Abstract
This study determined the trends in the quantities and patterns of nationwide antibiotic consumption in the Republic of Korea (ROK).
This nationwide descriptive epidemiological study was conducted in the ROK between 2008 and 2012. The quantities and patterns of total systemic antibiotic prescriptions were analyzed using National Health Insurance claims data collected through the Health Insurance Review and Assessment service. Data concerning systemic antibiotics were collected using measurement units of the defined daily dose (DDD) per 1000 people per day according to the Anatomical Therapeutic Chemical classification.
Over the 5-year study period, the annual consumption of systemic antibiotics ranged from 21.68 to 23.12 DDD per 1000 people per day. Outpatient antibiotic use accounted for 80.9% of total consumption. A regression model with autoregressive errors showed significant increased consumption of major antibiotic subgroups, including 3rd-generation cephalosporins, carbapenems, and glycopeptides (P < 0.001). However, the antibiotic use of 1st- (P = 0.003), 2nd- (P = 0.004), and 3rd-generation (P = 0.018) cephalosporins among patients who underwent surgery under monitoring by the antimicrobial stewardship programs for perioperative prescription was significantly lower than in those who underwent surgery without monitoring programs. In time-series analysis, total antibiotic consumption demonstrated significant seasonality (P < 0.001).
The consumption of broad-spectrum antibiotics was noted to have increased in the ROK from 2008 to 2012, providing a possible explanation for the changing epidemiology of multidrug resistance. Larger prospective studies are needed to investigate the impact on public health of monitoring programs of perioperative antibiotic usage.
INTRODUCTION
Antimicrobial resistance is an increasingly serious problem recognized as one of the greatest global threats to human health.1 Seven decades of medical advances enabled by the introduction of antibiotics are currently threatened by the rise of multidrug-resistant and extremely resistant hospital pathogens such as Staphylococcus aureus, Enterobacteriaceae, and Acinetobacter baumannii.2–5 Infections caused by multidrug-resistant bacteria result in high morbidity and mortality in critically ill patients and incur massive healthcare costs.6 The annual financial burden of antimicrobial resistance is estimated to exceed US$20 billion in the USA and €1.5 billion in Europe.7,8
Antibiotics use is the most important factor responsible for antibiotic resistance. Overuse or misuse of antibiotics can increase selective pressure, which is an important determinant of the emergence and dissemination of resistant organisms.9 Therefore, the measurement of antibiotic consumption is important to enhance understanding of the epidemiology of antimicrobial resistance and to implement appropriate policies.10 Surveillance programs for antibiotic consumption have been initiated in several countries, and, consequently, national antibiotic policies have been formulated.11–14 In 2012, the World Health Organization (WHO) established a worldwide strategy encompassing the 5 most important fields for the containment of antibiotic resistance: surveillance, rational use in humans, rational use in animals, infection prevention and control, and innovation.
The prevalence of antimicrobial resistance has increased gradually among major bacterial pathogens in the Republic of Korea (ROK) in recent years. According to the Korean Nationwide Surveillance of Antimicrobial Resistance (KONSAR) study, the prevalence of extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae and carbapenem-resistant A baumannii increased to 32% to 46% and 59% to 64%, respectively, among clinical isolates collected in 2011.15 However, no nationwide surveillance program on antibiotic consumption has been established. The limited nationwide data available on antibiotic consumption are based solely on sales data from the Korean Pharmaceutical Manufacturers Association.
The purpose of this study was to determine the quantities and patterns of antibiotic consumption in the ROK from 2008 to 2012 on the basis of population-wide reimbursement data and to provide highly reliable nationwide data on antibiotic consumption. In addition, this study investigated the trends in consumption of major antibiotic subgroups in the context of changing multidrug resistance epidemiology and evaluated the respective antibiotic prescriptions after implementation of the public health policy of antibiotic stewardship programs. For future antibiotic stewardship, this study can provide baseline information on the recent status of antibiotics overuse or misuse.
METHODS
Study Design and Setting
A retrospective nationwide population-based descriptive study was conducted in the ROK from January 1, 2008 to December 31, 2012. The quantities and patterns of total systemic antibiotic prescription were analyzed using National Health Insurance (NHI) claims data collected through the Health Insurance Review and Assessment (HIRA) service. Systemic antibiotics are available only with a prescription issued by a physician and are dispensed by pharmacies. Antiviral agents, antifungal agents, parasiticides, antituberculosis agents, and antileprosy agents were not included in the present analysis.
The ROK established universal population coverage through NHI in 1989. The NHI covers 97.1% of the population (∼50 million people), and the Medical Aid program covers the other 2.9%.16
The HIRA service has implemented antimicrobial stewardship programs to monitor antibiotic prescription patterns and to report performance feedback for upper-respiratory infections and surgical antimicrobial prophylaxis started in 2006 and 2007, respectively, for all medical institutions except private clinics.17,18 Assessment of the optimal surgical prophylaxis included the optimal timing, type, and duration of perioperative antibiotics for patients undergoing gastric surgery, colorectal surgery, gallbladder surgery, hip and knee total arthroplasty, hysterectomy, cesarean section, cranioplasty, prostatectomy, and glaucoma treatment.
