Abstract
Stem cell therapies have opened new frontiers in medicine with the possibility of regenerating lost or damaged cells. Embryonic stem cells, induced pluripotent stem cells, hematopoietic stem cells, and mesenchymal stem cells have been used to derive mature cell types for tissue regeneration and repair. However, the endometrium has emerged as an attractive, novel source of adult stem cells that are easily accessed and demonstrate remarkable differentiation capacity. In this review, we summarize our current understanding of endometrial stem cells and their therapeutic potential in regenerative medicine.
Keywords: cell transplantation, endometrium, mesenchymal stem cells, regenerative medicine, stem cells
INTRODUCTION
In the 21st century, arguably nothing has greater potential to improve health than stem cell therapies and regenerative medicine. Increasingly prevalent chronic degenerative diseases and organ failure are unlikely to be cured with existing drug therapies alone. While pharmacologic therapies provide valuable symptomatic relief and may assist in tissue repair by releasing necessary trophic factors, they do not directly replace the cells lost during the disease process [1]. Transplantation of whole organs, including heart, kidney, pancreas, lung, and liver, has been long practiced in medicine. However, limited availability of transplantable organs requires investigators to develop novel strategies for restoring tissue function [2]. Hence, stem cell therapies have emerged as a feasible option to replace cells lost or damaged during various disease processes. After the first report of successful hematopoietic stem cell (HSC) transplantation in 1957 [3], stem cell therapies have garnered substantial public and scientific attention [2]; numerous types of stem cells have been studied for use in numerous therapeutic applications. Thousands of clinical trials using stem cells are currently in progress [4].
REGENERATIVE MEDICINE AND MESENCHYMAL STEM CELLS
The potential of embryonic stem cells (ESCs), induced pluripotent stem cells (iPSs), stem cells derived from somatic cell nuclear transfer, and adult mesenchymal stem cells (MSCs) in regenerative medicine has been widely investigated. The risk of tumor formation after ESC or iPS transplant and genetic manipulation, in addition to ethical controversies surrounding the use of ESCs, has hampered potential clinical application. However, MSCs represent a promising tool for both autologous and heterologous cell replacement therapies.
According to the definition by the Committee of the International Society for Cellular Therapy, MSCs are multipotent cells that are plastic adherent, and express CD73, CD90, and CD105, while not expressing CD11b, CD14, CD19, CD79α, CD34, CD45, and HLA-DR, and must be able to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro [5]. MSCs have been identified in many adult tissues, including bone marrow, umbilical cord, dental pulp, periosteum, skeletal muscle, fat, pancreas, placenta, and endometrium [6–10]. Since MSCs can readily differentiate into chondrocytes and osteocytes, they have been used for cartilage and bone repair using tissue-specific scaffolds [11]. As discussed in the following sections in detail, accumulating evidence suggests that MSCs, especially MSCs derived from the endometrium, can generate a greater repertoire of mature cell types than was previously assumed. It is increasingly recognized that MSCs may be a valuable therapeutic tool in the regenerative medicine field. In addition to their differentiation potential, the discovery of a broad spectrum of bioactive molecules secreted by MSCs has opened the possibility of identifying trophic factors that mediate the reparative properties of stem cells. To date, this identification process has primarily relied upon RT-PCR, ELISA, and HPLC quantification of trophic factors of interest. Future attempts to identify these bioactive molecules may look towards high-throughput methods, such as RNA and protein microarray or whole transcriptome shotgun sequencing.
The majority of the existing evidence on the immunomodulatory properties of MSCs comes from bone marrow-derived MSCs (BM-MSC). Many studies have demonstrated that MSCs suppress the adaptive and innate immune systems [12]. In particular, MSCs inhibit T cell proliferation and differentiation of these cells into proinflammatory T helper (Th) 1 and Th17 cells, and promote T cell differentiation into tolerogenic T regulatory cells [13]. Moreover, MSCs can induce dendritic cells to acquire a tolerogenic phenotype and “switch” proinflammatory type 1 macrophages to anti-immunomodulatory type 2 macrophages [14, 15]. They may also inhibit natural killer (NK) cell activation, proliferation, and cytotoxicity, thereby reducing a key initial step in the inflammatory response [16]. MSCs have been shown to secrete a variety of cytokines and signaling molecules, which can largely be divided into three categories: antiapoptotic, supportive, and angiogenic trophic factors. Antiapoptotic molecules secreted by MSCs include IGF-1, TGF-β, bFGF, and HGF. Factors supportive of proliferation and differentiation include IL6, SDF-1, and M-colony-stimulating factor (CSF). Angiogenic molecules identified in MSC secretions include VEGF, PIGF, MCP-1, and bFGF. MSCs have additionally been shown to not only home to sites of injury via chemokine gradients, but also release chemoattractant cytokines, such as CXCL12, CCL2, and CXCL8, thereby increasing further MSC recruitment. Although most experiments working to characterize MSC secretions have relied on in vitro models, systemic MSC infusion has provided an effective treatment in humans for graft-versus-host disease, and has reduced inflammation in mouse models of bleomycin-induced lung injury [17]. The immunomodulatory nature of MSCs may allow their clinical use to be expanded from autologous to heterologous application; MSCs have been demonstrated to suppress immune response in vitro and in vivo, particularly when exposed to proinflammatory cytokines [12].
STEM CELLS IN ENDOMETRIUM
The human endometrium is a highly dynamic tissue that undergoes approximately 400 menstrual cycles during a woman's lifetime. This level of tissue regeneration is comparable to other tissues with high cellular turnover, such as epidermis, gut epithelium, and bone marrow. Although the presence of stem cells within the endometrium was first speculated by Prianishnikov in 1978 [18], the majority of studies constituting our current knowledge of endometrium-derived stem cells (EDSCs) has been published within the last decade.
A study in 2004 first identified and characterized stem cells in endometrial tissue [19, 20]. This study demonstrated that 0.22% of endometrial epithelial and 1.25% of endometrial stromal cells exhibit clonogenicity, suggesting the presence of stem/progenitor cells in the human endometrium [19]. Characterization of endometrial stromal cells revealed MSC properties of these cells through clonogenicity assays, adherence to plastic, fibroblast-like morphology, and differentiation to adipogenic, chondrogenic, and osteogenic fates in vitro [8, 9, 21–26]. Further characterization of human endometrial stromal cells using antibody panels showed the expression of CD29, CD44, CD73, CD90, CD105, MSI1, and NOTCH1 [8, 22, 25]. Others have suggested that EDSCs are c-kit- (CD117) and CD34-expressing cells in the basalis layer of endometrium [27]. Although unequivocal proof of adult stem cell character has generally relied on repopulation of a tissue after transplantation of labeled putative stem cells, studies of EDSCs from stromal tissue have largely involved characterization by cell surface markers and in vitro validations of clonogenicity and multipotency. Studies using cell surface markers to confirm the presence of stem cells have been constrained in their scope due to broad expression of certain stem cell markers in the endometrium, such as c-kit and CD133 in the epithelial compartment and CD90 in the functionalis stroma [22, 28]. A small number of transplantation experiments of EDSCs have been completed to date, including those on the differentiation of EDSCs into neuron-like cells [29–31].
Current research on EDSCs has primarily focused on stromal tissue. While cells within the epithelial compartment displaying clonogenic properties have been isolated, further study of these cells has been limited by the lack of widely accepted specific markers for epithelial progenitor and stem cells [32]. Recent research by Janzen et al. has proposed the EpCAM+CD44+ITGA6hiThy1−PECAM1−PTPRC−Ter119− genotype as unique to endometrial epithelial progenitor cells [33]. The especially dynamic nature of the epithelium during the menstrual cycle in response to steroid hormones has similarly complicated cellular research, which must be controlled for these changes. The relatively small population of clonogenic epithelial endometrial cells, paired with the requirement of a fibroblast feeder layer for culture, has thus shifted the focus of EDSC experiments with potential clinical application to stromal tissue-derived cells [34].
