Abstract
The purpose of this meta-analysis was to investigate whether bisphosphonates are a key therapy for bone metastases in lung cancer, breast cancer, and prostate cancer by comparing all randomized controlled trials that appraised the effects of bisphosphonates on risk of skeletal-related events (SREs).
PubMed, Embase, and Medline databases (up to December 2014) were used to search all related articles. Using the data from 19 available publications, the authors examined the efficacy in treating or reducing the risk of SREs in lung cancer, breast cancer, and prostate cancer by meta-analysis.
Bisphosphonates have demonstrated efficacy in treating or reducing the risk of SREs in lung cancer [odds ratio (OR) = 0.81, 95% confidence interval (CI) = 0.69–0.95, P = 0.008], breast cancer (OR = 0.62, 95% CI = 0.54–0.71, P = 0.000), and prostate cancer (OR = 0.62, 95% CI = 0.45–0.86, P = 0.004).
This meta-analysis suggests that bisphosphonates have demonstrated efficacy in treating or reducing the risk of SREs in lung cancer, breast cancer, and prostate cancer.
INTRODUCTION
Lung cancer, breast cancer, and prostate cancer remain the leading cause for cancer mortality worldwide. Lung cancer has an incidence in Europe of 417,000 new cases per year, and a mortality/incidence ratio of 0.88. Breast cancer is the most common cancer type in women worldwide and is the main cause of cancer mortality in women in the world. Prostate cancer is the most common cancer in men in many western countries, and the second leading cause of cancer death in men.
The skeleton is common site of the metastatic cancer, especially in patients with breast, lung, or prostate cancer.1 The most common complications of skeletal occurring from bone metastases are named skeletal-related events (SREs), including severe pain of bone, hypercalcemia of malignancy, and pathologic fractures.1 The bone metastasis of metastatic cancers is associated with a reduced survival rate and a low patients’ quality of life.2 In addition, cancerous patients with bone metastases and SREs have been shown to cost more hospital resources and money.3
Bisphosphonates, such as zoledronate, pamidronate, and alendronate, have been shown to inhibit osteoclast-mediated bone resorption4,5 and be effective for the treatment and prevention of SREs in breast cancer,6 prostate cancer,7 and lung cancer.8 The efficacy and safety of bisphosphonates, however, are still uncertain. As a result, we carried out a meta-analysis using data from randomized controlled trials to identify the effectiveness and safety of bisphosphonates in patients with breast, lung, and prostate cancer.
This meta-analysis aims to support the use of bisphosphonates in patients with lung cancer, breast cancer, or prostate-developing bone metastases. Its efficacy and safety, as well as its cost-effectiveness, are highlighted.
MATERIAL AND METHODS
Publication Search
We obtained relevant randomized controlled trials from PubMed, Embase, and Chinese biomedicine database that treated with either zoledronic acid or another bisphosphonates to prevent SREs in breast, lung, or prostate cancer. For the computer searches, we used the following key words: “bisphosphonates,” “ibandronate,” “ibandronic acid,” “zoledronic acid,” “zoledronate,” “SREs,” “breast cancer,” “prostate cancer,” or “lung cancer” in the title or abstract, and was limited by "clinical trials, meta-analysis, randomized controlled trial, and review" published in English between 2003 and 2014. Studies contained available data, which showed the association of bisphosphonates treatment in breast cancer, prostate cancer, and lung cancer between SREs. Among the studies with overlapping data published by the same author, only the complete study was included in this meta-analysis. Furthermore, included studies had to show their results as an odds ratio (OR) and 95% confidence interval (CI).
Data Extraction and Classification
For each study characteristics, data were extracted, including the first author, publication year, patients’ country, disease characteristics, treatment medication, OR, and risk estimates with corresponding 95% CI. Skeletal-related events were defined as pathologic bone fracture, the bone surgery, the bone radiation therapy, or change in anticancer therapy to relieve bone pain.
Statistical Analysis
The measure of effect of interest is the OR and the corresponding 95% CI. We showed all results as OR for simplicity and quantified the association of bisphosphonates treatment in breast cancer, prostate cancer, and lung cancer between SREs using random effects models9 of OR comparing the highest with the lowest category. The summary OR estimates were obtained from random effects models.9
For all analysis, P values <0.05 were considered significant. Publication bias was assessed by a Begg-adjusted rank correlation test (funnel plot method) and Egger linear regression asymmetry test.10 All meta-analyses were carried out using Stata software (version9.0, StataCorp, College Station, TX).
