Abstract
Detection of antiphospholipid antibodies represents the first-line approach for diagnosis of antiphospholipid syndrome (APS). In this study, we evaluated the clinical performance of a novel chemiluminescence assay (CIA) in detection of IgG/IgM/IgA anti-cardiolipin (aCL) and IgG/IgM/IgA anti-β2 glycoprotein 1 (aβ2GP1) antibodies and to compare it with commercial enzyme-linked immunosorbent assay (ELISA) kits from the same manufacturer.
A total of 227 sera were tested in this study, including 84 samples from patients with APS, 104 samples from patients with non-APS diseases as disease controls, and 39 healthy controls. Serum IgG/IgM/IgA aCL and IgG/IgM/IgA aβ2GP1 were determined by both ELISA (QUANTA Lite™ ELISA) and CIA (QUANTA Flash®assays).
Significant quantitative correlations were identified between ELISA and CIA in IgG/IgM/IgA aCL and IgG/IgM/IgA aβ2GP1 autoantibodies detection (P < 0.001), with the rho value ranging from 0.51 to 0.87. In addition, ELISA and CIA demonstrated good qualitative agreements in IgG/IgM/IgA aCL and IgM/IgA aβ2GP1 autoantibodies determination with kappa coefficient ranged from 0.52 to 0.77. In contrast, ELISA and CIA showed a moderate qualitative agreement in IgG aβ2GP1 detection with a kappa value of 0.2. Notably, significantly higher IgG aβ2GP1 positive sera were detected by CIA, compared to those detected by ELISA in both primary APS (52.9% vs. 8.8%) and APS associated to other diseases sera (70.0% vs. 8.0%). For diagnosis of APS, IgG aβ2GP1 detection by CIA (IgG aβ2GP1 CIA) demonstrated the highest sensitivity (63.1%), followed by IgG aCL CIA (48.8%). More importantly, IgG aβ2GP1 CIA demonstrated the highest ability to predict the thrombotic events in patients with APS, with an OR of 3 (95% CI: 1.1–7.9).
Our data suggest that this novel CIA assay had good performance in detecting aCL and aβ2GP1 antibodies, especially in the detection of IgG aβ2GP1 antibodies. Our findings could shed insight on the application of CIA in the laboratory diagnosis of APS in China.
INTRODUCTION
Antiphospholipid syndrome (APS) is a heterogeneous group of autoimmune disease characterized by recurrent arterial/venous thrombosis, and/or pregnancy morbidity, as well as the presence of antiphospholipid (aPL) antibodies. Primary APS (PAPS) is defined by no evidence of any underlying systemic autoimmune disorder, while APS associated to other diseases is associated with other systemic autoimmune syndromes, especially with systemic lupus erythematosus (SLE).1
As aPLs being a hallmark feature of APS, detection of aPLs represents the first-line approach for diagnosis of APS. According to 2006 updated consensus criteria of APS, the diagnosis of APS requires the persistent presence of at least one of the following aPLs, including lupus anticoagulant (LA), IgG and/or IgM anti-cardiolipin (aCL), and IgG and/or IgM anti-β2 glycoprotein 1 (aβ2GP1) antibodies.1,2 IgA aCL and IgA aβ2GP1 antibodies are not currently included in the laboratory criteria for APS, but are suggested as “noncriteria” antibodies for seronegative patients with clinical suspicion of APS.2,3
Of note, the diagnosis of APS relies predominantly on laboratory findings, as characteristic clinical features of thrombosis and pregnancy morbidity also occur in many other diseases. In addition, these laboratory results are critical for predicting and stratifying the risks to develop the clinical manifestations of the syndrome. Unfortunately, the routinely used assays in clinical settings, particularly enzyme-linked immunosorbent assay (ELISA), lack standardized kits, resulting in substantial variations in the antibody positivity between different laboratories.4–6
Recently, the chemiluminescence technology has been applied for autoantibody testing.7–15 Several studies indicated that this novel assay had similar performance to commercial ELISAs and had a good agreement of results among laboratories regarding the detection of IgG/IgM aCL and IgG/IgM aβ2GP1 autoantibodies,9–15 suggesting that the chemiluminescence assay (CIA) could be a promising tool to improve the reproducibility and reduce interlaboratory variations. However, those studies have been performed in heterogeneous groups of patients in terms of different ethnic/geographic background, and most of these studies compared HemosIL® AcuStar CIA system or Zenit RA CIA system with either homemade ELISA11,12–15 or ELISA kit from another manufacturer.9,13,14
With the introduction of QUANTA Flash®system, it is possible to compare the CIA system with ELISA kit from the same manufacturer. In addition, as CIA being a promising viable alternative, it is of paramount importance to evaluate this novel fully automated assay in aCL and aβ2GP1 autoantibodies detection in Chinese patients with APS. In the present study, we evaluated the analytical and clinical performance of a novel CIA assay (QUANTA Flash®assays) in the detection of IgG/IgM/IgA aCL and IgG/IgM/IgA aβ2GP1antibodies and to compare it with commercial ELISA kits from the same manufacturer.
