Abstract
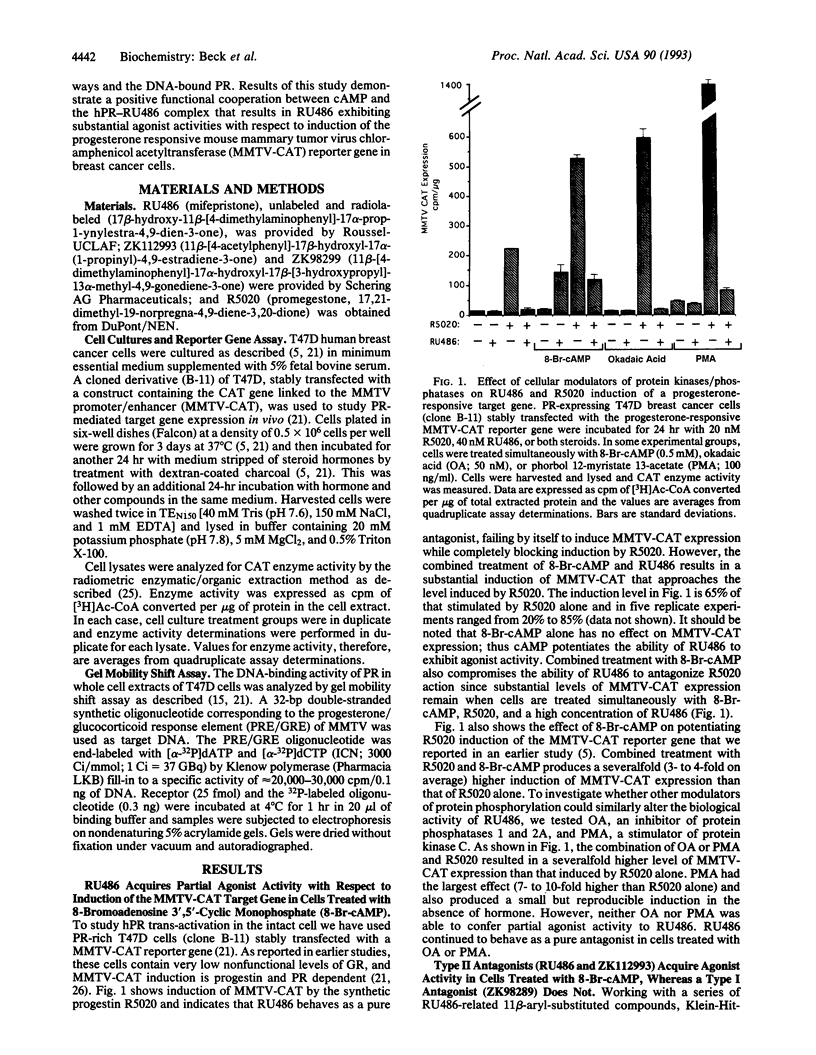
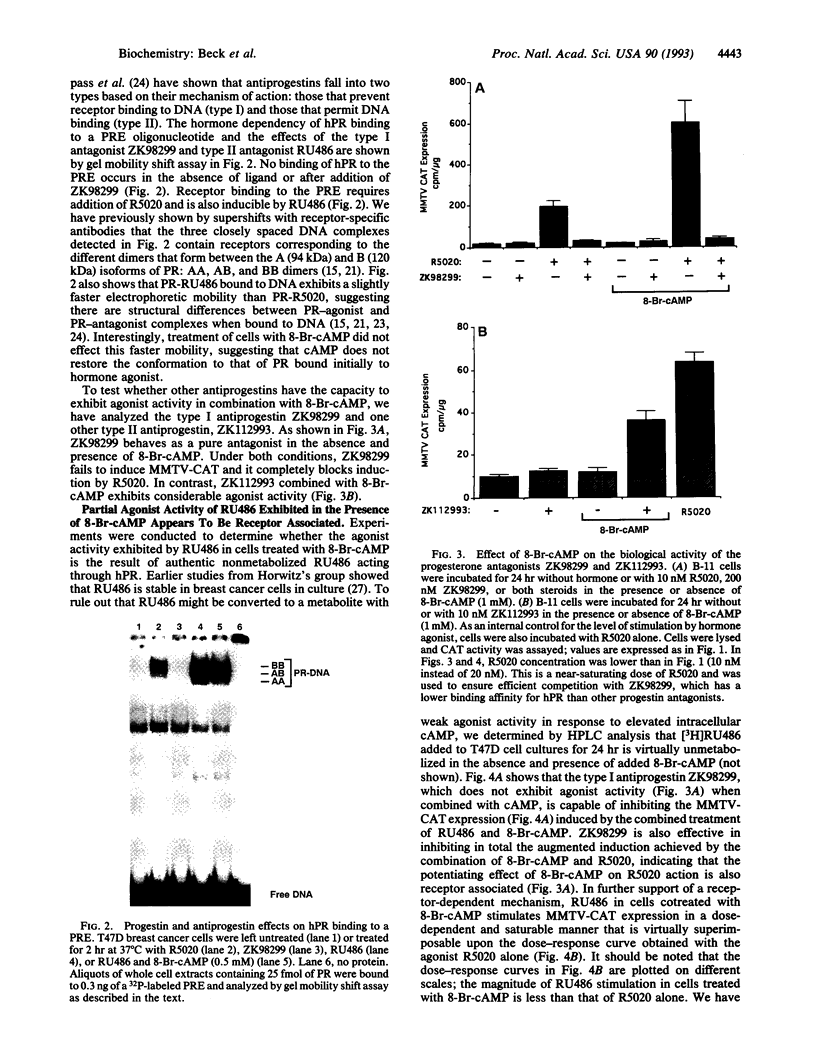
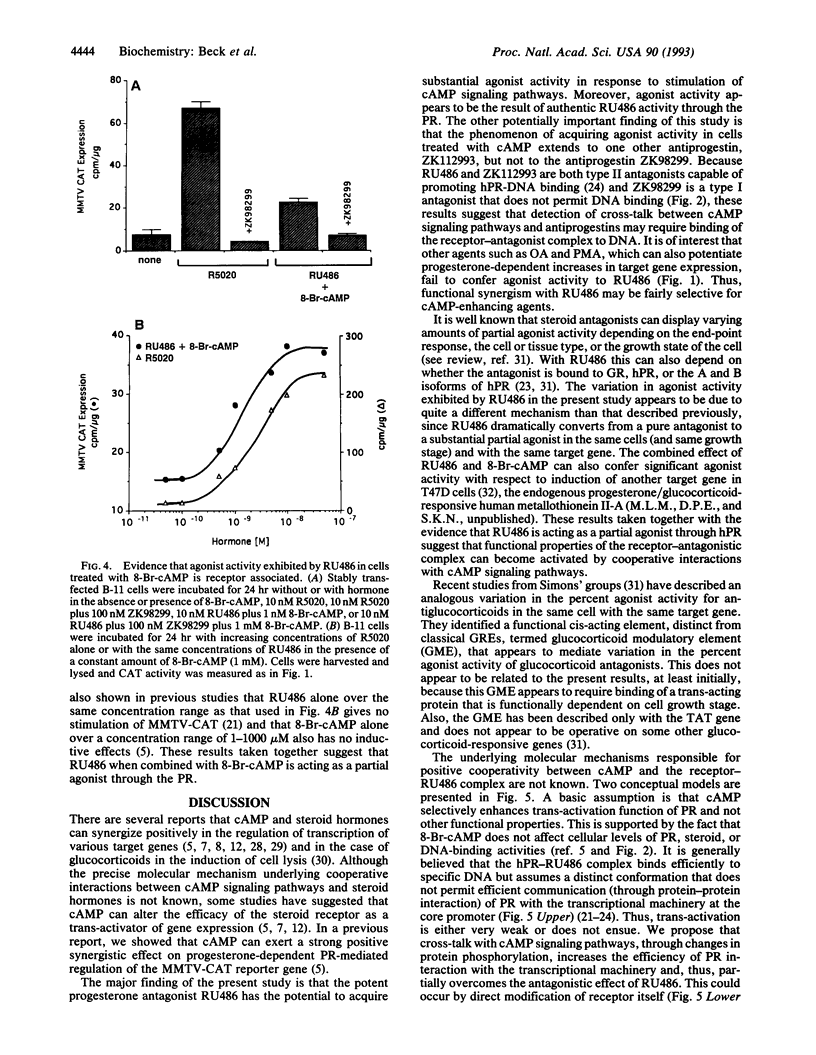
The protein kinase A stimulator cAMP can potentiate the ability of progestins to induce the transactivation function of the human progesterone receptor (hPR). We questioned in the present study whether cAMP could functionally cooperate with the progestin antagonist RU486. In T47D human breast cancer cells, RU486 behaves as a pure antagonist with respect to induction of the progesterone-responsive mouse mammary tumor virus chloramphenicol acetyltransferase (MMTV-CAT) reporter gene. It fails to stimulate MMTV-CAT expression and completely inhibits induction by the synthetic progestin R5020. However, when RU486 is combined with 8-bromoadenosine 3',5'-cyclic monophosphate (8-Br-cAMP), MMTV-CAT is induced to levels approaching that stimulated by R5020 alone. Also, RU486 in the presence of 8-Br-cAMP is only partially effective in antagonizing R5020 action. The agonist activity exhibited under these conditions appears to be due to RU486 acting through hPR as evidenced by the fact that 8-Br-cAMP alone has no effect on MMTV-CAT, whereas induction by the combination of 8-Br-cAMP and RU486 is dose responsive to RU486 in a saturable manner and can be inhibited by the type I antiprogestin (prevents hPR-DNA binding) ZK98299, which does not exhibit positive functional cooperation with cAMP. Acquisition of agonist activity in the presence of 8-Br-cAMP also extends to the type II antiprogestin (permits hPR-DNA binding) ZK112993. Since RU486 is also a type II antagonist, these results suggest that detection of functional synergism between cAMP and antiprogestins may require binding of the hPR-antagonist complex to DNA. We propose that cross-talk between second messenger and steroid receptor signal transduction pathways may be one mechanism for resistance to steroid antagonists that frequently develops in breast cancer.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronica S. M., Katzenellenbogen B. S. Progesterone receptor regulation in uterine cells: stimulation by estrogen, cyclic adenosine 3',5'-monophosphate, and insulin-like growth factor I and suppression by antiestrogens and protein kinase inhibitors. Endocrinology. 