An Ethics Committee
The ethical approval was not necessary. Our research does not involve ethics.
RESULTS
Characteristics of Studies for Meta-Analysis
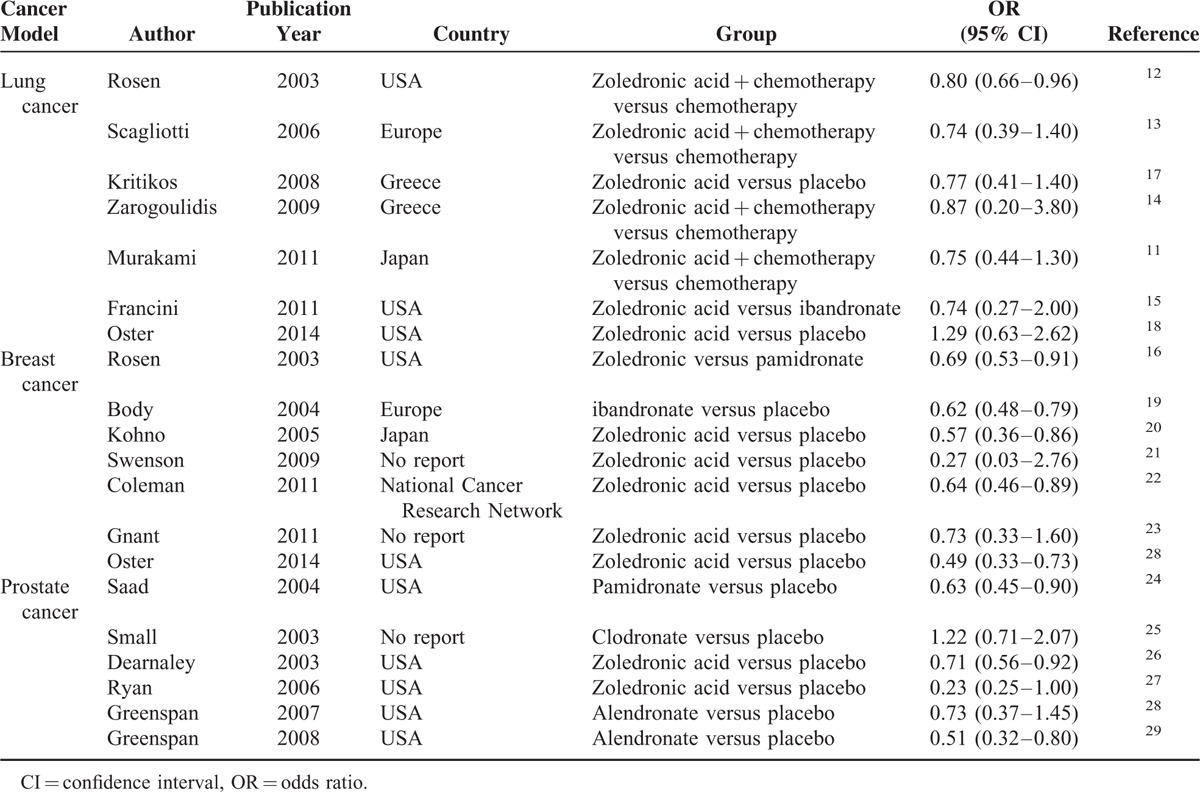
A total of 19 publications were identified for inclusion in the SREs for the 3 malignancies (Table 1), Among the 19 studies, 11 were conducted in the United States, 2 in Europe, 2 in Japan, and the other 4 were not reported. We separate the results by different malignancies, including lung cancer, breast cancer, and prostate cancer. With the exception of 7 studies comparing bisphosphonates (zoledronic acid + chemotherapy versus chemotherapy,11–14 zoledronic acid versus ibandronate15 in lung cancer, and zoledronic versus pamidronate in breast cancer),16 all studies compared bisphosphonates with placebo. Of the 19 placebo-controlled studies, 16 showed that bisphosphonates were effective in reducing the incidence of SREs in lung cancer, breast cancer, and prostate cancer.
TABLE 1.
