Abstract
The effects of the inflammatory mediators involved in systemic lupus erythematous (SLE) on subsequent Parkinson disease have been reported, but no relevant studies have focused on the association between the 2 diseases. This nationwide population-based study evaluated the risk of Parkinson disease in patients with SLE.
We identified 12,817 patients in the Taiwan National Health Insurance database diagnosed with SLE between 2000 and 2010 and compared the incidence rate of Parkinson disease among these patients with that among 51,268 randomly selected age and sex-matched non-SLE patients. A Cox multivariable proportional-hazards model was used to evaluate the risk factors of Parkinson disease in the SLE cohort.
We observed an inverse association between a diagnosis of SLE and the risk of subsequent Parkinson disease, with the crude hazard ratio (HR) being 0.60 (95% confidence interval 0.45–0.79) and adjusted HR being 0.68 (95% confidence interval 0.51–0.90). The cumulative incidence of Parkinson disease was 0.83% lower in the SLE cohort than in the non-SLE cohort. The adjusted HR of Parkinson disease decreased as the follow-up duration increased and was decreased among older lupus patients with comorbidity.
We determined that patients with SLE had a decreased risk of subsequent Parkinson disease. Further research is required to elucidate the underlying mechanism.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disease affecting any organ system of the body, and occurs predominantly in women of gestation age.1 Neuropsychiatric symptoms affect nearly half of the patients with SLE, and the 3 most common features are seizures, psychoses, and cerebrovascular diseases.2,3 Parkinson disease (PD)—an unusual manifestation of central nervous system lupus4—is the most common neurodegenerative disorder, and clinical signs of this disease are rigidity, bradykinesia, postural instability, and tremor.5 This disease is characterized by self-perpetuating damage of dopaminergic neurons in the substantia nigra.6,7 In addition, some immune system-mediated mechanisms have been proposed to clarify the possible mechanisms through which autoantibodies induce dopaminergic cell death.8,9
Patients with autoimmune diseases frequently produce high concentrations of inflammatory mediators and autoantibodies over long periods. Peripheral immunity contributes to the degenerative process of PD and may be responsible for the progressive nature of the disease.10 A study hypothesized that patients with antidopaminergic antibodies experience the destruction of domapinergic cells because of a humoral immune response.11 Associations between the presence of such autoantibodies (antineuronal cells, antibrain lysate, and anti-dsDNA) and clinical manifestations of PD (particularly dyskinesia and depression) confirmed that the autoantibodies play a role in the autoimmune mechanisms involved in the pathogenesis of the disease.12
We conducted this study to determine the risk of PD in patients with SLE and to identify related risk factors. We examined the relationship between SLE and the risk of PD, and unexpectedly observed a lower risk of PD in patients with SLE.
METHODS
Data Source
Taiwan inaugurated its National Health Insurance (NHI) program on March 1, 1995 to provide comprehensive and easily accessible medical care for all of its citizens (23.74 million people) (http://www.nhi.gov.tw/english/index.aspx). The National Health Insurance Research Database (NHIRD) is an administrative database containing the claims records from Taiwan's universal NHI program. The NHIRD includes complete information on inpatient care, ambulatory care, dental care, and prescription drugs, and provides researchers with scrambled identification numbers associated with the relevant claims information, including patients’ sex, date of birth, medical service records, and medication prescriptions. The diagnoses and procedures recorded in the NHIRD are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The records of patients were de-identified before conducting the analysis. This cohort study was conducted in compliance with the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of China Medical University (CMU-REC-101-012).