1991 Apr;128(4):2045–2052. doi: 10.1210/endo-128-4-2045. [DOI] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Ligand and DNA-dependent phosphorylation of human progesterone receptor in vitro. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2664–2668. doi: 10.1073/pnas.89.7.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Progesterone enhances target gene transcription by receptor free of heat shock proteins hsp90, hsp56, and hsp70. Mol Cell Biol. 1991 Oct;11(10):4998–5004. doi: 10.1128/mcb.11.10.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. A., Weigel N. L., Edwards D. P. Effects of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding, and phosphorylation of human progesterone receptors. Mol Endocrinol. 1992 Apr;6(4):607–620. doi: 10.1210/mend.6.4.1316549. [DOI] [PubMed] [Google Scholar]
- Carson-Jurica M. A., Schrader W. T., O'Malley B. W. Steroid receptor family: structure and functions. Endocr Rev. 1990 May;11(2):201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- Curtis S. W., Korach K. S. Uterine estrogen receptor interaction with estrogen-responsive DNA sequences in vitro: effects of ligand binding on receptor-DNA complexes. Mol Endocrinol. 1990 Feb;4(2):276–286. doi: 10.1210/mend-4-2-276. [DOI] [PubMed] [Google Scholar]
- DeMarzo A. M., Oñate S. A., Nordeen S. K., Edwards D. P. Effects of the steroid antagonist RU486 on dimerization of the human progesterone receptor. Biochemistry. 1992 Nov 3;31(43):10491–10501. doi: 10.1021/bi00158a012. [DOI] [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Maxwell B. L., Schrader W. T., O'Malley B. W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990 Dec 21;250(4988):1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- Denton R. R., Koszewski N. J., Notides A. C. Estrogen receptor phosphorylation. Hormonal dependence and consequence on specific DNA binding. J Biol Chem. 1992 Apr 15;267(11):7263–7268. [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M. Regulation of gene expression by the thyroid hormone receptor. Biochim Biophys Acta. 1990 Dec 11;1032(2-3):157–176. doi: 10.1016/0304-419x(90)90002-i. [DOI] [PubMed] [Google Scholar]