The Distribution and Odds Ratios (95% Confidence Interval) for Studies on 3 Cancer Models and Bisphosphonates
Lung Cancer
Most patients with bone metastases from lung cancer experience SRE. The association of bisphosphonates treatment of lung cancer between SREs was identified in 7 studies, including comparisons of zoledronic acid versus ibandronate and zoledronic acid versus placebo (Table 1). Pooled estimates showed a statistically significant 19% reduction in the risk of developing new SREs with bisphosphonates (OR = 0.81, 95% CI 0.69–0.95, P = 0.008; Figure 1; Table 2). This data indicate that bisphosphonates were associated with a reduction in skeletal mortality rate.
FIGURE 1.
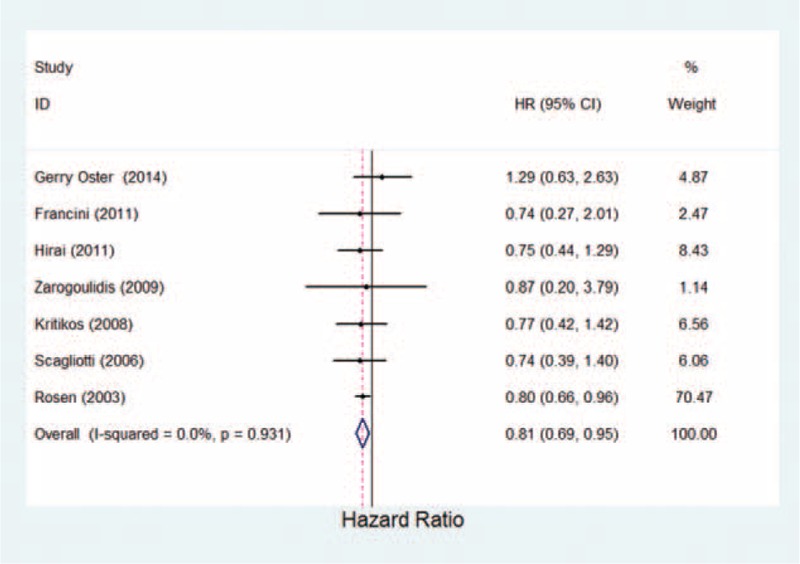
Estimated odds ratio of risk for skeletal-related events for patients with lung cancer under bisphosphonates therapy.
TABLE 2.
Summary Odds Ratios and 95% Confidence Interval for Bisphosphonates and Skeletal-Related Events Rate Under Different Cancer Models
Breast Cancer
Seven studies in breast were identified in the analysis, including comparisons of zoledronic versus pamidronate and zoledronic acid/ibandronate versus placebo (Table 1). The data analysis showed a statistically significant 38% reduction in the risk of developing new SREs with bisphosphonates (OR = 0.62, 95% CI 0.54–0.71, P = 0.000; Fig. 2; Table 2). It suggested that bisphosphonates demonstrate a statistically significant decrease in the risk of developing SREs compared with placebo.
FIGURE 2.
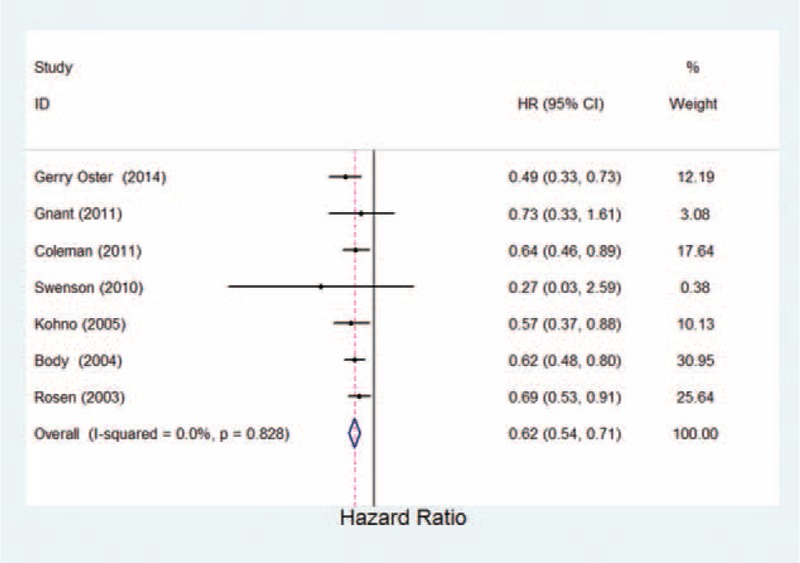
Estimated odds ratio of risk for skeletal-related events for patients with breast cancer under bisphosphonates therapy.
