Abstract
It has been shown that triple antiplatelet therapy with cilostazol results in better clinical outcomes than dual therapy in patients treated with a first-generation drug-eluting stent (DES); however, it is unclear whether triple antiplatelet therapy has a similar efficacy after the implantation of second-generation DES.
In the COACT (Cath Olic medical center percutAneous Coronary in Tervention) registry, 1248 study subjects who underwent percutaneous coronary intervention with an everolimus- or zotarolimus-eluting stent (Endeavor, Xience V, or Promus) were analyzed. The patients were divided into 2 groups after propensity score matching (n = 724; M = 422 [58.3%]; mean age = 66.1 ± 11.0 years): Group 1: patients treated with dual antiplatelet drugs (aspirin and clopidogrel; n = 362; M = 213 [58.8%]; mean age = 65.6 ± 11.7 years); Group 2: patients treated with triple antiplatelet drugs (aspirin, clopidogrel, and cilostazol; n = 362; M = 209 [57.7%]; mean age = 65.6 ± 11.7 years). The mean follow-up duration was 13 ± 10 months, and the cumulative incidence of major cardiovascular events (MACE) was 6.3% in Group 1 and 7.7% in Group 2. There were no significant differences in MACE (death, nonfatal myocardial infarction, and stroke) between the 2 groups (OR, 1.210; 95% CI: 0.772–1.898; P = 0.406). Kaplan–Meier curves for MACE did not show any survival benefit for triple antiplatelet therapy, even in patients with acute coronary syndrome.
In patients treated with a second-generation DES implantation, there is no added clinical benefit to using triple rather than dual antiplatelet therapy.
INTRODUCTION
Dual antiplatelet therapy with aspirin and clopidogrel is recommended after a percutaneous coronary intervention (PCI) related to stent thrombosis. In addition, newer antiplatelet agents, such as prasugrel, ticagrelor, or cilostazol, are considered for high-risk patients to prevent repeat revascularization. Cilostazol is a phosphodiesterase-3 inhibitor that inhibits platelet aggregation and causes an antiplatelet effect and vasodilation.1,2 Research has demonstrated improved clinical outcomes when triple rather than dual antiplatelet therapy is used with a first-generation drug-eluting stent (DES).3,4 Triple antiplatelet therapy has significantly reduced the rates of major adverse cardiac events (MACE, including with death, myocardial infarction, and stroke) by inhibiting neointima formation.3,4 In patients with acute coronary syndrome, triple-antiplatelet therapy has been shown to reduce long-term cardiac and cerebral events after a PCI.5 However, it remains unclear whether triple antiplatelet therapy has a similar additive efficacy after implantation with a second-generation DES. Recently, concerns have been raised regarding platelet reactivity as a result of cytochrome p450 polymorphisms. Some investigators have suggested that double- or triple-dose antiplatelet therapy can combat this platelet resistance.6 With improvements in pharmacologic management and the evolving mechanical attributes of drug stents, the need for antiplatelet therapy has been decreased. Therefore, we compared the efficacy of triple antiplatelet therapy and dual antiplatelet therapy in patients undergoing PCI with a second-generation DES.
METHODS
Study Population
The COACT (CathOlic medical center percutAneous Coronary inTervention) registry is a multicenter, observational registry of clinical data on patients who underwent PCI at the Catholic University of Korea between January 2004 and December 2009. A total of 1248 patients were eligible for this study. All subjects had angina pectoris or documented myocardial ischemia and an angiographically proven stenosis of ≥50% diameter. These patients received a PCI with 1 of the following second-generation DESs: Xience V everolimus-eluting stent (EES) (Abbott Vascular, Santa Clara, CA), Promus everolimus-eluting stent (EES) (Boston Scientific, Natick, MA), or Endeavor zotarolimus-eluting stent (ZES) (Medtronic Inc., Santa Rosa, CA). In addition, we did not include patients who had received repeated revascularizations, those who had previously used a first-generation DES. Study was firmly approved by Catholic Institutional Review Board. And we also got a informed consent.
