Abstract
The efficacy of pregabalin in acute postsurgical pain has been demonstrated in numerous studies; however, the analgesic efficacy and adverse effects of using pregabalin in various surgical procedures remain uncertain. We aim to assess the postsurgical analgesic efficacy and adverse events after pregabalin administration under different surgical categories using a systematic review and meta-analysis of randomized controlled trials.
A search of the literature was performed between August 2014 to April 2015, using PubMed, Ovid via EMBASE, Google Scholar, and ClinicalTrials.gov with no limitation on publication year or language. Studies considered for inclusion were randomized controlled trials, reporting on relevant outcomes (2-, 24-hour pain scores, or 24 hour morphine-equivalent consumption) with treatment with perioperative pregabalin.
Seventy-four studies were included. Pregabalin reduced pain scores at 2 hours in all categories: cardiothoracic (Hedge's g and 95%CI, −0.442 [−0.752 to −0.132], P = 0.005), ENT (Hedge g and 95%CI, −0.684 [−1.051 to −0.316], P < 0.0001), gynecologic (Hedge g, 95%CI, −0.792 [−1.235 to −0.350], P < 0.0001), laparoscopic cholecystectomy (Hedge g, 95%CI, –0.600 [–0.989 to –0.210], P = 0.003), orthopedic (Hedge g, 95%CI, −0.507 [−0.812 to −0.202], P = 0.001), spine (Hedge g, 95%CI, −0.972 [−1.537 to −0.407], P = 0.001), and miscellaneous procedures (Hedge g, 95%CI, −1.976 [−2.654 to −1.297], P < 0.0001). Pregabalin reduced 24-hour morphine consumption in gynecologic (Hedge g, 95%CI, −1.085 [−1.582 to −0.441], P = 0.001), laparoscopic cholecystectomy (Hedge g, 95%CI, –0.886 [–1.652 to –0.120], P = 0.023), orthopedic (Hedge g, 95%CI, −0.720 [−1.118 to −0.323], P < 0.0001), spine (Hedge g, 95%CI, −1.016 [−1.732 to −0.300], P = 0.005), and miscellaneous procedures (Hedge g, 95%CI, −1.329 [−2.286 to −0.372], P = 0.006). Pregabalin resulted in significant sedation in all surgical categories except ENT, laparoscopic cholecystectomy, and gynecologic procedures. Postoperative nausea and vomiting was only significant after pregabalin in miscellaneous procedures.
Analgesic effects and incidence of adverse effects of using pregabalin are not equal in different surgical categories.
INTRODUCTION
Pregabalin is a structural analogue of gamma-aminobutyric acid that acts as a potent ligand for alpha 2-delta subunits of the voltage-gated calcium channels in the nervous system. Such action results in a reduction in the depolarization-induced influx of calcium, hence a reduction in the release of excitatory neurotransmitters including glutamate, noradrenaline, dopamine, and serotonin.1 Compared with gabapentin, pregabalin is more potent, is associated with fewer adverse effects, and has a more predictable and linear pharmacokinetic profile.1,2 Its absorption is extensive, rapid, and proportional to dose.1,2 Pregabalin is an attractive adjuvant for perioperative analgesia in this regard as it can be taken on an empty stomach, does not lead to gastrointestinal bleeding, and is generally well-tolerated.3
A multimodal analgesic technique is now often employed in acute postsurgical pain management in an attempt to improve analgesic efficacy and decrease requirement for opioids that are associated with undesirable adverse effects.4 Uses of pregabalin therefore range from treatment of neuropathic pain to being an adjunct in the multimodal management of postsurgical pain.4
The efficacy of pregabalin in treating acute postsurgical pain has been demonstrated in numerous studies. A recent meta-analysis has suggested that pregabalin, at all doses and administration regimens, has opioid-sparing effects and reduces pain scores in the postsurgical setting,5 at the expense of increased sedation and visual disturbances; however, the efficacy of pregabalin in providing such in various surgical categories remains uncertain, and it is not known whether the risk : benefit ratio is greater for certain surgical categories. Therefore, the aim of this meta-analysis was to evaluate the analgesic efficacy of pregabalin in reducing postsurgical pain in terms of 2- and 24-hour postsurgical visual analogue scale (VAS) pain scores and 24-hour accumulative morphine-equivalent consumption, in various surgical categories to provide a useful reference in perioperative care.
MATERIALS AND METHODS
Protocol
This review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for reporting meta-analyses.6 Approval by ethics committee or written consent were not required for the extraction of data on studies already conducted for the purposes of this meta-analysis. Before commencing this meta-analysis, all authors agreed on the inclusion and exclusion criteria, which were articles with at least the abstract published in English, and gave data on at least 1 of the primary outcomes. This protocol was not published.
Eligibility Criteria
Studies considered for inclusion in the meta-analysis were randomized, double-blinded, controlled trials (RCTs) that investigated a minimum of 10 subjects in each group6 and reported on relevant pain outcomes with intervention or treatment with perioperative pregabalin. These studies had to present data for at least 1 of our prespecified outcome variables, which were 2- or 24-hour postsurgical pain or 24-hour morphine-equivalent consumption.
Systematic Search
A comprehensive search for literature for pregabalin was performed between August 2014 to April 2015, using PubMed, Ovid via EMBASE, Google Scholar, and ClinicalTrials.gov with no limitation on the year of publication or language. Attempts were made at accessing www.clinicalstudyresults.org to identify potentially relevant studies that have not been published in medical journals, but the website is no longer in use. The keywords used in the search included “pregabalin,” “lyrica,” “analgesia,” “acute pain,” “post-surgical pain,” and “post-operative pain.” Identified references were screened using title, abstract, and keywords. Searches of the reference lists of identified studies were also made.
Study Selection and Data Collection
Two primary investigators (D.M.H.L. and S.W.C.) screened the titles independently and removed the studies that did not meet the specified screening criteria. Abstracts, literature reviews, and meta-analyses were excluded. Potentially eligible trials were analyzed in detail on the basis of the full text and disagreements were discussed between D.M.H.L. and S.W.C. Data extraction was performed by the 2 reviewers (D.M.H.L. and S.W.C.) independently and included data on the patient (number of subjects, type of surgery, and type of anesthesia), data on the intervention and control (dose and frequency of pregabalin administered), and data on the outcomes (pain intensity, given as acute pain scores at rest, total opioid-equivalent consumption, and adverse effects including nausea, vomiting, sedation, and visual disturbance). Assessing each study for surgical category was performed by D.M.H.L. and C.-W.C.
Data Extraction
The pain intensity measured by either VAS or numeric rating scale (NRS) was extracted as pain scores. These scales have been shown to correlate well.7 The cumulative opioid consumption at the closest time to 24 hours postsurgery was extracted and converted to an equianalgesic dose of parenteral morphine in mg, based on the following scale: 15 : 1 for hydromorphone, 1.3 : 1 for oxycodone, 1 : 100 for fentanyl, 20 : 1 for codeine, 10 : 1 for tramadol, 10 : 1 for pethidine, 4 : 1 for hydrocodone, 1 : 100 for remifentanil, 1 : 1 for piritramide, and 1 : 1 for nalbuphine.8–11 If results were presented as the number of doses given, data were extracted from the methods section to ascertain the dosage and then converted to equianalgesic dose of parenteral morphine in mg for inclusion in the meta-analysis. Data regarding postsurgical analgesic consumption were not included from studies that did not utilize opioids during the postsurgical period12–14 or if data were presented as the number of patients who required rescue analgesics, although pain scores and other information from these studies were included in the analysis whenever given.
The primary outcomes of this present study were pain scores at rest at 2 and 24 hours postsurgery, and morphine-equivalent consumption in the first 24 hours postsurgery. Secondary outcomes were sedation at first assessment and adverse effects. Pain scores at 2 hours postsurgery were selected as the first time point for analysis because pain prior to that time point might be reduced by the effects of analgesics administered during anesthesia. Where pain scores were not available at 2 hours postsurgery, the closest time point was used. Pain scores and opioid consumption at 24 hours postsurgery were chosen in this study as most trials assessed here ceased data collection after 24 hours. Where data were presented graphically, the originals were obtained from the authors or extracted from graphs if no response was obtained from the authors. Twenty-eight corresponding authors were emailed for further details regarding data in the published studies. Seventeen of the emailed authors replied with further data not available in the published articles.
Studies were classified according to surgical categories, these were gynecologic, orthopedic (not including spine surgery), spine, ear, nose and throat (ENT), cardiothoracic surgery, and laparoscopic cholecystectomy. Where studies reported cumulative data on several different surgical categories or if the authors were only able to find 1 or 2 studies of that surgical category (eg, eye surgeries and breast surgeries), these were included in a miscellaneous, or >1 surgical category, group.
Assessment of Risk of Bias
The quality of the studies was assessed by 2 investigators (D.M.H.L. and S.W.C.) independently, using the Cochrane Collaboration's tool for assessing risk of bias.15
Statistical Analysis
Meta-analysis was used to assess the pooled effects of pregabalin 2 hours and 24 hours postsurgery. If the study included different doses of pregabalin, the higher dosage was used in this analysis. Data were analyzed using Comprehensive Meta-Analysis software (version 2.2.064, Englewood, NJ). Meta-regression was not performed in this review as a minimum number of 10 studies per subgroup is required.16
VAS pain scores or NRS pain scores were extracted from each study. Mean and standard deviation (SD) values were used when available, but when median and range data were presented, the mean was estimated using the median value, or the median value itself was used if the sample size exceeded 25 subjects in each group.17 In addition to the various different scoring methods used to assess pain, another major consideration was the heterogeneity of the studies, which included different types of patients, different pregabalin regimens in terms of time, dose and frequency, and method of administration. To take into consideration the heterogeneity of the studies, Hedge g standardized mean difference, which is the difference between the 2 means divided by the pooled SD, with a correction for small sample bias, using a random-effects model was computated and reported as the effect size between the pregabalin and the control groups. Hedge g was chosen as most of the studies investigated in this meta-analysis were small (<40 subjects per group). Hedge g is also an index of treatment efficacy independent of the scoring system used to measure efficacy, which is particularly useful in the present study as VAS 0–10, VAS 0–100 and NRS have all been used as pain scoring systems.
With regard to the analysis of adverse effects of pregabalin, in studies that have categorized patients according to a score (eg, sedation score) and if continuous data were available, this was inputted as means (SD). For studies that have categorized patients according to none, slight, moderate, or severe sedation, all patients, except those who had been classified as “none” by the investigators were regarded as being sedated for the purposes of this present meta-analysis, and these data were inputted using dichotomous data handling techniques. A Forest plot was generated for each endpoint and Hedge g with 95% confidence intervals (CIs) were reported. Effects on dichotomous outcomes such as visual disturbances, nausea, vomiting, and postsurgical nausea and vomiting were reported using odds ratio (OR) with a random-effects model. Publication bias was assessed using Funnel plots (Comphrensive Meta-Analysis).18 Sensitivity analysis was assessed using the 1 study removed technique. For all tests, statistical significance was defined as a 2-tailed P value of < 0.05.
RESULTS
Our primary search strategy identified 1700 publications. Seventy-four studies were included in this meta-analysis (Supplementary Figure 1). Results here were presented as all included studies and then according to the surgical category.
Risk of Bias
The results of the risk of bias assessment are summarized in Supplementary Table 1.
Study Protocols
The study protocols of the included trials varied significantly and led to considerable heterogeneity.
It is important to note that the primary outcomes as defined in this meta-analysis were not necessarily the primary outcomes of the published trials, and therefore those trials might not be powered to detect significant differences for the variables included in this meta-analysis. The primary outcomes of the trials are given in Tables 1–7 .
TABLE 1.
Characteristics of Studies in the Cardiothoracic Surgery Category
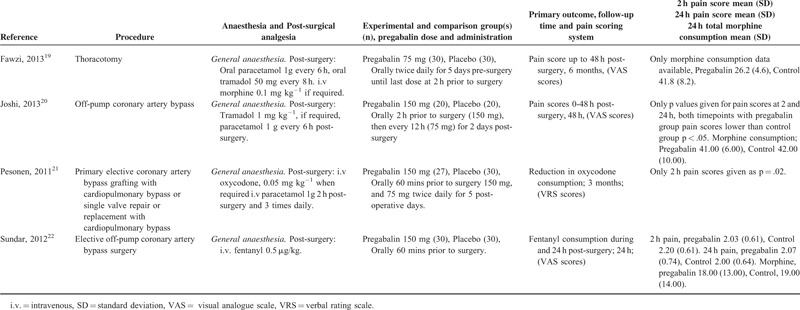
TABLE 5 (Continued).
