Supplemental Digital Content is available in the text
Abstract
Because it has been suggested that food rich in γ-aminobutyric acid (GABA) or angiotensin-converting enzyme inhibitor (ACEI) peptides have beneficial effects on blood pressure (BP) and other cardiovascular risk factors, we tested the effects of low-sodium bread, but rich in potassium, GABA, and ACEI peptides on 24-hour BP, glucose metabolism, and endothelial function.
A randomized, double-blind, crossover trial was conducted in 30 patients with pre or mild-to-moderate hypertension, comparing three 4-week nutritional interventions separated by 2-week washout periods. Patients were randomly assigned to consume 120 g/day of 1 of the 3 types of bread for each nutritional intervention: conventional wheat bread (CB), low-sodium wheat bread enriched in potassium (LSB), and low-sodium wheat bread rich in potassium, GABA, and ACEI peptides (LSB + G). For each period, 24-hour BP measurements, in vivo endothelial function, and biochemical samples were obtained.
After LSB + G consumption, 24-hour ambulatory BP underwent a nonsignificant greater reduction than after the consumption of CB and LSB (0.26 mm Hg in systolic BP and −0.63 mm Hg in diastolic BP for CB; −0.71 mm Hg in systolic BP and −1.08 mm Hg in diastolic BP for LSB; and −0.75 mm Hg in systolic BP and −2.12 mm Hg in diastolic BP for LSB + G, respectively). Diastolic BP at rest decreased significantly during the LSB + G intervention, although there were no significant differences in changes between interventions. There were no significant differences between interventions in terms of changes in in vivo endothelial function, glucose metabolism, and peripheral inflammatory parameters.
Compared with the consumption of CB or LSB, no greater beneficial effects on 24-hour BP, endothelial function, or glucose metabolism were demonstrated after the consumption of LSB + G in a population with pre or mild-to-moderate hypertension. Further studies are warranted to clarify the effect of GABA on BP, preferably using a specific design for noninferiority trials and ambulatory BP monitoring as a measure of BP.
This study was registered at Current Controlled Trials as ISRCTN31436822
INTRODUCTION
Hypertension is an important risk factor for cardiovascular disease (CVD) and all-cause mortality, which accounts for an estimated 7.5 million deaths per year, or 13.5% of total annual deaths worldwide.1,2 In addition to coronary heart diseases and stroke, other complications of hypertension include heart failure, peripheral vascular disease, renal failure, retinal hemorrhage, and visual impairment. Clinical management of hypertension is clearly associated with a reduction in cardiovascular complications, but treating prehypertension has also been thought to have several potential health benefits.3 The prehypertensive condition has been recognized as a risk factor for coronary heart disease, frank hypertension, and stroke.4 Unfortunately, current preventive approaches to prehypertension are not entirely effective at preventing or controlling this condition, and many prehypertensive patients will eventually develop hypertension.5
Several studies have shown that lifestyle modification, such as weight loss for the overweight and obese, giving up smoking, physical activity promotion, and adherence to a healthy diet rich in fruit, vegetables, and low-fat milk or milk products is a cornerstone in the prevention and the treatment of this condition.6 A reduction in the sodium content of the diet has also been emphasized to prevent hypertension and its complications.7,8 It has been estimated that lowering salt intake to 5 g/day could decrease the risk of stroke and CVD by 23% and 17%,9,10 respectively, contributing to a significant reduction in mortality. Despite this, recent observations of the Centers for Disease Control and Prevention and WHO estimated that sodium and salt intake surpasses the current dietary recommendations worldwide.11
As bread is one of the principal sources of salt in Europe and many other developed countries, it has been a leading target of strategies for blood pressure (BP) control.12 The content of salt in bread can be reduced to a limit at which its organoleptic properties decrease consumer's acceptance. For this reason, it has been suggested that functional molecules with a capacity for reducing BP levels may be incorporated into reduced salt bread.
γ-Aminobutyric acid (GABA) is one of the most important inhibitory neurotransmitters in the central nervous system. It is produced by irreversible α-decarboxylation of L-glutamic acid by the action of glutamic acid decarboxylase enzyme, which is dependent on the pyridoxal 5’-phospate molecule or vitamin B6.13
γ-Aminobutyric acid is naturally present in many foods, especially in fermented products, and can be absorbed by mammals and the human intestinal cells.14 GABA may undergo rapid transepithelial transport across the intestinal wall. Accumulation through the apical membrane and generation of overall net absorption result from the transport activity of a pH-dependent and Na+-independent H+/GABA symporter.14 Unfortunately, GABA bioavailability of food enriched with GABA is largely unknown.
Nevertheless, to date, several studies have examined the effects of GABA-enriched food on BP in animal models15–18 and humans,19–23 but the results are controversial. Moreover, recent studies suggest that GABA may be involved in the regulation of glucose metabolism because it has been demonstrated that GABA stimulates insulin secretion in the pancreas.24,25
In addition, several angiotensin-converting enzyme inhibitor (ACEI) peptides from a variety of food sources such as tripeptides valine-proline-proline (VPP) and isoleucine-proline-proline (IPP) from milk, ovokinin (FRADHPFL) from egg proteins, or threonine-glycine-valine-tyrosine (TGVY) from a rice protein hydrolysate26 have proved to have antihypertensive effects. A recent meta-analysis of 19 randomized, placebo-controlled clinical intervention trials conducted on prehypertensive or mildly hypertensive patients showed an overall BP-lowering effect,27 suggesting that small daily doses of lactotripeptides (which are small peptides derived from milk that mechanistically mimics ACEIs)28 in various functional food products may be a valuable nonpharmacological option for treating prehypertension or mild hypertension as part of lifestyle advice. Of note, this antihypertensive effect was more evident in Asian than in European populations.29
Only few studies have analyzed the effect of GABA and lactotripeptides on BP, most of which have been conducted in Asian populations, Therefore, the aim of the present study was to conduct a randomized, double-blinded, crossover study to evaluate the effect of replacing bread with bread low in sodium chloride and rich in GABA and ACEI peptides on BP, insulin resistance, inflammation, and endothelial function in Spanish patients with prehypertension and mild or moderate hypertension.