Sampled Patients
Patients newly diagnosed with SLE (ICD-9-CM code 710.0) from 2000 to 2010 were identified in the Registry of Catastrophic Illnesses Patient Database, a subset of the NHIRD. In the NHI program, patients with SLE receive a catastrophic illness certificate and are exempt from copayment for their SLE-related medical care. The diagnoses of SLE were based on the ICD-9 codes, which were judged and determined by related specialists and physicians according to the standard clinical/laboratory criteria (malar rash, discoid rash, photosensivity, oral ulcer, serositis, arthritis, nephritis, immunological disorder, hematological disorder, neurological disorder, antinuclear antibody, which met at least four American College of Rheumatology SLE criteria). The first-time SLE diagnosis date served as the index date. Patients who had a history of PD (ICD-9-CM 332), were younger than 20 years, and had incomplete information were excluded. To create a non-SLE cohort, we randomly selected patients without a history of SLE and frequency-matched them with the SLE patients in a 4:1 ratio according to age group, with an interval of 5 years, sex, and the year of the index date. The exclusion criteria applied to the SLE cohort were also applied to the non-SLE cohort.
Outcome and Comorbidities
All patients were followed up from the index date until the date of PD diagnosis, withdrawal from the NHI program, or December 31, 2011. The baseline comorbidity history for each patient was determined according to the claims data. We analyzed several well known risk factors of PD, namely diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), head injury (ICD-9-CM codes 310.2, 800, 801, 803, 804, 850, 851, 853, and 854), depression (ICD-9-CM codes 296.2, 296.3, 296.82, 300.4, and 311), stroke (ICD-9-CM codes 430–438), dementia (ICD-9-CM codes 290, 294.1, and 331.0), and chronic kidney disease (ICD-9-CM code 585).
Statistical Analysis
We used the chi-square test to compare descriptive statistics on demographic status and baseline comorbidities between the patients with and those without SLE. Differences in continuous variables between the cohorts were tested using the Student t test. To estimate the cumulative incidence of PD, we used the Kaplan–Meier method to conduct a survival analysis of the SLE cohort and non-SLE cohort, and the log-rank test to compare the 2 cohorts. The incidence densities of PD were calculated. Univariable and multivariable Cox proportional-hazards regression models were used to calculate the hazard ratios (HRs) with stratification based on sex, age, comorbidity, and follow-up duration. The multivariable Cox models were simultaneously adjusted for age, sex, and the comorbidities, namely diabetes, hypertension, hyperlipidemia, coronary artery disease, head injury, depression, and stroke. All analyses were conducted using SAS Version 9.3 (SAS Institute Inc., Cary, NC), with the significance level set to 0.05 in 2-tailed tests.
RESULTS
We included 12,817 patients in the SLE cohort and 51,268 patients in the non-SLE cohort. The age and sex distributions of the cohorts were similar (Table 1). In both cohorts, 44.2% of the patients were 20–34 years of age, and the mean age was approximately 40 years. Women constituted 88.1% of the patients with SLE. All comorbidities were more prevalent in the SLE cohort than in the non-SLE cohort (all P < 0.05). The mean durations until the development of PD in the SLE and non-SLE cohorts were 6.82 and 7.27 years, respectively. The Kaplan–Meier survival analysis showed that the cumulative incidence of PD was 0.83% lower in the SLE cohort than in the non-SLE cohort (Fig. 1; P ≤ 0.001).
TABLE 1.
