Abstract
Objective
Neonatal ICU care involves use of opiates to treat post-operative, ventilated or chronically ill infants. Opiates provide necessary analgesia and sedation, but the morbidities include prolonged Neonatal Abstinence Syndrome (NAS) and extended length of stay for dose tapering. Our objective was to quantify trends in opiate exposure in a tertiary care NICU. We hypothesize that medical opiate exposure and resultant ICU-acquired NAS would increase over time.
Design
retrospective cross-sectional cohort study
Setting
tertiary care NICU
Patients
high risk inborn infants admitted in fiscal years 2003–2004, 2007–2008 and 2010–2011
Main Outcome Measure
Average cumulative morphine exposure (all opiate doses converted to morphine equivalents) per time epoch was compared in cohorts of clinically similar infants. Linear regression was used to assess the primary outcome, assessing changes in opiate exposure over time.
Results
63 infants were included in the final analysis. The primary analysis assessing cumulative opiate exposure per infant showed an increase of 134 mg per time epoch (95% CI −12, 279 mg, p-value 0.071). There was a statistically significant increase in the percent of infants with a diagnosis of iatrogenic NAS, increasing from 9 to 35 to 50% (p-value 0.012).
Conclusion
Medical opiate exposure is increasing over time in ICU infants. In association, there are increased diagnoses of ICU-acquired NAS. This trend should be monitored closely and further studies to assess interventions including more strident pain and sedation monitoring, weaning protocols and other efforts to decrease opiate exposure are warranted.
Keywords: neonate, opiate, neonatal abstinence syndrome, iatrogenic exposure, pharmacoepidemiology, withdrawal
Introduction
Neonates are commonly exposed to opiates and benzodiazepines for analgesic and sedative effects in the intensive care unit (ICU). Although their use is warranted, there are few evidence-based resources to guide initiation, maintenance and weaning of analgesic and sedative medications. Secular trends in opinions about need to treat pain in neonates have shifted over time, from thoughts that infants could not feel pain to a realization that neonatal pain perception is intact1. Recently, the growing clinical perception is that the use of opiates in the Neonatal and Pediatric Intensive Care Unit is rapidly increasing and this increasing opiate exposure in the neonatal period is not without consequence2.
There is a fine balance between treating pain and avoiding the adverse events associated with opiate exposure, both clinical and cellular. There is animal evidence that pain control with morphine attenuates long-term negative consequences such as hyperalgesia3. A recent systematic review4 compiled multiple studies which associate painful procedures in the neonatal period to adverse neurologic outcomes; so there is indeed a need to minimize the experience of pain during a window of critical central and peripheral nervous system development. Conversely, there is animal data which suggests that opiates given in the absence of pain may cause adverse cellular changes. In the rat, repeated morphine administration leads to long-term alterations in neurochemicals in the hippocampus5. Morphine administration for six consecutive days in neonatal rats leads to increased supraspinal neuronal apoptosis in distinct anatomic brain regions, namely the cortex and the amygdala6. The negative effects of long-term opiate treatment in the developing human brain are not currently understood outside of clinical manifestations of tolerance and physiologic dependence.
Although it is understood that pain cannot go untreated in the Neonatal ICU, the growing concern that long-term and high dose opiate therapy is likely not benign prompted our group to look more closely at trends in opiate exposure in a tertiary referral NICU over one decade. The aim of this study was to investigate changes in the use of analgesic-sedative therapy and the rates of iatrogenic NAS over time in critically ill infants over three time epochs: fiscal years 2003, 2007 and 2010.