PATIENTS AND METHODS
Patients
The study population consisted of men and women from Spain with pre or mild-to-moderate hypertension,30 who were not taking antihypertensive drugs. The inclusion criteria were as follows: 18 to 65 years of age; body mass index (BMI) <35 kg/m2; systolic BP ranging between 120 and 159 mm Hg or diastolic BP between 80 and 99 mm Hg; and daily consumption of bread. The exclusion criteria were as follows: severe hypertension (systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg); antihypertensive medication, presence of diabetes mellitus or another endocrine disease, significant renal or hepatic disease; alcohol, drug, or tobacco abuse; pregnancy or desire to be pregnant, voluntary or involuntary significant weight loss (>5 kg) in the previous 3 months; a vegetarian diet or other dietary restrictions for disease control; previous history of atherosclerotic disease or target organ damage; use of dietary supplements, mineral or vitamin complexes, or sterols; or the presence of medical conditions that may affect participation in the study.
To discard target organ damage, in the recruitment visit before randomization, carotid echography was performed and renal function was determined by evaluation of biochemistry measurements.
All the patients provided written informed consent for their participation, and the study was approved by the Ethical Committee of the Hospital Universitari de Sant Joan de Reus.
Randomization and Masking
Investigators randomly assigned the volunteers who accomplished inclusion criteria to 1 of the 6 possible intervention sequences using a computer-generated randomization number table.
Study investigators, research coordinators, and participants were blinded to intervention group assignments.
Study Design
The present study is a randomized, double-blind, crossover trial comparing three 4-week nutritional intervention periods separated by a washout period of 2 weeks (Fig. 1). Two weeks before the intervention, the patients were asked to follow a standardized Mediterranean diet (MedDiet) until the start of the first intervention (run-in period). This diet was adapted to the energy requirements of the volunteers calculated by the WHO equations31 and adjusted for individual physical activity. After the 2-week run-in period, patients started with 1 of the 3 interventions. They were advised to follow the Mediterranean isocaloric diet and received 120 g of bread, corresponding to the Spanish per capita daily consumption12 (60 g of which should be eaten for breakfast). We randomly assigned participants to 6 intervention sequences which depended on the 3 types of bread to be consumed: conventional wheat bread (CB) with 1.4 g/100 g of sodium chloride, low-sodium chloride (1 g/100 g) wheat bread enriched with 0.4 g of potassium citrate (LSB), and low-sodium chloride (1 g/100 g) wheat bread enriched with 0.4 g of potassium citrate and containing 22.8 mg/100 g of GABA (LSB + G).
FIGURE 1.
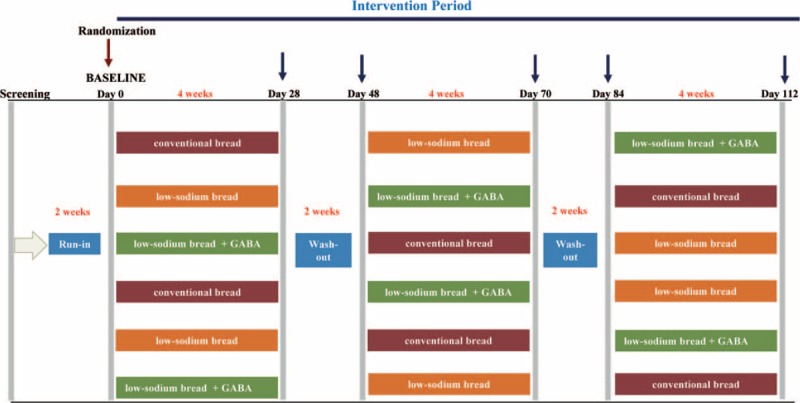
Study design.
During the study, the participants were advised to maintain the same level of physical activity, to keep their body weight stable, and to stop consuming bread not provided in the study.
Types of Bread Tested
The 3 types of bread were produced on an industrial line (Europastry, Sarral, Spain). The traditional bread was produced in normal production conditions. The sodium chloride in the LSB was replaced by potassium citrate.
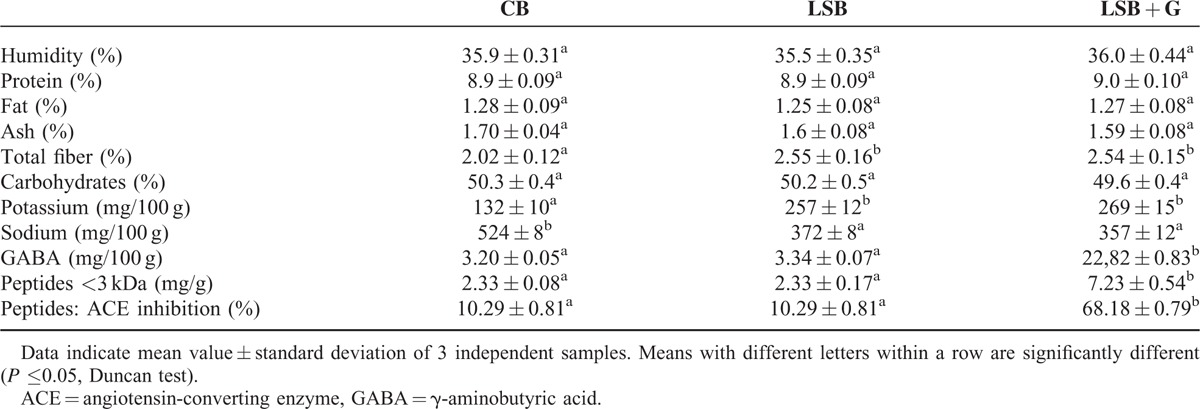
The LSB + G bread was produced from a liquid sourdough with a flour-to-water ratio of 30%. This sourdough was obtained with a Lactobacillus brevis CECT 818332 fermentation starter in a 20% solution of wheat flour in water. An additional 3% of soya protein concentrate rich in glutamic acid and a protease were added to the sourdough to ensure the presence of free amino acids. The fermentation was performed in a Biostat bioreactor (Sartorius, Germany) and the temperature of fermentation was maintained at 30°C for 48 hours. Finally, we obtained bread with a GABA concentration of 22.8 mg/100 g and a peptide concentration of 7.23 mg/g33 (Table 1). The 3 types of bread were partially baked and immediately frozen for storage. For distribution, bread was thawed, cooked at 180°C during 12 to 14 Minutes, and dispensed free, once a week, throughout the intervention.
