Abstract
Background
The burden estimation studies for respiratory syncytial virus (RSV) have been based on varied case definitions, including case–definitions designed for influenza surveillance systems. We used all medical admissions among children aged 0–59 months to study the effect of case definitions on estimation of RSV–associated hospitalizations rates.
Methods
The hospital–based daily surveillance enrolled children aged 0–59 months admitted with acute medical conditions from July 2009–December 2012, from a well–defined rural population in Ballabgarh in northern India. All study participants were examined and nasal and throat swabs taken for testing by real–time polymerase chain reaction (RT–PCR) for RSV and influenza virus. Clinical data were used to retrospectively evaluate World Health Organization (WHO) case definitions (2011) commonly used for surveillance of respiratory pathogens, ie, acute respiratory illness (WHO–ARI), severe ARI (SARI) and influenza–like illness (ILI), for determination of RSV–associated hospitalization. RSV–associated hospitalization rates adjusted for admissions at non–study hospitals were calculated.
Findings
Out of 505 children enrolled, 82 (16.2%) tested positive for RSV. Annual incidence rates of RSV–associated hospitalization per 1000 children were highest among infants aged 0–5 months (15.2; 95% confidence interval (CI) 8.3–26.8), followed by ages 6–23 months (5.3, 95% CI 3.2–8.7) and lowest among children 24–59 months (0.5, 95% CI 0.1–1.5). The RSV positive children were more likely to have signs of respiratory distress like wheeze, chest in–drawing, tachypnea, and crepitation compared to RSV–negative based on bivariate comparisons. Other less commonly seen signs of respiratory distress, ie, nasal flaring, grunting, accessory muscle usage were also significantly associated with being RSV positive. Compared to the estimated RSV hospitalization rate based on all medical hospitalizations, the WHO–ARI case definition captured 86% of the total incidence, while case definitions requiring fever like ILI and SARI underestimated the incidence by 50–80%.
Conclusions
Our study suggests that RSV is a substantial cause of hospitalization among children aged <24months especially those aged <6 months. The WHO–ARI case definition appeared to be the most suitable screening definition for RSV surveillance because of its high sensitivity.
Globally, respiratory syncytial virus (RSV) is a leading cause of acute lower respiratory infection (ALRI) including bronchiolitis and pneumonia among young children [1,2]. Studies indicate that most children are infected by RSV in the first two years of life with infants bearing the highest rates of RSV–associated lower respiratory illness [3,4]. A recent meta–analysis estimated 3.4 million hospitalizations and 66 000–199 000 RSV–associated deaths among children <5 years of age with ALRI, with 99% of the deaths occurring in developing countries [5]. While there are some studies on burden of pneumonia and viral etiology in India and other developing countries [2,6], recent data from a community–based and hospital–based studies have further emphasized the importance of RSV among children <5 years of age [7-9].
Systematic data are needed to better understand seasonality, burden and mortality associated with RSV infection in children. RSV infection has a different clinical presentation, age distribution, risk factors, and seasonality compared to influenza infection and requires studies specifically designed to detect and evaluate RSV [3,4]. For example, RSV illness may present without fever particularly among infants, whereas influenza is more likely to present as a febrile illness and thus, fever may be included in the case definitions [10]. However, existing surveillance networks for influenza, with protocols and case definitions designed for influenza have also often been used to generate burden estimations for RSV [11]. Thus, there is a need to identify appropriate case–definitions for epidemiologic field studies to accurately estimate the RSV burden among children.
The presence of a broad platform to estimate the rates of hospitalized influenza which captured all–cause hospitalization in a well–defined population with health demographic surveillance system [12] enabled us to evaluate the sensitivity and specificity of different case definitions and RSV burden among children <5 years in a rural setting in northern India. The current paper utilizes data from this hospital–based surveillance study to evaluate case definitions for RSV detection and the impact of choice of case definitions on RSV hospitalization rate estimates among children aged <5 years in a rural setting in India.
METHODS
Study site
The Ballabgarh Health and Demographic Surveillance System (HDSS) site is about 40 km south of Delhi and comprised a population of about 90 000 in June 2011 including 9500 children 0–59 months of age in 28 villages [13]. Based on health utilization survey of the site population conducted in April 2009, three public hospitals and 30 private facilities (ranging in size from 5–35 beds) in Ballabgarh and Faridabad towns were included for daily surveillance for patients from the catchment area seeking inpatient care [12,14]. Immunization coverage for EPI vaccines (BCG, DPT, OPV, Hepatitis B and Measles) provided through public health facilities was >95% in the study villages [13]; Hib vaccine was introduced into public health program in 2012–2013. Coverage for pneumococcal and rota vaccines are not known but likely to be low as they are available only in private facilities.
Enrolment and data collection
During July 2009 – December 2012, hospital–based daily surveillance–enrolled children aged 0–59 months from Ballabgarh–HDSS area who were hospitalized overnight with any acute medical illness or acute exacerbation of chronic illness at participating medical facilities [8,12]. Trained study physicians collected data using a standardized form on demographics, medical history and clinical symptoms by interview of the caregivers, and extracted data on clinical signs at admission from the medical record followed by clinical examination of cases for additional clinical information.