Characteristics of Studies in the Orthopedic Surgery Category
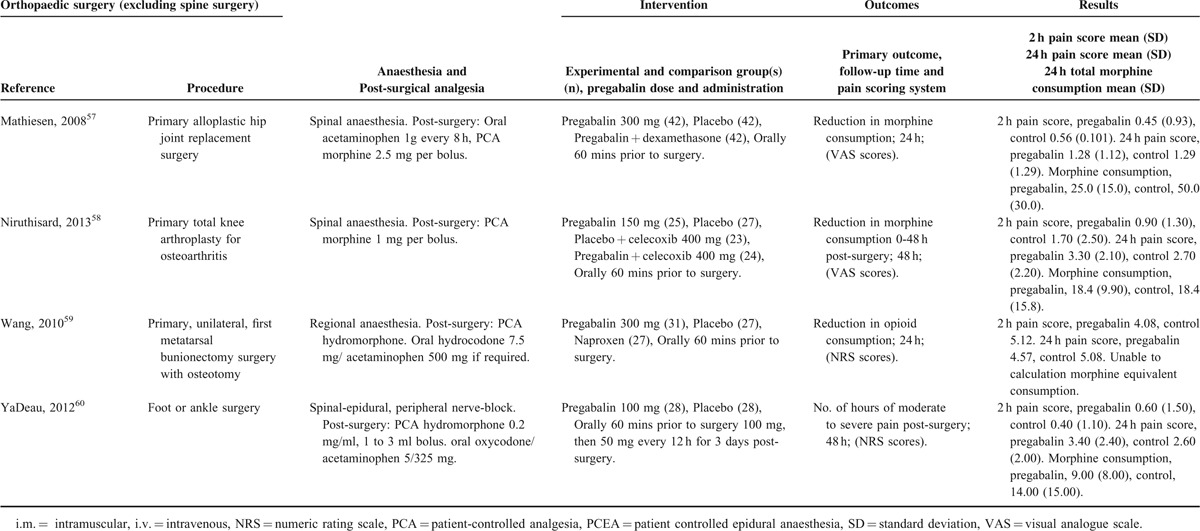
TABLE 2.
Characteristics of Studies in the ENT Surgery Category
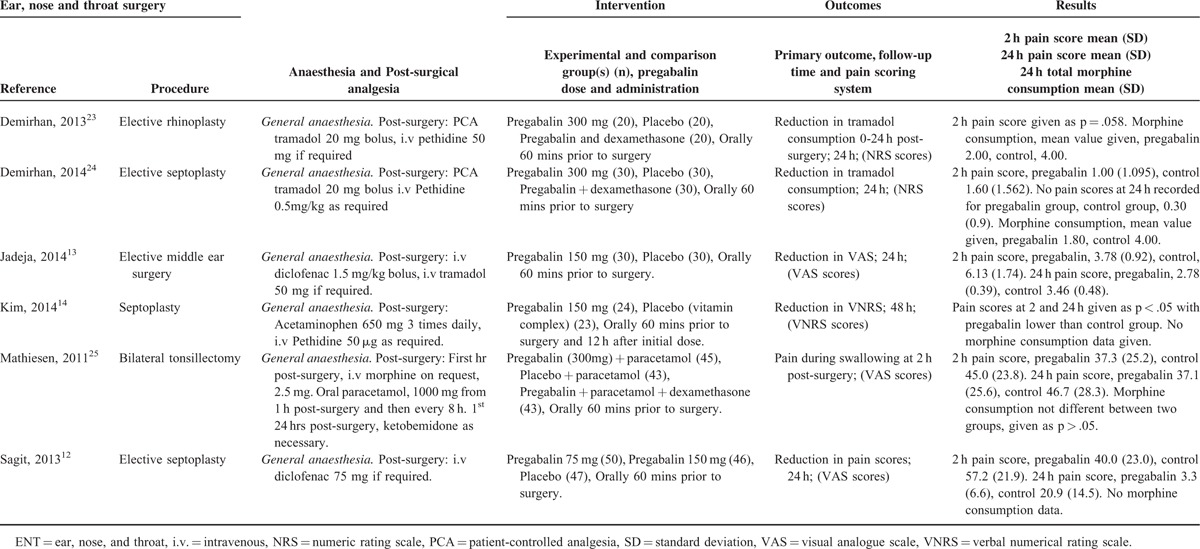
TABLE 3.
Characteristics of Studies in the Gynecologic Surgery Category
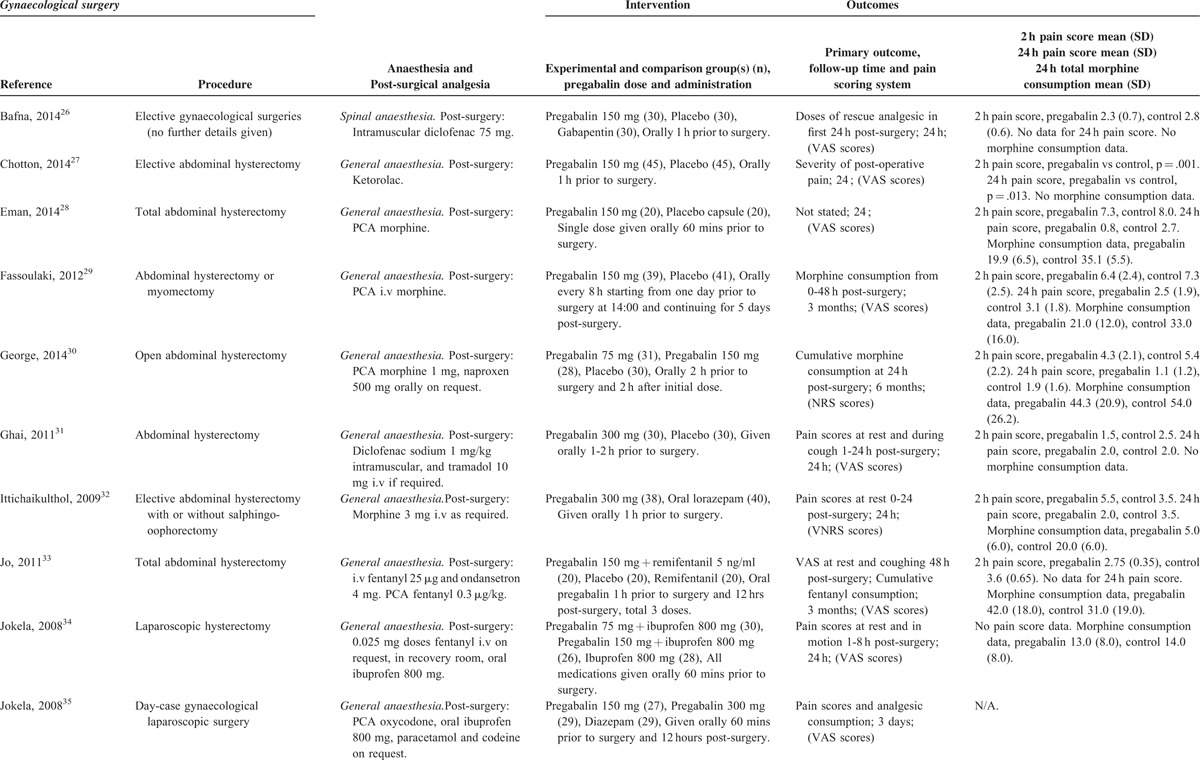
TABLE 3 (Continued).
Characteristics of Studies in the Gynecologic Surgery Category
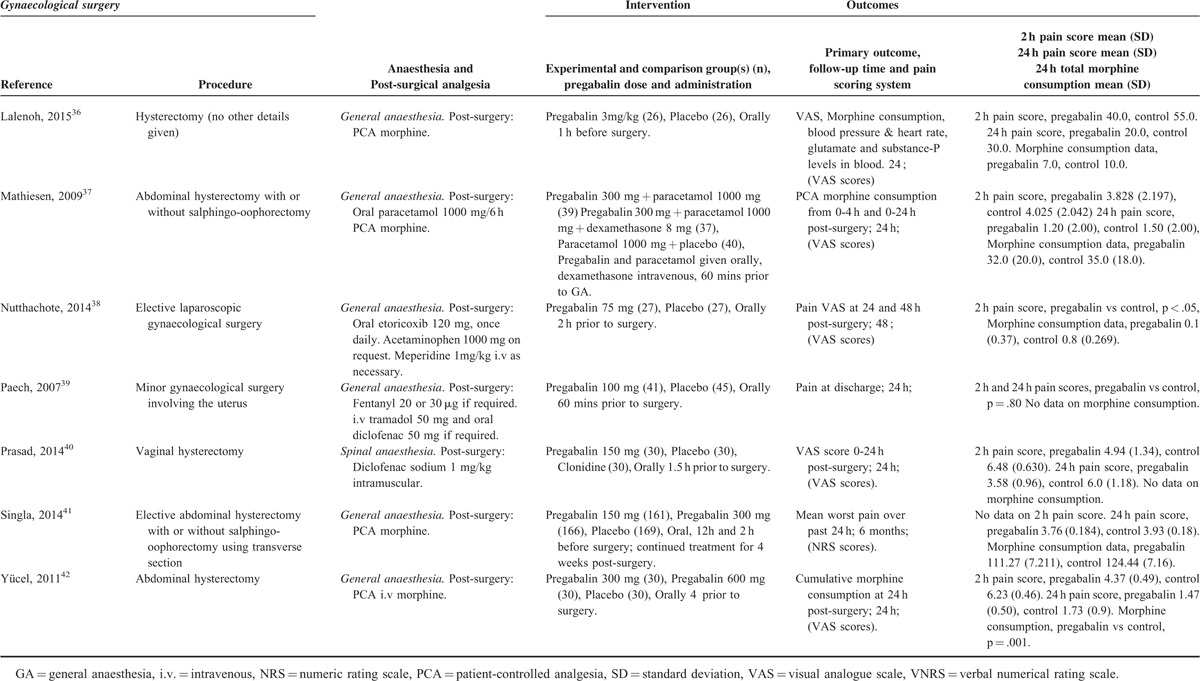
TABLE 4.
Characteristics of Studies in the Laparoscopic Cholecystectomy Category
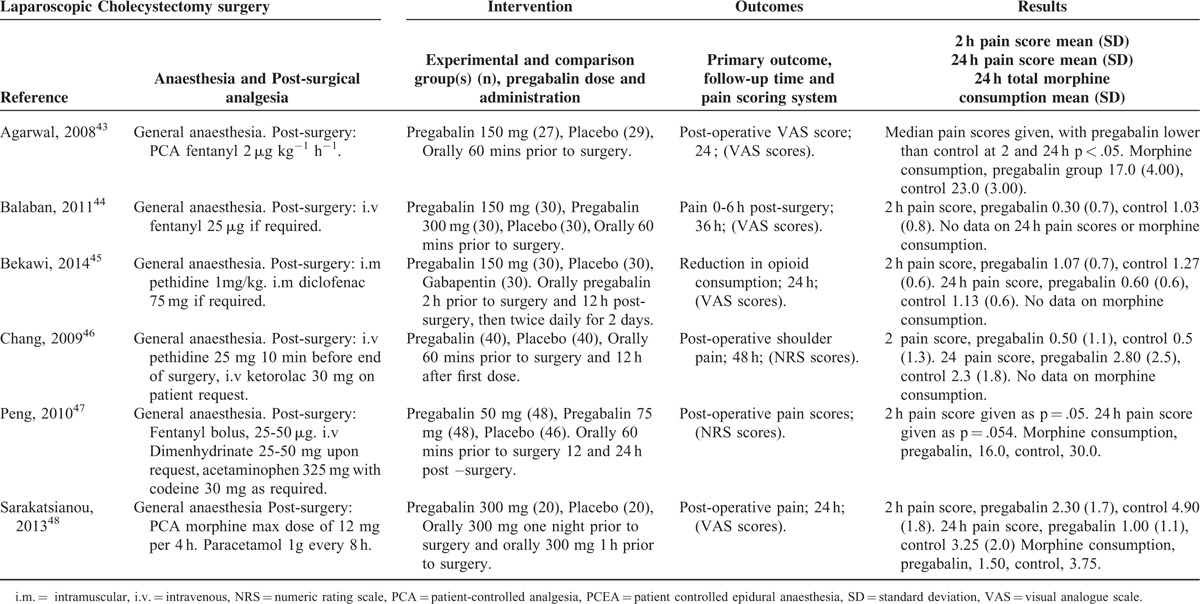
TABLE 5.