Methods
Patients
This study was a retrospective cross sectional cohort study which included medical record extractions from fiscal years 2003–2004, 2007–2008 and 2010–2011 of all inborn infants admitted to Johns Hopkins Hospital with high risk diagnoses. After IRB review and approval, the billing office queried discharge diagnosis ICD-9 codes including 746.7 and 745.11 representing Hypoplastic Left Heart Syndrome (HLH) and Double Outlet Right Ventricle (DORV), 756.6 representing congenital diaphragmatic hernia (CDH), 747.83 representing persistent fetal circulation (PPHN), 756.73 and 756.72 representing gastroschisis and omphalocele (G/O), 765.21 and 765.22 representing 24 completed weeks of gestation and less than 24 completed weeks of gestation (<25 weeks). These diagnoses were chosen because they include infants who are likely to require longer duration of mechanical ventilation, have had major surgeries and multiple painful procedures and thus are likely to have received opiate treatment. The infants identified by diagnostic billing codes then underwent discharge summary review (in the electronic medical record (EMR)) to decide if they met inclusion criteria.
Inclusion criteria included all inborn infants at Johns Hopkins Hospital, carrying one of the afore-mentioned ICD-9 codes, and living for a minimum of seven days. Infants who died before seven days of life were excluded because they represent an extreme form of clinical severity that is not representative of the typical ICU infant. In addition, infants who were transferred to an outside hospital in less than seven days were excluded because the primary outcome of the study, chronic cumulative opiate exposure, could not be measured in these infants. Outborn infants were excluded because any opiate exposure at an outside institution or in transport would have been difficult to accurately quantify. In order to make the groups across time comparable, we also used disease-specific exclusion criteria. For HLH and DORV, the infant had to undergo open-heart surgery during their initial inpatient stay (including pulmonary artery banding). Infants discharged with no procedures or cardiac catheterizations alone were excluded. For PPHN, infants had to be full-term at birth, have cardiorespiratory failure requiring intubation but not necessarily nitric oxide therapy. Infants with only nasal canula, BiPAP or oxyhood therapy were excluded. Also, infants with PPHN associated with structural cardiac defect, genetic syndrome or pulmonary hypoplasia were excluded. The infants with severe secondary PPHN are not representative of the majority of this cohort who had transient PPHN precipitated by meconium aspiration syndrome, Hypoxic Ischemic Encephalopathy, or sepsis.
Chart Review
Every eligible patient chart was thoroughly reviewed and demographic data, length of stay and need for transfer to step-down facility, pertinent secondary medical diagnoses, surgical procedures, and need for Extra Corporeal Membrane Oxygenation (ECMO) were extracted. In addition, every dose of opiate written as either a one-time, standing intermittent, continuous infusion or continuous background infusion as part of Parent/Nurse-Controlled Analgesia (PNCA) orders was extracted and converted to morphine equivalents. Opiates extracted include morphine, fentanyl, hydromorphone, methadone and diluted tincture of opium (DTO). The conversion metrics used to convert non-morphine opiates to morphine equivalents are listed in Table 1. The medication orders were in electronic form for the 2010 cohort, but in paper form for two prior cohorts. One time or standing PRN orders or “as needed” opiate doses were excluded because the documentation was not always available in the paper charts.
Table 1. Opiate Conversions to Morphine Equivalents.
Although there are multiple published opiate conversion algorithms, for the purpose of this study, we chose the conversions used clinically in our NICU. We used the same conversions in all three time epochs to make drug exposure comparable. It is possible that with other conversion metrics the absolute numbers would be different, but the trend would be similar.
In addition to opiate orders, orders for other types of medications were extracted. These included one-time or standing orders for paralytics (vecuronium and pancuronium) and one-time or standing orders for benzodiazepines (midazolam, diazepam, lorazepam). In an effort to identify a group of medications that would indicate overall degree of intensity of medical intervention, it was decided that antimicrobials, and specifically days on antimicrobials, would be a marker of changes in medical intervention intensity over time. We chose antimicrobials as a “control” for medicalization because their use is potentially more resistant to secular trends than other markers of medical intervention such as days of ventilation or days of intravenous nutrition. Orders for ampicillin, gentamicin, cefotaxime, vancomycin, cefepime, clindamycin, piperacillin, amoxicillin, metronidazole, acyclovir, fluconazole, and amphotericin were extracted. For paralytics, benzodiazepines and antibiotics, total days treated with these medications were calculated for each patient.
