Abstract
Mycobacteria of the Mycobacterium tuberculosis complex (MTBC) greatly affect humans and animals worldwide. The life cycle of mycobacteria is complex and the mechanisms resulting in pathogen infection and survival in host cells are not fully understood. Recently, comparative genomics analyses have provided new insights into the evolution and adaptation of the MTBC to survive inside the host. However, most of this information has been obtained using M. tuberculosis but not other members of the MTBC such as M. bovis and M. caprae. In this study, the genome of three M. bovis (MB1, MB3, MB4) and one M. caprae (MB2) field isolates with different lesion score, prevalence and host distribution phenotypes were sequenced. Genome sequence information was used for whole-genome and protein-targeted comparative genomics analysis with the aim of finding correlates with phenotypic variation with potential implications for tuberculosis (TB) disease risk assessment and control. At the whole-genome level the results of the first comparative genomics study of field isolates of M. bovis including M. caprae showed that as previously reported for M. tuberculosis, sequential chromosomal nucleotide substitutions were the main driver of the M. bovis genome evolution. The phylogenetic analysis provided a strong support for the M. bovis/M. caprae clade, but supported M. caprae as a separate species. The comparison of the MB1 and MB4 isolates revealed differences in genome sequence, including gene families that are important for bacterial infection and transmission, thus highlighting differences with functional implications between isolates otherwise classified with the same spoligotype. Strategic protein-targeted analysis using the ESX or type VII secretion system, proteins linking stress response with lipid metabolism, host T cell epitopes of mycobacteria, antigens and peptidoglycan assembly protein identified new genetic markers and candidate vaccine antigens that warrant further study to develop tools to evaluate risks for TB disease caused by M. bovis/M.caprae and for TB control in humans and animals.
Author Summary
Mycobacteria belonging to the Mycobacterium tuberculosis complex infect humans and animals since pre-history and are a serious health problem worldwide. Whole-genome sequencing and comparative genomics generate information on the evolution and molecular basis of pathogenicity and transmissibility. However, while genomic information is increasingly available for the main human pathogens such as Mycobacterium tuberculosis, little is known about closely related bacteria, Mycobacterium bovis and Mycobacterium caprae. These mycobacteria infect humans causing zoonotic tuberculosis and are the main causative agents of animal tuberculosis. Although human-to-human transmission of zoonotic tuberculosis is limited, the infection often causes extra-pulmonary disease in humans and is still a major public health concern in developing countries, causing not only human disease but also severe effects on livelihoods. In this study, whole-genome sequences and targeted comparative genomics of three Mycobacterium bovis and one Mycobacterium caprae field isolates generated new information on the evolution and phenotypic variation of these mycobacteria. The results identified new genetic markers and candidate vaccine antigens that warrant further study to develop tools to evaluate risks for tuberculosis caused by M. bovis/M.caprae and for disease control in humans and animals.
Introduction
Mycobacterium tuberculosis has infected more than 2.5 billion people worldwide with approximately 9 million new tuberculosis (TB) cases reported every year [1]. Animal TB is caused by infection with Mycobacterium bovis and closely related members of the M. tuberculosis complex (MTBC) such as M. caprae. Although cattle are the main concern regarding animal TB in industrialized countries, several other mammals including humans are also infected [2,3]. Eurasian wild boar (Sus scrofa) are a natural reservoir for M. bovis in some regions and thus vaccination strategies are being developed for TB control in this species [4–8]. Several other domestic and wild animals are also infected with M. bovis and may act as reservoir species [9–13].
The life cycle of mycobacteria is complex and the mechanisms resulting in pathogen infection and survival in host cells are not fully understood [14]. Nevertheless, it is generally accepted that after inhalation into the lung or entry to the oropharyngeal cavity, the principal entry routes, mycobacteria of the MTBC are phagocytized by macrophages, which constitute the main host cell. As with other intracellular bacteria, mycobacteria survive inside macrophages by escaping host immune response, which results in the formation of a granuloma that effectively contains infected cells. A change in the host-bacterial equilibrium of granulomas is thought to result in the release of infected cells outside containment and onward transmission of mycobacteria to susceptible hosts [14].
The association between M. bovis spoligotypes and TB lesions in cattle has been used to correlate bacterial genotype with virulence [15]. However, these genotyping methods cover only a small portion of the approximately 4,000 genes contained in the 4.4 Mb mycobacterial genome [16]. Recently, whole-genome sequencing and comparative genomics analyses have provided new insights into the evolution and adaptation of the MBTC to survive inside the host and explained phenotypic traits related with transmissibility and virulence [16–21]. Although the first M. tuberculosis genome sequence was reported in 1998 [16], it was not until 2003 when the first M. bovis genome was sequenced [19]. Presently, a large number of M. tuberculosis but few M. bovis (except for BCG strains) genome sequences are available [18]. The relative paucity of M. bovis genome sequence information limits the possibility of characterizing mycobacterial evolution and correlation with virulence at the whole-genome level.
In this study, the genome of three M. bovis (MB1, MB3, MB4) and one M. caprae (MB2) field isolates with different lesion score, prevalence and host distribution phenotypes were sequenced. Genome sequence information was used for whole-genome and protein-targeted comparative genomics analysis with the aim of finding correlates of phenotypic variation with potential implications for TB disease risk assessment and control.
Materials and Methods
Ethics statement
All animal sampling was post-mortem. Wildlife samples came from hunter-harvested individuals that were shot during the legal hunting season independently and prior to our research while livestock samples were obtained at the slaughterhouse where they were being processed as part of the normal work and submitted to the reference laboratory by the slaughterhouse veterinarian. According to EU and National legislation (2010/63/UE Directive and Spanish Royal Decree 53/2013) and to the University of Castilla–La Mancha guidelines no permission or consent is required for conducting this type of study. Field isolates used come from the EU Reference Laboratory for Animal Tuberculosis (VISAVET).
Mycobacteria isolates
Three M. bovis (MB1, MB3, MB4) and one M. caprae (MB2) field isolates were selected for this study (Table 1). These isolates were originally obtained from wild boar (MB3, MB4), cattle (MB1) and goat (MB2). The study focused on Ciudad Real Province, Spain. This is a high ungulate density area, the west side of the province composed by interspersed game ranges and protected nature areas, with persistent TB infection in extensive livestock farms [22]. Nine hundred MTBC isolates collected from wild ungulates and livestock from 2000 to 2011 were spoligotyped resulting in 62 different spoligotypes ([23] and S1 Fig).
Table 1. Mycobacteria isolates used in the study.
| Species, spoligotype (ID) | Distribution (isolation frequency) | Hosts | TB lesion score |
|---|---|---|---|
| M. bovis, SB0339 (MB1) | High | C, G, D, F, W, P | Low |
| M. caprae, SB0157 (MB2) | Medium | C, G, S, D, W, P | High |
| M. bovis, SB0134 (MB3) | High | C, G, D, F, W, P, S, B | High |
| M. bovis, SB0339 (MB4) | High | C, G, D, F, W, P | Low |
Summary of the results obtained after the analysis of different MTBC spoligotypes fulfilling selection criteria (i-iii) described in Materials and Methods. The spoligotypes SB0134, SB0339 and SB0157 were among the most frequent spoligotypes found in both livestock and wild ungulates in the study area. The spoligotypes SB0134 and SB0339 clustered in space and time and showed high abundance. Findings in Iberian red deer showed a higher lesion score caused by spoligotype SB0134 when compared to spoligotype SB0339 while these spoligotypes and particularly SB0134 and SB0157 suggested an association with high TB severity in Eurasian wild boar.
Abbreviations: C, cattle; G, goat; D, deer; F, fallow deer; W, wild boar; P, pig; S, sheep; B, badger.
The criteria for selection of the MTBC spoligotypes included in the study were based on:
Inclusion into an emergent spoligotype cluster defined considering the estimated evolutionary history of the strains belonging to it. Spacer oligonucleotide typing, or spoligotyping, is a genotyping method used to study the epidemiology of the MTBC and it is based on the presence or absence of 43 spacers at the direct repeat locus [24]. The visualization of spoligotype patterns based on an estimated evolutionary history is a procedure to predict emerging strains or genotypes associated with elevated transmission and can be appropriate for medium-large spatio-temporal scale analysis. This method was used to determine if M. tuberculosis strains were spreading faster than the background transmission rate [25] and implemented in DESTUS (Detecting Emerging Strains of Tuberculosis Using Spoligotypes), which is included in spolTools (http://www.emi.unsw.edu.au/spoltools/). This method was designed to be used with self-contained datasets corresponding to specific regions, rather than composite data from different countries or collection periods. We conducted these analyses for the total and for each host species separately.
Inclusion into a spoligotype cluster that successfully clusters in space and time. We combined the approach (i) with cluster analyses (purely spatial and spatio-temporal as implemented in SatScan software version 5.1.3; [26]) at the population (wildlife management area or livestock farm) level to evidence emerging strains to select spoligotypes that successfully clustered in space and time. The Bernoulli purely spatial and spatio-temporal models were used to detect spoligotype clusters in individual farms or wildlife management areas. Spoligotypes from non-clustered strains were used as controls.