Characteristics of Studies in the Orthopedic Surgery Category
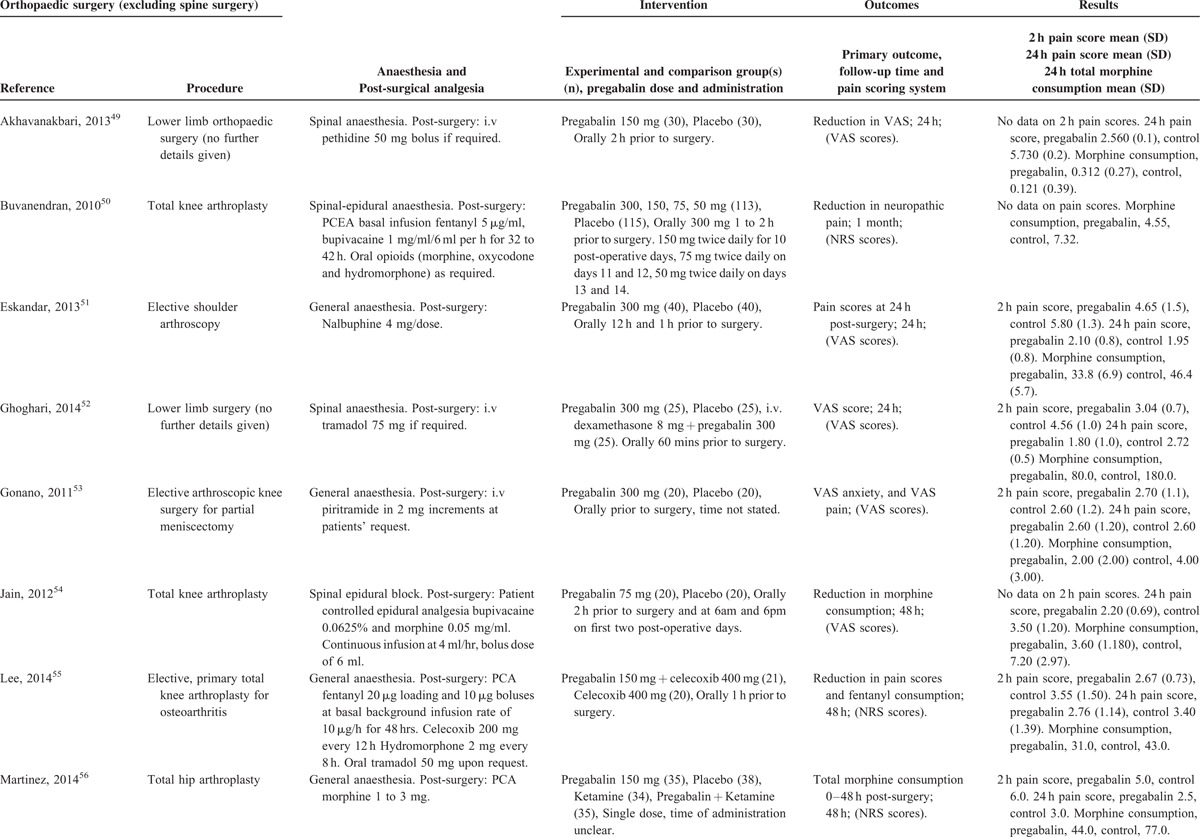
TABLE 7.
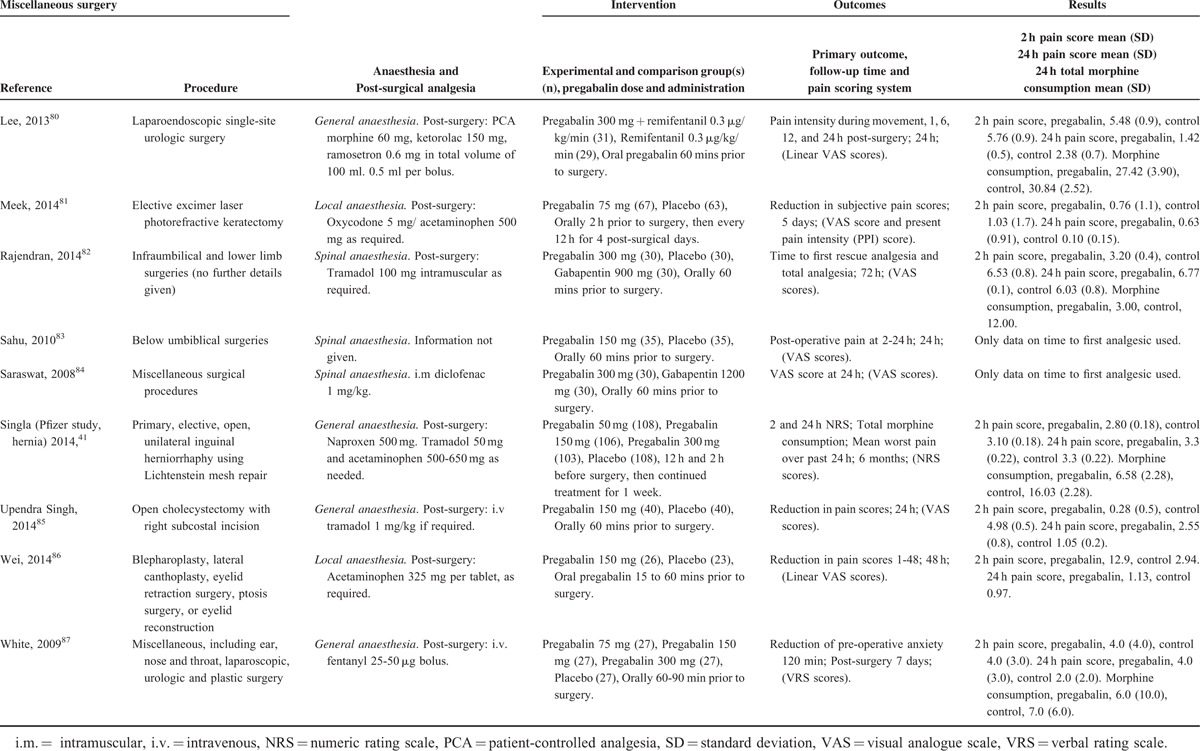
Characteristics of Studies in the Miscellaneous Surgery Category
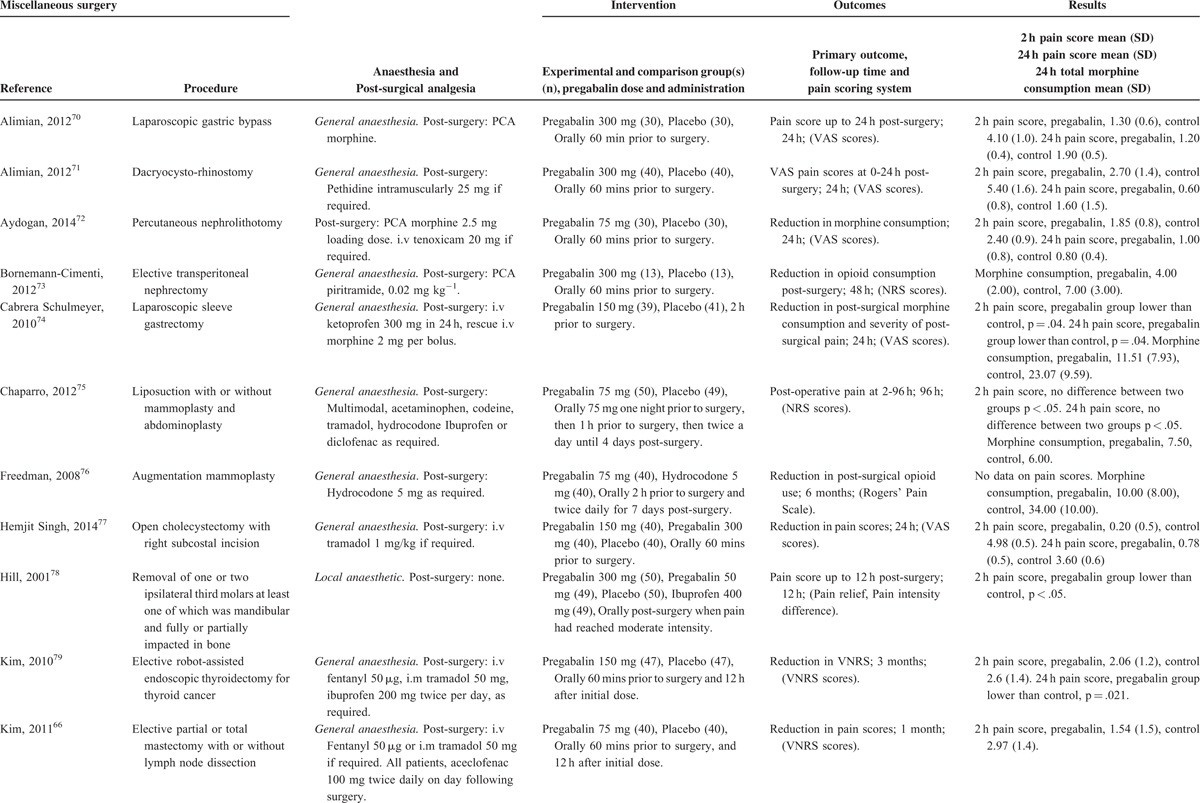
TABLE 7 (Continued).
Characteristics of Studies in the Miscellaneous Surgery Category
Effect of Pregabalin on Primary Outcomes in all Surgical Categories
Two-Hour VAS pain scores
A total of 60 studies with a total of 2019 patients taking pregabalin and 2019 patients on the control treatment that reported pains scores at or around 2 hours postsurgery were included. Overall, pregabalin reduced VAS pain scores at 2 hours postsurgery (Hedge g and 95%CI, −0.970 [−1.197 to −0.743], z score −8.389, P < 0.0001), Figure 1.
FIGURE 1.
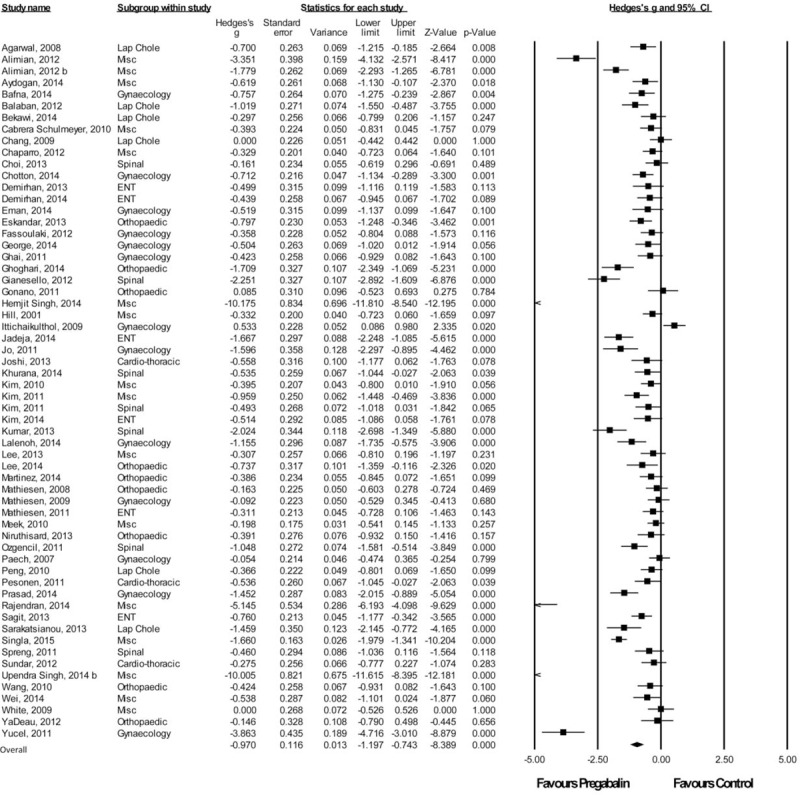
Forest plot for 2-hour pain scores.
Twenty-Four Hour VAS Pain Scores
A total of 57 studies with a total of 2033 patients taking pregabalin and 2033 patients on the control treatment that reported pains scores at 24 hours postsurgery were included. Overall, pregabalin reduced pain scores at 24 hours postsurgery (Hedge g and 95%CI, −0.442 [−0.665 to −0.220], z score −3.894, P < 0.0001), Figure 2.
FIGURE 2.