Study Procedure
All patients received a loading dose of 250 to 500 mg of aspirin and 300 to 600 mg of clopidogrel before the procedure. Following the procedure, maintenance doses of 100 mg/day of aspirin and 75 mg/day of clopidogrel were administered for at least 6 months. At the physician's discretion, 200 mg/day of cilostazol (Otsuka Pharmaceutical, Seoul, Republic of Korea) was added and continued for at least 3 months days after the PCI. Patients were placed into a dual therapy group (aspirin and clopidogrel; n = 873) or a triple therapy group (aspirin, clopidogrel, and cilostazol; n = 375). All interventions were performed in a conventional manner, and the infusion of glycoprotein IIb/IIIa inhibitors was performed according to the operator's discretion. All patients were given unfractionated heparin during the procedure. In addition, all patients were provided with unrestricted, optimal pharmacological therapy, including statins. A successful PCI was defined as a residual stenosis of ≤30% and recovery of normal flow.
Study End Points
The primary end point was the incidence of major adverse cardiovascular events, defined as death, nonfatal myocardial infarction (MI), or stroke during the follow-up period. Death was defined as death from any cause. MI was defined as a cardiac enzyme elevation (creatine kinase-myocardial band elevation >3 times normal) with an ST change in consecutive leads. Stroke was defined as a new neurologic deficit of vascular origin lasting at least 24 hr. The secondary end point was the occurrence of target lesion revascularization (TLR) or target vessel revascularization (TVR). TLR was defined as a ≥50% diameter stenosis confirmed with quantitative coronary angiography at the target lesion and requiring repeat revascularization. TVR was defined as any revascularization involving the target vessel. All the patients were monitored at the out-patients clinics periodically. We performed the routine angiographic follow up at 9 months (72%). The patients who declined to get a routine angiographic follow up were tested by noninvasive stress test such as exercise treadmill or coronary CT imaging.
Statistical Analysis
Baseline characteristics were summarized as the mean ± SD for continuous variables and as frequency with percentages for categorical variables. Comparisons between the 2 groups were analyzed by the Student t test or Mann–Whitney U test for continuous variables and the Chi-squared test or Fisher's exact test for categorical variables as appropriate. To reduce the effect of selection bias and potential confounding effects in an observational study, we performed rigorous adjustments for differences in baseline patient characteristics by propensity score matching. The propensity scores were estimated without regard to outcome using multiple logistic regression analysis. Adjusted covariates, including 23 variables, were used for the calculation of propensity scores (Table 1). All model discriminations using 23 variables and calibration were assessed (c-statistic = 0.658; Hosmer and Lemeshow statistic P = 0.791). We used the Greedy matching algorithm to create propensity score-matched pairs (1:1 match). After propensity score matching, we reassessed the balance in baseline covariates between the 2 groups with the paired t-test or the Wilcoxon signed rank test for continuous variables and McNemar's test for categorical variables. The comparisons between 2 groups were analyzed with Cox regression models for overall populations and propensity score-matched pairs. For the inverse probability of treatment weighting (IPTW), the weights for the dual therapy group were the inverse of (1-the propensity score), and the weights for the triple therapy group were the inverse of the propensity score. Cumulative incidence rates were obtained by Kaplan–Meier analysis and compared with the log-rank test for propensity score-matched pairs. All statistical tests were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC) and were 2-sided. Results were considered significant at a P value <0.05.
