Abstract
Single progesterone receptor positive (PgR+), especially in form of ER−/PgR+/HER2−, is a nonnegligible phenomenon. Little is known about the characteristics and the role of single PgR positive in this phenotype. Therefore, we explore the significance of single PgR positivity by comparing ER−/PgR+/HER2− breast cancers with triple negative breast cancers (TNBCs).
Three thousand nine hundred sixty-six cases of primary invasive breast carcinoma operated consecutively from January 2005 to May 2008 in Cancer Hospital, Chinese Academy of Medical Sciences were examined. Two hundred forty (6%) cases were identified as ER−/PgR+/HER2− breast cancers and 348 (8.8%) cases as TNBCs. Clinicopathological characteristics and survivals were analyzed respectively and then compared between 2 subtypes.
Compared with patients with TNBCs, ER−/PgR+/HER2− tumor tended to have lower tumor grade (Grade 3: 45.7% vs. 37.5%, P = 0.051) and smaller tumor size (P = 0.036). However, no differences were found between ER−/PgR+/HER2− and TNBC patients in relapse-free survival (RFS) and OS. The 5-year RFS rates were 80.7% and 77.4%, respectively (P = 0.330) and the 5-year OS rates were 88.0% and 85.2%, respectively (P = 0.290). ER−/PgR+/HER2− patients receiving adjuvant endocrine treatment had better RFS (P = 0.016) and overall survival (OS) (P < 0.0001) than patients receiving no endocrine therapy.
This exclusive analysis of patients with ER−/PgR+/HER2− breast cancers showed that this subtype exhibited an aggressive behavior as TNBC, suggesting that it should also be regarded as biologically distinctive group and single PgR positive itself is not a good prognostic factor. However, adjuvant endocrine therapy could still benefit this group of patients. Further investigations should be done to elucidate the underlying mechanism.
INTRODUCTION
Breast cancer shows distinctly diverse clinicopathological characteristics, different therapeutic responsiveness, and variable outcomes in different subtypes based on gene expression signature.1–3 However, gene profiling is still limited in current practice. Therefore, immunohistochemical surrogate markers have been developed including estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2). Among them, ER and HER2 have secured their positions as prognostic factors, however, there is still lack of agreement on the role of PgR in breast cancer.4,5
PgR belongs to a large superfamily of ligand-activated nuclear receptors. The binding of progesterone to PgR induces conformational changes that lead to the formation of homo- or heterodimers, increased receptor phosphorylation, interaction with target gene promoters by binding to progesterone response elements, and to specific coactivators and general transcription factors.6,7
Further researches also showed that PgR is a protein in which synthesis is positively regulated by ER. The presence of a functional ER is required for PgR synthesis in the cell. Therefore, the presence of PgR may indicate a more functionally intact ER pathway.8,9 A recent published paper10 demonstrated that in IHC-defined luminal A tumors, more than 20% of PR-positive tumor cells predicted significantly better survival. This was then adopted by St Gallen guideline to use PgR cut off of 20% to distinguish Luminal A-like with Luminal B-like breast cancer. However, the implication of PgR in ER negative breast cancer is still unknown. Previously, single PgR positivity was believed to be a rare phenomenon and may be false positive in IHC examination. There once was a debate on whether it is time to stop PgR testing in breast cancer management.11–13 However, more and more researches supported that it was not insignificant, accounting for 3.4% to 7% of total cases.14–17 In addition, some even found that PgR status was a strong prognostic factor for survivals.14–17 Although previous literatures suggested that ER−/PgR+ tumors might have biologic characteristics somewhere in between ER+/PgR+ and ER+/PgR−,14–17 HER2 was not taken into consideration at that time. Thus, up until now, very little was known about the clinical–pathological characteristics and outcomes of ER−/PgR+/HER2− phenotype.
