Abstract
The aim of this study was to identify conceptus-derived proteins, in addition to IFNT, that may facilitate pregnancy recognition in cattle. Analysis of the protein content of the uterine luminal fluid (ULF) from cyclic heifers on Day 16 by nano liquid chromatography tandem mass spectrometry identified 334 proteins. Comparison of these data with 299 proteins identified in the ULF of pregnant heifers on Day 16 identified 85 proteins only present in the ULF of pregnant heifers. Analysis of Day 16 conceptus-conditioned culture medium revealed the presence of 1005 proteins of which 30 proteins were unique to ULF from Day 16 pregnant heifers. Of these 30 proteins, 12 had mRNA expression values at least 2-fold higher in abundance (P < 0.05) in the conceptus compared to the endometrium (ARPC5L, CAPG, CKMT1, CSTB, HSPA8, HSPE1, LGALS3, MSN, NUTF2, P4HB, PRKAR2A, TKT) as determined by RNA sequencing. In addition, genes that have a significant biological interaction with the proteins (ACO2, CKMT1, CSTB, EEF2, GDI1, GLB1, GPLD1, HNRNPA1, HNRNPA2B1, HNRNPF, HSPA8, HSPE1, IDH2, KRT75, LGALS3, MSN, NUTF2, P4HB, PRKAR2A, PSMA4, PSMB5, PSMC4, SERPINA3, TKT) were differentially expressed in the endometrium of pregnant compared to cyclic heifers during the pregnancy recognition period (Days 16–18). These results indicate that 30 proteins unique to ULF from pregnant heifers and produced by short-term in vitro cultured Day 16 conceptuses could potentially be involved in facilitating the interactions between the conceptus and the endometrium during the pregnancy recognition period.
Keywords: conceptus, endometrium, gene expression, proteomics, uterine luminal fluid
INTRODUCTION
Successful establishment of pregnancy during the preimplantation period in cattle is dependent on timely interactions between the developing embryo/conceptus and the uterine environment. In cattle, progesterone (P4) from the corpus luteum (CL), induces both temporal and spatial (cell-specific) changes in the endometrial transcriptome necessary to establish uterine receptivity when implantation in the uterus is possible. These changes include down-regulation of the nuclear progesterone receptor (PGR) in the luminal and then glandular epithelium, which allows expression of genes and secretion of their protein products, as well as active transport of other molecules, required for conceptus elongation [1]. The capacity of the uterus to stimulate conceptus elongation is primarily dependent on secretions from the luminal and glandular epithelium [2]. However, the timing of conceptus elongation is clearly associated with concentrations of P4 in circulation, which acts via the uterus to alter the timing of PGR down-regulation and thus onset of expression of key genes required for elongation in cattle and sheep [3–6]. Consequently, P4 has an indirect effect on the onset of secretion of interferon tau (IFNT) by the conceptus, the pregnancy recognition signal in cattle, given the strong positive correlation between conceptus development or elongation and IFNT production [7]. In order for P4 output from the CL to be maintained, sufficient quantities of IFNT must be produced by the conceptus by Day 16 to abrogate the luteolytic mechanism and maintain CL function [8–10] and induce expression of both classical and nonclassical interferon-stimulated genes (ISGs) in the different cellular compartments of the endometrium that are proposed to regulate conceptus elongation [11–14].
In cattle, temporal changes in the endometrial transcriptome in cyclic (i.e., nonpregnant) and pregnant females are similar prior to Days 15–16 [11, 15]; it is only at the time of pregnancy recognition that differences between cyclic and pregnant animals become apparent. However, RNA sequencing data revealed subtle changes in the endometrium as early as Day 13 of pregnancy that are independent of IFNT [16]. Moreover, Bauersachs et al. [15] compared the transcriptomic signature of the endometrium from pregnant heifers to those from cyclic heifers exposed to IFNA (which has similar biological activity to IFNT) for 3 days, which revealed differences in the endometrium of pregnant heifers that were independent of IFNT secretion from the conceptus. Data from Bartol et al. [17] demonstrated that the fully elongated bovine conceptus produces a significant number of proteins when cultured in vitro, similar to observations in other species in which the conceptus undergoes elongation prior to implantation such as sheep and pigs [18–20]. Further, there is now a growing body of evidence in sheep and cattle to show that conceptus-derived factors other than IFNT, including prostaglandins and cortisol, modify the endometrium both alone and in tandem with IFNT [21, 22]. In cattle, using a model of multiple embryo transfer to magnify any conceptus-derived signals present on Day 13, Spencer et al. [23] demonstrated that the conceptus produces prostaglandins, which can modify the endometrium prior to pregnancy recognition. Given this emerging body of evidence, we hypothesized that the bovine conceptus on Day 16 produces molecules, in addition to IFNT, that may play a role in the pregnancy recognition signal and facilitate uterine receptivity to conceptus elongation and implantation. To address this hypothesis, we first characterized the protein content of uterine luminal fluid (ULF) from pregnant and cyclic heifers on Day 16 postestrus using nano liquid chromatography tandem mass spectrometry (nano-LC-MS/MS). We then cross-referenced these data to our previously generated RNA-sequencing gene expression data from similarly staged tissues to identify a putative tissue source for these proteins (i.e., endometrium, conceptus, or both). To further confirm that the conceptus was indeed a potential source of some of the proteins in ULF, we characterized the proteins produced by Day 16 conceptuses following short-term culture in vitro.
MATERIALS AND METHODS
All the experimental procedures involving animals were licensed by the Department of Health and Children, Ireland, in accordance with the Cruelty to Animals Act (Ireland 1876) and the European Community Directive 86/609/EC and were sanctioned by the Animal Research Ethics Committee of University College Dublin. Unless otherwise stated, all the chemicals were sourced from Sigma (Dublin, Ireland).
Study 1: Analysis of the Protein Content of the ULF of Pregnant and Cyclic Heifers at the Time of Pregnancy Recognition (Day 16)
The estrous cycles of crossbred beef heifers (n = 25) were synchronized using an 8-day intravaginal P4 device (CIDR, 1.38 g P4; Pfizer, Sandwich, Kent, UK.). One day prior to CIDR removal, all the heifers received a 2 ml intramuscular injection of a prostaglandin F2 alpha analog (Estrumate; Intervet, Dublin, Ireland; equivalent to 0.05 mg Cloprostenol) to regress the endogenous CL. Only those heifers observed in standing estrus were either artificially inseminated (n = 11) to generate pregnant animals or left as noninseminated cyclic controls (n = 6). Following slaughter at a commercial abattoir, the uteri were collected and processed for sample collection within 30 min. The uterine horn ipsilateral to the CL was flushed with 20 ml of 10 mM Tris solution (pH 7.2). Samples were centrifuged at 1000 × g for 15 min at 4°C, the supernatant was removed from the pelleted debris, snap frozen in 1 ml aliquots in liquid nitrogen, and stored at −80°C prior to analysis. In the inseminated group, only the reproductive tracts from which an appropriately developed conceptus was recovered (n = 6, 10.0 ± 0.7 mm in length) were processed for further analysis.
Proteomic Analysis of ULF
Analysis of the ULF was carried out via nano-LC-MS/MS by Applied Biomics (Haywood, CA) as previously described [24]. Briefly, nano-LC-MS/MS was carried out on four individual samples of ULF from Day 16 cyclic control heifers and from heifers confirmed pregnant on Day 16 of pregnancy. All eight samples were exchanged into 50 mM ammonium bicarbonate buffer, and dithiothreitol (DTT) was added to a final concentration of 10 mM. Samples were incubated at 60°C for 30 min and allowed to cool at room temperature (RT). Iodoacetamide (IAA; 10 mM) was added, and the samples incubated in the dark for 30 min at RT. Following overnight tryptic digestion at 37°C, nano-LC was carried out using a Dionex Ultimate 3000 (Milford, MA). Tryptic peptides were loaded into an α-Precolumn Cartridge and separated using a 5%–60% acetonitrile gradient on the nano-LC column. Fractions were collected at 20 sec intervals followed by MS analysis on AB SCIEX time of flight (TOF) TOF/TOF 5800 System (AB SCIEX, Framingham, MA). Mass spectra were acquired in a reflectron positive ion mode. TOF/TOF tandem MS fragmentation spectra were acquired for each ion, using an average of 4000 laser shots per fragmentation spectrum (excluding trypsin autolytic peptides and other known background ions. To identify the resulting peptides, both peptide mass and associated fragmentation spectra were submitted to a GPS Explorer workstation equipped with the MASCOT search engine (Matrix Science, London, U.K.) to search the nonredundant database of National Center for Biotechnology Information. Searches were performed without constraining protein molecular weight or isoelectric point, with variable carbamidomethylation of cysteine and oxidation of methionine residues. Only one missed cleavage was allowed in the search parameters, and a false discovery rate (FDR) of 3.11% was identified by submitting the list of identified peptides to a decoy database. Significant hits were designated when P < 0.05.