The protocol of the present study was approved by the HIRA Institutional Review Boards. The boards waived the need for informed consent owing to the nature of the research.
Data Source
All data were obtained from the electronic database of the HIRA service. Patient information linked to the health insurance claims included demographic characteristics, administrative data, medicine claims, and medical conditions. Patient names and national registration numbers were not provided to protect patient confidentiality. Medical institutions were categorized according to the number of licensed beds, facilities, and types of specialty care as follows: private clinic (<30 beds, n = 42,434), hospital (30–99 beds, n = 2513), general hospital (≥100 beds and 6–9 types of specialty care, n = 272), and tertiary care hospital (≥500 beds and ≥20 specialties, n = 44).
Measures of Antibiotic Consumption
Prescriptions for systemic antibiotics for ambulatory and hospital care were collected with additional patient information in a separate database using measures on a monthly or yearly basis from 2008 to 2012. Antibiotic utilization data were standardized according to Anatomical Therapeutic Chemical (ATC) classification with the defined daily dose (DDD) as a measurement unit, as recommended by the WHO Collaborating Centre for Drug Statistics Methodology.19 The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults. Antibiotics consumption expressed in DDDs per 1000 people per day was used as a proxy for prescriptions in order to multilaterally interpret current trends on a national level, thereby enabling international comparison. Population-weighted antibiotics consumption was calculated as follows: [total antibiotics consumption amount (g)/DDD (g) × duration of consumption (days) × total population] × 1000. The estimated total population of the ROK was 49,404,648–50,948,272 from 2008 to 2012.20
Statistical Analysis
Baseline characteristics, such as age, sex, and prescription information, were categorized as appropriate. Continuous variables are expressed as mean ± standard deviation or median (range), while categorical variables are expressed as percentages within each group. Nonparametric methods such as the Kruskal–Wallis test and Mann–Whitney U test were performed to assess differences in antibiotic use by age group, medical institution category, and sex. A regression model with autoregressive errors and a linear regression model using measures on a per-month and per-year basis, respectively, were used to determine the antibiotic consumption trends. Time-series analysis with an autoregressive integrated moving average model was performed to assess the seasonality of the monthly consumption of antibiotics. Statistical significance was defined a priori as P < 0.05. All analyses were performed using IBM SPSS Statistics version 20.0 (IBM Corporation, Armonk, NY), R 2.15.2 (The R Foundation for Statistical Computing, Vienna, Austria), and SAS 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Overall Consumption of Systemic Antibiotics
During the study period, the total consumption of antibiotics for systemic use (DDD per 1000 people per day) increased from 21.68 in 2008 to 23.12 in 2012, representing a stable upward trend with a 1.1-fold difference (Table 1). The ROK population-weighed mean antibiotic consumption was 22.28 ± 0.58 DDD per 1000 people per day during the 5-year study period. The antibiotic consumption rate was significantly higher in outpatient settings. The antibiotic consumption rate attributable to outpatient use was 80.9% with a mean quantity of 18.02 DDD per 1000 people per day. The rate of inpatient antibiotics use was 19.1% with a mean quantity of 4.26 DDD per 1000 people per day (Table 1).
TABLE 1.
Population-Weighted Consumption and Distribution of Antimicrobial Agents for Systemic Use in the Republic of Korea From 2008 to 2012
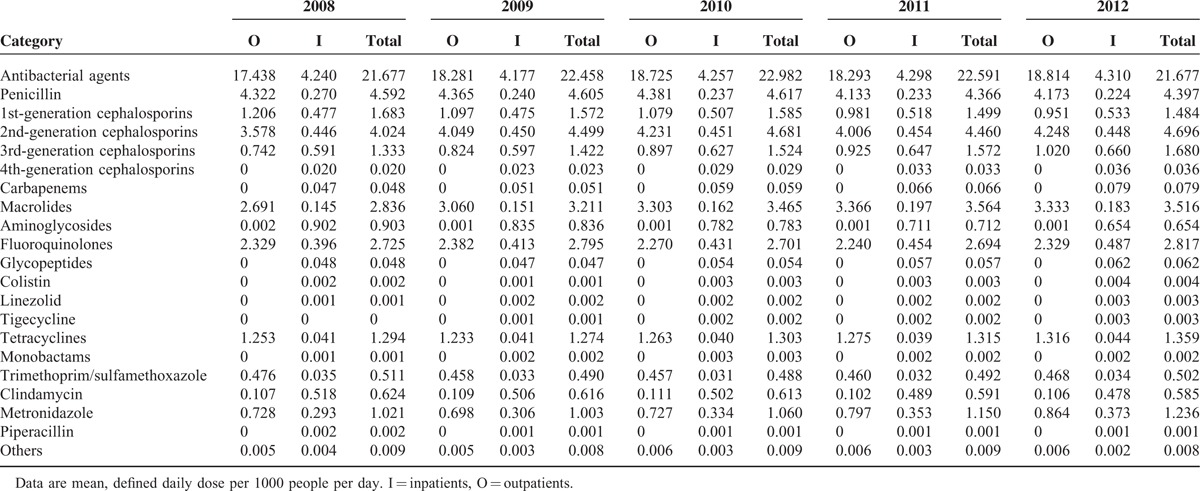
Consumption by Antibiotic Subgroup
Penicillins (population-weighted mean consumption: 4.52 DDD per 1000 people per day) were the most commonly used antibiotics subgroup, followed by 2nd-generation cephalosporins (4.47 DDD per 1000 people per day), macrolides (3.32 DDD per 1000 people per day), and fluoroquinolones (2.75 DDD per 1000 people per day). Similar antibiotics consumption patterns were observed in outpatient settings (Table 1). However, aminoglycosides and 3rd-generation cephalosporins were used more frequently in inpatient settings (0.78 and 0.62 DDD per 1000 people per day, respectively).
