Abstract
Objectives
The objective of this study was to attain a better understanding of infectious diseases (ID) physicians' experience with MDR organism (MDRO) urinary tract infections (UTIs) by means of a survey on disease perception, diagnostic management and treatment preferences.
Methods
A nine-question survey was developed and distributed to members of the North American Emerging Infections Network (EIN) in September 2013.
Results
Seven hundred and fourteen out of 1461 EIN members responded to the survey (49%). The responses of 603 responders were studied. Most providers perceived an increase in the incidence of MDRO UTIs over the past 3 years (75% of adult ID responders and 63% of paediatric ID responders). One hundred and thirty-four (22%) responders prefer intravenous over oral administration of antimicrobials when both are available, 171 (28%) prefer longer durations of therapy when comparing an MDRO with a susceptible isolate of the same species and 142 (24%) order a repeat urine culture as ‘proof of cure’ after treating an MDRO UTI. Nevertheless, 530 (88%) responders perceived MDRO UTIs to be of similar severity as non-MDRO UTIs. Fifty-five percent of providers prescribed fosfomycin for MDRO UTI at least once; the most common prescribing pattern (among a wide spectrum of approaches) was a single dose (16%).
Conclusions
Future studies on MDRO UTIs should clarify the role of resistance in patient outcomes and the comparative efficacy of different antimicrobials. Of particular interest is fosfomycin, which is unrelated to other antibiotic classes and may take a more prominent role in treating MDRO cystitis.
Introduction
Urinary tract infections (UTIs) involving MDR organisms (MDROs) appear to be increasing.1,2 There is little guidance on how to best manage MDRO UTIs as many aspects of clinical care, including when to initiate empirical treatment that covers MDROs, which antimicrobial to select and the preferable route of administration, and optimal treatment duration have not been thoroughly studied.3 The objective of this survey was to better understand infectious diseases (ID) physicians' perceptions and behaviours regarding MDRO UTIs using the CDC-funded, IDSA-hosted Emerging Infections Network (EIN), a provider-based sentinel network.4 We wanted to determine: (i) perceived trends of MDRO UTIs; (ii) the perceived severity of these infections; (iii) preferred antimicrobials, route of administration and treatment duration; and (iv) experience with fosfomycin for MDRO UTIs.
Methods
The EIN is an online network established through a Cooperative Agreement Program Award from the CDC. It consists of physicians who practice adult and/or paediatric ID and belong to the IDSA and/or the Pediatric Infectious Diseases Society.
A nine-question survey was developed through collaboration between EIN leadership (S. E. B. and P. M. P.) and the Division of Infectious Diseases at Washington University School of Medicine (all other authors). It was distributed to 1461 EIN members on 9 September 2013 (with two follow-up reminders for non-responders). For the survey, we defined multidrug resistance as resistance to at least three antibiotic classes (unless specified otherwise). The generic ‘UTI’ was intended to encompass lower and upper UTI. Survey questions 1–3 focused on perceived incidence of MDRO UTIs, commonly encountered pathogens and microbiological information routinely available. Questions 4 and 5 addressed diagnostic and management preferences for MDRO UTIs. Question 6 concerned the perceived likelihood of clinical resolution with appropriate therapy for specific organisms, and questions 7 and 8 concerned the preferences for antimicrobial selection and treatment duration for specific pathogens. Question 9 was about the experience of respondents utilizing fosfomycin for the treatment of MDRO UTIs. Fosfomycin, recommended as first-line therapy in uncomplicated UTI, was specifically addressed due to its perceived underutilization and the potential use in MDRO UTIs. No institutional review board review was required for this survey.
Results
Overall, 714 (49%) of 1461 EIN members responded to the survey. In terms of professional experience in ID, responders had <5 years (18%), 5–14 years (28%), 15–24 years (25%) or ≥25 years (28%) of experience. Providers were from all geographical areas, including the South Atlantic (18%), Pacific (18%), East North Central (15%) and Mid-Atlantic (14%). The most common employers were universities/medical schools (40%), followed by private practice groups (28%), hospitals/clinics (26%), Veteran Affairs/military (6%) and state government (<1%). The responders represent adult ID (74%), paediatric ID (22%) or combined adult/paediatric ID (4%). Significantly more paediatric than adult ID physicians reported not having managed MDRO UTIs during their last 3 years of clinical practice (25% versus 13%; P = 0.001). We excluded these 111 (16%) ‘non-exposed’ responders from subsequent analyses and studied the responses of the remaining 603 providers (the EIN report with full results is available as Supplementary data at JAC Online).