Gene Expression Analysis of the Endometrium and Conceptus
In order to determine a putative tissue source (endometrium or conceptus) of the proteins identified in ULF, previously generated RNA-sequencing data from an independent group of heifers [24] (GSE56392 and GSE56513) were interrogated in an attempt to determine the likely origin. Briefly, RNA was extracted from intercaruncular endometrial or conceptus tissues from pregnant heifers on Day 16 (n = 5) as previously described [16, 25]. Library preparation and cluster generation was performed as per manufacturer's instructions (www.illumina.com), and gene expression analysis was carried out using the Illumina GA2 sequencer using the standard Illumina protocol for sequencing cDNA samples. The resulting 32 base pair reads were processed through the standard software pipeline for the Genome Analyzer and aligned against the Bos taurus 4 genome. A pseudochromosome containing potential splice junction sequences was generated. The ensGene table from the University of California Santa Cruz genome browser (http://hgdownload.cse.ucsc.edu/goldenPath/bosTau4/database/ensGene.txt.gz: October 2007 BosTau4) was used to provide exon location information to the CASAVA module. Lists of expressed transcripts were generated using the moderated negative binomial test from the edgeR Bioconductor library [26]. All the data were displayed as transcripts per million with a FDR-adjusted P < 0.05 used as the cut-off for determining expression of a gene in at least one tissue. The comparative analysis was restricted to the 26 957 protein-coding transcripts in version 52 of Ensembl (www.ensembl.org).
Study 2: Analysis of Conceptus-Derived Proteins During Pregnancy Recognition
The estrous cycles of crossbred beef heifers (n = 12) were synchronized as described for Study 1. Following estrus detection (Day 0: n = 10), heifers were inseminated and slaughtered on Day 16 of pregnancy. At slaughter, the uterine horn ipsilateral to the CL was flushed with 20 ml of phosphate buffered saline (pH 7.3) supplemented with 5% fetal calf serum. Following recovery, the conceptuses were cultured individually in vitro in 500 μl of synthetic oviduct fluid medium [7] for 6 h (n = 4) or 24 h (n = 4) along with contemporaneous controls, that is, no conceptus. The spent culture medium was recovered, clarified by centrifugation, and analyzed for protein content by nano-LC-MS/MS.
Proteins were precipitated from the medium using the ProteoExtract Protein Precipitation Kit (Calbiochem, Darmstadt, Germany). The protein pellet was solubilized in 100 μl of 6 M urea; 200 mM DTT was added to a final concentration of 5 mM, and samples were incubated for 30 min at 37°C. A 20 mM solution of IAA was added to a final concentration of 15 mM and incubated for 30 min at RT, followed by 20 μl of DTT to quench the IAA reaction. Samples were then subjected to tryptic digestion (trypsin/lys-C; Promega, Madison, WI) for 4 h at 37°C. Samples were diluted to >1M urea by the addition of 50 mM ammonium bicarbonate, digested overnight at 37°C, and desalted using a Macro Spin Column (Nest Group, Southborough, MA).
Digested peptides were analyzed by LC-MS/MS on a Thermo Scientific Q Exactive Orbitrap Mass Spectrometer in conjunction Proxeon Easy-nLC II HPLC (Thermo Scientific, Waltham, MA) and Proxeon nanospray source. The digested peptides were loaded onto a 100 μm × 25 mm Magic C18 100-A 5U reverse phase trap and were desalted online prior to separation via a 75 μm × 150 mm Magic C18 200-A 3U reverse phase column. Peptides were eluted using a 90 min gradient with a flow rate of 300 nl/min. An MS survey scan was obtained for the m/z range 300–1600, MS/MS spectra were acquired using a top 15 method, where the top 15 ions in the MS spectra were subjected to high-energy collision dissociation. An isolation mass window of 2.0 m/z was for the precursor ion selection, and normalized collision energy of 27% was used for fragmentation with a 5 sec duration used for the dynamic exclusion.
Tandem MS were extracted and charge state deconvoluted by Proteome Discoverer (Thermo Scientific). All MS/MS samples were analyzed using X! Tandem (thegpm.org; version TORNADO [2013.02.01.1]). X! Tandem was set up to search the bovine_20140114_CjFpWL database (unknown version, 62692 entries), the cRAP database of common laboratory contaminants (www.thegpm.org/crap; 114 entries), plus an equal number of reverse protein sequences assuming digestion by trypsin. X! Tandem was searched with a fragment ion mass tolerance of 20 parts per million (PPM) and a parent ion tolerance of 20 PPM. IAA derivative of cysteine was specified in X! Tandem as a fixed modification. Deamidation of asparagine and glutamine, oxidation of methionine and tryptophan, sulphonation of methionine, oxidation of tryptophan to formylkynurenin of tryptophan, and acetylation of the N-terminus were specified in X! Tandem as variable modifications. Scaffold (version Scaffold 4.0.6.1; Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they exceeded specific database search engine thresholds. X! Tandem identifications required at least −Log (expect scores) of greater than 1.2 with a mass accuracy of 5 PPM. Protein identifications were accepted if they contained at least two identified peptides. Using the parameters above, the Decoy FDR was calculated at 1.1% on the protein level and 0.0% on the spectrum level [27]. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Isobaric Tags for Relative and Absolute Quantification Analysis of Quantitative Changes in Proteins Abundance During Early Pregnancy
In order to examine whether or not proteins identified in the ULF during pregnancy recognition as well as those produced by the Day 16 conceptus in vitro increased coordinately with conceptus elongation, quantitative changes in the protein content of the ULF were determined by isobaric tags for relative and absolute quantification (iTRAQ) analysis as previously described whereby the side chain amines of peptides are covalently labelled with tags of various sizes that can allow quantification of the resultant peptide fragments [24]. All iTRAQ analyses were carried out by the Proteome Factory (Berlin, Germany) via iTRAQ 8-plex analysis. Two milliliters of each individual ULF sample (n = 4 per day from heifers confirmed pregnant on Days 10, 13, 16—the same animals that were used for nano-LC-MS/MS analysis in study 1—and 19 of pregnancy) were precipitated overnight in 10 ml of 100% ethanol. The resulting pellet was washed twice and resuspended in 40 μl of lysis buffer—20 mM tetraethylammonium bromide, 5 mM tris(2-carboxyethyl)phosphine, 0.1% SDS, 2 mM Pefablock, 2.1 μM leupeptin, and 2 mM benzamidin—and centrifuged at 13 000 × g. The supernatant was transferred into a clean tube, to which 10 mM IAA was added and incubated for 30 min at RT in the dark. The concentration of proteins in each sample was determined by Bradford assay, and 100 μg of total protein was subjected to trypsin digestion (Promega, Mannheim, Germany) at 37°C overnight. Additional trypsin was added and the reaction continued for a further 3 h. Acidification of the resulting peptides was performed with formic acid (pH 2.0), then desalted with Macro spin tips containing Vydac C18 material (Nest Group) and lyophilized.
To carry out iTRAQ analyses, lyophilized samples were dissolved in 45 μl of iTRAQ buffer (AB SCIEX, Framingham, MA), and 30 μl of each sample were reacted with appropriate iTRAQ reagents for 2 h at RT as per the manufacturer's protocol. The reaction was stopped with 50 μl of 20% formic acid, pH 2.0, and dried by lyophilization. Strong cation exchange (SCX) was performed on a Polysulfoethyl A column (200 × 2.1 mm, 5 μm, 200 A; PolyLC, Columbia, MD) using an Agilent 1100 HPLC system (Agilent, Karlsruhe, Germany) with 18 fractions collected per sample. Identification and quantification of proteins was performed for each one of the iTRAQ reporter ions for each of the 18 SCX by nano-LC-electrospray ionization-MS/MS. The MS system consisted of an Agilent 1100 nano-LC system (Agilent), PicoTip emitter (New Objective, Langhorne, PA), and a QExactive Quadrupole-Orbitrap Mass Spectrometer (ThermoFisher Scientific, Bremen, Germany). The dried SCX peptide fractions were resuspended in 80 μl of MilliQ water containing 0.1% formic acid and 1% acetonitrile. After trapping 40 μl of each sample, the peptides were desalted for 5 min on an enrichment column (Zorbax SB C18, 0.3 × 5 mm; Agilent) using a solution of 1% acetonitrile and 0.1% formic acid. Peptide separation was performed on a Zorbax 300 SB C18, 75 μm × 150 mm column (Agilent), for 110 min using a 5%–25% acetonitrile gradient in 0.1% formic acid. The MS was operated in a data-dependent mode by subjecting the 10 most abundant ions of each survey spectrum (nominal resolution 35 000 at m/z 200) to high-energy collision dissociation fragmentation (normalized collision energy at 40%, resolution 17 500 at m/z 200). MS/MS peak lists were extracted to mascot generic format files and searched by the Mascot search algorithm against the curated bovine International Protein Index (IPI) database (version 3.66). The mass tolerance was set to 5 PPM for peptide masses and 0.02 Da for fragment ions. Protein identification and quantification was performed using Mascot version 2.2 (Matrix Science). For protein quantification, a significance threshold of P < 0.05 (FDR 1%) and at least two peptides were required with the additional settings of protein ratio type = weighted, normalization = summed intensities, and an automatic outlier removal was used.