Five-Year Trends in Antibiotic Consumption
In the regression model with autoregressive errors, the monthly overall antibiotic consumption trends remained stable throughout the study period (coefficient for time = 0.002, P = 0.2029). However, significantly increased trends were observed for 3rd-generation cephalosporins (coefficient for time = 0.0005, P < 0.001), carbapenems (coefficient for time = 0.00005, P < 0.001), and glycopeptides (coefficient for time = 0.00002, P < 0.001; Figure 1A). In addition, there were significantly increased trends for consumption of other antibiotic subgroups used for therapeutic options against multidrug-resistant microorganisms, including colistin (coefficient for time = 0.000005, P < 0.001), linezolid (coefficient for time = 0.000002, P < 0.001), and tigecycline (coefficient for time = 0.000005, P < 0.001; Figure 1B). Metronidazole, a first-line antimicrobial agent against Clostridium difficile infection, also exhibited a significantly increased consumption trend (coefficient for time = 0.0003, P < 0.001; Figure 1C). In contrast, the consumption of aminoglycosides showed a significantly decreased trend (coefficient for time = −0.0004, P < 0.001; Figure 1D).
FIGURE 1.
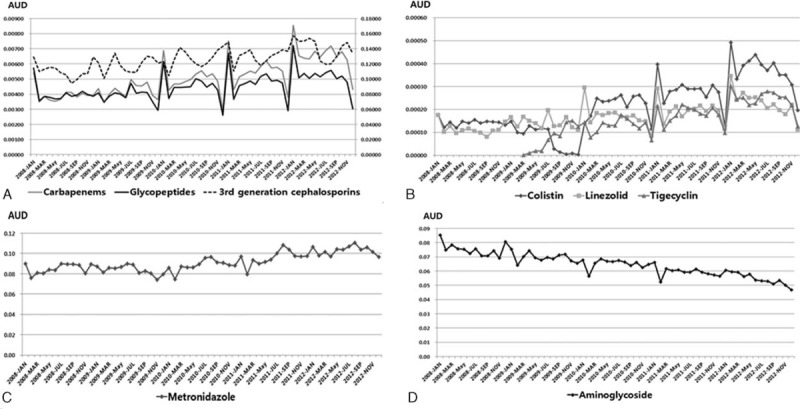
Monthly consumption trends of antibiotic subgroups in the Republic of Korea from 2008 to 2012 (defined daily dose per 1000 people per day). (A) Broad-spectrum antibiotics; (B) colistin, linezolid, and tigecycline; (C) metronidazole; (D) aminoglycosides.
The rate of consumption of antibiotics for systemic use appeared to be significantly lower during summer months than during other seasons, reflecting seasonality of antibiotic use. The average monthly consumption of antibiotics (DDD per 1000 people per day) in spring, summer, fall, and winter was 1.98 ± 0.12, 1.70 ± 0.16, 1.95 ± 0.13, and 1.96 ± 0.20, respectively (P < 0.001). Furthermore, the consumption rate was significantly higher during the influenza season (ie, November through April) than otherwise (1.98 ± 0.16 vs 1.81 ± 0.18 DDD per 1000 people per day, P < 0.001). This was evidenced by more frequent prescription of commonly used antibiotics such as penicillins, macrolides, and fluoroquinolones during the influenza season (Fig. 2). Antibiotic consumption was noted to fluctuate with the seasons in the time-series analysis. The monthly antibiotic consumption in the preceding 12 months (ie, the independent variable, time t − 12) was significantly associated with antibiotic consumption of the corresponding month (ie, the dependent variable, time t).
FIGURE 2.
