Abstract
Brain endocannabinoid signaling influences the motivation for natural rewards (such as palatable food, sexual activity and social interaction) and modulates the rewarding effects of addictive drugs. Pathological forms of natural and drug-induced reward are associated with dysregulated endocannabinoid signaling that may derive from pre-existing genetic factors or from prolonged drug exposure. Impaired endocannabinoid signaling contributes to dysregulated synaptic plasticity, increased stress responsivity, negative emotional states, and craving that propel addiction. Understanding the contributions of endocannabinoid disruptions to behavioral and physiological traits provides insight into the endocannabinoid influence on addiction vulnerability.
The brain reward system is critical for survival. The hedonic effects produced by eating, exercise and sexual activity provide important motivational effects that increase the likelihood of future engagement in these critical activities (e.g. positive reinforcement). The reward system is also essential for important negative hedonic responses in which aversive or unpleasant events (e.g sickness, bodily harm) increase the likelihood of behaviors that will avoid or relieve these negative states (e.g. negative reinforcement).
Seminal discoveries demonstrating that the effects of cannabis are mediated by cannabinoid receptors in the brain propelled significant research initiatives that expanded knowledge about the body’s endogenous cannabinoid system (termed the endocannabinoid system; ECS) that is now acknowledged to play a prominent role in modulating brain reward function and maintaining emotional homeostasis. This Review examines the evidence for an endocannabinoid (EC) influence in the positive reinforcing effects of natural rewards and drugs of abuse. In contrast to the initial pleasurable experience of rewarding stimuli, prolonged drug exposure contributes to aberrant synaptic plasticity, negative emotional states and impaired learning and memory processes that sustain compulsive drug consumption characteristic of the addicted state. We explore dysregulated ECS signaling underlying these maladaptive processes and provide an overview of the existing literature regarding genetic factors associated with the ECS to gain insight about the potential contribution to addiction disorders.
Endocannabinoid system and reward circuits
The ECS is comprised of G protein-coupled receptors, small neuromodulatory lipid ligands and biosynthetic and metabolic enzymes for the synthesis and degradation of the ligands, respectively. Two major types of cannabinoid receptor have been characterized and cloned: CB1 and CB2. CB1 receptors (CB1Rs) are the most abundant G protein-coupled receptor expressed in the adult brain, with particularly dense expression in regions with known involvement in reward, addiction and cognitive function including amygdala, cingulate cortex, prefrontal cortex (PFC), ventral pallidum, caudate putamen, nucleus accumbens (NAc), ventral tegmental area (VTA), lateral hypothalamus1, 2. CB2 receptors (CB2Rs) are mainly expressed by immune cells with recent evidence also suggesting CB2R expression in neurons, glia and endothelial cells in brain3. CB1R and CB2R are coupled to similar transduction systems primarily through Gi or Go proteins. CB1Rs directly inhibit the release of GABA, glutamate and acetylcholine that produce widespread effects on neural signaling across many neurotransmitter systems.
At present the best characterized endocannabinoid (EC) ligands are N-arachidonylethanolamide (anandamide, AEA) and 2-arachidonoylglycerol (2-AG). Due to their lipid nature AEA and 2-AG are not stored in vesicles but are synthesized on an “on demand” basis by cleavage from membrane precursors and immediate release through Ca2+-dependent mechanisms. AEA is derived from the phospholipid precursor N-arachidonoyl-phosphatidylethanolamine (NAPE), and while the precise mechanisms for AEA formation are not known, a very likely candidate is through N-acyl-phosphatidylethanolamine phospholipase D (NAPE-PLD). 2-AG derives primarily from the hydrolytic metabolism of 1,2-diacylglycerol (DAG) by sn-1-selective DAG lipases (DAGLα, DAGLβ). AEA is primarily catabolized through fatty acid amide hydrolase 1 (FAAH1) and 2-AG is catabolized through monoacylglycerol lipase (MAGL) and to a lesser extent α,β-hydrolase 6 (ABHD6), cyclooxygenase 2 (COX2) and FAAH1. The EC catabolic enzymes have distinct cellular anatomical locations with MAGL localized predominantly in presynaptic terminals and FAAH1 to the postsynaptic domain of neurons (Figure 1). AEA and 2-AG each exert agonist activity at CB1R and CB2R. AEA binds with slightly higher affinity to CB1R vs. CB2R, and like Δ9-tetrahydrocannabinol (Δ9-THC, the main psychoactive component of the cannabis plant) AEA exhibits low efficacy as an agonist at both receptors, producing sub-maximal signaling upon binding. 2-AG binds with essentially equal affinity at CB1R and CB2R and exhibits greater agonist efficacy than AEA. AEA and 2-AG also exhibit agonist properties at several secondary receptors including peroxisome proliferator-activated-receptors (PPARs), GPR55 and GPR119, and AEA exerts potent agonist effects at transient receptor potential ion channels including TRPV1.
Figure 1. Endocannabinoid biosynthesis, signaling and clearance.
The most commonly accepted route for AEA synthesis is from catalysis of N-arachidonoyl-phosphatidylethanolamine (NAPE) via a specific phospholipase D (NAPE-PLD). 2-AG derives from the hydrolysis of 1,2-diacylglycerol (DAG) via the sn-1-selective DAG lipases DAGLα and DAGLβ. DAGLα is found on the plasma membranes of both dopaminergic and non-dopaminergic neurons in the VTA, opposite CB1R-expressing glutamate and GABA axon terminals200. Termination of EC signaling is initiated by cellular reuptake followed by enzyme-mediated hydrolytic cleavage. 2-AG hydrolysis is primarily mediated by presynaptic monoacylglycerol lipase (MAGL), though post-synaptic enzymes including ABHD6 also contribute to 2-AG clearance. AEA hydrolysis occurs in postsynaptic cells through fatty acid amide hydrolase (FAAH). Although these mechanisms are depicted here in the VTA, the pre- and post-synaptic organization of EC biosynthetic and hydrolytic enzymes is generally conserved throughout the brain.
Neurobiology of reward
Mesocorticolimbic dopamine (DA) pathways, which arise from the midbrain ventral tegmental area (VTA), play a critical role in the mediation of reward. In particular, the VTA DA projection to the nucleus accumbens (NAc; also known as the ventral striatum) plays a prominent role in positive reinforcement (Figure 2), i.e. the recognition of rewards in the environment and promotion of goal-directed behavior (that is, ‘approach behaviour’) resulting in reward acquisition. Natural rewards such as food, sex and exercise and drugs of abuse including psychostimulants (such as cocaine and amphetamine), nicotine, alcohol, opiates and cannabinoids each increase NAc DA and this neurochemical response contributes to subjective reward and positive reinforcement4. Other components of the limbic system are also innervated by VTA DA neurons including the amygdala, hippocampus, orbitofrontal cortex and aspects of the prefrontal cortex (PFC). These regions are interconnected in complex circuits that involve excitatory (primarily glutamatergic) and inhibitory (primarily GABAegic) projections5. In broad, simplistic terms amygdala circuits contribute to the formation of associative reward- and fear-related memories, hippocampal circuits are critical for declarative memory functions and frontal cortical circuits mediate control of executive functions. In turn, innervation of the NAc by each of these circuits allows sensory and emotional information to be converted into motivational actions through the output to extrapyramidal motor systems. DA signaling in the dorsal striatum does not have a major influence in processing acute reward but plays a key role in the development of compulsive forms of reward seeking and consumption6.