RESULTS
Conceptus-Induced Differences in the Protein Content of ULF During Pregnancy Recognition
Analysis of the protein content of the ULF from cyclic heifers on Day 16 identified 1805 peptides, in at least one of the four heifers analyzed, with a range of 688–818 peptides per heifer. Proteins (334) were present in the ULF from at least three of four cyclic heifers (Supplemental Table S1). These data were compared to the list of 299 proteins identified in the ULF of pregnant heifers [24]. This comparison allowed the generation of a list of proteins present in the ULF from both pregnant and cyclic heifers as well as proteins unique to ULF from pregnant heifers (i.e., produced by the conceptus or produced by the endometrium in response to the presence of a conceptus). Comparison of these lists of proteins identified 85 proteins only present in the ULF of pregnant heifers, 214 that were present in both pregnant and cyclic heifers, and 120 that were only detected in ULF from cyclic heifers (Fig. 1). Full details of proteins identified in each of the three comparisons are provided in Supplemental Table S2, A–C.
FIG. 1.
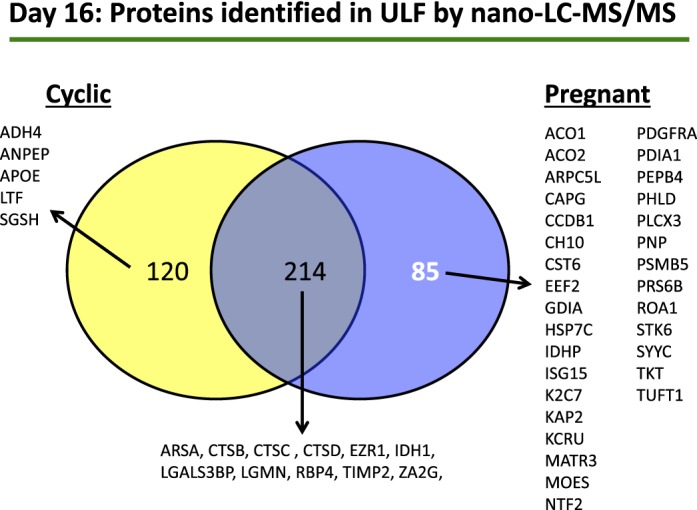
A comparison of the number of proteins identified in the ULF of cyclic (n = 4) and confirmed pregnant heifers (n = 4) on Day 16 (Day 0 = estrus). Proteins were determined by total peptide counts following nano liquid chromatography tandem mass spectrometry (nano-LC-MS/MS) and had to be present in at least three out of four heifers in each group. The subsets of proteins listed are on the basis of those described previously as localizing to luminal epithelium/glandular epithelium via in situ hybridization and/or are known in other species to be components of ULF. Full details of all the proteins are listed in Supplemental Table S2, A–C.
Of the 214 proteins identified in the ULF of both pregnant and cyclic heifers on Day 16, the expression of 163 transcripts that encode these proteins was detected in either the endometrial or conceptus tissues on Day 16. Fifty-eight of these genes displayed significantly higher expression (P < 0.05) of more than 2-fold in the endometrium compared to conceptus tissue on Day 16, including phosphatidylethanolamine-binding protein 4 (PEPB4), immunoglobulin light chain, lambda gene cluster (IGL), angiotensinogen (AGT), zinc-alpha-2-glycoprotein precursor (AZGP1), cathepsin S precursor (CTSS), plastin 3 (PLS3), CD59 molecule (CD59), nicotinate-nucleotide pyrophosphorylase (carboxylating) (LOC101929880), excitatory amino acid transporter 3 (SLC1A1), acid sphingomyelinase-like phosphodiesterase 3B (SMPDLB3), hepatocyte growth factor receptor precursor (MET), cystatin E/M (CST6), branched chain aminotransferase 1, cytosolic (BCAT1), lysosomal alpha-glucosidase precursor (GAA), purine nucleoside phosphorylase (PNP), and superoxide dismutase 3, extracellular (SOD3) as well as five genes with unknown function (IPI00692782.3, IPI00716555.3, IPI00685969.2, IPI00711414.1, and IPI00685930.2), all of which had an expression value of >10-fold higher in the endometrium compared to the conceptus (Fig. 2A).
FIG. 2.
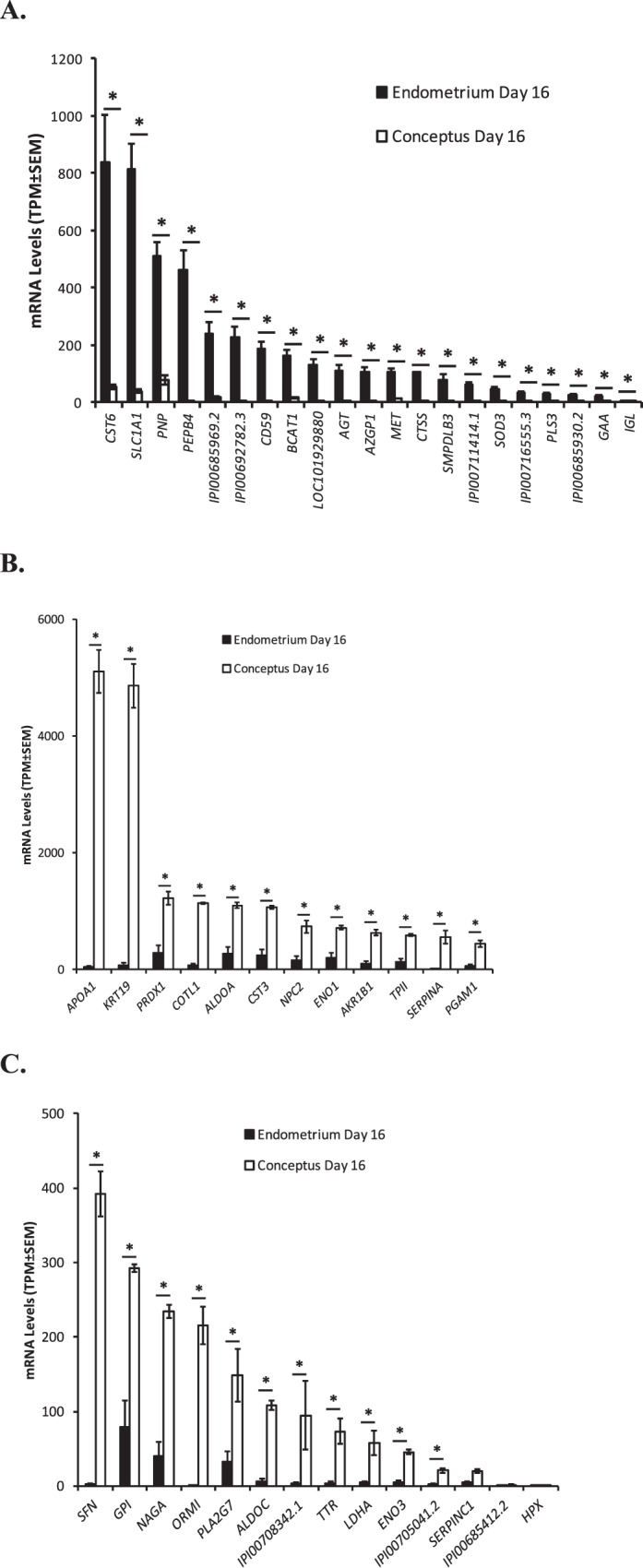
RNA sequencing data for genes whose proteins were detected in ULF from both cyclic and pregnant heifers on Day 16, with largest fold change difference in either the endometrium compared to the conceptus (A) or vice versa (B, C). Gene expression values in the endometrium (n = 5, black bars) and conceptus (n = 5, open bars) are given in average transcripts per million (T.P.M. ± SEM). Significant differences (P < 0.05) in expression values between the endometrium and conceptus are denoted by an asterisk.
