Supplemental Digital Content is available in the text.
Keywords: comparative effectiveness research, lower extremity bypass surgery, peripheral arterial disease
Background—
Treatment for symptomatic peripheral artery disease includes lower extremity bypass surgery (LEB) and peripheral endovascular interventions (PVIs); however, limited comparative effectiveness data exist between the 2 therapies. We assessed the safety and effectiveness of LEB and PVI in patients with symptomatic claudication and critical limb ischemia.
Methods and Results—
In a community-based clinical registry at 2 large integrated healthcare delivery systems, we compared 883 patients undergoing PVI and 975 patients undergoing LEB between January 1, 2005 and December 31, 2011. Rates of target lesion revascularization were greater for PVI than for LEB in patients presenting with claudication (12.3±2.7% and 19.0±3.5% at 1 and 3 years versus 5.2±2.4% and 8.3±3.1%, log-rank P<0.001) and critical limb ischemia (19.1±4.8% and 31.6±6.3% at 1 and 3 years versus 10.8±2.5% and 16.0±3.2%, log-rank P<0.001). However, in comparison with PVI, LEB was associated with increased rates of complications up to 30 days following the procedure (37.1% versus 11.9%, P<0.001). There were no differences in amputation rates between the 2 groups. Findings remained consistent in sensitivity analyses by using propensity methods to account for treatment selection.
Conclusions—
In patients with symptomatic peripheral artery disease, in comparison with LEB, PVI was associated with fewer 30-day procedural complications, higher revascularization rates at 1 and 3 years, and no difference in subsequent amputations.
Peripheral artery disease (PAD) is characterized by atherosclerotic stenosis and occlusions of the peripheral arteries and affects up to 30% of the US adult population.1,2 Claudication and critical limb ischemia (CLI) are symptomatic manifestations of PAD and are often considered indications for revascularization therapy with either lower extremity bypass surgery (LEB) or peripheral endovascular intervention (PVI). There is limited evidence comparing LEB versus PVI and, as a result, the optimal approach to revascularization remains uncertain for many patients.3
Clinical Perspective on p 2011
Over the past decade, PVI has become the dominant revascularization strategy for treating symptomatic PAD, increasing 1000% during this time period.4 PVI, in comparison with LEB, has been reported to have lower procedural morbidity and mortality,5,6 reduced costs,7 and shortened hospital lengths of stay.8 However, these findings are derived from small, single-center retrospective, observational analyses, and there is a paucity of corroborating evidence from large randomized, controlled trials or published data from large multicenter registries. In fact, the British Angioplasty versus Surgery in Ischemic Legs (BASIL) trial, a large randomized clinical trial comparing PVI versus LEB in patients with CLI did not find a difference in amputation-free survival.9 In the absence of robust efficacy data, proponents for an endovascular-first approach cite increased safety; however, a direct comparison with LEB has been lacking.
To address this issue we assembled a clinical registry of PAD patients undergoing lower extremity peripheral revascularization (LEB or PVI) in 2 large integrated healthcare delivery systems. We then used this registry to assess the safety and effectiveness of PVI and LEB in patients presenting with claudication and CLI.
Methods
Study Setting
The study was conducted in 2 large, integrated healthcare delivery systems that collectively care for >3.75 million people: Kaiser Permanente Colorado and Kaiser Permanente Northern California. Kaiser Permanente Colorado has >600 000 enrollees in the Denver, CO, metropolitan area and contracts with >1000 physicians to deliver care in 20 outpatient clinics. Kaiser Permanente Northern California provides care to >3.2 million members and contracts with a medical group of >6000 physicians who treat patients at 39 clinics. Data on vital signs, medication dispensing, laboratory test results, diagnoses, and healthcare use were available from electronic health records and administrative databases at both sites dating back to January 2000. Data from each of the health plans were restructured into a common, standardized format with identical variable names, formats, specifications and identical variable definitions, labels, and coding. Institutional review board approval was obtained in both systems.
Study Population
The study population included patients ≥18 years of age undergoing lower extremity revascularization procedures (LEB or PVI) from Kaiser Permanente Colorado or Kaiser Permanente Northern California between January 1, 2005, and December 31, 2011. We used a published algorithm of International Classification of Diseases, Ninth Revision diagnosis and Current Procedural Terminology procedural codes to identify 3800 patients undergoing PAD revascularization for preliminary chart review.10 Vascular experts confirmed lower extremity procedures in 2161 charts after detailed review. Because we were comparing endovascular with surgical intervention, patients whose initial revascularization procedure involved concomitant surgical and endovascular (hybrid) procedures (n=265) were excluded. We also excluded 29 patients who had <6 months enrollment before the identified procedure because there was insufficient time to assess for baseline comorbidities. Nine patients without the key stratifying variable of procedural indication (CLI versus claudication) were also excluded. The final cohort included 1858 patients: 975 patients treated with surgery (LEB) and 883 patients treated with endovascular therapy (PVI).
PAD Registry Development
To allow for a more robust assessment of the comparative effectiveness of PVI versus LEB, we conducted detailed chart abstraction to obtain key clinical and procedural variables. From the chart, study personnel collected a copy of the preprocedure history and physical, diagnostic testing reports, procedure reports, discharge summary, and follow-up visits within 30 days of discharge. Preprocedural assessments and procedure notes were collected for all cases through December 2012. This information was then reviewed the use of a standardized abstraction tool by a 6-member, multidisciplinary clinical abstraction team of vascular therapy physicians from interventional cardiology, interventional radiology, and vascular surgery.
Data abstracted from chart review in patients undergoing revascularization included: diagnostic study findings (eg, catheter-based angiography, computed tomography angiography, or magnetic resonance angiography), indication for procedure (claudication, critical limb ischemia), lesion characteristics (lesion length, diameter, and presence of total occlusion), procedure(s) performed (surgical or endovascular approach including procedural details such as devices used), and periprocedural events (eg, need for blood transfusion, surgical conversion, emergent reintervention, or myocardial infarction). To assess the reliability of the data collected from chart abstraction, each member of the chart abstraction team reviewed the same 20 charts. Agreement was good (κ 0.6–0.8) or very good (κ 0.8–1.0) for all variables assessed.
Clinical Outcomes
Primary outcomes were: (1) target lesion revascularization (TLR), (2) target limb revascularization (TLimb), (3) major (above-the-ankle) or minor (below-the-ankle) lower extremity amputation, and (4) mortality. Mortality data were obtained by using health plan mortality databases and by linking to state mortality databases and the National Death Index. Patients were followed for the occurrence of these outcomes from the time of the initial revascularization procedure until December 31, 2012. Longitudinal data on clinical events (eg, reintervention) were obtained from the electronic data sources at each site, including hospitalizations and procedures performed at non-Kaiser facilities (see online-only Data Supplement Table I for the criteria and sources of data used to identify these events). All outcomes were examined separately; thus, a patient could contribute to each of the primary end points.