Figure 2. Distribution of EC signaling mechanisms within the brain reward circuits.

CB1Rs are expressed throughout the regions implicated in reward and addiction including the basolateral amygdala (BLA), prefrontal cortex (PFC), hippocampus (HIPP), ventral pallidum (VP), globus pallidus (GP), dorsolateral striatum (DSTr), NAc, VTA, bed nucleus of the stria terminalis (BNST) and central nucleus of the amygdala (CeA)1, 2, 9. In general, the expression patterns of EC biosynthetic enzymes (e.g. NAPE-PLD and DAGLα) and hydrolytic EC clearance enzymes (e.g. FAAH and MAGL) are similar to that for CB1Rs across the regions depicted here201, 202. Within the amygdala, CB1, DAGLα, MAGL and FAAH expression is highest in the lateral and basolateral nuclei, with substantially lesser expression in the central nucleus56, 201. In the dorsal striatum there is a comparable medial-lateral gradient of CB1 and DAGLα expression with greater levels of expression evident in lateral aspects201, 203. Comparatively weaker CB1, DAGLα and FAAH expression is observed in the NAc201. Although little to no CB1 expression is found in dopamine cells in the NAc, DAGLα has been found in both dopaminergic and non-dopaminergic cells in this region204.
These same circuits participate in negative reinforcement mechanisms that promote behaviors for avoiding or relieving aversive states. In general, NAc DA levels are decreased by aversive conditions such as unavoidable shock, chronic pain, certain patterns of over- or under-eating and withdrawal from addictive drugs and the resultant increased activity of medium spiny output neurons contributes to aversive states5, 7. Negative reinforcement mechanisms associated with abstinence from long-term access to palatable food or abused drugs are mediated in part by excessive influence of pro-stress signaling systems (corticotropin releasing factor, dynorphin) and impaired function of anti-stress signaling systems (Neuropeptide Y, nociceptin) in stress circuits involving the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), frontal cortex and medial shell of the NAc5, 8.
Thus, reward processing is mediated in large part through an interconnected network of structures including the VTA, NAc, ventral pallidum, CeA, BNST and PFC. In addition to the well-known involvement of DA described above, reward processing is also heavily influenced by many other systems including the cholinergic, opioid peptide, glutamatergic, and GABAergic systems. CB1Rs are present in each of the interconnected structures involved in reward1, 2, 9 where they exert widespread modulatory influences on excitatory and inhibitory signaling in a manner that influences reward processing10, 11 In particular, ECs play a prominent role in fine-tuning the activity of the VTA-NAc DA projection and its influence on approach and avoidance behaviors that govern reward acquisition (Figure 3).
Figure 3. Endocannabinoid influences in the VTA and NAc contributing to approach and avoidance behavior.
a. EC influences on VTA synaptic signaling. Endocannabinoids produced by dopaminergic VTA neurons act on CB1Rs on nearby glutamatergic and GABAergic terminals before being degraded by ABHD6 or MAGL. CB1Rs mediate robust inhibition of GABA inputs arising from the pallidus, RMTg nucleus and local interneurons onto VTA DA cells 205 and most evidence points to a role for 2-AG but not AEA in these processes109, 206. CB1Rs are also localized on glutamatergic terminals synapsing on VTA DA neurons, with relatively greater expression on VGLUT1-positive terminals of cortical origin compared with VGLUT2-expressing terminals of subcortical origin207. Extensive evidence demonstrates EC-mediated suppression of glutamate signaling in VTA208. Thus, ECs play a prominent role in fine-tuning the activity of the mesolimbic DA projection through modulation of both excitatory and inhibitory signaling in the VTA. b. EC influences on NAc synaptic signaling. The majority of NAc neurons (>90%) are GABAergic medium spiny neurons (MSNs) that comprise the direct and indirect projection pathways. Direct pathway MSNs (dMSNs) project to midbrain regions including the VTA and activation of this pathway increases behavior toward a stimulus (approach or appetitive behavior). Indirect pathway MSNs (iMSNs) project to the ventral pallidum (VP) and activation of this pathway increases the stimulus avoidance209. dMSNs express excitatory D1 DA receptors and iMSNs express inhibitory D2 DA receptors, and thus reward-related phasic DA release activates the direct pathway and inhibits the indirect pathway, thereby increasing approach behavior and reducing avoidance behavior210. NAc MSN activity is also heavily modulated by glutamatergic inputs from the PFC, BLA and ventral hippocampus that express CB1Rs211, CB1-mediated suppression of excitatory signaling (EC-LTD) is preferentially active at iMSN synapses212 possibly resulting from D2 receptor-mediated EC production from iMSN cell bodies213. Thus, increased NAc EC formation preferentially reduces excitatory input to iMSNs vs. dMSNs, resulting in decreased avoidance behavior. Through these mechanisms, increased EC signaling in the NAc increases approach behavior while reducing avoidance-related processing thereby enhancing appetitive responding toward a stimulus. CB1Rs are also expressed on terminals of fast-spiking interneurons (FSIs) in the NAc, the majority of which are electrically and chemically coupled and provide direct innervation to adjacent MSNs214. FSIs exert important influence on the synchronization of neural ensemble activity and thus EC signaling may also exert critical influence on NAc output through feed-forward modulation of MSN network activity214.
Endocannabinoid signaling and reward
Both exogenous AEA and 2-AG increase extracellular DA levels in the NAc in a CB1R-dependent manner12 and the ECS exerts a strong influence on the fine-tuning of midbrain DA cell activity13. Through these and other interactions the ECS has a prominent influence on the hedonic effects of natural rewards such as food14, sexual activity15, and social interaction16. This is mediated in part through a direct CB1R modulation of the mesolimbic DA response to natural reward16 and through the interactions between the ECS and other signaling systems (endogenous opioids, hypothalamic signaling molecules, etc.)17, 18.
The rewarding effects of cannabinoid receptor activation are underscored by the fact that cannabis is one of the most widely used illicit substances worldwide. Δ9-THC is the primary psychoactive constituent in cannabis, and exhibits low efficacy as an agonist at CB1R and CB2R19. In animal models both Δ9-THC and synthetic CB1R agonists enhance brain reward function (indexed by intracranial self-stimulation), produce rewarding effects in the conditioned place-preference (CPP) paradigm and are voluntarily self-administered (intravenously and also directly into the NAc shell and posterior VTA)20. These effects are critically reliant on CB1R signaling and are highly dose-sensitive with a rapid shift to negative reinforcing effects with increasing dose.
In contrast to the effects of exogenous CB agonists, pharmacological enhancement of EC levels generally does not produce rewarding effects per se. For example, in most animal studies, selective EC clearance inhibitors do not support operant self-administration, do not produce conditioned place-preference and do alter brain stimulation reward thresholds in rats and mice21. Similarly, neither exogenously administered AEA nor 2-AG, nor selective FAAH or MAGL inhibitors produce Δ9-THC-like discriminative stimulus effects. However, exogenous AEA and 2-AG each support operant self-administration in squirrel monkeys21 and produce rewarding and Δ9-THC-like effects in rats when administered following EC clearance inhibition9, 22. Concurrent FAAH and MAGL inhibition in mice produces Δ9-THC-like discriminative stimulus and behavioral effects22, 23. These findings suggest that robust engagement of EC signaling is needed to evoke rewarding effects. However, recent evidence indicates that squirrel monkeys with a history of AEA, nicotine or cocaine self-administration will self-administer the FAAH inhibitor URB694, though this compound does not produce Δ9-THC- or nicotine-like discriminative stimulus effects and does not increase mesolimbic dopamine release24. Although it remains to be determined whether URB694 will be self-administered by drug-naïve monkeys or other species, this observation indicates that FAAH inhibition is not aversive and may produce mildly rewarding effects.