In contrast, the expression of 47 genes was at least 2-fold higher (P < 0.05) in the conceptus compared to the endometrium while the expression of an additional four genes approached significance (P < 0.10). The genes with greatest fold change difference in the conceptus compared to the endometrium included: keratin, type I cytoskeletal 19 (KRT19), apolipoprotein A-I precursor (APOA1), 14-3-3 protein sigma (SFN), alpha-1-antiproteinase precursor (SERPINA), alpha-1-acid glycoprotein precursor (ORM1), L-lactate dehydrogenase A chain (LHDA), coactosin-like protein (COTL1), aldolase C (ALDOC), transthyretin precursor (TTR), hemopexin precursor (HPX), phosphoglycerate mutase 1 (PGAM1), beta-enolase (ENO3), alpha-enolase (ENO1), glucose-6-phosphate isomerase (GPI), aldolase A (ALDOA), peroxiredoxin-1 (PRDX1), cystatin-C precursor (CST3), antithrombin-III precursor (SERPINC1), platelet-activating factor acetylhydrolase precursor (PLA2G7), epididymal secretory protein E1 precursor (NPC2), triosephosphate isomerase (TPI1), alpha-N-acetylgalactosaminidase precursor (NAGA), aldose reductase (AKR1B1), as well as three genes of unknown function (IPI00708342.1, IPI00685412.2, and IPI00705041.2) (Fig. 2, B and C).
Of the 85 proteins identified only in the ULF of pregnant heifers, 71 had transcript expression in either the endometrium or conceptus on Day 16 as detected by RNA sequencing. The transcripts with significantly greater fold change difference in endometrium compared to the conceptus were heterogeneous nuclear ribonucleoprotein A1 (HNRNPA1), heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1), retinoic acid receptor gamma protein (RARG), matrin 3 (MATR3), cyclin-D1-binding protein 1 (CCNDBP1), cytoplasmic aconitate hydratase (aconitase) (ACON1), isocitrate dehydrogenase (NADP), mitochondrial precursor (IDH2), PDGFRA protein fragment (PDGFRA), and ubiquitin cross-reactive protein (ISG 17) (ISG15) (Fig. 3A).
FIG. 3.
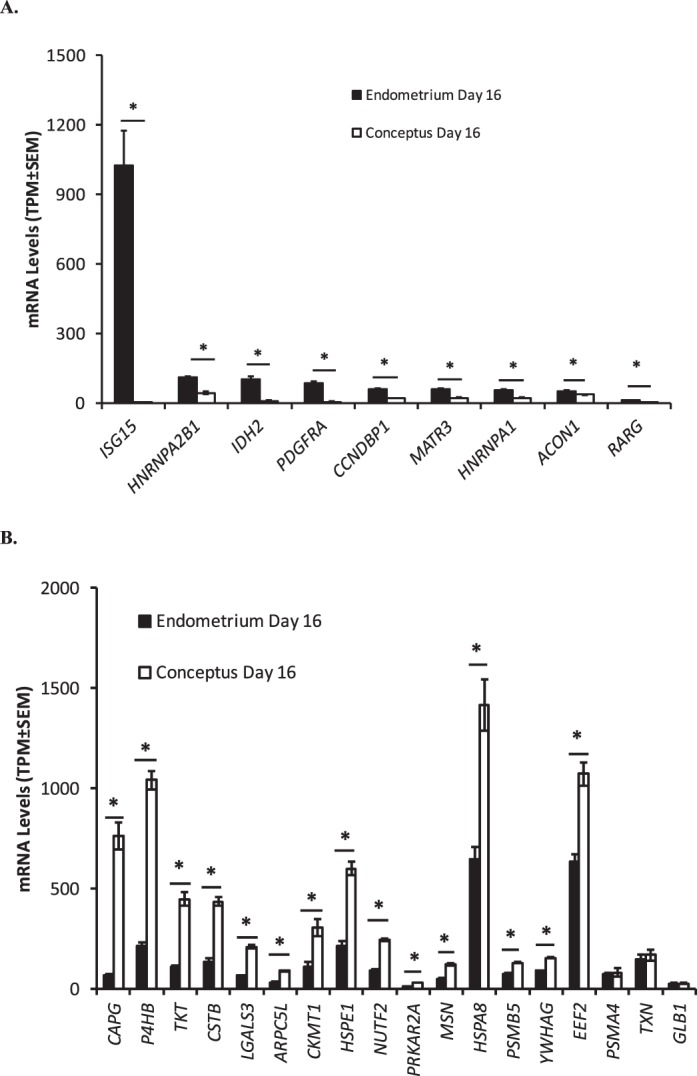
RNA sequencing data for genes whose proteins were detected in ULF only from pregnant heifers on Day 16 of pregnancy and in Day 16 conceptus-cultured medium, with largest fold change differences in the endometrium compared to the conceptus (A) or vice versa (B). Gene expression values in the endometrium (n = 5, black bars) and conceptus (n = 5, open bars) are given in average transcripts per million (T.P.M. ± SEM). Significant differences (P < 0.05) in the expression values between the endometrium and conceptus are denoted by an asterisk.
Identification of Conceptus-Produced Proteins
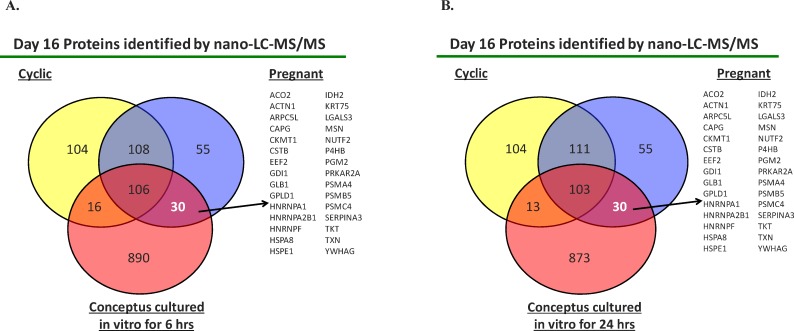
Analysis of Day 16 conceptus-conditioned culture medium for conceptuses cultured for 6 h revealed the presence of 1005 proteins (including IFNT) that were not detected in the contemporaneous blanks (Supplemental Table S3). Similar numbers were observed for Day 16 conceptus-conditioned medium recovered following 24 h of culture (1012 proteins, Supplemental Table S4). A comparison of the proteins identified following 6 and 24 h of culture identified a total of 875 proteins in the Day 16 conceptus-conditioned medium. Of these, 106 proteins were common to ULF of both pregnant and cyclic heifers as well as Day 16 conceptus-conditioned culture medium (Fig. 4A). Most interestingly, 30 proteins were unique to ULF from Day 16 pregnant heifers (i.e., not detected in cyclic heifers) and were also produced by the Day 16 conceptuses following 6 h of culture in vitro (Fig. 4A and Table 1). Similar numbers of proteins were observed following the comparison to 24 h culture, with 30 common to only ULF from pregnant heifers and Day 16 conceptus-conditioned culture medium and 103 were detected in culture medium following 24 h of culture and ULF from both pregnant and cyclic heifers (Fig. 4B and Supplemental Table S2).
FIG. 4.
Venn diagram comparing the number of proteins identified in ULF from cyclic (n = 4) and confirmed pregnant (n = 4) heifers on Day 16 (Day 0 = estrus) as well as Day 16 conceptus-conditioned cultured medium after culture for 6 h (A) or 24 h (B). Proteins were determined by total peptide counts following nano liquid chromatography tandem mass spectrometry (nano-LC-MS/MS) and had to be present in at least three of four heifers in each group. The 30 proteins listed were those identified only in pregnant ULF (i.e., not cyclic) on Day 16 and in spent culture medium. Full details of all the proteins are listed in Supplemental Tables S3 and S4.