Statistical Analysis
We first stratified patients into 2 analytic cohorts based on the clinical indication of the procedure: CLI or claudication. Within the CLI and claudication cohorts, we then classified patients into treatment groups based on whether they underwent LEB or PVI procedures. For the purposes of this study, patients were grouped into suprainguinal, infrainguinal, and tibial revascularization procedures. Stenting or bypasses of the aortoiliac, iliac, and external iliac vessels were considered suprainguinal procedures. Stenting, endarterectomy, or bypasses of the common femoral, superficial femoral, and popliteal arteries were considered infrainguinal procedures. Revascularization procedures below the popliteal were considered tibial procedures. Patients with multiple procedures were categorized based on the most distal revascularization (eg, patient with suprainguinal and infrainguinal PVI would be categorized into the infrainguinal group).
To adjust for potential confounding we used logistic regression to estimate propensity scores for the receipt of surgical therapy. The propensity score we constructed included variables reflecting patient age, sex, smoking status, comorbidities, previous vascular procedures, indicators of anatomic PAD severity, medical care use (ie, clinic visits), year of procedure, and site (Northern California or Colorado). The PAD severity variables were constructed from chart reviews of diagnostic testing documentation, which recorded the severity of vessel obstruction at 5 levels, which approximated regions of the iliac arteries, common femoral arteries, profunda femoris arteries, superficial femoral arteries, and tibial arteries. Stenosis severity was categorized as: other (<50%), severe (50%–99%), and occluded (100%) based on diagnostic imaging reports. Missing data categories were used to retain persons who did not have severity information at all levels.
We calculated Kaplan-Meier estimates of incidence rates for the end points and present 95% confidence intervals (CIs) or rates+standard errors by type of intervention (LEB versus PVI) stratified by clinical indication. For persons without an event, follow-up time was censored at the time of death, disenrollment from the health plan, or end of the study (December 2012). We estimated the relative hazard by using Cox proportional hazards regression models. These models included a term for intervention (LEB was the reference) and additionally included a term to account for clustering of patients within the 2 study sites. One set of models used inverse propensity score adjustment methods with the stabilized weights trimmed (<10) to limit the impact of a few outliers.11–13 Propensity score models were highly predictive of treatment (c statistics 0.90 and 0.87 for claudication and CLI indication groups, respectively). In addition to the inclusive inverse probability of treatment weighted (IPTW) models, we also estimated PVI versus LEB hazard ratios based on a matched sample (matched 1:1 on propensity scores using a greedy matching algorithm to identify the closest match within a +0.05 maximum distance). For the matched analyses, the Cox proportional hazards regression models accounted for matching by stratifying on the matched pairs. We used both IPTW and matched analyses because these techniques typically have dissimilar population inferences and different weaknesses. Typically, IPTW aims to estimate the average effect of an entire population being given treatment A in comparison with the entire population being given treatment B (average treatment effect). Matched analyses estimate the average effect of being given treatment B, versus A, among persons who are similar to persons receiving treatment A (average treatment effect of treated). When treatments are randomly assigned, average treatment effect and average treatment effect of treated will be equal. In observational studies, matching 1:1 may offer strong covariate control but will restrict sample sizes based on the least common treatment. The broader inference of IPTW is desirable, but even with careful examination and trimming of propensity scores, weighting may ignore effect modification within the larger population. We provide results from both analyses here. Follow-up outcomes for minor and major amputation were determined via published International Classification of Diseases, Ninth Revision /Current Procedural Terminology coding algorithms (online-only Data Supplement Table I). We estimated cumulative incidence curves from Cox proportional hazards models. Graphs presented in the article provide crude outcome estimates for the whole cohort.
Results
Baseline Characteristics by Presentation (Claudication versus CLI)
Between January 1, 2005, and December 31 2011, 1858 patients underwent lower extremity procedures. The study population had a mean age of 69.6±10.4 years, was 59.4% male, and had a high burden of hypertension (90.6%) and lipid disorders (84.6%) (Table 1). Overall, 27.4% were current smokers, 29.9% had undergone previous coronary revascularization, and 44.5% had diabetes mellitus. Only 7.5% and 8.2% of patients, respectively, had undergone previous PVI or LEB. Of the 1858 patients undergoing revascularization, 934 patients were treated for claudication and 924 patients were treated for CLI. Median follow-up time was 3.2 years up to a maximum of 8 years. Persons treated for CLI had less follow-up time than persons treated for claudication (median, 2.8 years versus 3.6 years).
Table 1.
Characteristics of Patients With Claudication versus CLI
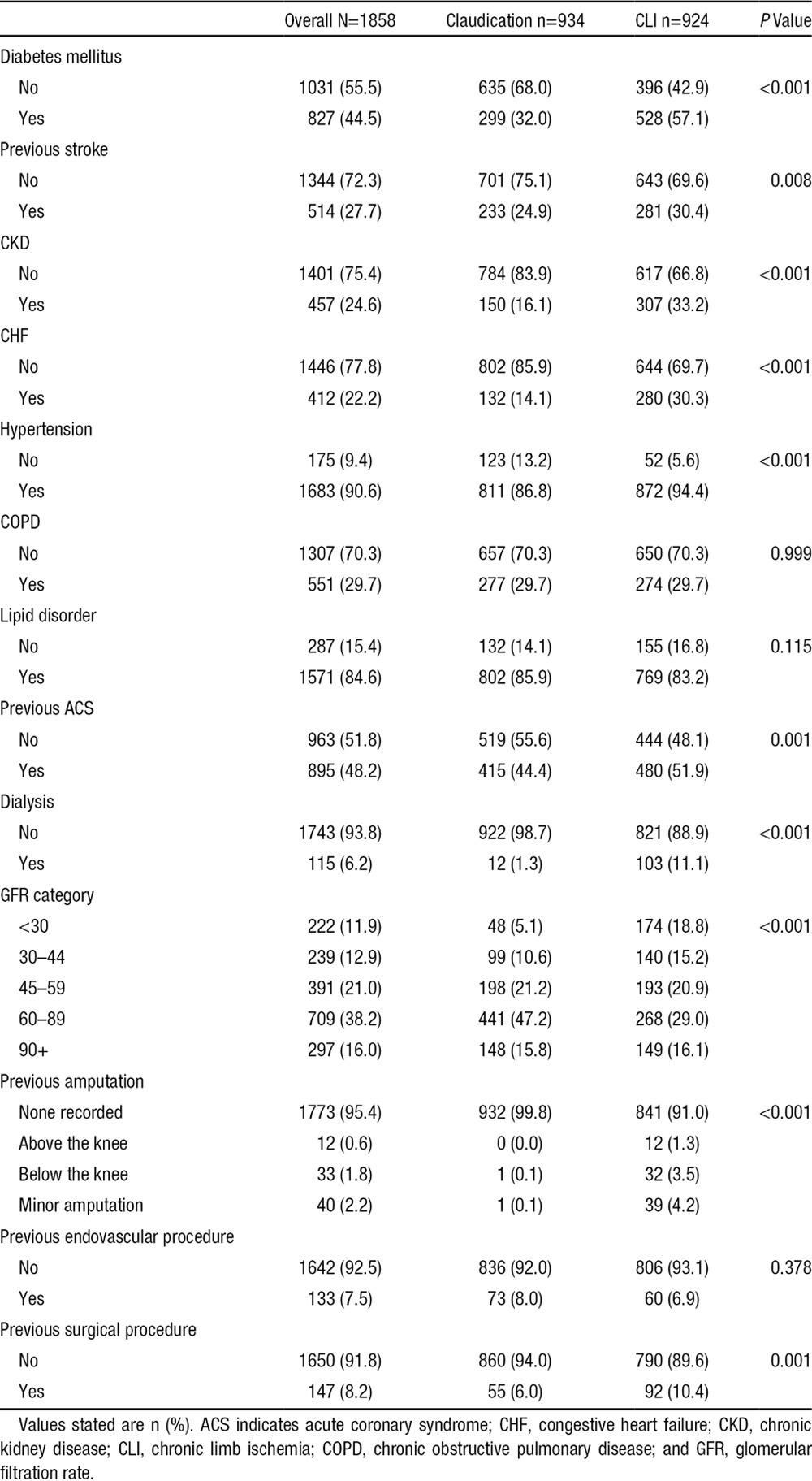
In comparison with the patients presenting with claudication, CLI patients were significantly older with a higher comorbidity burden including previous myocardial infarction, diabetes mellitus, previous stroke, chronic kidney disease, congestive heart failure, hypertension, and dialysis (Table 1). LEB was performed more commonly in patients presenting with CLI versus patients presenting with claudication (68.5% versus 36.6%, P=0.001).