The label-retaining cell (LRC) approach, which takes advantage of the relative quiescence of stem/progenitor cells, has also been used to identify potential populations of stem cells in the endometrium. After pulse administration of the thymidine analog bromodeoxyuridine (BrdU), the number of BrdU+ LRCs progressively decreases over time, and only slow-cycling cells, such as stem/progenitor cells, retain BrdU. Approximately 3% of the uterine epithelial and 6% of uterine stromal cells were identified as LRCs in murine endometrium in a follow-up period of 8 and 12 wk, respectively [35]. Epithelial LRCs were not observed after 8 wk, whereupon only the uterine stroma contained LRCs. Epithelial LRCs were primarily located in the luminal epithelium, and were not present in the glands, except for occasional LRCs in the neck of a gland. Stromal LRCs were observed adjacent to luminal and glandular epithelium, as well as at the endometrial-myometrial junction. Epithelial LRCs did not express estrogen receptor alpha (ER-α), stem cell antigen-1 (Sca-1), or CD45. The majority of the stromal LRCs were also negative for ER-α, Sca-1, and CD45, but occasional ER-α-expressing cells were observed [36]. Absence of ER-α expression was considered to be suggestive of an undifferentiated phenotype. In a separate study, 1.7% of stromal cells were identified as LRCs in murine endometrium 16 wk after BrdU administration [35]. The LRCs were located in the uterine stroma, and 0.32% of them expressed the stem cell marker c-KIT, while 0.19% of them expressed the pluripotency marker POU5F1 (OCT-4) [35]. Other studies investigated the LRCs in the context of their proliferation potential under the influence of estrogen and their contribution to endometrial decidualization, breakdown, and repair [37, 38]. The quiescent nature of LRCs and their proliferation potential identified them as potential stem/progenitor cells in the endometrium. Further studies are needed to delineate their exact differentiation potential and surface markers.
In order to avoid the mutagenic potential of BrdU labeling and difficulties in culturing viable BrdU LRCs, the LRC approach has also been used with the H2B-green fluorescent protein (GFP) transgenic mouse model, which labels cells at the embryonic, undifferentiated stage. In this model, sexually mature adult mice expressing Tet-inducable histone 2B-GFP were given a pulse of doxycycline through their drinking water for 7 days, thereby activating the expression of GFP [39]. Upon the removal of the doxycycline-laced water, referred to as the chase, cells were observed and characterized by a variety of techniques, including immunohistochemistry and immunofluorescence. After 7 days of doxycycline pulse, the majority of the uterine cells in each mouse expressed GFP, especially in the epithelial tissue of the endometrium. As regular cell division occurred, the GFP signal was expected to be lost in the cells with highest mitotic activity. In contrast, quiescent cells, and thus potential stem cells, would retain their label for longer periods. Although epithelial tissue initially showed high expression of GFP signal, within 2–4 wk all GFP expression was lost. In the stromal tissue, GFP expression was lost between 8 and 12 wk, indicating a slower division rate compared to the epithelial tissue. After more than 12 wk of chase, LRCs were found in the distal oviducts of the mice, and, as such, were termed long-term LRCs (LT-LRCs). These LT-LRCs were then isolated by fluorescence-activated cell sorting (FACS) and shown to form spheroids in culture, much like undifferentiated stem cells. Furthermore, they were later differentiated to a phenotype similar to proximal oviduct and endometrial tissue.
The H2B-GFP model has also been used to characterize LRCs in the distal oviduct and endocervical transition zone (TZ) during periods of natural as well as mechanically induced endometrial regeneration [40]. Mice were pulsed with doxycycline from Embryonic Day 13.5 to Postnatal Day 21, encompassing Müllerian duct development and postnatal uterine maturation. LRCs were located in the endometrial epithelium after up to 3 wk of chase, and located in the stroma and myometrium after up to 5 wk of chase. LT-LRCs in the distal oviduct and endocervical TZ were confirmed to lack ER-α and PGR, while expressing c-kit and P-p63, thus having an undifferentiated phenotype. These LT-LRCs often colocalized with cells expressing ER-α and PGR, corresponding to findings by Janzen et al. [33]. However, these cells did not contribute to endometrial re-epithelialization in a mouse model of endometrial shedding and proliferation (i.e., menses), implying that LRCs in the distal oviduct and endocervical TZ do not act as repopulating stem or progenitor cells during short-term re-epithelialization. When the mice were pulsed peripubertally, these cells were found to be present in endometrial glandular tissue up to 9 mo postpulse. Though these cells could not be definitively called stem cells, these data lend support to the idea that the endometrium maintains a source of endogenous stem cells.
Prospective isolation of endometrial stromal PDGF-Rβ+/CD146+ double-positive cells using FACS resulted in 8- to 10-fold enrichment for MSC-like cells compared to unfractioned endometrium [8]. In vitro assays showed that PDGF-Rβ+/CD146+ cells are able to differentiate into adipocytes, chondrocytes, and osteocytes, and express MSC markers CD29, CD44, CD73, CD90, and CD105 [8, 9, 26]. Therefore, PDGF-Rβ+/CD146+ double positivity was suggested as a marker for endometrial MSCs (eMSCs). PDGF-Rβ+/CD146+ eMSCs exhibit pericyte characteristics and are localized adjacent to endometrial blood vessels [26, 41]. Recently, W5C5 (SUSD2) was introduced as an alternative single marker to purify the same PDGF-Rβ+/CD146+ eMSC population. W5C5+ eMSCs reconstituted endometrial stromal tissue after transplantation under the kidney capsule of nonobese diabetic/severe combined immunodeficient/IL2 receptor γ-deficient (NOD/SCIDγ) mice [41].
Alternatively, potential populations of EDSCs may be initially selected based on the ability of side population (SP) cells and stem cells to exclude Hoechst 33342 dye. Hoechst dye-excluding SP cells are characterized by the expression of ATP binding cassette (ABC) subtypes ABCB1 and ABCG2 on the cytoplasmic membrane that pump the DNA-binding Hoechst dye out of the cells. SP cells can be isolated from both the epithelial and stromal fractions of the human endometrium [42, 43]. Most SP cells have been shown to be negative for CD9 and CD13 [44]. Flow cytometric analysis showed that endometrial SP cells preferentially express endothelial cell markers CD31, CD34, and CD144; some also express MSC markers CD90, CD105, or CD146, and epithelial cell marker EMA, which indicates that SP cells in the endometrium are a heterogeneous population [43, 45, 46]. Additionally, the expression of typical undifferentiated cell markers, including OCT-4, telomerase, GDF3, DNMT3B, Nanog, and GABR3, was detected in SP cells [42, 43]. SP cells displayed greater clonogenic efficiency compared with an unselected population of cells [42, 46]. When cultured in vitro, endometrial SP cells differentiated to epithelial, stromal, and endothelial cells, whereas the non-dye-excluding cells differentiated only to stromal cells [45]. Estrogen receptor and progesterone receptor expression was not detected in SP cells with immunohistochemical analysis [43]. However, in other studies, SP cells expressed decidualization markers prolactin and IGFBP-1, and displayed features of decidualization, such as polygonal morphology and abundant cytoplasm after 2 wk in culture. This suggests that SP cells were responsive to estrogen and progesterone, though the presence of required receptors was not confirmed [46]. As studies have not yet been able to detect ER and progesterone receptor expression in SP cells, this responsiveness may be the result of paracrine action. Adipogenic and osteogenic differentiation were observed under appropriate culture conditions [43]. Human endometrial SP cells formed glandular, stromal, and endothelial structures when transplanted under the kidney capsule of immunodeficient NOD/SCIDγ mice [45]. In vivo studies have also suggested that human endometrial SP cells may be able to differentiate to a smooth muscle cell-like phenotype [47]. Reconstitution of endothelial cells was not observed in other studies [43], which may have been a result of the heterogeneity of SP cells. The differentiation potential and quiescent nature of SP cells suggest that they are another distinct stem cell population in the endometrium.
We suggested an exogenous source of stem cells contributing to the endometrium by demonstrating that 2%–52% of endometrial stroma and epithelium was bone marrow derived in women who had undergone single HLA mismatched bone marrow transplantation for cancer treatment [20]. In a similar study, approximately 8% of the uterine epithelium and 9% of the uterine stroma cells were bone marrow derived in women who underwent bone marrow transplantation for cancer therapy [48]. Bone marrow-derived cells (BMDCs) were also detected in the endothelial layer of blood vessels [49]. We have further studied bone marrow's contribution to uterine endometrium in a murine model [50]. At 6 mo after bone marrow transplantation from male mice, Y chromosomes were observed in endometrial cells of the recipient mice. Y chromosome-containing cells represented 1 in 5000 cells within the uterine epithelium. The extent of contribution to uterine stroma was nearly twice that of the epithelium [50]. Various studies have additionally reported the migration of bone marrow-derived stem cells after transplantation to distal sites; one such study demonstrated migration of BMDCs to the spleen from the site of injection at the heart [51].