For those spoligotypes fulfilling criteria (i) and (ii), a third criterion based on TB severity in natural infections was considered. Firstly, TB severity in wild boar, the key reservoir host for M. bovis and M. caprae in Iberia [5, 27] was used based on the distribution of spoligotypes SB0134, SB0157, and SB0339 by age-class in naturally infected wild boar from Montes de Toledo, Spain. Secondly, we also considered the relationship between TB lesion scores and spoligotypes in red deer (Cervus elaphus), also a reservoir species for M. bovis in Iberia [28].
Following these three criteria, the M. bovis spoligotypes SB0339 (MB1, MB4) and SB0134 (MB3) were selected. The M. caprae spoligotype SB0157 (MB2) was included in the study as an outgroup but closely related species [29] and for the increased proportion of M. caprae isolated from bovine samples during 2004–2009 [30]. The MB4 isolate was included because although it has the same spoligotype as MB1, it served as a model to characterize possible differences between MTBC isolates otherwise classified with the same spoligotype.
Bacterial growth and genomic DNA extraction
The four isolates were grown in 15 ml of Middlebrook 7H9 liquid media supplemented with 0.36% sodium pyruvate and 10% OADC (Oleic Albumin Dextrose Catalase) for 5 weeks. Chromosomal DNA samples were obtained as described by van Soolingen et al. [31]. Briefly, cultures were centrifuged and pellets were washed twice in 5 ml water. Mycobacteria were heated at 100°C for 15 min to kill the cells. After centrifugation, the cells were resuspended in 5 ml TE buffer (0.01 M Tris-HCl, 0.001 M EDTA, pH 8.0). Lysozyme was added to a final concentration of 1 mg/ml and the tube was incubated over night at 37°C. Eighty hundred and seventy-five microliters of 10% sodium dodecyl sulfate (SDS) with 62.5 μl of proteinase K (at a 10-mg/ml concentration) were added, and the mixture was incubated for 1 h at 60°C. The extract was transferred to a phase lock gel tube (prime5, Fisher Scientific SL, Madrid, Spain) for a phenol/chloroform DNA extraction.
Genome sequencing
Genomic DNA (2–5 μg) was subjected to mechanical fragmentation using a BioRuptor (Life Technologies, Carlsbad, CA, USA). The number of cycles was adjusted to obtain DNA fragments of a final average size of about 500 pb. Samples were then used to prepare sequencing-amenable TruSeq libraries (NEB-Next, New England Biolabs, Ipswich, MA, USA). Briefly, DNA fragments were made blunt-ended, phosphorylated, adenylated and Illumina-compatible adapters were ligated. After purification, barcoded sequences as well as Illumina-specific sequences were introduced by PCR, followed by quantitation of individual libraries. Libraries were then pooled and quantified again. A quality control of the pooled library made in bioanalyzer is shown in the figure, including an estimation of the percentage of non-overlapping reads that could be obtained using a 2x250 paired-end sequencing protocol. Library was qPCR-quantitated and brought to a final concentration of 10 nM. DNA was then denatured and equilibrated so that a final concentration of 18 pM of library was loaded onto a MiSeq v.3 flowcell (Illumina, San Diego, CA, USA) and sequenced using a 2x250 paired-end sequencing protocol to obtain more than 400x high quality coverage (1.9–2.5 Gb) with 84% of the bases showing a Q30 factor > 30. Reads were finally split according to barcodes and used for bioinformatics analysis.
Genome sequence assembly and annotation
High quality overlapping reads were merged using FLASH (Magoc et al., 2011) and then assembled using Velvet [32] with k-value = 97 (S1 Table). Contigs were annotated using BG7 [33,34] (S2 Table). For annotation, a set of 191,017 reference proteins was used including (a) all Uniprot proteins from M. bovis and M. tuberculosis, (b) a set of bacterial antibiotic resistance related Uniprot proteins selected using the GO annotation terms “antibiotic resistance” and “response to antibiotic” and a selection of proteins based on similarity to the proteins of ARDB [35], and (c) all Uniprot proteins with Enzyme Code (EC) from MTBC. For whole genome comparative analysis, the 4 genomes were then aligned against the M. bovis reference genome sequence (AF2122/97; http://www.ncbi.nlm.nih.gov/nuccore/31791177) using Differences program (Turrientes et al., 2010) that allows comparisons at the whole genome level and particularly the detection of substitutions, insertions or deletions of any length and at any region of the genome. Genome sequence information and annotation was deposited in GenBank under the accession numbers CDHF01000001-CDHF01000049, CDHG01000001-CDHG01000059, CDHH01000001-CDHH01000094 and CDHE01000001-CDHE01000118 for MB1-MB4 isolates, respectively [36].
Whole genome phylogenetic analysis
A phylogenetic tree was constructed based on the SNPs found at the core genome sequence shared with a similarity over a threshold between all genomes included in the analysis using Harvest [37] and visualized with EvolView (v 198.3) [38]. Harvest defines the SNPs aligning whole assembled genomes and not reads like in other approaches, thus allowing the identification of the gene or the intergenic region where differences are allocated. The SNPs are included in the.vcf file provided by Harvest suite. The genomes used in the SNP phylogenetic analysis using Harvest included MB1-MB4, M. bovis AF2122/97 (http://www.ncbi.nlm.nih.gov/nuccore/31791177), M. bovis ATCC BAA-935 (http://www.ncbi.nlm.nih.gov/nuccore/690294709), M. bovis BCG Pasteur 1173P2 (http://www.ncbi.nlm.nih.gov/nuccore/121635883), and M. tuberculosis H37Rv (http://www.ncbi.nlm.nih.gov/nuccore/448814763).
Multilocus SNP phylogenetic analysis
The multilocus sequence analysis was conducted using 18 genes coding for the proteins linking stress response with lipid metabolism (S3 Table). The nucleotide sequences of the genes were obtained from the genomes of MB1-MB4 isolates used in this study. For comparison, the same sequences were obtained from the reference M. bovis BCG Pasteur 1173P2, M. bovis AF2122/97 and M. tuberculosis H37Rv. M. canettii (NCBI reference sequence NC_019950) was used as outgroup. The nucleotide sequences were concatenated and then aligned with MAFFT (v7), configured for the highest accuracy [39]. After alignment, regions with gaps were removed and 20080 gap-free sites were used in maximum likelihood phylogenetic analysis as implemented in PhyML (v3.0 aLRT) [40,41]. The reliability for the internal branches was assessed using the approximate likelihood ratio test (aLRT–SH-Like) [41]. Graphical representation and editing of the phylogenetic trees were performed with EvolView (v 198.3) [38]. The alignments obtained by MAFFT were used to perform codon alignments using HIV database website (www.hiv.lanl.gov; [42]). Non-synonymous (dN) and synonymous (dS) nucleotide substitutions were classified using the SNAP method [43] implemented in HIV database website [42]. SNPs were identified by pairwise comparison of MB1-MB4 and M. bovis AF2122/97 using SNAP.
Nucleotide substitution rate analysis
The dN/dS ratio was calculated for the 18 genes coding for the proteins linking stress response with lipid metabolism (S3 Table) and for 81 antigen-coding genes (S4 Table) present on each of the MB1-MB4 isolates included in the study. Under the Datamonkey server (http://www.datamonkey.org; [44]), the algorithm SLAC [45] was used to detect which nucleotide substitution site were positively or negatively selected. For each dS and non-synonymous dN substitution site, four measurements were made: normalized expected (ES and EN) and observed numbers (NS and NN). The SLAC algorithm then calculated: dN = NN/EN and dS = NS/ES. If dN < dS a codon was negatively selected and if dN > dS a codon was positively selected. A two-tailed extended binomial distribution set at P<0.05 was used to assess significance of the algorithm. The SLAC algorithm uses a neighbor-joining tree with a maximum likelihood for branch lengths and substitution rates.
Sequence analysis of Rv0050
To identify non-synonymous mutations that may be associated with virulence and/or transmission, the orthologs of peptidoglycan assembly protein locus Rv0050 (H37Rv) in strains MB1-MB4 were compared to the equivalent locus in animal isolates (BCG Pasteur 1173P2, AF2122/97, 09–1192) and human isolates (Bz 3115, B2 7505) of M. bovis available in the GenBank. The M. bovis strains were selected among all strains for having distinct Rv0050 locus and/or distinct source of isolation. The presence of a putative signal peptide and cleavage site in the Rv0050 locus was analyzed with a previously validated program for their prediction in M. tuberculosis (SignalP; http://www.cbs.dtu.dk/services/SignalP-3.0/) [46]. Results were also confirmed with two other programs, Signal-Blast (http://sigpep.services.came.sbg.ac.at/signalblast.html) and Phobious (http://phobius.sbc.su.se/).