- Gruol D. J., Rajah F. M., Bourgeois S. Cyclic AMP-dependent protein kinase modulation of the glucocorticoid-induced cytolytic response in murine T-lymphoma cells. Mol Endocrinol. 1989 Dec;3(12):2119–2127. doi: 10.1210/mend-3-12-2119. [DOI] [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Ragot T., Bailly A., Atger M., Misrahi M., Perricaudet M., Milgrom E. Receptors bound to antiprogestin from abortive complexes with hormone responsive elements. Nature. 1988 Dec 15;336(6200):695–698. doi: 10.1038/336695a0. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Pike A. W., Gonzalez-Aller C., Fennessey P. V. Progesterone metabolism in T47Dco human breast cancer cells--II. Intracellular metabolic path of progesterone and synthetic progestins. J Steroid Biochem. 1986 Dec;25(6):911–916. doi: 10.1016/0022-4731(86)90323-7. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. The molecular biology of RU486. Is there a role for antiprogestins in the treatment of breast cancer? Endocr Rev. 1992 May;13(2):146–163. doi: 10.1210/edrv-13-2-146. [DOI] [PubMed] [Google Scholar]
- Ignar-Trowbridge D. M., Nelson K. G., Bidwell M. C., Curtis S. W., Washburn T. F., McLachlan J. A., Korach K. S. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan V. C., Murphy C. S. Endocrine pharmacology of antiestrogens as antitumor agents. Endocr Rev. 1990 Nov;11(4):578–610. doi: 10.1210/edrv-11-4-578. [DOI] [PubMed] [Google Scholar]
- Joshi J., Sabol S. L. Proenkephalin gene expression in C6 rat glioma cells: potentiation of cyclic adenosine 3',5'-monophosphate-dependent transcription by glucocorticoids. Mol Endocrinol. 1991 Aug;5(8):1069–1080. doi: 10.1210/mend-5-8-1069. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Cato A. C., Henderson D., Ryffel G. U. Two types of antiprogestins identified by their differential action in transcriptionally active extracts from T47D cells. Nucleic Acids Res. 1991 Mar 25;19(6):1227–1234. doi: 10.1093/nar/19.