Characteristics of Patients With Systemic Lupus Erythematosus and Those Without Systemic Lupus Erythematosus
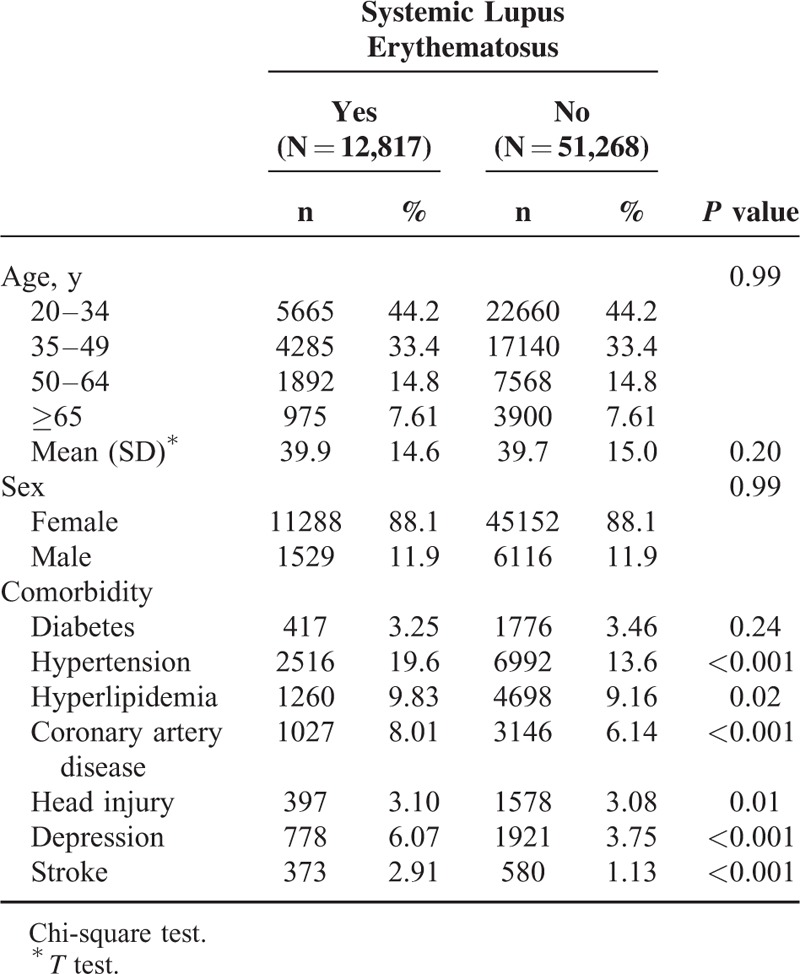
FIGURE 1.
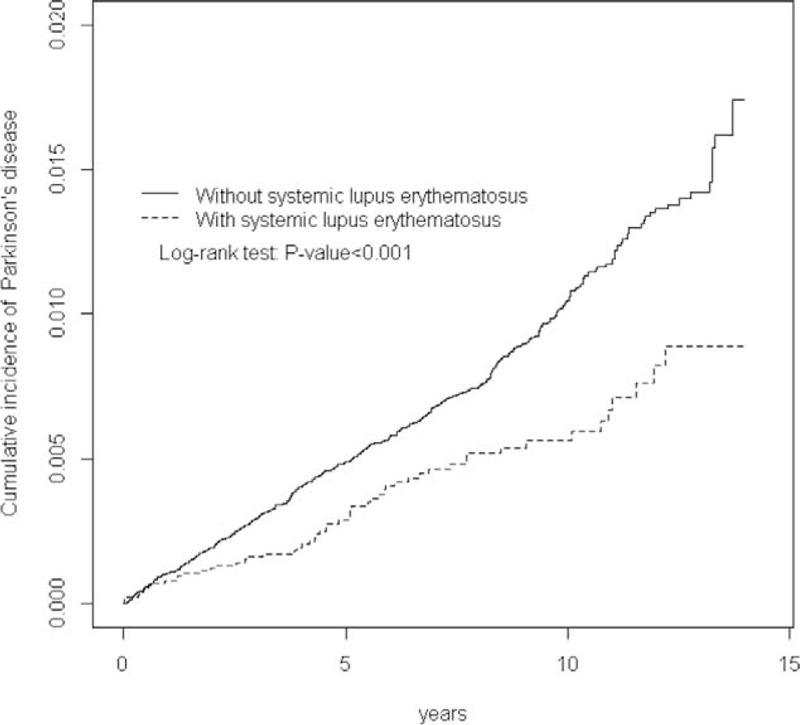
Cumulative incidence of Parkinson disease in the systemic lupus erythematosus and comparison cohorts.