PCR and sequence analysis
To confirm selected SNPs identified in the mycobacteria genomes sequenced in this study, sequence-specific oligonucleotide primers were design using reference genomes M. bovis AF2122/97 (BX248333.1) or M. tuberculosis H37Rv (AL123456.3) for PCR and sequencing of the amplicons. Selected loci and direct and reverse primers used for analysis included Rv0050 (ponA1; 5´-GACTTTCCCCAAACCGACCGAGG-3’ and 5’-GATCGGTCCCCCGACCACCATT-3’), Rv0589 (MCE2a; 5´-GTGCCAACGCTGGTGACGAG-3’ and 5’-AGAACACGATCAACCCATGA-3’), Rv1198 (ESAT-6; 5´-ATGACCATCAACTATCAATT-3’ and 5’-TCGGCTCCAGCTGGGCCTGA-3’), and Rv1860 (FAP-B; 5´-ATGCATCAGGTGGACCCCAA-3’ and 5’- AGCGGACCTTACCGGCCTGA-3’). The PCR was conducted using 2 μl of DNA with 20 pmol of each primer in a 50 μl reaction PCR Master Mix (Promega, Madison, WI, USA) using a GeneAmp PCR System 2700 thermocycler (Applied Biosystems, Carlsbad, CA, USA). PCR products were electrophoresed on 1.5% agarose gels to check the size of the amplified fragments by comparison to molecular weight marker GeneRuler 1kb DNA ladder (Thermo Scientific, Waltham, MA, USA). Amplified DNA fragments were purified with a PureLink Quick PCR Purification Kit (Thermo Scientific, Waltham, MA, USA) and sequenced using the reverse primer on each locus. Amplicons from at least two independent PCR reactions were sequenced.
Results and Discussion
Selection of mycobacteria isolates
Among the 900 MTBC field isolates analyzed, 62 different spoligotypes were identified suggesting that the study area is one of the regions with the highest diversity of MTBC spoligotypes described in the literature [47]. The high genetic diversity of MTBC in this area supported an important natural scenario where MTBC and particularly M. bovis have diversified, thus offering an interesting epidemiological and evolutionary context where new genotypes can emerge and diversify in terms of adaption to host under a range of environmental and human driven factors.
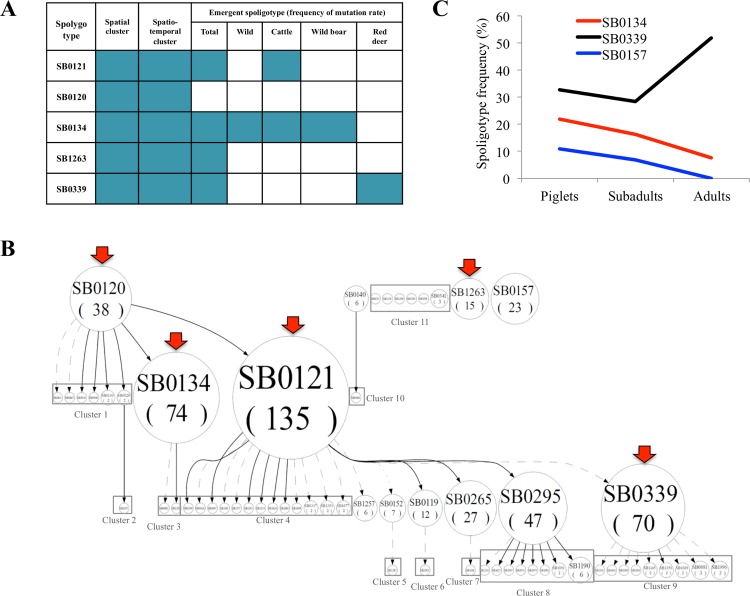
The spoligotypes SB0121, SB0134, SB0339, SB0120, SB1263 and SB0157 were among the most frequent ones found in both livestock and wild ungulates in the study area (S1 Fig). The spoligotypes found at a higher frequency than expected given a predicted mutation rate were identified (Fig 1A) and represented in a hierarchical tree (Fig 1B). The spoligotypes SB0134 (MB3) and SB0339 (MB1 and MB4), which clustered in space and time and showed low mutation rates but high abundance, were selected as emergent under study conditions. The output hierarchical tree suggested a history of mutation events and a relationship between these spoligotypes with different levels of delection for spoligotypes SB0134 (MB3) and SB0339 (MB1 and MB4) (Fig 1B). These spoligotypes were therefore chosen for this study as fulfilling selection criterion (i) and (ii) described above. Additionally, findings in Iberian red deer showed a higher lesion score caused by spoligotype SB0134 when compared to spoligotype SB0339 (Mann-Whitney U test; p = 0.04) while these spoligotypes and particularly SB0134 and SB0157 suggested an association with high TB severity in Eurasian wild boar thus also fulfilling selection criteria (iii) (Fig 1C).
Fig 1. Selection of mycobacteria isolates.
(A) Potentially emerging spoligotypes according to different criteria of emergence: (i) spatial and spatio-temporal clustering and (ii) the frequency of mutation rate (abundance for the expected mutation rate) considering all the hosts together and separately. Both criteria are indicative of high transmissibility and emergence. (B) The hierarchical tree showing a history of mutation events and relationship among spoligotypes in a sample of isolates from south central Spain (62 different spoligotypes out of 900 different MTBC isolates from livestock and wildlife). The size of each node is proportional to the number of isolates (the cluster size); edges between nodes reflect evolutionary relationships between spoligotypes with arrowheads pointing to descendants. Spoligotypes that are spreading faster than the background rate are marked with arrows. Spoligotypes that are inferred to be derived from another spoligotype are placed below the inferred parent. Cluster 1: SB0831, SB0867, SB0920, SB0948, SB0130(2), SB0828 (2); Custer 2: SB1675; Cluster 3: SB0885, SB1299; Cluster 4: SB0294, SB0426, SB0967, SB1141, SB1177, SB1195, SB1254, SB1624, SB1681, SB1998, SB0337(2), SB1353(2), SB1677(2); Cluster 5: SB1387; Cluster 6: SB2003; Cluster 7: SB1018; Cluster 8: SB1230, SB1627, SB1967, SB1972, SB1979, SB1981, SB1091 (2), SB1190 (6); Cluster 9: SB1316, SB1642, SB2000, SB2001, SB1145(2), SB1350 (2), SB1689 (2), SB0881 (3), SB1996 (3); Cluster 10: SB0484; Cluster11: SB0933, SB1174, SB1390, SB1391, SB1991, SB0341(3). (C) Distribution of spoligotypes SB0134, SB0157 and SB0339 by age-class in naturally infected Eurasian wild boar from Montes de Toledo, Spain. The percentage of detection of SB0339 in adult wild boar was higher than in yearlings and juveniles, indicating an age-increasing infection. By contrast, the percentage of adults infected with SB0134 was lower than expected, and no SB0157 infected adults were found, indicating that yearling or juvenile wild boar infected with these strains had a low chance of survival.
In summary, three M. bovis (MB1, MB3, MB4) and one M. caprae (MB2) field isolates with different prevalence, lesion score and host distribution phenotypes were selected for this study (Table 1). MB3 showed high distribution and lesion score while MB1 and MB4 were highly distributed but with low lesion score. The M. caprae (MB2) isolate had moderate distribution and high lesion score and was selected for comparison with the M. bovis isolates. These phenotypic variations are relevant for pathogen transmission and virulence and could be correlated with genome sequence information with implications for TB disease risk assessment and control.
Phylogenetic analysis and comparative genomics
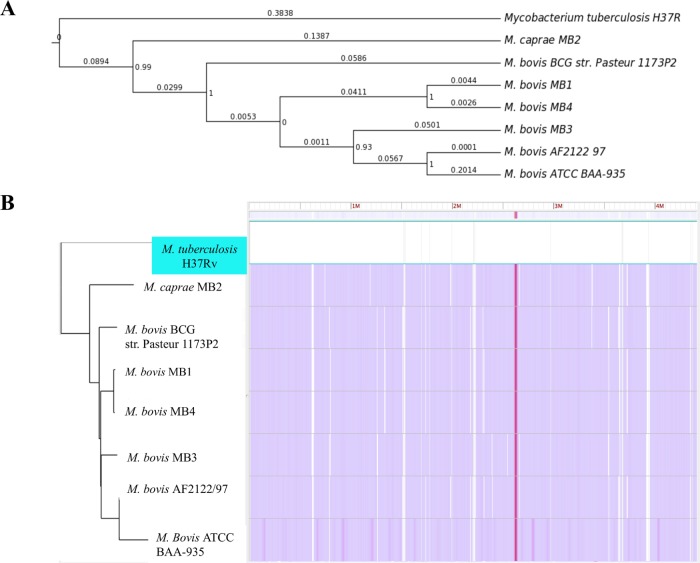
The results of the phylogenetic analysis showed that the MB1 and MB4 isolates with the same spoligotype were the most closely related isolates (Figs 2A and S2). The MB2 isolate (M. caprae) clustered separately from the other isolates, which clustered together with M. bovis sequences. Nevertheless, M. caprae (MB2) was closely related to M. bovis when compared to M. tuberculosis (Fig 2A).