Prostate Cancer
The 7 prostate cancer studies included in the analysis compared zoledronic acid, pamidronate, clodronate, and alendronate with placebo (Table 1). Because the study by Bhoopalam et al30 of zoledronic acid versus placebo had a higher SRE rate than the other placebo-controlled studies (OR = 4.38, 95% CI = 0.53–6.13). Removing the Bhoopalam study30 from the analysis resulted in a lower SRE rate for bisphosphonates. The meta-analysis had a statistically significant result of 38% reduction in the risk of SREs with bisphosphonates (OR = 0.62, 95% CI 0.45–0.86, P = 0.004; Fig. 3; Table 2). The result indicated that the bisphosphonates favors a decrease in SREs.
FIGURE 3.
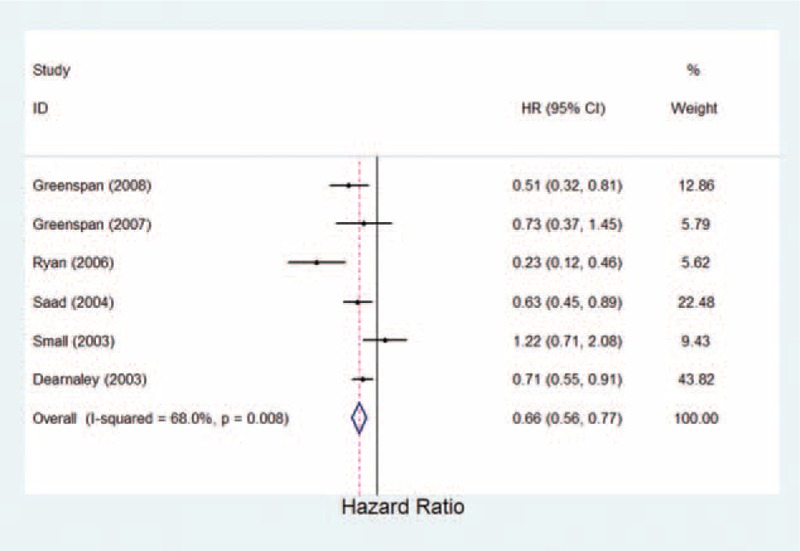
Estimated odds ratio of risk for skeletal-related events for patients with prostate cancer under bisphosphonates therapy.
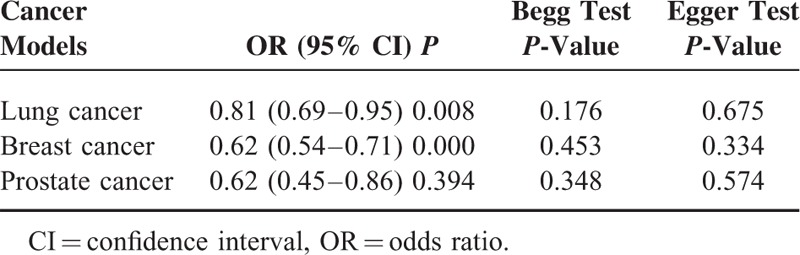
Publication Bias
We evaluated publication bias by Egger test and Begg test. The results of the Egger test (P > 0.05) and the Begg test (P > 0.05) provided statistical evidence for funnel plot symmetry in the overall results, suggesting the absence of publication bias (Table 2).
DISCUSSION
Our meta-analysis suggests that bisphosphonates have demonstrated efficacy in treating or reducing the risk of SREs in lung cancer, breast cancer, and prostate cancer. Bisphosphonates have a protective effect for SREs, with a 19% lower risk in lung cancer and a 38% lower risk in breast cancer and prostate. The intense inhibition of osteoclast function precipitated by bisphosphonate therapy can lead to inhibition of normal bone turnover.