For the purposes of data analysis, NAS was defined in two ways: 1) the presence of a billing code or discharge diagnosis of iatrogenically acquired NAS in the patient record, or 2) the need for weaning of opiate medications over > 1 day. NAS is measured with the Modified Finnegan Score in the NICU, and individual NAS scores were not extracted.
Statistical Analysis
The primary outcome is cumulative mg of morphine equivalent per infant. The per infant result is then transitioned to a population measure such as mean or median per time epoch. The secondary outcomes are the need for opiate weaning (a surrogate marker for NAS) and a discharge diagnosis of NAS. STATA version 10.0 was used for all statistical analyses. Exploratory data analysis was performed and then continuous variables were compared between groups with Kruskal-Wallis one-way analysis of variance. Categorical variables were compared using chi squared test. Univariate regression was then used to test if time epoch was a statistically significant predictor of cumulative Mg of morphine equivalents in both a linear (primary) and quintile (secondary) analysis. A p-value of less than 0.05 was considered statistically significant.
Results
Billing inquiry revealed 82, 68, and 88 unique patients for FYs 2003, 2007 and 2010 respectively. Please see Figure 1 for a flow diagram of inclusion and exclusion criteria. After multiple rounds of chart review, the final cohort for each year was finalized and the demographic information is presented in Table 2. The length of inpatient stay, the percentage of patients who underwent major surgery and who were placed on ECMO did not differ statistically between groups. Days treated with any antibiotics, a marker of overall medicalization, did not differ between groups (Figure 2). Although it did not reach statistical significance, there were more infants transferred to outside facilities while still treated with opiate medications in the later time period.
Figure 1. Study Participant Selection Workflow.
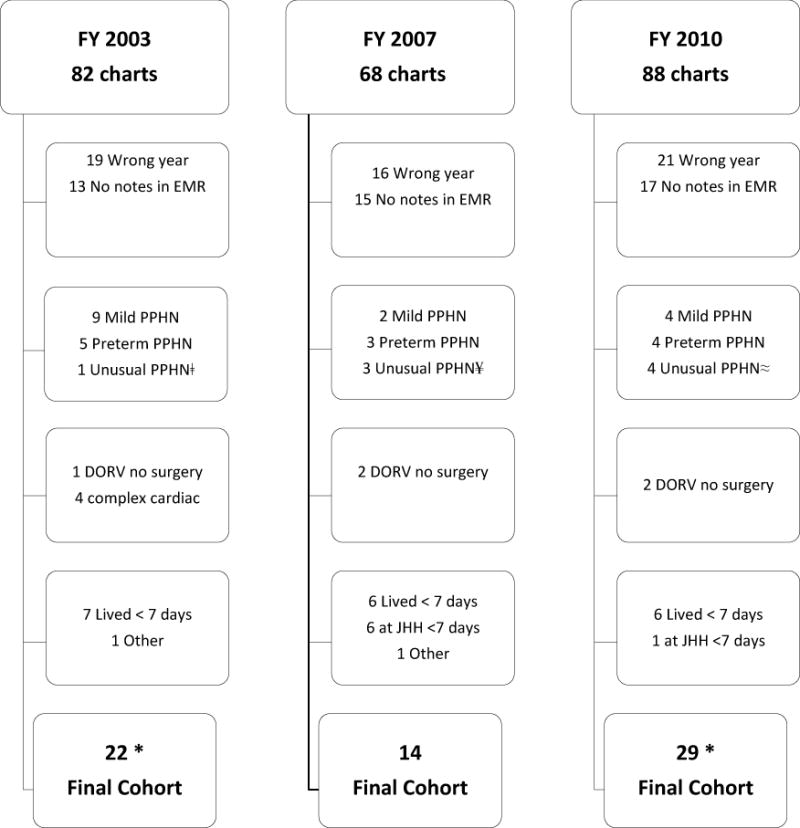
*One outlier excluded from final analysis from this group (see Results section)
ǂUnusual PPHN was a full-term infant with alveolar capillary dysplasia
¥Unusual PPHN were one full-term infant with PPHN after arterial switch for Transposition of the Great Arteries, one full-term infant with diagnosis of severe surfactant deficiency, and one infant with long-standing anhydramnios
≈Unusual PPHN were one infant with severe GU anomaly, oligohydramnios and pulmonary hypoplasia, one infant with critical coarct, one infant with trisomy 21 and AV canal and one infant with severe Pulmonary Stenosis
Table 2.