Triple negative breast cancer (TNBC) is a distinct subtype of breast cancer, which is characterized as ER negative, PR negative, and HER2 negative, featuring rapid progression and poor prognosis.18,19 To illustrate the true significance of single PgR positivity, we compared a group of ER−/PgR+/HER2− patients with TNBC patients. To our knowledge, this is the first study to investigate the differences and similarities between ER−/PgR+/HER2− and TNBC patients. Also this is the largest series of ER−/PgR+/HER2 tumors ever explored to evaluate the efficacy of adjuvant endocrine therapy exclusively in this group of patients.
METHODS
Patient Selection and Data Collection
A consecutive cohort of 3966 breast cancer patients operated from January 1, 2005 to May 31, 2008 at Cancer Hospital and Institute, Chinese Academy of Medical Sciences which is the National Cancer Center of China were retrospectively collected.
All the immunohistochemistry slides for ER/PgR/HER2 were reviewed again by 2 independent pathologists. Immunohistochemistry staining of 4 μm sections of formalin-fixed paraffin-embedded tissue was performed with anti-ER (clone SP1, Ventana), anti-PR (clone 1E2, Ventana), anti-HER2 (clone 4B5, Ventana) primary monoclonal antibodies. Universal secondary antibody (Dako) was applied for 15 minutes. Diaminobenzidine was used as chromogens and slides were counterstained with hematoxylin before mounting. Tumors with ≥1 % nuclear-stained cells were considered ER and/or PgR positive according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines.20 The magnitude of PgR positivity were classified into +/++/+++ by percentage of PgR staining. + was defined as PgR positive if finding of 1% to 25% of tumor cell nuclei were immunoreactive. ++ was defined as PgR positive if 25% to 50% of tumor cell nuclei were immunoreactive. +++ was defined as PgR positive if more than 50% of tumor cell nuclei were immunoreactive. HER2 staining was evaluated from 0 to 3+ according to the ASCO/CAP guidelines and 3+ was considered positive while 0 and 1+ was considered negative.21 Samples with HER2 2+ were either confirmed by FISH or excluded. Totally, 273 patients were identified as ER−/PgR+/HER2− cases and 398 patients were diagnosed with TNBC cases. Eighty-three patients were excluded from the final analysis (see Fig. 1).
FIGURE 1.
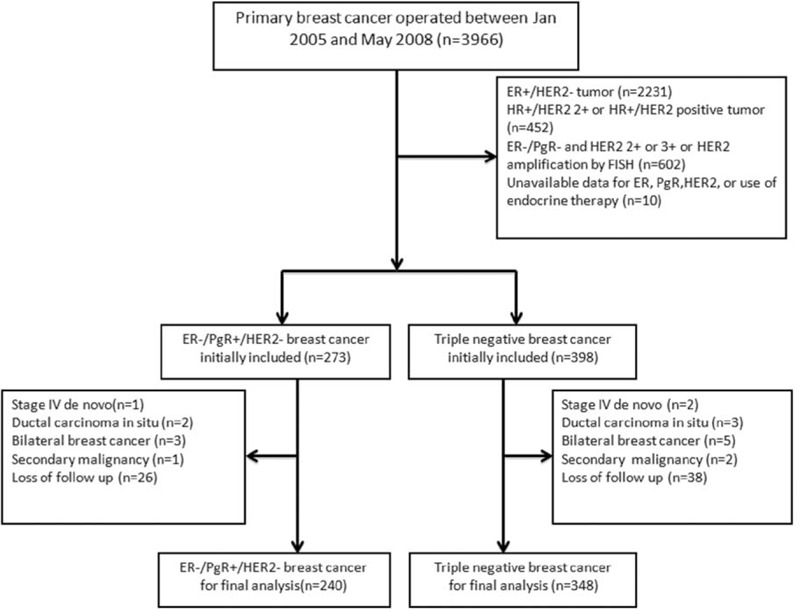
Study schema.
Staging of primary tumors was based on the TNM system of the Seventh American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) manual.22
Ethics Statement
The retrospective study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences (No. 12-123/657). Detailed demographic, clinical, pathologic, and treatment information and follow-ups were obtained from clinic records or pathologic reports. Patient records/information was anonymized and deidentified before analysis. Therefore, the informed consent was remitted by the Ethics Committee.