In total, 55 patients in the SLE cohort and 393 patients in the non-SLE cohort developed PD, with the incidence rates being 0.63 per 1000 person-years and 1.06 per 1000 person-years, respectively, yielding a crude HR of 0.60 (95% confidence interval [CI] 0.45–0.79) and an adjusted HR of 0.68 (95% CI 0.51–0.90) (Table 2).
TABLE 2.
Incidence and Hazard Ratio of Parkinson Disease in Patients With Systemic Lupus Erythematosus and Those Without Systemic Lupus Erythematosus
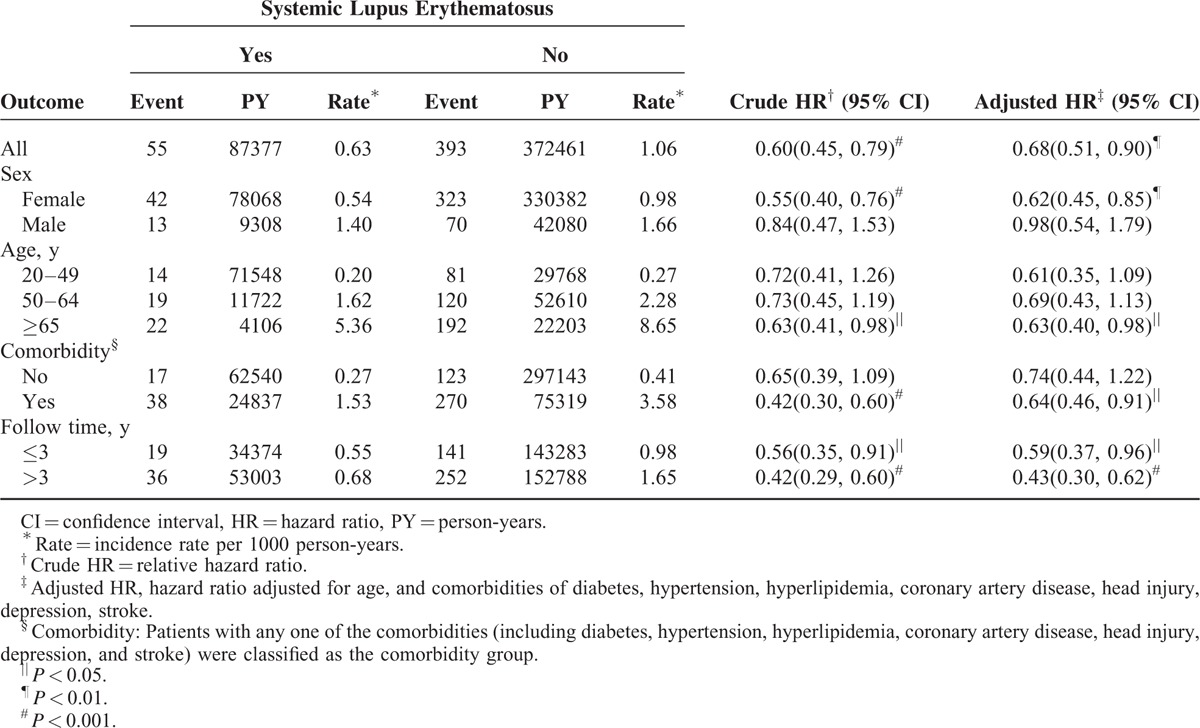
The PD incidence was greater in the men than in the women in both cohorts. However, the sex-specific SLE cohort to non-SLE cohort relative risk of PD was significantly lower for the women (adjusted HR 0.62, 95% CI 0.45–0.85). The PD incidence increased with age and comorbidity in both cohorts. Patients aged older than 65 years with SLE had a 0.63-fold lower risk of PD than the non-SLE cohort did (adjusted HR 0.63, 95% CI 0.40–0.98). Patients with SLE and comorbidity also had a lower risk than did the non-SLE cohort. The adjusted HR of PD slightly decreased as the follow-up duration increased. The SLE cohort was associated with a lower risk of PD compared with the non-SLE cohort throughout the follow-up period.