Study definitions
The presence of fever or key respiratory signs or symptoms was determined among all hospitalized children aged 0–59 months. Fever was defined as either measured temperature >38.0°C at admission or parental report of fever because antipyretic use is known to be common in the study community [15]. Key respiratory symptoms or signs were defined as parental report of cough or fast breathing or physician exam findings of tachypnea, crepitation, wheezing, nasal flaring, chest in–drawing, grunting, or stridor. Tachypnea was defined based on the definition used by the Indian Integrated Management of Neonatal and Childhood Illness (IMNCI) as ≥60 breaths/min in children aged 0–2 months of age, ≥50 breaths/min in children aged 2–12 months of age, and ≥40 breaths/min in children aged 12 months –5 years of age [16]. Data on clinical signs and symptoms were used post hoc to classify each patient using standard case definitions specified by WHO (May 2011, see Box 1), ie, acute respiratory infection (ARI), severe acute respiratory infection (SARI), and influenza–like illness (ILI), and evaluated them for RSV positivity.
Box 1. Case definitions for respiratory syndromes [17,18].
ARI – acute respiratory infection (WHO, 2011):
Acute onset of at least one of the following four respiratory symptoms: cough or sore throat or shortness of breath or coryza and a clinician’s judgment that illness is due to infection.
ILI – influenza–like illness (WHO, 2011):
An acute respiratory illness with onset during the last 7 days with measured temperature ≥38°C, AND cough. (Dropped sore throat).
ILI (old):
Sudden onset of fever (>38°C) with cough or sore throat, in absence of other diagnoses.
SARI – severe ARI (WHO, 2011):
An acute respiratory illness with onset during the previous 7 days requiring overnight hospitalization that includes history of fever or measured fever of ≥38°C, AND cough, AND shortness of breath or difficulty breathing.
SARI (old):
Meets ILI (old) definition (sudden onset of fever (>38°C) with cough or sore throat) and has shortness of breath or difficulty breathing and requires overnight hospitalization.
Pneumonia (IMCI):
Fast breathing or chest indrawing
Severe pneumonia (IMCI):
General danger signs – Not able to drink, persistent vomiting, convulsions, lethargic or unconscious, stridor, or severe malnutrition
Specimen collection and laboratory methods
Nasal and throat samples were collected by a study nurse from all enrolled patients within 24 hours of admission to the hospital using polyester swabs; in infants only nasal swabs were collected. The swabs were placed immediately into viral transport media and transported on ice to laboratory on the same day for processing. Specimens were tested for RSV and influenza using US Centers for Disease Control and Prevention (CDC) real–time reverse transcription polymerase chain reaction (rRT–PCR) protocols, as described previously [8,12].
Data analysis
We assessed different signs and symptoms associated with RSV positivity using bivariate analysis for different age–groups and backward stepwise logistic regression adjusted for age–groups. We also assessed the ability of standard case definitions (ARI, SARI, ILI) for respiratory illness to capture RSV–associated hospitalizations by calculating sensitivity and specificity for each case definition using all RSV positive hospitalized patients as the gold standard. We assessed the impact of standard case definitions on average annual incidences of RSV–associated hospitalizations using available 3 calendar years’ data from 2010 to 2012.
The population of June 2011 in the HDSS was considered the mid–term population denominator for calculations. Annual health utilization surveys were used to estimate the average proportion of hospitalization in enrolled facilities [14]. The annual hospitalization rates based on enrollment were adjusted for missed hospitalizations in non–study facilities and were multiplied by the positivity rate of the viruses to get an estimate of virus specific hospitalization rates. We calculated incidence rates for four age groups (0–5 months, 6–11 months, 12–23 months and 24–59 months) for RSV and influenza because clinical manifestations, as well as viral etiologies are likely to be different in these age groups. The average annual incidence was calculated separately for each case definition among all medical and respiratory admissions, to evaluate the effect of using different screening definitions on the estimations of RSV–associated hospitalizations. The data analysis was done using STATA 12 (College Station, Texas, USA) [17] and Micosoft Excel. A P–value of <0 · 05 was considered statistically significant for all analyses. The 95% confidence intervals (CI) for odds ratios, sensitivity and specificity were calculated.
RESULTS
Background characteristics
During the study period, 505 children aged 0–59 months from the HDSS area hospitalized with acute medical illness in the health facilities under surveillance were enrolled; of these 79.6% (402/505) were aged 0–24 months and 71.7% (362/505) were males (Table 1). RSV was detected in 82 (16%) hospitalized patients with 89% (73/82) of detections among children <2 years old (P < 0.001). There was no significant difference in gender, time from symptom onset to specimen collection or any underlying medical condition among RSV negative and positive children (with the exception of chronic diarrhea observed among RSV negative children, data not shown). The RSV detections among hospitalized children occurred with seasonal peaks between September–October and then again in January–February of each year (data not shown).
Table 1.