Endpoints and Statistical Analysis
Relapse-free survival (RFS) was measured from the date of diagnosis to the date of first documented local or distant recurrence. Patients who were still relapse free or died before recurrence were censored at their dates of last follow-up or dates of death. Overall survival (OS) was defined as the time from the date of diagnosis to the date of death or last follow-up.
Clinicopathological characteristics were compared between 2 phenotypes by the Chi-square test or Fisher exact test. RFS and OS were computed and compared by Kaplan–Meier method using log-rank test. Cox proportional hazard regression model was used to identify variables that were independently associated with survival. P values less than 0.05 were considered statistically significant; all tests were 2 sided. All the statistical analyses were carried out using SPSS17.0 (SPSS, Inc., Chicago, IL).
RESULTS
Characteristics of Patient cohort
Characteristics of 240 (6%) ER−/PgR+/HER2− cases and 348 (8.7%) TNBC cases are listed in Table 1. For PgR positivity, 164 (68.3%) tumors were PgR+, 55 (22.9%) were PgR++, and 21 (8.8%) were PgR+++. Compared with patients with ER−/PgR+/HER2− tumor, TNBC patients tended to have higher tumor grade (Grade 3: 45.7% vs. 37.5%, P = 0.051) and larger tumor size (P = 0.036). Other baseline characteristics were comparable between the 2 groups (Table 1).
TABLE 1.
Patient Characteristics
In terms of systemic treatment, undoubtedly, more ER−/PgR+/HER2− patients (76.3%) received adjuvant endocrine therapy than TNBC patients (4.6%). On the contrary, more TNBC patients received taxane-based chemotherapy than ER−/PgR+/HER2 patients (54.0% vs. 45.4%, P = 0.031). Forty-eight cases in ER−/PgR+/HER2 group and 80 cases in TNBC group presented with BC-specific recurrence including regional relapse and distant metastasis. The metastatic pattern was similar between the 2 groups (P > 0.05), that is, recurrent cases in both groups tended to have visceral metastasis with lung as the most common metastatic site, and less likely to develop bone metastasis (Table 1).
Survivals
Median follow-up of the entire cohort was 66 months (range, 22 months to 96 months). The 5-year RFS rate and OS rate for the entire cohort were 79.1% and 86.4%, respectively. In the recurrent cases, the median RFS time of ER−/PgR+/HER2− and TNBC was 20.0 and 18.3 months, respectively, and no significant difference was demonstrated (P = 0.984).
There were also no significant differences in RFS and OS between ER−/PgR+/HER2− patients and TNBC patients. The 5-year RFS rates were 80.7% and 77.4%, respectively (P = 0.330) and the 5-year OS rates were 88.0% and 85.2%, respectively (P = 0.290) (Fig. 2A and B).
FIGURE 2.
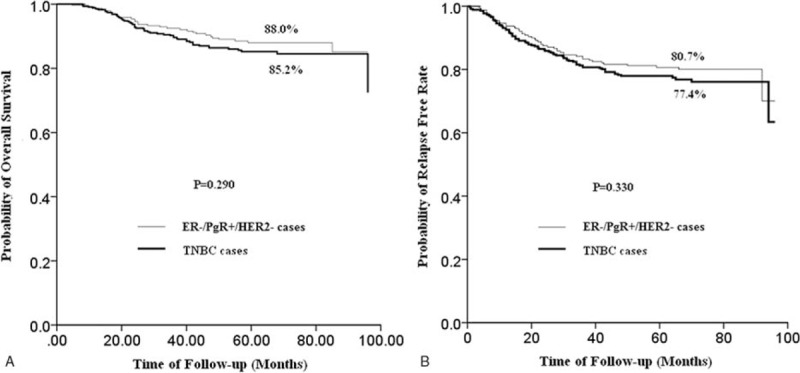
(A) RFS curves of ER−/PgR+/HER2− patients (n = 240) and TNBC (n = 348) patients. (There was no significant difference in RFS between ER−/PgR+/HER2− patients and TNBC patients. The 5-year RFS rates were 80.7% and 77.4%, respectively (P = 0.330).) (B) OS curves of ER−/PgR+/HER2− patients (n = 240) and TNBC (n = 348) patients. (There was no significant difference in RFS between ER-/PgR+/HER2− patients and TNBC patients. The 5-year OS rates were 88.0% and 85.2% respectively (P = 0.290).)