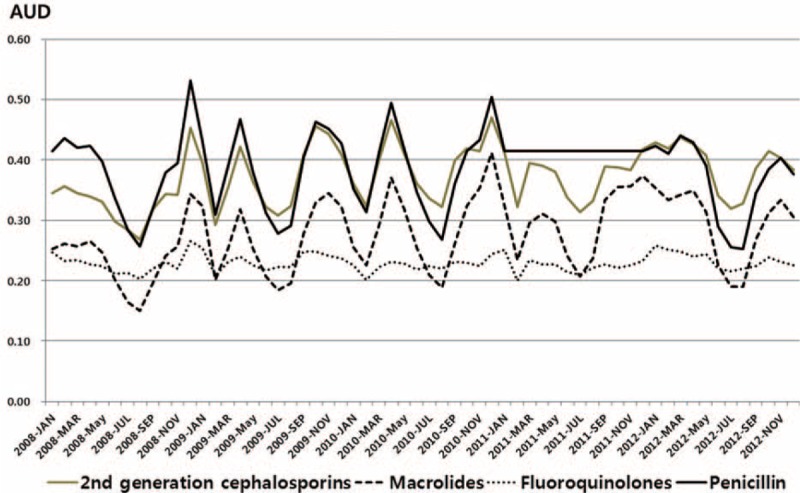
Seasonal trends of consumption among subgroups of antibiotics commonly used in the Republic of Korea from 2008 to 2012 (defined daily dose per 1000 people per day).
Consumption of Antibiotics Stratified by Age Group, Sex, and Type of Medical Institution
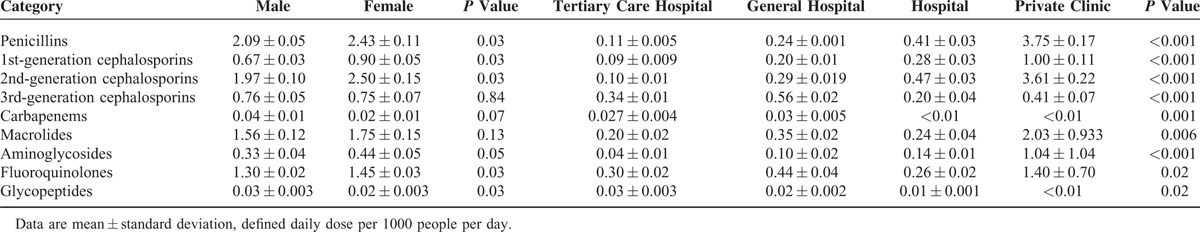
Systemic antibiotic consumption with respect to age group is shown in Table 2. Children younger than 9 years of age old exhibited disproportionately higher rates of consumption of penicillins, macrolides, and 3rd-generation cephalosporins (2.2-, 2.0-, and 2.6-fold greater, respectively) when compared with people ages 10 to 59 years old. Use of fluoroquinolones was very low in patients’ ages 0 to 19 years old, as their use is not generally recommended in pediatric patients. The rate of consumption of systemic antibiotics was higher in women than in men. However, the proportional consumption of the most commonly used antibiotics did not differ between sexes (Table 3). Private clinics had the highest usage rate of several classes of antibiotics, including penicillins, 1st- and 2nd-generation cephalosporins, macrolides, aminoglycosides, and fluoroquinolones. Third-generation cephalosporins and fluoroquinolones were more frequently used in general and tertiary care hospitals (Table 3).
TABLE 2.
Consumption and Distribution of Major Antibiotic Subgroups by Age Group in the Republic of Korea From 2008 to 2012
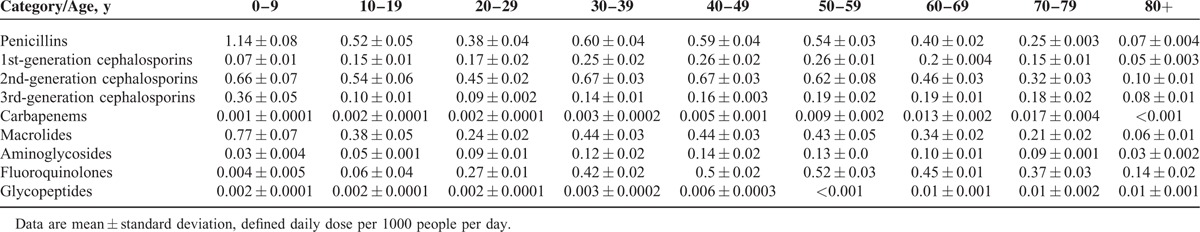
TABLE 3.