Characteristics of children <5 years of age enrolled for all cause hospitalization in Ballabgarh, Haryana, India from July 2009 – December 2012
| Characteristics (n, %) | Total (n = 505) | RSV+ (n = 82) | RSV– (n = 423) | P–value |
|---|---|---|---|---|
|
Age group (months): | ||||
| 0–5 |
114 (22.6) |
35 (42.7) |
79 (18.7) |
<0.001 |
| 6–11 |
146 (28.9) |
16 (19.5) |
130 (30.7) |
|
| 12–23 |
142 (28.1) |
22 (26.8) |
120 (28.4) |
|
| 24–35 |
43 (8.5) |
4 (4.9) |
39 (9.2) |
|
| 36–59 |
60 (11.9) |
5 (6.1) |
55 (13) |
|
|
Sex: | ||||
| Female |
143 (28.3) |
24 (29.3) |
119 (28.1) |
0.834 |
| Male |
362 (71.7) |
58 (70.7) |
304 (71.9) |
|
|
Time from symptom onset to specimen collection:* | ||||
| 0–2 days |
127/468 (27.1) |
16/79 (20.2) |
111/389 (28.5) |
0.497 |
| 3–4 days |
135/468 (28.8) |
26/79 (32.9) |
109/389 (28.0) |
|
| 5–7 days |
116/468 (24.8) |
21/79 (26.6) |
95/389 (24.4) |
|
| 8–10 days |
39/468 (8.3) |
9/79 (11.4) |
30/389 (7.7) |
|
| ≥11 days | 51/468 (10.9) | 7/79 (8.9) | 44/389 (11.3) | |
*Data on 37 cases, including 3 RSV–positive cases, were missing.
Clinical characteristics
Among the enrolled children, 347 (68.7%) had some respiratory illness. Further, those with symptoms of cough (OR = 5.3, 95% CI 2.8–10.1) and fast breathing (OR = 3.9, 95% CI 2.4–6.3) were more likely to test positive than negative for RSV (Table 2). The presence of signs of wheeze, chest in–drawing, tachypnea, and crepitation had significantly higher odds of being RSV positive vs RSV–negative based on bivariate comparisons. Other less commonly seen signs of respiratory distress, ie, nasal flaring, grunting, accessory muscle usage were also significantly associated with being RSV positive. Stepwise backward logistic regression (Table 3) analysis of all clinical features identified the presence of cough, fast–breathing, crepitation and hypoxia as significant predictors for RSV–associated hospitalization, while history of fever and diarrhea were significantly associated with non–RSV–associated hospitalization.
Table 2.
Age–specific clinical signs and symptoms significantly associated with laboratory–confirmed respiratory syncytial virus infection among hospitalized patients <5 y of age, in Ballabgarh, India, July 2009 – December 2012; (n = 505)*
| 0–5 months | 6–23 months | 24–59 months | 0–59 months | |||||
|---|---|---|---|---|---|---|---|---|
|
RSV+ (n = 35) |
OR (95% CI) |
RSV+ (n = 38) |
OR (95% CI) |
RSV+ (n = 9) |
OR (95% CI) |
RSV+ (n = 82) |
OR (95% CI) |
|
|
Symptoms: | ||||||||
| Fever |
27 (77.1) |
0.6 (0.2–1.6) |
32 (84.2) |
0.8 (0.3–2.2) |
9 (100.0) |
– |
68 (82.9) |
0.8 (0.4–1.5) |
| Cough |
32 (91.4) |
4.4 (1.2–15.7) |
32 (84.2) |
6.5 (2.6–16.0) |
6 (66.7) |
1.6 (0.4–6.8) |
70 (85.4) |
5.3 (2.8–10.1) |
| Breathing difficulty |
20 (57.1) |
3.5 (1.5–7.9) |
15 (39.5) |
3.3 (1.6–6.9) |
2 (22.2) |
1.5 (0.3–8) |
37 (45.1) |
1.8 (1.1–2.7) |
| Nasal discharge |
13 (37.1) |
1.4 (0.6–3.1) |
20 (52.6) |
2.3 (1.1–4.5) |
4 (44.4) |
2 (0.5–8) |
37 (45.1) |
1.8 (1.1–2.9) |
| Sore throat (>2years) |
0 (0) |
– |
0 (0) |
– |
2 (22.2) |
1.5 (0.3–8) |
2 (16.7) |
1.4 (0.3–7.1) |
| Ear discharge |
1 (2.9) |
1.1 (0.1–12.9) |
4 (10.5) |
2.3 (0.7–7.6) |