In ER−/PgR+/HER2− group, cases with adjuvant endocrine therapy had significantly better RFS (5-year RFS rate, 84.0% vs. 70.1%, P = 0.016) and also significantly longer OS (5-year OS rate, 93.0% vs. 71.9%, P < 0.0001) than cases receiving no adjuvant endocrine therapy (Fig. 3A). The magnitude of PgR positivity, whether it is +, ++, or +++ was associated neither with PFS (P = 0.656) or OS (P = 0.608). When compared with TNBC, ER−/PgR+/HER2− patients who were not given endocrine drugs had a worse prognosis (5-year OS rate, 71.9% vs. 85.2%, P = 0.005) while those treated with endocrine therapy had a better prognosis (5-year OS rate, 93.0% vs. 85.2%, P = 0.006) (Fig. 3B).
FIGURE 3.
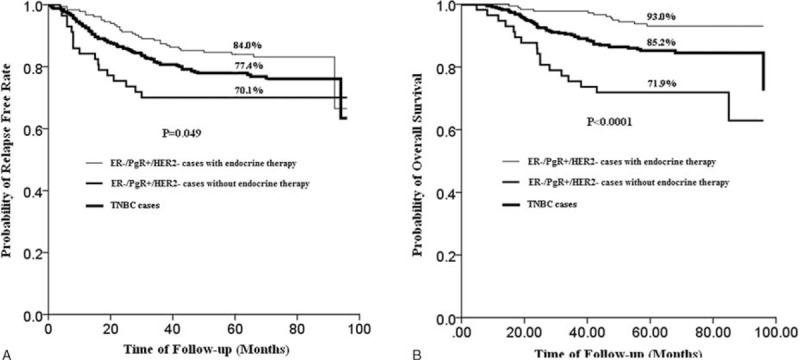
(A) RFS curves of TNBC patients and ER−/PgR+/HER2− patients (n = 240) with or without endocrine therapy (ER−/PgR+/HER2− cases with endocrine therapy vs. TNBC cases (n = 348): HR 0.686, 95% CI: 0.453–1.038, P = 0.075; ER−/PgR+/HER2− cases without endocrine therapy versus TNBC cases: HR 1.392, 95% CI: 0.824–2.353, P = 0.217; ER−/PgR+/HER2− cases with endocrine therapy versus ER-/PgR+/HER2− cases without endocrine therapy: HR 0.491, 95% CI: 0.271–0.888, P = 0.019). (B) OS curves of TNBC patients (n = 348) and ER−/PgR+/HER2− patients (n = 240) with or without endocrine therapy (ER−/PgR+/HER2− cases with endocrine therapy versus TNBC cases: HR 0.410, 95% CI: 0.219–0.768, P = 0.005; ER−/PgR+/HER2− cases without endocrine therapy versus TNBC cases: HR 2.166, 95% CI: 1.252–3.746, P = 0.006; ER−/PgR+/HER2− cases with endocrine therapy versus ER−/PgR+/HER2− cases without endocrine therapy: HR 0.190, 95% CI: 0.091–0.397, P < 0.0001).