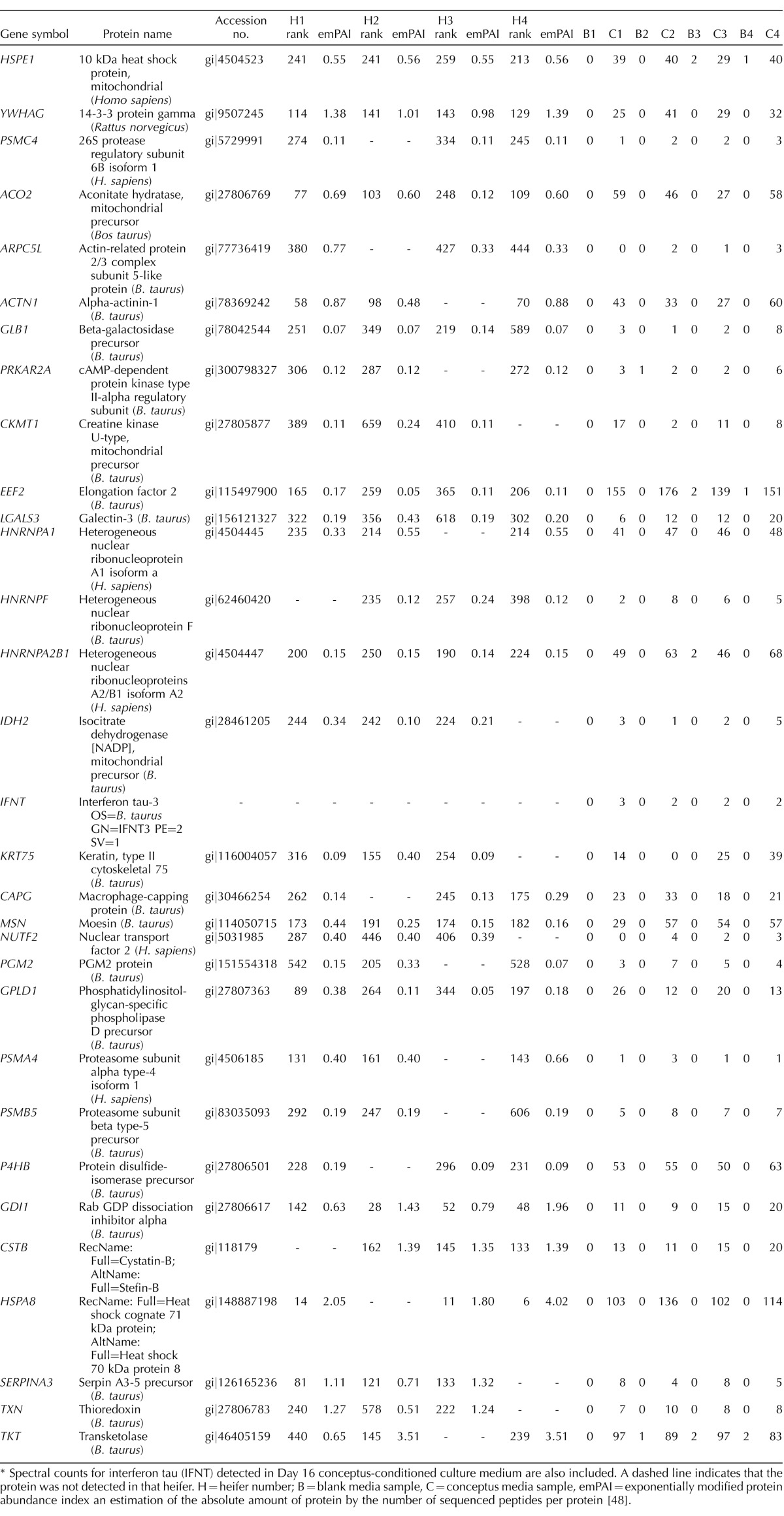
TABLE 1.
Protein name, accession number, and total spectral counts for proteins identified in both uterine luminal fluid from pregnant heifers on Day 16 (i.e., not detected in cyclic heifers on Day 16) and in medium following 6 h in vitro culture of Day 16 conceptuses.*
Spectral counts for interferon tau (IFNT) detected in Day 16 conceptus-conditioned culture medium are also included. A dashed line indicates that the protein was not detected in that heifer. H = heifer number; B = blank media sample, C = conceptus media sample, emPAI = exponentially modified protein abundance index an estimation of the absolute amount of protein by the number of sequenced peptides per protein [48].
Gene ontology (GO) analysis for cellular localization of the 30 proteins produced by the conceptus in vitro and also present in ULF from pregnant heifers determined that 16 of the proteins localized to the extracellular region (GO:0005576), extracellular space (GO:0005615), extracellular vesicular exosome (GO:0070062), and extracellular matrix (GO:0031012). The expression of the transcripts encoding 12 of the 30 proteins had at least 2-fold higher abundance (P < 0.05) in the conceptus compared to the endometrium, including ARPC5L, CAPG, CKMT1, CSTB, HSPA8, HSPE1, LGALS3, MSN, NUTF2, P4HB, PRKAR2A, and TKT (Fig. 3B). Of the other proteins whose transcripts were detected by RNA sequencing, seven had higher expression in the endometrium (P < 0.05) or the expression was not different (P > 0.05) between the endometrium and conceptus (n = 8 genes).
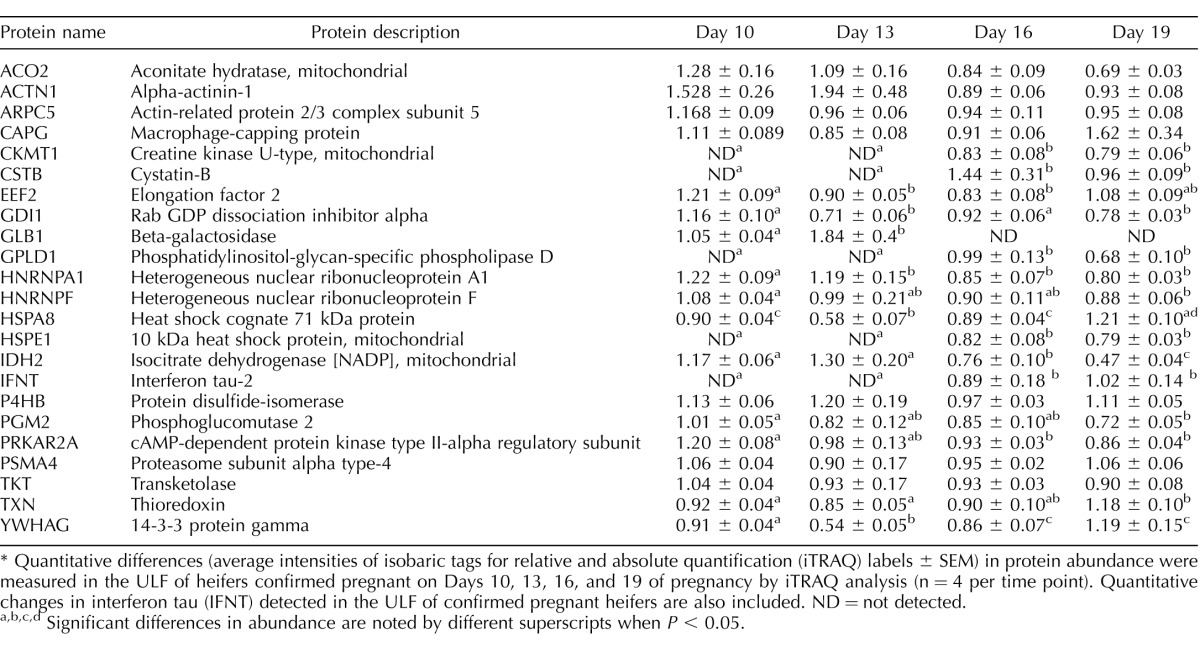
Analysis by iTRAQ of the quantitative temporal changes in the protein content of ULF of pregnant heifers during the period in which the conceptus undergoes elongation and initiates implantation detected the presence of 22 of the 30 candidate conceptus-derived proteins on one or more of Days 10, 13, 16, or 19 (Table 2). The proteins CKMT1, CSTB, GLPD1, and HSPE1 were not detected on Days 10 or 13, but were detectable on Days 16 and 19. Moreover, the abundance of HSPA8, TXN, and YWHAG, although present during earlier stages of pregnancy, increased significantly in the ULF from pregnant heifers on Day 19.
TABLE 2.
Quantitative changes in the abundance of proteins identified in uterine luminal fluid (ULF) from pregnant heifers only and produced by in vivo derived Day 16 conceptuses following short-term culture in vitro.*
Quantitative differences (average intensities of isobaric tags for relative and absolute quantification (iTRAQ) labels ± SEM) in protein abundance were measured in the ULF of heifers confirmed pregnant on Days 10, 13, 16, and 19 of pregnancy by iTRAQ analysis (n = 4 per time point). Quantitative changes in interferon tau (IFNT) detected in the ULF of confirmed pregnant heifers are also included. ND = not detected.
Significant differences in abundance are noted by different superscripts when P < 0.05.