0 (0) |
– |
5 (6.1) |
1 (0.4–2.7) |
| Fast breathing |
18 (51.4) |
2.9 (1.3–6.7) |
18 (47.4) |
6.1 (2.9–12.8) |
3 (33.3) |
3.8 (0.8–17.3) |
39 (47.6) |
3.9 (2.4–6.3) |
| Lethargy |
9 (25.7) |
1.5 (0.6–3.9) |
3 (7.9) |
0.3 (0.1–0.9) |
3 (33.3) |
1.2 (0.3–5) |
15 (18.3) |
0.7 (0.4–1.2) |
| Refusal to feed |
15 (42.9) |
1 (0.5–2.3) |
10 (26.3) |
0.6 (0.3–1.3) |
3 (33.3) |
0.8 (0.2–3.3) |
28 (34.2) |
0.8 (0.5–1.3) |
| Seizure |
2 (5.7) |
0.9 (0.2–4.9) |
0 (0) |
– |
2 (22.2) |
6.4 (1–41.4) |
4 (4.9) |
1.6 (0.5–4.7) |
| Unconsciousness |
2 (5.7) |
0 (0–0) |
0 (0) |
– |
0 (0) |
– |
2 (2.4) |
1.5 (0.2–11) |
| Vomiting |
10 (28.6) |
0.4 (0.2–0.9) |
22 (57.9) |
0.4 (0.2–0.8) |
4 (44.4) |
0.5 (0.1–2) |
36 (43.9) |
0.4 (0.2–0.6) |
| Diarrhea |
13 (37.1) |
0.5 (0.2–1.2) |
16 (42.1) |
0.2 (0.1–0.4) |
1 (11.1) |
0.2 (0–1.7) |
30 (36.6) |
0.3 (0.2–0.5) |
| Rash |
1 (2.9) |
2.3 (0.1–37.8) |
0 (0) |
– |
0 (0) |
– |
1 (1.2) |
0.3 (0–2.6) |
| Jaundice |
1 (2.9) |
1.1 (0.1–12.9) |
0 (0) |
– |
0 (0) |
– |
1 (1.2) |
0.7 (0.1–4.2) |
|
Signs: | ||||||||
| Fever |
1 (2.9) |
0.2 (0–1.3) |
10 (26.3) |
1.8 (0.8–4) |
6 (66.7) |
5 (1.2–21.3) |
17 (20.7) |
1.1 (0.6–2) |
| Stridor |
4 (11.4) |
2.4 (0.6–10.3) |
5 (13.2) |
9.3 (2.4–36.5) |
0 (0) |
0 (0–0) |
9 (11) |
5.7 (2.2–14.8) |
| Nasal flaring |
5 (14.3) |
2.4 (0.6–8.9) |
4 (10.5) |
9.7 (2.1–45.2) |
0 (0) |
0 (0–0) |
9 (11) |
5.1 (2–12.9) |
| Chest in–drawing |
13 (37.1) |
5.1 (1.2–13.9) |
6 (15.8) |
3.7 (1.3–10.6) |
0 (0) |
0 (0–0) |
19 (23.2) |
5.7 (2.9–11.3) |
| Grunting |
5 (14.3) |
3 (0.8–12.1) |
4 (10.5) |
7.2 (1.7–30.3) |
0 (0) |
0 (0–0) |
9 (11) |
5.6 (2.2–14.7) |
| Accessory muscle use |
5 (14.3) |
4.2 (0.9–18.8) |
1 (2.6) |
0.8 (0.1–6.7) |
0 (0) |
0 (0–0) |
6 (7.3) |
2.5 (0.9–6.8) |
| Crepitation |
21 (60) |
2.9 (1.3–6.6) |
17 (44.7) |
5.0 (2.4–10.3) |
4 (44.4) |
5.5 (1.3–23.2) |
42 (51.2) |
5 (3–8.2) |
| Wheeze |
24 (68.6) |
5 (2.1–11.8) |
10 (26.3) |
2.8 (1.2–6.4) |
2 (22.2) |
2.2 (0.4–11.7) |
36 (43.9) |
4.5 (2.7–7.5) |
| Tachypnoea |
25 (71.0) |
1.4 (0.6–3.3) |
16 (42.1) |
1.9 (0.9–3.8) |
1 (11.1) |
0.6 (0.1–4.8) |
42 (51.2) |
2.2 (1.4–3.5) |
| Hypoxia* | 8 (22.9) | 1.5 (0.6–4) | 8 (22.9) | 2.6 (1.0–6.6) | 1 (11.1) | 2.8 (0.3–28.3) | 17 (20.7) | 2.5 (1.3–4.8) |
OR – odds ratio, CI – confidence interval
*Defined as oxygen saturation <90% on room air or <95% on oxygen therapy.
Table 3.
Clinical predictors of respiratory syncytial virus associated hospitalization among children aged <5 y by stepwise backward logistic regression adjusted for age–groups*
| Symptoms and signs | Odds ratio (95% CI) | P > z |
|---|---|---|
| Cough |
1.85 (1.13–0.46) |
0.013 |
| History of fever |
0.4 (0.23–0.11) |
0.001 |
| History of fast breathing |
3.44 (2.26–0.73) |
<0.001 |
| Diarrhea |
0.62 (0.41–0.12) |
0.021 |
| Inability/refusal to feed |
0.57 (0.39–0.1) |
0.004 |
| Unconsciousness |
0.07 (0.01–0.07) |
0.005 |
| Nasal flaring |
3.13 (1.41–1.27) |
0.005 |
| Stridor |
2.73 (1.22–1.12) |
0.014 |
| Accessory muscle use |
0.22 (0.08–0.11) |
0.003 |
| Crepitation |
2.79 (1.81–0.61) |
<0.001 |
| Hypoxia | 2.71 (1.58–0.74) | <0.001 |
CI – confidence interval
*Age groups: 0–5 months, 6–11 months, 12–23 months and 24–59 months.