Infant Demographics
| FY 2003–2004 | FY 2007–2008 | FY 2010–2011 | P-Value | |
|---|---|---|---|---|
| Total Number Infants | 21 | 14 | 28 | |
| Primary Diagnosis, N (%) | 0.429 | |||
| GA < 25 weeks | 7 (33) | 7 (50) | 6 (21) | |
| PPHN | 6 (29) | 2 (14) | 6 (21) | |
| HLH/DORV | 5 (24) | 4 (29) | 6 (21) | |
| Gastroschisis/Omphalocele | 1 (5) | 0 | 4 (14) | |
| CDH | 2 (9) | 1 (7) | 6 (21) | |
| Birthweight, Median (IQR) | 2860 (800,3331) | 1450 (740,3045) | 2675 (1998,3285) | 0.554 |
| Inpatient Stay, days Mean (SD) Median (IQR) |
56.2 (48.3) 36 (25,87) |
60.6 (48.9) 43 (19,84) |
68.9 (46.8) 50 (34,99) |
0.412 |
| Thoracic/Abdominal50 (34,99) Surgery, N (%) |
14 (67) | 9 (65) | 24 (86) | 0.191 |
| ECMO, N (%) | 4 (19) | 1 (7) | 4 (14) | 0.615 |
| Days on Antibiotics Mean (SD) Median (IQR) |
26.8 (26.6) 15 (10,47) |
23.1 (20.6) 16.5 (8,39) |
31.9 (23.6) 22 (13,45) |
0.308 |
| Transferred on Opiate Medications, N (%) |
1 (5) | 2 (14) | 6 (21) | 0.256 |
| Died prior to hospital Discharge, N |
3 | 1 | 1 | 0.387 |
Inpatient stay does not include days spent at step-down facilities
Figure 2.
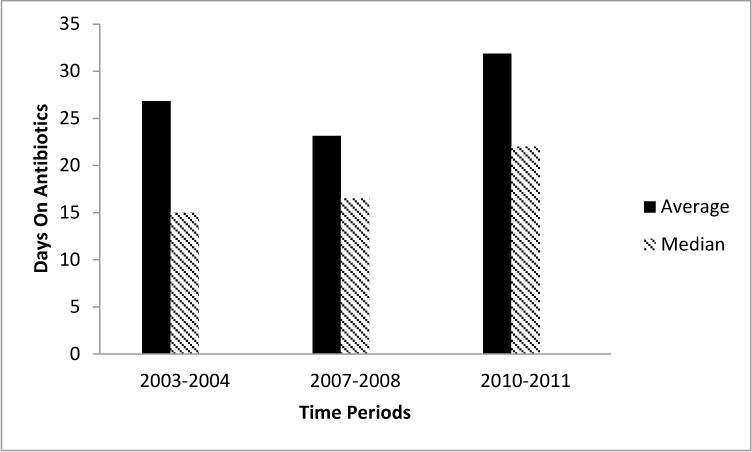
Days on Antibiotics by Time Period
The primary analysis of average cumulative opiate exposure modeled as a continuous variable in a linear regression did not reach statistical significance. On average, there was a 134 mg increase in opiate exposure for each subsequent time period (95% CI −12, 279, p-value 0.071). Because the data was not normally distributed with a strong right skew, we undertook a secondary analysis comparing the median exposures. This secondary analysis involved regression modeling of the median exposure per time epoch. The median cumulative opiate exposure per infant increased from 10 mg to 25 mg to 114 mg, and this was statistically significant with an average increase of 45 mg (95% CI 2.6, 88, p-value 0.038). Trends in cumulative opiate exposure are displayed in Figure 3.