MATERIALS AND METHODS
Sera
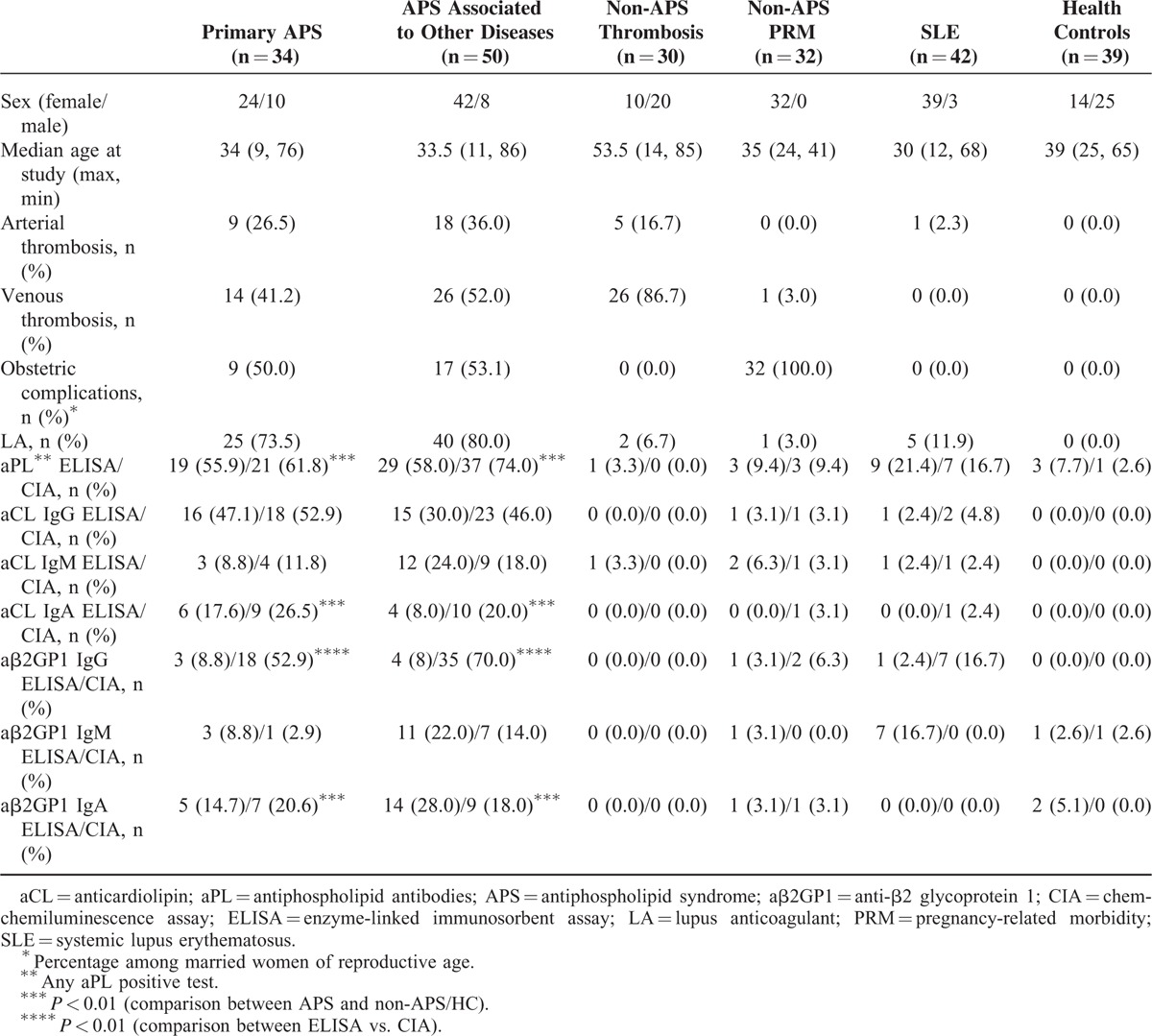
A total of 227 sera were tested in this study, including 84 samples from patients with APS, 104 samples from patients with non-APS diseases as disease controls (non-APS), and 39 healthy controls (HC). The APS samples included 34 samples from patients with PAPS and 50 samples from patients with APS associated to other diseases. The non-APS samples included 30 samples from patients with non-APS thrombosis, 32 samples from patients with non-APS pregnancy-related morbidity (PRM), and 42 samples from patients with SLE. HC included subjects without any signs of infection or inflammation or other significant illnesses. The diagnosis of APS was determined according to the Sydney revised Sapporo guidelines.2 Specifically, subjects were diagnosed with APS based on a combination of one positive clinical criterion and one positive laboratory criterion (LA, aCL or aβ2G1 antibodies determined by ELISA) on 2 different occasions separated by 12 weeks.2 The demographics and clinical characteristics of all subjects are shown in Table 1. All of samples were tested for LA. Study protocols were reviewed and approved by the Ethical Committee of Peking Union Medical College Hospital (PUMCH) and informed consents were obtained from all participants. All sera were stored at −20 °C until analysis.