Consumption and Distribution of Major Antibiotic Subgroups by Sex and Type of Medical Facility in the Republic of Korea From 2008 to 2012
Five-Year Changes in the Consumption of Perioperative Antibiotics
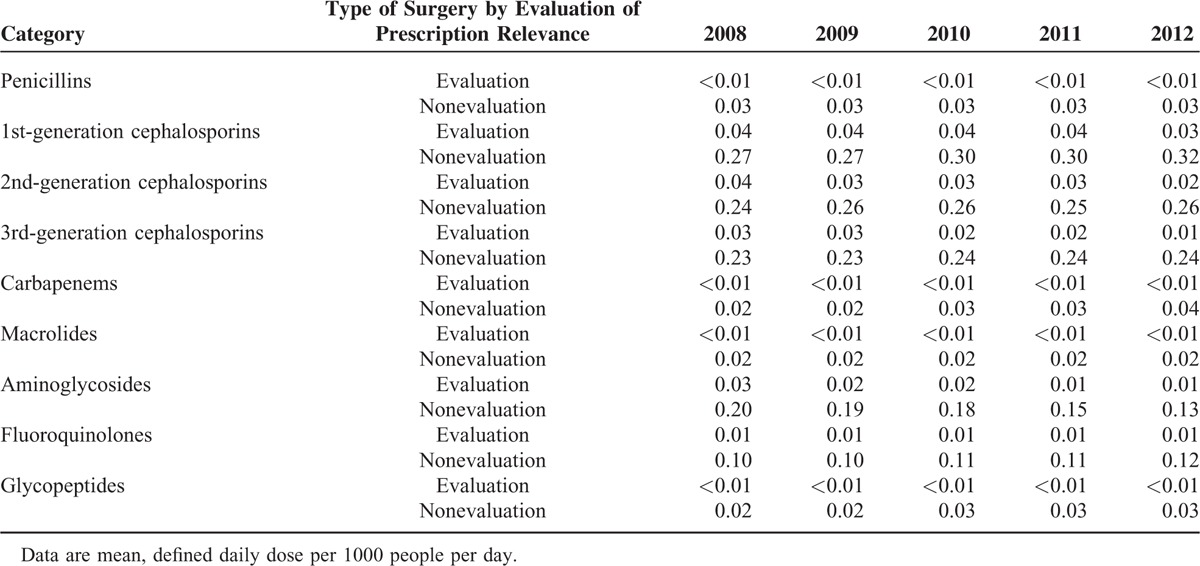
The HIRA service's policy aiming to improve the optimal surgical prophylaxis was being continuously implemented throughout the study period. Therefore, the changes in perioperative antibiotics consumption were assessed in patients who underwent surgery under monitoring by the antimicrobial stewardship programs on an annual basis. The overall prescription patterns of antibiotic subgroups differed between surgical and nonsurgical patients. First-, second-, or third-generation cephalosporins were most frequently used in surgical patients, whereas penicillins, 2nd-generation cephalosporins, and macrolides were prescribed most commonly in nonsurgical patients (Table 4). Although antibiotic usage by surgical patients being monitored by the antimicrobial stewardship programs accounted for only a small proportion of the total antibiotic consumption by surgical patients, prescription of perioperative antibiotics for those patients decreased significantly for 1st- (P = 0.003), 2nd- (P = 0.004), and 3rd-generation (P = 0.018) cephalosporins (Table 5). In contrast, the prescription of 1st-generation cephalosporins (P = 0.008), carbapenems (P = 0.002), and fluoroquinolones (P = 0.006) increased significantly for surgical patients who were not being monitored by antimicrobial stewardship programs (Table 5). Aminoglycosides consumption rates decreased continuously in surgical patients overall during the 5-year study period (Table 5).
TABLE 4.
Antibiotic Consumption of Major Antibiotic Subgroups by Perioperative Prescription in the Republic of Korea From 2008 to 2012
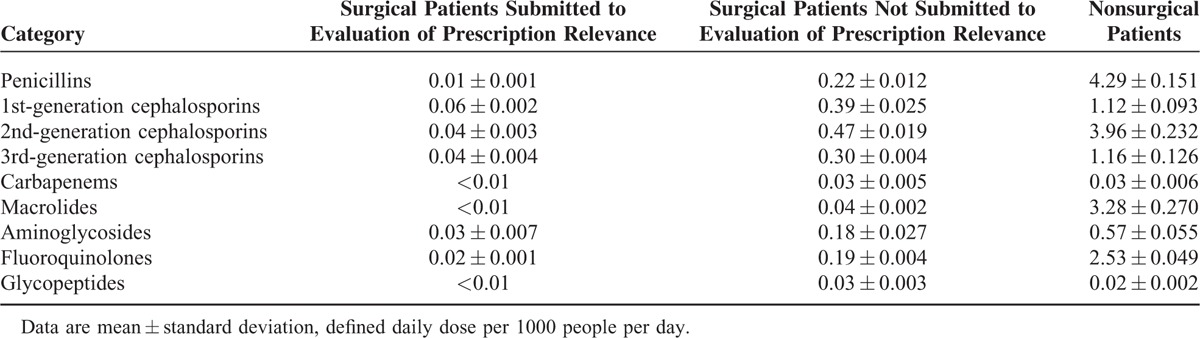
TABLE 5.