Fig 2. Phylogenetic analysis.
(A) The phylogenetic tree showing the bootstrap scores (at the bifurcations) and the branchlength values (over the branches) was based on the SNPs included in the.vcf file provided by Harvest suite as the result of analyzing the core-genome alignment of the 7 genomes included in the phylogenetic analysis (M. tuberculosis H37Rv was used as reference). The image was built using EvolView. (B) Screenshot of Harvest suite tool displaying the phylogenetic tree and the core-genome multiple alignment for the 7 genomes included in the analysis. Regions with sequence differences and discarded from the SNP analysis are shown in white. The regions included in the SNP analysis are colored with a similarity dependent color scale.
The genome of M. bovis BCG Pasteur 1173P2 was the most similar to all sequenced mycobacteria genomes (Figs 2A, 2B and 3). It has been proposed that during evolution, a clone of M. tuberculosis that was originally adapted to cause human TB evolved to infect a non-human mammal and thus began the transition into non-human ecotypes such as M. bovis, which in turn spread to cattle, goats, oryx, seals and pigs [20]. Our results supported a close relationship between M. bovis isolates and suggested that M. caprae is one of the M. bovis-related mycobacteria adapted to infect goats and sheep as well as other hosts such as wild boar, red deer, cattle and humans [29,48,49].
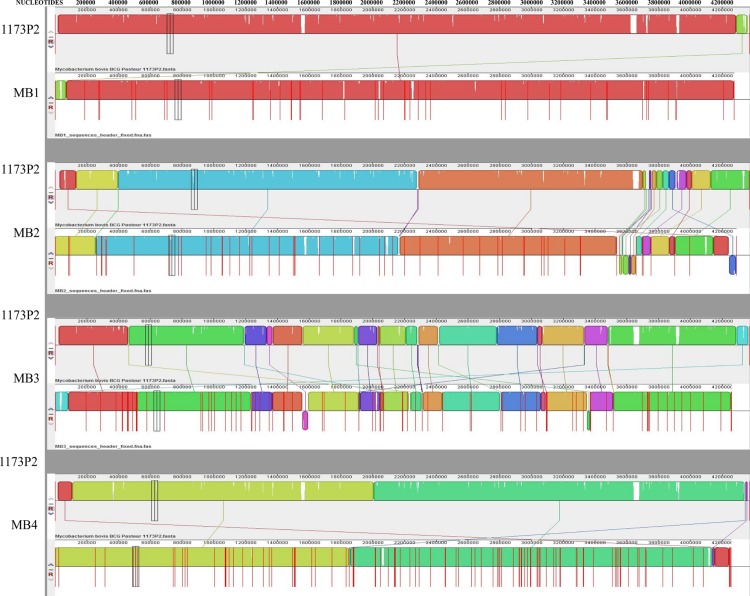
Fig 3. Comparative genomics of MB1-MB4 isolates.
Genome sequence comparison between mycobacteria isolates MB1-MB4 and the most similar reported sequence of M. bovis BCG Pasteur 1173P2 using Differences program.
To better understand the relationship between these isolates, a comparative genomics approach was used. The results showed the presence of translocations, deletions of small genomic regions and SNPs between genomic sequences (Figs 2B, 3 and S2). Large-scale polymorphism studies have demonstrated that the MTBC shows a large number of deletions of small genomic regions consistent with the reductive evolution typical of intracellular bacteria [20]. Nevertheless, sequential chromosomal nucleotide substitutions are considered to be the main driver in the M. tuberculosis genome evolution [20]. The results reported here supported these findings for M. bovis isolates and suggested that the isolates with high lesion score, MB2 and MB3, contained the largest number of polymorphisms when compared to the MB1 and MB4 isolates with low lesion score (Figs 2B and 3). However, a clear correlation between phenotype and genome sequence requires a protein-targeted comparative analysis between the different isolates. For protein-targeted analysis, the study was focused on proteins that are known to play an important role in mycobacterial viability or virulence.
Protein-targeted comparative analysis
Protein-targeted comparative analysis was conducted for (a) the ESX or type VII secretion system, (b) proteins linking stress response with lipid metabolism, (c) host T cell epitopes of mycobacteria, (d) antigens and (e) peptidoglycan assembly protein to define possible correlates with bacterial virulence and viability or distribution.
(a) ESX secretion system family proteins
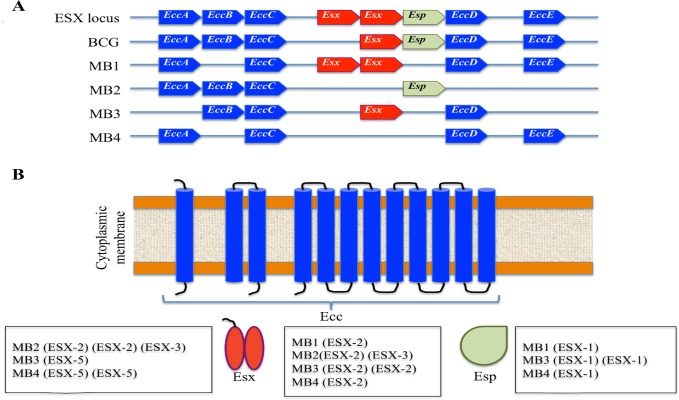
The ESX or type VII secretion system (ESX-1 through ESX-5) proteins were targeted for analysis due to their role in pathogen-host interactions affecting mycobacterial viability (ESX-3, ESX-5 loci) or virulence (ESX-1, ESX-5 loci) and as candidate protective antigens [50,51]. The ESX locus exists as either complete or partial cluster of ESX-coding genes and other components [50–52]. Each ESX complete locus contains 2 ESX secreted protein Esx genes, 4 ESX core component Ecc genes and additional ESX secretion-associated protein Esp genes [50]. Based on the current model described for the ESX system, Esx heterodimers are recognized by EccC, which provides energy to propel substrates through the transmembrane export channel EccD [50].
ESX-coding genes were annotated in the four genomes analyzed resulting in 53 proteins (S5 Table). Of these, 12 ESX-coding genes were differentially represented due to deletions in the 4 mycobacteria genomes characterized here (Table 2 and Fig 4A).
Table 2. Differentially represented ESX-coding genes.
| MB1 | MB2 | MB3 | MB4 | Description | ESX locus |
|---|---|---|---|---|---|
| H8HUI3 | NF | H8HUI3 | H8HUI3 | Putative ESAT-6 like protein EsxD | ESX-2 |
| H8HW13 | H8HW13 | H8HW13 | NF | ESAT-6 like protein 4 (EsxL) | ESX-5 |
| H8I294 | H8I294 | NF | H8I294 | Putative ESAT-6 like protein EsxV | ESX-5 |
| L0PV18 | L0PV18 | L0PV18 | NF | Putative Esat-6 like protein EsxW (ESAT-6 Like Protein 10) | ESX-5 |
| M1J229 | NF | M1J229 | M1J229 | ESAT-6 like protein 11 EsxC | ESX-2 |
| NF | O05449 | O05449 | NF | VESX-2 secretion system protein EccB2 | ESX-2 |
| O05459 | NF | NF | O05459 | ESX-2 secretion system protein EccE2 | ESX-2 |
| O05460 | O05460 | NF | O05460 | ESX-2 secretion system protein EccA2 | ESX-2 |
| NF | O69742 | NF | NF | ESX-1 secretion-associated protein EspJ | ESX-1 |
| S5EZJ9 | S5EZJ9 | NF | S5EZJ9 | Secretion protein EspD | ESX-1 |
| V2V7U1 | NF | V2V7U1 | V2V7U1 | Secretion protein EccD | ESX-3 |
| V2VSA7 | NF | V2VSA7 | V2VSA7 | EsxH | ESX-3 |
Uniprot accession numbers are shown for each reference sequence. Abbreviation: NF, not found.
Fig 4. ESX secretion system.
(A) Organization of Ecc, Esx and Esp genes in the ESX complete ESX-1 –ESX-5 loci, M. bovis BCG Pasteur 1173P2 and the genome of MB1-MB4 mycobacteria isolates. (B) Localization of Ecc, Esx and Esp proteins with reference to the ESX loci with deletions in the genome of MB1-MB4 mycobacteria isolates.