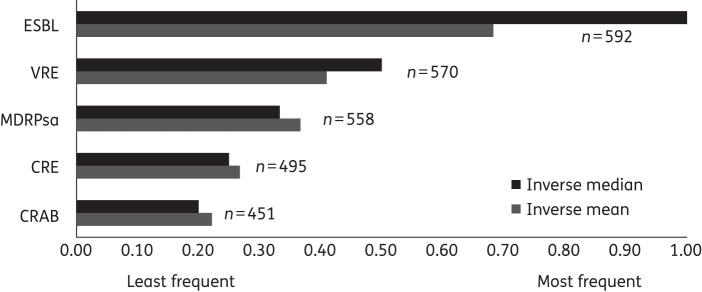
Most adult and paediatric ID providers perceived an increase in the incidence of MDRO UTIs over the past 3 years (75% and 63%, respectively). The most frequently encountered MDROs were reported to be ESBL-producing Enterobacteriaceae, followed by VRE, MDR Pseudomonas aeruginosa (MDRPsa), carbapenem-resistant Enterobacteriaceae (CRE) and carbapenem-resistant Acinetobacter baumannii (CRAB) (Figure 1). When eliciting the practices of the local clinical laboratories used by respondents, most screen for and report VRE (576, 96%), ESBL-producing organisms (573, 95%) and carbapenemase producers (486, 81%).
Figure 1.
Provider ranking of the most frequently encountered organisms in MDRO UTIs. Inverse median and inverse mean = the median and mean values were inverted 1/x to yield higher values for more important criteria; n = number of respondents for each category of antibiotic-resistant organisms shown. ESBL, ESBL-producing Enterobacteriaceae.
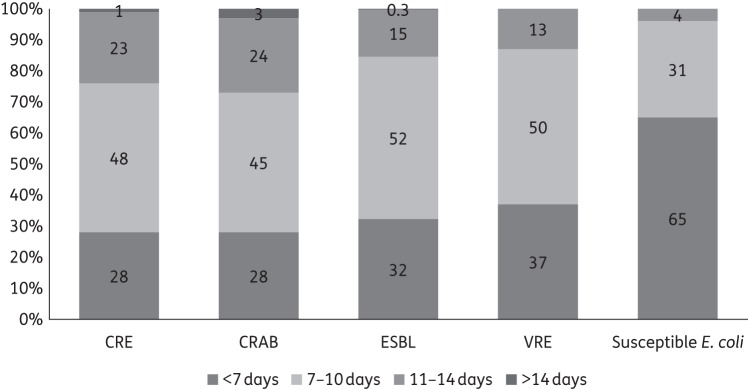
Regarding management preferences, 134 (22%) responders prefer intravenous over oral antibiotic administration when both are available, 171 (29%) prefer longer treatment durations when comparing an MDRO with a susceptible isolate of the same species and 142 (24%) order repeat urine cultures as ‘proof of cure’ after treating MDRO UTIs. To inform treatment decisions, 568 (94%) providers routinely use urinalysis results in addition to culture findings; 581 (96%) do not feel compelled to administer antibiotics if there is low suspicion for infection (i.e. asymptomatic bacteriuria with MDRO); and 530 (88%) believe MDRO UTIs are not more severe than non-MDRO infections. The likelihood of clinical resolution with appropriate therapy was perceived as high for all organisms: susceptible Escherichia coli (>99%), ESBL producers (99%), VRE (95%), MDRPsa (90%), CRE (90%) and CRAB (81%). When asked about the preferred duration of therapy specifically for lower UTI, 65% reported treating susceptible E. coli for <7 days, while the majority preferred 7–14 days for CRE (71%), CRAB (69%), ESBL producers (67%) and VRE (63%) (Figure 2).
Figure 2.
Preferred treatment duration for lower UTI due to different organisms. ESBL, ESBL-producing Enterobacteriaceae.