TABLE 1.
Proximate Composition and γ-Aminobutyric Acid and Angiotensin-converting Enzyme Inhibitors Peptide Content of Conventional Wheat Bread (CB), Low-sodium Wheat Bread (LSB), and Low-sodium Wheat Bread Rich in GABA and ACEI peptides (LSB + G)33
Measurements
Energy and nutrient intake, physical activity, and compliance with the intervention were determined at baseline and before and after each intervention period. Possible adverse events were recorded throughout the study. BP measurements, fasting blood samples, and 24-hour urine excretion samples were obtained at baseline, and before and at the end of each intervention period. Furthermore, the 24-hour ambulatory BP monitoring (ABPM) measurements, and small artery endothelial function determined by peripheral artery tonometry were measured at baseline and at the end of each intervention period.
The compliance with the intervention was determined by recording the bread consumed by each participant.
Fasting Blood Pressure and 24-Hour ABPM Measurements
The primary outcome was the daily mean systolic and diastolic BP. Patients attended the research facility of each institution on a week day between 8:00 a.m. and 10:00 a.m. At the screening visit, the systolic BP and diastolic BP were taken in both arms from seated participants using a validated oscillometer (Omron HEM705CP; Hoofddorp, the Netherlands). Measurements were taken in triplicate with a 1-minute interval between each measurement. The first measurement was discarded and the mean of the other 2 was recorded. The arm with the highest value was used through the study for measuring BP.
Ambulatory BP monitoring was performed using Spacelabs 90207/90217 devices (SpacelabsW Inc.; Richmond, WA), with readings scheduled every 20 minutes during the activity period and every 40 minutes during the rest period. The periods of activity and rest were determined on an individual basis according to hours of sleep and waking. The duration of the procedure in hours, the percentage of valid readings, and mean systolic BP and diastolic BP during periods of activity and rest, and for the whole 24-hour period, were measured. We considered all ABPM recordings with twenty or more measurements during the activity period and seven or more measurements during the rest period.34
Anthropometry and Body Composition
Body weight and waist circumference were measured with the patient in fasting conditions, with light clothing and no shoes. The waist circumference was measured midway between the lowest rib and the iliac crest using an anthropometric tape. The body composition and the water content were estimated by tetrapolar bioelectrical impedance (Human-Im-Scan, Dietosystem, Barcelona, Spain).
Assessment of Dietary and Physical Activity
Food consumption was evaluated by 3-day dietary records (including 1 nonworking day). Energy and nutrient intake were estimated using Spanish food composition tables.35 Leisure-time physical activity was assessed by the Minnesota questionnaire validated for the Spanish population.36
Assessment of Endothelial Function
The small artery reactive hyperemia index (saRHI) was measured using peripheral artery tonometry (PAT) technology (EndoPAT-2000, Itamar Medical Ltd., Israel). Participants’ determinations were performed under fasting conditions in a quiet room with a controlled temperature (22°C–24°C) and in the absence of smoking or strenuous exercise during the preceding 24 hours. The PAT technique has been described elsewhere.37 Briefly, it consisted in 15 minutes exploration divided in 5-minute baseline measurement, 5-minute measurement after inducing ischemia by placing a cuff on the forearm and inflating enough to produce above baseline systolic pressure, and an additional 5-minute measurement after occlusion once the cuff was rapidly deflated. Blood flow measurements from 2 fingertips (1 from each hand, one a test and the other a control) were compared after a stabilization period, and a second comparison pair of measurements was taken before and after 5 minutes of brachial ischemia in the test arm. The results were processed using specific software to calculate the postischemic reflex vasodilation. The value obtained was termed saRHI. Arterial stiffness (AIx), expressed as an augmentation index, was also determined during the same exploration analyzing the differences in pulse wave amplitude before and after ischemia. Augmentation index adjusted to 75 beats/minute (AI75) was subsequently calculated.
Assessment of Carotid Intima-media Thickness and Presence or Absence of Atheroma Plaque
To assess carotid intima-media thickness (cIMT), we used a My Lab 50 X-Vision sonograph (EsaoteSpA, Indianapolis, IN) with a linear array ultrasound probe of 8–12 MHz transducer. Determination of the maximum IMT (maxIMT) was recorded according to standardized methodology described elsewhere.38,39 Images were obtained and measured by a single operator to reduce observer variability.
Biochemical Measurements
Blood samples were obtained after an overnight fast at baseline and at the end of each intervention period. Plasma CRP (IBL International, Hamburg, Germany), VCAM, and IL-6 and serum sE-Selectine (R&D Systems, Minneapolis, MN) were measured using ELISA commercial kits. Serum aldosterone (ZenTech, Angleur, Belgium) and plasma angiotensin I (Immunotech, Prague, Czech Republic) were measured using radioimmunoassay (RIA) kits. The 24-hour urine noradrenaline was measured using high-performance liquid chromatography (HPLC). Serum glucose and insulin were analyzed using standard enzymatic methods. Homeostatic model assessment of insulin resistance (HOMA-IR) and homeostatic model assessment of β-cell function (HOMA-β) were estimated using the formulas published by Matthews.40
All the laboratory technicians were blinded to intervention group assignments.