CB receptor involvement in non-cannabinoid drug reward
The presence of CB1Rs throughout brain reward circuits and the rewarding effects produced by CB1R activation allows for the possible influence of EC signaling on the acute rewarding effects produced by non-cannabinoid substances. The effects of CB1R and FAAH manipulations on non-cannabinoid drug reward are summarized in Table 1. In general, drugs that activate CB1Rs do indeed appear to facilitate the rewarding effects of non-cannabinoid drugs. CB1R agonists increase the motivational and reinforcing effects of alcohol, nicotine and opiates indexed by animal models of drug reward including the CPP and operant self-administration assays while diminished CB1R signaling (either genetic deletion or pharmacological antagonism) attenuates the motivational and rewarding effects of these drugs11, 25, 26. The effects of CB1R antagonism on alcohol and nicotine reward result in part from a diminished ability of these drugs to increase NAc DA release27. Blockade of CB1Rs specifically in the VTA decreases both alcohol and nicotine self-administration28, 29 and blockade of CB1Rs specifically in the NAc reduces alcohol consumption28, 30. However, while nicotine reward is critically dependent on the mesolimbic DA system31, the motivational and rewarding effects of alcohol and opiates are less DA-dependent32, 33 and the CB1R modulation of the rewarding effects of these drugs likely involves non-dopaminergic mechanisms. Indeed, CB1R antagonism does not alter opiate-induced increases in NAc DA but reduces opiate reward through prevention of opiate-induced reductions in ventral pallidal GABA release34. In comparison to these drugs, the effects of CB1R manipulations on psychostimulant reward are modest and less consistent. CB1R agonists reduce the facilitation of brain stimulation reward produced by cocaine and reduce cocaine self-administration35, 36. Most reports indicate that CB1R antagonism does not affect psychostimulant reward (cocaine-induced enhancement of brain stimulation reward, CPP, self-administration) or cocaine-induced increases in NAc DA37 (but see; 27, 37).
Table 1.
Summary of CB1R and FAAH influence on non-cannabinoid drug reward.
| EtOH | Nicotine | Opiates | Stimulants | |
|---|---|---|---|---|
| CB1 R KO |
 CPP CPP Self-Admin. Self-Admin. EtOH-induced NAc DA EtOH-induced NAc DA |
 CPP CPP Self-Admin. Self-Admin. |
 CPP CPP Self-Admin. Self-Admin. |
n/c CPP n/c Self-Admin. |
| CB1R Antagonist |
 Self-Admin. Self-Admin. Preference Preference EtOH-induced NAc DA EtOH-induced NAc DA |
 CPP CPP Self-Admin. Self-Admin. NIC-induced NAc DA NIC-induced NAc DA |
 CPP CPP Self-Admin. Self-Admin.n/c MORPH-induced NAc DA |
 Self-Admin (AM251) Self-Admin (AM251) COC effects on ICSS COC effects on ICSSn/c COC-induced NAc DA |
| CB1R Agonist |
 Self-Admin. Self-Admin. Motivation for EtOH Motivation for EtOH |
 CPP CPP |
 CPP CPP Motivation for heroin Motivation for heroin |
 Self-Admin Self-Admin COC effects on ICSS COC effects on ICSS |
| FAAH Inhibition |
 Self-Admin. Self-Admin. Preference Preference |
 CPP (mouse, CB1R) CPP (mouse, CB1R) CPP (rat, non-CB1R) CPP (rat, non-CB1R) Self-Admin (rat, non-CB1R) Self-Admin (rat, non-CB1R) NIC-induced NAc DA (rat, non-CB1R) NIC-induced NAc DA (rat, non-CB1R) |
n/c Self-Admin n/c MORPH-induced VTA DA excitation |
n/c COC-induced VTA DA excitation n/c Self-Admin |
EtOH, ethanol; NIC, nicotine; MORPH, morphine; COC, cocaine; CPP, conditioned place preference; n/c. no change; Self-Admin., operant self-administration; Preference, relative consumption of EtOH vs. water; ICSS, intracranial self-stimulation (an index of brain reward function);
Recent evidence in mice also implicates CB2Rs in the modulation of drug reward, including an inhibitory influence on cocaine and alcohol reward38, 39 but a facilitatory influence on nicotine reward40, 41. However, disparate observations have been made in rats39, 42, 43 and it is possible these findings are influenced by species differences in CB2R gene splicing that confer district CB2R structure, function or pharmacology39.
Alterations in brain EC levels by drugs of abuse
Given the “on demand” nature of EC production and associated modest EC signaling tone under baseline conditions, the robust influence of CB receptor signaling on non-cannabinoid drug reward has led to the hypothesis that drug exposure increases brain EC formation. Substantial evidence demonstrates alcohol-induced alterations in postmortem EC content in rodent brain, though inconsistencies among studies cloud definitive conclusions regarding the direction of change and regional nature of the effects 44. For example, alcohol exposure increases extracellular 2-AG levels in rat NAc (measured by in vivo microdialysis), and this is more pronounced following voluntary self-administration as compared to non-contingent alcohol exposure28, 30. In contrast, extracellular AEA levels in the NAc are unaltered by alcohol self-administration and decreased by non-contingent alcohol administration. Alcohol also appears to induce region-specific changes in brain tissue EC levels, with alcohol-induced disruptions consistently observed in striatal regions30, 45, 46 but not frontal cortical areas28 consistent with evidence that alcohol consumption is reduced by CB1R antagonism in the VTA and NAc, but not PFC28, 30, 47.
Similar to alcohol, nicotine also alters EC levels in rodent brain with factors such as the brain region evaluated and the voluntary nature of drug exposure having important relevance to the effects observed. Repeated non-contingent nicotine injections increase AEA levels in rat limbic forebrain and dorsal striatal tissue, but decrease both AEA and 2-AG levels in cortical tissue48. Intravenous nicotine self-administration increases extracellular levels of both AEA and 2-AG in rat VTA, and the effect on 2-AG is sensitized by chronic nicotine exposure49. Interestingly, while VTA 2-AG levels are elevated by either voluntary or non-contingent nicotine exposure, VTA AEA levels are increased only by voluntary nicotine self-administration49. Together with the evidence of distinct patterns of brain EC levels induced by volitional vs. non-contingent alcohol exposure28, 30 these data suggest that brain EC production is influenced not only by drug-related pharmacological effects but also by neural activity engaged by active drug self-administration (possibly related to the motivation for drug consumption).
Relatively less is known regarding the effects of other rewarding drugs on brain EC levels. Available evidence consistently indicates that opiates increase AEA, but decrease 2-AG tissue concentrations in the striatum, limbic forebrain and hippocampus.50, 51 Similarly heroin self-administration increases extracellular AEA with a concomitant decrease of extracellular 2-AG levels in the rat NAc30. Psychostimulants generally produce modest disruptions in brain EC content with subtle increases and decreases in 2-AG concentration in forebrain following non-contingent acute and chronic cocaine exposure, respectively (no other alterations evident regardless of region analyzed)48, 52. Moreover, voluntary cocaine self-administration does not alter rat extracellular NAc EC levels30 but decreases 2-AG content in frontal cortex and hippocampal tissue53–55.