Bisphosphonates have been proven to display high affinity for inhibiting bone resorption by osteoclasts.31 Many nitrogen-containing bisphosphonates, such as alendronate, risedronate, pamidronate, ibandronate, and zoledronic acid are considered as an important part of treatment to reduce the risk for SREs in cancer patients with bone metastases.32–34 A great number of studies have identified the effect of bisphosphonates to inhibit tumor cell adhesion, invasion, proliferation, and interplay with the microenvironment components of bone such as matrix metalloproteinases.35,36 In addition, it reported that bisphosphonates interfere with signaling pathways required for osteoclast function and survival.37 Malignant cells, such as lung cancer, breast cancer, and prostate cancer have a unique predilection to metastasize to bone, and the microenvironment is highly receptive to metastatic tumor cell.38 Therefore, early diagnosis and therapy of bone metastases will delay the development of common clinical complications associated to bone metastases.39 Our meta-analysis showed bisphosphonates are a key therapy for bone metastases and have a proved efficacy in lung cancer, breast cancer, and prostate cancer, resulting in reduced risk of SREs.
There are many limitations that are present in our study. For example, this analysis was based on case-control studies, and the limitation of these studies could influence our results. Furthermore, our findings were likely to be affected by different measurement and range of drug. It will need larger and wider case-control studies to confirm that bisphosphonates reduced the risk of SREs in lung cancer, breast cancer, and prostate cancer.
In summary, our meta-analysis provides some support for the hypothesis that bisphosphonates have demonstrated efficacy in treating or reducing the risk of SREs in lung cancer (OR = 0.81, 95 % CI = 0.69–0.95, P = 0.008), breast cancer (OR = 0.62, 95% CI = 0.54–0.71, P = 0.000), and prostate cancer (OR = 0.62, 95% CI = 0.45–0.86, P = 0.004). Future well-designed large studies might be necessary and should consider the interrelations between different bisphosphonates.
Footnotes
Abbreviations: CI = 95%confidence interval, MMPs = matrix metalloproteinases, OR = odds ratio, SREs = skeletal-related events.
This work was financially supported by the Nature Science Foundation of Yunnan Province (No. 2013FC006), the National Nature Science Foundation of Yunnan Province (No. 2014FD011), and the Nature Science Foundation of Yunnan Province (No. KKSY201460043).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001; 27:165–176. [DOI] [PubMed] [Google Scholar]
- 2.Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007; 110:1860–1867. [DOI] [PubMed] [Google Scholar]
- 3.Pockett RD, Castellano D, McEwan P, et al. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) 2010; 19:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aapro M, Abrahamsson PA, Body JJ, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol 2008; 19:420–432. [DOI] [PubMed] [Google Scholar]
- 5.Terpos E, Sezer O, Croucher PI, et al. The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol 2009; 20:1303–1317. [DOI] [PubMed] [Google Scholar]
- 6.Wong MH, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev 2012; 2:CD003474. [DOI] [PubMed] [Google Scholar]
- 7.Yuen KK, Shelley M, Sze WM, et al. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev 2006; 18:CD006250. [DOI] [PubMed] [Google Scholar]
- 8.Price N. Bisphosphonates to prevent skeletal morbidity in patients with lung cancer with bone metastases. Clin Lung Cancer 2004; 5:267–269. [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 10.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami H, Yamanaka T, Seto T, et al. Phase II study of zoledronic acid combined with docetaxel for non-small-cell lung cancer: West Japan Oncology Group. Cancer Sci 2014; 105:989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen LS, Gordon D, Tchekmedyian S, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial: the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol 2003; 21:3150–3157. [DOI] [PubMed] [Google Scholar]
- 13.Scagliotti G, Manegold C, Kosmidis P, et al. Efficacy of zoledronic acid for the prevention of bone metastases in patients with non-small cell lung cancer. Bone 2006; 38:S83. [Google Scholar]
- 14.Zarogoulidis K, Boutsikou E, Zarogoulidis P, et al. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int J Cancer 2009; 125:1705–1709. [DOI] [PubMed] [Google Scholar]