Baseline Characteristics by Treatment (PVI versus LEB)
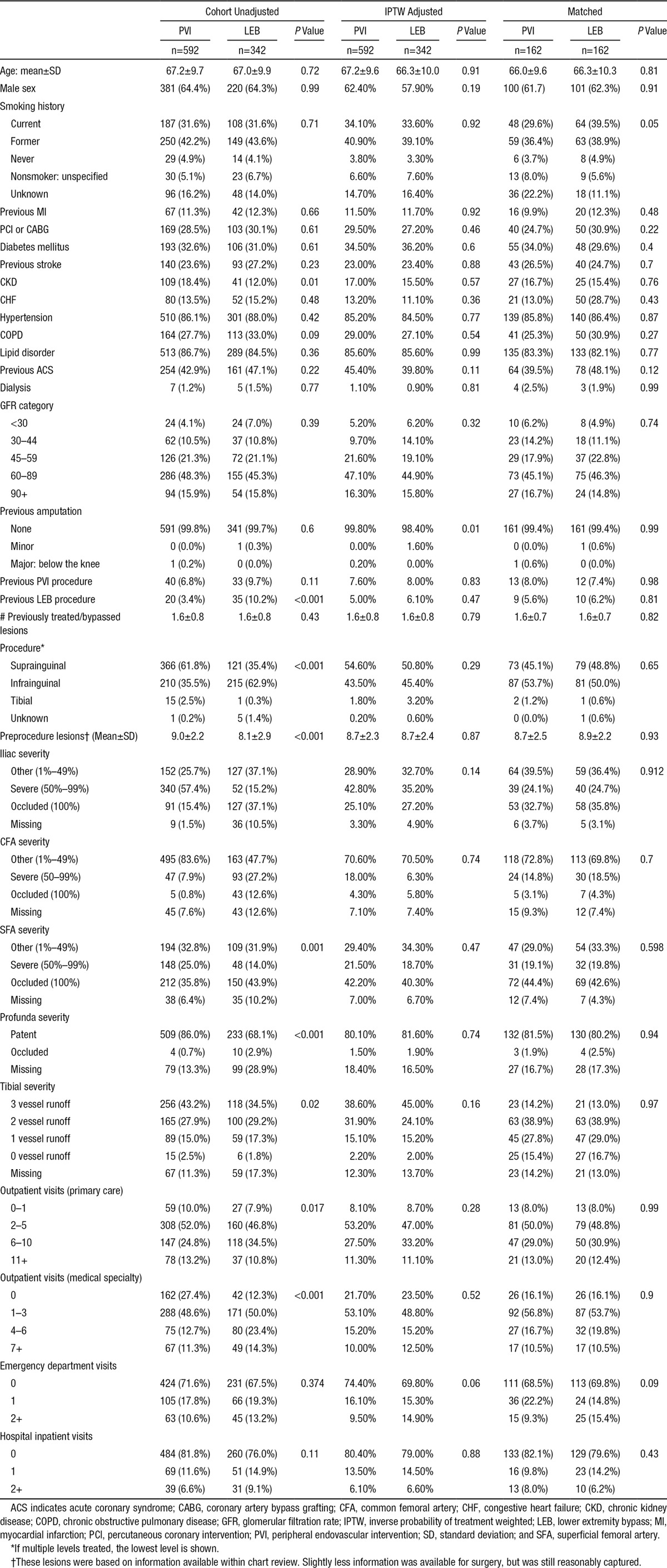
Among patients undergoing revascularization for claudication, demographic and comorbidity variables were not significantly different with the exception of a higher percentage of patients with chronic kidney disease in the PVI versus LEB group (18.4% versus 12.0%, P=0.01). Patients who underwent LEB were more likely to have undergone previous LEB or PVI procedures versus patients treated with PVI (Table 2). Burden of disease scores suggested more severe PAD disease for LEB patients (ie, more occlusions and less vessel runoff). The most common level for PVI procedures was suprainguinal, whereas for LEB the most common level was infrainguinal. Most covariate differences were reduced with propensity score weighting.
Table 2.