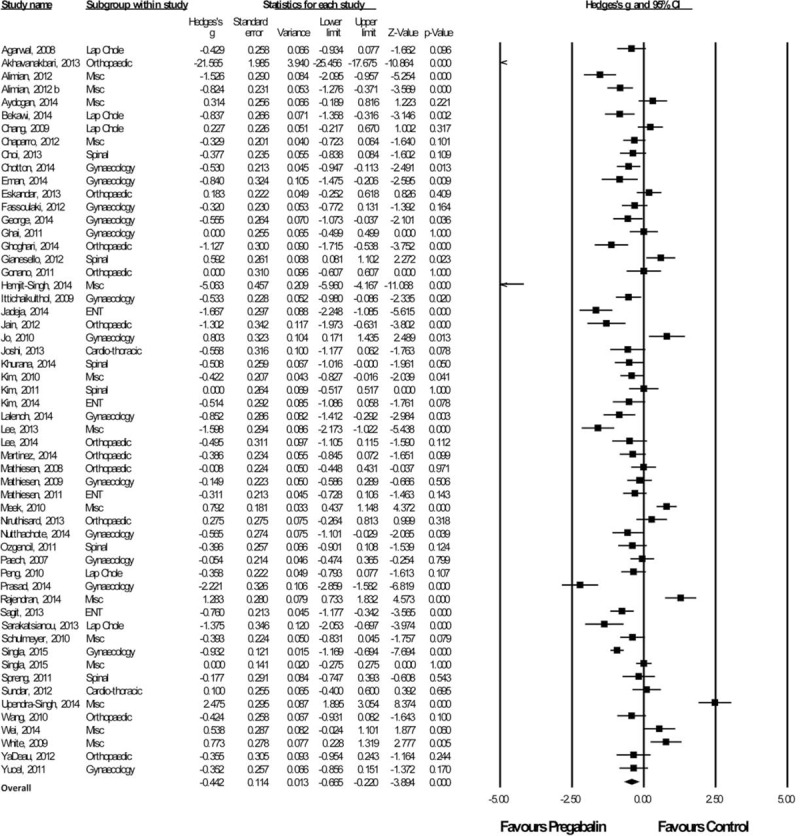
Forest plot for 24-hour pain scores.
Subgroup Analysis According to Dosing Regimen
Fifty-five studies that provided information on 24-hour pain scores were categorized according to whether a single dose (prior to surgery) or multiple doses (starting from the night, or days prior to surgery) were administered. There was no difference seen in 24-hour pain scores in these 2 subgroups. Pregabalin reduced pain scores at 24 hours postsurgery regardless of whether a single dose (Hedge g and 95%CI, −0.566 [−0.914 to −0.218], z score −3.191, P = 0.001), or multiple doses were administered (Hedge g and 95%CI, −0.322 [−0.571 to −0.073], z score −2.536, P = 0.011).
Twenty-Four Hour Morphine-Equivalent Consumption
Forty-six studies with a total of 1610 patients taking pregabalin and 1636 patients on the control treatment that reported morphine-equivalent consumption at 24 hours postsurgery were included. Overall, pregabalin reduced morphine-equivalent consumption at 24 hours postsurgery (Hedge g and 95%CI, −0.932 [−1.212 to −0.652], z score −6.519, P < 0.0001), Figure 3.
FIGURE 3.
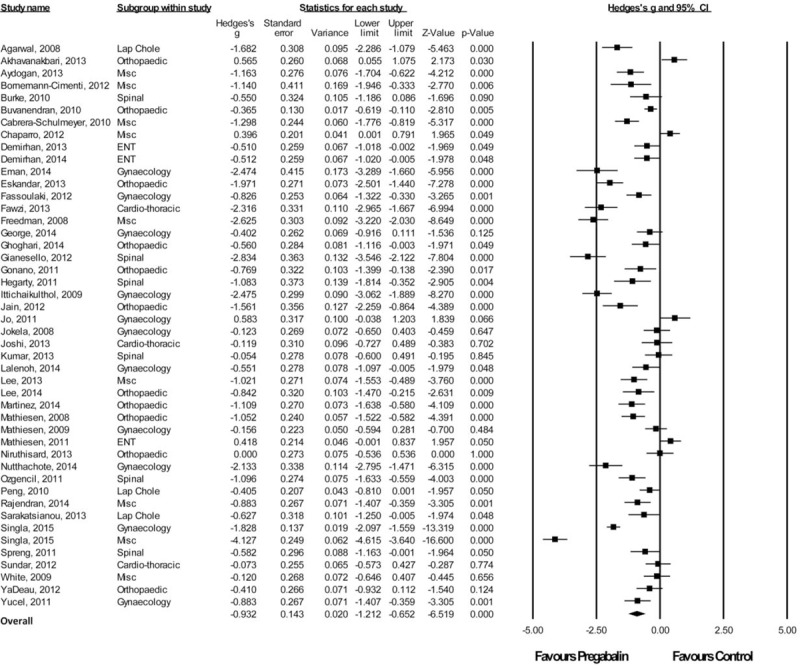
Forest plot for 24-hour morphine-equivalent consumption.
Effect of Pregabalin on Primary Outcomes in Different Surgical Categories
Cardiothoracic Procedures
There were 4 studies19–22 with a total of 107 patients taking pregabalin and 110 patients on the control treatment in this group. Pregabalin reduced the pain score at rest 2 hours postsurgery (P = 0.005). No significant difference was seen in pain score at rest at 24 hours postsurgery (P = 0.537) or morphine-equivalent consumption (P = 0.239), Figure 4 (Table 8).
FIGURE 4.
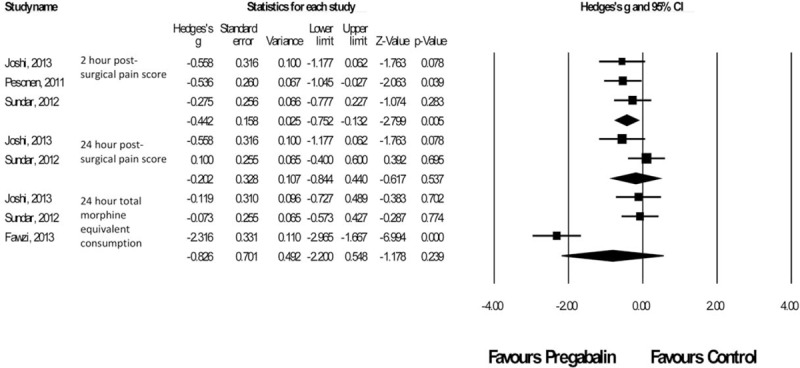
Forest plot for primary outcomes of studies under the cardiothoracic surgery category.
TABLE 6.
Characteristics of Studies in the Spine Surgery Category
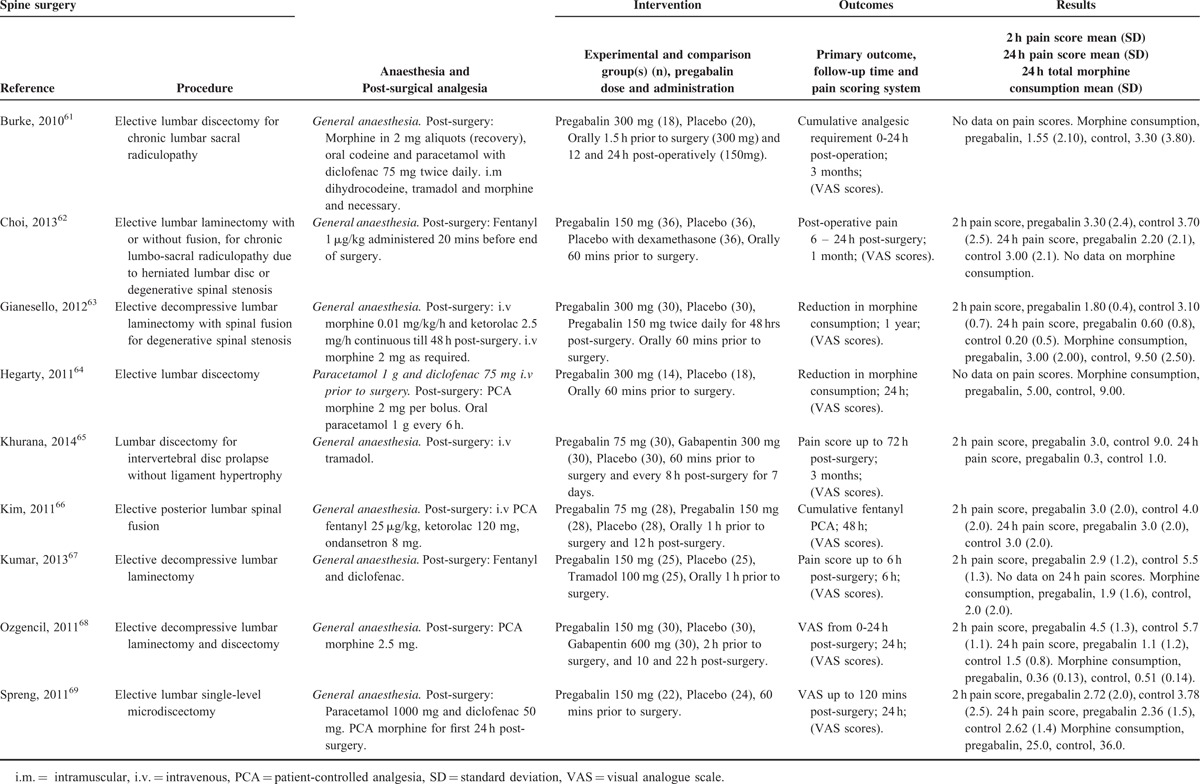
TABLE 8.
Summary of Results According to Surgical Type
ENT Procedures
There were 6 studies12–14,23–25 with a total of 265 patients taking pregabalin and 266 patients on the control treatment in this group. Pregabalin reduced the pain score at rest 2 hours postsurgery (P < 0.0001) and pain score at rest at 24 hours postsurgery (P = 0.004). No statistically significant reduction in morphine-equivalent consumption was seen (P = 0.568), Figure 5.
FIGURE 5.
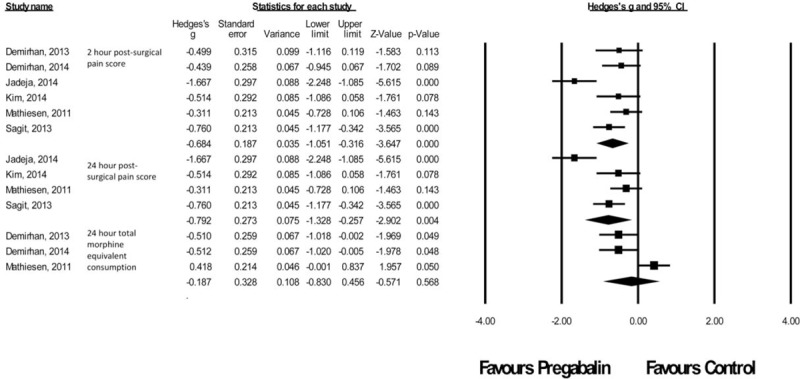
Forest plot for primary outcomes of studies under the ear, nose and throat surgery category.
Gynecologic Procedures
There were 17 studies26–42 with a total of 980 patients taking pregabalin and 730 patients on the control treatment in this group. Pregabalin reduced the pain score at rest 2 hours postsurgery (P < 0.0001), pain score at rest at 24 hours postsurgery (P = 0.001), and the morphine-equivalent consumption (P = 0.001), Figure 6A.
FIGURE 6.