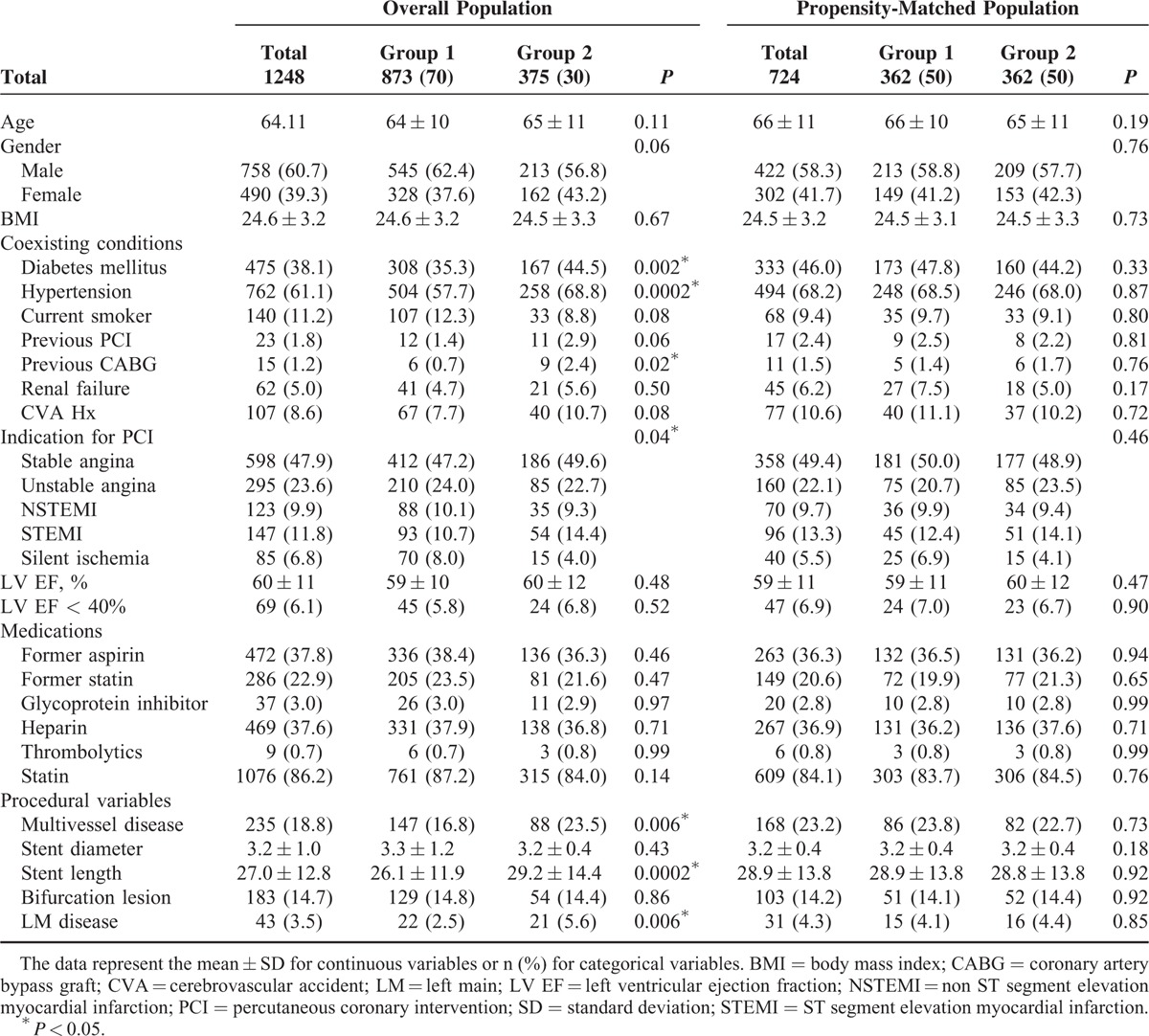
TABLE 1.