Figure 3.
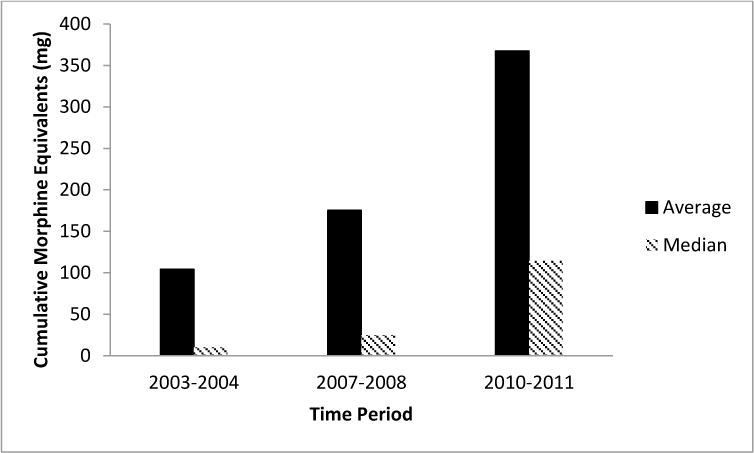
Cumulative Morphine Equivalents by Time Period
The percentage of infants who carried a discharge diagnosis of iatrogenic NAS significantly increased over the three time periods from 9% to 36% to 50% (p-value 0.012), commensurate with the increased opiate exposure. There were no statistically significant increases in days of paralytic or benzodiazepine exposure over the three time epochs (Table 3), suggesting that opiate exposure was the main difference in sedato-analgesic use. In addition, there was no significant increase in days of antimicrobial exposure, a metric we chose as a benchmark of overall therapeutic intensity.
Table 3.
Iatrogenic Medication Exposure and Neonatal Abstinence Syndrome
| β (95% CI) | FY 2003–2004 | FY 2007–2008 | FY 2010–2011 | p-value≠ | |
|---|---|---|---|---|---|
|
| |||||
| Cumulative Morphine Equivalents per Infant, Mg | |||||
| Mean (SD) | 104 (162) | 175 (470) | 367 (670) | ||
| linear regression | 134 (−12, 279) | 0.071 | |||
| Median (Range) | 10 (0–620) | 25 (0–1794) | 114 (0–2593) | ||
| quartile regression | 45 (2.6, 88) | 0.038 | |||
|
| |||||
| Days on Continuous Opiate Infusion | |||||
| Mean (SD) | 15.2 (27.5) | 17.0 (13.8) | 23.6 (22.3) | 0.094 | |
| Median (IQR) | 8 (0,18) | 18.5 (4,25) | 18 (7,38) | ||
|
| |||||
| Days Paralytics | |||||
| Mean (SD) | 1.5 (2.1) | 1.8 (5.0) | 3.1 (4.7) | 0.434 | |
| Median (IQR) | 1 (0,2) | 0 (0,1) | 1 (1,4.5) | ||
|
| |||||
| Days Benzodiazepine | |||||
| Mean (SD) | 13.3 (34.8) | 15.4 (17.2) | 18.8 (25.5) | 0.305 | |
| Median (IQR) | 3 (1,5) | 9 (1,30) | 8 (1,28) | ||
|
| |||||
| Ever Required Weaning of Opiate, N (%)* | 11 (53) | 10 (71) | 23 (82) | 0.068 | |
|
| |||||
| Discharge Diagnosis of NAS, N (%) | 2 (9) | 5 (36) | 14 (50) | 0.012 | |
Statistically significant p-values are displayed in bold.