Table 3 lists the results of the univariable and multivariable Cox proportional-hazards analyses of the association between SLE and PD. The risk of PD was 1.08-fold (95% CI 1.07–1.09) greater for each 1-year increase in patient age. The risk of PD was increased for patients with comorbidities of hypertension (adjusted HR 1.51, 95% CI 1.19–1.91), coronary artery disease (adjusted HR 1.32, 95% CI 1.04–1.66), and depression (adjusted HR 1.75, 95% CI 1.27–2.42).
TABLE 3.
Hazard Ratios and 95% Confidence Intervals for Parkinson Disease in Patients With Systemic Lupus Erythematosus and Covariates

DISCUSSION
According to a review of relevant literature, no other large-scale cohort studies have focused on the possible association between PD and SLE. This large study investigated this possible association through an extensive analysis of a nationwide database and complete follow-up assessment. We determined that PD is inversely associated with SLE, and that the adjusted HR of PD decreased as the follow-up duration increased and is decreased among patients aged older than 65 years. In this study, the PD cohort exhibited an unexpected link to SLE. We observed a crude HR of 0.60 (95% CI 0.45–0.79), adjusted HR of 0.68 (95% CI 0.51–0.90), and low incidence (0.83%) of SLE among the patients with SLE after controlling for other critical covariates.
Results from previous studies have suggested that inflammation plays a crucial role in the pathogenesis of PD,13,14 and an animal study demonstrated that the inflammatory mediators tumor necrosis factor α and interleukin 1β can cause degeneration of dopaminergic neurons.15 It is well known that interferon plays a role in the development of SLE,16 which might contribute to the pathogenesis of PD.17 The hypothesis that patients with SLE are at an increased risk of PD,18–20 because circulating inflammatory mediators produced by some autoimmune diseases may trigger an inflammatory process in the brain that leads to degeneration of the dopaminergic neurons of the substantia nigra,21 contrasts the observation of a reduced risk of PD in patients with SLE. Some longitudinal studies have reported that the use of immunosuppressive agents for lupus activity in patients with PD benefits clinical recovery.18,20,22–24
Some may argue that neuropsychiatric lupus and PD have overlapping symptoms and that the possible association between these diseases can be attributed to diagnostic confusion or misclassification. Three types of autoantibodies, namely antineuronal, antibrain lysate, and anti-dsDNA antibodies, were strongly associated with clinical manifestations of PD, particularly dyskinesia and depression.11 Therefore, we performed a sensitivity analysis. The frequency of lupus visits over the follow-up duration, which represents lupus severity, correlated negatively with the risk of PD. This finding confirms that lupus is associated with a significantly lower risk of PD (32%), which can be explained by the existence of a protective factor. Regular use of immunosuppressive agents could prevent neurodegeneration and, thus, the development of PD. Lupus-related immunosuppressive therapy, including corticosteroids, mycophenolate mofetil, and cyclophosphamide, was effective in preventing lupus flare and may alleviate rigidity-akinesia-tremor syndrome by inhibiting autoimmune-based neuroinflammation. Consistent with our findings, the rheumatoid arthritis patients who received immunosuppressant therapy had 30% reduction in the risk of PD, which further supports the potential beneficial effects of immunosuppressant therapy in the development of PD.25
Unexpectedly, patients aged older than 65 years with SLE had a 0.63-fold lower risk of PD. We observed a seemingly paradoxical harmful effect of immunosuppressive agent use on the risk of PD among older patients. However, because older people are traditionally more likely to use immunosuppressive agents for longer periods and have longer follow-up durations, awareness of PD in patients with SLE may prevent incorrect diagnosis and maladministration resulting in severe disability. The risk of PD was increased for patients with comorbidities of hypertension, coronary artery disease, and depression. Older patients and patients with comorbidities are less tolerant of steroid-related side effects, more severe disease, and higher rates of treatment toxicity and increased susceptibility to opportunistic infections. Thus, the association between immunosuppressive agent use and PD could be noncausal, and further research is required to determine the relative effects of specific agents, despite the nationwide population-based design of this study and unbiased ascertainment and confirmation of SLE among patients and controls through linkage to the hospital register.