Comparative Consumption of Major Antibiotic Subgroups With Respect to the Presence of an Evaluation of Perioperative Prescription Relevance Among Surgical Patients in the Republic of Korea From 2008 to 2012
DISCUSSION
The present study investigated the quantities and patterns of systemic antibiotic consumption in the ROK by analyzing the reimbursement claims data provided by the HIRA service from 2008 to 2012. During the study period, the rate of consumption of major antibiotic subgroups used primarily for serious multidrug-resistant gram-negative and -positive bacterial infections increased significantly. On the other hand, the rate of perioperative cephalosporins use was noted to have a significant decrease for a subgroup of surgical patients being monitored by antibiotic stewardship programs. Our study represents the first reliable data for the population-weighted consumption of major classes of individual antibiotics in the ROK using measurement of DDD per 1000 people per day in accordance with the ATC classification system. Therefore, results from the present study reflect the recent status of antibiotic consumption in the ROK and they can be used for implementation of public health policies to prevent development of antimicrobial resistance resulting from overuse of antibiotics.
The levels of antibiotic consumption in the European Union (EU) and 12 non-EU European countries (DDD per 1000 people per day) were variable, ranging from 11.1 in Estonia to 42.3 in Turkey.21 However, the antibiotic consumption in the ROK (22.28 ± 0.58 DDD per 1000 people per day) was similar to those of certain European countries such as Spain (20.87), Slovakia (22.04), Iceland (22.11), and Poland (22.91) in 2012, based on the data from the European Surveillance of Antibiotic Consumption (ESAC) projects.22,23
Analysis of antibiotic subgroups showed that penicillins were the most frequently used antibiotic subgroups in the ROK. Broad-spectrum penicillins (eg, amoxicillin, ampicillin, amoxicillin/clavulanic acid, and ampicillin/sulbactam) were used more frequently than narrow-spectrum penicillins, which was similarly observed in EU countries.22
Compared to the ESAC-Net countries, the ROK consumed higher volumes of 2nd-generation cephalosporins and macrolides.22 Owing to current high rates of resistance to 2nd-generation cephalosporins (61.3%) and macrolides (77.7–83.1%) by Streptococcus pneumoniae as well as to macrolides (62.9%) by Mycoplasma pneumoniae in the ROK,24–26 routine use of 2nd-generation cephalosporins and macrolides must be carefully evaluated based on local antibiotic susceptibility patterns to prevent indiscriminate antibiotic usage, particularly in treatment of community-acquired pneumonia.
The increasing prevalence of multidrug-resistant bacteria has recently become a serious issue in healthcare settings in the ROK. After the Infectious Disease Control and Prevention Act was enacted in December of 2010, infections caused by 6 types of multidrug-resistant bacteria have become legally reportable diseases for a sentinel surveillance program.27 The increasing trend of carbapenem consumption observed in the present study is comparable to the increasing prevalence of ESBL-producing Escherichia coli and K pneumoniae isolates in the ROK.15 Increased use of carbapenems invariably exerts selective pressure, leading to the development of inactivating carbapenemase with emergence of carbapenem-resistant organisms. These have been particularly complicated in the management of multidrug-resistant gram-negative infections where carbapenem has been regarded as a last resort for effective treatment.28–30 Likewise, other antibiotics used for treating multidrug-resistant gram-negative bacterial infections such as colistin and tigecycline exhibited increasing consumption trends. The use of glycopeptides in the ROK also appeared to be increasing, similar to their use in many countries.31 Our findings of the increased consumption of antibiotic subgroups used to treat multidrug-resistant organisms are consistent with a recent report by Kim et al32 on the changing patterns of antibiotic usage in Korea based on wholesalers’ data provided by the Korea Pharmaceutical Manufacturers Association.
In the present study, private clinics had the highest rates of penicillins, 2nd-generation cephalosporins, and macrolides usage. In addition, private clinics had approximately 8 to 9 times higher consumption rates of penicillins, 3rd-generation cephalosporins, and macrolides than did hospitals. Thus, the development and implementation of an antimicrobial stewardship program for private clinics should be considered for further guidance of rational use of antibiotics without overuses.33–35 Of note, the proportion of antibiotics consumption in the inpatient settings (19.1%) was found to be higher in the ROK than in many other European countries (5–10%).31
High volumes of antibiotics were administered during winter months in the seasonal trends analysis of this study. This might be associated with an increased prevalence of bacterial infections following respiratory virus infections and/or misuse of antibiotics to treat seasonal viral infections. It is noteworthy that a periodic pattern of increasing antibiotic consumption in winter months has not been seen since 2011 (Fig. 2), reflecting the impact of the antimicrobial stewardship program for upper-respiratory infections conducted by the HIRA service. In fact, the HIRA service began to penalize hospitals that received unfavorable scores due to public disclosure of antibiotic prescription quality since 2011. Chun and Cheong36 evaluated the Korean policy and reported that the prescription rate of antibiotics for upper-respiratory tract infections decreased from roughly 50% in 2005 to 40% in 2009 in hospitals, including tertiary care and general hospitals. In addition, the decreased consumption of perioperative antibiotics in the subgroup of surgical patients under monitoring by antibiotic stewardship programs suggests the potential efficacy and impact of a monitoring program itself to result in a substantial benefit.