There were three children who died before opiate weaning in the 2003–2004 cohort
There were two infants identified as outliers, both by statistical (undue leverage and Cooks D test) and clinical measures. These two infants both had cumulative exposures of over 5000 mg which is greater than 50 times the median in the highest exposure group. The first infant born in 2003 was a full-term infant with CDH and severe PPHN who required greater than two weeks of ECMO support, was treated for MRSA meningitis and received a Nissen/GT prior to discharge to step-down facility after an 102 day admission. The second infant born in 2010 was also full-term with CDH, severe PPHN who was treated with ECMO for > 2 weeks, and was transferred to step-down facility after an 102 day admission with continuing treatment with methadone, valium and clonidine. As a comparison, all other infants on ECMO in the cohorts were canulated for fewer than 7 days.
Discussion
This is the first study of which we are aware that attempts to quantify exact amounts of opiate exposure in the neonatal ICU population and to compare this exposure over time. The most important finding of this study is that medical opiate exposure is increasing over time in very high risk neonates in the ICU setting. Although Neonatal Infant Pain Scores (NIPS) were recorded during opiate therapy, they are not solely used to titrate medications because other factors including agitation, movement during wound healing with multiple invasive lines and tubes, endotracheal tube stability and synchronicity with mechanical ventilation, and oxygenation and ventilation status are often considered when titrating opiates. It is possible that pain control also improved concomitantly over this time period, but this is speculative as data on pain scores were not extracted from the charts for the reasons mentioned above. There were no systemic changes in pain management or pain protocols implemented during the time period studied. In addition, the practitioners in the NICU did not use pain treatment protocols or opiate weaning guidelines over the time period under study.
Although this study is limited to one tertiary care ICU, it is possible that this trend is more widespread than currently appreciated. The goal of this study is to provide a first glimpse into the cumulative amount of opiate received by an infant during an ICU stay. Because our study is limited in sample size and the data are not normally distributed, we were unable to perform meaningful regression analyses adjusting for all factors which might contribute to increasing opiate therapy, so definitive conclusions cannot be made until this study is replicated in a larger cohort. The sharp increase in cumulative opiate exposure is supportive of the impression among physicians that we are seeing an increase in the number and severity of iatrogenic Neonatal Abstinence Syndrome. These infants with prolonged, high dose opiate exposures become physiologically dependent and have withdrawal once weaning is initiated, leading to prolonged inpatient and, at times, outpatient weaning programs.
One prior study has investigated medical opiate exposure in neonates weighing less than 1500 grams who were ventilated from day of life one across six different NICUs7. Unlike our study which aims to quantify cumulative exposure, this prior study addressed exposure as binary on three different hospital days. Opiate exposure varied by birth weight, illness severity and site. There was a 28-fold variation in opiate administration between the six study sites.
Sedato-analgesic medications are an important part of ICU care. Studies have shown decreased markers of physiologic stress8, improved hemodynamic stability9 and improved synchrony with the ventilator10, but these are all short-term outcomes and the long term effects of substantial opiate exposure in the immediate neonatal period are unknown. Given the lack of long-term studies, medical opiate exposure should be closely examined and efforts to curtail further increases may be warranted. The increase in exposure could be due to many clinical possibilities. For example, there could be secular trends in using more opiates for sedation, aggressiveness of opiate weaning, or tolerance of withdrawal symptoms vs markedly slow and prolonged weans in an effort to keep infants symptom free. Although we were unable to explain the etiology behind the increase in medical opiate exposure with this retrospective study, we still feel this is a trend worth discussing. Both clinical and research paradigms that are geared towards a lowest effective dose strategy and incorporate a multi-modal pain control approach might lessen the exposure to opiate medications. One barrier to weaning opiate medications is reliance on withdrawal scores which have not been well validated in sick, ventilated and post-operative infants. In addition, there are few well-validated sedation scores in the neonatal population, the use of which may prompt clinicians to recognize over-sedation or over-narcotization and wean opiate medications more readily.