TABLE 1.
Demographic Characteristics and Antibody Profiles of APS Patients and Controls
Serum Antibodies Determination
Serum aCL autoantibodies (IgG, IgM, and IgA) and aβ2GP1 (IgG, IgM, and IgA) were determined by both ELISA (QUANTA Lite™ ELISAs, INOVA Diagnostic, Inc., San Diego, CA) and CIA (QUANTA Flash®assays, INOVA Diagnostic, Inc.) according to the manufacturer's instructions. The QUANTA Flash®assays were performed on BIO-FLASH® instrument (Biokit S. A., Barcelona, Spain). The principle of the QUANTA Flash® assay system was previously described by Mahler et al7 and Bentow et al.16 The cut-off values for positivity were set based on the recommendations by the manufacturer.
Statistical Analysis
SPSS 20.0 statistical software package (SPSS, Inc., Chicago, IL) and Prism 5.02 (GraphPad Software, San Diego, CA) were utilized for all statistical tests. Cohen kappa agreement test and Spearman correlation test were performed to analyze the qualitative and quantitative agreement between ELISA and CIA. Serial receiver-operating characteristic (ROC) curves were used to calculate the area under the ROC (AUC) for defining optimal cut-off values and analyzing the performance of different assays. Average linkage clustering by Heml 1.0 Heatmap illustrator (The CUCKOO Workgroup, Hefei, Anhui, China) was used for cluster analysis. Hierarchical clustering was utilized to illustrate the relationship between different assays and to display the reactivity patterns of the patients. P values of less than 0.05 were considered statistical significant.
RESULTS
Clinical Characteristics
Clinical characteristics and laboratory findings of all subjects are listed in Table 1. Specifically, the incidence of arterial thrombosis in patients with PAPS, APS associated to other diseases, non-APS thrombosis, non-APS PRM, and SLE were 26.5%, 36.0%, 16.7%, 0, and 2.3%, respectively. The presence of venous thrombosis in patients with PAPS, APS associated to other diseases, non-APS thrombosis, non-APS PRM, and SLE were 41.2%, 52.0%, 86.7%, 3.0%, and 0, respectively. For calculation of the incidence of obstetric complications, we excluded male patients and nonmarried female patients. Thus, the calibrated incidence of obstetric complications in patients with PAPS, APS associated to other diseases, non-APS thrombosis, non-APS PRM, and SLE were 50.0%, 53.1%, 0%, 100%, and 0, respectively. LA was detected in 73.5% of PAPS patients, 80% of patients with APS associated to other diseases, 6.7% of patients with non-APS thrombosis, 3% of patients with non-APS PRM, and 11.9% of SLE patients.
aCL (IgG, IgM, and IgA) and aβ2GP1 (IgG, IgM, and IgA) Autoantibodies Detection by ELISA and CIA Assays
Table 1 shows the results of IgG/IgM/IgA aCL and IgG/IgM/IgA aβ2GP1 autoantibodies detection by ELISA and CIA from all tested sera. Except for IgM/IgA aβ2GPI autoantibodies, all HC samples were negative by both assays. Similar percentages of positive results for IgG/IgM/IgA aCL and IgM/IgA aβ2GP1 autoantibodies were found in both assays. However, significantly higher IgG aβ2GP1 positive sera were detected by CIA, compared to those detected by ELISA in both PAPS (52.9% vs. 8.8%, P < 0.001) and APS associated to other diseases sera (70.0% vs. 8.0%, P < 0.001). IgA aCL and IgA aβ2GP1 autoantibodies have been considered as “noncriteria” antibodies for seronegative patients with clinical suspicion of APS.2 Importantly, both IgA aCL and IgA aβ2GP1 antibodies detected by either assay were significantly higher in patients with APS than those in non-APS disease controls or HC (Table 1).
Qualitative Agreements and Quantitative Agreements Between ELISA and CIA Assays in aCL (IgG, IgM, and IgA) and aβ2GP1 (IgG, IgM, and IgA) Autoantibodies Determination
Generally, ELISA and CIA demonstrated good overall agreements (>90%) in IgG/IgM/IgA aCL and IgM/IgA aβ2GP1 autoantibodies determination. The positive agreement and negative agreement between ELISA and CIA in detection of these autoantibodies ranged from 40.9% to 67.4% and 90.7% to 96.8%, respectively (Table 2). kappa coefficient was calculated to assess the qualitative agreements between ELISA and CIA, and the kappa coefficient for those antibodies ranged from 0.52 to 0.77 (Table 2). Interestingly, ELISA and CIA showed a moderate overall agreement (76.7%) in IgG aβ2GP1 detection, with the positive agreement, negative agreement, and kappa value of 14.8%, 75.7%, and 0.2, respectively (Table 2). Quantitative agreements between ELISA and CIA assays were determined by Spearman correlation test. Importantly, significant quantitative correlations were identified between ELISA and CIA assays in IgG/IgM/IgA aCL and IgG/IgM/IgA aβ2GP1 autoantibodies detection (P < 0.001), with the rho value ranging from 0.51 to 0.87 (Fig. 1).