This study has some limitations. First, as with the reimbursement claims database elsewhere, the data source analyzed in this study might have limited information on record of care received, diagnoses, microbiological data, and inconsistencies in use of coding systems, among others. Thus, confounding factors from unmeasured clinical variables might have affected our antibiotic consumption for specific types of infections (eg, multidrug-resistant bacterial infections). In addition, our data source did not contain nonreimbursed antibiotic use. Nonetheless, antibiotic usage data driven from wholesalers’ data in the previous study were roughly comparable to those drawn from reimbursement claims data in this study except use of fluoroquinolones.32 Disparity of fluoroquinolones usage between the 2 studies requires further examination. Second, this study evaluated the potential impact of antimicrobial stewardship on perioperative antibiotic prescriptions, which was an explanatory investigation. Larger prospective studies are needed to evaluate the impact of antimicrobial stewardship on surgical prophylaxis. Third, the DDD measurement was equally applied to pediatric patients and adult patients with diminished renal function. As the DDD is determined on the basis of antibiotic use in adult patients with normal renal function, the volumes of the relevant antibiotics expressed in DDDs in those patients might have been underestimated.37,38
In conclusion, the present study provides the first comprehensive data on population-weighted systemic antimicrobial consumption in the ROK. The findings highlight the necessity of antimicrobial stewardship, especially for very young children and for private clinics in outpatient settings. Furthermore, this study provides valuable baseline data for establishment of public health strategies for the containment of antibiotic resistance as well as the guidance of antimicrobial prescription. Considering the increasing prevalence of antimicrobial resistance in clinical practice, national surveillance projects to monitor antimicrobial consumption and resistance are urgently required in order to formulate appropriate antibiotic prescription policies in the ROK.
Footnotes
Abbreviations: ATC = Anatomical Therapeutic Chemical, DDD = defined daily dose, ESAC = European Surveillance of Antibiotic Consumption, ESBL = extended-spectrum β-lactamase, EU = European Union, HIRA = Health Insurance Review and Assessment, KONSAR = Korean Nationwide Surveillance of Antimicrobial Resistance, NHI = National Health Insurance, ROK = Republic of Korea, WHO = World Health Organization.
The present affiliation for an author, GCP, has been changed to Gyeonggi Infectious Disease Control Center, Seongnam, Republic of Korea.
YKY and GCP have contributed equally and are considered joint first authors.
This study was supported by a 2012 grant from the Korean Academy of Medical Sciences. The funder had no role in study design, data collection, data analysis, data interpretation, or writing the report.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Spellberg B, Blaser M, Guidos RJ, et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis 2011; 52:S397–S428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakoulas G, Moellering RC., Jr Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis 2008; 46:S360–S367. [DOI] [PubMed] [Google Scholar]
- 3.Yu WL, Chuang YC, Walther-Rasmussen J. Extended-spectrum beta-lactamases in Taiwan: epidemiology, detection, treatment and infection control. J Microbiol Immunol Infect 2006; 39:264–277. [PubMed] [Google Scholar]
- 4.Pogue JM, Mann T, Barber KE, et al. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev Anti Infect Ther 2013; 11:383–393. [DOI] [PubMed] [Google Scholar]
- 5.Temkin E, Adler A, Lerner A, et al. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci 2014; 1323:22–42. [DOI] [PubMed] [Google Scholar]
- 6.Filice GA, Nyman JA, Lexau C, et al. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2010; 31:365–373. [DOI] [PubMed] [Google Scholar]
- 7.Roberts R, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 2009; 49:1175–1184. [DOI] [PubMed] [Google Scholar]
- 8.ECDC/EMEA Joint Technical Report. The bacterial challenge: time to react. 2009. EMEA/576176/2009. http://ecdc.europa.eu/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf Accessed June 23, 2015. [Google Scholar]
- 9.Angebault C, Andremont A. Antimicrobial agent exposure and the emergence and spread of resistant microorganisms: issues associated with study design. Eur J Clin Microbiol Infect Dis 2013; 32:581–595. [DOI] [PubMed] [Google Scholar]
- 10.Gravatt LA, Pakyz AL. Challenges in measuring antibiotic consumption. Curr Infect Dis Rep 2013; 15:559–563. [DOI] [PubMed] [Google Scholar]
- 11.Fridkin SK, Steward CD, Edwards JR, et al. Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: project ICARE phase 2. Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals. Clin Infect Dis 1999; 29:245–252. [DOI] [PubMed] [Google Scholar]
- 12.Bager F. DANMAP: monitoring antimicrobial resistance in Denmark. Int J Antimicrob Agents 2000; 14:271–274. [DOI] [PubMed] [Google Scholar]
- 13.Mölstad S, Erntell M, Hanberger H, et al. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish Strama programme. Lancet Infect Dis 2008; 8:125–132. [DOI] [PubMed] [Google Scholar]