This study has potential limitations. First, mild PD was theoretically omitted from our study population. Patients with SLE in stable condition were treated at outpatient departments, and more severely affected patients were treated at hospitals, indicating that the SLE patients with PD are not completely representative of all patients with the disease. Second, the NHIRD does not contain detailed information regarding clinical, laboratory, and image examinations including genetic factors (alpha-synuclein), declining estrogen levels (use of hormone replacement therapy), low levels of B vitamin folate, autoantibodies that can be used as biomarkers (such as antineuronal, antibrain lysate, and anti-dsDNA antibodies), and a family history of PD or records on smoking habits, alcohol consumption, and agricultural work, all of which may be risk factors for PD. We cannot make more profound implications regarding the pathogenesis of PD in patients with SLE. Third, because of possible biases related to adjustments for confounding variables, the data derived from a cohort study are generally lower in statistical quality than those from randomized trials. Fourth, prescription records of anti-inflammatory and immunosuppressive agents including doses, timing, and durations of use are unavailable. The mechanism of autoimmune-based neuroinflammation in PD cannot be conclusively identified, unless additional well designed and well conducted epidemiologic studies confirm the relationship between immunosuppressive agents and PD by investigating the relative effects of specific agents.
In conclusion, we determined that patients with SLE had a decreased risk of subsequent PD. The underlying mechanism is not clearly understood and further research is warranted.
ACKNOWLEDGMENTS
Author contributions: Conceived and designed the experiments: F-CL, W-YH, C-HK; performed the experiments: all authors; analyzed the data: all authors; contributed reagents/materials/analysis tools: C-HK; wrote the manuscript: all authors; approval of the manuscript: all authors.
Footnotes
Abbreviations: CIs = confidence interval, HRs = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, IRR = incidence rate ratio, NHI = Taiwan's National Health Insurance, NHIRD = National Health Insurance Research Database, PD = Parkinson disease, SLE = systemic lupus erythematous.
Funding: This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212–113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103–2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. No additional external funding was received for this study.
Conflicts of interest: All authors report no conflicts of interest. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Pramanik B. Diagnosis of systemic lupus erythematosus in an unusual presentation: what a primary care physician should know. Curr Rheumatol Rev 2015; 10:81–86. [DOI] [PubMed] [Google Scholar]
- 2.Chiewthanakul P, Sawanyawisuth K, Foocharoen C, et al. Clinical features and predictive factors in neuropsychiatric lupus. Asian Pac J Allergy Immunol 2012; 30:55–60. [PubMed] [Google Scholar]
- 3.Kivity S, Agmon-Levin N, Zandman-Goddard G, et al. Neuropsychiatric lupus: a mosaic of clinical presentations. BMC Med 2015; 13.43:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan EK, Chan LL, Auchus AP. Reversible Parkinsonism in systemic lupus erythematosus. J Neurol Sci 2001; 193:53–57. [DOI] [PubMed] [Google Scholar]
- 5.Ali K, Morris HR. Parkinson's disease: chameleons and mimics. Pract Neurol 2015; 15:14–25. [DOI] [PubMed] [Google Scholar]