Statistical Analysis
Sample size was estimated by taking into account 2 previously published clinical trials evaluating the effect of GABA supplementation on BP.20,23 For a unilateral alpha risk of 0.05, a statistical power of 80% (beta = 0.20) and a difference between 2 interventions of 5 mm Hg (and 15.5 mm Hg of standard deviation), it was estimated that 30 individuals were required for the crossover trial.
The Kolmogorov–Smirnov test was used to determine the normal distribution of continuous variables. The variables that were non-normally distributed were logarithm-transformed before the statistical analyses. The continuous variables are presented as the mean ± standard deviation and categorical variables were presented as numbers and percentages. The PKcross command in the STATA statistical package was used to evaluate differences in daily ABPM and different secondary outcomes between treatments. A paired t test was used to evaluate differences in each outcome from baseline to the end of the period.
The P values <0.05 were considered significant. Statistical analyses were made with the SPSS statistical package (version 19.0, SPSS Inc, Chicago, IL) and STATA 12.0 (StataCorp, College Station, TX).
RESULTS
A total of 74 participants were screened for eligibility, and of the 45 potential participants, 32 were effectively randomized to the study between February 2013 and January 2014. One participant was excluded from the analysis because new-onset diabetes was diagnosed in the second visit, and another participant dropped out for personal reasons. Therefore, 30 participants completed the 3 different interventions (see Fig. 2). Table 1 shows the nutrient and GABA and ACEI peptide content of the 3 breads used for the intervention. No serious side effects were observed during the trial.
FIGURE 2.
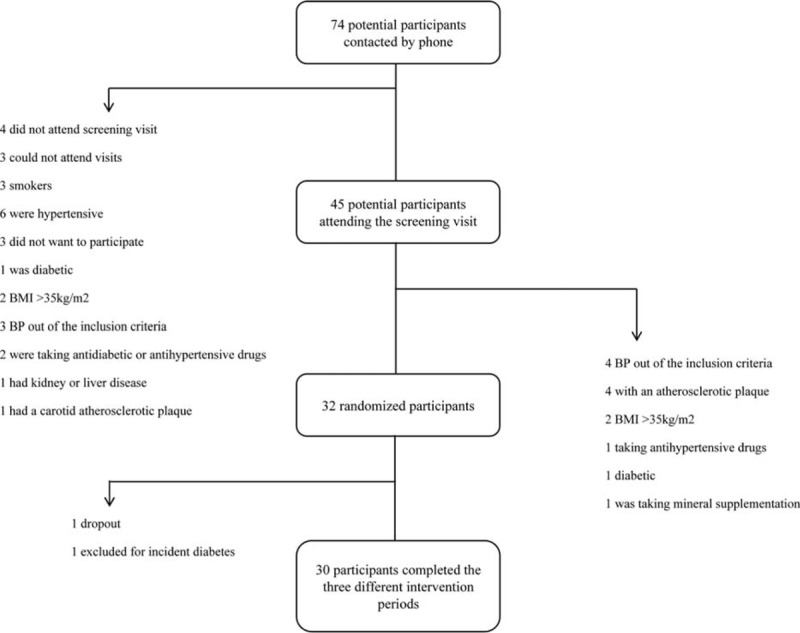
Flow chart of participants.
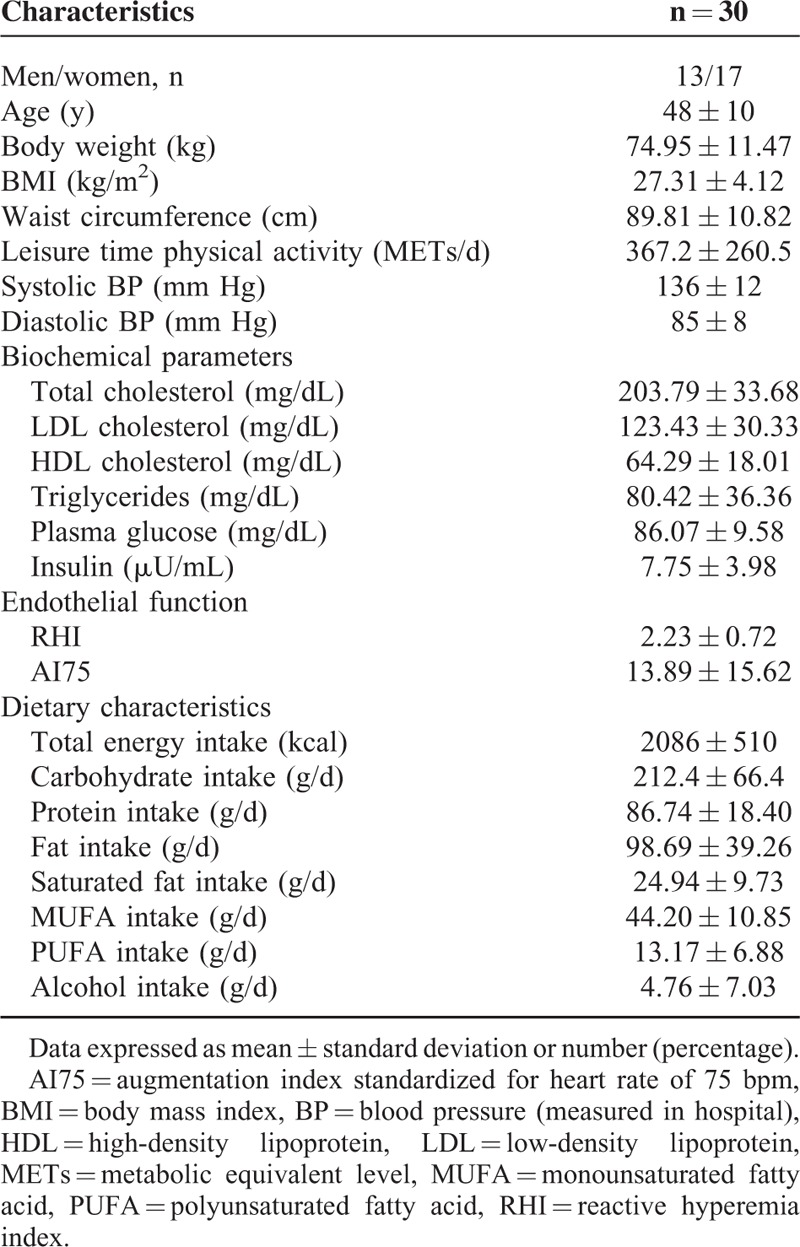
The baseline characteristics of the study population are presented in Table 2. A total of 43% of the total study population were men. The mean systolic BP and diastolic BP were 136 and 85 mm Hg, respectively.