TABLE 2.
Qualitative Agreements Between ELISA and CIA Assays in aCL (IgG, IgM, and IgA) and aβ2GP1 (IgG, IgM, and IgA) Detection
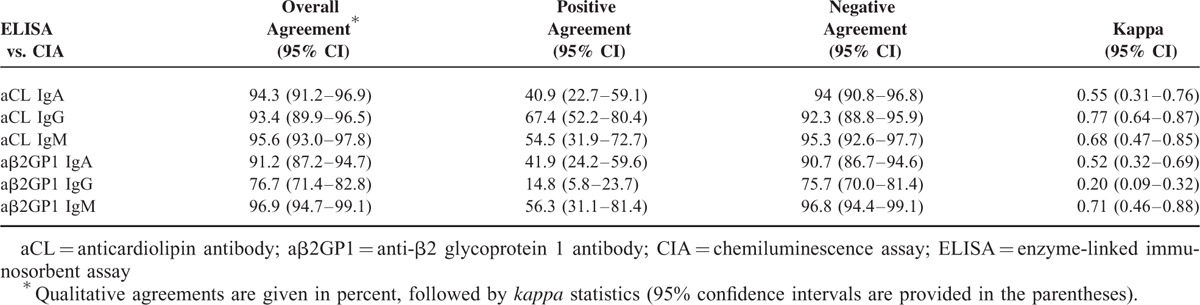
FIGURE 1.
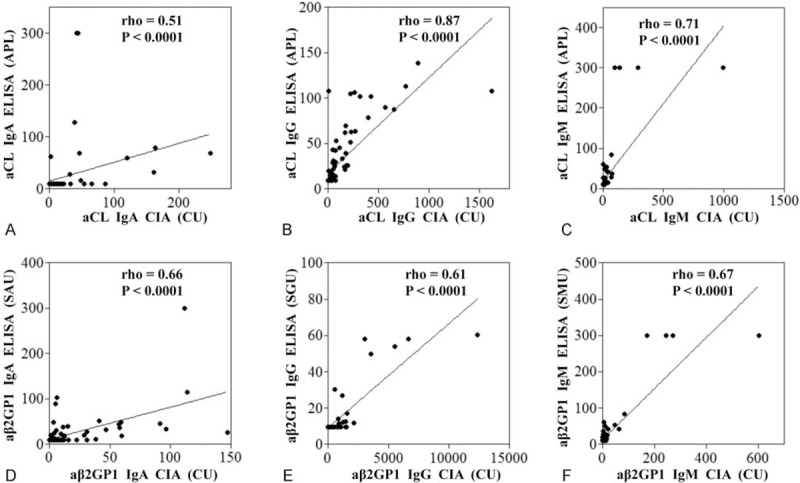
Quantitative correlation between ELISA and CIA. Correlations between ELISA and CIA for IgA aCL (A), IgG aCL (B), IgM aCL (C), IgA aβ2GP1 (D), IgG aβ2GP1 (E), and IgM aβ2GP1 (F) antibodies detection are shown. Quantitative correlation between the ELISA and CIA for each individual antibody detection was calculated by Spearman correlation test. aβ2GP1 = anti-β2 glycoprotein 1, aCL = anti-cardiolipin, CIA = chemiluminescence assay.