Baseline Characteristics of Patients Undergoing Peripheral Intervention for Claudication
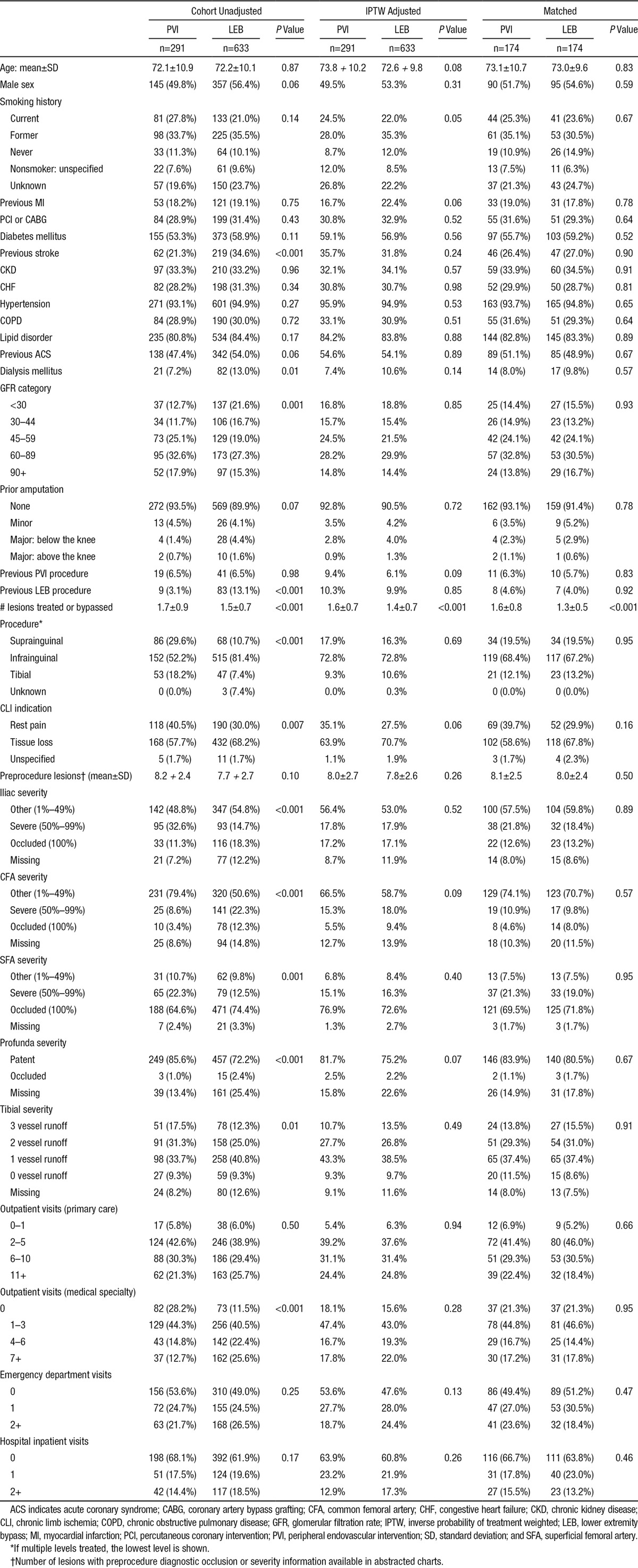
In the CLI cohort, patients undergoing LEB were more likely to have glomerular filtration rate levels <30, to have a history of stroke, and to be on dialysis (Table 3). Infrainguinal procedures were the most frequent anatomic location for both LEB and PVI. Vessel severity indicators suggested more severe PAD disease for LEB patients (ie, more occlusions and poorer vessel runoff). Most covariate differences were reduced with propensity score weighting.
Table 3.
Baseline Characteristics of Patients Undergoing Peripheral Intervention for CLI
Procedural Complications
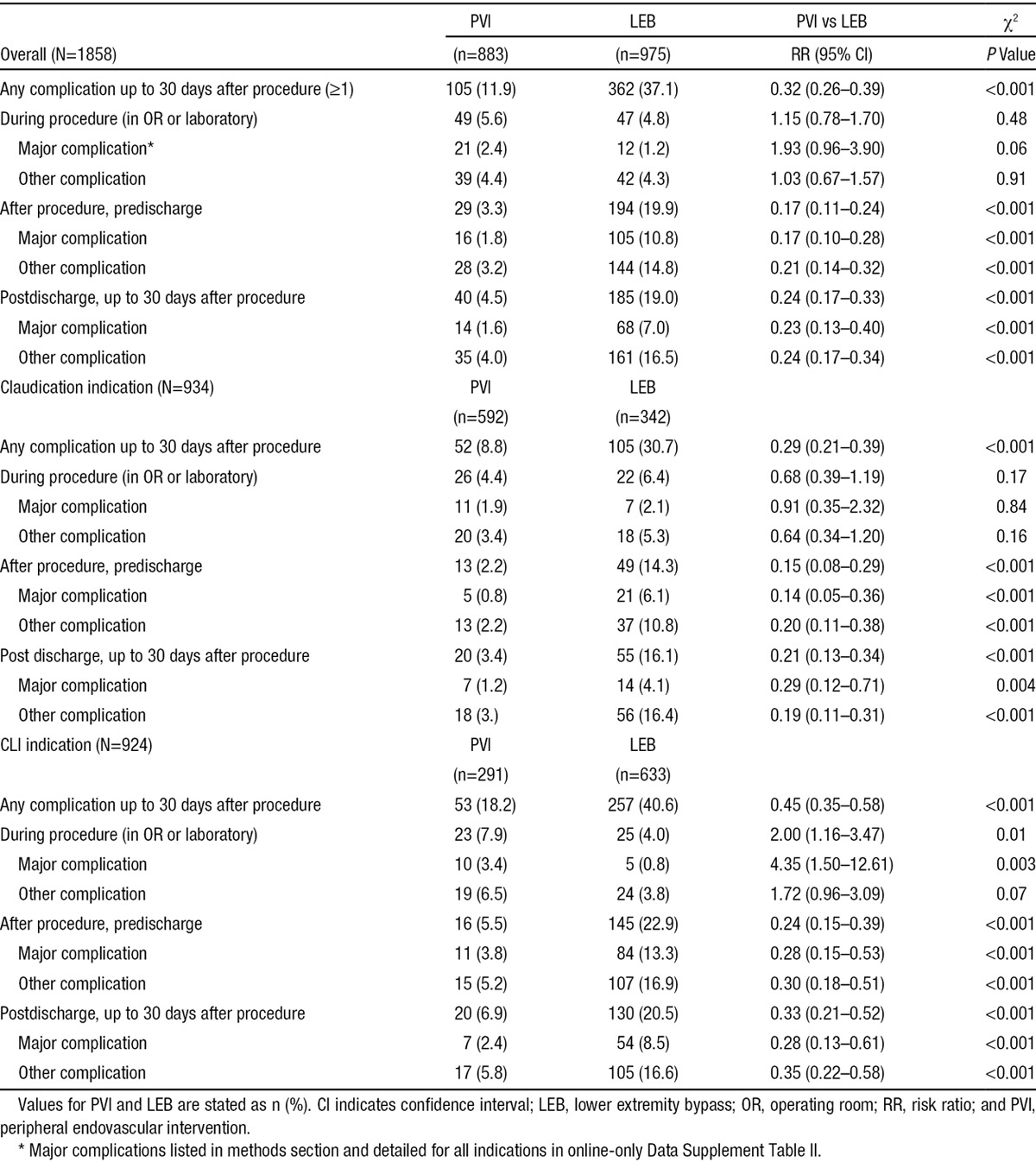
Overall, in comparison with PVI, patients undergoing LEB were more likely to have ≥1 complications from the time of the procedure to 30 days follow-up (37.1% versus 11.9%, Table 4). The majority of the complications occurred early; ie, before discharge or within 30 days following the procedure. In the periprocedural phase, an embolic event during the procedure (1.9%) was the most common complication for PVI, whereas a blood transfusion (3.3%) was the most common complication for LEB. During the postprocedure, predischarge phase, worsening limb ischemia (1.9%) was the most common complication for PVI, whereas an unplanned additional vascular procedure/return to the operating room (5.4%) was the most common complication for LEB. Finally, for the postdischarge to 30-day follow-up phase, worsening ischemia (2.8%) was the most common complication for PVI, whereas a surgical site infection (11.0%) was the most common complication for LEB.
Table 4.
Frequency of One or More Complications After PVI or LEB Interventions, Overall and by Indication and Timing of Complication
Revascularization
Figure 1 shows the cumulative incidence rates of revascularization for patients presenting with claudication or CLI estimated by the Kaplan-Meier method stratified by treatment with LEB or PVI. Rates of TLR were greater for PVI than for LEB in patients presenting with claudication (12.3±2.7% and 19.0±3.5% at 1 and 3 years versus 5.2±2.4% and 8.3±3.1%, log-rank P<0.001) (Figure 1A) and CLI (19.1±4.8% and 31.6±6.3% at 1 and 3 years versus 10.8±2.5% and 16.0±3.2%, log-rank P<0.001) (Figure 1B).