Baseline Characteristics of the Overall Population and Propensity Score-Matched Population According to Antiplatelet Therapy
RESULTS
Baseline Characteristics
The baseline characteristics of the overall populations are listed in Table 1. The prevalence of diabetes, hypertension, and a history of previous coronary artery bypass graft were significantly greater in the triple therapy group. The triple therapy group had more complex lesions, including multivessel disease, longer stent length per lesion, and left main disease. The patients were divided into the following 2 groups after propensity score matching (n = 724; M = 422 [58.3%]; mean age = 66.1 ± 11.0 years): Group 1: patients treated with dual antiplatelet drugs (aspirin and clopidogrel; n = 362; M = 213 [58.8%]; mean age = 65.6 ± 11.7 years); Group 2: patients treated with triple antiplatelet drugs (aspirin, clopidogrel, and cilostazol; n = 362; M = 209 [57.7%]; mean age = 65.6 ± 11.7 years). Mean duration of cilostazol was 8 ± 4 months. The propensity model included multiple factors that could influence coronary artery disease. After propensity matching, there were no significant differences in any of the covariates (Table 1).
Comparison of Clinical Outcomes in the Dual and Triple Therapy Groups
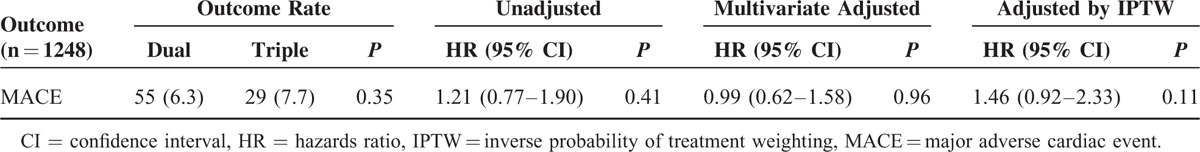
The mean follow-up duration was 13 ± 10 months, and the cumulative incidence of MACE was 55 (6.3%) in Group 1 and 29 (7.7%) in Group 2 (P = 0.354). There were no significant differences in MACE (death, nonfatal MI, and stroke) between the 2 groups (odds ratio [OR]: 1.210; 95% CI: 0.772–1.898; P = 0.406).
Multivariable Analysis
After multivariate adjustment, there was no difference in the risk of MACE between the 2 groups (OR: 0.987; 95% CI: 0.617–1.578; P = 0.955). In addition, the IPTW-adjusted risk of MACE did not differ between the 2 groups (OR: 1.464; 95% CI: 0.919–2.332; P = 0.109; Table 2).
TABLE 2.
Hazards Ratio for Clinical Outcomes in the Overall Population According to Antiplatelet Therapy
Similar clinical outcomes were observed in the propensity score-matched groups, with no differences in death, nonfatal MI, or stroke (OR: 0.911; 95% CI: 0.549–1.512; P = 0.719; Table 3). In addition, similar results were obtained for each of the following: death (OR: 0.720; 95% CI: 0.404–1.284; P = 0.265), recurrent nonfatal MI (OR: 3.886; 95% CI: 0.434–34.770; P = 0.225), and stroke (OR: 2.297; 95% CI: 0.594–8.881; P = 0.288). The Kaplan–Meier curves for MACE did not demonstrate any survival benefit for triple antiplatelet therapy (Figure 1).
TABLE 3.
Hazard Ratios for Clinical Outcomes in the Propensity-Matched Patients According to Antiplatelet Therapy
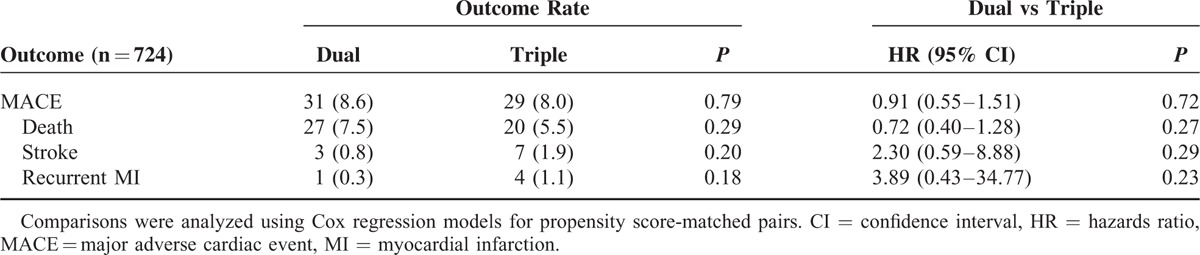
FIGURE 1.