We further investigated the factors that influence recruitment of BMDCs to the uterus. Ischemia reperfusion uterine injury 1 wk after bone marrow transplantation increased BMDC recruitment to the uterine stroma by two-fold compared to that in noninjured animals [52]. Without ischemic injury, an average of 22 and 13 Y chromosome signals were detected per 100 000 cells in the stromal and epithelial compartments, respectively. In contrast, when ischemic injury was applied, an average of 42 and 14 Y chromosome signals were detected per 100 000 cells in the stromal and epithelial compartments, respectively. BMDCs in the uterus expressed the mature stromal cell marker vimentin, but not CD45, suggesting that BMDCs fully differentiated and mimicked expression patterns of endogenous cells in the uterine stroma. Interestingly, ischemia-reperfusion injury did not increase the epithelial contribution of BMDCs, suggesting that the stroma is the main target of injury and repair. Increased HSC mobilization with granulocyte-CSF (G-CSF) after uterine ischemia-reperfusion injury reduced the BMDC contribution to the uterus, likely by selectively mobilizing HSCs over MSCs. Similarly, systemic administration of the proinflammatory cytokine IL1β, which induces proliferation and differentiation of myeloid and hematopoietic precursors, did not alter the uterine engraftment of BMDCs. These observations suggest that a distinct cellular population within bone marrow that behaves differently from HSC, likely a subset of bone marrow MSCs, contributes to uterine regeneration after injury [52]. Although the numbers of engrafted BMDCs in the endometrial stroma were seemingly small, at 0.042%, after ischemic injury, populations of stem cells need not reach large numbers in order to be physiologically relevant. Only 0.01% (1 in 10 000) of cells in a sample of bone marrow are likely to be HSCs, and yet this small cell population is functionally capable of replenishing needed myeloid and lymphoid cells [53]. It is unknown if these BMDCs are involved directly in repopulation of injured endometrium, or if they act only as support systems for surrounding cells by releasing various trophic factors, as has been well reported in the literature [9]. MSCs have been shown to transiently release cytokines and growth factors that stimulate tissue growth while inhibiting fibrosis and apoptosis at sites of injury. These trophic factors have also been shown to have a role in the mobilization and differentiation of tissue-specific adult stem cells. However, given that, in this study, the uteri were collected 8 mo after bone marrow transplant, thereby indicating that the engraftment of the BMDCs was long term, this may suggest a regenerative role beyond the transient support of growth and repair [33].
We have also evaluated the effect of sex steroids on BMDC migration to the uterus. When busulfan and cyclophosphamide chemotherapy were used to ablate endogenous bone marrow in female mice before they received bone marrow transplants from male mice, recipient animals developed ovarian failure and did not cycle. Ovarian transplantation successfully salvaged estrus cycling in these animals. However, the number of BMDCs in the uterus was not different between the cycling and noncycling animals. These findings indicate that the local uterine injury is the key factor recruiting BMDCs to the uterus, and that the engraftment is independent of the estrous cycle, sex steroids, or G-CSF [52].
Stem cells with high proliferation and differentiation capacity were isolated from menstrual blood and named endometrial regenerative cells (ERCs); it is likely that these cells are the same or a subset of those discussed above. A fraction of EDSCs may be shed with menses in reproductive-aged women. ERCs expressed CD9, CD29, CD41a, CD44, CD59, CD73, CD90, and CD105, and not CD14, CD34, CD38, CD45, CD133, or STRO-1 [54]. Interestingly, ERCs were also positive for the ESC marker OCT-4 and for telomerase (hTERT). The cells had substantially faster replicative potential when compared with bone marrow MSCs, a unique cytokine and matrix metalloproteinase (MMP) profile, as well as the ability to differentiate into cardiomyocytic, respiratory epithelial, neurocytic, myocytic, endothelial, pancreatic, hepatic, adipocytic, and osteogenic lineages [54].
REGENERATION OF ENDOMETRIUM WITH BMDCS
Congenital malformations of the uterus and acquired disorders of the endometrium are associated with infertility and adverse pregnancy outcomes. A common acquired endometrial disorder is Asherman syndrome, which is associated with destruction of the basal-layer endometrium and formation of intrauterine synechiae, often following curettage or miscarriage. Current treatment strategies for Asherman syndrome aim to break up the synechiae and induce endometrial proliferation in order to restore functionality to the uterine cavity. Unfortunately, the risk of treatment failure is high. These fibrotic synechiae often lack endometrial lining entirely, or, if present, the lining is thin and largely nonfunctional [55]. Given that endogenous EDSCs are likely responsible for regenerating the endometrium, it follows that EDSC may be damaged or lost in women with Asherman syndrome. Motivated by our previous observations, we have tested the contribution of bone marrow-derived stem cells to uterine regeneration in a mouse model of Asherman syndrome [56]. Following unilateral uterine horn injury, mice were randomized to receive either allogeneic bone marrow from male mice or sham transplantation. At 3 mo after surgery, 55 out of 10 000 and 14 out of 10 000 cells in the damaged uterine horn were Y chromosome+/CD45− bone marrow-derived stromal and epithelial cells, respectively. Y chromosome+/CD45− bone marrow-derived stromal and epithelial cells were detectable in both uterine horns of the recipient animals. Although there was no significant histological difference in levels of fibrosis between the sham and treatment groups as determined by hematoxylin and eosin staining, pregnancy rates were substantially higher in the bone marrow recipient mice compared with those that were transplanted with PBS, which suggests a functional role of BMDCs in supporting endometrial regeneration, possibly by trophic means [56].
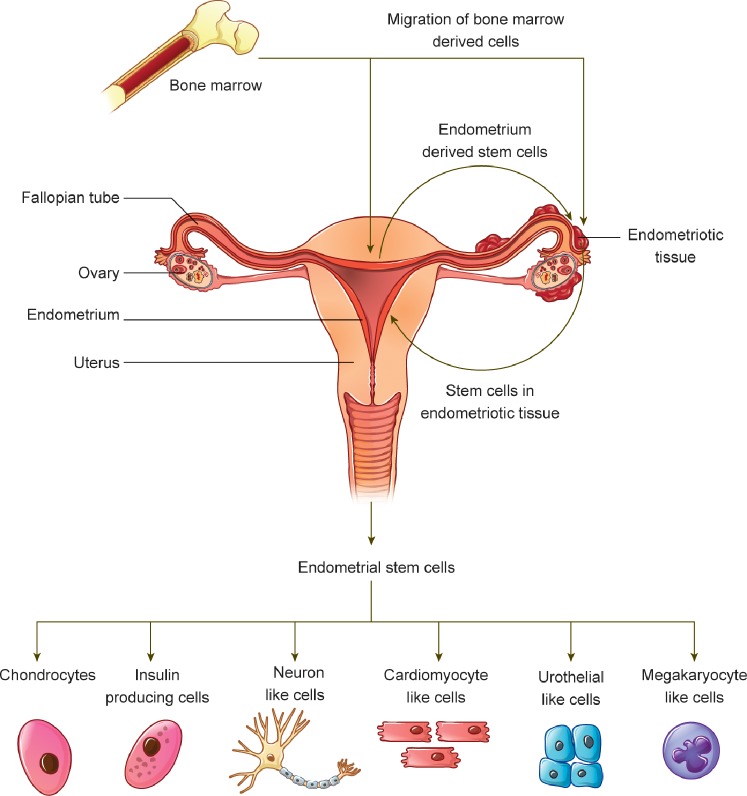
Additional evidence for the role of circulating BMDCs in endometrial regeneration was demonstrated in an endometriosis mouse model [57]. We demonstrated that, after endometriosis induction and bone marrow transplantation, Y chromosome+/CD45− BMDCs were competitively attracted to both endometriotic lesions and the eutopic endometrium. As a result of this competition, the mice with endometriotic lesions had reduced numbers of BMDCs in the eutopic endometrium compared with their lesion-free counterparts [57]. However, bazedoxifene, a selective ER modulator, can shrink the endometriotic lesions [57–59] and diminish the flux of BMDCs to them, thereby redirecting these cells to the eutopic endometrium [57]. In fact, treatment with bazedoxifene completely restored the BMDC engraftment to the eutopic endometrium in mice with endometriotic lesions. Bazedoxifene acts as an ER antagonist, and thus likely decreased cell proliferation mediated by estradiol at sites of endometriotic lesion. BDMCs may be selectively targeted toward regions of increased proliferation or inflammation; accordingly, as proliferation within endometriotic lesions was reduced, BDMCs were redirected to eutopic sites. These findings suggest a novel mechanism for endometriosis-induced infertility, in which the endometriotic lesions compete for the flux of BMDCs and reduce their engraftment to the eutopic endometrium (Fig. 1).