Figure 1.
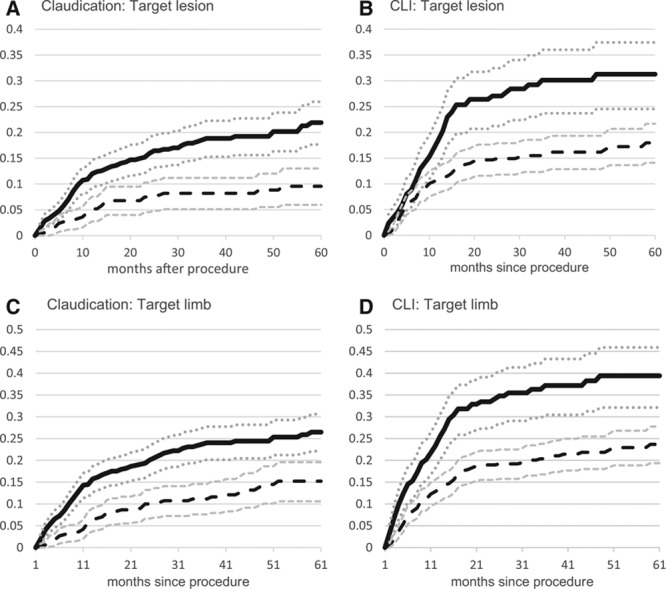
Cumulative incidence by indication and procedure type. Peripheral endovascular intervention, solid line (95% CI dotted lines); lower extremity bypass, long dashed lines (95% CI smaller dashed lines); and unadjusted cumulative incidence using Kaplan-Meier survival models. CI indicates confidence interval; and CLI, chronic limb ischemia.
Rates of TLimb were also greater for PVI than for LEB in patients presenting with claudication (16.1±3.0% and 24.4±3.8% at 1 and 3 years versus 6.4±2.6% and 11.3±3.5%, log-rank P<0.001) (Figure 1C) and CLI (26.5±5.3% and 38.9±6.5% at 1 and 3 years versus 13.4±2.8% and 21.0±3.6%, log-rank P<0.001) (Figure 1D).
In IPTW propensity-adjusted analyses (Table 5), patients presenting with claudication treated with PVI were significantly more likely than LEB patients to require TLR (hazard ratio [HR], 2.49; 95% CI, 1.62–3.81) and TLimb (HR, 2.59; 95% CI, 1.79–3.74). Similarly, patients presenting with CLI treated with PVI were also significantly more likely than LEB patients to require TLR (HR, 2.29; 95% CI, 1.69–3.12) and TLimb (HR, 2.17; 95% CI, 1.65–2.84). In sensitivity analyses using a propensity-matched cohort, the results were similar.
Table 5.
Revascularization, Amputation, and Death Outcomes for Claudication and CLI Indication Cohorts
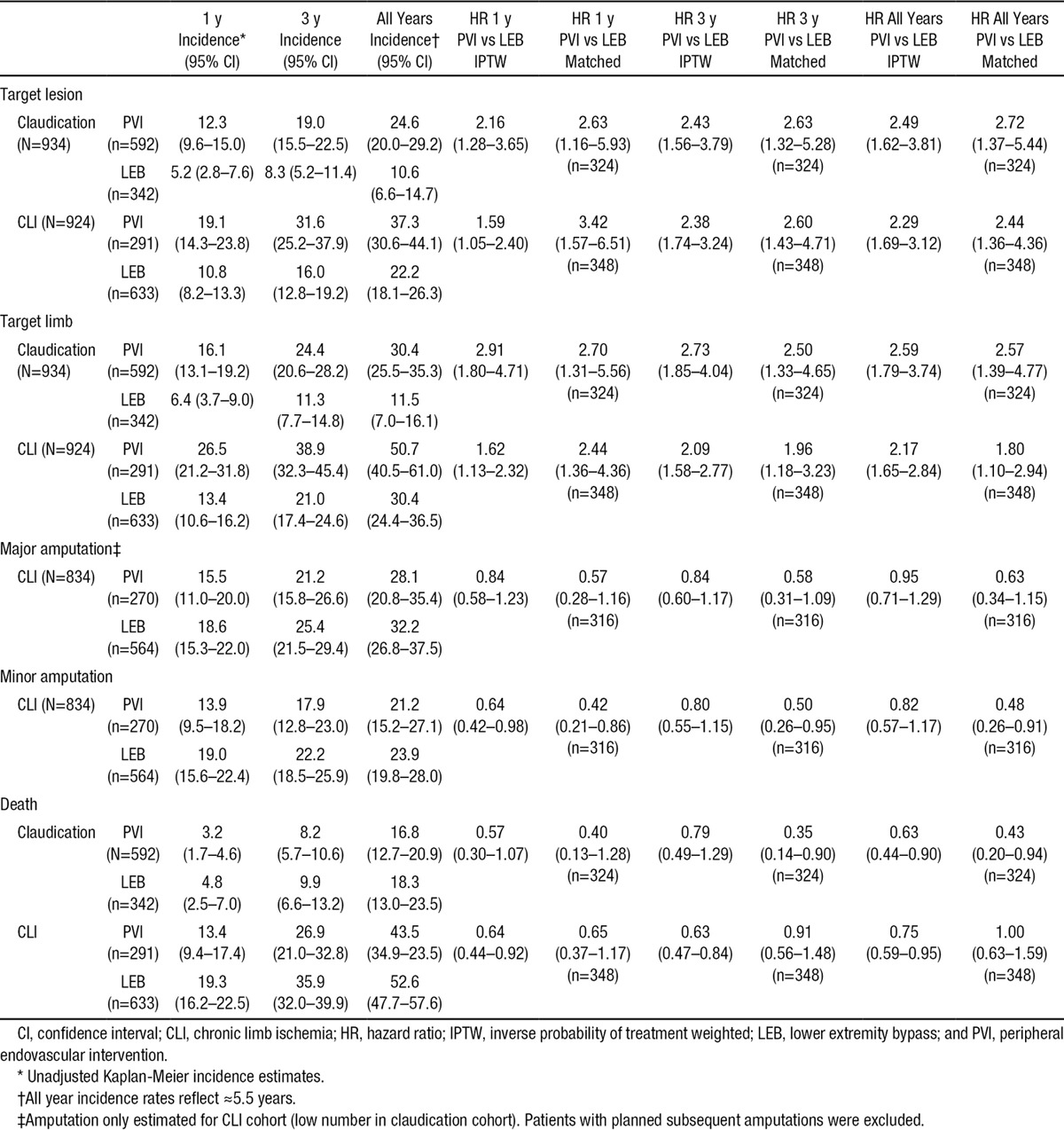
Amputation
The 1- and 3-year major amputation rates for PVI (15.5±4.5% and 21.2±5.4%) were not significantly different from those for LEB (18.6±3.4% and 25.4±4.0%) (Figure 2). There remained no significant difference in amputation rates after IPTW propensity adjustment (HR, 0.95; 95% CI, 0.71–1.29).