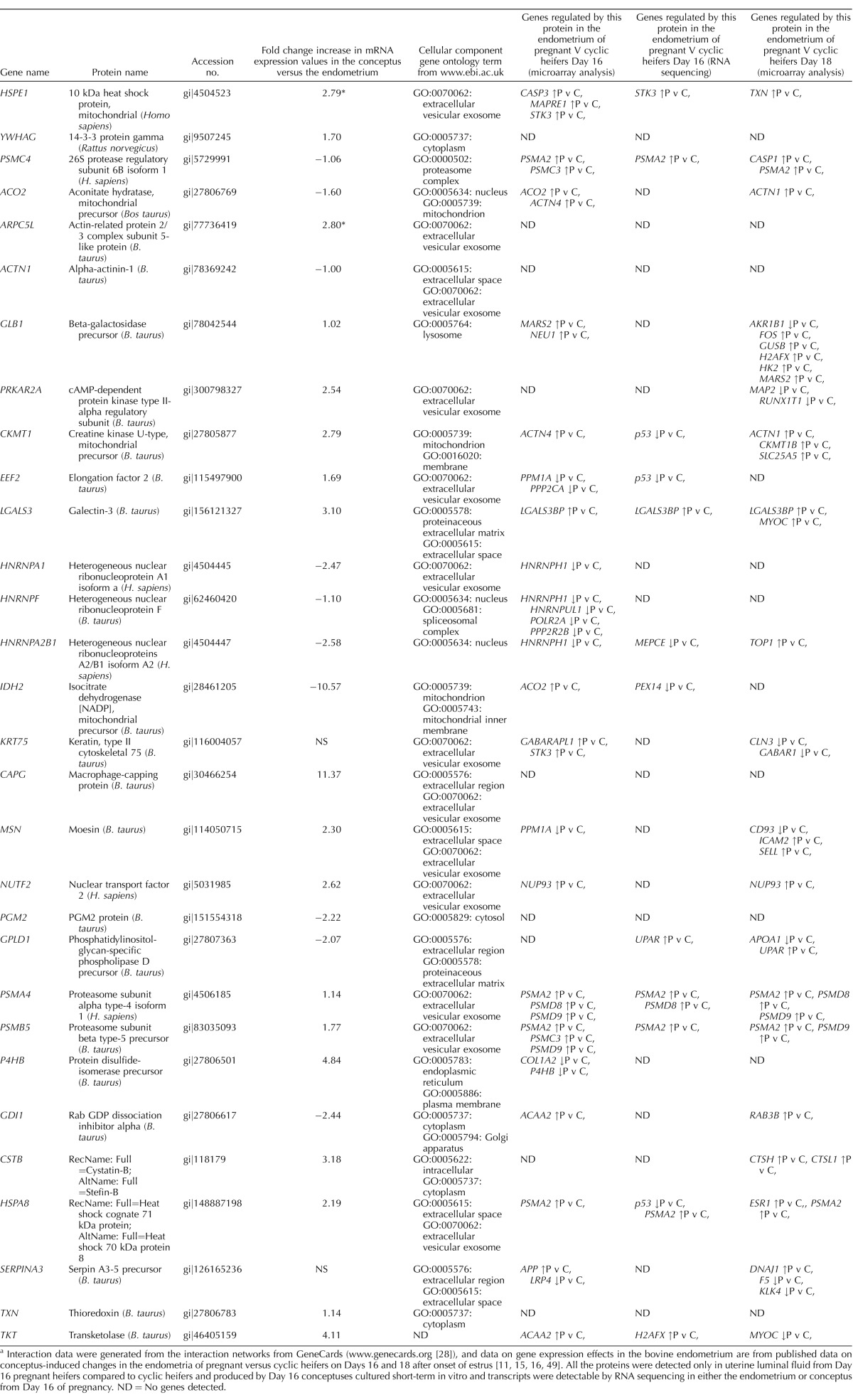
To further examine the potential function of these 30 proteins that were only detected in pregnant ULF and were produced by Day 16 conceptus-conditioned culture medium, we examined what genes/proteins they interact with in other systems using the string interaction network analysis in GeneCards (www.genecards.org; Table 3 [28]). This list of interacting genes for each of the 30 conceptus-derived proteins were then used to screen available transcriptomic data sets to determine if their expression was modified in the endometrium of pregnant compared to cyclic heifers during the time of pregnancy recognition [11, 15, 16]. Six of the 30 conceptus-derived proteins had no interacting genes represented on any of the three transcriptomic data set lists. Ten of the conceptus-derived proteins interact with genes that are modified in the endometrium during pregnancy recognition. Five conceptus-derived proteins interact with genes that are down-regulated in the endometrium of pregnant compared to cyclic heifers, while nine conceptus-derived proteins interact with genes that are up-regulated in pregnant compared to cyclic endometria during pregnancy recognition. Full details of the interacting genes and gene expression changes in pregnant compared to cyclic endometria are given in Table 3.
TABLE 3.
Summary table of conceptus-derived proteins and how they may potentially affect endometrial gene expression.a
Interaction data were generated from the interaction networks from GeneCards (www.genecards.org [28]), and data on gene expression effects in the bovine endometrium are from published data on conceptus-induced changes in the endometria of pregnant versus cyclic heifers on Days 16 and 18 after onset of estrus [11, 15, 16, 49]. All the proteins were detected only in uterine luminal fluid from Day 16 pregnant heifers compared to cyclic heifers and produced by Day 16 conceptuses cultured short-term in vitro and transcripts were detectable by RNA sequencing in either the endometrium or conceptus from Day 16 of pregnancy. ND = No genes detected.
DISCUSSION
The results of this study indicate that proteins unique to ULF from pregnant heifers and produced by Day 16 conceptuses following short-term culture in vitro could potentially be involved in mediation of conceptus-endometrial interactions and/or pregnancy recognition. Specifically, 1) a subset of proteins are predominantly expressed by the conceptus at the mRNA level, 2) their abundance in ULF increases coordinately with conceptus elongation, and 3) they have GO terms that are associated with extracellular vesicular exosome and extracellular region/matrix. Moreover, genes that have a significant biological interaction with these proteins are differentially expressed in the endometrium of pregnant compared to cyclic heifers during the period of pregnancy recognition (Days 16–18) (Table 3) [11, 15, 16]. We propose that those proteins may play a role in mediating conceptus-maternal interactions during early pregnancy.
The vast majority of proteins (214) identified in ULF from Day 16 were present in both pregnant and cyclic heifers. The main purpose of this study was to identify conceptus-derived proteins that may mediate conceptus-maternal interactions. As such, those proteins are unlikely to be specifically produced by the conceptus given their detection in the cyclic uterus as well. However, these are likely important endometrial-derived mediators of the cross-talk between the uterus and the conceptus during the preimplantation period of pregnancy. Indeed, a number of these proteins have been characterized in endometria of different species, and the genes encoding them are expressed in both pregnant and cyclic endometria prior to pregnancy recognition. Similarly, a number of proteins purported to mediate the process of conceptus elongation and that are secreted into the uterine lumen prior to pregnancy recognition were detected. These fall into the category of nonclassical ISGs and proteins, that is, those genes and proteins whose expression in the endometrium is stimulated by IFNT but are not associated with a typical type I interferon response. These included APOA1 [29], ARSA [29], CA2 [30], CSTB, CSTD, CSTS, and CSTZ [31], CST6 [30], EZRIN [30], IGFBP1[32], LGMN [30], LGALS3BP [33], MIF [34], PRDX1 [30], PNP [24, 30], and RBP4 [35–37], and are associated with the capacity of the uterus to support conceptus elongation and/or to have a direct effect on trophoblast proliferation and migration, which are essential processes governing conceptus elongation [38]. Indeed, the expression and abundance of these proteins in ULF from both cyclic and pregnant heifers supports the notion that the bovine uterus, at least up to the time of pregnancy recognition, drives changes that support conceptus elongation, irrespective of whether an appropriately developed conceptus is present [11].
Of the 85 proteins detected only in the ULF from pregnant heifers, 54 were not detected following in vitro culture of Day 16 conceptuses. This finding suggests that these proteins are derived from the endometrium in response to the presence of the conceptus, and this idea is supported by evidence for expression levels of genes that encode these proteins (Fig. 3). A classical ISG, ISG15 mRNA expression in the pregnant endometrium, is stimulated during pregnancy recognition in response to IFNT [11, 15] with release of its protein from the endometrium detectable on Day 15 of pregnancy in cattle [39] and in sheep [40]. Its detection only in the ULF of pregnant heifers in this study is likely due to the stimulatory nature of IFNT on ISG expression during pregnancy recognition and its secretion from the endometrial epithelia, a phenomenon that has been previously reported [39]. In addition, stimulation of expression of PPA1 in pregnant and IFNA-exposed endometria [15] and its detection in the ULF from pregnant heifers (this study) indicates that it may be an ISG released from the endometrium via an unconventional secretory pathway similar to other ISGs such as MX1 [41]. Other proteins such as cytoplasmic tyrosyl-tRNA synthetase and cytochrome C have been reported to be up-regulated in pregnant endometria but not following exposure to IFNA [15], indicating that the presence of these proteins in pregnant ULF may be due to the actions of other conceptus-derived molecules stimulating their expression and release from the endometrium.