- 15.Francini F, Pascucci A, Bargagli G, et al. Effects of intravenous zoledronic acid and oral ibandronate on early changes in markers of bone turnover in patients with bone metastases from non-small cell lung cancer. Int J Clin Oncol 2011; 16:264–269. [DOI] [PubMed] [Google Scholar]
- 16.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 2003; 98:1735–1744. [DOI] [PubMed] [Google Scholar]
- 17.Kritikos K, Heras P, Hatzopoulos A, et al. Efficacy and safety of intravenous zoledronic acid 4 mg infused over 15 minutes: results from a 2-year study of lung cancer patients with metastatic bone disease. Ann Oncol 2008; 19:286–287. [Google Scholar]
- 18.Oster G, Lamerato L, Glass AG, et al. Use of intravenous bisphosphonates in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer 2014; 22:1363–1373. [DOI] [PubMed] [Google Scholar]
- 19.Body JJ, Diel IJ, Lichinitzer M, et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies. Br J Cancer 2004; 90:1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol 2005; 23:3314–3321. [DOI] [PubMed] [Google Scholar]
- 21.Swenson KK, Nissen MJ, Anderson E, et al. Effects of exercise vs bisphosphonates on bone mineral density in breast cancer patients receiving chemotherapy. J Support Oncol 2009; 7:101–107. [PubMed] [Google Scholar]
- 22.Coleman RE, Marshall H, Cameron D, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 2011; 365:1396–1405. [DOI] [PubMed] [Google Scholar]
- 23.Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol 2011; 12:631–641. [DOI] [PubMed] [Google Scholar]
- 24.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004; 96:879–882. [DOI] [PubMed] [Google Scholar]
- 25.Small EJ, Smith MR, Seaman JJ, et al. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol 2003; 21:4277–4284. [DOI] [PubMed] [Google Scholar]
- 26.Dearnaley DP, Sydes MR, Mason MD, et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J Natl Cancer Inst 2003; 95:1300–1311. [DOI] [PubMed] [Google Scholar]
- 27.Ryan CW, Huo D, Demers LM, et al. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol 2006; 176:972–978.discussion 8. [DOI] [PubMed] [Google Scholar]
- 28.Greenspan SL, Nelson JB, Trump DL, et al. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med 2007; 146:416–424. [DOI] [PubMed] [Google Scholar]
- 29.Greenspan SL, Nelson JB, Trump DL, et al. Skeletal health after continuation, withdrawal, or delay of alendronate in men with prostate cancer undergoing androgen-deprivation therapy. J Clin Oncol 2008; 26:4426–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhoopalam N, Campbell SC, Moritz T, et al. Intravenous zoledronic acid to prevent osteoporosis in a veteran population with multiple risk factors for bone loss on androgen deprivation therapy. J Urol 2009; 182:2257–2264. [DOI] [PubMed] [Google Scholar]
- 31.Ohba T, Cole HA, Cates JM, et al. Bisphosphonates inhibit osteosarcoma-mediated osteolysis via attenuation of tumor expression of MCP-1 and RANKL. J Bone Miner Res 2014; 29:1431–1445. [DOI] [PubMed] [Google Scholar]
- 32.Holen I, Coleman RE. Bisphosphonates as treatment of bone metastases. Curr Pharm Design 2010; 16:1262–1271. [DOI] [PubMed] [Google Scholar]
- 33.Kanis JA, Powles T, Paterson AH, et al. Clodronate decreases the frequency of skeletal metastases in women with breast cancer. Bone 1996; 19:663–667. [DOI] [PubMed] [Google Scholar]
- 34.Kavanagh KL, Guo K, Dunford JE, et al. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci U S A 2006; 103:7829–7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neville-Webbe HL, Gnant M, Coleman RE. Potential anticancer properties of bisphosphonates. Semin Oncol 2010; 37:S53–65. [DOI] [PubMed] [Google Scholar]
- 36.Winter MC, Holen I, RE C. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev 2008; 34:453–475. [DOI] [PubMed] [Google Scholar]
- 37.Hadji P, Bundred N. Reducing the risk of cancer treatment-associated bone loss in patients with breast cancer. Semin Oncol 2007; 34:S4–S10. [DOI] [PubMed] [Google Scholar]
- 38.Singh T, Kaur V, Kumar M, et al. The critical role of bisphosphonates to target bone cancer metastasis: an overview. J Drug Target 2015; 23:1–15. [DOI] [PubMed] [Google Scholar]
- 39.De Marinis F, Eberhardt W, Harper PG, et al. Bisphosphonate use in patients with lung cancer and bone metastases: recommendations of a European expert panel. J Thorac Oncol 2009; 4:1280–1288. [DOI] [PubMed] [Google Scholar]