Figure 2.
Cumulative incidence by indication and procedure type. Peripheral endovascular intervention, solid line (95% CI dotted lines); lower extremity bypass, long dashed lines (95% CI smaller dashed lines); and unadjusted cumulative incidence using Kaplan Meier survival models. CI indicates confidence interval; and CLI, chronic limb ischemia.
Mortality
Figure 3 shows the mortality rates for patients presenting with claudication or CLI estimated by the Kaplan-Meier method stratified by treatment with LEB or PVI. Mortality rates were similar for LEB in comparison with PVI in patients presenting with claudication (4.8±2.3% and 9.9±3.3% at 1 and 3 years versus 3.2±1.4% and 8.2±2.5%, log-rank P=0.34) (Figure 3A), whereas mortality rates were higher for LEB than for PVI patients presenting with CLI (19.3±3.1% and 35.9±3.9% at 1 and 3 years versus 13.4±4.0% and 26.9±5.9%, log-rank P=0.003) (Figure 3B).
Figure 3.
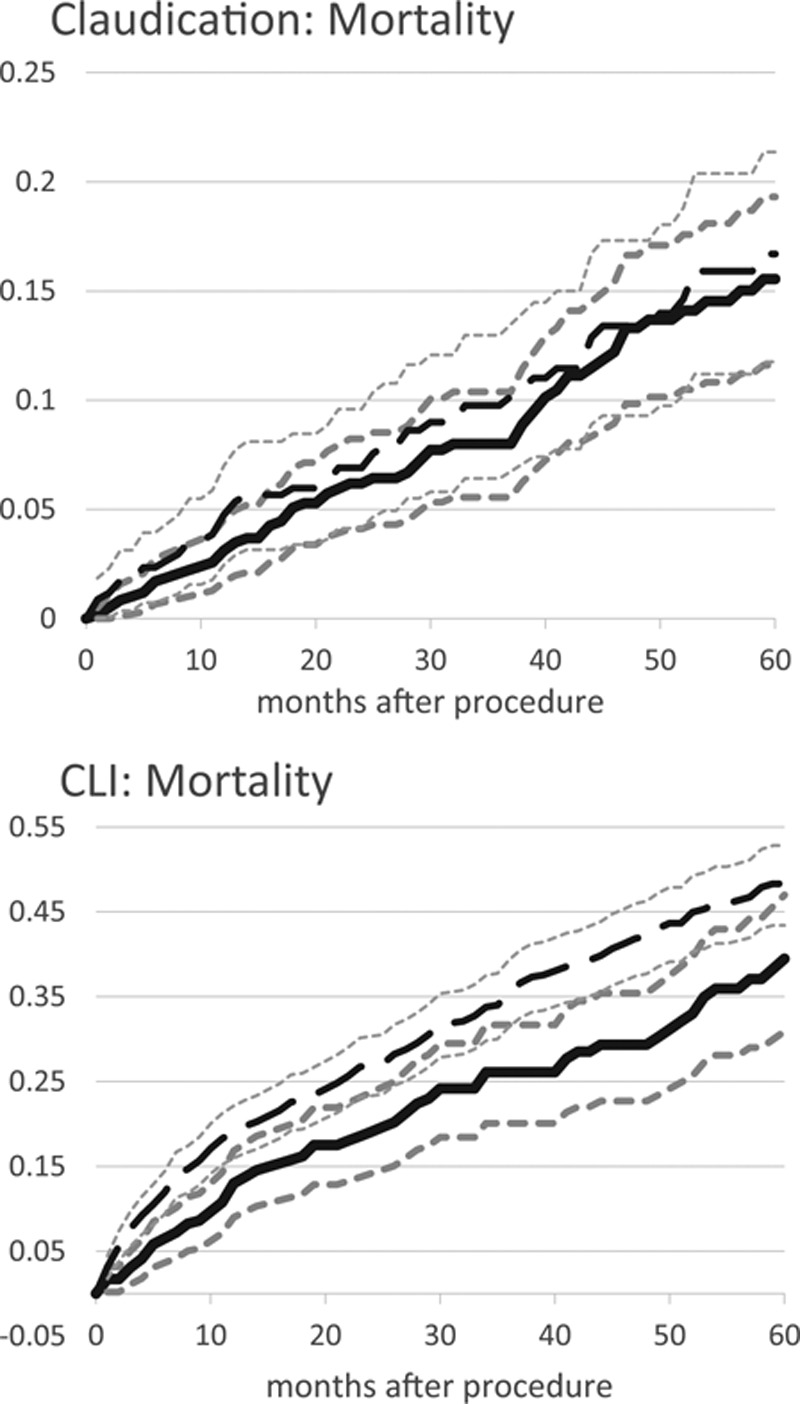
Cumulative incidence by indication and procedure type. Peripheral endovascular intervention, solid line (95% CI dotted lines); lower extremity bypass, long dashed lines (95% CI smaller dashed lines). CI indicates confidence interval; and CLI, chronic limb ischemia.
In IPTW propensity-adjusted analyses over the duration of follow-up (Table 5), patients presenting with claudication treated with PVI were significantly less likely than LEB patients to die (HR, 0.63; 95% CI, 0.44–0.90). Similarly, patients presenting with CLI treated with PVI were also significantly less likely than LEB patients to die (HR, 0.75; 95% CI, 0.59–0.95). In sensitivity analyses using a propensity-matched cohort, the findings in the claudication cohort were similar, but the findings in the CLI cohort were not significant.
Discussion
From this large cohort of PAD patients undergoing peripheral revascularization from 2 integrated healthcare delivery systems, we found that PVI was associated with lower rates of 30-day procedural complications, higher rates of reintervention (TLR and TLimb), and no significant difference in amputation rates. The outcomes were consistent for patients undergoing these procedures for claudication or CLI. These findings add to the available literature for the management of patients with PAD and highlight areas that need future research.
Specifically, the clinical uncertainty over the best approach for treating patients with symptomatic PAD is attributable in part to the paucity of data favoring 1 treatment modality over another. There is an urgent need for contemporary comparative trials to guide treatment decisions. In the absence of randomized clinical trials, comparative effectiveness analysis applied to population-based registries can provide some guidance.14 Using stratified propensity adjustment methods, we found that PVI in comparison with LEB for the treatment of patients with claudication or CLI was associated with increased target lesion and target limb revascularization but had similar rates of minor and major amputation. Accordingly, the unadjusted Kaplan-Meier results depict real-world practice patterns and outcomes as treated in a large, integrated healthcare system.