Substantially more proteins were identified following conceptus culture in vitro than were identified in the ULF of pregnant or cyclic heifers. The significance of this observation is unclear but likely reflects the diluted nature of the ULF (20 ml) compared with the culture medium (500 μl), or indeed it is possible that conceptus-secreted proteins are bound to the luminal epithelium and, as such, are not detectable in the ULF. Of the 30 proteins present in ULF that are also produced by the conceptus in vitro, a significant proportion of them (15) are associated with GO terms of extracellular region, extracellular space, and/or extracellular vesicular exosome (Table 1). The mode of microvesicular transfer of retroviral sequences from the sheep endometrium to the conceptus has been established [42] with more recent studies carried out in sheep demonstrating the presence of microRNAs, mRNAs, as well as proteins in the microvesicles of ULF recovered from cyclic and pregnant sheep [43, 44]. Indeed, there were a number of significant differences reported in the composition of the microvesicular fraction between pregnant and cyclic sheep, indicating that it is possible that the conceptus itself may produce microvesicles that can mediate communication between the conceptus and the endometrium. Moreover, data from humans and cattle demonstrated there are microRNAs released during culture in vitro that may be derived from microvesicles released from or secreted by the developing blastocyst [45, 46]. Comparison of the list of proteins identified as conceptus-derived in this study and those identified in the microvesicular fraction of the pregnant sheep ULF revealed that, while there was little overlap of orthologous proteins (only cAMP-dependent protein kinase catalytic subunit alpha and HSP70, a marker of exosomes), proteins in the same families were identified, for example, keratins, galectins, and actins. This finding may be due to the inconsistency between the samples, as in the study of Burns et al. [44], the ULF was processed specifically for microvesicle analysis. Despite the similarities between the bovine and ovine uterine environment during early pregnancy, differences do exist, for example, while LGALS15 is present in both the ovine and bovine genome, it is only expressed in sheep and is silent in cattle [47].
This study aimed to identify additional conceptus-derived proteins that may function to modify endometrial function and/or mediate the pregnancy recognition response. Of the 30 proteins that were present only in ULF from pregnant heifers and were produced by the conceptus following short-term culture in vitro, we examined what genes/proteins they interact with in other systems using the string interaction network analysis in GeneCards (www.genecards.org, Table 3). Each protein generated a list of interacting genes/proteins that we used to screen available transcriptomic data sets to see if their expression was modified in the endometrium of pregnant compared to cyclic heifers during the time of pregnancy recognition [11, 15, 16]. Twenty-six of those proteins interact with genes in other model systems that are also modified due to pregnancy status in endometrium of pregnant versus cyclic heifers on Days 16–18 (Table 3). This gives a strong indication that these conceptus-derived proteins may indeed act, either in concert with IFNT or independent of it, to modify endometrial function in vivo, though further experiments will clearly be required to prove this in vivo.
In conclusion, we propose that some of the 30 proteins only identified in ULF from pregnant heifers and produced by Day 16 conceptuses following short-term culture in vitro are involved in interactions between the conceptus and the endometrium during pregnancy recognition. These proteins, some of which are not classically secreted in nature, may facilitate interactions between the conceptus and the endometrium via a microvesicular transport mechanism; however, the exact transport mechanism of those proteins remains to be elucidated.
ACKNOWLEDGMENT
We wish to acknowledge the help of graduate students and technical staff that assisted in sample collection.
Footnotes
This work was supported by Science Foundation Ireland under grant number 13/IA/1983 and grant R01 HD072898 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
REFERENCES
- Forde N, Lonergan P. Transcriptomic analysis of the bovine endometrium: what is required to establish uterine receptivity to implantation in cattle? J Reprod Dev. 2012;58:189–195. doi: 10.1262/jrd.2011-021. [DOI] [PubMed] [Google Scholar]
- Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction. 2002;124:289–300. [PubMed] [Google Scholar]
- Carter F, Forde N, Duffy P, Wade M, Fair T, Crowe MA, Evans AC, Kenny DA, Roche JF, Lonergan P. Effect of increasing progesterone concentration from Day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod Fertil Dev. 2008;20:368–375. doi: 10.1071/rd07204. [DOI] [PubMed] [Google Scholar]
- Forde N, Beltman ME, Duffy GB, Duffy P, Mehta JP, O'Gaora P, Roche JF, Lonergan P, Crowe MA. Changes in the endometrial transcriptome during the bovine estrous cycle: effect of low circulating progesterone and consequences for conceptus elongation. Biol Reprod. 2011;84:266–278. doi: 10.1095/biolreprod.110.085910. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Bazer FW, Spencer TE. Progesterone regulation of preimplantation conceptus growth and galectin 15 (LGALS15) in the ovine uterus. Biol Reprod. 2006;75:289–296. doi: 10.1095/biolreprod.106.052944. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Fox TC, Morgan GL, Wells ME, Wettemann RP, Zavy MT. Survival of bovine embryos transferred to progesterone-treated asynchronous recipients. J Reprod Fertil. 1991;92:475–482. doi: 10.1530/jrf.0.0920475. [DOI] [PubMed] [Google Scholar]
- O'Hara L, Forde N, Carter F, Rizos D, Maillo V, Ealy AD, Kelly AK, Rodriguez P, Isaka N, Evans AC, Lonergan P. Paradoxical effect of supplementary progesterone between Day 3 and Day 7 on corpus luteum function and conceptus development in cattle. Reprod Fertil Dev. 2014;26:328–336. doi: 10.1071/RD12370. [DOI] [PubMed] [Google Scholar]
- Betteridge KJ, Eaglesome MD, Randall GC, Mitchell D. Collection, description and transfer of embryos from cattle 10-16 days after oestrus. J Reprod Fertil. 1980;59:205–216. doi: 10.1530/jrf.0.0590205. [DOI] [PubMed] [Google Scholar]
- Northey DL, French LR. Effect of embryo removal and intrauterine infusion of embryonic homogenates on the lifespan of the bovine corpus luteum. J Anim Sci. 1980;50:298–302. doi: 10.2527/jas1980.502298x. [DOI] [PubMed] [Google Scholar]
- Bott RC, Ashley RL, Henkes LE, Antoniazzi AQ, Bruemmer JE, Niswender GD, Bazer FW, Spencer TE, Smirnova NP, Anthony RV, Hansen TR. Uterine vein infusion of interferon tau (IFNT) extends luteal life span in ewes. Biol Reprod. 2010;82:725–735. doi: 10.1095/biolreprod.109.079467. [DOI] [PubMed] [Google Scholar]
- Forde N, Carter F, Spencer TE, Bazer FW, Sandra O, Mansouri-Attia N, Okumu LA, McGettigan PA, Mehta JP, McBride R, O'Gaora P, Roche JF, et al. Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biol Reprod. 2011;85:144–156. doi: 10.1095/biolreprod.110.090019. [DOI] [PubMed] [Google Scholar]
- Mansouri-Attia N, Aubert J, Reinaud P, Giraud-Delville C, Taghouti G, Galio L, Everts RE, Degrelle S, Richard C, Hue I, Yang X, Tian XC, et al. Gene expression profiles of bovine caruncular and intercaruncular endometrium at implantation. Physiol Genomics. 2009;39:14–27. doi: 10.1152/physiolgenomics.90404.2008. [DOI] [PubMed] [Google Scholar]
- Bauersachs S, Ulbrich SE, Gross K, Schmidt SE, Meyer HH, Wenigerkind H, Vermehren M, Sinowatz F, Blum H, Wolf E. Embryo-induced transcriptome changes in bovine endometrium reveal species-specific and common molecular markers of uterine receptivity. Reproduction. 2006;132:319–331. doi: 10.1530/rep.1.00996. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Burghardt RC, Johnson GA, Spencer TE, Wu G. Interferons and progesterone for establishment and maintenance of pregnancy: interactions among novel cell signaling pathways. Reprod Biol. 2008;8:179–211. doi: 10.1016/s1642-431x(12)60012-6. [DOI] [PubMed] [Google Scholar]
- Bauersachs S, Ulbrich SE, Reichenbach HD, Reichenbach M, Buttner M, Meyer HH, Spencer TE, Minten M, Sax G, Winter G, Wolf E. Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol Reprod. 2012;86:46. doi: 10.1095/biolreprod.111.094771. [DOI] [PubMed] [Google Scholar]