FIG. 1.
Schematic illustration of the migration and engraftment of BMDCs, EDSCs, and the differentiation potential of EDSCs. BMDCs migrate and engraft in the endometrium and endometriotic lesions. The presence of endometriotic lesions can decrease the flux of these cells to the eutopic endometrium. Furthermore, cells from endometriotic lesions can migrate back to the endometrium. Both of these mechanisms present a novel mechanism of endometriosis-related infertility. Once harvested from the endometrium and cultured in appropriate conditions, EDSCs can differentiate into a variety of mature cell types with therapeutic potential, including chondrocytes, insulin-producing cells, neuron-like cells, cardiomyocyte-like cells, urothelial cells, and megakaryocyte-like cells.
In a case report of Asherman syndrome, a 33-yr-old woman, who had not responded to medical or surgical therapy, underwent autologous CD9+, CD44+, and CD90+ bone marrow transplantation. Bone marrow transplantation resulted in increased endometrial vascularization and endometrial thickness. The patient then underwent in vitro fertilization and embryo transfer, which resulted in a successful pregnancy [60].
DIFFERENTIATION OF EDSCS TO TREAT NONENDOMETRIAL DISEASE
As discussed above, MSCs have emerged as an alternative source for cell replacement therapies. MSCs derived from bone marrow, adipose tissue, and other sources have been long used for cell replacement therapies with varying degrees of success. Studies from our laboratory and others have recently concentrated on the endometrium, which contains a rich supply of MSC-like cells [61]. In fact, the endometrium is the only source of tissue-derived MSCs that can be obtained without analgesic requirements, thereby rendering it a feasible and functional source for stem cell therapies. Recent studies have shown that the stem cells in the endometrium exhibit greater differentiation potential than was previously assumed, and these cells could be used for regeneration of other organs. Finally, in females, a woman's own EDSCs may be used, eliminating any concerns over immune rejection.
Differentiation of Human EDSCs into Neuronal Phenotypes
Differentiation to dopamine-producing cells.
A promising example of a therapeutic role for eMSC differentiation came from our laboratory in 2010. We differentiated CD90, CD146, and PDGF receptor (PDGFR) β expressing human EDSCs (HEDSCs) to neuron-like cells in vitro [30]. In vitro-differentiated cells exhibited neurite-like processes and expressed the neuronal cell markers nestin and tyrosine hydroxylase (TH), the enzyme that catalyzes the rate-limiting step in dopamine production. Further characterization of these cells with electrophysiological studies showed the expression of barium-sensitive inward rectifier potassium channels, which are seen in central neurons, including those within the substantia nigra. Differentiation and transplantation of HEDSCs were tested in a 1-methyl 4-phenyl 1,2,3,6-tetrahydro pyridine (MPTP) treated mouse model of Parkinson disease (PD). MPTP is a selective neurotoxin of dopaminergic cells, and is used widely to mimic PD in animal models [62, 63]. Undifferentiated HEDSCs were transplanted into the striatum of the recipient mice. These stem cells migrated to substantia nigra, expressed nestin, and adopted a neuron-like morphology in vivo. Additionally, the brains of HEDSC-transplanted mice had significantly higher concentrations of dopamine and its metabolite, DOPAC, when compared with sham transplant mice.
The dramatic difference in striatal dopamine concentrations between the sham and the HEDSC-transplanted mice is likely a combination of the protective mechanism of the HEDSCs against endogenous neuronal death and the active contribution of in vivo-differentiated HEDSCs to dopamine production, as evidenced by the increased expression of TH [30].
We repeated these studies in a nonhuman primate model of PD [31]. After unilateral injection into the striatum of MPTP-treated male monkeys, allogeneic EDSCs: engrafted into the striatum; migrated to the substantia nigra, where they exhibited neuron-like morphology; expressed TH; and increased the numbers of TH-positive cells on the transplanted side. Most impressively, EDSC transplantation was associated with a mean increase of 27% in dopamine metabolite concentration on the transplanted side of the brain [31]. Although the exact molecular mechanism behind this therapeutic benefit remains to be elucidated, these two proof-of-concept studies show the promise of EDSCs in PD research.
Differentiation to cholinergic neuron-like cells.
In addition to dopamine-producing cells, other groups have reported differentiation of CD146-expressing HEDSCs into cholinergic neuron-like cells in vitro. Upon stimulation with nerve growth factor and bFGF, HEDSCs increased expression of choline acetyl transferase, microtubule-associated protein 2, and neurofilament L, while demonstrating neuron-like morphology. However, detailed electrophysiological studies and thorough characterization of the differentiated cells have not yet been completed [64].
Differentiation to oligodendrocyte-like cells.
Inflammatory diseases of the central nervous system (CNS), like multiple sclerosis, typically result in the loss of oligodendrocytes, which are myelinating neuroglial cells. Thus, derivation of oligodendrocytes has been of central interest in cell replacement therapies for CNS disorders that affect the brain and the spinal cord. Previous studies have shown that human endometrial mesenchymal cells expressing CD105, CD90, CD146, and CD44 can be induced into oligodendrocyte-like cells in a neuron-conditioned medium containing bFGF, epidermal growth factor (EGF), PDGF-AA, and thyroid hormone [65]. Differentiated cells exhibited bipolar oligodendrocyte-like morphology and expressed oligodendrocyte lineage markers—nestin, PDGFRα, SOX10, and OLIG2—on the mRNA level. Moreover, the expression of oligodendrocyte cell markers A2B5, O4, and OLIG2 was observed via immunocytochemistry [65].
In addition to differentiating into oligodendrocyte-like cells, HEDSCs may exhibit a beneficial effect in demyelinating diseases by modulating the inflammatory response within the CNS. In an animal model of autoimmune encephalitis, intraperitoneal administration of HEDSCs that expressed CD29, CD73, CD90, HLA-ABC, and SH4 exhibited a neuroprotective effect by reducing the absolute number of proinflammatory Th1 and Th17 CD4 infiltrating cells within the CNS, increasing anti-inflammatory cytokines IL10, IL27, indolamine 2,3 dioxygenase, and Foxp3, and increasing regulatory T cells in the spleen of the recipient mice [66]. These findings suggest that HEDSC transplantation confers favorable systemic immunomodulatory effects and alleviates the infiltration of proinflammatory cells into the CNS.
Applications in stroke.
Stroke is the third leading cause of death in United States and, if not fatal, results in significant disability [67]. Stem cell therapies aim to supplant lost neurons, reduce neuronal death, or increase vasculogenesis after stroke. The therapeutic potential of ERCs expressing CD117 has been tested in a rat model of stroke [68]. After middle cerebral artery occlusion and subsequent intracerebral or intravenous injection of ERCs, ERC recipient rats demonstrated increased neuronal survival in the ischemic penumbra when compared with control. Moreover, ERC recipient rats had better functional outcomes, as demonstrated by significantly reduced behavioral abnormalities, such as motor asymmetry. The treated mice additionally displayed superior motor coordination compared to vehicle recipient rats. Interestingly, the intracerebral or intravenous route of delivery of ERCs resulted in similar functional outcomes in the recipient rats. Nonetheless, significantly less graft survival was observed after intravenous injection. Although the exact mechanism of increased neuronal survival after ERC transplantation observed in vivo was not elucidated, cultured ERCs secreted neurotrophic factors like BDNF and NT3, and decreased hypoxia-induced neuronal death in vitro [68]. The increased neuronal survival after ERC transplantation, thus, may have been conferred by ERC-released neurotrophic factors. Further studies are needed to exactly delineate the neuroprotective mechanisms of ERC transplantation and determine whether they are able to differentiate into neuronal phenotypes in vivo.
