab. Bone Dis. Relat. Res. 1978;1:89–95. [Google Scholar]
- 65.Anderson H.C., Stechschulte D.J., Collins D.E., Jacobs D.H., Morris D.C., Hsu H.H., Redford P.A., Zeiger S. Matrix vesicle biogenesis in vitro by rachitic and normal rat chondrocytes. Am. J. Pathol. 1990;136:391–398. [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson K.A., Hessle L., Vaingankar S., Wennberg C., Mauro S., Narisawa S., Goding J.W., Sano K., Millan J.L., Terkeltaub R. Osteoblast tissue-nonspecific alkaline phosphatase antagonizes and regulates PC-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1365–R1377. doi: 10.1152/ajpregu.2000.279.4.R1365. [DOI] [PubMed] [Google Scholar]
- 67.Moss D.W., Eaton R.H., Smith J.K., Whitby L.G. Association of inorganic-pyrophosphatase activity with human alkaline-phosphatase preparations. Biochem. J. 1967;102:53–57. doi: 10.1042/bj1020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yadav M.C., Huesa C., Narisawa S., Hoylaerts M.F., Moreau A., Farquharson C., Millan J.L. Ablation of osteopontin improves the skeletal phenotype of phospho1(−/−) mice. J. Bone Miner. Res. 2014;29:2369–2381. doi: 10.1002/jbmr.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruff C.B., Hayes W.C. Cross-sectional geometry of Pecos Pueblo femora and tibiae—a biomechanical investigation: II. Sex, age, side differences. Am. J. Phys. Anthropol. 1983;60:383–400. doi: 10.1002/ajpa.1330600309. [DOI] [PubMed] [Google Scholar]
- 70.Mullender M.G., Tan S.D., Vico L., Alexandre C., Klein-Nulend J. Differences in osteocyte density and bone histomorphometry between men and women and between healthy and osteoporotic subjects. Calcif. Tissue Int. 2005;77:291–296. doi: 10.1007/s00223-005-0043-6. [DOI] [PubMed] [Google Scholar]
- 71.Robling A.G., Turner C.H. Mechanotransduction in bone: genetic effects on mechanosensitivity in mice. Bone. 2002;31:562–569. doi: 10.1016/s8756-3282(02)00871-2. [DOI] [PubMed] [Google Scholar]
- 72.Skedros J.G., Grunander T.R., Hamrick M.W. Spatial distribution of osteocyte lacunae in equine radii and third metacarpals: considerations for cellular communication, microdamage detection and metabolism. Cells Tissues Organs. 2005;180:215–236. doi: 10.1159/000088938. [DOI] [PubMed] [Google Scholar]
- 73.Qiu S., Rao D.S., Palnitkar S., Parfitt A.M. Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone. 2002;31:313–318. doi: 10.1016/s8756-3282(02)00819-0. [DOI] [PubMed] [Google Scholar]
- 74.Mullins L.P., McGarry J.P., Bruzzi M.S., McHugh P.E. Micromechanical modelling of cortical bone. Comput. Meth. Biomech. Biomed. Eng. 2007;10:159–169. doi: 10.1080/10255840601110802. [DOI] [PubMed] [Google Scholar]
- 75.Mullender M.G., Huiskes R. Osteocytes and bone lining cells: which are the best candidates for mechano-sensors in cancellous bone? Bone. 1997;20:527–532. doi: 10.1016/s8756-3282(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 76.Skedros J.G., Hunt K.J., Hughes P.E., Winet H. Ontogenetic and regional morphologic variations in the turkey ulna diaphysis: implications for functional adaptation of cortical bone. Anat. Rec. A: Discov. Mol. Cell. Evol. Biol. 2003;273:609–629. doi: 10.1002/ar.a.10073. [DOI] [PubMed] [Google Scholar]
- 77.Lane N.E., Yao W., Balooch M., Nalla R.K., Balooch G., Habelitz S., Kinney J.H., Bonewald L.F. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J. Bone Miner. Res. 2006;21:466–476. doi: 10.1359/JBMR.051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iwamoto J., Matsumoto H., Takeda T., Sato Y., Yeh J.K. Effects of vitamin K2 on cortical and cancellous bone mass, cortical osteocyte and lacunar system, and porosity in sciatic neurectomized rats. Calcif. Tissue Int. 2010;87:254–262. doi: 10.1007/s00223-010-9387-7. [DOI] [PubMed] [Google Scholar]
- 79.Vashishth D., Gibson G., Kimura J., Schaffler M.B., Fyhrie D.P. Determination of bone volume by osteocyte population. Anat. Rec. 2002;267:292–295. doi: 10.1002/ar.10114. [DOI] [PubMed] [Google Scholar]
- 80.McCreadie B.R., Hollister S.J., Schaffler M.B., Goldstein S.A. Osteocyte lacuna size and shape in women with and without osteoporotic fracture. J. Biomech. 2004;37:563–572. doi: 10.1016/S0021-9290(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 81.van Hove R.P., Nolte P.A., Vatsa A., Semeins C.M., Salmon P.L., Smit T.H., Klein-Nulend J. Osteocyte morphology in human tibiae of different bone pathologies with different bone mineral density—is there a role for mechanosensing? Bone. 2009;45:321–329. doi: 10.1016/j.bone.2009.04.238. [DOI] [PubMed] [Google Scholar]