- Forde N, Duffy GB, McGettigan PA, Browne JA, Mehta JP, Kelly AK, Mansouri-Attia N, Sandra O, Loftus BJ, Crowe MA, Fair T, Roche JF, et al. Evidence for an early endometrial response to pregnancy in cattle: both dependent upon and independent of interferon tau. Physiol Genomics. 2012;44:799–810. doi: 10.1152/physiolgenomics.00067.2012. [DOI] [PubMed] [Google Scholar]
- Bartol FF, Roberts RM, Bazer FW, Lewis GS, Godkin JD, Thatcher WW. Characterization of proteins produced in vitro by periattachment bovine conceptuses. Biol Reprod. 1985;32:681–693. doi: 10.1095/biolreprod32.3.681. [DOI] [PubMed] [Google Scholar]
- Godkin JD, Bazer FW, Moffatt J, Sessions F, Roberts RM. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13-21. J Reprod Fertil. 1982;65:141–150. doi: 10.1530/jrf.0.0650141. [DOI] [PubMed] [Google Scholar]
- Godkin JD, Bazer FW, Lewis GS, Geisert RD, Roberts RM. Synthesis and release of polypeptides by pig conceptuses during the period of blastocyst elongation and attachment. Biol Reprod. 1982;27:977–987. doi: 10.1095/biolreprod27.4.977. [DOI] [PubMed] [Google Scholar]
- Masters RA, Roberts RM, Lewis GS, Thatcher WW, Bazer FW, Godkin JD. High molecular weight glycoproteins released by expanding, pre-attachment sheep, pig and cow blastocysts in culture. J Reprod Fertil. 1982;66:571–583. doi: 10.1530/jrf.0.0660571. [DOI] [PubMed] [Google Scholar]
- Dorniak P, Bazer FW, Spencer TE. Prostaglandins regulate conceptus elongation and mediate effects of interferon tau on the ovine uterine endometrium. Biol Reprod. 2011;84:1119–1127. doi: 10.1095/biolreprod.110.089979. [DOI] [PubMed] [Google Scholar]
- Dorniak P, Welsh TH, Jr, , Bazer FW, Spencer TE. Endometrial HSD11B1 and cortisol regeneration in the ovine uterus: effects of pregnancy, interferon tau, and prostaglandins. Biol Reprod. 2011;86:124. doi: 10.1095/biolreprod.111.097063. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Forde N, Dorniak P, Hansen TR, Romero JJ, Lonergan P. Conceptus-derived prostaglandins regulate gene expression in the endometrium prior to pregnancy recognition in ruminants. Reproduction. 2013;146:377–387. doi: 10.1530/REP-13-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde N, McGettigan PA, Mehta JP, O'Hara L, Mamo S, Bazer FW, Spencer TE, Lonergan P. Proteomic analysis of uterine fluid during the pre-implantation period of pregnancy in cattle. Reproduction. 2014;147:575–587. doi: 10.1530/REP-13-0010. [DOI] [PubMed] [Google Scholar]
- Mamo S, Mehta JP, Forde N, McGettigan P, Lonergan P. Conceptus-endometrium crosstalk during maternal recognition of pregnancy in cattle. Biol Reprod. 2012;87:6. doi: 10.1095/biolreprod.112.099945. [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ. Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb DL. What's driving false discovery rates? J Proteome Res. 2008;7:45–46. doi: 10.1021/pr700728t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeneCards. Rehovot, Israel: Weizmann Institute of Science; 2015. www.genecards.org. Accessed 20 January. [Google Scholar]
- Forde N, Mehta JP, McGettigan PA, Mamo S, Bazer FW, Spencer TE, Lonergan P. Alterations in expression of endometrial genes coding for proteins secreted into the uterine lumen during conceptus elongation in cattle. BMC Genomics. 2013;14:321. doi: 10.1186/1471-2164-14-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgard AM, Lee RS, Peterson AJ. Bovine endometrial legumain and TIMP-2 regulation in response to presence of a conceptus. Mol Reprod Dev. 2009;76:65–74. doi: 10.1002/mrd.20931. [DOI] [PubMed] [Google Scholar]
- Song G, Spencer TE, Bazer FW. Cathepsins in the ovine uterus: regulation by pregnancy, progesterone, and interferon tau. Endocrinology. 2005;146:4825–4833. doi: 10.1210/en.2005-0768. [DOI] [PubMed] [Google Scholar]
- Simmons RM, Erikson DW, Kim J, Burghardt RC, Bazer FW, Johnson GA, Spencer TE. Insulin-like growth factor binding protein one in the ruminant uterus: potential endometrial marker and regulator of conceptus elongation. Endocrinology. 2009;150:4295–4305. doi: 10.1210/en.2009-0060. [DOI] [PubMed] [Google Scholar]
- Okumu LA, Fair T, Szekeres-Bartho J, O'Doherty AM, Crowe MA, Roche JF, Lonergan P, Forde N. Endometrial expression of progesterone induced blocking factor and galectins-1, -3, -9 and -3 binding protein in the luteal phase and early pregnancy in cattle. Physiol Genomics. 2011;43:903–910. doi: 10.1152/physiolgenomics.00251.2010. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Song G, Kochan KJ, Riggs PK, Simmons RM, Elsik CG, Adelson DL, Bazer FW, Zhou H, Spencer TE. Discovery of candidate genes and pathways in the endometrium regulating ovine blastocyst growth and conceptus elongation. Physiol Genomics. 2009;39:85–99. doi: 10.1152/physiolgenomics.00001.2009. [DOI] [PubMed] [Google Scholar]
- Dore JJ, Roberts MP, Godkin JD. Early gestational expression of retinol-binding protein mRNA by the ovine conceptus and endometrium. Mol Reprod Dev. 1994;38:24–29. doi: 10.1002/mrd.1080380105. [DOI] [PubMed] [Google Scholar]
- MacKenzie SH, Roberts MP, Liu KH, Dore JJ, Godkin JD. Bovine endometrial retinol-binding protein secretion, messenger ribonucleic acid expression, and cellular localization during the estrous cycle and early pregnancy. Biol Reprod. 1997;57:1445–1450. doi: 10.1095/biolreprod57.6.1445. [DOI] [PubMed] [Google Scholar]
- Mullen MP, Forde N, Parr MH, Diskin MG, Morris DG, Nally JE, Evans AC, Crowe MA. Alterations in systemic concentrations of progesterone during the early luteal phase affect RBP4 expression in the bovine uterus. Reprod Fertil Dev. 2012;24:715–722. doi: 10.1071/RD11246. [DOI] [PubMed] [Google Scholar]
- Dorniak P, Bazer FW, Spencer TE. Biological role of interferon tau in endometrial function and conceptus elongation. J Anim Sci. 2013;91(4):1627–1638. doi: 10.2527/jas.2012-5845. [DOI] [PubMed] [Google Scholar]
- Austin KJ, Ward SK, Teixeira MG, Dean VC, Moore DW, Hansen TR. Ubiquitin cross-reactive protein is released by the bovine uterus in response to interferon during early pregnancy. Biol Reprod. 1996;54:600–606. doi: 10.1095/biolreprod54.3.600. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Spencer TE, Hansen TR, Austin KJ, Burghardt RC, Bazer FW. Expression of the interferon tau inducible ubiquitin cross-reactive protein in the ovine uterus. Biol Reprod. 1999;61:312–318. doi: 10.1095/biolreprod61.1.312. [DOI] [PubMed] [Google Scholar]
- Toyokawa K, Carling SJ, Ott TL. Cellular localization and function of the antiviral protein, ovine Mx1 (oMx1): I. Ovine Mx1 is secreted by endometrial epithelial cells via an ‘unconventional' secretory pathway. Am J Reprod Immunol. 2007;57:13–22. doi: 10.1111/j.1600-0897.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- Black SG, Arnaud F, Burghardt RC, Satterfield MC, Fleming JA, Long CR, Hanna C, Murphy L, Biek R, Palmarini M, Spencer TE. Viral particles of endogenous betaretroviruses are released in the sheep uterus and infect the conceptus trophectoderm in a transspecies embryo transfer model. J Virol. 2010;84:9078–9085. doi: 10.1128/JVI.00950-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gonzalez I, Xu J, Wang X, Burghardt RC, Dunlap K, Bazer FW. Exosomes, endogenous retroviruses and toll-like receptors: pregnancy recognition in ewes. Reproduction. 2015;149(3):281–291. doi: 10.1530/REP-14-0538. [DOI] [PubMed] [Google Scholar]
- Burns G, Brooks K, Wildung M, Navakanitworakul R, Christenson LK, Spencer TE. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS One. 9:e90913. doi: 10.1371/journal.pone.0090913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp J, Salih SM, Khatib H. Expression of microRNAs in bovine and human pre-implantation embryo culture media. Front Genet. 2014;5:91. doi: 10.3389/fgene.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth EM, Shelton DN, Wells LM, Sparks AE, Van Voorhis BJ. Human embryos secrete microRNAs into culture media–a potential biomarker for implantation. Fertil Steril. 2014;101:1493–1500. doi: 10.1016/j.fertnstert.2014.01.058. [DOI] [PubMed] [Google Scholar]
- Lewis SK, Farmer JL, Burghardt RC, Newton GR, Johnson GA, Adelson DL, Bazer FW, Spencer TE. Galectin 15 (LGALS15): a gene uniquely expressed in the uteri of sheep and goats that functions in trophoblast attachment. Biol Reprod. 2007;77:1027–1036. doi: 10.1095/biolreprod.107.063594. [DOI] [PubMed] [Google Scholar]
- Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, Mann M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics. 2005;4:1265–1272. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- Mamo S, Mehta JP, McGettigan P, Fair T, Spencer TE, Bazer FW, Lonergan P. RNA sequencing reveals novel gene clusters in bovine conceptuses associated with maternal recognition of pregnancy and implantation. Biol Reprod. 2011;85:1143–1151. doi: 10.1095/biolreprod.111.092643. [DOI] [PubMed] [Google Scholar]