TABLE 2.
Baseline Characteristics of the Study Population
No significant differences were observed in BMI, waist circumference, or dietary variables changes between treatments (see Table, Supplemental Content, http://links.lww.com/MD/A522, which illustrates dietary characteristics and anthropometric measurements according to different interventions).
Table 3 shows the changes in systolic BP and diastolic BP between the baseline values and after the consumption of different types of bread. The 24-hour BP with fewer than 20 daytime measurements or fewer than 7 night-time measurements was not considered to be valid and was excluded from the analysis of BP. The analyses were conducted on 24, 24, and 25 participants in the CB, LSB, and LSB + G groups, respectively.
TABLE 3.
Effect of Conventional Wheat Bread, Low-sodium Wheat Bread (LSB), and Low-sodium Wheat Bread Rich in GABA and ACEI Peptides (LSB + G) on Continuous 24-hour Ambulatory Blood Pressure Measurements
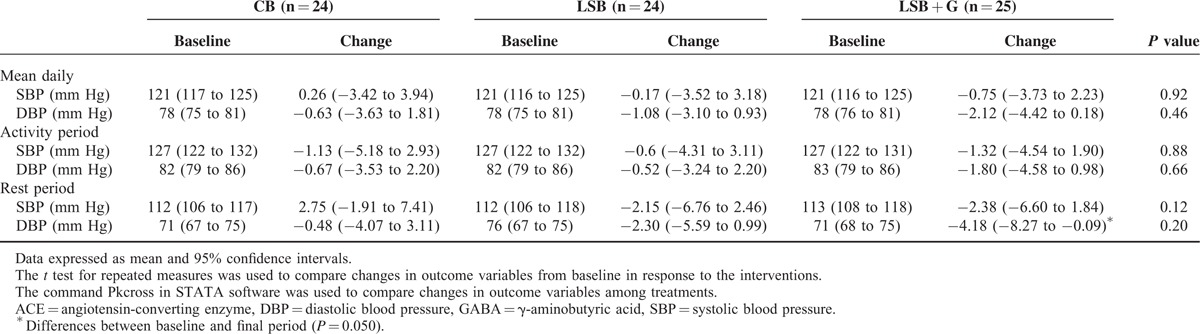
The decrease in BP after the intervention period with LSB + G, although not significant, tended to be higher than after the intervention with CB or LSB (0.26 mm Hg in systolic BP and −0.63 mm Hg in diastolic BP for CB; −0.71 mm Hg in systolic BP and −1.08 mm Hg in diastolic BP for LSB; and −0.75 mm Hg in SBP and −2.12 mm Hg in DBP for LSB + G). However, the differences in changes between treatments were not statistically significant for either daily systolic or diastolic BP.
When systolic BP and diastolic BP differences between treatments were analyzed during the activity and rest periods, although nonstatistically significant (P = 0.88 for systolic BP and 0.66 for diastolic BP in the activity period; P = 0.12 for systolic BP and 0.20 for diastolic BP in the rest period), the highest decrease in BP was observed not only in the activity period but also in the rest period after the consumption of LSB + G bread.
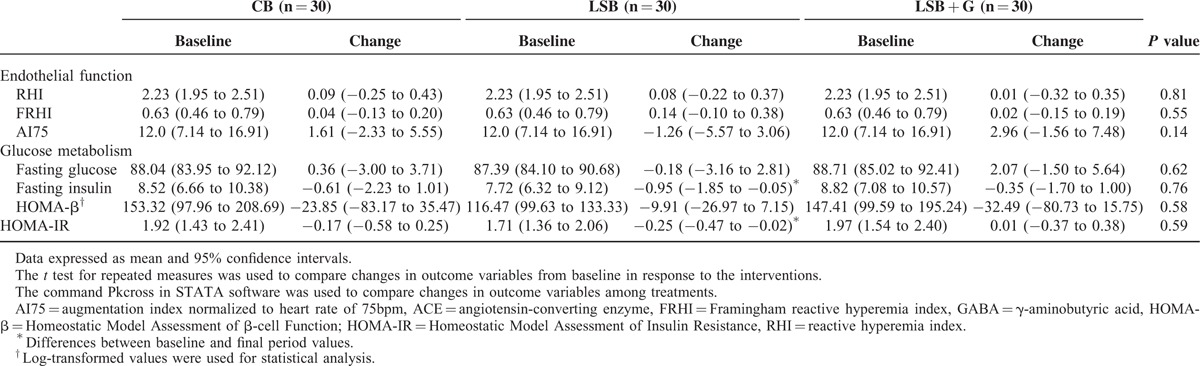
Table 4 shows changes in the baseline values of endothelial function and glucose metabolism parameters and the values after the consumption of the 3 different types of bread. No significant differences were found between treatments for saRHI, Framingham RHI (FRHI), and AI75 parameters. Along the same line, when the effect of the 3 different types of bread on fasting glucose, insulin, HOMA-IR, and HOMA-β were analyzed, nonstatistical significant differences were observed between interventions.
TABLE 4.