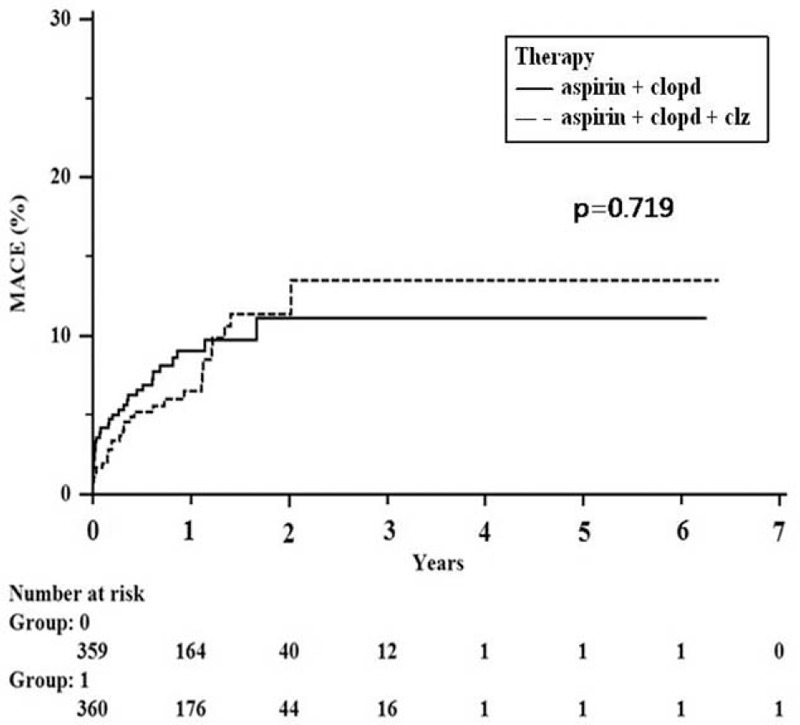
Kaplan–Meier curves for outcomes in propensity matched patients who underwent dual antiplatelet therapy or triple antiplatelet therapy.
Acute Coronary Syndrome
We performed a subgroup analysis for patients with acute coronary syndrome (n = 565; M = 354 [62.6%]; mean age = 64.7 ± 12.2 years). Patients were divided into 2 groups: Group 1 (dual therapy; n = 391; M = 245 [62.7%]; mean age = 64.8 ± 12.2 years) and Group 2 (triple therapy; n = 174; M = 109 [62.6%]; mean age = 64.3 ± 12.3 years). No significant differences were observed in the composite event rate (11.0% vs 10.3%; P = 0.818; Table 4). A 1:1 propensity matching analysis (n = 322; M = 206 [64.0%]; mean age = 65.1 ± 11.8 years) demonstrated no difference in MACE (12.4% vs 10.6%, P = 0.600; OR, 0.798 [0.418–1.525], P = 0.495) and in TLR or TVR (6.2% vs 11.8%, P = 0.080; OR: 1.725 [0.801–3.717], P = 0.164) between the 2 groups (Figure 2).
TABLE 4.
Hazard Ratios for Clinical Outcomes in Patients With Acute Coronary Syndrome According to Antiplatelet Therapy
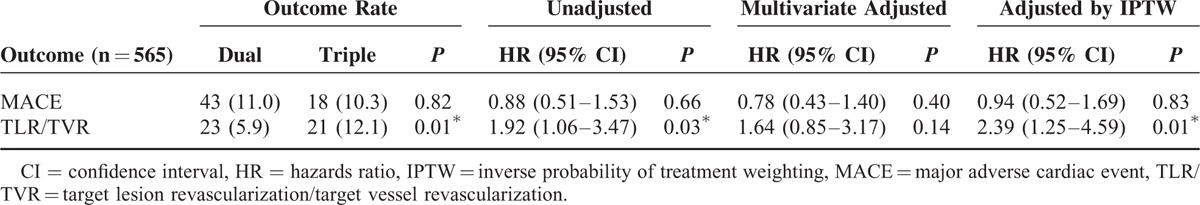
FIGURE 2.