Case definitions
We then examined the sensitivity and specificity of standard case definitions (ARI, SARI, ILI) for detection of RSV–associated hospitalization (Figure 1). Among the standard case definitions, ARI had the highest sensitivity (87.8%) and specificity (40%) based on receiver–operating characteristics. All other case definitions (ILI, and SARI, (both old and revised 2011 versions) had lower sensitivity and variable specificity, with older the definition of SARI showing higher specificity). We also evaluated different clinical syndromes including IMCI definitions of pneumonia/severe pneumonia [18] and other syndromes. The sensitivity of IMCI either pneumonia or severe pneumonia was high (75.6%) but specificity was low (31.9%). Among other combination of symptoms and signs, we found history of cough or crepitation along with presence of any one of following– history of fast–breathing, breathing difficulty, nasal discharge, sore–throat, chest–in–drawing, wheeze or hypoxia, had high sensitivity (81 · 7%) and specificity (60%).
Figure 1.
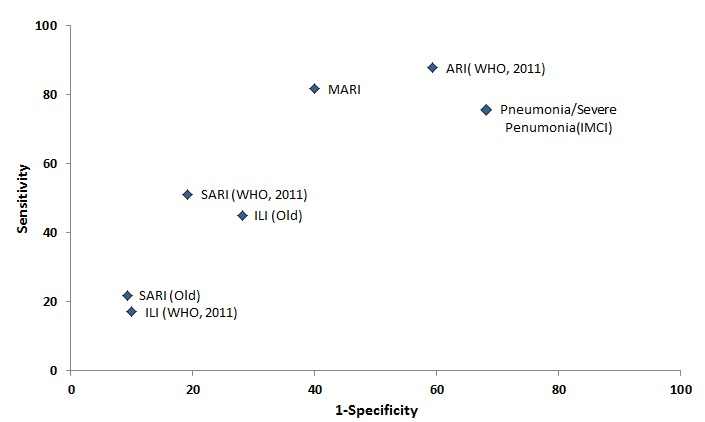
Receiver operator characteristic (ROC) chart for screening definitions for respiratory syncytial virus associated hospitalization with 1–specificity on x-axis and sensitivity on y-axis. Each dot represents the sensitivity and 1–specificity of the case definition for detection of RSV–associated hospitalization. See Box 1 for case definitions details of each syndrome.
Burden of RSV in children
The average annual incidence of RSV–associated hospitalizations was found to be higher at 7 · 4 (95% CI: 4.9–10 · 5) per 1000 child–years in those 0–23 months as compared to 0.5 (95% CI 0.1–1.5) among 24–59 months population signifying that most of burden of RSV–associated hospitalization is among children under two years of age. Further breakdown of the age–specific annual incidence of RSV–associated hospitalization per 1000 children revealed that the highest rate occurred in young infants 0–5 months (OR = 15.2, 95% CI 8.3–26.8), followed by 6–23 months (OR = 5.3, 95% CI 3.2–8.7) with comparable rates for the 6–11 months and 12–23 months age groups (Figure 2). Incidence rates for influenza were lower across all age groups.
Figure 2.
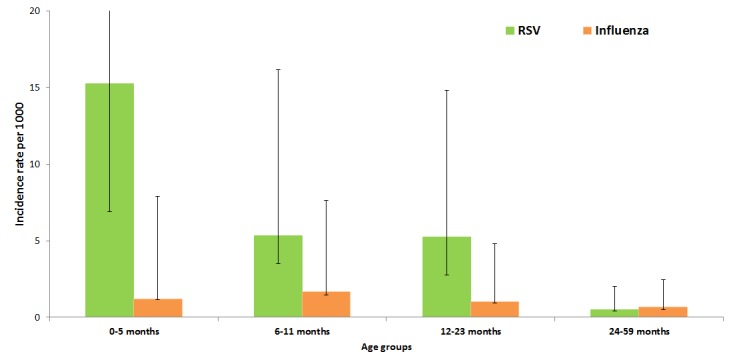
Age–specific incidence rates using all–cause hospitalization for respiratory syncytial virus (RSV) and influenza–associated hospitalization rates in north India (2009–2012). The age groups are on the x–axis and incidence rate of RSV–associated hospitalization per 1000 children denoted on the y–axis. Green and orange colored bars denote incidence rates of RSV and influenza associated hospitalizations respectively with error bars for 95% confidence intervals.
Impact of case definitions on RSV burden
We assessed the impact of the use of different standard case definitions on RSV burden estimates by comparing the definitions with RSV hospitalization based on all–cause hospitalization (Table 4). Use of the ARI case definition among hospitalized children would have detected 90% and 86% of the RSV–associated hospitalization rates in children aged <2 years and <5 years, respectively. In contrast, use of definitions which require presence of fever and most commonly used for influenza surveillance platforms, ie, SARI or ILI definitions, (both old and revised) would have under estimated RSV burden by as much as 50–85% in both <2 as well as <5–year age groups (Table 4).
Table 4.