Collectively these findings indicate that alcohol, nicotine, and opiates alter brain EC content, consonant with the CB1R influence on the behavioral effects produced by these drugs. The generally modest effects of psychostimulants on brain EC levels are in line with the subtle CB1R influence on psychostimulant-induced behaviors. Similar to that seen with multiple biological conditions, drug exposure often produces distinct and sometimes opposite effects on brain AEA and 2-AG levels, respectively. This suggests differential regulation of the synthesis and/or degradation of these EC moieties at specific synapses that may arise from the segregation of MAGL and FAAH in the pre- and post-synaptic compartments56, or the hypothesized role of AEA and 2-AG in regulating ‘tonic’ and ‘phasic’ signaling in the EC system, respectively57. Although a general picture of drug-induced alterations in brain EC levels is emerging, experimental differences between studies including drug dose, method of drug exposure and the duration of treatment make it difficult to draw strong conclusions and additional studies are warranted.
Influence of EC tone on drug reward
ECs are rapidly degraded, and thus strategies that reduce EC clearance have been employed as a means to further investigate the EC influence on drug reward. Most investigations have focused on the effects of FAAH inhibition because selective tools for inhibiting MAGL and other EC clearance enzymes were not available until recently. Such studies have shed light on important species differences that confounds the overall conclusions that can be made from existing data. For example, FAAH inhibition in mice increases nicotine reward in the CPP paradigm58, 59, though in rats FAAH inhibition prevents nicotine-induced CPP, diminishes nicotine self-administration and blunts nicotine-induced increases in NAc DA release60. The potentiation of nicotine reward in mice by FAAH inhibition is CB1R-mediated, whereas the reduction in nicotine reward in rats results from activation of PPAR-α by non-cannabinoid lipids such as oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) that are hydrolytically cleared by FAAH61. FAAH inhibition also produces distinct species-related alterations in alcohol consumption, with increased intake observed in mice but not rats62–65. The mechanisms underlying these differences are not understood. Brain region-specific disruptions in FAAH activity may be an important factor. In regard to alcohol reward as inhibition of FAAH activity specifically in the PFC results in increased alcohol consumption, and rats selectively bred for high alcohol intake and preference are characterized by reduced FAAH activity specifically in the PFC64, 65. The effects of FAAH inhibition on opiate and psychostimulant reward have primarily been studied in rats. FAAH inhibition does not alter morphine- or cocaine-induced disruptions in VTA DA cell firing or the self-administration of either drug66, 67. However, FAAH inhibition diminishes cocaine-induced alterations in NAc medium-spiny neuron activity103 and this may contribute to enhanced sensitization of both cocaine-induced motor activity and mesolimbic dopamine responses following repeated cocaine exposure68. Other studies have investigated the effects of putative EC transport inhibitors such as AM404 and VDM11, and the findings thus far suggest that these compounds produce subtle and inconsistent effects on nicotine and cocaine reward69–72.
While growing evidence implicates ECS influences in the modulation of acute drug reward, additional efforts are needed to further clarify the nature of EC disruptions caused by different classes of abused drug and the neural mechanisms through which these EC influences are mediated. Selective inhibitors of 2-AG clearance have recently been developed, but studies are still in their infancy so there are presently no published reports on the effects of enhanced 2-AG tone on drug reward and related physiological events. As such there remains a substantial gap of knowledge given the prominent 2-AG influence on neural signaling and plasticity related to both drug and natural rewards. Nevertheless, the role of the CB1R in drug reward is unequivocal and although there is evident complexity related to the effects produced by EC clearance inhibition (producing discrete modulation of EC tone in specific synapses/circuits as compared with broad CB1R activation by exogenous CB1R agonists) the extant evidence strongly supports an EC influence on the sensitivity to and motivation for several drugs of abuse.
Endocannabinoid Signaling in Addiction
A number of factors influence the transition from intermittent, controlled drug use to the compulsive forms of drug-seeking and drug-taking that characterize addiction. Substantial evidence implicates genetic influences in the development of substance use disorders (SUDs) and pathological forms of eating, sexual behavior and gambling73 and it is increasingly recognized that epigenetic mechanisms drive lasting changes in addiction-related gene expression74. Long-term drug exposure induces lasting neuroadaptations in motivational mechanisms that propel drug-seeking and use. Although initial drug use is motivated by hedonic processes, prolonged drug exposure progressively blunts reward system function thereby leading to escalated frequency and amount of drug consumption resulting in a dependent state wherein negative affective symptoms emerge during abstinence (e.g. dysphoria, anxiety, irritability). These negative emotional states arise from the recruitment of stress signaling systems (such as corticotropin releasing factor and dynorphin) and dysregulation of mechanisms that constrain these responses (such as neuropeptide Y and nociceptin) within the extended amygdala5. Renewed drug consumption alleviates these negative affective states, and this is conceptualized to motivate compulsive drug use through negative reinforcement5. Superimposed on these processes is a dysregulation of corticostriatal mechanisms mediating stimulus-response learning, constraint of impulsivity, conditioned reinforcement and incentive motivation resulting in a narrowed focus on drug-seeking at the expense of natural rewards6. ECs exert prominent modulatory influence in the extended amygdala and corticostriatal circuits5, 32, and increasing evidence suggests that pre-existing genetic influences on the ECS and/or drug-induced dysregulation of EC function participates in the development and maintenance of addiction, including pathological forms of eating (Box 1). The following sections consider the consequences of chronic drug exposure on EC signaling within the reward circuitry and related disruptions in synaptic plasticity, affective state and learning and memory mechanisms related to extinction and relapse. Lastly, evidence for an influence of innate disruptions in ECS function (EC gene polymorphisms) as vulnerability factors for substance abuse and addictive disorders in humans is discussed.
Box 1. Endocannabinoid signaling and eating disorders.
Food and drug addiction derive in part from aberrant brain reward function and share overlapping neuroadaptations in the mesolimbic system176. Similar to drug addiction, food addiction is defined by compulsive over- or under-eating, aberrant feeding despite negative consequences and unsuccessful attempts to “normalize” dysfunctional eating.
The rewarding effects of self-starvation or binge eating may involve disrupted EC function177. Anorexia nervosa and binge eating disorder are associated with increased blood AEA levels and diminished levels of leptin178 (an anorectic hormone that inhibits AEA synthesis). Anorexia is associated with a CNR1 AAT repeat polymorphism179 (but see180) and a synergistic association between the C385A FAAH gene and CNR1 rs1049353 polymorphisms181. The C385A polymorphism is also associated with obesity182, though it is unknown whether this may influence hedonic mechanisms, metabolism or both. Anorexia and bulimia nervosa are also associated with elevated plasma CB1R mRNA levels183, increased CB1R availability in frontal cortical areas184 and a nonsynonymous CNR2 polymorphism185. Preclinical models of anorexia suggest therapeutic effects Δ9-THC186 (but see187). Although two small clinical trials did not show Δ9-THC efficacy176 a larger trial demonstrated small but significant weight gain in women with severe anorexia nervosa following 4 weeks treatment with Dronabinol188. Conversely, in binge eating disorder the CB1R antagonist Rimonabant significantly reduces binge eating and promotes weight loss with only modest presentation of psychiatric side effects189, 190.