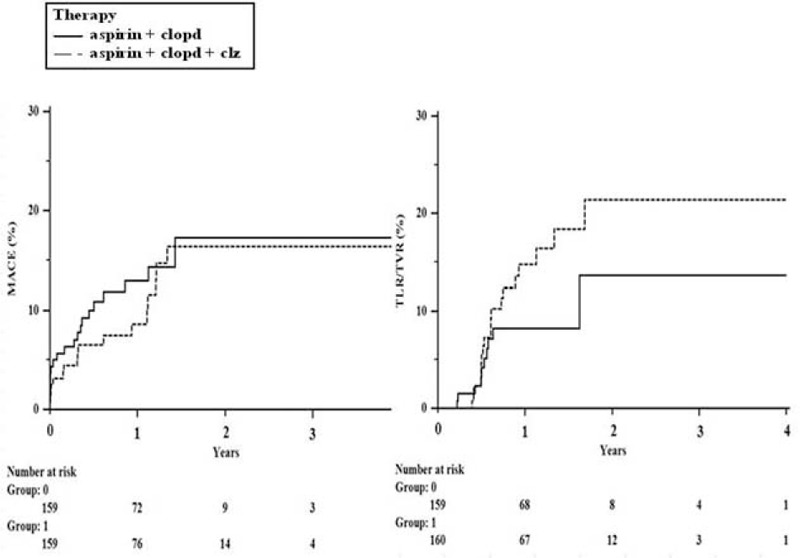
Kaplan–Meier curves for outcomes in propensity matched patients who underwent dual antiplatelet therapy or triple antiplatelet therapy in acute coronary syndrome population.
DISCUSSION
The implantation of a DES is more effective and stable than a bare metal stent (BMS), which suppresses restenosis and adverse cardiac events; however, there are still unresolved clinical issues, such as very late stent thrombosis.7 Some stent thrombosis symptoms are fatal, especially in patients with acute coronary syndrome and/or other high-risk conditions (long stent length per lesion, small vessels, diabetes). Some studies have indicated that cilostazol-based triple antiplatelet therapy decreases MACE in these patient groups.3–5 To resolve these issues, researchers have begun to investigate the use of second-generation DESs; for example, novel drugs and stent materials (cobalt–chromium or platinum–chromium alloy) and biocompatible polymer undergoing laboratory and clinical tests to improve the effectiveness and safety of second-generation DESs.8,9 A recent meta-analysis,10 which included 22 randomized trials with 12,453 STEMI patients, compared the 1-year event rate (mortality, MI, and TVR) of BMSs, first-generation DESs and second-generation DESs. The results showed that cobalt–chromium everolimus-eluting stents (CoCr-EES) were associated with significantly lower rates of cardiac death or MI and stent thrombosis than BMSs. In addition, first-generation DESs resulted in a significant reduction in TVR compared with BMSs; however, there were no significant differences in the risk of overall cardiac death and MI, which demonstrated the safety and efficacy of second-generation DESs.11
Based on these results, we hypothesized that there is no additive benefit when triple rather than dual antiplatelet therapy is used with a second-generation DES. To the best of our knowledge, this is the first study comparing the effectiveness of triple versus dual antiplatelet therapy using second-generation DESs. It has been reported that triple antiplatelet therapy is superior to dual antiplatelet therapy with first-generation DESs; however, even in patients with acute coronary syndrome, there was no benefit to using triple antiplatelet therapy with a second-generation DES. In the present study, patients were followed for a mean of 13 months with no evidence of a decrease in the risk of very late stent thrombosis for patients treated with triple therapy. These findings suggest that triple antiplatelet therapy is less attractive of using with second-generation stents.