- 14.Zarb P, Goossens H. European Surveillance of Antimicrobial Consumption (ESAC): value of a point-prevalence survey of antimicrobial use across Europe. Drugs 2011; 71:745–755. [DOI] [PubMed] [Google Scholar]
- 15.Yong D, Shin HB, Kim Y, et al. Increase in the prevalence of carbapenem-resistant Acinetobacter isolates and ampicillin-resistant non-typhoidal Salmonella species in Korea: A KONSAR study conducted in 2011. Infect Chemother 2014; 46:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health Insurance Service and Health Insurance Review and Assessment, Service, 2013 National Health Insurance Statistical, Yearbook, 2014. http://www.129.go.kr/news/news02_view.jsp?n=8076 Accessed June 23, 2015. [Google Scholar]
- 17.Kim SY, Kim HE, Back MS, et al. The effect of public report on antibiotics prescribing rate. Kor J Clin Parm 2010; 20:242–247. [Google Scholar]
- 18.Health Insurance Review and Assessment Service. 2009 Report of prophylactic antibiotics for preventing surgical infection. 2009. http://www.hira.or.kr/ebook/af1fb128-3502-40bf-b45c-60cd1dfddfac/110_Page_img/extra/110.pdf Accessed June 23, 2015. [Google Scholar]
- 19.WHO Collaborating Centre for Drug Statistics Methodology. http://www.whocc.no/atc_ddd_index/ Accessed June 23, 2015. [Google Scholar]
- 20.Statistics Korea. Korean Statistical Information Service. http://kosis.kr/statisticsList/statisticsList_01List.jsp?vwcd=MT_ZTITLE&parmTabId=M_01_01#SubCont Accessed June 23, 2015. [Google Scholar]
- 21.Versporten A, Bolokhovets G, Ghazaryan L, et al. Antibiotic use in Eastern Europe: a cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect Dis 2014; 14:381–387. [DOI] [PubMed] [Google Scholar]
- 22.European Centre for Disease Prevention and Control. Summary of the latest data on antibiotic consumption in the European Union. European Antibiotic Awareness Day. November, 2012. http://www.ecdc.europa.eu/en/eaad/documents/eaad-2011-summary-antimicrobial-consumption-data.pdf Accessed June 24, 2015. [Google Scholar]
- 23.European Centre for Disease Prevention and Control. Country overview of antimicrobial consumption. http://ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/esac-net-database/Pages/overview-country-consumption.aspx Accessed June 24, 2015. [Google Scholar]
- 24.Song JH, Jung SI, Ko KS, et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother 2004; 48:2101–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong KB, Choi EH, Lee HJ, et al. Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000–2011. Emerg Infect Dis 2013; 19:1281–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Song JH, Chung DR, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother 2012; 56:1418–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korea Centers for Disease Control. 2011 Case definitions for National Notifiable Infectious Diseases. http://www.cdc.go.kr/CDC/contents/CdcKrContentView.jsp?cid=21153&menuIds=HOME001-MNU1132-MNU1138-MNU1065 Accessed June 23, 2015. [Google Scholar]
- 28.Yoon YK, Yang KS, Lee SE, et al. Effects of Group 1 versus Group 2 carbapenems on the susceptibility of Acinetobacter baumannii to carbapenems: a before and after intervention study of carbapenem-use stewardship. PLoS ONE 2014; 9:e99101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee K, Yong D, Jeong SH, et al. Multidrug-resistant Acinetobacter spp.: increasingly problematic nosocomial pathogens. Yonsei Med J 2011; 52:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Alliance for the Prudent Use of Antibiotics. Nightmare bacteria and the worldwide threat to carbapenem. APUA Clinical Newsletter. 2013;31:1–28. http://www.tufts.edu/med/apua/news/newsletter.shtml Accessed June 23, 2015. [Google Scholar]
- 31.Vander Stichele RH, Elseviers MM, Ferech M, et al. European Surveillance of Antibiotic Consumption (ESAC) Project Group. Hospital consumption of antibiotics in 15 European countries: results of the ESAC Retrospective Data Collection (1997–2002). J Antimicrob Chemother 2006; 58:159–167. [DOI] [PubMed] [Google Scholar]
- 32.Kim YJ, Chun HJ, Lee JW, et al. The changing patterns of antibiotics usage in Korea during 1981–2008. Infect Chemother 2012; 44:411–418. [Google Scholar]
- 33.Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med 2013; 173:267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao L, Peng R, Wang Y, et al. Significant reduction of antibiotic consumption and patients’ costs after an action plan in China, 2010–2014. PLoS ONE 2015; 10:e0118868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Wang P, Wang X, et al. Use and prescription of antibiotics in primary health care settings in China. JAMA Intern Med 2014; 174:1914–1920. [DOI] [PubMed] [Google Scholar]
- 36.Chun DS, Cheong KH. Analysis about effects of public information: the proportion of antibiotic prescription for upper respiratory tract infection in Seoul. Korean Policy Stud Rev 2011; 20:109–142. [Google Scholar]
- 37.Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 2007; 44:664–670. [DOI] [PubMed] [Google Scholar]
- 38.Muller A, Monnet DL, Talon D, et al. Discrepancies between prescribed daily doses and WHO defined daily doses of antibacterials at a university hospital. Br J Clin Pharmacol 2006; 61:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]