There are potential confounders to our study results. Although not statistically significant, there was an increased length of stay, increased use of paralytics to medically manage, and an increased rate of thoracic or abdominal surgery over time, all of which could partially account for the increase in exposure to opiates. Despite these potentially clinically meaningful trends, the degree of increase in opiate exposure over time cannot be fully explained by these factors. A multimodal approach to pain and sedation therapy and to minimizing opiate withdrawal symptoms often includes use of a benzodiazepine. We considered that the decreased use of adjunct benzodiazepines for pain and sedation might explain the increase in opiate exposure we observed, but there was no difference in the days of benzodiazepine exposure over time in our data. The measure of benzodiazepine exposure we chose to extract from the charts was “days of exposure” as opposed to cumulative doses, and it is possible that if we had assessed benzodiazepines in a more granular fashion, the increase in opiate exposure would be explained by a decrease in cumulative benzodiazepine exposure.
There are limitations to our study. In regards to opiate, benzodiazepine and paralytic exposure, the charts were extracted for standing doses only – meaning that due to heterogeneity and missing data in charting of PRNs over the three time epochs, we chose not to include PRNs in our calculations of exposure. A potential interpretation of our results is that in the earlier time epochs, more PRN opiate doses may have been used instead of standing orders or infusions. In addition, other non-opiate medications used for pain control were not extracted (i.e. NSAIDs, Acetaminophen), so it is possible that a reverse trend in non-opiate use could explain the rise in medical opiate use. Lastly, the increase in opiate exposure could partially be explained by changes in prescribing patterns, i.e. doctors using more methadone in later epochs and using more lengthy opiate weaning strategies. Even if these potential scenarios were the case, such a sharp increase in exposure to opiates from any form of standing orders and infusions is still worth investigating.
Regarding secondary endpoints, there was no very clear way to retrospectively capture every infant who manifested signs of physical opiate dependence, so we chose to measure both rates of diagnosis of NAS and also the number of infants who required weaning of their medications. These are imperfect measures and we acknowledge that weaning of opiates may be a preventative strategy to mitigate signs of NAS. Additionally, because we did not analyze cumulative benzodiazepine exposure, it is possible that the increase in NAS observed in these infants was a result of a combined withdrawal from increasing opiates and benzodiazepines. There are issues with current methods of quantifying withdrawal from medical opiate exposure in newborns. First, many scoring tools used in the NICU11,12 are only validated for withdrawal from in utero opiate exposure but are used because the clinical syndromes of withdrawal are thought to be sufficiently similar. Second, there are scoring tools validated for medical opiate and benzodiazepine withdrawal in pediatrics13, but they are not well studied in infants less than 6 months of age.
It is also possible that opiate exposure is increasing over time because the care is “more intensive” overall. In an effort to measure intensity of care, we collected the number of days on the most commonly used antibiotics and antifungals. There was no significant change in antibiotic exposure in the time frame studied. This could imply that opiate exposure is increasing not as a function of overall increased medicalization, but as a unique entity.
Although the increase in discharge diagnosis of NAS could be due to changes in accuracy of billing or increased recognition of this syndrome, given the increase in opiate exposure, it is more likely secondary to a true increase in the incidence of physical withdrawal behaviors. The fact that the cumulative opiate exposure per infant during the inpatient stay rose despite the fact that an increasing number of infants were transferred to outside hospitals still treated with opiates is a testament to the overall increasing opiate exposure.
Conclusion
Although analgesia with opiates is important in the most critically ill neonates, it may be time to readdress our current sedative-analgesic practices and move towards more aggressive attempts to limit the amount and duration of opiate exposure in the ICU. Studies which address long-term neurodevelopmental outcomes stratified by opiate exposure are difficult due to many confounding factors, but novel techniques for monitoring medical opiate exposure and identifying children with the highest cumulative doses might help inform future developmental studies.