Differentiation to Insulin-Producing Cells
The potential of generating insulin-producing cells from stem cells has led to exciting new avenues in diabetes research. Previous research from our laboratory has shown that HEDSC expressing MSC markers PDGFβ-R, CD146, and CD90 can be successfully induced to insulin-producing cells [69]. After incubation for 1 wk in differentiation media, HEDSCs expressed early pancreatic developmental genes NGN3 and PDX1. After 3 wk, expression of mature pancreatic beta cell markers PAX4, GLUT2, and insulin was increased, which suggests pancreatic beta cell-like differentiation of HEDSCs. Moreover, HEDSCs changed their morphology and formed islet-like clusters in culture. Functional analysis of the HEDSC-derived beta-like cells showed that these cells are responsive to glucose in vitro and increase insulin secretion when exposed to increasing concentrations of glucose. We have further shown the therapeutic potential of the HEDSC-derived beta-like cells in a mouse model of diabetes. After being treated with streptozocin to induce diabetes, mice that were transplanted with endometrial stromal cells developed more severe hyperglycemia compared to the mice that had been transplanted with HEDSC-derived pancreatic beta-like cells. Notably, transplantation of pancreatic beta-like cells prevented worsening of hyperglycemia in the recipient mice, whereas stromal cell transplantation failed to do so. Although beta-like cell transplantation did not normalize glucose levels, it prevented and slowed down the development of diabetes-associated complications, such as dehydration, weight loss, and cataracts [69].
Li et al. [70] used a different approach to differentiate CD29-, CD44-, CD81-, and CD105-expressing human endometrial mesenchymal stem-like cells (EMSCs) to pancreatic beta-like cells. In this study, EMSCs were cultured in a specific differentiation medium and ultimately formed spheroid bodies (SB-EMSCs) that secreted insulin and expressed pancreatic islet cell markers NKX2, GLUT2, glucagon, and somatostatin on the mRNA level. Moreover, SB-EMSCs showed glucose-dependent insulin secretion in vitro. After transplantation, SB-EMSCs normalized glucose levels in streptozocin-treated diabetic mice. Interestingly, SB-EMSCs showed resistance to oxidative stress and conferred prolonged survival on the recipient mice.
These pioneer studies have shown the clinical potential of the human endometrium to generate insulin-secreting cells. Further research should lead to large-scale and efficient production of insulin-secreting cells.
Differentiation to Cardiomyocyte-Like Cells
Heart disease is the most common cause of hospitalization and death in adults. Not surprisingly, cell-based therapies to replenish lost cardiomyocytes has generated great interest. ESCs, BM-MSCs, and endothelial progenitor cells have been employed as a stem cell source in murine cardiac infarction models. A novel source of cardiac precursor-like cells was proposed in 2008. Hida et al. [71] successfully differentiated human menstrual blood-derived MSCs (MMCs) into spontaneously beating cardiomyocyte-like cells. These cells are easily collectible in menses, and were shown to retain their differentiation potential for approximately 28 generations in vitro. MMCs expressed CD29 and CD59, but not CD14, CD34, CD45, or Flk-1. Interestingly, mRNA expression of cardiac progenitor cell marker GATA4 was observed in human MMCs, suggesting the cardiomyogenic differentiation potential of these cells. After being cocultured with murine cardiac cells, human MMC-derived cells exhibited strong contractility. Moreover, immunofluorescence analysis revealed the expression of cardiac-specific troponin I and connexin 43 in the MMC that were cocultured with murine cardiomyocytes, but not in the control group. Similarly, characteristic striations of the intermediate filament α-actinin were observed in the differentiated MMCs, indicating cardiomyogenic differentiation. Electrophysiological studies on differentiated cells showed pacemaker potential and action potentials characteristic of cardiomyocytes. The clinical potential of MMCs was also tested in murine models of cardiovascular disease; 2 wk after ligation of the left coronary artery and resultant left ventricular infarction, either undifferentiated MMCs or BM-MSCs were injected into the center and margin of the infarct zone. Notably, left ventricular systolic functions were superior and the infarct area was smaller in MMC compared to BM-MSC recipients. Moreover, EGFP-tagged MMCs were detectable in the infarct area and demonstrated classical striations of α-actinin and troponin I, which suggests spontaneous cardiomyocyte-like differentiation in vivo [71]. Many investigators speculate that the favorable effect of stem cell transplantation after myocardial infarction is due to increased angiogenesis. However, as the transplantation was done after myocardial necrosis had been completed, improved cardiac function was not merely due to MMC-induced angiogenesis. These findings suggest that MMCs have a substantial capacity to differentiate into cardiomyocyte-like cells. However, many studies using MSCs for cardiac repair suggest that the stem cells improve overall cardiac function by releasing soluble factors that induce angiogenesis, improving cardiomyocyte survival, and fusing with the endogenous cardiomyocytes [72–75]. Further research is needed to understand whether HEDSCs secrete such factors, which could increase their therapeutic potential.
Differentiation to Megakaryocyte-Like Cells
The vast differentiation potential of HEDSCs was further supported by an in vitro study, in which HEDSCs were differentiated into megakaryocyte-like cells that are able to produce functional platelets [76]. When cultured in media containing thrombopoietin, up to 30% of the cells expressed megakaryocyte markers CD41a and CD42b. Moreover, the differentiated megakaryocytes were able to release functional platelets that can bind fibrinogen after thrombin stimulation in vitro. Ultrastructural assessment of these platelets showed the expression of alpha granules, and a dense tubular system similar to their endogenous circulating counterparts [76]. Further in vivo studies will help to delineate the functionality of HEDSC-derived platelets and their potential clinical utility.
Differentiation to Urothelial Cells
The urinary bladder is a musculocutaneous organ, the primary function of which is to store and expel waste products in the form of urine. Developmental and acquired abnormalities of the bladder result in urinary incontinence and potential damage to the upper urinary tract, which may result in renal failure. Therefore, efforts to restore the anatomical integrity and function of the bladder with tissue engineering have been of central interest in regenerative medicine. Previously, MSCs derived from bone marrow and endothelial progenitors were used as a source of smooth muscle cells and vasculature in efforts to regenerate bladder in vivo [77, 78]. Recently, a report of the derivation of urinary-epithelial cells from HEDSCs suggested that the human endometrium may be an alternative autologous cell source for bladder regeneration [80]. In this study, CD146+, CD105+, CD90+, and CD44+ HEDSCs were isolated and plated on collagen-V, silk, and silk-collagen nanofibers, which provided a suitable three-dimensioanl scaffold for HEDSC proliferation and tissue engineering applications. Stimulation of HEDSCs by keratinocyte growth factor and EGF in vitro resulted in increased expression of urinary bladder epithelium (urothelium) characteristic genes and proteins: uroplakin-Ia, uroplakin-Ib, uroplakin-II, uroplakin-III, and cytokeratin 20 [79]. Although this study provided promising preliminary results, further studies are needed to determine the applicability and function of the HEDSC-derived urothelial cells.
Differentiation to Other Mesenchymal Cell Types
Cartilage tissue is composed of chondrocytes and abundant extracellular matrix, which is adopted to provide mechanical stability and absorb trauma in the musculoskeletal system. However, cartilage tissue has limited regenerative capability, and an injury beyond its self-repair capacity may result in permanent disability. Many studies have investigated the potential of MSCs seeded on mechanically stable scaffolds to renew damaged cartilage. HEDSCs have been shown to differentiate into chondrocytes that can produce abundant extracellular matrix in vitro [9, 80]. Recently, the chondrogenic differentiation potential of HEDSCs derived from menstrual blood has been investigated using electrospun nanofibers, which are polymer-based biomaterials acting like extracellular matrix to support chondrocyte differentiation and growth. After seeding on poly-ε-caprolactone (PCL) nanofibers and incubation in chondrogenic differentiation media, menstrual blood-derived HEDSCs differentiated to chondrocyte-like cells that produced abundant collagen II and stained positively with alizarin red, which is characteristic for chondrocyte differentiation [81]. However, the long-term integrity of PCL fibers and HEDSC-derived chondrocytes should be tested in vivo to demonstrate clinical utility.