Target lesion revascularization is a common end point in randomized clinical trials of peripheral revascularization and reflects the failure of lesion-specific treatment as opposed to progression in nontreated segments.15 This end point has traditionally been difficult to ascertain in observational registries because it requires clinical adjudication and is not captured by administrative coding. By using a physician abstraction team of vascular therapy experts to review the revascularization procedures, we were able to distinguish between target lesion and target limb revascularization procedures, thereby delineating between treatment failures and the progression of disease. Also, the decision to proceed to a repeat procedure in our study reflects contemporary real-world practice patterns. The TLR rates for PVI were 12.3% at 1 year and 19% at 3 years which are comparable to the 1-year TLR rate of 13% in the recent randomized controlled Randomized Study Comparing the Edwards Self-Expanding Lifestent versus Angioplasty Alone In Lesions Involving the SFA and/or Proximal Popliteal Artery (RESILIENT) trial comparing infrainguinal stenting with balloon angioplasty in patients with claudication.16 Furthermore, the TLR rates for LEB of 10.8% at 1 year and 16.0% at 3 years in patients with CLI are numerically better than a meta-analysis of lower extremity bypass procedures for CLI where 1- and 3-year TLR rates were 15.7% and 23.4%, respectively.17 Therefore, in comparison with the existing literature, the PVI and LEB revascularization rates for both claudication and CLI patients cared for in the 2 integrated healthcare delivery systems in this study were excellent with relatively favorable long-term patency rates. In Kaplan-Meier analysis with propensity adjustment, patients receiving PVI were >2 times more likely to require a repeat procedure than patients receiving LEB. These observed differences reflect the median effect over the 7-year duration of the study period where PVI was under constant evolution with different iterations of new devices and techniques, but LEB, as a procedure, was well established.
Target limb revascularization is a broader definition that includes revascularization of the previously treated lesion in addition to the progression of disease in the nontreated segment that results in repeat LEB or PVI. Across the spectrum of disease in our study, lesion-specific revascularization comprised between 70% and 80% of the revascularization procedures. Therefore, it is far more common for revascularization procedures to be related to the previously treated lesion (restenosis or graft failure) as opposed to treatment of de novo disease, emphasizing the need for better treatment of the incident lesion to minimize the need for repeat procedures.
Although PVI was associated with higher TLR and TLimb rates in comparison with LEB in patients presenting with CLI, there was no difference in the rate of major or minor amputations. In comparison with the historic literature, where 30% of patients with CLI undergo a major amputation within the first year,18 our CLI cohort undergoing revascularization procedures had much lower amputation rates of 15.5% with PVI and 18.6% with LEB. There continues to be conflicting evidence from the existing literature regarding the contemporary rates of lower extremity amputation procedures nationwide,4 but our cohort comprised chart-adjudicated CLI patients undergoing revascularization and therefore represents a well-defined clinical population. Recent data from the Centers for Medicare and Medicaid Services between 2000 and 2008 showed a decrease in lower extremity amputations, which was attributed to improved screening and PAD care in general.19 This supports an underlying principle in the treatment for CLI that prioritizes revascularization with successful limb perfusion to achieve wound healing and less emphasis on the long-term patency of the index intervention.20
A major argument for the decision to treat with PVI over LEB is the consideration for an increased risk of complications with LEB. LaMuraglia et al21 recently reported a high incidence of complications related to bypass surgery with a 2.7% mortality and 18.7% major complications, including 7.4% graft thrombosis. However, PVI has been reported to have variable complication rates of 7% to 17%.20,22,23 A challenge in comparing complication rates from 2 different procedures is the difficulty in weighing the severity of complications unique to each of the procedures as evidenced from recent randomized trials of carotid intervention.24–26 In our study, intraprocedural complication rates were similar for PVI and LEB, whereas after the procedure, predischarge and postdischarge complications were significantly higher with LEB than with PVI. How these increased risks of LEB are weighed against the benefit or decreased reinterventions requires judgment by both patients and treating physicians. Perhaps risk prediction and decision-making tools can better quantify the risks versus benefits of LEB in comparison with PVI to help patients and clinicians make thoughtful decisions regarding the best course of treatment.
Finally, mortality rates in patients undergoing lower extremity revascularization for claudication and CLI are high, approaching 18% and 53%, respectively, with 5.5 years of follow-up. The overall mortality is consistent with the literature and represents the severe prognosis, especially in patients presenting with CLI. Differential survival by treatment is difficult to assess given the observational nature of this study and the lack of information on concomitant medical therapy for cardiovascular disease. Nonetheless, these results emphasize the importance of aggressive secondary prevention therapies in our patients with peripheral arterial disease.
Limitations
We would like to acknowledge several potential limitations of this study. Because this is an observational comparative effectiveness study, patients were not randomly assigned to LEB or PVI. The decision to choose 1 approach versus the other may be related to unmeasured factors associated with the clinical outcomes. To reduce potential confounding by indication bias we used 2 different propensity methods to balance the covariates among patients undergoing the 2 revascularization strategies. We found comparable results by using IPTW and matched propensity score analyses. The matched analyses estimated treatment differences selecting patients with characteristics of those who received the less common treatment (LEB for patients with claudication and PVI for patients with CLI). In contrast, the IPTW models aimed to estimate treatment effects for a full population. The similar results imply that patterns seen in this study did not depend on the patient population selected. Nevertheless, despite the methodological rigor of our study design and analysis, we may not have been able to eliminate the impact of unmeasured confounding.
Second, this study included patients undergoing peripheral revascularization within 2 integrated healthcare delivery systems, the findings of which may not be generalizable to other healthcare settings. However, the demographics, comorbidities, and extent of vascular disease of patients in this cohort are comparable to other observational and randomized controlled studies of peripheral revascularization.
Third, this study represents an era before the approval of drug-eluting balloons, the routine use of drug-eluting stents in the tibial arteries, and advanced retrograde tibial techniques. These advances may narrow the gap between PVI and LEB in target lesion and target limb revascularization rates.
Finally, our study did not evaluate health status outcomes (symptoms, function, and quality of life). The extent to which PVI and LEB impact patient short- and long-term health status is an important factor to consider in selecting a procedure. To date, most studies of revascularization have focused on patency, which does not necessarily correlate with patient-reported symptoms or measured exercise impairment.27 Therefore, measurement of the impact of different revascularization strategies on health status is critical to support future comparative effectiveness studies of PAD treatment.
Conclusions
In conclusion, among patients undergoing revascularization for PAD in 2 large integrated healthcare delivery systems, PVI was associated with higher rates of reintervention (TLR and TLimb) at 1 and 3 years following the procedure in comparison with LEB. However, PVI was associated with lower rates of complications up to 30 days following the procedure. There were no differences in amputation rates between PVI and LEB. These findings provide additional evidence to the risk-benefit discussion regarding the optimal strategy for revascularization of PAD.
Acknowledgments
This project was funded under Contract No. 290201000008I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
Disclosures
None.
Supplementary Material
Footnotes
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.114.013440/-/DC1.