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. E., Pornon A., Ji J. W., Bocquel M. T., Chambon P., Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990 Dec;9(12):3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen S. K., Green P. P., 3rd, Fowlkes D. M. A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA. 1987 Apr;6(2):173–178. doi: 10.1089/dna.1987.6.173. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K., Kühnel B., Lawler-Heavner J., Barber D. A., Edwards D. P. A quantitative comparison of dual control of a hormone response element by progestins and glucocorticoids in the same cell line. Mol Endocrinol. 1989 Aug;3(8):1270–1278. doi: 10.1210/mend-3-8-1270. [DOI] [PubMed] [Google Scholar]
- Ortí E., Bodwell J. E., Munck A. Phosphorylation of steroid hormone receptors. Endocr Rev. 1992 Feb;13(1):105–128. doi: 10.1210/edrv-13-1-105. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Coronado E., Allred D. C., Wiebe V., DeGregorio M. Acquired tamoxifen resistance: correlation with reduced breast tumor levels of tamoxifen and isomerization of trans-4-hydroxytamoxifen. J Natl Cancer Inst. 1991 Oct 16;83(20):1477–1482. doi: 10.1093/jnci/83.20.1477. [DOI] [PubMed] [Google Scholar]
- Power R. F., Lydon J. P., Conneely O. M., O'Malley B. W. Dopamine activation of an orphan of the steroid receptor superfamily. Science. 1991 Jun 14;252(5012):1546–1548. doi: 10.1126/science.2047861. [DOI] [PubMed] [Google Scholar]
- Power R. F., Mani S. K., Codina J., Conneely O. M., O'Malley B. W. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991 Dec 13;254(5038):1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- Rangarajan P. N., Umesono K., Evans R. M. Modulation of glucocorticoid receptor function by protein kinase A. Mol Endocrinol. 1992 Sep;6(9):1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- Reese J. C., Katzenellenbogen B. S. Differential DNA-binding abilities of estrogen receptor occupied with two classes of antiestrogens: studies using human estrogen receptor overexpressed in mammalian cells. Nucleic Acids Res. 1991 Dec 11;19(23):6595–6602. doi: 10.1093/nar/19.23.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R., Weigel N. L., O'Malley B. W., Schrader W. T. Dimerization of the chicken progesterone receptor in vitro can occur in the absence of hormone and DNA. Mol Endocrinol. 1990 Dec;4(12):1782–1790. doi: 10.1210/mend-4-12-1782. [DOI] [PubMed] [Google Scholar]