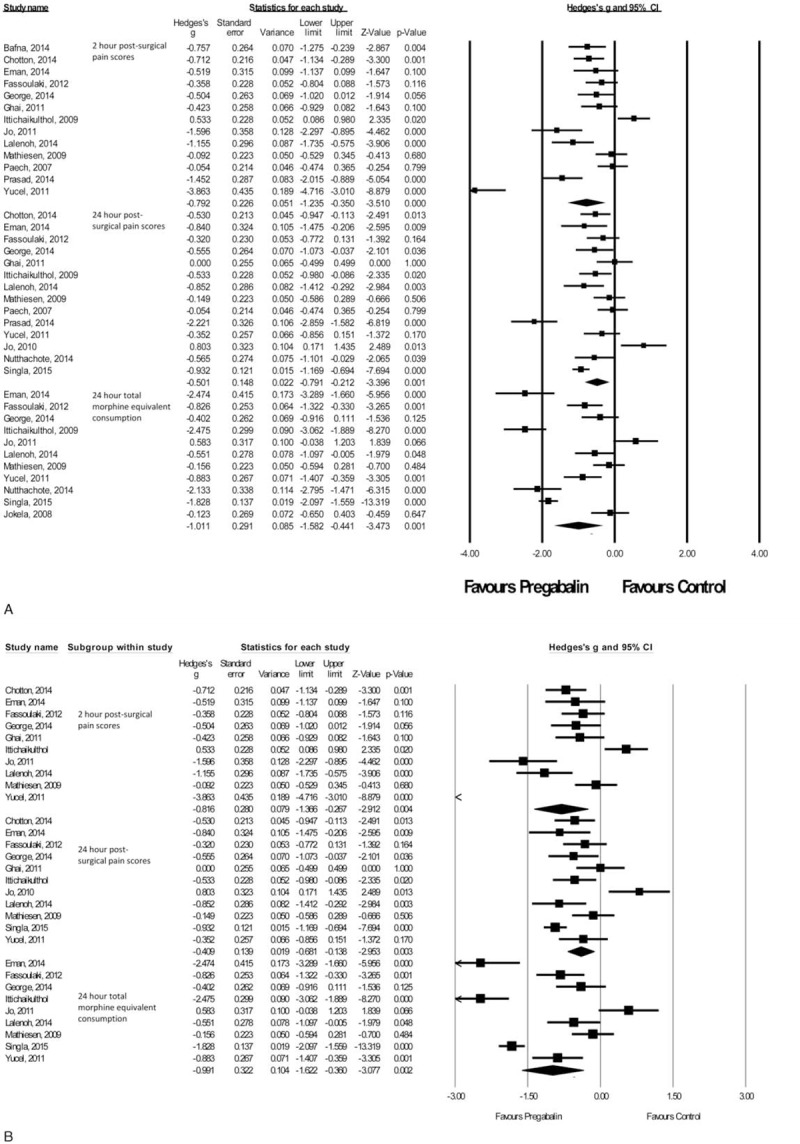
A,B Forest plot for primary outcomes of studies under the gynecologic surgery category.
Due to the heterogeneity within the gynecologic group, a subanalysis was performed on open hysterectomy studies only.27–33,36,37,41,42 There were 11 studies with a total of 468 patients taking pregabalin and 485 patients on the control treatment in this group. Pregabalin reduced the pain score at rest 2 hours postsurgery (P = 0.004), pain score at rest at 24 hours postsurgery (P = 0.003), and the morphine-equivalent consumption (P = 0.002), Figure 6B.
Laparoscopic Cholecystectomy Procedures
There were 6 studies43–48 with a total of 273 patients taking pregabalin and 225 patients on the control treatment in this group. Pregabalin reduced the pain score at rest 2 hours postsurgery (P = 0.003), pain score at rest at 24 hours postsurgery (P = 0.036), and the morphine-equivalent consumption (P = 0.023), Figure 7.
FIGURE 7.
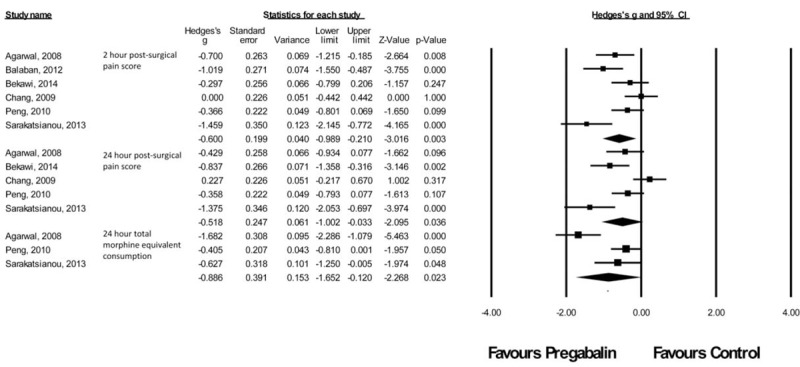
Forest plot for primary outcomes of studies under the laparoscopic cholecystectomy category.
Orthopedic Procedures
There were 12 studies49–60 with a total of 430 patients taking pregabalin and 642 patients on the control treatment in this group. Pregabalin reduced the pain score at rest 2 hours postsurgery (P = 0.001), pain score at rest at 24 hours postsurgery (P = 0.013), and the morphine-equivalent consumption (P < 0.0001), Figure 8.
FIGURE 8.
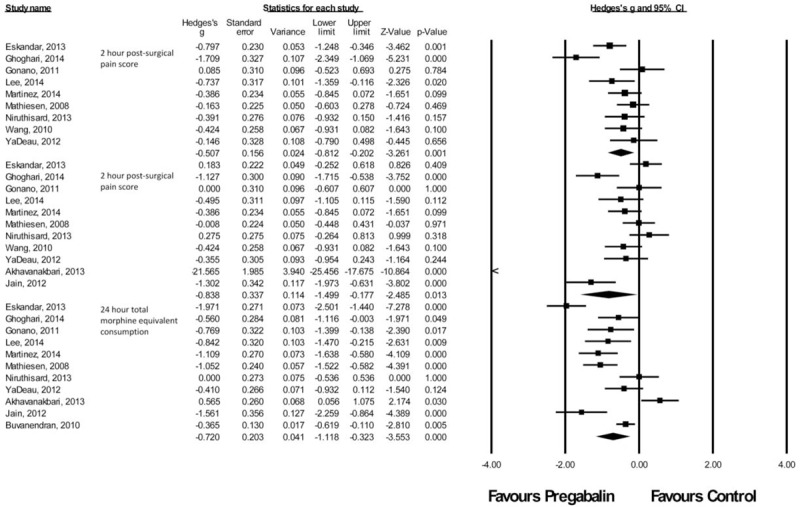
Forest plot for primary outcomes of studies under the orthopedic surgery category.
Spine Procedures
There were nine studies61–65,67–69,88 with a total of 291 patients taking pregabalin and 332 patients on the control treatment in this group. Pregabalin reduced the pain score at rest 2 hours postsurgery (P = 0.001) and the morphine-equivalent consumption (P = 0.005). No significant difference was seen in pain score at rest at 24 hours postsurgery (P = 0.373), Figure 9.
FIGURE 9.
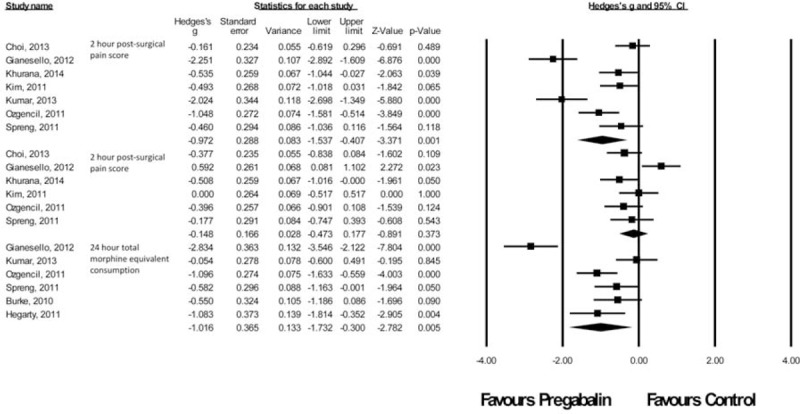
Forest plot for primary outcomes of studies under the spine surgery category.
Miscellaneous Procedures
There were 20 studies41,66,70–87 with a total of 1165 patients taking pregabalin and 884 patients on the control treatment in this group. Pregabalin reduced the pain score at rest 2 hours postsurgery (P < 0.0001) and the morphine-equivalent consumption (P = 0.006). No significant difference was seen in pain score at rest at 24 hours postsurgery (P = 0.422), Figure 10.
FIGURE 10.
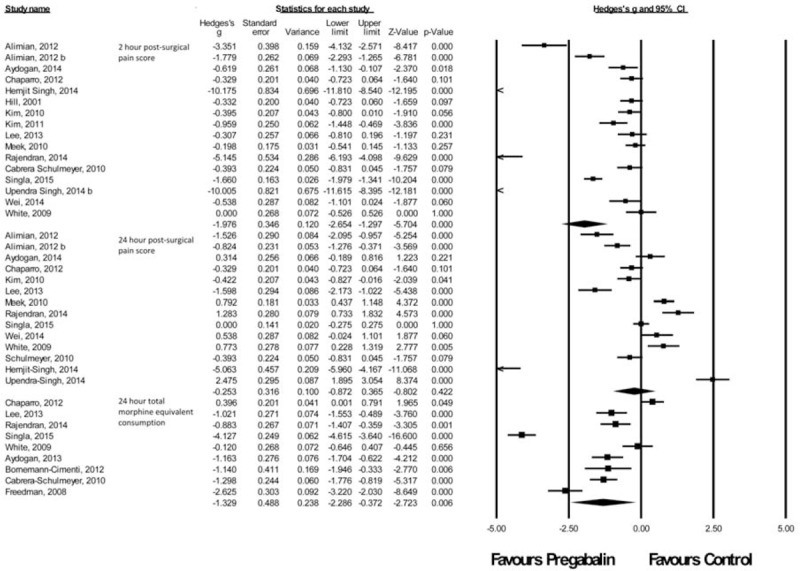
Forest plot for primary outcomes of studies under the miscellaneous surgery category.
Common Adverse Effects of Pregabalin
Sedation Effects of Pregabalin
Thirty studies had included data on the sedative effects of pregabalin,13,14,21,22,25,29,30,32,37,39,43,50–52,55–57,59,60,62,65–69,77,79,82,85,87,88 with a total of 1147 patients taking pregabalin and 1170 patients on the control treatment. Subgroup analysis was performed on studies according to the surgical categories (number of studies) under cardiothoracic surgery (2), ENT surgery (3), gynecologic surgery (4), laparoscopic cholecystectomy (2), orthopedic surgery (7), spine surgery (6), and miscellaneous surgery (6). Data from George et al30 could not be included in the analysis as there was no difference in sedation between the treatment and control group. With the exception of ENT surgery, laparoscopic cholecystectomy and gynecologic surgery, pregabalin was associated with sedation in all other surgical categories (overall OR and 95% CI, 2.144 [1.640–2.803], z score 5.574, P < 0.0001), Table 8.
Visual Disturbances
Fifteen studies had included data on incidence of visual disturbance (including blurred vision) after pregabalin administration,24,34,35,39,46,48,51,62,64,68,77,79,80,85,88 with a total of 491 patients taking pregabalin and 498 patients on control treatment. There were not enough studies under different surgical categories for subgroup analyses to be performed. Overall, pregabalin was found to be associated with an increased incidence of visual disturbances (OR and 95%CI, 6.215 [3.317–11.646], z score 5.702, P < 0.0001).
Nausea
Thirty-one studies had included data on nausea prevalence after pregabalin administration,13,21,24,26,28,30,33,37,44,46,47,50–52,54,56,57,59,60,63–65,67,68,70,71,77,85,86,88 with a total of 1067 patients taking pregabalin and 1038 patients on the control treatment. It was found that there was no difference in nausea incidence in cardiothoracic surgery, ENT surgery, gynecologic surgery, laparoscopic cholecystectomy, and spine surgery between pregabalin and control treatment groups. Pregabalin administration was associated with reduced incidence of nausea in miscellaneous surgery (OR and 95% CI, 0.138 [0.073–0.262], z score −6.085, P < 0.0001) and orthopedic surgery (OR and 95% CI, 0.586 [0.377–0.911], z score −2.373, P < 0.018). Overall results showed that pregabalin reduced postsurgical nausea (OR and 95% CI, 0.478 [0.365–0.626], z score −5.364, P < 0.0001).
Vomiting
A total of 22 studies provided information on vomiting incidence after pregabalin administration,13,21,23,25,44,46,47,50–52,54,56,59,60,63,65,67,68,70,71,77,85 with 826 patients treated with pregabalin and 816 on control treatment. The different surgical categories were cardiothoracic surgery (1), ENT surgery (3), laparoscopic cholecystectomy (3), miscellaneous surgery (4), orthopedic surgery (7), and spine surgery (4). In subgroup analysis, pregabalin was associated with reduced vomiting only after miscellaneous procedures (OR and 95% CI, 0.163 [0.073–0.368], z score −4.375, P < 0.0001), but pregabalin was found to be associated with reduced postsurgical vomiting in overall analysis (OR and 95% CI, 0.468 [0.328–0.668], z score −4.173, P < 0.0001).