Effect of Conventional Wheat Bread, Low-sodium Wheat Bread (LSB), and Low-sodium Wheat Bread Rich in GABA and ACEI Peptides (LSB + G) on In Vivo Endothelial Function, and Glucose Metabolism
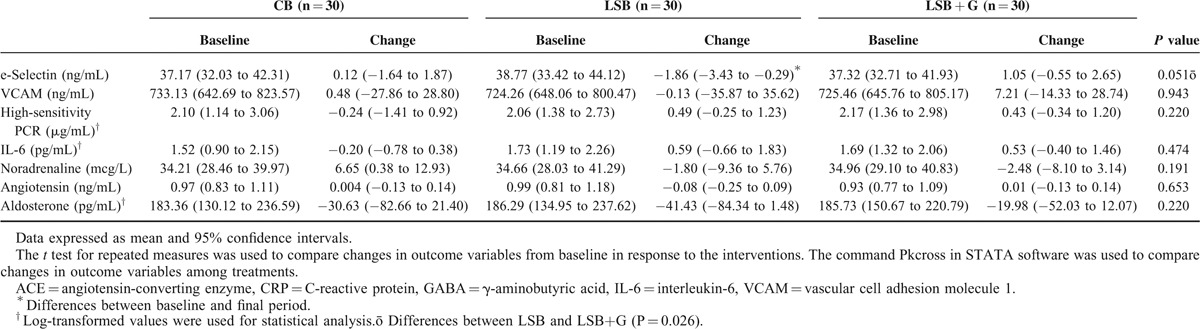
Regarding peripheral changes in endothelial and inflammatory parameters (Table 5), only the E-selectin changes showed a borderline significant trend of decrease between the LSB and LSB + G treatments (P = 0.051). The changes in other inflammatory markers between interventions were nonsignificant. Moreover, nonstatistically significant differences were observed after the consumption of the 3 different types of breads in relation to peripheral concentrations of angiotensin, aldosterone, and noradrenalin (Table 5).
TABLE 5.
Effect of Conventional Bread (CB), Low-sodium Wheat Bread (LSB), and Low-sodium Wheat Bread Rich in GABA and ACEI Peptides (LSB + G) on Peripheral Endothelial and Inflammatory Markers
DISCUSSION
This is the first randomized, double-blinded, crossover study to evaluate the effect of a bread naturally enriched in potassium, GABA, and ACEI peptides on BP, endothelial function, inflammation, and glucose metabolism in a population with pre or mild-to-moderate hypertension. The results showed that the consumption of a bread reduced in sodium and rich in potassium, GABA, and ACEI did not affect BP, endothelial function, or glucose metabolism compared with the conventional wheat bread, although a trend toward a higher decrease in both daily and rest 24-hour systolic BP and diastolic BP was observed.
The magnitude in the 24-hour diastolic BP decrease observed in our study during the GABA-enriched bread period, although nonsignificant, was about 2 mm Hg, a reduction that has been associated to a 17% decrease in the prevalence of hypertension, 6% decrease in the risk of coronary heart disease, and 15% reduction risk of stroke when measured in office BP.41
As far as we know, 5 intervention studies have evaluated the effect of foods enriched with GABA on BP, and the results are controversial. One of them was conducted in a Canadian population19 and the others in Asian populations.20–23 In contrast to our results, the study conducted by Inoue et al23 showed a significant decrease in systolic BP and diastolic BP after the daily consumption of a novel fermented milk containing 10 to 12 mg of GABA in mildly hypertensive patients. In line, after the ingestion of GABA-rich Chlorella tablets, participants with pre and mild-to-moderate hypertension showed a significant decrease in systolic BP and diastolic BP, and the decrease was greatest in those individuals with higher BP levels at baseline.20 Moreover, a recent study showed that the consumption of 150 g of rice containing 16.8 mg of GABA improved self-measured BP in the morning compared with placebo rice.22 In 2 other studies, a decrease in systolic BP and diastolic BP was observed after the consumption of vinegar and dried bonito enriched with GABA,21 and after the consumption of cheese rich in GABA.19 However, no significant differences were observed between treatment and placebo, which suggests that the decrease in BP cannot be attributed to the GABA intake alone.
Although the amount of daily GABA ingested in our study was similar to that ingested in previous studies, we found that for the difference between the diastolic BP baseline values and the values in the rest period, only a borderline significant trend to reduction was observed after the consumption of LSB + G, but no significant differences were observed in BP changes between breads. The differences between the aforementioned studies and our results could be explained by the method of measuring BP. In all the studies BP was measured in the office, whereas in our study we used 24-hour BP which gives a mean daily BP instead of one particular measure. This gives a more realistic approach of total daily pressure levels and exposure to damage.
Another possible explanation for the differences in the results between studies is the baseline BP values of the participants. It has been demonstrated that reductions in BP were greater in hypertensive rats (SHR/Izm) than in normotensive rats,42 suggesting an important interaction between baseline BP values and the impact of GABA on changes in BP. In this respect, the mean systolic BP and diastolic BP baseline values of our participants were considerably lower than those of individuals from previously published studies20,23 using foods enriched with GABA. In the only 2 studies that showed that the consumption of food enriched with GABA had no effect on BP, baseline values were comparable with our study.19,21 Thus, the relatively low baseline BP levels in our study may have influenced the low impact of GABA on BP.
It has been suggested that the positive action of GABA on BP may be due to its ability to inhibit the release of noradrenaline through the activation of presynaptic receptors leading to suppressing the increase in perfusion pression.42 In our study, although not significantly, it has been observed that there is a greater reduction in 24-hour urine excretion of noradrenaline after the consumption of LSB + G bread. As far as the possible effect of GABA on glucose metabolism is concerned, it has been suggested that in rats and humans, GABA increases the secretion of insulin in pancreas.24,25 However, only 1 trial has examined the effect of GABA supplementation on glucose metabolism in humans, showing a significant increase in plasma insulin levels after the oral administration between 5 and 10 g of GABA, more than what was administered in our study.43 Therefore, more studies are needed aiming to test if GABA administration can affect glucose metabolism in humans.