A withdrawal state similar to that associated with drugs of abuse is evident in rats during forced abstinence from highly palatable food191, 192 including increased anxiety and excessive consumption upon renewed palatable food access. These symptoms result from increased CRF1 signaling in the CeA8 and are reversed by increased CeA 2-AG-CB1R signaling193. This suggests that dysregulated CeA function is a common factor contributing to pathological motivation for drugs and food, and that CeA EC signaling may counter withdrawal-related stress signaling.
Chronic drug exposure and EC function
It is unsurprising that chronic cannabis use disrupts brain cannabinoid receptor availability and function. Using the in vivo technique of positron emission tomography (PET) imaging, Hirvonen et al.75 reported down-regulation of brain CB1Rs correlating with years of use by daily cannabis users, which were reversed after one month’s monitored abstinence. Another PET study reported a global reduction in CB1R availability76 driven by differences in the temporal lobe, anterior and posterior cingulate cortex and nucleus accumbens. Similarly, animals given non-contingent chronic cannabinoid exposure exhibit decreased CB1R function throughout the brain77, 78. Recent experiments employing stochastic optical reconstruction microscopy (STORM) demonstrate that chronic exposure to clinically-relevant doses of Δ9-THC results in a startling loss of CB1Rs on terminals of perisomatically projecting GABA interneurons in the mouse hippocampus, and internalization of the remaining CB1Rs79. The resulting deficits in inhibitory CB1R control over hippocampal GABA release persisted during several weeks of Δ9-THC abstinence, and this may underlie the enduring loss of hippocampal LTP in rodents and memory deficits in humans evident following chronic cannabinoid exposure80. Surprisingly little is known of the effect of chronic cannabinoid exposure on other facets of ECS function. Chronic cannabinoid exposure increases enzymatic clearance of AEA and reduces brain tissue AEA content in rodents81, 82 and frequent cannabis smokers present decreased AEA and increased 2-AG levels in blood83, 84, though increased serum AEA levels are evident following at least 6 months of cannabis abstinence85. The contribution of these disruptions to cannabis use disorder and related physiological and behavioral disruptions is presently unexplored. However, as discussed below, ECs provide important homeostatic constraint over emotional state86 and sleep function87, and it’s conceivable that Δ9-THC-induced impairment of endocannabinoid signaling contributes to negative emotional states and sleep disturbances present during protracted cannabis abstinence88, 89.
Several findings support the hypothesis that chronic exposure to non-cannabinoid drugs disrupts EC signaling and processing. Chronic alcohol exposure in rodents alters EC-related gene expression in a manner sensitive to the intermittent nature of alcohol exposure and post-alcohol abstinence period90 and down-regulates CB1R expression and function45, 91. Post-mortem studies of alcohol dependent humans also demonstrate disrupted CB1R expression in the ventral striatum and cortical regions92 and in vivo imaging studies demonstrate decreased CB1R availability in heavy drinking alcoholics that persist for at least 1 month of abstinence93, 94 (but see95). Although a potential contribution of CNR1 gene variants to these observations cannot be excluded, a common interpretation based on animal studies is that these CB1R adaptations in alcoholic humans are a consequence of prolonged alcohol-induced increases in brain EC levels. This is supported by evidence of transient recovery (and perhaps eventual upregulation) of CB1R function in humans during protracted alcohol abstinence91, 96. In rodents, chronic nicotine exposure induces distinct age-related disruptions in CB1R binding, with increased levels evident in the PFC, VTA and hippocampus of adolescent but not adult rats97, and increased hippocampal and decreased striatal CB1R binding in adult rats during protracted nicotine abstinence98. Few studies have investigated altered CB1R binding following chronic opiate or psychostimulant exposure but findings in rodents implicate impaired CB1R function in the development and expression of opiate dependence99, 100 and demonstrate that chronic cocaine increases CB1R binding in dorsal striatum, NAc and cortical areas101. Interestingly, detoxified cocaine addicts present significant increases in plasma AEA and decreases in plasma 2-AG content102 but the functional consequence of these disturbances is not known. Overall, accruing data suggests that long-term exposure to a variety of drug classes compromises EC processing and CB1R expression and function. As discussed below these perturbations may contribute to aberrant neural signaling during acute and protracted drug abstinence.
Addiction-related synaptic plasticity
The development and persistence of addiction is attributed to maladaptive synaptic plasticity evident in the neuronal reorganization (molecular, cellular and functional activity) of mesocorticolimbic and striatal pathways. EC signaling at CB1Rs is implicated in several forms of synaptic plasticity, most commonly (i) depolarization-induced suppression of excitatory or inhibitory transmission (DSE/DSI), (ii) short-term depression (STD) and (iii) long-term depression (LTD, a prolonged form of weakened synaptic strength)103, 104. STD and DSE/DSI are mediated primarily by 2-AG signaling, typically persist for a minute or less, and have been observed in brain areas relevant to reward and addiction including the VTA, basolateral amygdala, hippocampus, neocortex and substantia nigra10. In comparison, EC-mediated LTD can persist for hours to weeks, is particularly important in learning and memory, and has also been observed in addiction-related regions including the NAc, VTA, amygdala, PFC, hippocampus and dorsal striatum10.
Acute and chronic alcohol exposure reduces CB1R-dependent plasticity resulting in long-lasting disinhibition of striatal output neurons and diminished EC-mediated LTD at inhibitory striatal synapses105, 106. Because the dorsal striatum mediates reward-guided learning and habitual behavior these EC disruptions may contribute to maladaptive habitual behavior that perpetuates addiction107. Cocaine diminishes EC-LTD of excitatory transmission in the NAc108 and facilitates EC-LTD of inhibitory signaling at VTA DA synapses109, 110, resulting in diminished inhibitory control over VTA DA cell activity and heightened excitatory signaling in the NAc (Figure 4). Cocaine also disrupts EC-LTD of excitatory transmission in the BNST111, a component of the extended amygdala, and this may contribute to aberrant stress-reward interactions (via projections to the VTA)112 and excessive anxiety-like behavior. Similarly, chronic Δ9-THC or synthetic CB1R agonist exposure abolishes EC-LTD of excitatory and inhibitory signaling in the NAc and hippocampus113, 114 which may significantly impact reward processing mediated by these regions. Little is known regarding opiate- or nicotine-induced disruptions in EC-mediated synaptic plasticity, though cue-induced reinstatement of nicotine-seeking behavior (an animal model of relapse) relies in part on the induction of CB1R-mediated LTP of cortical synapses in the BNST115. Thus, chronic drug exposure disrupts EC-mediated forms of synaptic plasticity in several regions involved in reward processing. As will be discussed, impaired EC-mediated plasticity may also contribute to dependence-related affective disruptions that serve to sustain drug dependence.
Figure 4. Drug-induced alterations in EC-mediated synaptic plasticity.