More evidence for the feasibility of using HEDSCs on synthetic biomaterials for tissue engineering applications has been demonstrated in a rat skin injury model. Endometrial MSCs expressing W5C5/SUSD2 were seeded on synthetic gelatin-coated polyamide (PA-G; a synthetic material that can be used for tissue engineering [81–83]) knit meshes, and transplanted subcutaneously after skin dissection [85]. Endometrial MSCs were detectable around the PA-G mesh up to Day 14 after implantation; 7 days after implantation, PA-G meshes coated with W5C5+ eMSCs demonstrated increased angiogenesis compared to the mesh alone. Transplantation of both eMSC-coated and uncoated PA-G meshes caused an inflammatory response, which was more abundant at Day 7 and subsequently decreased until Day 90 after transplantation. At Day 90, the inflammatory cells around the eMSC-coated PA-G meshes were less than the noncoated meshes. Although eMSCs were not detectable around the meshes after 14 days of implantation, their immunomodulatory effects around the mesh persisted. Endometrial MSC coating was associated with increased proinflammatory M1 macrophages at Day 7 compared to the uncoated mesh; however, anti-inflammatory M2 macrophages predominated proinflammatory M1 macrophages at Day 30. There was no difference in collagen deposition between the groups. Endometrial MSC-coated PA-G demonstrated reduced stiffness compared to the PA-G mesh alone. These results indicate that W5C5+ eMSCs may favorably modulate the tissue response to implanted foreign materials, and that W5C5+ eMSC-coated meshes could be used for regenerative purposes, such as pelvic organ prolapse repair [84, 85].
The myogenic potential of menstrual blood-derived stem cells has been tested in a mouse model of Duchenne muscular dystrophy (DMD) [85]. DMD results from mutations in the DMD gene, which causes impaired function or production of the membrane-stabilizing glycoprotein dystrophin. The absence of dystrophin leads to destabilization of the skeletal muscle fibers and progressive muscle degeneration. Like DMD, mdx mice lack dystrophin expression on the skeletal muscle fiber membrane, and exhibit phenotypic similarities with DMD [85, 86]. Human menstrual blood-derived stem cells, expressing high levels of MSC markers CD29, CD44, CD59, and CD90, were injected into the right thigh muscle of the immunodeficient mdx mice [85]. At 3 wk after injection, immunofluorescence analysis showed that the menstrual blood-derived stem cells fused with the host myocytes, and human dystrophin was detectible in approximately 1.5% of the muscle fibers. Similarly, coculture assays have shown that the menstrual blood-derived stem cells fused with C2C12 murine myoblasts and increased dystrophin expression. Upon induction with 5-azacytidine, menstrual blood-derived stem cells were able to differentiate into myocytes that express myogenin and dystrophin, which further demonstrates the differentiation and therapeutic potential of menstrual blood-derived stem cells in DMD [85].
STUDIES ON THE ANGIOGENIC ROLE OF EDSCS
The human endometrium is shed every month upon reaching puberty under physiological conditions, and endometrial regeneration requires a high degree of angiogenesis to support tissue growth. Given the high demand of regeneration, it was speculated that the stem cells in endometrium have a natural propensity to support vascular growth. In fact, it has been demonstrated that ERCs express high levels of angiogenic factors like PDGF, EGF, VEGF, and MMP, and can induce human umbilical vein endothelial cell proliferation in vitro [29, 54, 87]. The clinical potential of ERCs to induce angiogenesis was demonstrated in a mouse model of peripheral vascular disease. After ligation of the femoral artery and nerve excision to stimulate critical limb ischemia in mice, subcutaneously transplanted ERCs were able to rescue the limb in the recipient mice, whereas the control group suffered limb necrosis. Although, the human-derived ERCs were transplanted to the immune-competent BALB/c mice in this study, these results suggest that the ERCs demonstrated significant levels of immune privilege. The immunomodulatory effects of ERCs were demonstrated in mixed lymphocyte reactions, where they were able to reduce IFNgamma, TNF, and lymphocyte proliferation, while simultaneously inducing the immunosuppressant cytokine IL4 [87].
HUMAN STUDIES USING ENDOMETRIAL STEM CELLS
The first anecdotal case report on using ERCs in human subjects was published in 2009. Four patients suffering from multiple sclerosis underwent either intrathecal or intravenous administration of ERCs [88]. The follow-up period ranged between 6 and 17 mo, and no major adverse events were reported. However, baseline patient characteristics, the fate of the transplanted ERCs, and follow-up characteristics for all subjects were not reported in detail.
A case report of EDSC therapy used to treat DMD was published in 2009, where a 22-yr-old male received a total of 116 × 106 intramuscular and 6 × 106 intravenous ERCs, along with a total of 9 × 106 CD34+ cord blood cells and 3 × 106 placental matrix-derived MSCs [90]. At 3 mo after transplantation, muscle strength in all muscle groups increased significantly, and the frequency of respiratory infections decreased. Muscle biopsy 5 mo after transplantation showed an increased level of muscle dystrophin [89].
A 74-yr-old patient, suffering from heart failure with an ejection fraction of 30%, received a total of 15 × 106 ERCs and 13 × 106 CD34+ cells by intravenous injection. A 2-yr follow up with echocardiogram revealed that the patient's ejection fraction increased to 40%, and the blood concentrations of heart failure marker brain natriuretic peptide decreased in parallel with the improved cardiac function. No adverse events were reported during the course of follow up [90]. A privately funded trial on ERCs was launched in 2012, in which patients with advanced congestive heart failure received ERCs via a retrograde coronary artery delivery technique. Preliminary safety results suggested that the retrograde delivery of ERCs was not associated with major adverse events [29].
A search of the clinical trial database provided by the U.S. National Institutes of Health lists two phase I/II trials using ERCs for end-stage liver cirrhosis and critical limb ischemia. To date, there are no interim results available from either trial [91, 92].
CONCLUSION AND FUTURE DIRECTIONS
The endometrium has emerged as a novel source of stem cells with remarkable differentiation capacity. New research venues have been opened by the discovery of the differentiation potential and wide variety of growth factors secreted by EDSCs. SP cells in the endometrium as well as LRCs have provided populations from which stem cells may be isolated. ERCs, isolated from menstrual blood, have shown vast potential for differentiation, as have MSCs isolated from the endometrial stroma. While cells from the endometrial epithelium have demonstrated clonogenic potential, the multipotent or pluripotent potential of these cells has not yet been verified. BMDCs have engrafted in endometrium after bone marrow transplant, likely contributing to endometrial regeneration. More research is needed to understand the biological regulation and mechanism of action of EDSCs, as well as the alterations in the microenvironment after transplantation. Elucidation of the role of EDSCs in the reproductive system may also shed light on the mechanisms behind uterine diseases in which uterine proliferation is abnormal, thereby potentially leading to clinical treatments. The interplay between bone marrow-derived stem cells and eMSCs in physiological and disease states may provide new therapeutic opportunities in reproductive disorders, such as endometriosis. The initial preclinical and clinical studies of the therapeutic applications of EDSCs are promising. However, stem cell therapies are in their relative infancy, and we still have limited understanding of the therapeutic mechanisms or safety of these treatments. Future research should also look toward optimizing the isolation of EDSCs, and, if necessary, subsequent differentiation to a desired phenotype for transplantation. Further understanding of the molecular mechanisms of the effects of EDSCs in preclinical studies will help in designing safe trials and making these therapies a viable therapeutic option. This is an exciting avenue for regenerative medicine, as EDSCs may provide an autologous and easily attainable source of stem cells for women suffering from a variety of conditions, as well as a potential heterologous source of stem cells, given the differentiation potential and the immunomodulatory nature of MSCs.
Footnotes
This work was supported by National Institutes of Health grant NIH U54 HD052668.