Deletions in the ESX genes included the main components of the ESX system, Ecc, Esx and Esp genes in the ESX-1, ESX-2, ESX-3 and/or ESX-5 loci [50] (Fig 4 and 4B). All Esp gene deletions occurred in MB1, MB3 and MB4 isolates and affected the ESX-1 locus (Fig 4A and 4B). EspD of ESX-1 locus has been shown to be critical for virulence in M. tuberculosis [53], thus suggesting that retention of the Esp gene in the M. caprae MB2 isolate may correlate with its high lesion score when compared to the other sequenced isolates. However, deletion of the Esp gene in the MB3 isolate with high lesion score suggested that this locus might be dispensable for virulence in M. bovis. The attenuation of M. bovis BCG strains has been associated with deletion of the EsxA gene in the ESX-1 locus (Fig 4A) but EsxA or early secreted antigen target protein 6 (ESAT-6) of M. tuberculosis promotes protective T helper 17 cell responses in a Toll-like receptor-2 dependent manner [17,54]. Recently, M. tuberculosis EsxH protein of the ESX-3 system was shown to target a host component of the endosomal sorting complex required for transport (ESCRT) to disrupt delivery of mycobacteria to lysosomes [55]. Interestingly, Esx gene deletions in MB2-MB4 isolates occurred at ESX-2, ESX-3 or ESX-5 loci but not at the ESX-1 locus (Fig 4A and 4B) suggesting that Esx gene deletions in these loci do not affect lesion score produced by M. caprae and M. bovis. Immunity conferred by antigen-specific CD4+ T cells is critical for controlling infection with M. tuberculosis and the ESX-3 locus has been shown to induce maximal responses in the CD4+ T cells screen [50]. This result suggested that deletion in the ESX-3 locus that occurred only in the M. caprae MB2 isolate might affect the host immune protective response to increase lesion score.
In summary, the analysis of the ESX secretion system in the MB1-MB4 isolates suggested a correlation with MB2 mycobacterial viability or distribution and lesion score. While deletions in the ESX-3 locus of M. caprae MB2 isolate might affect mycobacterial viability, they could also increase lesion score in conjunction with retention of the Esp gene of ESX-1. However, these results suggested that these differences could be host-specific so that the same isolate may behave differently in different hosts.
(b) Proteins linking stress response with lipid metabolism
Host stress response induces metabolic changes in mycobacteria that include a switch to the catabolism of host lipids, particularly cholesterol [56]. Recent results have shown that host cells and mycobacteria may interact partly through positive feedback loops, in which responses to the host environment and the digestion of host lipids lead to the production of bacterial immunomodulatory lipids that shape the host environment to increase the availability of host lipids [20]. Following the model recently proposed by Galagan [20] for selected regulatory interactions linking stress responses with changes in lipid metabolism in M. tuberculosis, genes coding for proteins involved in stress sensing (phoP, whiB3, dosR or devR), stress adaptation (lsr2, sigE, Rv0081, Rv0324, Rv3249c), lipid catabolism (kstR, kstD, mcs, kshA) and lipid production (Rv1353c, Rv1776c, pks2, pks3, drrA, drrB, drrC, mmpL7) were selected for analysis (S3 Table).
All genes but the methylcitrate synthase gene (mcs) were found in all genomes analyzed. The mcs gene was not found in any of the MB1-MB4 isolates analyzed nor in the M. bovis reference genome, thus suggesting a deletion of this gene in M. bovis/M. caprae. The mcs gene is important for lipid catabolism [20]. Furthermore, mycobacterial metabolism of propionyl-CoA is also important because accumulated propionate as well as MCS/MCD-generated propionate metabolites are toxic and exert a dominant inhibitory effect on bacterial growth [57]. We speculate that this deletion increases M. bovis susceptibility to lipid deprivation, correlating with the TB-resistant phenotype associated with higher methylmalonyl CoA mutase (MUT) levels in wild boar [56, 58–60]. High MUT levels will reduce host cholesterol and thus put an additional pressure on M. bovis survival in this species [56].
The multilocus sequence analysis was conducted using 18 genes coding for the proteins linking stress response with lipid metabolism (S3 Table). SNPs were found in 6 genes only with few non-synonymous substitutions (Fig 5A). Furthermore, no evidence was found for a positive or negative selection (P>0.05) in the codons where SNPs were identified. Interestingly, the highest number of non-synonymous substitutions was found in the MB2 and MB3 isolates with high lesion score (Fig 5A). The phylogenetic analysis corroborated the results at genome level (Fig 2A) with a strong support for the M. bovis/M. caprae clade (Fig 5B).
Fig 5. Genes coding for the proteins linking stress response with lipid metabolism.
(A) SNPs detected in mycobacteria isolates MB1-MB4 with M. bovis AF2122/97 reference genome sequence. Non-synonymous substitutions are shown in red. Gene No. refers to row number in S3 Table. (B) Multilocus sequence analysis. The nucleotide sequences of the selected genes were concatenated and aligned to construct a maximum likelihood phylogenetic tree. Numbers of internal branches represent genetic distances. The reliability for the internal branches was assessed using the approximate likelihood ratio test (aLRT–SH-Like). The clade of M. bovis/M. caprae (red dot) is supported by an aLRT–SH-Like value of 87. M. canettii was used as outgroup. The image was built using EvolView.
These results showed that genes coding for the proteins linking stress response with lipid metabolism are highly conserved in M. bovis/M.caprae and suggested that deletion of the mcs gene may increase susceptibility to lipid deprivation in these mycobacteria. However, some of the non-synonymous substitutions found in MB2 and MB3 may provide an advantage resulting in higher lesion score for these isolates.
(c) Host T cell epitopes of mycobacteria
The experimentally confirmed human T cell epitopes of M. tuberculosis [17] were analyzed in the MB1-MB4 genomes. These epitopes are hyperconserved in M. tuberculosis and M. africanum strains consistent with strong purifying selection acting on these epitopes. As discussed by Comas et al. [17], MTBC might benefit from recognition by human T cells because this essential response for host survival may be necessary for mycobacteria to establish latent infection. Thus, T lymphocyte recognition is an important factor in hyperconservation of these sequences and hence other structural or functional constraints are unlikely to fully account for the lack of sequence variation in these domains.
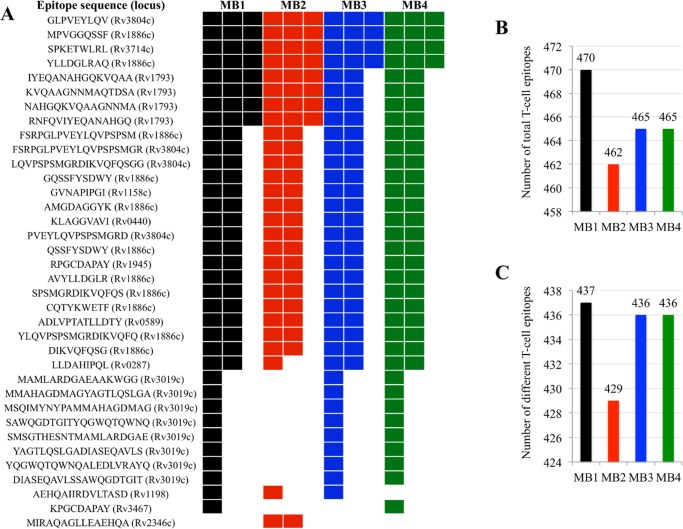
Of the 491 epitopes included in the analysis [17], 438 were identified in the MB1-MB4 isolates sequenced here with 100% sequence identity to M. tuberculosis sequences (S6 Table). These results extended the hyperconservation of these epitopes to M. bovis/M. caprae and suggested that similar mechanisms may function in other species of the MTBC. However, several specific patterns of epitope repetitions and deletions were found in the studied isolates (Fig 6A). Interestingly, MB2 was the isolate with the lowest number of both total (Fig 6B) and different (Fig 6C) T cell epitopes, correlating with the lowest distribution of this isolate (Table 1) thus providing additional support for the role of the T cell response in mycobacterial transmission. One limitation of this analysis is the fact that the T cell epitopes used are confirmed in humans but not in other hosts susceptible to M. bovis/M caprae infection. However, the approach used in this study provided an initial assessment of the presence and possible role of the host T cell epitopes in M. bovis/M. caprae infection and transmission.
Fig 6. Host T cell epitopes of mycobacteria.
(A) Organization of the repeated/deleted epitopes in the different sequenced mycobacteria isolates. The locus name of the antigen in the H37Rv genome for T-cell epitope containing antigens [17] is shown in parenthesis after epitope sequence. The protein annotation according to the Immune Epitope Database (IEDB; http://www.iedb.org) can be found in S4 Table. Each column represents epitope repeat or deletion. (B) Total number of T cell epitopes including repetitions identified on each mycobacteria. (C) Number of different T cell epitopes identified on each mycobacteria.