Univariate and Multivariate Analysis of ER−/PgR+/HER2− Disease and TNBC
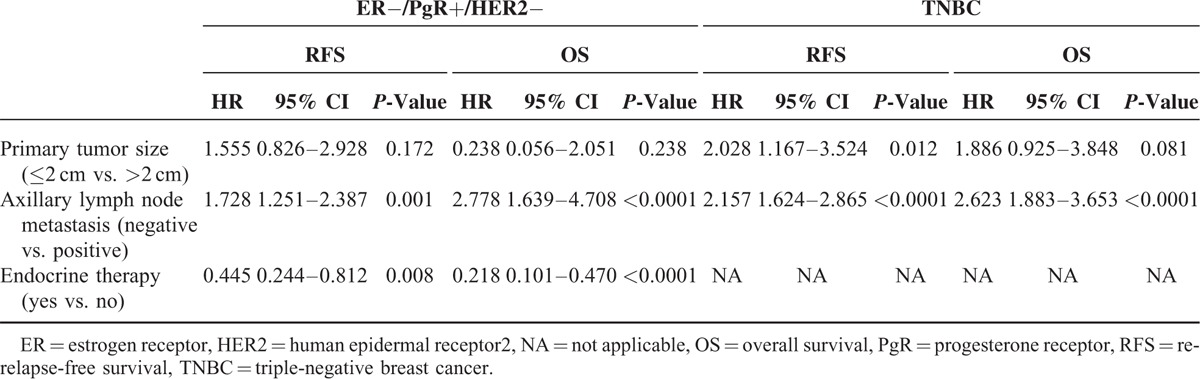
Prognostic factors which were significantly correlated with PFS and OS in univariate analysis are highlighted in Table 2. Multivariate analysis suggested that axillary lymph node metastasis status was an independent adverse prognostic factor for both RFS and OS in ER−/PgR+/HER2− disease (HR 1.728, 95% CI: 1.251–2.387, P = 0.001; HR 2.778, 95% CI: 1.639–4.078, P < 0.0001). Adjuvant endocrine therapy is also an independent prognostic factor, significantly decrease the risk of recurrence (HR 0.4454, P = 0.008) and death (HR 0.218, P < 0.0001). In TNBC, Unlike ER−/PgR+/HER2− disease, tumor size >2 cm was identified as a poor prognostic factor for RFS (HR2.028, 95% CI: 1.167–3.524, P = 0.012). Similar to ER−/PgR+/HER2− disease, multivariate Cox regression models showed that positive lymph node was an independent adverse prognostic factor for both RFS (HR 2.157, 95% CI: 1.624–2.865, P < 0.0001) and OS (HR 2.623, 95% CI: 1.883–3.653, P < 0.0001) in TNBC tumors (Table 3).
TABLE 2.
Univariate Analyses of RFS and OS in ER−/PgR+/HER2− Patients and TNBC Patients
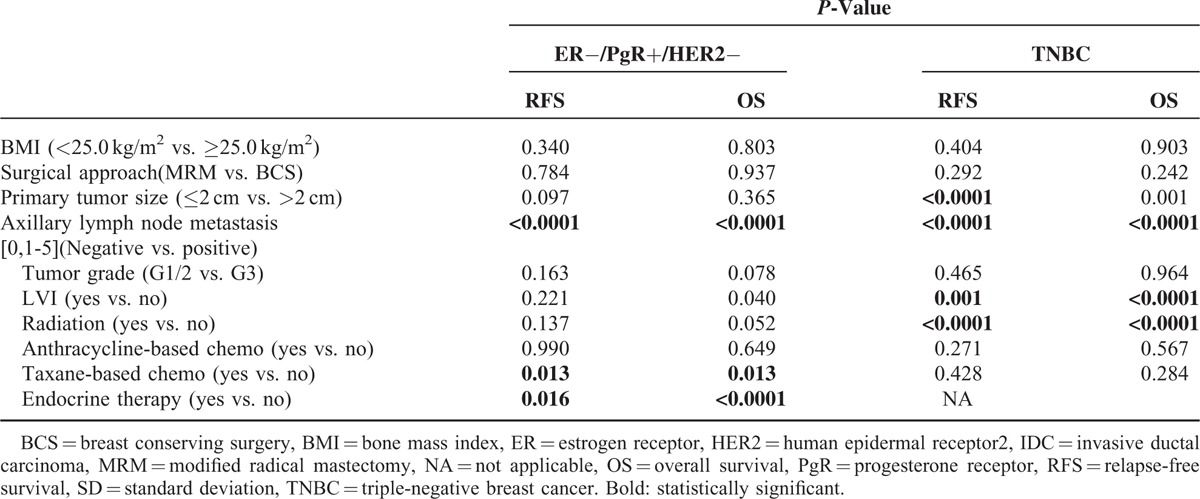
TABLE 3.
