Abstract
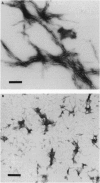
We synthesized polypeptides corresponding to sequences encoded by normal and mutant alleles in the regions of codon 178 (Asp-->Asn) and codon 200 (Glu-->Lys) of the chromosome 20 amyloid gene that have been linked to familial Creutzfeldt-Jakob disease. Peptide suspensions from both regions spontaneously formed amyloid fibrils with different morphological characteristics and aggregation tendencies. Fibrillar arrays were denser and more profuse in mutant than in normal peptide suspensions and were even more marked when the homologous mutant and normal peptides were mixed together. Preparations from the region of codon 200 were in all cases more fibrillogenic than corresponding peptides from the region of codon 178. These in vitro observations support the hypothesis that amino acid changes from pathogenic single-allele point mutations in Creutzfeldt-Jakob disease may nucleate the in vivo folding behavior of the normal host protein to favor formation of insoluble amyloid fibrils.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow C. J., Zagorski M. G. Solution structures of beta peptide and its constituent fragments: relation to amyloid deposition. Science. 1991 Jul 12;253(5016):179–182. doi: 10.1126/science.1853202. [DOI] [PubMed] [Google Scholar]
- Brown P. The phenotypic expression of different mutations in transmissible human spongiform encephalopathy. Rev Neurol (Paris) 1992;148(5):317–327. [PubMed] [Google Scholar]
- Burdick D., Soreghan B., Kwon M., Kosmoski J., Knauer M., Henschen A., Yates J., Cotman C., Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/beta amyloid peptide analogs. J Biol Chem. 1992 Jan 5;267(1):546–554. [PubMed] [Google Scholar]
- Castaño E. M., Ghiso J., Prelli F., Gorevic P. D., Migheli A., Frangione B. In vitro formation of amyloid fibrils from two synthetic peptides of different lengths homologous to Alzheimer's disease beta-protein. Biochem Biophys Res Commun. 1986 Dec 15;141(2):782–789. doi: 10.1016/s0006-291x(86)80241-8. [DOI] [PubMed] [Google Scholar]
- Colon W., Kelly J. W. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992 Sep 15;31(36):8654–8660. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- Colon W., Kelly J. W. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992 Sep 15;31(36):8654–8660. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- Englebretsen D. R., Harding D. R. Solid phase peptide synthesis on hydrophilic supports. Part II--Studies using Perloza beaded cellulose. Int J Pept Protein Res. 1992 Dec;40(6):487–496. [PubMed] [Google Scholar]
- Fraser P. E., Nguyen J. T., Inouye H., Surewicz W. K., Selkoe D. J., Podlisny M. B., Kirschner D. A. Fibril formation by primate, rodent, and Dutch-hemorrhagic analogues of Alzheimer amyloid beta-protein. Biochemistry. 1992 Nov 10;31(44):10716–10723. doi: 10.1021/bi00159a011. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Transmissible and non-transmissible amyloidoses: autocatalytic post-translational conversion of host precursor proteins to beta-pleated sheet configurations. J Neuroimmunol. 1988 Dec;20(2-3):95–110. doi: 10.1016/0165-5728(88)90140-3. [DOI] [PubMed] [Google Scholar]
- Gasset M., Baldwin M. A., Lloyd D. H., Gabriel J. M., Holtzman D. M., Cohen F., Fletterick R., Prusiner S. B. Predicted alpha-helical regions of the prion protein when synthesized as peptides form amyloid. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10940–10944. doi: 10.1073/pnas.89.22.10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb L. G., Petersen R. B., Tabaton M., Brown P., LeBlanc A. C., Montagna P., Cortelli P., Julien J., Vital C., Pendelbury W. W. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: disease phenotype determined by a DNA polymorphism. Science. 1992 Oct 30;258(5083):806–808. doi: 10.1126/science.1439789. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Castano E. M., Sarma R., Frangione B. Ten to fourteen residue peptides of Alzheimer's disease protein are sufficient for amyloid fibril formation and its characteristic x-ray diffraction pattern. Biochem Biophys Res Commun. 1987 Sep 15;147(2):854–862. doi: 10.1016/0006-291x(87)91008-4. [DOI] [PubMed] [Google Scholar]
- Halverson K., Fraser P. E., Kirschner D. A., Lansbury P. T., Jr Molecular determinants of amyloid deposition in Alzheimer's disease: conformational studies of synthetic beta-protein fragments. Biochemistry. 1990 Mar 20;29(11):2639–2644. doi: 10.1021/bi00463a003. [DOI] [PubMed] [Google Scholar]
- Hilbich C., Kisters-Woike B., Reed J., Masters C. L., Beyreuther K. Aggregation and secondary structure of synthetic amyloid beta A4 peptides of Alzheimer's disease. J Mol Biol. 1991 Mar 5;218(1):149–163. doi: 10.1016/0022-2836(91)90881-6. [DOI] [PubMed] [Google Scholar]
- Hollosi M., Otvos L., Jr, Kajtar J., Percel A., Lee V. M. Is amyloid deposition in Alzheimer's disease preceded by an environment-induced double conformational transition? Pept Res. 1989 Jan-Feb;2(1):109–113. [PubMed] [Google Scholar]
- Kirschner D. A., Inouye H., Duffy L. K., Sinclair A., Lind M., Selkoe D. J. Synthetic peptide homologous to beta protein from Alzheimer disease forms amyloid-like fibrils in vitro. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6953–6957. doi: 10.1073/pnas.84.19.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Yamaguchi K., Doh-ura K., Tateishi J. A prion protein missense variant is integrated in kuru plaque cores in patients with Gerstmann-Sträussler syndrome. Neurology. 1991 Feb;41(2 ):306–310. doi: 10.1212/wnl.41.2_part_1.306. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Nurmiaho-Lassila E. L. Creation of amyloid fibrils from mutant Asn187 gelsolin peptides. Biochem Biophys Res Commun. 1992 Feb 28;183(1):227–231. doi: 10.1016/0006-291x(92)91632-z. [DOI] [PubMed] [Google Scholar]
- McPherson A., Shlichta P. Heterogeneous and epitaxial nucleation of protein crystals on mineral surfaces. Science. 1988 Jan 22;239(4838):385–387. doi: 10.1126/science.239.4838.385. [DOI] [PubMed] [Google Scholar]
- Prelli F., Levy E., van Duinen S. G., Bots G. T., Luyendijk W., Frangione B. Expression of a normal and variant Alzheimer's beta-protein gene in amyloid of hereditary cerebral hemorrhage, Dutch type: DNA and protein diagnostic assays. Biochem Biophys Res Commun. 1990 Jul 16;170(1):301–307. doi: 10.1016/0006-291x(90)91274-v. [DOI] [PubMed] [Google Scholar]
- Westermark P., Sletten K., Olofsson B. O. Prealbumin variants in the amyloid fibrils of Swedish familial amyloidotic polyneuropathy. Clin Exp Immunol. 1987 Sep;69(3):695–701. [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T., Ghiso J., Frangione B. Peptides homologous to the amyloid protein of Alzheimer's disease containing a glutamine for glutamic acid substitution have accelerated amyloid fibril formation. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1247–1254. doi: 10.1016/0006-291x(91)91706-i. [DOI] [PubMed] [Google Scholar]