Postsurgical Nausea and Vomiting
A total of 20 studies provided data on postsurgical nausea and vomiting (PONV) incidence after pregabalin administration,12,14,27,31,32,34,35,38,40,42,43,45,62,66,69,73,79,80,82 with 638 patients treated with pregabalin and 644 on control treatment. The different surgical categories were ENT surgery (2), gynecologic surgery (8), laparoscopic cholecystectomy (2), miscellaneous surgery (6), and spine surgery (2). In subgroup analysis, pregabalin was associated with reduced PONV in miscellaneous surgery only (OR and 95% CI, 0.528 [0.309–0.902], z score −2.339, P < 0.019) but pregabalin was found to be associated with reduced PONV in overall analysis (OR and 95% CI, 0.592 [0.415–0.845], z score −2.887, P < 0.004).
No evidence of publication bias was seen using funnel plot analysis with regard to 2- and 24-hour pain scores and 24-hour morphine-equivalent consumption (Supplementary Figures 2A to C), or with regard to adverse effects (Supplementary Figures 2D to h).
DISCUSSION
This present meta-analysis shows that perioperative administration of pregabalin significantly reduced VAS pain scores at 2 hours postsurgery in all surgical categories, and at 24 hours postsurgery in all surgical categories with the exception of cardiothoracic and spine procedures. Total morphine consumption at 24 hours postsurgery was significantly reduced in all surgical categories with the exception of cardiothoracic and ENT procedures. Adverse effects include significant sedation after pregabalin in cardiothoracic, orthopedic, spine, and miscellaneous procedures. PONV was significantly reduced after pregabalin in all, except miscellaneous procedures. Taken together, results of this meta-analysis show that pregabalin is useful in reducing postsurgical pain as well as reducing morphine consumption, with concomitant reduction in PONV.
It has long been recognized that different surgical procedures require procedure-specific pain management.89–92 It is evident that the degree of pain experienced by patients after different surgical procedures is not universal, and even some laparoscopic approaches might result in unexpectedly high levels of postsurgical pain.93,94 Moreover, the analgesic efficacy of different pain medications might also be different in different types of surgery. The analgesic efficacy of paracetamol is 2-fold less in orthopedic compared with dental procedures.95 It has also been found that the analgesic efficacy between nonsteroidal anti-inflammatory agents and paracetamol depends on the magnitude of the surgical procedure.96 In addition to differing analgesic effects of the same drug under different conditions, a 50% decrease in pain might have a different clinical relevance depending if it were a reduction from 4 to 2, or 8 to 4 on the VAS pain scale.97 Therefore, specific recommendations for surgical procedures including abdominal hysterectomy, laparoscopic cholecystectomy, and total knee arthroplasty have been made.98 It is in recognition that pain management should be procedure-specific that provided the insight to take this approach of subgroup analysis for this current investigation.
A previous meta-analysis of 11 RCTs10 concluded that presurgical pregabalin administration did reduce 2-hour pain scores and postsurgical opioid requirement. The authors divided the studies under investigation by pregabalin dose, <300 or ≥300 mg and found that the higher dose reduced opioid consumption more than the lower dose. Pregabalin also reduced opioid-related adverse effects such as vomiting, but the risk of visual disturbance was greater. Another recently conducted meta-analysis on 55 RCTs5 concluded that when all doses and administration regimens were combined, pregabalin was associated with a significant reduction in pain scores at rest and during movement and opioid consumption at 24 hours compared with placebo. Pregabalin was also associated with less postsurgical nausea, vomiting, and pruritus, although it was associated with higher incidence of sedation, dizziness, and visual disturbance. These previous meta-analyses have been criticized for not having investigated surgical specific-opioid consumption as different procedures will result in different opioid requirements.99 Hence, this caveat has been addressed in the present meta-analysis. This meta-analysis is the first study to investigate the efficacy of pregabalin when used under different surgical procedures in acknowledgment that different surgical procedures result in variable pain intensity and different opioid requirements,94 and that the efficacy of perioperative analgesia varies according to surgical type.98 By identifying the types of surgery that would benefit from pregabalin, clinicians can improve efficiency in treating acute postsurgical pain and can better allocate resources.
This present meta-analysis is the first to show that the analgesic effect of perioperative pregabalin is procedure specific. With regard to the cardiothoracic procedure category, pain at 2 hours postsurgery was significantly lower in the pregabalin group, but no difference was seen at 24 hours postsurgery. It should be noted that only 2 studies showed data for 24-hour VAS pain scores, therefore there are insufficient data to draw definitive conclusions, and the only study showing reduction in morphine consumption after pregabalin did not show either 2-, or 24-hour VAS pain scores. No data on PONV were given and significant sedation was seen after pregabalin, so although overall, pregabalin appears to be efficacious for acute postsurgical pain in cardiothoracic procedures, caution should be exercised when deciding to use pregabalin.
In the ENT category, although both 2- and 24-hour postsurgical pain was shown to be reduced in the pregabalin group, there was no difference in total morphine-equivalent consumption at 24 hours between pregabalin and the control group. PONV is more common in patients undergoing ENT, compared with other procedures,100 and as no difference was seen in either sedation or PONV, pregabalin can be recommended for use in ENT procedures.
There is strong evidence to recommend the use of pregabalin in gynecologic procedures, due to the large effects sizes with regard to pain reduction, and no evidence of increased sedation and PONV.
With regard to laparoscopic cholecystectomy, caution should be exercised when considering pregabalin, as although pain scores at 2 and 24 hours, and morphine-equivalent consumption are reduced, the OR seen for sedation was extremely high, even though, due to the heterogeneity of the studies, this was not statistically significant. Pain scores tend to be low after laparoscopic cholecystectomy procedures (not >5 on the VAS at 2 hours postsurgery according to the studies included here), and as pain reduction at 24 hours postsurgery and total morphine-equivalent consumption is modest in terms of effect-size, the risk–benefit ratio should be carefully considered.
Although pain scores at 2 and 24 hours, and morphine-equivalent consumption are reduced in orthopedic surgery, the reduction of pain scores at 2 hours is modest and sedation was significantly increased in the pregabalin group. The increased risk of sedation might be preferable when weighed with the significantly decreased morphine-equivalent consumed. Considering that many orthopedic procedures are performed in the elderly101 the risk of sedation might outweigh the benefit of modest decrease in pain scores.
With regard to spinal, and also miscellaneous surgeries, a large decrease in pain at 2 hours and total morphine consumption was seen, although there was no reduction in pain at 24 hours postsurgery. Considering the high incidence of sedation, in both spinal and miscellaneous surgical procedures, pregabalin should be used with caution.
It should be noted that although statistically significant reductions in the pain scores were noted in all surgical procedures in this meta-analysis, the magnitude of effect is relatively small. For example, in Bafna et al,26 Balaban et al,44 Aydogan et al,72 Eskandar and Ebeid,51 and Lee et al,55 statistically significant decreases in pain scores at 2 hours postsurgery were reported, although the standard difference in mean pain scores between pregabalin and the control group was only <1 point on the VAS pain score. Studies on clinically significant decreases in VAS/NRS pain scores have demonstrated that an average decrease in pain score of at least 1.80 points on NRS scores or 1.3 to 2.8 points on VAS pain scores are required for the decrease to be considered clinically meaningful.102,103 The reduction in pain scores demonstrated in the studies included in this meta-analysis may reach statistical significance, but might be too small to be considered of clinical significance.
An interesting finding from study by Mishriky et al5 was that a single preoperative dose was as effective as multiple doses, and that smaller doses (≤75 mg) were as effective as larger (300 mg) doses in terms of reducing opioid consumption. It was beyond the scope of this present meta-analysis to subdivide the studies according to surgical-type as well as dosages and dosing regimens, although an analysis of single versus multiple doses did not reveal any differences in efficacy regarding 2-hour postsurgical pain. Subgroup analyses performed in this present meta-analysis according to whether single or multiple doses of pregabalin were used showed a statistically significant reduction in 24-hour postsurgical pain for both single and multiple dose, contrary to previous studies.10 In particular, with regard to the gynecologic category, it was noted, that 8 out of 13 studies showed significant reduction in 24-hour postsurgical pain score, of which, 6 studies used a single-dose of pregabalin and 2 used multiple dose. The dose of pregabalin used included low dose (≤75 mg), intermediate dose (100–150 mg), and high dose (>150 mg). Sensitivity analysis data from this present meta-analysis do not show that higher doses were more effective at reducing pain scores when compared with lower doses (data not shown). There is no evidence from this current meta-analysis to recommend multiple dosing, or dosages >75 mg, in any of the surgical procedures that has investigated dosing.
Well established adverse effects of pregabalin are sedation, dizziness, and headache, and so pregabalin should be used with caution in an ambulatory setting.104 As shown in previous meta-analyses, pregabalin is associated with increased incidence of visual disturbances and sedation; but reduced incidence of PONV.5,10 Of the 15 studies included in this analysis that showed such an association, only 4 provided information on morphine-equivalent consumption. Two of these 4 studies showed a pregabalin-associated reduction in morphine-equivalent consumption, whereas the remaining 2 showed no reduction. Due to the limited data available, it is not possible to ascertain whether the reduction in incidence of PONV is due to a direct effect of pregabalin or a result of reduced opioid consumption. Opioids are considered the primary analgesic therapy in postsurgical pain, but are associated with many dose-related adverse effects such as sedation, respiratory depression, postsurgical nausea and vomiting, urinary retention, ileus, and constipation.105 This meta-analysis shows that administration of pregabalin reduced morphine-equivalent consumption in most surgical categories, and looking at the effect size data show that there is up to 30% reduction. These data indicate pregabalin is useful to reduce opioid induced adverse effects, as seen by the reduced incidence of nausea and vomiting.
Meta-analyses have been conducted to assess the effects of perioperative gabapentin on postoperative pain,106–108 and although all the studies concluded that perioperative gabapentin was able to reduce postsurgical pain and 24-hour morphine consumption, a recent meta-regression on RCTs of perioperative gabapentin that included 133 trials, found that these effects of gabapentin might have been overestimated by statistically significant small study effects.109 Small study effects may also explain the difference in findings between our current meta-analysis and previously published work on pregabalin.
A problem inherent with meta-analyses using the random-effects model is the assumption that the effects underlying different studies are drawn from a normal distribution.110 This is seldom true, especially in the case of pain scores, which commonly show a skewed distribution. Much data used in this present meta-analysis were drawn from median, rather than the mean values required. Efforts were made to reduce the impact of clinical heterogeneity by analyzing data according to the type of surgery. Some studies are heterogeneous in themselves in that the investigators had included different surgical types in their own analysis.84 Methodologic heterogeneity also exists in the assessment of pain and sedation. In addition to the commonly used VAS pain scores and NRS pain scores, which have been shown to correlate well,7 Roger Pain Scale was used in 1 study.76 Similarly, with regard to assessing sedation, both the Ramsay Sedation Scale and Richmond Agitation Sedation Scale were used. Although these scales have been shown to correlate well,111 some studies have neither stated with which method they have assessed sedation, nor at which time postsurgery, was the assessment carried out. Some studies have instead reported on either presence or absence of somnolence and these data were excluded in the analysis for sedation effects. It is noted here although that none of the studies included in this present meta-analysis were powered to assess pregabalin-associated adverse effects, as these were secondary outcomes of the studies.
In the setting of an ideal RCT, subjects are placed in a closely monitored environment, where their pain intensity is regularly assessed. Analgesia is provided on demand by the nursing staff in the form of nursing-controlled analgesia or delivered by the subjects themselves using patient-controlled analgesia (PCA). The pain intensity of both control and treatment group should therefore be titrated to similar levels, although total opioid consumption and time to first analgesic would differ between the 2 groups based on the effectiveness of the treatment. Limitations certainly exist for both nursing-controlled analgesia and PCA in providing adequate analgesia. For the former, inadequacy of nursing staff can result in delay in delivering analgesics; for the latter, malfunctioning, poor initial titration, or incorrect setup of the PCA instruments can also prevent timely delivery of analgesics. Such limitations, however, would apply to both control and treatment group in a well-conducted trial and the pain scores of both control and treatment group will therefore be similar. It is proposed here that pain scores should only be 1 of the primary outcomes in such trials, whereas the more pertinent parameters would be changes in analgesic consumption and in time to first analgesic requirement.
Pain is not only affected by gender, age, and psychologic well-being, but also by polygenetic elements. The current list of genetic polymorphisms that may affect the action of analgesics is growing rapidly, but 1 of the enzyme systems of high relevance to opioids is the cytochrome P450 system.112 As it has been shown that polymorphisms that affect opioid metabolism are found in up to 30% of the general population,112 future clinical trials utilizing opioid consumption as an outcome could take genetic variability into consideration. The fact that none of the studies included here have factored in the genetic variability in opioid metabolism brings in another layer of heterogeneity, especially when an increase in opioid requirement in 1 or 2 patients can have substantial impact in the overall results.