Multivariate Analyses of RFS and OS in ER−/PgR+/HER2− Patients and TNBC Patients
DISCUSSION
Determination of ER and PgR expression by IHC analysis is fundamental for daily clinical practice and is routinely used to predict the prognosis and to identify patients who are most likely to benefit from endocrine therapy. Although the clinical significance of ER positivity has been firmly established, the implication of PgR positivity, especially single PgR positivity has long been questioned. Olivotto et al11 reported that among 192 patients with ER− disease on IHC testing, 191 (99.5%) were also PgR−, they recommended it was time to stop PgR testing in breast cancer management. However, this was opposed by Rakha et al14 and Colomer et al12 that in their studies, ER−/PgR+ patients is not insignificant (3.4% and 7%). Similar results was found in our study that 6% of patients presented with ER−/PgR+/HER2− phenotype, therefore, single PgR entity is definitely not a negligible phenomenon and needs to be further investigated.
Although the presence of single PgR have been more and more widely accepted, studies evaluating its role as an independent prognostic factor yielded conflicting results.7–9 Banerjee et al23 applied a tree-based model for breast cancer prognostication and found PgR positive is a good prognostic factor. More recently, Rakha et al14 also examined a large series of 1944 breast cancer cases, focusing on single hormone-receptor positive phenotype. They found that when compared with the double-negative phenotype, ER+/PgR-showed better outcome but no such survival advantage was detected in ER−/PgR+ tumors. The latter exhibited more aggressive behavioral characteristics. This result was echoed in another study reported by Keshgegian and Cnaan,24 but slightly different from Bernoux et al's25 study which showed ER−PgR+ patients had a small but significant better OS compared with ER−PgR− patients. However, significant difference was also not found in disease-free interval and the metastasis-free survival. These studies all presented with 2 issues: first, the prognostic value of PgR was evaluated by analyzing mixed population including both ER− and ER+ patients. When ER is negative, whether PgR is prognostic is an interesting issue that has not been addressed yet; more importantly, the status of HER2 was not taken into consideration, however, we know now ER pathway has crosstalk with HER2 pathway. There might also be inconsistency of antibodies for IHC testing, but it is doubtful whether this will bring dramatic changes in results. Thus, our study might be more informative by limiting population into ER−/PgR+/HER2− patients so we cannot only provide some insights of clinicopathological characteristics of truly single PgR positive, but also further explore the potential role of PgR by comparing ER−/PgR+/HER2− patients with TNBC patients.
As the matter of fact, results of our study revealed that Chinese breast cancer patients with ER−/PgR+/HER2− tumors had similar clinicopathological features with TNBC patients except that ER−/PgR+/HER2− tumors tend to present with lower tumor grade (P = 0.051) and smaller tumor size (P = 0.036). These data suggested that ER−/PgR+/HER2− might have a less aggressive biologic feature than TNBC. However, no significant differences were found in RFS or OS between ER−/PgR+/HER2− and TNBC cases. This is just as expected since lymph node status, as the strongest prognostic indictor, showed no difference. They both tended to relapse within 2 years of diagnosis and had lungs as the most common site of visceral metastases. All these implied that ER−/PgR+/HER2− tumor should also be regarded as biologically and clinically distinctive subgroup of breast cancer and PgR still cannot be established as a prognostic marker.