Clinical Performance Characteristics of ELISA and CIA Assays in aCL (IgG, IgM, and IgA) and aβ2GP1 (IgG, IgM, and IgA) Determination
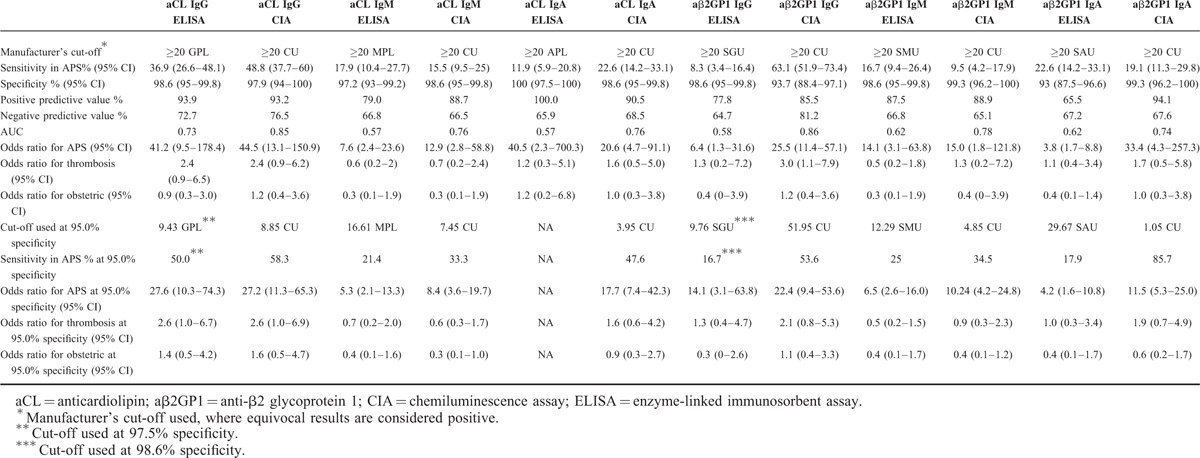
Assay performance characteristics for detection of IgG/IgM/IgA aCL and IgG/IgM/IgA aβ2GP1 autoantibodies were evaluated on both ELISA and CIA assays, and the results are summarized in Table 3. For diagnosis of APS, IgG aβ2GP1 detection by CIA (IgG aβ2GP1 CIA) demonstrated the highest sensitivity (63.1%), followed by IgG aCL CIA (48.8%), IgG aCL ELISA (36.9%), IgA aβ2GP1 ELISA (22.6%), and IgA aCL CIA (22.6%). Interestingly, IgG aβ2GP1 ELISA exhibited the lowest sensitivity (8.3%). Of note, the highest sensitivity observed in IgG aβ2GP1 CIA did not result in loss of specificity (93.7%) (Table 3). In addition, IgA aCL ELISA showed the highest positive predictive value (100%), and IgG aβ2GP1 CIA showed the highest negative predictive value (81.2%) (Table 3).
TABLE 3.
Clinical Performance Characteristics for ELISA and CIA Assays in aCL (IgG, IgM, and IgA) and aβ2GP1 (IgG, IgM, and IgA) Detection
ROC analysis was performed to evaluate the discrimination power of ELISA and CIA for distinguishing patients with APS and controls. IgG aβ2GP1 CIA exhibited the best discrimination power with the area under the curves (AUC) of 0.86, followed by IgG aCL CIA (AUC of 0.85) and IgM aβ2GP1 CIA (AUC of 0.78) (Fig. 2 and Table 3). Interestingly, IgM aCL ELISA, IgA aCL ELISA and IgG aβ2GP1 ELISA showed poor discrimination power with the ACU of 0.57, 0.57, and 0.58, respectively (Fig. 2 and Table 3).
FIGURE 2.
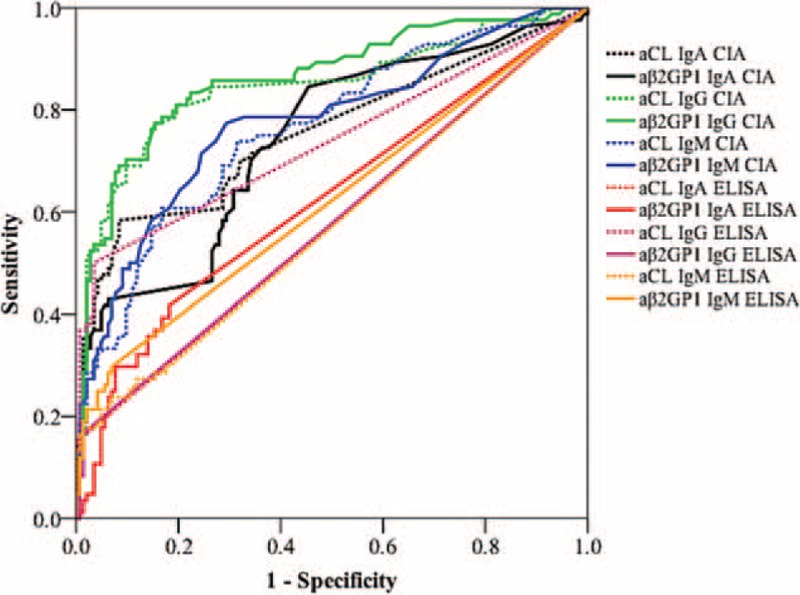
Receiver-operating characteristics (ROC) analysis. ROC analysis was performed to evaluate the discrimination power of aCL (IgA, IgG, and IgM) and aβ2GP1 (IgA, IgG, and IgM) antibodies detected either by ELISA or by CIA for distinguishing patients with APS (n = 84) and controls (n = 143). aβ2GP1 = anti-β2 glycoprotein 1, aCL = anti-cardiolipin, CIA = chemiluminescence assay.