Out of the 74 studies assessed in this meta-analysis, only 12 investigated the effects of pregabalin on chronic (≥3 months) postsurgical pain.19,21,29,30,33,41,61,63,65,76,79 Chronic postsurgical pain is an underexplored area and more studies are required to assess the efficacy of pregabalin in this regard.
CONCLUSIONS
In conclusion, the analgesic efficacy and adverse effects of pregabalin might not be similar under all surgical categories. Although sedation may be increased, especially in cardiothoracic, spinal, and miscellaneous procedures, this was not seen in ENT, gynecologic, or laparoscopic cholecystectomy procedures. Two-hour VAS scores were reduced in all procedures, but effect sizes varied greatly. Taken together, this meta-analysis shows strong evidence that consideration for the use of pregabalin in postsurgical pain should be procedure-specific.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, ENT = ear, nose and throat, NRS = numeric rating scale, OR = odds ratio, PCA = patient-controlled analgesia, PONV = postoperative nausea and vomiting, RCT = randomized controlled trial, SD = standard deviation, VAS = visual analogue scale.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia 2004; 45 suppl 6:13–18. [DOI] [PubMed] [Google Scholar]
- 2.Shneker BF, McAuley JW. Pregabalin: a new neuromodulator with broad therapeutic indications. Ann Pharmacother 2005; 39:2029–2037. [DOI] [PubMed] [Google Scholar]
- 3.Hindmarch I, Trick L, Ridout F. A double-blind, placebo- and positive-internal-controlled (alprazolam) investigation of the cognitive and psychomotor profile of pregabalin in healthy volunteers. Psychopharmacology 2005; 183:133–143. [DOI] [PubMed] [Google Scholar]
- 4.Schug SA, Zech D, Grond S. Adverse effects of systemic opioid analgesics. Drug Saf 1992; 7:200–213. [DOI] [PubMed] [Google Scholar]
- 5.Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 2015; 114:10–31. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth 2008; 101:17–24. [DOI] [PubMed] [Google Scholar]
- 8.Gammaitoni AR, Fine P, Alvarez N, et al. Clinical application of opioid equianalgesic data. Clin J Pain 2003; 19:286–297. [DOI] [PubMed] [Google Scholar]
- 9.Dopfmer UR, Schenk MR, Kuscic S, et al. A randomized controlled double-blind trial comparing piritramide and morphine for analgesia after hysterectomy. Eur J Anaesthesiol 2001; 18:389–393. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth 2011; 106:454–462. [DOI] [PubMed] [Google Scholar]
- 11.Akshat S, Ramachandran R. Morphine versus nalbuphine for open gynaecological surgery: a randomized controlled double blinded trial. Pain Res Treat 2014; 2014:727952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagit M, Yalcin S, Polat H, et al. Efficacy of a single preoperative dose of pregabalin for postoperative pain after septoplasty. J Craniofac Surg 2013; 24:373–375.101097/SCS0b013e31827fece5. [DOI] [PubMed] [Google Scholar]
- 13.Jadeja CA, Khatri H, Oza V, et al. Comparative study of single dose pre-emptive pregabalin vs. Placebo for post-operative pain relief in middle ear surgery. Int J of Biomed and Adv Res 2014; 5. [Google Scholar]
- 14.Kim JH, Seo MY, Hong SD, et al. The efficacy of preemptive analgesia with pregabalin in septoplasty. Clin Exp Otorhinolaryngol 2014; 7:102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002; 21:1559–1573. [DOI] [PubMed] [Google Scholar]
- 17.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343:d40022011. [DOI] [PubMed] [Google Scholar]
- 19.Fawzi H, El-Tohamy S. Effect of perioperative oral pregabalin on the incidence of post-thoracotomy pain syndrome. Ains Shams J Anaesth 2014; 7:143–147. [Google Scholar]
- 20.Joshi SS, Jagadeesh AM. Efficacy of perioperative pregabalin in acute and chronic post-operative pain after off-pump coronary artery bypass surgery: a randomized, double-blind placebo controlled trial. Ann Card Anaesth 2013; 16:180–185. [DOI] [PubMed] [Google Scholar]
- 21.Pesonen A, Suojaranta-Ylinen R, Hammaren E, et al. Pregabalin has an opioid-sparing effect in elderly patients after cardiac surgery: a randomized placebo-controlled trial. Br J Anaesth 2011; 106:873–881. [DOI] [PubMed] [Google Scholar]
- 22.Sundar A, Kodali R, Sulaiman S, et al. The effects of preemptive pregabalin on attenuation of stress response to endotracheal intubation and opioid- sparing effect in patients undergoing off-pump coronary artery bypass grafting. Ann Card Anaesth 2012; 15:18–25. [DOI] [PubMed] [Google Scholar]
- 23.Demirhan A, Tekelioglu UY, Akkaya A, et al. Effect of pregabalin and dexamethasone addition to multimodal analgesia on postoperative analgesia following rhinoplasty surgery. Aesthetic Plast Surg 2013; 37:1100–1106. [DOI] [PubMed] [Google Scholar]
- 24.Demirhan A, Akkaya A, Tekelioglu UY, et al. Effect of pregabalin and dexamethasone on postoperative analgesia after septoplasty. Pain Res Treat 2014; 2014:850794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathiesen O, Jorgensen DG, Hilsted KL, et al. Pregabalin and dexamethasone improves post-operative pain treatment after tonsillectomy. Acta Anaesthesiol Scand 2011; 55:297–305. [DOI] [PubMed] [Google Scholar]
- 26.Bafna U, Rajarajeshwaran K, Khandelwal M, et al. A comparison of effect of preemptive use of oral gabapentin and pregabalin for acute post-operative pain after surgery under spinal anesthesia. J Anaesth Clin Pharm 2014; 30:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chotton T, Singh N, Singh L, et al. The effect of pregabalin for relief of postoperative pain after abdominal hysterectomy. J Med Soc 2014; 28:18–21. [Google Scholar]
- 28.Eman A, Bilir A, Beyaz S. The effects of preoperative pregabalin on postoperative analgesia and morphine consumption after abdominal hysterectomy. Acta Medica Meditrranea 2014; 30:481–485. [Google Scholar]
- 29.Fassoulaki A, Melemeni A, Tsaroucha A, et al. Perioperative pregabalin for acute and chronic pain after abdominal hysterectomy or myomectomy: a randomised controlled trial. Eur J Anaesthesiol 2012; 29:531–536. [DOI] [PubMed] [Google Scholar]
- 30.George RB, McKeen DM, Andreou P, et al. A randomized placebo-controlled trial of two doses of pregabalin for postoperative analgesia in patients undergoing abdominal hysterectomy. Can J Anaesth 2014; 61:551–557. [DOI] [PubMed] [Google Scholar]
- 31.Ghai A, Gupta M, Hooda S, et al. A randomized controlled trial to compare pregabalin with gabapentin for postoperative pain in abdominal hysterectomy. Saudi J Anaesth 2011; 5:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ittichaikulthol W, Virankabutra T, Kunopart M, et al. Effects of pregabalin on post operative morphine consumption and pain after abdominal hysterectomy with/without salphingo-oophorectomy: a randomized, double-blind trial. J Med Assoc Thai 2009; 92:1318–1323. [PubMed] [Google Scholar]
- 33.Jo HR, Chae YK, Kim YH, et al. Remifentanil-induced pronociceptive effect and its prevention with pregabalin. Korean J Anesthesiol 2011; 60:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jokela R, Ahonen J, Tallgren M, et al. Premedication with pregabalin 75 or 150 mg with ibuprofen to control pain after day-case gynaecological laparoscopic surgery. Br J Anaesth 2008; 100:834–840. [DOI] [PubMed] [Google Scholar]
- 35.Jokela R, Ahonen J, Tallgren M, et al. A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain 2008; 134:106–112. [DOI] [PubMed] [Google Scholar]
- 36.Lalenoh LAP, Lalenoh HJ, Tanra AH, et al. The antinociceptive effects of pregabalin on post-operative hysterectomy patient. J Anesth Clin Res 2014; 5:6. [Google Scholar]
- 37.Mathiesen O, Rasmussen ML, Dierking G, et al. Pregabalin and dexamethasone in combination with paracetamol for postoperative pain control after abdominal hysterectomy. A randomized clinical trial. Acta Anaesthesiol Scand 2009; 53:227–235. [DOI] [PubMed] [Google Scholar]
- 38.Nutthachote P, Sirayapiwat P, Wisawasukmongchol W, et al. A randomized double-blind placebo-controlled trial of oral pregabalin for relief of shoulder pain after laparoscopic gynecologic surgery. J Minim Invasive Gynecol 2014; 21:669–673. [DOI] [PubMed] [Google Scholar]
- 39.Paech MJ, Goy R, Chua S, et al. A randomized, placebo-controlled trial of preoperative oral pregabalin for postoperative pain relief after minor gynecological surgery. Anesth Analg 2007; 105:1449–1453. [DOI] [PubMed] [Google Scholar]
- 40.Prasad A, Bhattacharyya S, Biswas A, et al. A comparative study of pre-operative oral clonidine and pregabalin on post-operative analgesia after spinal anesthesia. Anesth Essays Res 2014; 8:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singla NK, Chelly JE, Lionberger DR, et al. Pregabalin for the treatment of postoperative pain: results from three controlled trials using different surgical models. J Pain Res 2015; 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yucel A, Ozturk E, Aydogan MS, et al. Effects of 2 different doses of pregabalin on morphine consumption and pain after abdominal hysterectomy: a randomized, double-blind clinical trial. Curr Ther Res Clin Exp 2011; 72:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal A, Gautam S, Gupta D, et al. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth 2008; 101:700–704. [DOI] [PubMed] [Google Scholar]
- 44.Balaban F, Yagar S, Ozgok A, et al. A randomized, placebo-controlled study of pregabalin for postoperative pain intensity after laparoscopic cholecystectomy. J Clin Anesth 2012; 24:175–178. [DOI] [PubMed] [Google Scholar]
- 45.Bekawi MS, El Wakeel LM, Al Taher WM, et al. Clinical study evaluating pregabalin efficacy and tolerability for pain management in patients undergoing laparoscopic cholecystectomy. Clin J Pain 2014; 30:944–952. [DOI] [PubMed] [Google Scholar]
- 46.Chang SH, Lee HW, Kim HK, et al. An evaluation of perioperative pregabalin for prevention and attenuation of postoperative shoulder pain after laparoscopic cholecystectomy. Anesth Analg 2009; 109:1284–1286. [DOI] [PubMed] [Google Scholar]
- 47.Peng PW, Li C, Farcas E, et al. Use of low-dose pregabalin in patients undergoing laparoscopic cholecystectomy. Br J Anaesth 2010; 105:155–161. [DOI] [PubMed] [Google Scholar]
- 48.Sarakatsianou C, Theodorou E, Georgopoulou S, et al. Effect of pre-emptive pregabalin on pain intensity and postoperative morphine consumption after laparoscopic cholecystectomy. Surg Endosc 2013; 27:2504–2511. [DOI] [PubMed] [Google Scholar]
- 49.Akhavanakbari G, Entezariasl M, Isazadehfar K, et al. The effects of oral pregabalin on post-operative pain of lower limb orthopedic surgery: a double-blind, placebo-controlled trial. Perspect Clin Res 2013; 4:165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buvanendran A, Kroin JS, Della Valle CJ, et al. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg 2010; 110:199–207. [DOI] [PubMed] [Google Scholar]
- 51.Eskandar AM, Ebeid AM. Effect of pregabalin on postoperative pain after shoulder arthroscopy. Eg J Anaesth 2013; 29:363–367. [Google Scholar]
- 52.Ghoghari DV, Parmar D P, Meera D. Pregabalin and dexamethasone for post operative pain relief in lower limb surgeries: a randomized controlled study. J Dent Med Sci 2014; 13:10–14. [Google Scholar]
- 53.Gonano C, Latzke D, Sabeti-Aschraf M, et al. The anxiolytic effect of pregabalin in outpatients undergoing minor orthopaedic surgery. J Psychopharmacol 2011; 25:249–253. [DOI] [PubMed] [Google Scholar]