Another issue that needs to be born in mind is that PgR exists in 2 isoforms, PR-A and PR-B.26 It was reported that high PR-A: PR-B ratio predicted shorter disease-free survival, indicating resistance to tamoxifen either intrinsically or a more rapid onset of acquired resistance. It is suggested that overexpression of PR-A could act as dominant repressor of PR-B and ER.27 Whether the expression of PgR in the ER−/RgR+/HER2− patients derives from PR-A or PR-B and their ratio remains unknown since the current method only detect total PR levels, but it is possible that the expression of PgR in this group is mainly contributed by PR-A, leading to the poor prognosis even PgR is positive. However, since it is still contradictory about the role of PR-A and PR-B and currently PgR expression was still a combination of PR-A and PR-B, it can only be regarded as a hypothesis.
Although PgR was not prognostic in our study, it does not mean it is useless and it is time to stop the PgR testing. In further analysis, patients with ER−/PgR+/HER2− tumors receiving endocrine agents did have a significantly more favorable prognosis in terms of both RFS and OS than those receiving no endocrine therapy. This was repeated in other studies28 including Colomer et al's12 study that when compared with ER−/PgR− subgroup, ER−/PgR+ subset had benefit from tamoxifen, In that case, even if the expression of PgR is not strong enough to improve the aggressiveness of breast cancer cells, endocrine agents can still work by interacting with somewhere in the ER pathway in the presence of PgR.
New technologies might help us to further understand the underlying biological traits of this special group. In a recent online published paper,29 distribution of molecular subtypes among the ER−/PgR+ patients by the PAM50 classifier was performed, 15% were luminal A, 5% were Luminal B, and 65% were basal like. Since TNBC had around 75% of patients were basal like subtype in gene profiling, it is reasonable that ER−/PgR+/HER2− tumors share similar characteristics and survivals with TNBC tumors.
In summary, this is the first retrospective analysis with a large sample size addressing the ER−/PgR+/HER2− tumors and discussing the role of PgR by comparing ER−/PgR+/HER2− patients with TNBC patients. The comparable clinicopathological features and survivals between these 2 groups suggest that single PgR positive is not a good prognostic factor and ER−/PgR+/HER2− tumors should also be regarded as biologically and clinically distinct group of breast cancer. However, single PgR positivity is still a predictive factor for endocrine treatment and hormonal therapy should be routinely used in this subset. Further studies are warranted to find possible methods to improve the poor outcome of this special subtype.
Acknowledgments
The authors would like to thank Dr. Shan Zheng and Dr. Liyan Xue for their excellent work in reviewing pathological slides. The authors would also like to thank the patients and their families for their cooperation in the follow-up.
Footnotes
Abbreviations: AJCC/UICC = American Joint Committee on Cancer/International Union Against Cancer, ASCO/CAP = American Society of Clinical Oncology/College of American Pathologists, BCS = breast conserving surgery, BMI = bone mass index, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, IDC = invasive ductal carcinoma, MRM = modified radical mastectomy, NA = not applicable, OS = overall survival, PgR = progesterone receptor, RFS = relapse-free survival, SD = standard deviation, TNBCs = triple negative breast cancers.
This paper was presented in part as poster at the 36th Annual San Antonio Breast Cancer Symposium, December 10 to 14, 2013, San Antonio, Texas, USA and Ying Fan received an Avon Foundation-AACR International Scholar-in-Training Grant.