Effect of screening case definition on respiratory syncytial virus (RSV) associated annual hospitalization rates (2010–2012)*
| Population under surveillance | Under–2 years (N = 3956) | Under–5 years (N = 9740) | ||||||
|---|---|---|---|---|---|---|---|---|
|
Case definitions |
No. met case definition |
No. of RSV positive cases (%) |
Incidence Rate (IR)† |
IR under–estimation (%) |
No. met case definition |
No. of RSV positive cases (%) |
IR† |
Under–estimation IR (%) |
| All Medical |
386 |
67 (17) |
7.4 |
NA |
484 |
74 (15) |
3.2 |
NA |
| ARI (WHO) |
239 |
60 (25) |
6.6 |
–10% |
299 |
64 (21) |
2.8 |
–14% |
| SARI (WHO) |
90 |
34 (38) |
3.8 |
–49% |
107 |
35 (33) |
1.5 |
–53% |
| SARI (Old) |
35 |
10 (29) |
1.1 |
–85% |
41 |
11 (27) |
0.5 |
–85% |
| ILI (WHO) |
37 |
10 (27) |
1.1 |
–85% |
54 |
14 (26) |
0.6 |
–81% |
| ILI (Old) | 106 | 26 (25) | 2.9 | –61% | 139 | 32 (23) | 1.4 | –57% |
ARI – acute respiratory illness, SARI – severe acute respiratory illness, ILI – influenza–like illness, WHO – World Health Organization
*For incidence rate calculations data for full calendar years were used. Data imputed for 6 weeks surveillance gap between 31 January 2012 and 13 March 2012.
†Per 1000 age–specific population.
DISCUSSION
The uniqueness of this study based on a comprehensive surveillance system designed to capture all–cause hospitalization in a well–defined population, together with the availability of highly sensitive molecular testing for RSV allowed us to estimate RSV–associated the hospitalization rates among children aged <5 years in rural northern India [8,12]. Most studies estimating RSV–associated burden rely on existing surveillance platforms for influenza in developing countries [11,19]. A very important aspect of our study was that we were able to evaluate the impact of using different case definitions on burden estimation. We provided evidence that the WHO–defined ARI case definition has the highest sensitivity for RSV–associated hospitalization and demonstrate the limitations of definitions like ILI and SARI commonly used for influenza surveillance. We found that testing only children meeting the SARI and ILI definitions would have under–estimated the burden of RSV–associated hospitalization by almost 50–85%, although specificity would have been significantly higher with the latter case definition. Several case definitions, including ARI, have been used in studies for RSV burden estimation in many countries [11,19-22]. This highlights the importance of the use of a sensitive case definition for surveillance of RSV to avoid underestimation of the burden.
The all–cause hospitalization surveillance also allowed us to compare the symptoms and signs of hospitalized children with or without RSV and thereby identify the clinical predictors for RSV–associated hospitalization in children; this would not have been possible if only standard case definitions were used to identify potential RSV patients. We found that the presence of cough, fast–breathing, crepitation and hypoxia are independent predictors of RSV infection. Of note, Durani et al. (2008) in their study among children hospitalized with ARI found the combination of cough, wheezing and retractions to be good clinical predictor for RSV infection [23]. We found that even though fever is a common presenting symptom and sign among children being hospitalized, it is not a good predictor of RSV–associated hospitalization in this population. The use of history or presence of fever in screening case definitions lowers the sensitivity of the definition. We also observed that two–thirds of hospitalized patients had some respiratory symptoms, suggesting that a very high proportion of hospitalizations are due to respiratory symptoms in rural India. This observation corroborates previous findings that ARI is a significant cause of morbidity in the developing world [4,24,25].
We found substantial incidence of RSV–associated hospitalization in the study community especially among <2–year old children. The RSV–associated incidence of hospitalization per 1000 child–years was 3.2 among <5–year children, and 7.4 among <2–year children, with highest incidence rate of 15.2 per 1000 child years among infants 0–5 months, which is similar to findings of an earlier community–based study from this area [7]. RSV–associated hospitalization rates among children <5years observed in our study were also comparable to what has been observed in Kenya (2.9/1000 child–years) and Guatemala (2–13.7/1000 child–years), although lower than in Thailand (9.8/1000 child–years), Indonesia (34/1000 child–years), Nigeria (94/1000 child–years) [20-22,26]. The highest risk group for RSV–associated burden was infants 0– to 5–month old, which was also observed in Thailand (15.4/1000 child–years), Indonesia (41/1000 child–years), Hong Kong (<6m, 23.4–31.1/1000 child–years), Guatemala (5.9–45.9/1000 child–years), Kenya (11.0/1000 child–years) and Nigeria (116/1000 child–years). Studies from Brazil, USA and Korea have established that infants are at high risk of RSV–associated burden both in terms of incidence in community and proportion of hospitalization [19-22,27-31]. Even though the rates of RSV–associated hospitalization vary in different countries, most of the burden is observed among children aged <2 years, therefore studies focusing on RSV–associated morbidity and mortality or high–risk group for RSV infections may consider children aged <2 years. Also, prioritizing this age–group for any preventive measure would likely have profound effect on prevention of RSV–associated hospitalization and deaths in India and other developing countries [32,33].