Study Limitations
This study was based on observational, nonrandomized trials. Therefore, there may be confounding factors, such as methodological biases and unmeasured covariates. It might be true that physician who attended in this study chose triple therapy for higher risk patients. Considering these limitations, we performed a propensity matching analysis; therefore, the number of patient groups was relatively small. So the confidence intervals of the primary endpoint are therefore quite wide. Recruitment into this registry was spread out over a 6-year period. One could imagine that the Endeavor stent was used more frequently during the early years, and the Xience and Promus more frequently later on. Is it also possible that the annual percentage of patients treated with triple antiplatelet therapy changed over time? Clinical symptoms and objective signs were observed, collected, and analyzed. However, routine angiography follow up was only performed for a limited number of subjects during the follow-up period, making it difficult to diagnose TLR and TVR. A longitudinal randomized study with a larger number of subjects will be necessary to further investigate the use of triple versus dual antiplatelet therapy in patients treated with a second-generation DES.
CONCLUSION
In patients treated with a second-generation DES, there is no added clinical benefit to using triple rather than dual antiplatelet therapy.
Key Message
After evolving the coronary drug coated stent, the duration and number of antiplatelet therapy are going to be more simple without any additional risk even in high risk patients.
Footnotes
Abbreviations: CABG = coronary artery bypass graft, DESd = rug-eluting stents, IPTW = inverted probability of treatments weighing, MACE = major adverse cardiac events, PCI = percutaneous coronary intervention, TLR = target lesion revascularization, TVR = target vessel revascularization.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Bonow RO, Mann DL, Zipes DP, et al. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 2012; Philadelphia, PA: Elsevier Suanders, 1349–1350. [Google Scholar]
- 2.Kambayashi J, Liu Y, Sun B, et al. Cilostazol as a unique antithrombotic agent. Curr Pharm DES 2003; 9:2289–2302. [DOI] [PubMed] [Google Scholar]
- 3.Lee SW, Park SW, Kim YH, et al. Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus the DECLARE-DIABETES Trial (A Randomized Comparison of Triple Antiplatelet Therapy with Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation in Diabetic Patients). J Am Coll Cardiol 2008; 51:1181–1187. [DOI] [PubMed] [Google Scholar]
- 4.Lee SW, Park SW, Kim YH, et al. Comparison of triple versus dual antiplatelet therapy after drug-eluting stent implantation (from the DECLARE-Long trial). Am J Cardiol 2007; 100:1103–1108. [DOI] [PubMed] [Google Scholar]
- 5.Han Y, Li Yi, Wang S, et al. Cilostazol in addition to aspirin and clopidogrel improves long-term outcomes after percutaneous coronary intervention in patients with acute coronary syndromes: a randomized, controlled study. Am Heart J 2009; 157:733–739. [DOI] [PubMed] [Google Scholar]
- 6.Park KW, Kang SH, Park JJ, et al. Adjunctive cilostazol versus double-dose clopidogrel after drug-eluting stent implantation: the HOST-ASSURE randomized trial (Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis-Safety & Effectiveness of Drug-Eluting Stents & Anti-platelet Regimen). JACC Cardiovasc Interv 2013; 6:932–942. [DOI] [PubMed] [Google Scholar]
- 7.Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol 2006; 48:2584–2591. [DOI] [PubMed] [Google Scholar]
- 8.Lim KS, Jeong MH, Bae IH, et al. Histopathological comparison among biolimus, zotarolimus and everolimus-eluting stents in porcine coronary restenosis model. Korean Circ J 2013; 43:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn SG, Yoon J, Kim J, et al. Genotype- and phenotype-directed personalization of antiplatelet treatment in patients with non-ST elevation acute coronary syndromes undergoing coronary stenting. Korean Circ J 2013; 43:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with drug-eluting and bare metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2013; 62:496–504. [DOI] [PubMed] [Google Scholar]
- 11.Baber U, Mehran R, Sharma SK, et al. Impact of the everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J Am Coll Cardiol 2011; 58:1569–1577. [DOI] [PubMed] [Google Scholar]