The study was supported in part by a grant from the National Natural Science Foundation of China for Young Scholars (no. 81202108).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000; 406:747–752. [DOI] [PubMed] [Google Scholar]
- 2.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006; 295:2492–2502. [DOI] [PubMed] [Google Scholar]
- 3.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 2003; 100:8418–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687–1717. [DOI] [PubMed] [Google Scholar]
- 5.Cui X, Schiff R, Arpino G, et al. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 2005; 23:7721–7735. [DOI] [PubMed] [Google Scholar]
- 6.Graham JD, Bain DL, Richer JK, et al. Thoughts on tamoxifen resistant breast cancer. Are coregulators the answer or just a red herring? J Steroid Biochem Mol Biol 2000; 74:255–259. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins RA, White G, Bundred NJ, et al. Prognostic significance of oestrogen and progestogen receptor activities in breast cancer. Br J Surg 1987; 74:1009–1013. [DOI] [PubMed] [Google Scholar]
- 8.Robertson JF, Cannon PM, Nicholson RI, et al. Oestrogen and progesterone receptors as prognostic variables in hormonally treated breast cancer. Int J Biol Markers 1996; 11:29–35. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Redmond C, Fisher ER, et al. Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node negative breast cancer patients: findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06. J Clin Oncol 1988; 6:1076–1087. [DOI] [PubMed] [Google Scholar]
- 10.Prat A, Cheang MC, Martin M, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol 2013; 31:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivotto IA, Truong PT, Speers CH, et al. Time to stop progesterone receptor testing in breast cancer management. J Clin Oncol 2004; 22:1769–1770. [DOI] [PubMed] [Google Scholar]
- 12.Colomer R, Beltran M, Dorcas J, et al. It is not time to stop progesterone receptor testing in breast cancer. J Clin Oncol 2005; 23:3868–3869.author reply 3869–3870. [DOI] [PubMed] [Google Scholar]
- 13.MacGrogan G, de Mascarel I, Sierankowski G, et al. Time for reappraisal of progesterone-receptor testing in breast cancer management. J Clin Oncol 2005; 23:2870–2871.author reply 2871. [DOI] [PubMed] [Google Scholar]
- 14.Rakha EA, El-Sayed ME, Green AR, et al. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol 2007; 25:4772–4778. [DOI] [PubMed] [Google Scholar]
- 15.Yang LH, Tseng HS, Lin C, et al. Survival benefit of tamoxifen in estrogen receptor-negative and progesterone receptor-positive low grade breast cancer patients. J Breast Cancer 2012; 15:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Chia SK, Mehl E, et al. Progesterone receptor is a significant factor associated with clinical outcomes and effect of adjuvant tamoxifen therapy in breast cancer patients. Breast Cancer Res Treat 2010; 119:53–61. [DOI] [PubMed] [Google Scholar]
- 17.Park S, Park BW, Kim TH, et al. Lack of either estrogen or progesterone receptor expression is associated with poor survival outcome among luminal A breast cancer subtype. Ann Surg Oncol 2013; 20:1505–1513. [DOI] [PubMed] [Google Scholar]
- 18.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol 2007; 8:235–244. [DOI] [PubMed] [Google Scholar]
- 19.Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist 2010; 15 Suppl. 5:39–48. [DOI] [PubMed] [Google Scholar]
- 20.Hammond ME, Hayes DF, Wolff AC, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 2010; 6:195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013; 31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee M, George J, Song EY, et al. Tree-based model for breast cancer prognostication. J Clin Oncol 2004; 22:2567–2575. [DOI] [PubMed] [Google Scholar]
- 24.Keshgegian AA, Cnaan A. Estrogen receptor-negative, progesterone receptor-positive breast carcinoma: poor clinical outcome. Arch Pathol Lab Med 1996; 120:970–973. [PubMed] [Google Scholar]
- 25.Bernoux A, de Cremoux P, Lainé-BidronC, et al. Estrogen receptor negative and progesterone receptor positive primary breast cancer: pathological characteristics and clinical outcome. Breast Cancer Res Treat 1998; 49:219–225. [DOI] [PubMed] [Google Scholar]
- 26.Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 1990; 9:1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopp TA, Weiss HL, Hilsenbeck SG, et al. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res 2004; 10:2751–2760. [DOI] [PubMed] [Google Scholar]
- 28.Dowsett M, Houghton J, Iden C, et al. Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol 2006; 17:818–826. [DOI] [PubMed] [Google Scholar]
- 29.Itoh M, Iwamoto T, Matsuoka J, et al. Estrogen receptor (ER) mRNA expression and molecular subtype distribution in ER-negative/progesterone receptor-positive breast cancers. Breast Cancer Res Treat 2014; 143:403–409. [DOI] [PubMed] [Google Scholar]