Acknowledgments
All phases of this study were supported by an NIH grant, 5T32GM066691
Abbreviations
- NAS
Neonatal Abstinence Syndrome
- NICU
Neonatal Intensive Care Unit
Footnotes
Contributor’s Statements:
Tamorah Lewis, MD: Dr. Lewis conceptualized and designed the study, collected the data, analyzed the data, drafted the initial manuscript, and approved the final manuscript as submitted.
Betty Erfe, BS and Tarrah Ezell, BS: Ms Erfe and Ms Ezell participated in data collection, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Estelle Gauda, MD: Dr Gauda oversaw the conceptualization and design of the study, critically reviewed the manuscript, and approved the final manuscript as submitted.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1.Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. The New England journal of medicine. 1987;317(21):1321–1329. doi: 10.1056/NEJM198711193172105. [DOI] [PubMed] [Google Scholar]
- 2.Anand KJ, Willson DF, Berger J, et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125(5):e1208–1225. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laprairie JL, Johns ME, Murphy AZ. Preemptive morphine analgesia attenuates the long-term consequences of neonatal inflammation in male and female rats. Pediatr Res. 2008;64(6):625–630. doi: 10.1203/PDR.0b013e31818702d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valeri BO, Holsti L, Linhares MB. Neonatal Pain and Developmental Outcomes in Children Born Preterm: A Systematic Review. The Clinical journal of pain. 2014 doi: 10.1097/AJP.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 5.Rozisky JR, Laste G, de Macedo IC, et al. Neonatal morphine administration leads to changes in hippocampal BDNF levels and antioxidant enzyme activity in the adult life of rats. Neurochemical research. 2013;38(3):494–503. doi: 10.1007/s11064-012-0941-8. [DOI] [PubMed] [Google Scholar]
- 6.Bajic D, Commons KG, Soriano SG. Morphine-enhanced apoptosis in selective brain regions of neonatal rats. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2013;31(4):258–266. doi: 10.1016/j.ijdevneu.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn DJ, Richardson DK, Gray JE, et al. Variation among neonatal intensive care units in narcotic administration. Archives of pediatrics & adolescent medicine. 1998;152(9):844–851. doi: 10.1001/archpedi.152.9.844. [DOI] [PubMed] [Google Scholar]
- 8.Gitto E, Pellegrino S, Manfrida M, et al. Stress response and procedural pain in the preterm newborn: the role of pharmacological and non-pharmacological treatments. European journal of pediatrics. 2012;171(6):927–933. doi: 10.1007/s00431-011-1655-7. [DOI] [PubMed] [Google Scholar]
- 9.Anand KJ, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. The New England journal of medicine. 1992;326(1):1–9. doi: 10.1056/NEJM199201023260101. [DOI] [PubMed] [Google Scholar]
- 10.Ancora G, Garetti E, Pirelli A, et al. Analgesic and sedative drugs in newborns requiring respiratory support. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(Suppl 4):88–90. doi: 10.3109/14767058.2012.715036. [DOI] [PubMed] [Google Scholar]
- 11.Finnegan LP, Connaughton JF, Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addictive diseases. 1975;2(1–2):141–158. [PubMed] [Google Scholar]
- 12.Zahorodny W, Rom C, Whitney W, et al. The neonatal withdrawal inventory: a simplified score of newborn withdrawal. Journal of developmental and behavioral pediatrics: JDBP. 1998;19(2):89–93. doi: 10.1097/00004703-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Franck LS, Harris SK, Soetenga DJ, Amling JK, Curley MA. The Withdrawal Assessment Tool-1 (WAT-1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2008;9(6):573–580. doi: 10.1097/PCC.0b013e31818c8328. [DOI] [PMC free article] [PubMed] [Google Scholar]