Simplified summary of the effects of cocaine, Δ9-THC, and possibly other drugs of abuse on EC-mediated long-term depression (LTD). a | Under normal circumstances, EC-mediated long-term depression (EC-LTD) is induced by afferent stimulation with or without postsynaptic depolarization resulting in the 2-AG formation from postsynaptic cells. 2-AG activates CB1Rs on stimulated or neighboring non-stimulated neurons, which together with other events (e.g. increased [Ca+2], NMDAR stimulation, DA D2R stimulation, etc.) results in persistently decreased neurotransmitter release. The presynaptic signaling mechanisms contributing to EC-LTD are not fully understood. Depending on brain region, EC-LTD of both excitatory (glutamatergic) and inhibitory (GABAergic) afferents has been described. b | Repeated cocaine exposure facilitates the EC-LTD of inhibitory signaling in the VTA109, 110 resulting in diminished inhibitory constraint of VTA DA cell activity and increased excitability. c | In contrast, EC-LTD of excitatory signaling is lost in the NAc medium spiny neurons (MSN) following exposure to either cocaine or Δ9-THC108, 113, 114, resulting in diminished constraint of glutamatergic release and increased excitation of NAc cells. Thus, drug exposure results in concurrent loss of EC-mediated plasticity that normally provides inhibitory control over VTA DA cell excitation and loss of EC-mediated plasticity that normally constrains excitatory signaling in the NAc terminal region, conferring an overall enhancement of mesolimbic signaling.
Withdrawal-related affective disruption
Stress plays a prominent role in the development of addiction116 and stress exposure disrupts EC-mediated plasticity in regions including the NAc, amygdala and BNST117 that participate in emotional control. Withdrawal from most drugs of abuse is associated with increased stress responsivity and persistent negative affective symptoms such as anxiety and depression, the severity of which are closely associated with relapse susceptibility. Comorbidity of affective disorders and SUDs is prevalent and preexisting negative affective traits may be an antecedent to addiction. The ECS participates in a negative feedback system that constrains emotional distress under stressful circumstances and contributes to the suppression of aversive memories117, 118. This function is reliant on EC-mediated forms of synaptic plasticity, and deficient EC signaling is associated with increased anxiety and depression. As such impaired EC function may contribute to negative affective states and increased stress responsivity that underlie negative reinforcement mechanisms driving drug use by dependent individuals and that contribute to drug relapse following periods of abstinence.
Mice lacking CB1Rs exhibit greater anxiety-like behavior than normal animals during nicotine withdrawal119, though innate anxiety-like behavior in the knockouts clouds interpretations. Studies evaluating EC clearance inhibition provide more direct insight into withdrawal-related EC disruption and negative affect. Acute FAAH inhibition reverses enhanced anxiety-like behavior normally present during both nicotine and alcohol withdrawal65, 120 and the EC transport inhibitor AM404 attenuates depression-like behavior during nicotine withdrawal121. Post-traumatic stress disorder is particularly prevalent among individuals with alcohol-use disorders and this is often modeled in rodents using the fear-potentiated startle paradigm to study reflexive physiological reaction to a stimulus. Rodents selectively bred for high alcohol consumption exhibit greater fear-potentiated startle than corollary lines bred for low alcohol consumption122, 123 and acute FAAH inhibition by LY2183240 reduces fear-potentiated startle in high alcohol-preferring, but not low alcohol-preferring mice124 consistent with the efficacy of FAAH inhibition for accelerating the extinction of aversive memory125. LY2183240 also enhances the conditioned rewarding effects of alcohol without altering alcohol consumption itself, suggesting that FAAH inhibition influences memory-related processes (conditioned fear and conditioned alcohol reward) in animals predisposed toward high alcohol consumption.
Addiction-related learning and memory
Both positive and negative memories and conditioned cues associated with drug use perpetuate drug seeking and the continued cycle of abuse. The ECS plays a prominent role in learning and memory processes126 and CB1R signaling is strongly linked to the conditioned rewarding effects of alcohol, nicotine and opiates11, 25. Although drug-induced conditioning effects are generally interpreted in the context of drug reward, a CB1R influence on the associative learning aspects of drug exposure is also possible, which as discussed below may have relevance to the persistent reactivity to drug-related memories that characterizes addiction.
Drug-seeking (relapse)
Drug exposure produces powerful interoceptive effects that become associated with environmental cues such that these cues alone can induce craving and promote relapse following periods of abstinence127. In addition to conditioned drug memories, acute exposure to a preferred drug or pharmacologically-related agent (e.g. drug priming) and stressful events can also precipitate relapse116.
Animal models of relapse demonstrate an important cannabinoid influence on the reinstatement of extinguished drug-seeking and drug-taking behaviors. Δ9-THC and synthetic CB1R agonists reinstate drug-seeking for cannabinoids, alcohol, nicotine, opiates and cocaine while CB1R antagonists attenuate drug-seeking behavior associated with each of these drugs25, 128, 129. CB1Rs in the PFC and NAc shell influence cue-induced reinstatement of both heroin- and nicotine-seeking behavior, while CB1Rs in the basolateral amygdala also contribute to cue-induced nicotine-, but not heroin-seeking behavior130, 131. Despite the subtle effects of CB1R inactivation on psychostimulant self-administration, CB1R antagonism attenuates drug-primed, cue-induced and some forms of stress-induced reinstatement of cocaine- and methamphetamine-seeking behavior in rats25. Thus, CB1R signaling modulates drug-seeking for a variety of pharmacologically distinct drugs. There is also evidence that CB1R antagonism also blocks both cue- and “priming”–induced reinstatement of seeking behavior for non-drug rewards such as sucrose and corn oil132, 133 (but see134). Accordingly CB1R signaling appears to participate in the modulation of conditioned reward in general.
Both drug-primed and cue-induced nicotine- and cocaine-seeking behavior are reduced following acute FAAH inhibition that leads to elevated AEA levels 25, 60, which may be surprising since CB1R agonists enhance both nicotine- and cocaine-seeking behavior25. However, inhibition of EC clearance likely amplifies EC signaling preferentially in circuits/synapses activated by a given stimulus (in this case drug-seeking behavior), rather than inducing more widespread indiscriminate CB1R activation as produced by exogenous CB1R agonists. Moreover, FAAH hydrolyzes a large variety of fatty acid moieties and the effects of FAAH inhibition on drug-seeking may involve non-cannabinoid lipid signaling. In this regard, it’s notable that the EC transport inhibitor VDM11 attenuates both nicotine- and cue-induced nicotine-seeking behavior70 and this compound may preferentially block AEA clearance with weaker effects on non-cannabinoid lipids135, 136. Similarly, the EC transport inhibitor AM404 dose-dependently attenuates nicotine- and cue-induced nicotine-seeking behavior without altering nicotine self-administration71.
In contrast to nicotine- and cocaine-seeking, neither FAAH inhibition nor EC transport inhibition alter cue- or stress-induced reinstatement of alcohol-seeking behavior65, 137. However, studies in human alcoholics suggest a relationship between EC tone and craving that may relate to the degree of dependence and possibly inherent factors contributing to alcoholism vulnerability. In social drinkers, alcohol-related cues increase both craving and plasma AEA levels and the relative magnitude of cue-induced increases in AEA is significantly correlated with the degree of craving138. However, recently detoxified alcoholics present significantly lower baseline plasma AEA levels than non-dependent social drinkers and while alcohol-related cues elicit more intense craving in alcoholics these subjects do not present significant cue-induced increases in plasma AEA. This blunted AEA response may reflect aberrant EC processing in alcoholics, but further investigations are needed to confirm a direct link between this potential peripheral biomarker and brain EC signaling, as well as possible causal relationships between dysregulated EC processing and behavior.