We then solicited the most frequently prescribed antibiotic for specific uropathogens while explicitly stating an absence of drug allergies, interacting medications and renal insufficiency. The first choice for UTIs caused by susceptible E. coli was trimethoprim/sulfamethoxazole (49%); for ESBL producers and MDRPsa, it was carbapenems (55% and 41%, respectively); for CRE, it was an aminoglycoside (28%) or fosfomycin (25%); and for VRE, it was linezolid (66%). For CRAB UTI, providers primarily selected an antibiotic ‘other’ (48%) than the ones offered (also including fluoroquinolones, nitrofurantoin and doxycycline).
Regarding clinical experience with fosfomycin for the treatment of MDRO UTIs, 332 (55%) providers reported prescribing fosfomycin at least once in this setting. The most common prescribing pattern was a single dose (16%), followed by 3 g once every 3 days for a total of 9 days (9%), 6 days (7%) or 3 days (7%). Success rates with fosfomycin for this indication were thought to be >50% by 282 (88%) responders, >75% by 188 (59%) and >90% by 63 (20%).
Discussion
Infections due to MDROs are on the rise across the globe and pose significant problems. Yet, we lack treatment guidelines given the limited evidence from comparative research. This is also true for UTIs, the most frequent bacterial infection in humans.5 For MDRO UTIs, there are a handful of studies with older drugs such as fosfomycin6 and newer options such as ceftazidime/avibactam;7 however, no additional, randomized studies are available for guidance. In this survey of ID physicians, responders perceived the frequency of MDRO UTIs to be increasing and revealed significant variation in their management approaches.
Interestingly, MDRO UTIs were perceived as similar in severity to UTIs caused by susceptible organisms. Some data suggest that the impact of resistance on clinical presentation and outcomes is not substantial in the case of UTIs.8,9 Consequently, one would expect treatment preferences to be similar for infections with resistant or susceptible organisms. However, about one-quarter of providers reported preferring intravenous over oral medication (assuming both options are available), choosing longer durations and obtaining ‘proof of cure’ cultures at the end of treatment. None of these practices is supported by evidence, but they may reflect the difficulties physicians encounter when treating non-UTI MDRO infections (or their anxiety when facing MDROs). To our knowledge, this mismatch between perceived clinical impact and preferred management approach has never been reported in the scientific literature. Currently, it is unknown whether longer treatment durations are indeed necessary for MDRO UTIs.
There is limited experience with fosfomycin as a treatment option for MDRO UTIs in the EIN and, apparently, many never used it at all. Fosfomycin is unrelated to other antibiotic classes, retaining its efficacy when organisms have acquired resistance to more commonly used antimicrobials. In the major treatment guideline for uncomplicated UTIs, Gupta et al.10 list fosfomycin as one option and laud it for being well-tolerated and largely unaffected by resistance problems. Moreover, in two studies, clinical success with fosfomycin for ESBL producers exceeded 90%.6,11 It is unclear, however, how often fosfomycin is utilized by ID physicians. From our survey, it appears to be regarded as a second-line treatment option, with fosfomycin resistance testing not readily available and the ID community uncertain about the best dosing regimen. While there is an established dose for uncomplicated cystitis, utility and dosage for complicated UTIs have not been determined and should be considered ‘off-label’.12 We hypothesize that one barrier to fosfomycin use is the longstanding quinolone and sulphonamide preference for UTI treatment. Suboptimal guideline adherence is another potential cause for low uptake of fosfomycin and is well described.13,14
A major limitation of surveys is that responses are self-reported perceptions and behaviours; thus, findings may not necessarily reflect how patients are managed in real life. Our questions did not differentiate between the selection of empirical versus targeted therapy or consider the effect of cross-resistance in the organisms involved. The survey addressed UTIs in general and only focused on lower UTI in one question on treatment duration. Furthermore, while the sample size of 603 participants was large, it may not be representative of the North American ID community. Findings should not be extrapolated to other geographical areas with different UTI management strategies.
In conclusion, there is a mismatch between perceived clinical severity of MDRO UTIs (i.e. no more severe than regular UTIs) and preferred treatment approach (i.e. longer than regular UTIs and via the intravenous route). Future studies on MDRO UTIs should clarify the role of resistance in patient outcomes and the comparative efficacy of different antimicrobials and/or durations. Among the treatment options of particular interest is fosfomycin, which may take a more prominent role in treating MDRO cystitis.