(d) Antigens
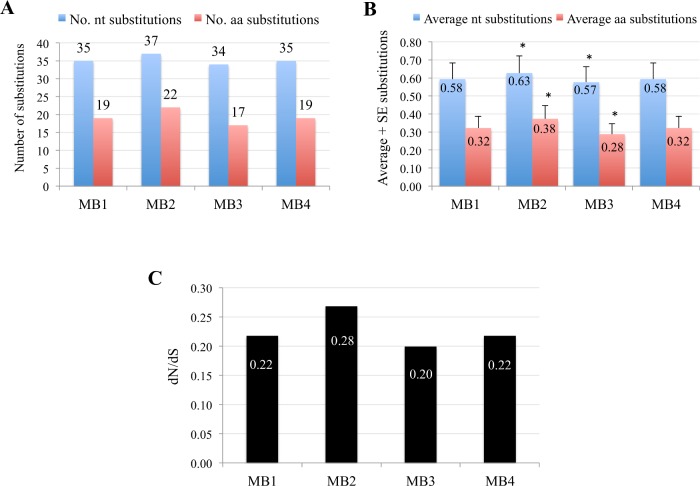
The 81 antigens conserved in MB1-MB4 isolates containing the confirmed human T cell epitopes of M. tuberculosis [17] or inducing an antibody response against inactivated M. bovis [8] were selected for further characterization using the number of nucleotide and amino acid substitutions in each of the isolates (S4 Table). Of them, 39 were conserved 100% at the nucleotide sequence and 33 showed polymorphisms when compared to the M. tuberculosis H37Rv sequence (S4 Table). The results showed that as in M. tuberculosis [17] the antigen-coding genes were evolutionarily hyperconserved with a low number of nucleotide and amino acid substitutions (Fig 7A). However, the average number of substitutions at both nucleotide and amino acid levels were significantly (P<0.0001) higher and lower for MB2 and MB3 isolates, respectively with no differences between MB1 and MB4 isolates (Fig 7B). However, the values for dN/dS ratios were higher for M. tuberculosis (0.5; [17]) than for M. bovis/M. caprae (0.20–0.28; Fig 7C) thus suggesting that these genes are under a strong purifying selection in these species.
Fig 7. Nucleotide substitution rate analysis in antigen-coding genes.
(A) The total number and (B) the average + standard error (SE) of nucleotide (nt) and amino acid (aa) substitutions were calculated in 81 antigen-coding genes conserved in MB1-MB4 isolates using M. tuberculosis H37Rv as a reference. Average values were compared between isolated using a χ2 test (*P<0.0001). (C) The dN/dS ratio was determined on the basis of the number of non-redundant synonymous and non-synonymous changes using the SLAC algorithm and a two-tailed extended binomial distribution was used to assess significance of the algorithm (P<0.05).
As reported for M. tuberculosis [17], despite high sequence conservation in these antigens, sequencing revealed a subset of antigens that did show variation (Table 3). These antigens included proteins that are involved in bacterial infection (MCE-family protein MCE2a, Fibronectin attachment protein; [61,62]), induction of host immune response to infection (ESAT-6 like protein 4 EsxL; [17,54]), clustered regularly interspaced short palindromic repeats (CRISPRs) bacterial adaptive immunity against mobile genetic elements (CRISPR-associated protein Cas10/Csm1; [63]) and recombination and DNA repair (ATP-dependent DNA helicase; [64]) and thus may warrant further study in the context of vaccine discovery.
Table 3. Antigens with high sequence variation.
| Sequence ID (H37Rv) | Description | Number of amino acids substitutions | |||
|---|---|---|---|---|---|
| MB1 | MB2 | MB3 | MB4 | ||
| Rv0589 | MCE-family protein MCE2a | 3 | 2 | 2 | 3 |
| Rv1198 | ESAT-6 like protein 4 EsxL | 2 | 1 | 2 | 2 |
| Rv1860 | Fibronectin attachment protein FAP-B | 1 | 2 | 1 | 1 |
| Rv2823c | CRISPR-associated protein Cas10/Csm1 | 2 | 2 | 0 | 2 |
| Rv3296 | ATP-dependent helicase | 2 | 1 | 3 | 2 |
Protein sequence diversity among all isolates was determined in comparison to M. tuberculosis H37Rv. Antigens selected showed an average amino acid diversity > 1.
(e) Peptidoglycan assembly protein ponA1 (Rv0050)
The M. tuberculosis hypervariable locus Rv0050 coding for PonA1 was targeted for analysis due to its role in glycan chain assembly and peptide cross-linking of bacterial peptidoglycan. PonA1 is a member of a family of penicillin binding proteins that may be essential to mycobacterial survival [65,66]. Although known to be hypervariable among bacteria [67,68], Rv0050 was very conserved (>99%; 698/704) among all M. bovis strains. A cluster of three non-synonymous mutations at the N terminus of the protein was identified for MB1 to MB4 compared to M. tuberculosis H37Rv (S3A Fig). The analysis of the N terminus region of PonA1 revealed a putative inner membrane domain and a cleavage site between amino acids 31 and 32 (S3A and S3B Fig), which exhibited homology to M. leprae LprE [46]. The isolate with high lesion score, MB3, had two mutations in the putative inner membrane domain P24L and T29M. The other isolate with high lesion score, MB2 (M. caprae), did not contain the mutation P24L but had instead mutation T27I in the putative inner membrane domain closer to the putative cleavage site located between amino acids 31 and 32. Mutations P24L, T27I, and T29M all exchanged small (P or T) residues with very hydrophobic residues (L, I, and M) that may favor insertion/delivery of PonA1 to the bacterial membrane. All three mutations may also change the way PonA1 may be cleaved as substitutions upstream of the cleavage site have a bigger penalty in mycobacteria [69].
MB1 and MB4 contained only mutation P24L that is present in all M. bovis isolates infecting animals. Only M. bovis human isolate BZ3115 contained mutation P24L (S3A Fig) while all other human isolates of M. bovis had a sequence identical to strains of M. tuberculosis in the complex (wild type P at position 24; S3A Fig). The selective pressure for mutations on PonA1 may affect uniquely strains of M. bovis infecting animals, as all other members of the MTBC including strains of M. bovis infecting humans have identical wild type sequences. A possible ancestry [20] in strains of M. bovis infecting humans and M. tuberculosis is also supported through strain BZ3115 PonA1 sequence (S3A Fig).
To confirm some of the SNPs identified in the mycobacteria genomes sequenced in this study, targeted PCR and sequence analysis were conducted for Rv0050 (ponA1), Rv0589 (MCE2a), Rv1198 (ESAT-6) and Rv1860 (FAP-B) loci with potential interest for TB disease risk assessment and control. The results corroborated the predictions of the genome sequencing for the MB1-MB4 isolates included in the study, thus providing additional support for the results presented and discussed in the paper (S4 Fig).
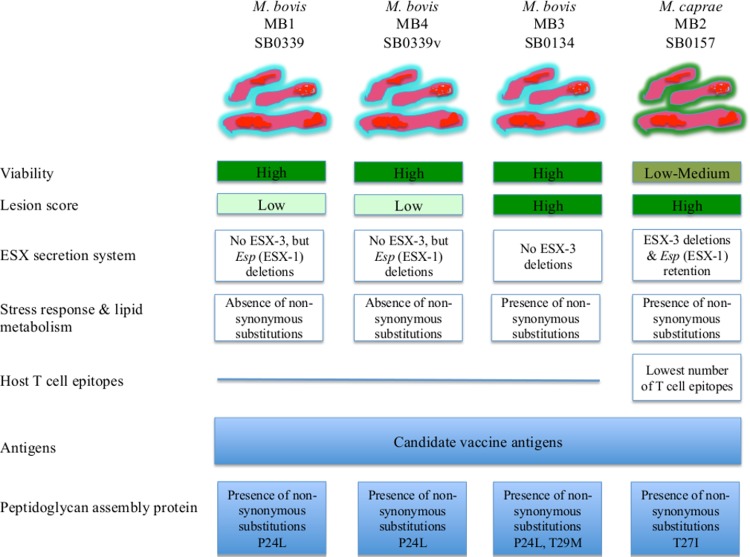
In summary, this is the first comparative genomics study of field isolates of M. bovis including for the first time M. caprae. Three M. bovis (MB1, MB3, MB4) and one M. caprae (MB2) isolates with different distribution and lesion score were selected for genome sequencing and annotation (Fig 8). Comparative genomics showed that as previously reported for M. tuberculosis, sequential chromosomal nucleotide substitutions were the main driver of the M. bovis genome evolution. The phylogenetic analysis provided a strong support for the M. bovis/M. caprae clade, but supported M. caprae as a separate species. The comparison of the MB1 and MB4 isolates revealed differences in genome sequence, including gene families that are important for bacterial infection and transmission, thus highlighting differences with functional implications between MTBC isolates otherwise classified with the same spoligotype. Protein-targeted comparative analysis was conducted for (a) the ESX or type VII secretion system, (b) proteins linking stress response with lipid metabolism, (c) host T cell epitopes of mycobacteria, (d) antigens and (e) penicillin binding protein to define possible correlates with bacterial distribution and lesion score. The analysis of the ESX secretion systems provided possible correlates for MB2 low-medium distribution (ESX-3 deletions) and high lesion score (retention of ESX-1 Esp) but suggested that these differences could be host-specific so that the same isolate might behave differently in different hosts (Fig 8). These results showed that genes coding for the proteins linking stress response with lipid metabolism are highly conserved in M. bovis/M.caprae and suggested that deletion of the mcs gene may increase susceptibility to lipid deprivation in these mycobacteria. However, some of the non-synonymous substitutions found in MB2 and MB3 may provide an advantage resulting in higher lesion score for these isolates (Fig 8). MB2 was the isolate with the lowest number of T cell epitopes, correlating with the lowest distribution of this isolate if we consider that recognition by T cells could be essential to establish mycobacterial latent infection [17] (Fig 8). The genes coding for mycobacterial antigens were highly conserved and under a strong purifying selection in M. bovis/M.caprae, thus extending previous results in M. tuberculosis. The polymorphisms provided new candidate vaccine antigens. The comparison of PonA1 sequences among MB1-MB4 isolates suggested a possible correlation with their phenotypic variation with possible implications for TB disease risk assessment and control (Fig 8). The accumulation of mutations at the putative inner membrane protein domain suggested that the retention of mutations that affected delivery/insertion or cleavage of ponA1 at the membrane might have been the result of the forces driving selection. Because of the role of PonA1 in cell wall assembly, the described mutations may have a link to environmental selective pressures such as transmission and pathogenesis. These results identified new genetic markers and candidate vaccine antigens that warrant further study providing additional experimental data to develop tools to evaluate risks for TB disease caused by M. bovis /M.caprae and for TB control in humans and animals.