Endothelial dysfunction is an early and critical event related to BP and hypertension. It plays an important role in the pathogenesis and progression of atherosclerosis and ensuing cardiovascular events. The digital peripheral artery tonometry used in our study enables the saRHI, a surrogate marker of peripheral small artery endothelial function that correlates with both traditional and novel cardiovascular risk factors,44 and subsequent cardiac events, to be measured in vivo.45,46 Our study is the first to evaluate the effect of bread rich in GABA and ACEI on endothelial function measured in vivo by plasma biomarkers. However, no effect was observed. Because endothelial function was a secondary outcome in this study, may be the statistical power was insufficient to demonstrate beneficial effects on endothelial function. Therefore, further studies, with larger samples, are warranted to understand the possible association between GABA consumption, endothelial function, and the subsequent risk of hypertension.
Our study has several strengths. First, the double-blind crossover design, which removes between-patient variation and requires fewer patients than a parallel study for an equal number of treatment comparisons because each experimental unit (ie, patient), can be used several times. Second, to our knowledge, this is the first study that has evaluated BP with ABPM, which has several advantages over office BP measurement: it gives a larger number of readings, provides a natural profile of BP behavior in the participants, and assesses BP variability over the 24-hour period.47
Some limitations, however, also deserve comment. First, glucose metabolism and endothelial function were secondary endpoints, so the statistical power may not be enough to demonstrate differences between interventions. Second, our results cannot be extrapolated to the general population because our sample consisted of patients with pre or mild-to-moderate hypertension. Third, we cannot discard the beneficial effects of this bread rich in GABA and ACEI peptides on Asiatic populations, because ACEI peptides have been shown to decrease BP in Asian individuals, but not in European populations.29 Fourth, the bioavailability of GABA and ACEI peptides from our bread LSB + G has not been evaluated in our study. Therefore, we cannot discard that the absence of the expected effects on several outcomes may be explained to the lack of bioavailability of these molecules. Fifth, unfortunately, we do not have measured serum angiotensin II concentrations or ACE activity throughout the study which could help us to better understand the role of ACEI peptides derived from LSB + G on BP. And finally, the size of our sample may be small to test our hypothesis so the statistical power may be too low for comparisons despite the fact that we performed sample size estimation before initiating the study based on studies evaluating the effect of GABA on office BP measurements.
In conclusion, the results of this study do not support the notion that bread low in sodium and enriched in potassium GABA and ACEI peptides has any beneficial effects on BP, endothelial function, or glucose metabolism. However, the present results should be interpreted with caution because absence of evidence does not mean evidence of no effect.48 Further studies are warranted to clarify the effect of GABA on BP, preferably using a specific design for noninferiority trials and ABPM as a measure of BP.
ACKNOWLEDGMENTS
The authors thank all the participants who agreed to participate in this study. Special thanks go to Núria Aguilera and Anna Varela for their nursing assistance during the study. We acknowledge Cristian Tebé for his help in the statistical analyses of the data.
Footnotes
Abbreviations: ABPM = ambulatory blood pressure monitoring, ACEI = angiotensin-converting enzyme inhibitory peptides, AI75 = augmentation index adjusted to 75 beats/minute, AIx = arterial stiffness, BP = blood pressure, CB = conventional bread, cIMT = carotid intima-media thickness, CVD = cardiovascular disease, GABA = γ-aminobutyric acid, HPLC = high-performance liquid chromatography, LSB = low-sodium wheat bread, LSB + G = low-sodium wheat bread rich in GABA and ACEI peptides, maxIMT = maximum intima-media thickness, MedDiet = Mediterranean diet, mIMT = mean intima-media thickness, PAT = peripheral artery tonometry, RIA = radioimmunoassay, saRHI = small artery reactive hyperemia index.
Funding: The present study was funded by Europastry.
Conflict of interest: Jordi Salas Institution has received a grant from Europastry. Joan Quilez is employed by Europastry. The rest of authors declare no conflicts of interest relevant to this article. The funders participated in the study design, but had no role in data collection and analysis.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: World Health Organization; 2009. [Google Scholar]
- 2.Lawes CM, Hoorn S, Vander, et al. Global burden of blood-pressure-related disease, 2001. Lancet 2008; 371:1513–1518. [DOI] [PubMed] [Google Scholar]
- 3.MacMahon S, Peto R, Collins R, et al. Blood pressure, stroke, and coronary heart disease. Lancet 1990; 335:765–774. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi AI, Suri MFK, Kirmani JF, et al. Is prehypertension a risk factor for cardiovascular diseases? Stroke 2005; 36:1859–1863. [DOI] [PubMed] [Google Scholar]
- 5.Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006; 354:1685–1697. [DOI] [PubMed] [Google Scholar]
- 6.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007; 28:1462–1536. [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ. ASH position paper: dietary approaches to lower blood pressure. J Am Soc Hypertens 2009; 3:321–331. [DOI] [PubMed] [Google Scholar]
- 8.Strazzullo P, D’Elia L, Kandala N-B, et al. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ 2009; 339 (nov24_1):b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis 49:59–75. [DOI] [PubMed] [Google Scholar]
- 10.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007; 334:885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powles J, Fahimi S, Micha R, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013; 3:e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quilez J, Salas-Salvado J. Salt in bread in Europe: potential benefits of reduction. Nutr Rev 2012; 70:666–678. [DOI] [PubMed] [Google Scholar]
- 13.Ueno H. Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal B Enzym 2000; 10:67–79. [Google Scholar]