The study’s limitations include first that it was designed to address influenza–associated burden, so data on variables such as gestation at birth were not collected; this data might have allowed us to also understand some of the risk–factors for RSV infection. Second, the active surveillance at health facilities (which were almost 30 plus facilities) did not capture all hospitalizations for the denominator population, and we had to make adjustments in rates of hospitalization using HDSS survey results. It is plausible that children seeking care at participating hospitals may be different from those who did not seek care in these hospitals, thus biasing the incidence rates for RSV. Third, there is a possibility that we have under–estimated the burden of RSV–associated hospitalization as some children might not have been hospitalized in spite of being diagnosed with severe respiratory illness [11,24]. Despite these limitations, we believe that this study enabled us to understand the effect of surveillance case definitions on population–based rates of RSV hospitalization in northern rural India. However, due to the study design where we captured all cause medical hospitalization instead of ILI or SARI, we are in unique position to not only address the burden of RSV, but also assess various screening case definition for RSV–associated hospitalization. Analysis of this broad platform for RSV case definition assessment allowed us to recommend the WHO–defined ARI case definition as the most appropriate screening case definition for RSV among those considered.
CONCLUSION
In conclusion, we observed that RSV is a substantial cause of hospitalization among children aged <2 years, and especially among infants aged <6 months. This is true regardless of the screening definition used, even though rates may be underestimated if an insensitive screening definition is used. These data will help support public health strategies and interventions, targeting young children to reduce the overall RSV–associated morbidity and mortality among children in the developing world.
Acknowledgements
We thank Marika Iwane, Eduardo Azziz–Baumgartner, and Marc–Alain Widdowson for their comments and suggestions for design of the study. We also thank Drs. Akhilesh C Mishra, Marc–Alain Widdowson, Josh Mott, Fatimah Dawood, Marika Iwane, Vivek Gupta, and Debjani Ram Purakayastha for their contribution to this study. The authors would also like to thank the study participants, their caregivers, the health facilities and the community of the Comprehensive Rural Health Services Project (CRHSP) villages for their cooperation for this study.
Funding: This study was funded through cooperative agreement 1U01IP000206 between the US Centers for Disease Control and the National Institute of Virology, Pune.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Ethics approval: Written informed consent was obtained from the parent/legal guardian of each participant prior to enrollment. The study protocol was reviewed and approved by the Institutional Review Boards of the Indian Council of Medical Research, All India Institute of Medical Sciences and US CDC.
Authorship declaration: SS, RBL, AK, and SB conceptualized the study design. SKR, and SB managed the surveillance platform. BG, AC, and SB performed sample processing and viral testing. SS and RBL analyzed the data and wrote the first draft. AK, MKI, SIG, PS, MSC reviewed the results and helped writing the manuscript. All authors reviewed the manuscript.
Competing interests: All authors have completed the Unified Competing Interest form at www.icme.org/coi_disclosure.pdf (available on request from the corresponding author). The authors declare no conflict of interest.
REFERENCES
- 1.Ogra PL.Respiratory syncytial virus: the virus, the disease and the immune responsePaediatr Respir Rev 20045Suppl AS119–26. 10.1016/S1526-0542(04)90023-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudan I, O'Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:010401. doi: 10.7189/jogh.03.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus––a comprehensive review. Clin Rev Allergy Immunol. 2013;45:331–79. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, et al. Respiratory syncytial virus–associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–8. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 5.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta–analysis. Lancet. 2010;375:1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathew JL, Patwari AK, Gupta P, Shah D, Gera T, Gogia S, et al. Acute respiratory infection and pneumonia in India: a systematic review of literature for advocacy and action: UNICEF–PHFI series on newborn and child health, India. Indian Pediatr. 2011;48:191–218. doi: 10.1007/s13312-011-0051-8. [DOI] [PubMed] [Google Scholar]
- 7.Broor S, Parveen S, Bharaj P, Prasad VS, Srinivasulu KN, Sumanth KM, et al. A prospective three–year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS ONE. 2007;2:e491. doi: 10.1371/journal.pone.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broor S, Dawood FS, Pandey BG, Saha S, Gupta V, Krishnan A, et al. Rates of respiratory virus–associated hospitalization in children aged <5 years in rural northern India. J Infect. 2014;68:281–9. doi: 10.1016/j.jinf.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh AK, Jain A, Jain B, Singh KP, Dangi T, Mohan M, et al. Viral aetiology of acute lower respiratory tract illness in hospitalised paediatric patients of a tertiary hospital: one year prospective study. Indian J Med Microbiol. 2014;32:13–8. doi: 10.4103/0255-0857.124288. [DOI] [PubMed] [Google Scholar]
- 10.Iwane MK, Farnon EC, Gerber SI. Importance of global surveillance for respiratory syncytial virus. J Infect Dis. 2013;208(Suppl 3):S165–6. doi: 10.1093/infdis/jit484. [DOI] [PubMed] [Google Scholar]