Extinction learning
The potent motivational effects of drug-related cues create substantial difficulties during periods of attempted drug abstinence, and are causal in the reinstatement of drug intake (e.g. relapse)127. One approach for reducing the motivational impact of drug-associated cues is through extinction training wherein a subject learns that these cues no longer have predictive value. However, extinction therapy is generally ineffective for reducing relapse in both humans139 and rodents140, and it is conceivable this is a consequence of diminished learning mechanisms required to override the original cue association memory. The ECS plays a prominent role in memory extinction, and deficient CB1R signaling results in impaired extinction of cued fear memory, contextual fear memory, fear-potentiated startle and spatial memory under mildly aversive conditions141, 142. Moreover, as previously noted FAAH inhibition facilitates the extinction of fearful memory in mice selectively bred for high levels of alcohol preference and consumption124. Because aversive memory may be involved in relapse to drug taking143 deficient EC signaling following long-term drug exposure may contribute to the limited efficacy of extinction therapy for addiction.
EC gene polymorphisms and addiction
Approaches to explore the contribution of the ECS to addiction disorders in humans often involve heritability considerations since it is now acknowledged that genetics play an important role in drug addiction vulnerability accounting ~30–80% for risk depending on the drug class144, 145. Based on the growing evidence of the ECS in regulating reward, mood and cognition and due to its prominent expression within neuronal systems related to these functions, the ECS has been viewed as a central target for candidate gene studies of addictive disorders. Similar to the preclinical animal studies described above, most investigations have focused on the CNR1 and FAAH genes that encode the CB1R and FAAH proteins, respectively146, 147. Consistent with most genetic investigations, factors including race, ethnicity, type of drug, polysubstance use and population sample size are important confounds. Nevertheless, although not all equivocal, what can be garnered from existing genetic studies (though limited) suggests that genomic heterogeneity of the EC-related genes may influence in part substance abuse vulnerability and relate to behavioral and pathophysiological traits that are highly associated with addictive disorders in humans (Figure 5).
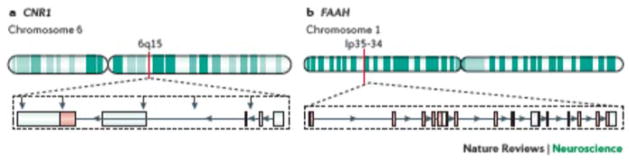
Figure 5. CNR1 and FAAH genes and genetic variants associated with addiction.
The EC genes primarily studied to date in relation to genetic associations with addiction are CNR1 and FAAH. The human CNR1 gene, which encodes the CB1R, maps to chromosome 6 specifically in the cytogenetic band 6q14-q15 and is transcribed from the minus strand (3′ to 5′ orientation) of the DNA. The gene contains four exons with the protein coding region located at the 5′ end of exon 4146–148. There are multiple mRNA variants of the CNR1 with the prominent form encoding the canonical 472 aa protein. The FAAH gene is located on human chromosome 1, 1p35-34, and is transcribed from the plus strand (5′ to 3′ orientation). The gene contains 15 exons with functional protein domains encoded across multiple exons. Recently, another FAAH gene, FAAH2, was identified on chromosome X in cytogenetic band Xp11.21 that is composed of a 532 amino acids protein that share about 20% sequence identity with the canonical FAAH1147. There is evidence, though not all consistent, that genetic variants (red arrows) associated with addiction and related phenotypes such as reward sensitivity, impulsivity and negative affect are located within exon 4 (rs1049353), introns (rs2023239, rs1535255, rs806380) and the 3′UTR (AATn triplet repeat, rs806368) of CNR1159,146–148. For the FAAH gene the polymorphic variant most associated with substance use disorders is rs324420 (exon 3)166,146, 147.
CNR1
The human CNR1 gene is located on the human chromosome 6 (6q14-q15) with the coding region situated at the 5′ end of exon 4. Several different CNR1 isoforms vary in expression across brain regions, though each of the main mRNA variants express the same exon 4 that encodes the CB1R protein148. Indeed, the CNR1 gene exhibits substantial functional conservation with few common missense variants in the CB1R protein148.
One of the first CNR1 variants explored in relation to drug abuse was the (AAT)n triplet repeat microsatellite polymorphism in the 3′ UTR located close to the exon 4 translational start site148. Unfortunately direct functional evidence is lacking to understand its relevance of EC processing, but the increased number of repeats is speculated to result in reduced CB1R expression149. Increased frequency of long (AAT)n repeats was initially observed in an intravenous drug-dependent non-Hispanic USA Caucasian population150, and this was partially supported in subsequent evaluations of Afro-Caribbean subjects151. Some reports failed to replicate the original finding148, 152, but meta-analysis of multiple variants of the CNR1 gene in Caucasian populations specifically identified the AAT polymorphism (n>16 repeats) as the only significant association with illicit substance use disorders153. Interestingly, the (AAT)n polymorphism has been linked with reduced amplitude of the frontal lobe P300 event-related brain potential, a disruption that has been suggested as a neurobiological endophenotype of impaired cortical processing in drug abusers146.
Additional SNPs of the CNR1 gene have been investigated, the most frequent of which is a silent intragenic biallelic polymorphism (G1359A; rs1049353). This exon 4 synonymous mutation does not change the amino acid sequence of the mature protein, but the SNP is speculated to affect mRNA stability or protein translation that could alter CB1R function. Several investigations, though not all congruent, suggest an association of the CNR1 G1359A polymorphism with substance abuse. For example, the A-allele is associated with severe alcoholism, specifically in relation to enhanced withdrawal delirium in Caucasian patients154 and enhanced impulsivity in Native Americans with a high lifetime prevalence of substance dependence155. The G1359A variant has also been associated with heroin abuse in Caucasians, but with the A-allele conferring protection and the G/G genotype addiction risk156. Additional studies are clearly needed to determine whether the risk/protection profile might depend on the drug class.
Aside from the G1359A SNP, most of the other CNR1 variants reported to be associated with addiction are not within the coding region; this is not surprising considering that it is now evident that most variants in the genome falls outside of protein-coding regions157. The rs2023239 variant representing a C/T polymorphism in the intronic region upstream of exon 3 has been shown to relate to CB1R levels measured both in postmortem brain tissue158 and in vivo using PET imaging93, with the C-allele associated with enhanced CB1R levels in the normal human brain. As discussed above, increased CB1R in animal models is predictive of addiction vulnerability and indeed the rs2023239 SNP has been linked to a general liability for substance abuse148. C allele individuals use greater amounts of marijuana, exhibit higher marijuana dependency and experience greater negative affect and craving for marijuana following withdrawal159. The rs2023239 minor allele also associates with increased activation in reward-associated brain areas [measured by blood oxygenation level dependent (BOLD) imaging] to marijuana-related cues 160. rs2023239 C-allele carriers also have enhanced alcohol cue-elicited brain activation in the PFC, NAc and midbrain (consistent with the VTA and surrounding regions), greater subjective reward when consuming alcohol, a strong correlation between cue-elicited brain activation and alcohol consumption measures, as well as a strong association with alcohol use disorder and craving measures161.