The odds ratios (OR) were calculated to evaluate the performance of each autoantibody tested by either ELISA or CIA in prediction of APS. Interestingly, all of the autoantibodies tested by both ELISA and CIA showed high ORs for predicting APS, ranging from 3.8 in IgA aβ2GP1 ELISA to 44.5 in IgG aCL CIA. Importantly, IgG aβ2GP1 CIA demonstrated the highest ability to predict the thrombotic events in patients with APS, with an OR of 3 (95% CI: 1.1–7.9) (Table 3). However, all the autoantibodies detected by either ELISA or CIA had little power to predict the obstetric risks (Table 3).
Cluster Analysis
To further illustrate the distribution of each autoantibody tested by either ELISA or CIA in patients with APS and controls, and to illustrate the relationships among these autoantibodies, a supervised cluster analysis with a dendrogram was performed. The cluster analysis indicates that the majority of PAPS and patients with APS associated to other diseases were positive for IgG aβ2GP1 CIA and IgG aCL CIA (Fig. 3). Some of the controls also showed positive results in some autoantibodies by different assays. In addition, IgG aCL CIA clustered were found closer to IgG aβ2GPI CIA than to IgG aCL ELISA (Fig. 3). More importantly, the dendrogram shows that IgG aβ2GPI CIA and IgG aCL CIA clusters, and to a less extent, IgG aCL ELISA cluster, were closest related the APS than other autoantibodies tested by either ELISA or CIA (Fig. 3).
FIGURE 3.
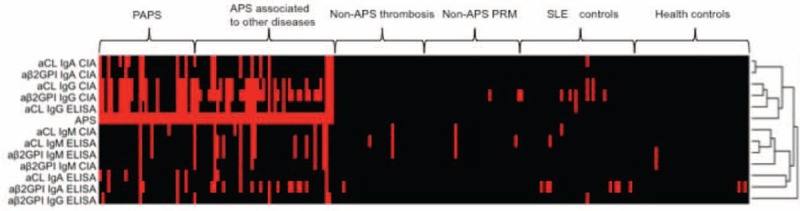
Supervised cluster analysis. Supervised centered cluster analysis according to disease cohorts (PAPS, APS associated to other diseases, non-APS thrombosis, non-APS PRM, SLE, and health controls) is shown. The dendrogram is shown to illustrate the relationships among these antibodies in the diagnosis of APS. APS = associated to other diseases, secondary antiphospholipid syndrome, non-APS PRM = non-APS pregnancy-related morbidity; SLE = systemic lupus erythematosus, PAPS = primary antiphospholipid syndrome.
DISCUSSION
The major findings in this study include: CIA strikingly increased the sensitivity in detection of IgG aβ2GP1 antibody without loss of specificity in patients with APS, compared with the results by ELISA; IgG aβ2GP1 detected by CIA demonstrated the ability to predict the thrombotic events in patients with APS, while IgG aβ2GP1 detected by ELISA or other antibodies detected by either ELISA or CIA showed poor ability in predicting the thrombotic risks; ELISA and CIA exhibited good overall qualitative agreements (>90%) in IgG/IgM/IgA aCL and IgM/IgA aβ2GP1 autoantibodies detection, while they only showed a moderate overall agreement (76.7%) in IgG aβ2GP1 detection; ELISA and CIA assays demonstrated significant quantitative correlations in IgG/IgM/IgA aCL and IgG/IgM/IgA aβ2GP1 autoantibodies detection. Our findings supported that CIA could serve as a promising viable alternative for ELISA in the detection of aCL and aβ2GP1 autoantibodies, especially in the detection of IgG aβ2GP1 autoantibody.
We found that CIA strikingly increased the sensitivity in detection of IgG aβ2GP1 antibodies compared with the results by ELISA. Importantly, the increased sensitivity of IgG aβ2GP1 by CIA did not sacrifice the specificity, PPV and NPV values. Several factors may contribute to the improved sensitivity by CIA, such as differences in detection system and antigens, as CIA utilized the full-length recombinant aβ2GP1 expressed in the insect cells as antigen in BIO-FLASH® instrument. Mondejar et al10 reported that IgG aβ2GP1 detected by CIA and IgG aβ2GP1 detected by ELISA had a comparable sensitivity in APS patients from Spain using the same CIA and ELISA systems, although a trend of higher sensitivity in CIA was observed. Interestingly, the sensitivity of IgG aβ2GP1 detected by CIA was higher in our study compared to that in Mondejar's study,10 although the sensitivity of IgG aβ2GP1 detected by ELISA was much lower.
Despite widespread use of ELISA for detection of aCL and aβ2GP1 autoantibodies in clinical settings, several limitations, such as low reproducibility, substantial interlaboratory variations, challenged the role of ELISA in accurately evaluating the risks of developing APS-related complications.4,18 aβ2GP1 autoantibodies have been recognized as the main pathogenic subset in aPLs, especially with thrombosis events.12,17 However, we did not observe any association between IgG aβ2GP1 autoantibodies detected by ELISA with thrombosis events. In contrast, we did identify an association between IgG aβ2GP1 autoantibodies and thrombosis events using the CIA assay. Interestingly, Moerloose et al,12 reported that IgG aβ2GP1 determined by both ELISA (QUANTA Lite INOVA) and CIA (HemosIL® AcuStar) significantly correlated with thrombosis events in patients with APS from Europe. As we used the same ELISA kit, the discrepancies may be due to different ethnic/geographic backgrounds or due to substantial interlaboratory variations in ELISA testing, as mentioned earlier.