- 11.Nasreen S, Luby SP, Brooks WA, Homaira N, Al Mamun A, Bhuiyan MU, et al. Population–based incidence of severe acute respiratory virus infections among children aged <5 years in rural Bangladesh, June–October 2010. PLoS ONE. 2014;9:e89978. doi: 10.1371/journal.pone.0089978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta V, Dawood FS, Rai SK, Broor S, Wigh R, Mishra AC, et al. Validity of clinical case definitions for influenza surveillance among hospitalized patients: results from a rural community in North India. Influenza Other Respir Viruses. 2013;7:321–9. doi: 10.1111/j.1750-2659.2012.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kant S, Misra P, Gupta S, Goswami K, Krishnan A, Nongkynrih B, et al. The Ballabgarh Health and Demographic Surveillance System (CRHSP–AIIMS). Int J Epidemiol. 2013;42:758–68. doi: 10.1093/ije/dyt055. [DOI] [PubMed] [Google Scholar]
- 14.Hirve S, Krishnan A, Dawood FS, Lele P, Saha S, Rai S, et al. Incidence of influenza–associated hospitalization in rural communities in western and northern India, 2010–2012: a multi–site population–based study. J Infect. 2015;70:160–70. doi: 10.1016/j.jinf.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses: 2nd ed. Geneva: WHO, 2013. [PubMed] [Google Scholar]
- 16.World Health Organization. WHO Regional Office for Europe guidance for sentinel influenza surveillance in humans: May 2011 Edition. Copenhagen, Denmark: WHO Regional Office for Europe, 2011. [Google Scholar]
- 17.StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP, 2011. [Google Scholar]
- 18.World Health Organization. Revised WHO classification and treatment of childhood pneumonia at health facilities: Quick Reference Guide. Geneva: WHO, 2014. [Google Scholar]
- 19.Haynes AK, Manangan AP, Iwane MK, Sturm–Ramirez K, Homaira N, Brooks WA, et al. Respiratory syncytial virus circulation in seven countries with Global Disease Detection Regional Centers. J Infect Dis. 2013;208(Suppl 3):S246–54. doi: 10.1093/infdis/jit515. [DOI] [PubMed] [Google Scholar]
- 20.Fry AM, Chittaganpitch M, Baggett HC, Peret TC, Dare RK, Sawatwong P, et al. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS ONE. 2010;5:e15098. doi: 10.1371/journal.pone.0015098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer Langley G, McCracken J, Arvelo W, Estevez A, Villarruel G, Prill M, et al. The epidemiology and clinical characteristics of young children hospitalized with respiratory syncytial virus infections in Guatemala (2007–2010). Pediatr Infect Dis J. 2013;32:629–35. doi: 10.1097/INF.0b013e318289e3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nokes DJ, Ngama M, Bett A, Abwao J, Munywoki P, English M, et al. Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin Infect Dis. 2009;49:1341–9. doi: 10.1086/606055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durani Y, Friedman MJ, Attia MW. Clinical predictors of respiratory syncytial virus infection in children. Pediatr Int. 2008;50:352–5. doi: 10.1111/j.1442-200X.2008.02589.x. [DOI] [PubMed] [Google Scholar]
- 24.Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta–analysis. Lancet. 2011;378:1917–30. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 25.Régnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta–analysis. Pediatr Infect Dis J. 2013;32:820–6. doi: 10.1097/INF.0b013e31829061e8. [DOI] [PubMed] [Google Scholar]
- 26.Robertson SE, Roca A, Alonso P, Simoes EA, Kartasasmita CB, Olaleye DO, et al. Respiratory syncytial virus infection: denominator–based studies in Indonesia, Mozambique, Nigeria and South Africa. Bull World Health Organ. 2004;82:914–22. [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu SS, Chan KH, Chen H, Young BW, Lim W, Wong WH, et al. Virologically confirmed population–based burden of hospitalization caused by respiratory syncytial virus, adenovirus, and parainfluenza viruses in children in Hong Kong. Pediatr Infect Dis J. 2010;29:1088–92. doi: 10.1097/INF.0b013e3181e9de24. [DOI] [PubMed] [Google Scholar]
- 28.Simöes EA, Mutyara K, Soh S, Agustian D, Hibberd ML, Kartasasmita CB. The epidemiology of respiratory syncytial virus lower respiratory tract infections in children less than 5 years of age in Indonesia. Pediatr Infect Dis J. 2011;30:778–84. doi: 10.1097/INF.0b013e318218ab9e. [DOI] [PubMed] [Google Scholar]
- 29.Madhi SA, Kuwanda L, Cutland C, Klugman KP. Five–year cohort study of hospitalization for respiratory syncytial virus associated lower respiratory tract infection in African children. J Clin Virol. 2006;36:215–21. doi: 10.1016/j.jcv.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Kim MR, Lee HR, Lee GM. Epidemiology of acute viral respiratory tract infections in Korean children. J Infect. 2000;41:152–8. doi: 10.1053/jinf.2000.0715. [DOI] [PubMed] [Google Scholar]
- 31.Lamarăo LM, Ramos FL, Mello WA, Santos MC, Barbagelata LS, Justino MC, et al. Prevalence and clinical features of respiratory syncytial virus in children hospitalized for community–acquired pneumonia in northern Brazil. BMC Infect Dis. 2012;12:119. doi: 10.1186/1471-2334-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray J, Saxena S, Sharland M. Preventing severe respiratory syncytial virus disease: passive, active immunisation and new antivirals. Arch Dis Child. 2014;99:469–73. doi: 10.1136/archdischild-2013-303764. [DOI] [PubMed] [Google Scholar]
- 33.Nair H, Verma VR, Theodoratou E, Zgaga L, Huda T, Simoes EA, et al. An evaluation of the emerging interventions against Respiratory Syncytial Virus (RSV)–associated acute lower respiratory infections in children. BMC Public Health. 2011;11(Suppl 3):S30. doi: 10.1186/1471-2458-11-S3-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]