- 54.Jain P, Jolly A, Bholla V, et al. Evaluation of efficacy of oral pregabalin in reducing postoperative pain in patients undergoing total knee arthroplasty. Indian J Orthop 2012; 46:646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JK, Chung KS, Choi CH. The effect of a single dose of preemptive pregabalin administered with cox-2 inhibitor: a trial in total knee arthroplasty. J Arthroplasty 2015; 30:38–42. [DOI] [PubMed] [Google Scholar]
- 56.Martinez V, Cymerman A, Ben Ammar S, et al. The analgesic efficiency of combined pregabalin and ketamine for total hip arthroplasty: a randomised, double-blind, controlled study. Anaesthesia 2014; 69:46–52. [DOI] [PubMed] [Google Scholar]
- 57.Mathiesen O, Jacobsen LS, Holm HE, et al. Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth 2008; 101:535–541. [DOI] [PubMed] [Google Scholar]
- 58.Niruthisard S, Earsakul A, Bunburaphong P, et al. Preoperative pregabalin and/or celecoxib for pain management after total knee arthroplasty under intrathecal morphine: a randomized controlled trial. Asian Biomedicine 2013; 7:579–585. [Google Scholar]
- 59.Wang H, Gargano C, Lukac S, et al. An enhanced bunionectomy model as a potential tool for early decision-making in the development of new analgesics. Adv Ther 2010; 27:963–980. [DOI] [PubMed] [Google Scholar]
- 60.Yadeau JT, Paroli L, Kahn RL, et al. Addition of pregabalin to multimodal analgesic therapy following ankle surgery: a randomized double-blind, placebo-controlled trial. Reg Anesth Pain Med 2012; 37:302–307. [DOI] [PubMed] [Google Scholar]
- 61.Burke SM, Shorten GD. Perioperative pregabalin improves pain and functional outcomes 3 months after lumbar discectomy. Anesth Analg 2010; 110:1180–1185. [DOI] [PubMed] [Google Scholar]
- 62.Choi YS, Shim JK, Song JW, et al. Combination of pregabalin and dexamethasone for postoperative pain and functional outcome in patients undergoing lumbar spinal surgery: a randomized placebo-controlled trial. Clin J Pain 2013; 29:9–14. [DOI] [PubMed] [Google Scholar]
- 63.Gianesello L, Pavoni V, Barboni E, et al. Perioperative pregabalin for postoperative pain control and quality of life after major spinal surgery. J Neurosurg Anesthesiol 2012; 24:121–126. [DOI] [PubMed] [Google Scholar]
- 64.Hegarty DA, Shorten GD, Randomised A. Placebo-controlled trial of the effects of preoperative pregabalin on pain intensity and opioid consumption following lumbar discectomy. Korean J Pain 2011; 24:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khurana G, Jindal P, Sharma JP, et al. Postoperative pain and long-term functional outcome after administration of gabapentin and pregabalin in patients undergoing spinal surgery. Spine 2014; 39:E363–E368. [DOI] [PubMed] [Google Scholar]
- 66.Kim SY, Song JW, Park B, et al. Pregabalin reduces post-operative pain after mastectomy: a double-blind, randomized, placebo-controlled study. Acta Anaesthesiol Scand 2011; 55:290–296. [DOI] [PubMed] [Google Scholar]
- 67.Kumar KP, Kulkarni DK, Gurajala I, et al. Pregabalin versus tramadol for postoperative pain management in patients undergoing lumbar laminectomy: a randomized, double-blinded, placebo-controlled study. J Pain Res 2013; 6:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozgencil E, Yalcin S, Tuna H, et al. Perioperative administration of gabapentin 1,200 mg day-1 and pregabalin 300 mg day-1 for pain following lumbar laminectomy and discectomy: a randomised, double-blinded, placebo-controlled study. Singapore Med J 2011; 52:883–889. [PubMed] [Google Scholar]
- 69.Spreng UJ, Dahl V, Raeder J. Effect of a single dose of pregabalin on post-operative pain and pre-operative anxiety in patients undergoing discectomy. Acta Anaesthesiol Scand 2011; 55:571–576. [DOI] [PubMed] [Google Scholar]
- 70.Alimian M, Imani F, Faiz SH, et al. Effect of oral pregabalin premedication on post-operative pain in laparoscopic gastric bypass surgery. Anesth Pain Med 2012; 2:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alimian M, Imani F, Hassani V, et al. Effects of single-dose pregabalin on postoperative pain in dacryocystorhinostomy surgery. Anesth Pain Med 2012; 2:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aydogan H, Kucuk A, Yuce HH, et al. Adding 75 mg pregabalin to analgesic regimen reduces pain scores and opioid consumption in adults following percutaneous nephrolithotomy. Braz J Anesthesiol 2014; 64:335–342. [DOI] [PubMed] [Google Scholar]
- 73.Bornemann-Cimenti H, Lederer AJ, Wejbora M, et al. Preoperative pregabalin administration significantly reduces postoperative opioid consumption and mechanical hyperalgesia after transperitoneal nephrectomy. Br J Anaesth 2012; 108:845–849. [DOI] [PubMed] [Google Scholar]
- 74.Cabrera Schulmeyer MC, de la Maza J, Ovalle C, et al. Analgesic effects of a single preoperative dose of pregabalin after laparoscopic sleeve gastrectomy. Obes Surg 2010; 20:1678–1681. [DOI] [PubMed] [Google Scholar]
- 75.Chaparro LE, Clarke H, Valdes PA, et al. Adding pregabalin to a multimodal analgesic regimen does not reduce pain scores following cosmetic surgery: a randomized trial. J Anesth 2012; 26:829–835. [DOI] [PubMed] [Google Scholar]
- 76.Freedman BM, O’Hara E. Pregabalin has opioid-sparing effects following augmentation mammaplasty. Aesthet Surg J 2008; 28:421–424. [DOI] [PubMed] [Google Scholar]
- 77.Singh TH, Thokchom R, Rajkumar G, et al. Pregabalin for post-cholecystectomy pain relief- a study on the response of two different doses. IJHSR 2014; 4:159–168. [Google Scholar]
- 78.Hill CM, Balkenohl M, Thomas DW, et al. Pregabalin in patients with postoperative dental pain. Eur J Pain 2001; 5:119–124. [DOI] [PubMed] [Google Scholar]
- 79.Kim SY, Jeong JJ, Chung WY, et al. Perioperative administration of pregabalin for pain after robot-assisted endoscopic thyroidectomy: a randomized clinical trial. Surg Endosc 2010; 24:2776–2781. [DOI] [PubMed] [Google Scholar]
- 80.Lee C, Lee H-W, Kim J-N. Effect of oral pregabalin on opioid-induced hyperalgesia in patients undergoing laparo-endoscopic single-site urologic surgery. Korean J Anesthesiol 2013; 64:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meek JM, Rosbolt MB, Taylor KR, et al. Pregabalin versus placebo in postoperative pain relief of patients’ status post photorefractive keratectomy: a double-masked, randomized, prospective study. J Ocul Pharmacol Ther 2014; 30:527–532. [DOI] [PubMed] [Google Scholar]
- 82.Rajendran I, Basavareddy A, Meher B, et al. Prospective, randomised, double blinded controlled trial of gabapentin and pregabalin as pre emptive analgesia in patients undergoing lower abdominal and limb surgery under spinal anaesthesia. Indian J Pain 2014; 28:155–159. [Google Scholar]
- 83.Sahu S, Sachan S, Verma A, et al. Evaluation of pregabalin for attenuation of postoperative pain in below umbilical surgeries under spinal anaesthesia. J Anaesth Clin Pharmacol 2010; 26:167–171. [Google Scholar]
- 84.Saraswat V, Arora V. Preemptive gabapentin vs pregabalin for acute postoperative pain after surgery under spinal anaesthesia. Indian J Anesth 2008; 52:829–834. [Google Scholar]
- 85.Singh U, Singh TH, Pratima K, et al. A randomized placebo controlled study of preoperative pregabalin on postcholecystectomy pain relief. J Evol Med Dent Sci 2014; 3:1573–1581. [Google Scholar]
- 86.Wei LA, Davies BW, Hink EM, et al. Perioperative pregabalin for attenuation of postoperative pain after eyelid surgery. Ophthal Plast Reconstr Surg 2015; 31:132–135. [DOI] [PubMed] [Google Scholar]
- 87.White PF, Tufanogullari B, Taylor J, et al. The effect of pregabalin on preoperative anxiety and sedation levels: a dose-ranging study. Anesth Analg 2009; 108:1140–1145. [DOI] [PubMed] [Google Scholar]
- 88.Kim JC, Choi YS, Kim KN, et al. Effective dose of peri-operative oral pregabalin as an adjunct to multimodal analgesic regimen in lumbar spinal fusion surgery. Spine 2011; 36:428–433. [DOI] [PubMed] [Google Scholar]
- 89.Ward CW. Procedure-specific postoperative pain management. Medsurg Nurs 2014; 23:107–110. [PubMed] [Google Scholar]
- 90.Joshi GP, Kehlet H. Procedure-specific pain management: the road to improve postsurgical pain management? Anesthesiology 2013; 118:780–782. [DOI] [PubMed] [Google Scholar]
- 91.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367:1618–1625. [DOI] [PubMed] [Google Scholar]
- 92.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology 2000; 93:1123–1133. [DOI] [PubMed] [Google Scholar]
- 93.Joshi GP, Bonnet F, Kehlet H. Evidence-based postoperative pain management after laparoscopic colorectal surgery. Colorectal Dis 2013; 15:146–155. [DOI] [PubMed] [Google Scholar]
- 94.Gerbershagen HJ, Aduckathil S, van Wijck AJ, et al. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013; 118:934–944. [DOI] [PubMed] [Google Scholar]
- 95.Gray A, Kehlet H, Bonnet F, et al. Predicting postoperative analgesia outcomes: NNT league tables or procedure-specific evidence? Brit J Anaesth 2005; 94:710–714. [DOI] [PubMed] [Google Scholar]
- 96.Hyllested M, Jones S, Pedersen JL, et al. Comparative effect of paracetamol, NSAIDs or their combination in postoperative pain management: a qualitative review. Brit J Anaesth 2002; 88:199–214. [DOI] [PubMed] [Google Scholar]
- 97.Kehlet H, Wilkinson RC, Fischer HB, et al. PROSPECT: evidence-based, procedure-specific postoperative pain management. Best Pract Res Clin Anaesthesiol 2007; 21:149–159. [DOI] [PubMed] [Google Scholar]
- 98.Joshi GP, Schug SA, Kehlet H. Procedure-specific pain management and outcome strategies. Best Pract Res Clin Anaesthesiol 2014; 28:191–201. [DOI] [PubMed] [Google Scholar]
- 99.Sahgal N, Banerjee A. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Brit J Anaesth 2011; 107:274.author reply 5. [DOI] [PubMed] [Google Scholar]
- 100.Erkalp K, Kalekoglu Erkalp N, Sevdi MS, et al. Gastric decompression decreases postoperative nausea and vomiting in ENT surgery. Int J Otolaryngol 2014; 2014:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kates SL. Geriatric orthopaedic surgery & rehabilitation: the imminent silver tsunami and the need for a new journal. Geriatr Orthop Surg Rehabil 2010; 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hanley MA, Jensen MP, Ehde DM, et al. Clinically significant change in pain intensity ratings in persons with spinal cord injury or amputation. Clin J Pain 2006; 22:25–31. [DOI] [PubMed] [Google Scholar]
- 103.Bird SB, Dickson EW. Clinically significant changes in pain along the visual analog scale. Ann Emerg Med 2001; 38:639–643. [DOI] [PubMed] [Google Scholar]
- 104.Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med 2014; 3:263–275. [DOI] [PubMed] [Google Scholar]
- 105.Berde C, Nurko S. Opioid side effects: mechanism-based therapy. NEJM 2008; 358:2400–2402. [DOI] [PubMed] [Google Scholar]
- 106.Hurley RW, Cohen SP, Williams KA, et al. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Region Anesth Pain M 2006; 31:237–247. [DOI] [PubMed] [Google Scholar]
- 107.Peng PWH, Wijeysundera DN, Li CCF. Use of gabapentin for perioperative pain control: a meta-analysis. Pain Res Manag 2007; 12:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seib RK, Paul JE. Preoperative gabapentin for postoperative analgesia: a meta-analysis. Can J Anaesth 2006; 53:461–469. [DOI] [PubMed] [Google Scholar]
- 109.Doleman B, Heinink TP, Read DJ, et al. A systematic review and meta-regression analysis of prophylactic gabapentin for postoperative pain. Anaesthesia 2015; 70:1186–1204. [DOI] [PubMed] [Google Scholar]
- 110.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009; 172:137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166:1338–1344. [DOI] [PubMed] [Google Scholar]
- 112.Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002; 3:229–243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