- Sheridan P. L., Evans R. M., Horwitz K. B. Phosphotryptic peptide analysis of human progesterone receptor. New phosphorylated sites formed in nuclei after hormone treatment. J Biol Chem. 1989 Apr 15;264(11):6520–6528. [PubMed] [Google Scholar]
- Simons S. S., Jr, Oshima H., Szapary D. Higher levels of control: modulation of steroid hormone-regulated gene transcription. Mol Endocrinol. 1992 Jul;6(7):995–1002. doi: 10.1210/mend.6.7.1324423. [DOI] [PubMed] [Google Scholar]
- Slater E. P., Cato A. C., Karin M., Baxter J. D., Beato M. Progesterone induction of metallothionein-IIA gene expression. Mol Endocrinol. 1988 Jun;2(6):485–491. doi: 10.1210/mend-2-6-485. [DOI] [PubMed] [Google Scholar]
- Somers J. P., DeFranco D. B. Effects of okadaic acid, a protein phosphatase inhibitor, on glucocorticoid receptor-mediated enhancement. Mol Endocrinol. 1992 Jan;6(1):26–34. doi: 10.1210/mend.6.1.1310797. [DOI] [PubMed] [Google Scholar]
- Takimoto G. S., Tasset D. M., Eppert A. C., Horwitz K. B. Hormone-induced progesterone receptor phosphorylation consists of sequential DNA-independent and DNA-dependent stages: analysis with zinc finger mutants and the progesterone antagonist ZK98299. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3050–3054. doi: 10.1073/pnas.89.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeeck M. A., Sutanto W., Burbach J. P. Regulation of vasopressin messenger RNA levels in the small cell lung carcinoma cell line GLC-8: interactions between glucocorticoids and second messengers. Mol Endocrinol. 1991 Jun;5(6):795–801. doi: 10.1210/mend-5-6-795. [DOI] [PubMed] [Google Scholar]
- el-Ashry D., Oñate S. A., Nordeen S. K., Edwards D. P. Human progesterone receptor complexed with the antagonist RU 486 binds to hormone response elements in a structurally altered form. Mol Endocrinol. 1989 Oct;3(10):1545–1558. doi: 10.1210/mend-3-10-1545. [DOI] [PubMed] [Google Scholar]