Our results revealed good qualitative and quantitative agreements between CIA and ELISA in IgG/IgM aCL and IgM aβ2GP1 autoantibodies determinations. In IgG aCL detection, CIA and ELISA showed good overall, positive and negative agreements of 93.4%, 67.4%, and 92.3%, respectively, which is similar to what has been previously described by Mondejar et al.10 (90.1%, 68.4%, and 95.1%, respectively). However, for IgM aCL and IgM aβ2GP1 antibodies detection, the overall and positive agreements between CIA and ELISA in our study were higher than what they have reported.10 Chung et al13 compared HemosIL® AcuStar CIA system with QUANTA Lite ELISA, and found an overall agreement of 86.2% in IgM aCL detection, which was lower than what we have found (95.6%). However, for IgM aβ2GP1 detection, they found an overall agreement of 93.6%, which is similar to what we reported (96.9%).13
The 2006 Sydney criteria for APS suggest IgA aCL and IgA β2GP1 antibodies as “noncriteria” antibodies for seronegative patients with clinical suspicion of APS.2 We also evaluated the clinical performance of CIA and ELISA in the determination of levels of IgA aCL and IgA aβ2GP1 antibodies. Our data demonstrated that CIA and ELISA had good overall qualitative agreements and good quantitative agreements, as determined by Cohen kappa agreement test and Spearman correlation test, respectively. Importantly, we found that both IgA aCL and IgA aβ2GP1 antibodies either detected by CIA or by ELISA in patients with APS were strikingly higher than those in non-APS disease controls or health controls, supporting the 2006 Sydney criteria that IgA aCL and IgA aβ2GP1 antibodies could contribute to the diagnosis of APS. However, we did not identify any associations either between IgA aCL and thrombosis events/obstetric complications or between IgA aβ2GP1 and thrombosis events/obstetric complications, which is different from the results from Despierres et al.19 Despierres et al19 found that IgA aβ2GP1 antibodies were significantly correlated with thrombosis events, but not obstetric complications.
It should be noted, however, that several limitations exist in our study. Patients with APS were diagnosed based on the 2006 updated consensus criteria, which requires presence of at least one of the LA, aCL, and aβ2GP1 autoantibodies. It has been proposed that seronegative APS, which refers as patients with clinical manifestations indicative of APS but with persistently negative results in the routinely used assays to detect the LA, aCL, and aβ2GP1 autoantibodies, does exist.20 Thus, we may ignore these seronegative APS patients in our study. Further studies on those patients are needed.
In addition, our findings need to be confirmed in other independent study, especially the greatly improved sensitivity of the IgG aβ2GPI assay by CIA as compared to the conventional ELISA. This information would be particularly important, as ELISA is currently widely used in Chinese hospitals in the detection of aPLs.
In summary, our data suggest that this novel CIA assay had good performance characteristics in detecting aCL and aβ2GP1 antibodies, especially in the detection of IgG aβ2GP1 antibodies. Of particular interest is the finding that CIA had a better prediction power of thrombosis events. In addition, considering the advantage of being fully automated, CIA allows for a decrease in interlaboratory variability and an increase in reproducibility. Our findings could shed insight on the introduction and application of CIA in the laboratory diagnosis of APS in Chinese hospitals.
Footnotes
Abbreviations: aβ2GP1 = anti-β2 glycoprotein 1, aCL = anti-cardiolipin, aPL = antiphospholipid antibodies, APS = antiphospholipid syndrome, CIA = chemiluminescence assay, ELISA = enzyme-linked immunosorbent assay, LA = lupus anticoagulant, OR = odds ratios, ROC = receiver-operating characteristic, SLE = systemic lupus erythematosus.
Shulan Zhang and Ziyan Wu contributed equally to this work.
The authors have no conflicts of interest to disclose.
This work was supported in part by the National Natural Science Foundation of China Grants No. 81373188, 81172857 (to YL), 81302592 (to SZ), the Chinese National High Technology Research and Development Program, Ministry of Science and Technology Grants No. 2011AA02A113, the National Science Technology Pillar Program in the 12nd Five-year Plan No. 2014BAI07B00, the capital health research and development of special grants No. 2014-1-4011 (to YL).