Funding
This work was supported by the National Institutes of Health (KL2RR024994 and KL2TR000450 to J. M.; KM1CA156708-01, UL1 TR000448, KL2 TR000450 and TL1 TR000449 to M. A. L.), the Burroughs-Wellcome Fund Career Award for Medical Scientists (to J. P. H.), the NIH (R01DK099534 to J. P. H.), the CDC Prevention Epicenters Program (#5U54CK000162 to J. M. and H. M. B.) and the CDC Emerging Infections Network (5U50CK000187 to P. M. P.). In addition, J. M. was supported by the Barnes-Jewish Hospital Patient Safety & Quality Fellowship Program.
Transparency declarations
None to declare.
Disclaimer
The work's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or CDC.
Supplementary data
Acknowledgements
We appreciate the time and effort dedicated by all EIN members who participated in this survey. We also thank the EIN Executive Committee for valuable suggestions during the design process of the survey tool.
References
- 1.Fan NC, Chen HH, Chen CL et al. . Rise of community-onset urinary tract infection caused by extended-spectrum β-lactamase-producing Escherichia coli in children. J Microbiol Immunol Infect 2014; 47: 399–405. [DOI] [PubMed] [Google Scholar]
- 2.Zilberberg MD, Shorr AF. Secular trends in Gram-negative resistance among urinary tract infection hospitalizations in the United States, 2000–2009. Infect Control Hosp Epidemiol 2013; 34: 940–6. [DOI] [PubMed] [Google Scholar]
- 3.Meier S, Weber R, Zbinden R et al. . Extended-spectrum β-lactamase-producing Gram-negative pathogens in community-acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection 2011; 39: 333–40. [DOI] [PubMed] [Google Scholar]
- 4.Pillai SK, Beekmann SE, Santibanez S et al. . The Infectious Diseases Society of America Emerging Infections Network: bridging the gap between clinical infectious diseases and public health. Clin Infect Dis 2014; 58: 991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pallett A, Hand K. Complicated urinary tract infections: practical solutions for the treatment of multiresistant Gram-negative bacteria. J Antimicrob Chemother 2010; 65 Suppl 3: iii25–33. [DOI] [PubMed] [Google Scholar]
- 6.Pullukcu H, Tasbakan M, Sipahi OR et al. . Fosfomycin in the treatment of extended spectrum β-lactamase-producing Escherichia coli-related lower urinary tract infections. Int J Antimicrob Agents 2007; 29: 62–5. [DOI] [PubMed] [Google Scholar]
- 7.Vazquez JA, Gonzalez Patzan LD, Stricklin D et al. . Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin 2012; 28: 1921–31. [DOI] [PubMed] [Google Scholar]
- 8.Khair HN, VanTassell P, Henderson JP et al. . Vancomycin resistance has no influence on outcomes of enterococcal bacteriuria. J Hosp Infect 2013; 85: 183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dayan N, Dabbah H, Weissman I et al. . Urinary tract infections caused by community-acquired extended-spectrum β-lactamase-producing and nonproducing bacteria: a comparative study. J Pediatr 2013; 163: 1417–21. [DOI] [PubMed] [Google Scholar]
- 10.Gupta K, Hooton TM, Naber KG et al. . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52: e103–20. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Bano J, Alcala JC, Cisneros JM et al. . Community infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Arch Intern Med 2008; 168: 1897–902. [DOI] [PubMed] [Google Scholar]
- 12.Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant gram-negative bacterial infections. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2014; 34: 845–57. [DOI] [PubMed] [Google Scholar]
- 13.Garcin F, Leone M, Antonini F et al. . Non-adherence to guidelines: an avoidable cause of failure of empirical antimicrobial therapy in the presence of difficult-to-treat bacteria. Intensive Care Med 2010; 36: 75–82. [DOI] [PubMed] [Google Scholar]
- 14.van der Velden LB, Tromp M, Bleeker-Rovers CP et al. . Non-adherence to antimicrobial treatment guidelines results in more broad-spectrum but not more appropriate therapy. Eur J Clin Microbiol Infect Dis 2012; 31: 1561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.