CLINICAL PERSPECTIVE
Treatment for symptomatic peripheral artery disease includes lower extremity bypass surgery (LEB) or peripheral vascular interventions (PVI). Given the paucity of randomized controlled trials in this area, there are limited data on the safety and effectiveness of LEB and PVI to help clinicians and patients decide on either therapy. In a large integrated healthcare system, we studied 975 patients undergoing LEB and 883 patients undergoing PVI for lower extremity claudication or chronic limb ischemia. The average patient was 70 years of age, and half of the patients were treated for claudication. The rates of target lesion revascularization were greater for PVI than for LEB in patients presenting with claudication and chronic limb ischemia; however, LEB was associated with an increased rate of complications up to 30 days following the procedure. There were no differences in amputation rates between the 2 groups. Therefore, although PVI is associated with fewer complications, rates of repeat revascularization are higher. Weighing the risks and benefits of each procedure will hopefully help clinicians and their patients decide on treatment. Future studies should focus on randomized comparisons and risk models that refine the risk/benefit ratio for each patient.
References
- 1.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 3.Valadares MC, Bincoletto C, Oliveira SC, de Melo A, Saad ST, Queiroz ML. Bone marrow progenitor cells from chemically exposed workers display an intrinsic ability for autonomous proliferation. Immunopharmacol Immunotoxicol. 2005;27:137–145. doi: 10.1081/iph-51761. doi: 10.1081/IPH-51761. [DOI] [PubMed] [Google Scholar]
- 4.Anderson PL, Gelijns A, Moskowitz A, Arons R, Gupta L, Weinberg A, Faries PL, Nowygrod R, Kent KC. Understanding trends in inpatient surgical volume: Vascular interventions, 1980–2000. J Vasc Surg. 2004;39:1200–1208. doi: 10.1016/j.jvs.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 5.Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. Eur J Vasc Endovasc Surg. 2005;30:164–175. doi: 10.1016/j.ejvs.2005.03.011. doi: 10.1016/j.ejvs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Regensteiner JG, Meyer TJ, Krupski WC, Cranford LS, Hiatt WR. Hospital vs home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology. 1997;48:291–300. doi: 10.1177/000331979704800402. [DOI] [PubMed] [Google Scholar]
- 7.Regensteiner JG. Exercise in the treatment of claudication: assessment and treatment of functional impairment. Vasc Med. 1997;2:238–242. doi: 10.1177/1358863X9700200313. [DOI] [PubMed] [Google Scholar]
- 8.Regensteiner JG, Gardner A, Hiatt WR. Exercise testing and exercise rehabilitation for patients with peripheral arterial disease: status in 1997. Vasc Med. 1997;2:147–155. doi: 10.1177/1358863X9700200211. [DOI] [PubMed] [Google Scholar]
- 9.Currie IC, Wilson YG, Baird RN, Lamont PM. Treatment of intermittent claudication: the impact on quality of life. Eur J Vasc Endovasc Surg. 1995;10:356–361. doi: 10.1016/s1078-5884(05)80057-7. [DOI] [PubMed] [Google Scholar]
- 10.Jaff MR, Cahill KE, Yu AP, Birnbaum HG, Engelhart LM. Clinical outcomes and medical care costs among Medicare beneficiaries receiving therapy for peripheral arterial disease. Ann Vasc Surg. 2010;24:577–587. doi: 10.1016/j.avsg.2010.03.015. doi: 10.1016/j.avsg.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Nicolaï SP, Kruidenier LM, Rouwet EV, Graffius K, Prins MH, Teijink JA. The walking impairment questionnaire: an effective tool to assess the effect of treatment in patients with intermittent claudication. J Vasc Surg. 2009;50:89–94. doi: 10.1016/j.jvs.2008.12.073. doi: 10.1016/j.jvs.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 12.Kügler CF, Rudofsky G. Do age and comorbidity affect quality of life or PTA-induced quality-of-life improvements in patients with symptomatic pad? J Endovasc Ther. 2005;12:387–393. doi: 10.1583/04-1449.1. doi: 10.1583/04-1449.1. [DOI] [PubMed] [Google Scholar]
- 13.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS PREVENT III Investigators. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 14.McDermott MM, Liu K, Guralnik JM, Martin GJ, Criqui MH, Greenland P. Measurement of walking endurance and walking velocity with questionnaire: validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg. 1998;28:1072–1081. doi: 10.1016/s0741-5214(98)70034-5. [DOI] [PubMed] [Google Scholar]
- 15.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg. 1996;23:104–115. doi: 10.1016/s0741-5214(05)80040-0. [DOI] [PubMed] [Google Scholar]
- 16.Wullink M, Stoffers HE, Kuipers H. A primary care walking exercise program for patients with intermittent claudication. Med Sci Sports Exerc. 2001;33:1629–1634. doi: 10.1097/00005768-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Landry GJ. Functional outcome of critical limb ischemia. J Vasc Surg. 2007;(suppl A):A141–A148. doi: 10.1016/j.jvs.2007.02.052. doi: 10.1016/j.jvs.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 18.Spertus J, Jones P, Poler S, Rocha-Singh K. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;147:301–308. doi: 10.1016/j.ahj.2003.08.001. doi: 10.1016/j.ahj.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Kalbaugh CA, Taylor SM, Cull DL, Blackhurst DW, Gray BH, Langan EM, 3rd, Dellinger MB, McClary GE, Jr, Jackson MR, Carsten CG, 3rd, Snyder BA, York JW, Youkey JR. Invasive treatment of chronic limb ischemia according to the Lower Extremity Grading System (LEGS) score: a 6-month report. J Vasc Surg. 2004;39:1268–1276. doi: 10.1016/j.jvs.2004.02.009. doi: 10.1016/j.jvs.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 21.Mehta T, Venkata Subramaniam A, Chetter I, McCollum P. Assessing the validity and responsiveness of disease-specific quality of life instruments in intermittent claudication. Eur J Vasc Endovasc Surg. 2006;31:46–52. doi: 10.1016/j.ejvs.2005.08.028. doi: 10.1016/j.ejvs.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Wann-Hansson C, Hallberg IR, Risberg B, Lundell A, Klevsgard R. Health-related quality of life after revascularization for peripheral arterial occlusive disease: long-term follow-up. J Adv Nurs. 2005;51:227–235. doi: 10.1111/j.1365-2648.2005.03499.x. doi: 10.1111/j.1365-2648.2005.03499.x. [DOI] [PubMed] [Google Scholar]
- 23.Martem’ianov SV, Uvarov EA, Safonova OV. Assessment of the patient’s quality of life in the long-term postoperative period after reconstructions on lower extremity arteries. Angiol Sosud Khir. 2004;10:129–135. [PubMed] [Google Scholar]
- 24.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ringleb PA, Allenberg J, Brückmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Stingele R, Zeumer H, Hacke W SPACE Collaborative Group. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247. doi: 10.1016/S0140-6736(06)69122-8. [Google Scholar]
- 26.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lièvre M, Leys D, Bonneville JF, Watelet J, Pruvo JP, Albucher JF, Viguier A, Piquet P, Garnier P, Viader F, Touzé E, Giroud M, Hosseini H, Pillet JC, Favrole P, Neau JP, Ducrocq X EVA-3S Investigators. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. doi: 10.1056/NEJMoa061752. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 27.Randomized placebo-controlled, double-blind trial of ketanserin in claudicants. Changes in claudication distance and ankle systolic pressure. Pack claudication substudy. Circulation. 1989;80:1544–1548. doi: 10.1161/01.cir.80.6.1544. [DOI] [PubMed] [Google Scholar]