REFERENCES
- 1.Gómez-Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. J Autoimmun 2014; 48–49:20–25. [DOI] [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306. [DOI] [PubMed] [Google Scholar]
- 3.Bertolaccini ML, Amengual O, Atsumi T, et al. ‘Non-criteria’ aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus 2011; 20:191–205. [DOI] [PubMed] [Google Scholar]
- 4.Devreese KM. Standardization of antiphospholipid antibody assays. Where do we stand? Lupus 2012; 21:718–721. [DOI] [PubMed] [Google Scholar]
- 5.Ruffatti A, Olivieri S, Tonello M, et al. Influence of different IgG anticardiolipin antibody cut-off values on antiphospholipid syndrome classification. J Thromb Haemost 2008; 6:1693–1696. [DOI] [PubMed] [Google Scholar]
- 6.Forastiero R, Papalardo E, Watkins M, et al. Evaluation of different immunoassays for the detection of antiphospholipid antibodies: report of a wet workshop during the 13th International Congress on Antiphospholipid Antibodies. Clin Chim Acta 2014; 428:99–105. [DOI] [PubMed] [Google Scholar]
- 7.Mahler M, Radice A, Yang W, et al. Development and performance evaluation of novel chemiluminescence assays for detection of anti-PR3 and anti-MPO antibodies. Clin Chim Acta 2012; 413:719–726. [DOI] [PubMed] [Google Scholar]
- 8.Webb T, Lakos G, Swart A, et al. Clinical evaluation of a novel chemiluminescent immunoassay for the detection of anti-citrullinated peptide antibodies. Clin Chim Acta 2014; 437:161–167. [DOI] [PubMed] [Google Scholar]
- 9.Persijn L, Decavele AS, Schouwers S, et al. Evaluation of a new set of automated chemiluminescense assays for anticardiolipin and anti-beta2-glycoprotein I antibodies in the laboratory diagnosis of the antiphospholipid syndrome. Thromb Res 2011; 128:565–569. [DOI] [PubMed] [Google Scholar]
- 10.Mondejar R, González-Rodríguez C, Toyos-Sáenz de Miera FJ, et al. Role of antiphospholipid score and anti-β2-glycoprotein I Domain I autoantibodies in the diagnosis of antiphospholipid syndrome. Clin Chim Acta 2014; 431:174–178. [DOI] [PubMed] [Google Scholar]
- 11.Meneghel L, Ruffatti A, Gavasso S, et al. Detection of IgG anti-Domain I beta2 Glycoprotein I antibodies by chemiluminescence immunoassay in primary antiphospholipid syndrome. Clin Chim Acta 2015; 446:201–205. [DOI] [PubMed] [Google Scholar]
- 12.DE Moerloose P, Reber G, Musial J, et al. Analytical and clinical performance of a new, automated assay panel for the diagnosis of antiphospholipid syndrome. J Thromb Haemost 2010; 8:1540–1546. [DOI] [PubMed] [Google Scholar]
- 13.Chung Y, Kim JE, Lim HS, et al. Clinical performance of anticardiolipin and antiβ2 glycoprotein I antibodies using a new automated chemiluminescent assay: superior thrombotic prediction of combined results measured by two different methods. Blood Coagul Fibrinolysis 2014; 25:10–15. [DOI] [PubMed] [Google Scholar]
- 14.Van Hoecke F, Persijn L, Decavele AS, et al. Performance of two new, automated chemiluminescence assay panels for anticardiolipin and anti-beta2-glycoprotein I antibodies in the laboratory diagnosis of the antiphospholipid syndrome. Int J Lab Hematol 2012; 34:630–640. [DOI] [PubMed] [Google Scholar]
- 15.Meneghel L, Ruffatti A, Gavasso S, et al. The clinical performance of a chemiluminescent immunoassay in detecting anti-cardiolipin and anti-β2 glycoprotein I antibodies. A comparison with a homemade ELISA method. Clin Chem Lab Med 2015; 53:1083–1089. [DOI] [PubMed] [Google Scholar]
- 16.Bentow C, Swart A, Wu J, et al. Clinical performance evaluation of a novel rapid response chemiluminescent immunoassay for the detection of autoantibodies to extractable nuclear antigens. Clin Chim Acta 2013; 424:141–147. [DOI] [PubMed] [Google Scholar]
- 17.Meroni PL, Borghi MO, Raschi E, et al. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol 2011; 7:330–339. [DOI] [PubMed] [Google Scholar]
- 18.Pierangeli SS, Harris EN. A quarter of a century in anticardiolipin antibody testing and attempted standardization has led us to here, which is? Semin Thromb Hemost 2008; 34:313–328. [DOI] [PubMed] [Google Scholar]
- 19.Despierres L, Beziane A, Kaplanski G, et al. Contribution of anti-β2glycoprotein I IgA antibodies to the diagnosis of anti-phospholipid syndrome: potential interest of target domains to discriminate thrombotic and non-thrombotic patients. Rheumatology (Oxford) 2014; 53:1215–1218. [DOI] [PubMed] [Google Scholar]
- 20.Cervera R, Conti F, Doria A, et al. Does seronegative antiphospholipid syndrome really exist? Autoimmun Rev 2012; 11:581–584. [DOI] [PubMed] [Google Scholar]