- 14.Thwaites DT, Basterfield L, McCleave PM, et al. Gamma-Aminobutyric acid (GABA) transport across human intestinal epithelial (Caco-2) cell monolayers. Br J Pharmacol 2000; 129:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayakawa K, Kimura M, Kasaha K, et al. Effect of a (-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar–Kyoto rats. Br J Nutr 2007; 92:411–417. [DOI] [PubMed] [Google Scholar]
- 16.Yamakoshi J, Fukuda S, Satoh T, et al. Antihypertensive and natriuretic effects of less-sodium soy sauce containing γ-aminobutyric acid in spontaneously hypertensive rats. Biosci Biotechnol Biochem 2007; 71:165–173. [DOI] [PubMed] [Google Scholar]
- 17.Liu CF, Tung YT, Wu CL, et al. Antihypertensive effects of Lactobacillus-fermented milk orally administered to spontaneously hypertensive rats. J Agric Food Chem 2011; 59:4537–4543. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura M, Toyoshi T, Sano A, et al. Antihypertensive effect of a gamma-aminobutyric acid rich tomato cultivar “DG03-9” in spontaneously hypertensive rats. J Agric Food Chem 2010; 58:615–619. [DOI] [PubMed] [Google Scholar]
- 19.Pouliot-Mathieu K, Gardner-Fortier C, Lemieux S, et al. Effect of cheese containing gamma-aminobutyric acid-producing lactic acid bacteria on blood pressure in men. PharmaNutrition 2013; 1:141–148. [Google Scholar]
- 20.Shimada M, Hasegawa T, Nishimura C, et al. Anti-hypertensive effect of g-aminobutyric acid (GABA)-rich chlorella on high-normal blood pressure and borderline hypertension in placebo-controlled double blind study. 2009;31:342–354. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H, Watanabe K, Ma M, et al. The effects of gamma-aminobutyric acid, vinegar, and dried bonito on blood pressure in normotensive and mildly or moderately hypertensive volunteers. J Clin Biochem Nutr 2009; 45:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura M, Yoshida S, Haramoto M, et al. Effects of white rice containing enriched gamma-aminobutyric acid on blood pressure. J Tradit Complement Med 2015; doi: 101016/jjtcme201411022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue K, Shirai T, Ochiai H, et al. Blood-pressure-lowering effect of a novel fermented milk containing g-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr 2003; 57:490–495. [DOI] [PubMed] [Google Scholar]
- 24.Braun M, Ramracheya R, Bengtsson M, et al. y-Aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic b-cells. Diabetes 2010; 59:1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adeghate E, Ponery AS. GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell 2002; 34:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Maqueda D, Miralles B, Recio I, et al. Antihypertensive peptides from food proteins: a review. Food Funct 2012; 3:350–361. [DOI] [PubMed] [Google Scholar]
- 27.Turpeinen AM, Järvenpää S, Kautiainen H, et al. Antihypertensive effects of bioactive tripeptides-a random effects meta-analysis. Ann Med 2013; 45:51–56. [DOI] [PubMed] [Google Scholar]
- 28.Qin L-Q, Xu J-Y, Dong J-Y, et al. Lactotripeptides intake and blood pressure management: a meta-analysis of randomised controlled clinical trials. Nutr Metab Cardiovasc Dis 2013; 23:395–402. [DOI] [PubMed] [Google Scholar]
- 29.Cicero AFG, Gerocarni B, Laghi L, et al. Blood pressure lowering effect of lactotripeptides assumed as functional foods: a meta-analysis of current available clinical trials. J Hum Hypertens 2011; 25:425–436. [DOI] [PubMed] [Google Scholar]
- 30.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 31.Organization WH. Human energy requirements: report of a joint FAO/WHO/UNU Expert Consultation, Rome 17–24 October 2001. Rome: Food Agric Organ United Nations; 2004. [Google Scholar]
- 32.Diana M, Tres A, Quílez J, et al. Spanish cheese screening and selection of lactic acid bacteria with high gamma-aminobutyric acid production. LWT - Food Sci Technol 2014; 56:351–355. [Google Scholar]
- 33.Peñas E, Diana M, Frias J, et al. A multistrategic approach in the development of sourdough bread targeted towards blood pressure reduction. Plant Foods Hum Nutr 2015; 70:97–103. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien E, Parati G, Stergiou G. Ambulatory blood pressure measurement: what is the international consensus? Hypertension 2013; 62:988–994. [DOI] [PubMed] [Google Scholar]
- 35.Mataix VJ. Tabla de composicion de alimentos [food composition tables]. 4th ed.Granada, Spain: Universidad de Granada; 2003. [Google Scholar]
- 36.Elosua R, Marrugat J, Molina L, et al. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am J Epidemiol 1994; 139:1197–1209. [DOI] [PubMed] [Google Scholar]
- 37.Ferré R, Plana N, Merino J, et al. Effects of therapeutic lifestyle changes on peripheral artery tonometry in patients with abdominal obesity. Nutr Metab Cardiovasc Dis 2012; 22:95–102. [DOI] [PubMed] [Google Scholar]
- 38.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascula. J Am Soc Echocardiogr 2008; 21:93–111.quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 39.Junyent M, Gilabert R, Núñez I, et al. [Carotid ultrasound in the assessment of preclinical atherosclerosis. Distribution of intima-media thickness values and plaque frequency in a Spanish community cohort]. Med Clin (Barc) 2005; 125:770–774. [DOI] [PubMed] [Google Scholar]
- 40.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 41.Cook NR. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med 1995; 155:701–709. [PubMed] [Google Scholar]
- 42.Hayakawa K, Kimura M, Kamata K. Mechanism underlying gamma-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. Eur J Pharmacol 2002; 438:107–113. [DOI] [PubMed] [Google Scholar]
- 43.Cavagnini F, Pinto M, Dubini A, et al. Effects of gamma aminobutyric acid (GABA) and muscimol on endocrine pancreatic function in man. Metabolism 1982; 31:73–77. [PubMed] [Google Scholar]
- 44.Ferré R, Aragonès G, Plana N, et al. High-density lipoprotein cholesterol and apolipoprotein A1 levels strongly influence the reactivity of small peripheral arteries. Atherosclerosis 2011; 216:115–119. [DOI] [PubMed] [Google Scholar]
- 45.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008; 117:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J 2010; 31:1142–1148. [DOI] [PubMed] [Google Scholar]
- 47.Parati G, Stergiou G, O’Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 2014; 32:1359–1366. [DOI] [PubMed] [Google Scholar]
- 48.Alderson P. Absence of evidence is not evidence of absence. BMJ 2004; 328:476–477. [DOI] [PMC free article] [PubMed] [Google Scholar]