Fig 8. Summary of the results of strategic protein-targeted comparative analysis.
Protein-targeted comparative analysis was conducted for (a) the ESX or type VII secretion system, (b) proteins linking stress response with lipid metabolism, (c) host T cell epitopes of mycobacteria, (d) antigens and (e) penicillin binding protein to define possible correlates with bacterial distribution and lesion score and to identify genetic markers and candidate antigens for TB disease risk assessment and control.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLS)
(XLSX)
(DOCX)
(DOCX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by grants AGL2014-56305 and IPT-2011-0735-010000 from Ministerio de Economía y Competitividad, Spain, and the European Union FP7 ANTIGONE grant 278976 and Horizon 2020 COMPARE Grant 377/14. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Parrish NM, Dick JD, Bishai WR. Mechanisms of latency in Mycobacterium tuberculosis . Trends Microbiol. 1998;6:107–12. [DOI] [PubMed] [Google Scholar]
- 2. Gortazar C, Torres MJ, Acevedo P, Aznar J, Negro JJ, De La Fuente J, et al. Fine-tuning the space, time, and host distribution of mycobacteria in wildlife. BMC Microbiol. 2011;11:27 10.1186/1471-2180-11-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gortazar C, Delahay RJ, McDonald RA, Boadella M, Wilson GJ, Gavier-Widen D, et al. The status of tuberculosis in European wild mammals. Mamm Rev. 2012;42:193–206. [Google Scholar]
- 4. Ryan TJ, Livingstone PG, Ramsey DSL, De Lisle GW, Nugent G, Collins DM, et al. Advances in understanding disease epidemiology and implications for control and eradication of tuberculosis in livestock: The experience from New Zealand. Vet Microbiol. 2006;112:211–9. [DOI] [PubMed] [Google Scholar]
- 5. Naranjo V, Gortazar C, Vicente J, de la Fuente J. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet Microbiol. 2008;127:1–9. [DOI] [PubMed] [Google Scholar]
- 6. Ballesteros C, Garrido JM, Vicente J, Romero B, Galindo RC, Minguijón E, et al. First data on Eurasian wild boar response to oral immunization with BCG and challenge with a Mycobacterium bovis field strain. Vaccine. 2009;27:6662–8. 10.1016/j.vaccine.2009.08.095 [DOI] [PubMed] [Google Scholar]
- 7. Garrido JM, Sevilla IA, Beltrán-Beck B, Minguijón E, Ballesteros C, Galindo RC, et al. Protection against tuberculosis in Eurasian wild boar vaccinated with heat-inactivated Mycobacterium bovis . PLoS ONE. 2011;6(9):e24905 10.1371/journal.pone.0024905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beltrán-Beck B, De La Fuente J, Garrido JM, Aranaz A, Sevilla I, Villar M, et al. Oral vaccination with heat inactivated Mycobacterium bovis activates the complement system to protect against tuberculosis. PLoS ONE. 2014;9(5): e98048 10.1371/journal.pone.0098048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmitt SM, Fitzgerald SD, Cooley TM, Bruning-Fann CS, Sullivan L, Berry D, et al. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J Wildl Dis. 1997;33:749–58. [DOI] [PubMed] [Google Scholar]
- 10. Delahay RJ, De Leeuw ANS, Barlow AM, Clifton-Hadley RS, Cheeseman CL. The status of Mycobacterium bovis infection in UK wild mammals: A review. Vet J. 2002;164:90–105. [DOI] [PubMed] [Google Scholar]
- 11. Vicente J, Barasona JA, Acevedo P, Ruiz-Fons JF, Boadella M, Diez-Delgado I, et al. Temporal trend of tuberculosis in wild ungulates from mediterranean Spain. Transbound Emerg Dis. 2013;60:92–103. 10.1111/tbed.12167 [DOI] [PubMed] [Google Scholar]
- 12. Nugent G, Buddle BM, Knowles G. Epidemiology and control of Mycobacterium bovis infection in brushtail possums (Trichosurus vulpecula), the primary wildlife host of bovine tuberculosis in New Zealand. N Z Vet J. 2015; 63:28–41. 10.1080/00480169.2014.963791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gortazar C, Diez-Delgado I, Barasona JA, Vicente J, de la Fuente J, Boadella M. The wild side of disease control at the wildlife-livestock-human interface: a review. Front Vet Sci. 2015;1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russell DG. Who puts the tubercle in tuberculosis? Nat Rev Microbiol. 2007;5:39–47. [DOI] [PubMed] [Google Scholar]
- 15. Garbaccio S, Macias A, Shimizu E, Paolicchi F, Pezzone N, Magnano G, et al. Association between spoligotype-VNTR types and virulence of Mycobacterium bovis in cattle. Virulence. 2014;5:297–302. 10.4161/viru.27193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–44. [DOI] [PubMed] [Google Scholar]
- 17. Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. 2010;42:498–503. 10.1038/ng.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. 2013;45:1176–82. 10.1038/ng.2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garnier T, Eiglmeier K, Camus J-C, Medina N, Mansoor H, Pryor M, et al. The complete genome sequence of Mycobacterium bovis . Proc Natl Acad Sci. 2003;100:7877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galagan JE. Genomic insights into tuberculosis. Nat Rev Gen. 2014;15:307–20. [DOI] [PubMed] [Google Scholar]
- 21. Gonzalo-Asensio J, Malaga W, Pawlik A, Astarie-Dequeker C, Passemar C, Moreau F, et al. Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc Natl Acad Sci U S A. 2014;111:11491–6. 10.1073/pnas.1406693111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martínez-López B, Barasona JA, Gortázar C, Rodríguez-Prieto V, Sánchez-Vizcaíno JM, Vicente J. Farm-level risk factors for the occurrence, new infection or persistence of tuberculosis in cattle herds from South-Central Spain. Prev Vet Med. 2014;116:268–78. 10.1016/j.prevetmed.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 23. Gortazar C, Vicente J, Samper S, Garrido JM, Fernandez-de-Mera IG, Gavin P, et al. Molecular characterization of Mycobacterium tuberculosis complex isolates from wild ungulates in south-central Spain. Vet Res. 2005;36:43–52. [DOI] [PubMed] [Google Scholar]
- 24. Kamerbeek J, Schouls L, Kolk A, vanAgterveld M, vanSoolingen D, Kuijper S, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanaka MM, Francis AR. Detecting emerging strains of tuberculosis by using spoligotypes. Proc Natl Acad Sci U S A. 2006;103:15266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulldorff M. SaTScan User Guide for version 6.0 2005. Available at: www.satscan.org.