REFERENCES
- Wang J, Lu J, Bond MC, Chen M, Ren XR, Lyerly HK, Barak LS, Chen W. Identification of select glucocorticoids as Smoothened agonists: potential utility for regenerative medicine. Proc Natl Acad Sci U S A. 2010;107:9323–9328. doi: 10.1073/pnas.0910712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10:740–749. doi: 10.1016/j.stem.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ED, Lochte HL, Jr, , Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov [Internet] Bethesda, MD: National Library of Medicine; (US) 2000. https://clincaltrials.gov/ct2/results?term=stem+cells&Search=Search. Accessed 10 March 2015. [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- Wolff EF, Wolff AB, Hongling D, Taylor HS. Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod Sci. 2007;14:524–533. doi: 10.1177/1933719107306896. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G, Noel D, Jorgensen C, Figueroa F, Djouad F, Carrion F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Yi S, Wang G, Cheng J, Zhang Y, Chen W, Tai Y, Chen S, Chen G, Liu W, Zhang Q, Yang Y. Umbilical cord-derived mesenchymal stem cells instruct dendritic cells to acquire tolerogenic phenotypes through the IL-6-mediated upregulation of SOCS1. Stem Cells Dev. 2014;23:2080–2092. doi: 10.1089/scd.2013.0559. [DOI] [PubMed] [Google Scholar]
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Prianishnikov VA. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception. 1978;18:213–223. doi: 10.1016/s0010-7824(78)80015-8. [DOI] [PubMed] [Google Scholar]
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–85. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Chan RW, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(suppl 2):1124–1130. doi: 10.1016/j.fertnstert.2005.02.056. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Hutchinson P, Gargett CE. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23:934–943. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- Dimitrov R, Timeva T, Kyurkchiev D, Stamenova M, Shterev A, Kostova P, Zlatkov V, Kehayov I, Kyurkchiev S. Characterization of clonogenic stromal cells isolated from human endometrium. Reproduction. 2008;135:551–558. doi: 10.1530/REP-07-0428. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuring AN, Schulte N, Kelsch R, Ropke A, Kiesel L, Gotte M. Characterization of endometrial mesenchymal stem-like cells obtained by endometrial biopsy during routine diagnostics. Fertil Steril. 2011;95:423–426. doi: 10.1016/j.fertnstert.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Spitzer TL, Rojas A, Zelenko Z, Aghajanova L, Erikson DW, Barragan F, Meyer M, Tamaresis JS, Hamilton AE, Irwin JC, Giudice LC. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod. 2012;86:58. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NH, Park YK, Kim YT, Yang H, Kim SK. Lifetime expression of stem cell markers in the uterine endometrium. Fertil Steril. 2004;81:403–407. doi: 10.1016/j.fertnstert.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Elmore LW, Domson K, Moore JR, Kornstein M, Burks RT. Expression of c-kit (CD117) in benign and malignant human endometrial epithelium. Arch Pathol Lab Med. 2001;125:146–151. doi: 10.5858/2001-125-0146-EOCKCI. [DOI] [PubMed] [Google Scholar]
- Bockeria L, Bogin V, Bockeria O, Le T, Alekyan B, Woods EJ, Brown AA, Ichim TE, Patel AN. Endometrial regenerative cells for treatment of heart failure: a new stem cell enters the clinic. J Transl Med. 2013;11:56. doi: 10.1186/1479-5876-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff EF, Gao XB, Yao KV, Andrews ZB, Du H, Elsworth JD, Taylor HS. Endometrial stem cell transplantation restores dopamine production in a Parkinson's disease model. J Cell Mol Med. 2011;15:747–755. doi: 10.1111/j.1582-4934.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff EF, Mutlu L, Massasa EE, Elsworth JD. Eugene Redmond D Jr, Taylor HS. Endometrial stem cell transplantation in MPTP-exposed primates: an alternative cell source for treatment of Parkinson's disease. J Cell Mol Med. 2015;19:249–256. doi: 10.1111/jcmm.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Nguyen HP, Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13:235–251. doi: 10.1007/s11154-012-9221-9. [DOI] [PubMed] [Google Scholar]
- Janzen DM, Cheng D, Schafenacker AM, Paik DY, Goldstein AS, Witte ON, Jaroszewicz A, Pellegrini M, Memarzadeh S. Estrogen and progesterone together expand murine endometrial epithelial progenitor cells. Stem Cells. 2013;31:808–822. doi: 10.1002/stem.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Chan RW, Schwab KE. Endometrial stem cells. Curr Opin Obstet Gynecol. 2007;19:377–383. doi: 10.1097/GCO.0b013e328235a5c6. [DOI] [PubMed] [Google Scholar]
- Cervello I, Martinez-Conejero JA, Horcajadas JA, Pellicer A, Identification Simon C. characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum Reprod. 2007;22:45–51. doi: 10.1093/humrep/del332. [DOI] [PubMed] [Google Scholar]
- Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529–1538. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- Chan RW, Kaitu'u-Lino T, Gargett CE. Role of label-retaining cells in estrogen-induced endometrial regeneration. Reprod Sci. 2012;19:102–114. doi: 10.1177/1933719111414207. [DOI] [PubMed] [Google Scholar]
- Kaitu'u-Lino TJ, Ye L, Salamonsen LA, Girling JE, Gargett CE. Identification of label-retaining perivascular cells in a mouse model of endometrial decidualization, breakdown, and repair. Biol Reprod. 2012;86:184. doi: 10.1095/biolreprod.112.099309. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sacchetti A, van Dijk MR, van der Zee M, van der Horst PH, Joosten R, Burger CW, Grootegoed JA, Blok LJ, Fodde R. Identification of quiescent, stem-like cells in the distal female reproductive tract. PLoS One. 2012;7:e40691. doi: 10.1371/journal.pone.0040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AL, Pru JK. Long-term label retaining cells localize to distinct regions within the female reproductive epithelium. Cell Cycle. 2013;12:2888–2898. doi: 10.4161/cc.25917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Anwar SS, Buhring HJ, Rao JR, Gargett CE. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 2012;21:2201–2214. doi: 10.3727/096368911X637362. [DOI] [PubMed] [Google Scholar]
- Cervello I, Gil-Sanchis C, Mas A, Delgado-Rosas F, Martinez-Conejero JA, Galan A, Martinez-Romero A, Martinez S, Navarro I, Ferro J, Horcajadas JA, Esteban FJ, et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5:e10964. doi: 10.1371/journal.pone.0010964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervello I, Mas A, Gil-Sanchis C, Peris L, Faus A, Saunders PT, Critchley HO, Simon C. Reconstruction of endometrium from human endometrial side population cell lines. PLoS One. 2011;6:e21221. doi: 10.1371/journal.pone.0021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Yoshimoto M, Kato K, Adachi S, Yamayoshi A, Arima T, Asanoma K, Kyo S, Nakahata T, Wake N. Characterization of side-population cells in human normal endometrium. Hum Reprod. 2007;22:1214–1223. doi: 10.1093/humrep/del514. [DOI] [PubMed] [Google Scholar]
- Masuda H, Matsuzaki Y, Hiratsu E, Ono M, Nagashima T, Kajitani T, Arase T, Oda H, Uchida H, Asada H, Ito M, Yoshimura Y, et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One. 2010;5:e10387. doi: 10.1371/journal.pone.0010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S, Yoshimoto M, Takahashi K, Noda Y, Nakahata T, Heike T. Side population cells contribute to the genesis of human endometrium. Fertil Steril. 2008;90:1528–1537. doi: 10.1016/j.fertnstert.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Maruyama T, Masuda H, Yamasaki A, Uchida S, Oda H, Uchida H, Yoshimura Y. Stem cell-like differentiation potentials of endometrial side population cells as revealed by a newly developed in vivo endometrial stem cell assay. PLoS One. 2012;7:e50749. doi: 10.1371/journal.pone.0050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma T, Kyo S, Maida Y, Ozaki S, Takakura M, Nakao S, Inoue M. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol. 2009;201:608, e601–608. doi: 10.1016/j.ajog.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Mints M, Jansson M, Sadeghi B, Westgren M, Uzunel M, Hassan M, Palmblad J. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum Reprod. 2008;23:139–143. doi: 10.1093/humrep/dem342. [DOI] [PubMed] [Google Scholar]
- Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25:2082–2086. doi: 10.1634/stemcells.2006-0828. [DOI] [PubMed] [Google Scholar]
- Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 2012;21:3324–3331. doi: 10.1089/scd.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domen JWA, Weissman IL. Bone Marrow (Hematopoietic) Stem Cells In Stem Cell Information [ Internet]. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services, 2011. http://stemcells.nih.gov/info/Regenerative_Medicine/Pages/2006Chapter2.aspx. Accessed 5 March 2015. [Google Scholar]
- Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, Thebaud B, Riordan NH. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Healy DL. Generating receptive endometrium in Asherman's syndrome. J Hum Reprod Sci. 2011;4:49–52. [PMC free article] [PubMed] [Google Scholar]
- Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS One. 2014;9:e96662. doi: 10.1371/journal.pone.0096662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr S, Naqvi H, Komm B, Taylor HS. Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology. 2014;155:1489–1497. doi: 10.1210/en.2013-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi H, Sakr S, Presti T, Krikun G, Komm B, Taylor HS. Treatment with bazedoxifene and conjugated estrogens results in regression of endometriosis in a murine model. Biol Reprod. 2014;90:121. doi: 10.1095/biolreprod.113.114165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak J, Jr, , Fischer C, Komm B, Taylor HS. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology. 2011;152:3226–3232. doi: 10.1210/en.2010-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman's syndrome. J Hum Reprod Sci. 2011;4:43–48. doi: 10.4103/0974-1208.82360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Ye L. Endometrial reconstruction from stem cells. Fertil Steril. 2012;98:11–20. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Porras G, Li Q, Bezard E. Modeling Parkinson's disease in primates: the MPTP model. Cold Spring Harb Perspect Med. 2012;2:a009308. doi: 10.1101/cshperspect.a009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet JF, Redmond DE, Jr, , Bloch J. Primate adult brain cell autotransplantation, a pilot study in asymptomatic MPTP-treated monkeys. Cell Transplant. 2009;18:787–799. doi: 10.3727/096368909X470847. [DOI] [PubMed] [Google Scholar]
- Noureddini M, Verdi J, Mortazavi-Tabatabaei SA, Sharif S, Azimi A, Keyhanvar P, Shoae-Hassani A. Human endometrial stem cell neurogenesis in response to NGF and bFGF. Cell Biol Int. 2012;36:961–966. doi: 10.1042/CBI20110610. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Barough S, Kouchesfahani HM, Ai J, Massumi M. Differentiation of human endometrial stromal cells into oligodendrocyte progenitor cells (OPCs) J Mol Neurosci. 2013;51:265–273. doi: 10.1007/s12031-013-9957-z. [DOI] [PubMed] [Google Scholar]
- Peron JP, Jazedje T, Brandao WN, Perin PM, Maluf M, Evangelista LP, Halpern S, Nisenbaum MG, Czeresnia CE, Zatz M, Camara NO, Rizzo LV. Human endometrial-derived mesenchymal stem cells suppress inflammation in the central nervous system of EAE mice. Stem Cell Rev. 2012;8:940–952. doi: 10.1007/s12015-011-9338-3. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali M, Allickson JG, Sanberg CD, Kuzmin-Nichols N, Sanberg PR. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19:439–452. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria X, Massasa EE, Feng Y, Wolff E, Taylor HS. Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol Ther. 2011;19:2065–2071. doi: 10.1038/mt.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Chen YJ, Chen SJ, Kao CL, Tseng LM, Lo WL, Chang CM, Yang DM, Ku HH, Twu NF, Liao CY, Chiou SH, et al. Induction of insulin-producing cells derived from endometrial mesenchymal stem-like cells. J Pharmacol Exp Ther. 2010;335:817–829. doi: 10.1124/jpet.110.169284. [DOI] [PubMed] [Google Scholar]
- Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T, Mori T, Miyado K, Ikegami Y, Cui C, Kiyono T, Kyo S, et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26:1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen S, Zhang C, Stegeman S, Pfaff-Amesse T, Zhang Y, Zhang W, Amesse L, Chen Y. Human endometrial stromal stem cells differentiate into megakaryocytes with the ability to produce functional platelets. PLoS One. 2012;7:e44300. doi: 10.1371/journal.pone.0044300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Bury MI, Marks AJ, Fuller NJ, Meisner JW, Tapaskar N, Halliday LC, Matoka DJ, Cheng EY. A nonhuman primate model for urinary bladder regeneration using autologous sources of bone marrow-derived mesenchymal stem cells. Stem Cells. 2011;29:241–250. doi: 10.1002/stem.568. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Fuller NJ, Sullivan RR, Fulton N, Hota PV, Harrington DA, Villano J, Hagerty JA, Cheng EY. Defined populations of bone marrow derived mesenchymal stem and endothelial progenitor cells for bladder regeneration. J Urol. 2009;182:1898–1905. doi: 10.1016/j.juro.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Shoae-Hassani A, Mortazavi-Tabatabaei SA, Sharif S, Seifalian AM, Azimi A, Samadikuchaksaraei A, Verdi J. Differentiation of human endometrial stem cells into urothelial cells on a three-dimensional nanofibrous silk-collagen scaffold: an autologous cell resource for reconstruction of the urinary bladder wall J Tissue Eng Regen Med 2013. DOI: 10.1002/term.1632. [DOI] [PubMed] [Google Scholar]
- Kazemnejad S, Zarnani AH, Khanmohammadi M, Mobini S. Chondrogenic differentiation of menstrual blood-derived stem cells on nanofibrous scaffolds. Methods Mol Biol. 2013;1058:149–169. doi: 10.1007/7651_2013_9. [DOI] [PubMed] [Google Scholar]
- Edwards SL, Werkmeister JA, Rosamilia A, Ramshaw JA, White JF, Gargett CE. Characterisation of clinical and newly fabricated meshes for pelvic organ prolapse repair. J Mech Behav Biomed Mater. 2013;23:53–61. doi: 10.1016/j.jmbbm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Edwards SL, White JF, Supit T, Ramshaw JA, Lo C, Rosamilia A, Werkmeister JA, Gargett CE. A preclinical evaluation of alternative synthetic biomaterials for fascial defect repair using a rat abdominal hernia model. PLoS One. 2012;7:e50044. doi: 10.1371/journal.pone.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su K, Edwards SL, Tan KS, White JF, Kandel S, Ramshaw JA, Gargett CE, Werkmeister JA. Induction of endometrial mesenchymal stem cells into tissue forming cells suitable for fascial repair. Acta Biomater. 2014;10:5012–5020. doi: 10.1016/j.actbio.2014.08.031. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Edwards SL, Su K, Tan KS, White JF, Ramshaw JA, Lo C, Rosamilia A, Werkmeister JA, Gargett CE. Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng Part A. 2014;20:785–798. doi: 10.1089/ten.tea.2013.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui CH, Uyama T, Miyado K, Terai M, Kyo S, Kiyono T, Umezawa A. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol Biol Cell. 2007;18:1586–1594. doi: 10.1091/mbc.E06-09-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G, Mavilio F. Myogenic stem cells for the therapy of primary myopathies: wishful thinking or therapeutic perspective? J Clin Invest. 2000;105:1669–1674. doi: 10.1172/JCI10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Wang H, Patel AN, Kambhampati S, Angle N, Chan K, Marleau AM, Pyszniak A, Carrier E, Ichim TE, Riordan NH. Allogeneic endometrial regenerative cells: an “Off the shelf solution” for critical limb ischemia? J Transl Med. 2008;6:45. doi: 10.1186/1479-5876-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Patel AN, Ichim TE, Riordan NH, Wang H, Min WP, Woods EJ, Reid M, Mansilla E, Marin GH, Drago H, Murphy MP, et al. Feasibility investigation of allogeneic endometrial regenerative cells. J Transl Med. 2009;7:15. doi: 10.1186/1479-5876-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichim TE, Alexandrescu DT, Solano F, Lara F, Campion Rde N, Paris E, Woods EJ, Murphy MP, Dasanu CA, Patel AN, Marleau AM, Leal A, et al. Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell Immunol. 2010;260:75–82. doi: 10.1016/j.cellimm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Ichim TE, Solano F, Lara F, Rodriguez JP, Cristea O, Minev B, Ramos F, Woods EJ, Murphy MP, Alexandrescu DT, Patel AN, Riordan NH. Combination stem cell therapy for heart failure. Int Arch Med. 2010;3:5. doi: 10.1186/1755-7682-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medistem Inc Phase I/II Trial of Endometrial Regenerative Cells (ERC) in Patients With Critical Limb Ischemia In: ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US); 2000. https://www.clinicaltrials.gov/ct2/show/NCT01558908?term=Phase+I%2FII+Trial+of+Endometrial+Regenerative+Cells+%28ERC%29+in+Patients+With+Critical+Limb+Ischemia&rank=1 of the record NLM Identifier: NCT01558908. Accessed 5 March 2015. [Google Scholar]
- S-Evans Biosciences Co, Ltd. Human Menstrual Blood-derived Mesenchymal Stem Cells for Patients With Liver Cirrhosis. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US); 2000. Mar 5, 2015. https://www.clinicaltrials.gov/ct2/show/NCT01483248?term=Human+Menstrual+Blood-derived+Mesenchymal+Stem+Cells+for+Patients+With+Liver+Cirrhosis&rank=1 In: [Internet]. of the record NLM Identifier: NCT01483248. Accessed . [Google Scholar]