- 6.Miller DB, O’Callaghan JP. Biomarkers of Parkinson's disease: present and future. Metabolism 2015; 64:S40–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol 2014; 71:499–504. [DOI] [PubMed] [Google Scholar]
- 8.Abramsky O, Litvin Y. Automimmune response to dopamine-receptor as a possible mechanism in the pathogenesis of Parkinson's disease and schizophrenia. Perspect Biol Med 1978; 22:104–114. [PubMed] [Google Scholar]
- 9.Chen S, Le WD, Xie WJ, et al. Experimental destruction of substantia nigra initiated by Parkinson disease immunoglobulins. Arch Neurol 1998; 55:1075–1080. [DOI] [PubMed] [Google Scholar]
- 10.Monahan AJ, Warren M, Carvey PM. Neuroinflammation and peripheral immune infiltration in Parkinson's disease: an autoimmune hypothesis. Cell Transplant 2008; 17:363–372. [PubMed] [Google Scholar]
- 11.Kunas RC, McRae A, Kesselring J, et al. Antidopaminergic antibodies in a patient with a complex autoimmune disorder and rapidly progressing Parkinson's disease. J Allergy Clin Immunol 1995; 96:688–690. [DOI] [PubMed] [Google Scholar]
- 12.Benkler M, Agmon-Levin N, Hassin-Baer S, et al. Immunology, autoimmunity, and autoantibodies in Parkinson's disease. Clin Rev Allergy Immunol 2012; 42:164–171. [DOI] [PubMed] [Google Scholar]
- 13.Orr CF, Rowe DB, Halliday GM. An inflammatory review of Parkinson's disease. Prog Neurobiol 2002; 68:325–340. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, O’Reilly EJ, Schwarzschild MA, et al. Peripheral inflammatory biomarkers and risk of Parkinson's disease. Am J Epidemiol 2008; 167:90–95. [DOI] [PubMed] [Google Scholar]
- 15.Carvey PM, Chen EY, Lipton JW, et al. Intra-parenchymal injection of tumor necrosis factor-alpha and interleukin 1-beta produces dopamine neuron loss in the rat. J Neural Transm 2005; 112:601–612. [DOI] [PubMed] [Google Scholar]
- 16.Yu SY, Zuo LJ, Wang F, et al. Potential biomarkers relating pathological proteins, neuroinflammatory factors and free radicals in PD patients with cognitive impairment: a cross-sectional study. BMC Neurol 2014; 14.113:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bezalel S, Guri KM, Elbirt D, et al. Type I interferon signature in systemic lupus erythematosus. Isr Med Assoc J 2014; 16:246–249. [PubMed] [Google Scholar]
- 18.Osawa H, Yamabe H, Kaizuka M, et al. Systemic lupus erythematosus associated with transverse myelitis and Parkinsonian symptoms. Lupus 1997; 6:613–615. [DOI] [PubMed] [Google Scholar]
- 19.Shahar E, Goshen E, Tauber Z, et al. Parkinsonian syndrome complicating systemic lupus erythematosus. Pediatr Neurol 1998; 18:456–458. [DOI] [PubMed] [Google Scholar]
- 20.Chacón J, García-Moreno JM, Valencia J, et al. Parkinson disease of juvenile onset with systemic lupus erythematosus in a pre-symptomatic stage. Rev Neurol 1999; 29:725–727. [PubMed] [Google Scholar]
- 21.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun 2004; 18:407–413. [DOI] [PubMed] [Google Scholar]
- 22.Sleegers MJ, Beutler JJ, Hardon WJ, et al. Reversible rapidly progressive dementia with Parkinsonism induced by valproate in a patient with systemic lupus erythematosus. J Am Geriatr Soc 2010; 58:799–801. [DOI] [PubMed] [Google Scholar]
- 23.Lachhab L, Regragui W, Hamaz S, et al. Parkinsonism as first manifestation of lupus. Rev Neurol (Paris) 2012; 168:990–991. [DOI] [PubMed] [Google Scholar]
- 24.Marino M, Morgante F, Montagnese F, et al. Acute parkinsonism as first manifestation of systemic lupus erythematosus unmasked by CMV infection. Neurol Sci 2014; 35:2019–2021. [DOI] [PubMed] [Google Scholar]
- 25.Rugbjerg K, Friis S, Ritz B, et al. Autoimmune disease and risk for Parkinson disease: a population-based case-control study. Neurology 2009; 73:1462–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]