- 27. Aranaz A, Liebana E, Gomez-Mampaso E, Galán JC, Cousins D, Ortega A, et al. Mycobacterium tuberculosis subsp. caprae subsp. nov.: A taxonomic study of a new member of the Mycobacterium tuberculosis complex isolated from goats in Spain. Int J Syst Bacteriol. 1999;49:1263–73. [DOI] [PubMed] [Google Scholar]
- 28. Gortazar C, Vicente J, Samper S, Garrido JM, Fernandez-de-Mera IG, Gavin P, et al. Molecular characterization of Mycobacterium tuberculosis complex isolates from wild ungulates in south-central Spain. Vet Res. 2005;36:43–52. [DOI] [PubMed] [Google Scholar]
- 29. Aranaz A, Cousins D, Mateos A, Domínguez L. Elevation of Mycobacterium tuberculosis subsp. caprae Aranaz et al. 1999 to species rank as Mycobacterium caprae comb. nov., sp. nov . Int J Syst Evol Microbiol. 2003;53:1785–9. [DOI] [PubMed] [Google Scholar]
- 30. Rodríguez S, Bezos J, Romero B, de Juan L, Álvarez J, Castellanos E, et al. Mycobacterium caprae infection in livestock and wildlife, Spain. Emerg Infect Dis. 2011;17:532–5. 10.3201/eid1703.100618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Soolingen D, Hermans PWM, De Haas PEW, Soll DR, Van Embden JDA. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: Evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zerbino DR. Using the Velvet de novo assembler for short-read sequencing technologies. Curr Protoc Bioinform. 2010; Chapter 11: Unit 11.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pareja-Tobes P, Manrique M, Pareja-Tobes E, Pareja E, Tobes R. BG7: A New Approach for Bacterial Genome Annotation Designed for Next Generation Sequencing Data. PLoS ONE. 2012;7(11): e49239 10.1371/journal.pone.0049239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tobes R, Pareja-Tobes P, Manrique M, Pareja-Tobes E, Kovach E, Alekhin A, et al. Gene calling and bacterial genome annotation with BG7. Methods Mol Biol: Humana Press Inc.; 2015. p. 177–89. 10.1007/978-1-4939-1720-4_12 [DOI] [PubMed] [Google Scholar]
- 35. Liu B, Pop M. ARDB—Antibiotic resistance genes database. Nucleic Acids Res. 2009;37:D443–7. 10.1093/nar/gkn656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de la Fuente J., Díez-Delgado I., Contreras M., Vicente J., Cabezas-Cruz A., Manrique M., Tobes R., López V., Romero B., Domínguez L., Garrido J.M., Juste R., Gortazar C. Complete genome sequence of field isolates of Mycobacterium bovis and Mycobacterium caprae from naturally infected Eurasian wild boar (Sus scrofa). Genome Announc. 2015;3(3):e00247–15. 10.1128/genomeA.00247-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Treangen TJ, Ondov BD, Koren S, Phillippy AM. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014;15:524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang H, Gao S, Lercher MJ, Hu S, Chen WH. EvolView, an online tool for visualizing, annotating and managing phylogenetic trees. Nucleic Acids Res. 2012;40:W569–72. 10.1093/nar/gks576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guindon S, Gascuel O. A Simple, Fast, and Accurate Algorithm to Estimate Large Phylogenies by Maximum Likelihood. Syst Biol. 2003;52:696–704. [DOI] [PubMed] [Google Scholar]
- 41. Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst Biol. 2006;55:539–52. [DOI] [PubMed] [Google Scholar]
- 42. Korber B. HIV Signature and Sequence Variation Analysis In: Rodrigo AG. and Learn GH, editors. Computational Analysis of HIV Molecular Sequences. Kluwer Academic Publishers; 2000. p. 55–72. [Google Scholar]
- 43. Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–26. [DOI] [PubMed] [Google Scholar]
- 44. Delport W, Poon AFY, Frost SDW, Kosakovsky Pond SL. Datamonkey 2010: A suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics. 2010;26:2455–7. 10.1093/bioinformatics/btq429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kosakovsky Pond SL, Frost SDW. Not so different after all: A comparison of methods for detecting amino acid sites under selection. Mol Biol Evol. 2005;22:1208–22. [DOI] [PubMed] [Google Scholar]
- 46. Leversen NA, de Souza GA, Målen H, Prasad S, Jonassen I, Wiker HG. Evaluation of signal peptide prediction algorithms for identification of mycobacterial signal peptides using sequence data from proteomic methods. Microbiol. 2009;155:2375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rodríguez S, Romero B, Bezos J, de Juan L, Álvarez J, Castellanos E, et al. High spoligotype diversity within a Mycobacterium bovis population: Clues to understanding the demography of the pathogen in Europe. Vet Microbiol. 2010;141:89–95. 10.1016/j.vetmic.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 48. Müller B, Dürr S, Alonso S, Hattendorf J, Laisse CJM, Parsons SDC, et al. Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg Infect Dis. 2013;19:899–908. 10.3201/eid1906.120543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prodinger WM, Indra A, Koksalan OK, Kilicaslan Z, Richter E. Mycobacterium caprae infection in humans. Expert Rev Anti-Infect Ther. 2014;12:1501–13. 10.1586/14787210.2014.974560 [DOI] [PubMed] [Google Scholar]
- 50. Kunnath-Velayudhan S, Porcelli SA. Recent Advances in Defining the Immunoproteome of Mycobacterium tuberculosis. Front Immunol. 2013;4:335 10.3389/fimmu.2013.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bottai D, Serafini A, Cascioferro A, Brosch R, Manganelli R. Targeting type VII/ESX secretion systems for development of novel antimycobacterial drugs. Curr Pharm Des. 2014; 20: 4346–56. [DOI] [PubMed] [Google Scholar]
- 52. Uplekar S, Heym B, Friocourt V, Rougemont J, Cole ST. Comparative genomics of ESX genes from clinical isolates of Mycobacterium tuberculosis provides evidence for gene conversion and epitope variation. Infect Immun. 2011;79:4042–9. 10.1128/IAI.05344-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen JM, Boy-Röttger S, Dhar N, Sweeney N, Buxton RS, Pojer F, et al. EspD is critical for the virulence-mediating ESX-1 secretion system in Mycobacterium tuberculosis . J Bacteriol. 2012;194:884–93. 10.1128/JB.06417-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chatterjee S, Dwivedi VP, Singh Y, Siddiqui I, Sharma P, van Kaer L, et al. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog. 2011;7(11): e1002378 10.1371/journal.ppat.1002378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mehra A, Zahra A, Thompson V, Sirisaengtaksin N, Wells A, Porto M, et al. Mycobacterium tuberculosis Type VII Secreted Effector EsxH Targets Host ESCRT to Impair Trafficking. PLoS Pathog. 2013;9(10): e1003734 10.1371/journal.ppat.1003734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de la Fuente J, Gortazar C, Vicente J, Villar M. Host expression of methylmalonyl-CoA mutase and tuberculosis: A missing link? Med Hypotheses. 2011;76:361–4. 10.1016/j.mehy.2010.10.040 [DOI] [PubMed] [Google Scholar]
- 57. Upton AM, McKinney JD. Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis . Microbiol. 2007;153:3973–82. [DOI] [PubMed] [Google Scholar]
- 58. Naranjo V, Höfle U, Vicente J, Martín MP, Ruiz-Fons F, Gortazar C, et al. Genes differentially expressed in oropharyngeal tonsils and mandibular lymph nodes of tuberculous and nontuberculous European wild boars naturally exposed to Mycobacterium bovis . FEMS Immunol Med Microbiol. 2006;46:298–312. [DOI] [PubMed] [Google Scholar]
- 59. Naranjo V, Ayoubi P, Vicente J, Ruiz-Fons F, Gortazar C, Kocan KM, et al. Characterization of selected genes upregulated in non-tuberculous European wild boar as possible correlates of resistance to Mycobacterium bovis infection. Vet Microbiol. 2006;116:224–31. [DOI] [PubMed] [Google Scholar]
- 60. Naranjo V, Acevedo-Whitehouse K, Vicente J, Gortazar C, De La Fuente J. Influence of methylmalonyl-CoA mutase alleles on resistance to bovine tuberculosis in the European wild boar (Sus scrofa). Anim Genet. 2008;39:316–20. 10.1111/j.1365-2052.2008.01725.x [DOI] [PubMed] [Google Scholar]
- 61. Haile Y, Caugant DA, Bjune G, Wiker HG. Mycobacterium tuberculosis mammalian cell entry operon (mce) homologs in Mycobacterium other than tuberculosis (MOTT). FEMS Immunol Med Microbiol. 2002;33:125–32. [DOI] [PubMed] [Google Scholar]
- 62. Secott TE, Lin TL, Wu CC. Fibronectin attachment protein is necessary for efficient attachment and invasion of epithelial cells by Mycobacterium avium subsp. paratuberculosis . Infect Immun. 2002;70:2670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hatoum-Aslan A, Maniv I, Samai P, Marraffini LA. Genetic characterization of antiplasmid immunity through a type III-A CRISPR-cas system. J Bacteriol. 2014;196:310–7. 10.1128/JB.01130-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sinha KM, Stephanou NC, Gao F, Glickman MS, Shuman S. Mycobacterial UvrD1 is a Ku-dependent DNA helicase that plays a role in multiple DNA repair events, including double-strand break repair. J Biol Chem. 2007;282:15114–25. [DOI] [PubMed] [Google Scholar]
- 65. Patru MM, Pavelka MS Jr. A role for the class a penicillin-binding protein PonA2 in the survival of Mycobacterium smegmatis under conditions of nonreplication. J Bacteriol. 2010;192:3043–54. 10.1128/JB.00025-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Qin L, Wang J, Zheng R, Lu J, Yang H, Liu Z, et al. Perspective on sequence evolution of microsatellite locus (CCG) nin Rv0050 gene from Mycobacterium tuberculosis . BMC Evol Biol. 2011;11:247 10.1186/1471-2148-11-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Goffin C, Ghuysen JM. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol Mol Biol Rev. 1998;62:1079–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zapun A, Contreras-Martel C, Vernet T. Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol Rev. 2008;32:361–85. 10.1111/j.1574-6976.2007.00095.x [DOI] [PubMed] [Google Scholar]
- 69. de Souza GA, Leversen NA, Målen H, Wiker HG. Bacterial proteins with cleaved or uncleaved signal peptides of the general secretory pathway. J Proteomics. 2011;75:502–10. 10.1016/j.jprot.2011.08.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLS)
(XLSX)
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.