A number of CNR1 haplotype blocks have also been linked with addiction. When analyzed as a haplotype (T-A-G), three SNPs (rs806379-rs1535255-rs2023239) in the distal region of intron 2 of the CNR1 gene were significantly associated with polysubstance abuse in adults from different ethnicities148. Moreover, Agrawal and colleagues162 demonstrated an association between a CNR1 haplotype (rs806380, rs806368, and rs754387) and cannabis dependence (the majority of those subjects also met criteria for alcohol dependence). The rs806380 SNP proximal to the T-A-G haplotype has also been linked with the development of cannabis dependence symptoms (protective effect of G-allele). Other haplotypes have been reported to associate with either low (rs6454674, rs806380, rs806377, rs1049353: G-G-C-C) or increased (T-A-C-C and G-A-C-C) risk for cannabis dependence163. However, inconsistent and nominal significance have been reported in replication studies of cannabis dependence in adolescent and young adult populations164 and for other haplotypes in substance abuse populations165. Overall, although the majority of genetic investigations suggest an association between CNR1 variants and aspects of SUD, the data are not definitive and no causative loci have been described to date. What appears most consistent in the human genetic studies is a relevance to cue and craving which would complement the preclinical animal studies that demonstrate their direct link to the ECS.
FAAH
In comparison to CNR1, few genetic investigations have focused on other components of the ECS with FAAH being the second gene most often studied in relation to addiction based on AEA’s important functional role. The human FAAH gene is located on chromosome 1p35–34 with 15 exons and functional protein domains encoded across multiple exons. A SNP that has been highly investigated is rs324420, located in exon 3 that results in a missense mutation of a C-to-A replacement at position 385, leading to a proline to threonine change at protein position 129166. This C385A SNP is functional, and believed to result in reduced FAAH expression and enzyme activity such that individuals with the A/A genotype have enhanced plasma concentrations of AEA and other N-acylethanolamine FAAH substrates167. Although some studies have not observed associations between the C385A polymorphism with substance use disorders, existing evidence implicates this genetic disruption in addiction-related behaviors in different races and ethnicities147. Specifically, the A/A genotype associates with reduced vulnerability for cannabis dependence in adult Caucasians while the C/C genotype associates with increased craving and negative affect during marijuana withdrawal. Initial studies failed to detect a link between the A/A genotype with alcohol or nicotine dependence168 though recently an over-representation of C/C carriers was observed among risky alcohol drinkers169. Carriers of the FAAH C385A SNP display increased ventral striatal reactivity associated with delay discounting, a behavioral index of impulsivity and reward sensitivity170 and a markedly decreased relationship between threat-related amygdala reactivity and trait anxiety, similar to patterns observed in individuals with high familial risk for alcoholism171. These findings suggest that dysregulation of FAAH function through the C385A polymorphism confers increased impulsivity and increased anxiety sensitivity.
Collectively, recent studies of CNR1 and FAAH genetic variants generally suggest an association with endophenotypes implicated in addiction susceptibility including reward sensitivity, impulsivity and negative affect consistent with preclinical evidence linking the ECS to such behaviors. Gene-gene interactions within the ECS may also be relevant to vulnerability as there appear to be additive interactions between variants of the FAAH (C385A/rs324420) and CNR1 (rs2023239) genes resulting in heightened neural responses in reward-related brain areas to marijuana cues and more severe negative affect during marijuana abstinence159, 160. Growing evidence of an EC influence on epigenetic mechanisms suggests an additional but understudied way in which EC signaling may contribute to addiction (Box 2).Clearly, a major confound of most investigations to date is the small population size emphasizing the need for replication studies and larger populations. The few existing agnostic genome-wide approaches have not identified EC-related genes in relation to substance use disorders interrogated thus far. However, the contribution of endophenotypes along the continuum between genotype and drug abuse phenotype has not been interrogated despite the complex nature of non-Mendelian addictive disorders. The lack of systematic consideration of behavioral traits limits the possibility to understand the full repertoire of the ECS to individual vulnerability to addition. Moreover the functional consequences of the variants (causal or correlated) are unknown which makes definitive conclusions challenging.
Box 2. Endocannabinoid influence on epigenetic mechanisms.
Epigenetic influences refer to functionally relevant changes to the genome that do not involve disruptions in the nucleotide sequence of DNA. Examples of epigenetic mechanisms include DNA methylation, posttranslational histone modification, nucleosome positioning and small non-coding RNAs (microRNA, small interfering RNA (siRNA)). Recent evidence demonstrates that epigenetic factors can regulate the expression of EC-related genes, and that ECs may themselves induce epigenetic alterations194. For example, DNA hypermethylation of the CNR1 gene results in down regulation of CB1R transcription in the CNS and immune system, while decreased DNA methylation can result in increased FAAH transcription; these processes have been implicated in several pathologies including colon cancer and late onset Alzheimer’s Disease. Conversely, EC-induced alterations in enzymes influencing histone modification may disrupt transcription of several genes including those encoding various neurotransmitter systems. In rodents, early life stress (maternal separation) is associated with elevated DNA methylation of the CNR1 promoter195, which through a resultant decrease in CB1R expression could contribute to affective dysregulation and addiction susceptibility later in life. Several studies implicate increased epigenetic influences following chronic Δ9-THC exposure. Marijuana dependent patients present robust methylation of the CNR1 promoter in association with diminished CNR1 mRNA in peripheral blood cells196. Further, offspring of maternal marijuana users exhibit histone methylation and dysregulated mesolimbic dopamine D2 receptor Drd2 expression197 and adolescent Δ9-THC exposure is associated with NAc chromatin modifications and concurrent upregulation of the opioid neuropeptide proenkephalin gene198. Prenatal alcohol exposure increases expression of the regulatory microRNA miR-26b (which targets the 3′-UTR of the CNR1 transcript) and decreased CNR1 transcription in the adult mouse brain199. Thus, growing evidence suggests EC-related epigenetic influences following drug exposure.
Summary and future directions
Although enhancement of EC levels does not produce rewarding effects per se, EC signaling at cannabinoid receptors participates in the mediation and modulation of both natural and drug-induced reward. Brain EC content is modulated by most drugs of abuse and natural rewards and a robust CB1R influence on the motivation to consume distinct classes of abused drugs and the association of CNR1 gene polymorphisms with aberrant reward processing and addictive behaviors strongly implicates CB1Rs in the etiology of addiction. Long-term drug use leads to neuroadaptive down-regulation of EC signaling resulting from diminished CB1R and/or CB2R function as well as possible disruptions in EC biosynthesis and/or clearance. This blunting of EC function may contribute to increased stress responsivity, increased negative affect, inefficient extinction of drug-related memories and drug seeking/craving that are known contributors to relapse. Recent preclinical evidence demonstrates the efficacy of EC clearance inhibitors for ameliorating these behavioral abnormalities that might offer future therapeutic interventions for addiction disorders. Importantly, because ECs are generally produced in a synapse-specific manner, EC clearance inhibitors may preferentially facilitate EC signaling in specific circuits engaged by distinct stimuli (e.g. stress, drug-associated cue, etc.) and therefore could present fewer unwanted behavioral effects than produced by exogenous agonists that produce widespread CB receptor activation.
Despite growing attention on cannabinoids, there continues to be notable gaps in our understanding of the EC influence on reward and addiction. The ECS plays a prominent role in neuronal guidance and brain development172 and as such disruptions in EC function at an early age likely has substantial consequences for adult brain function. This is underscored by increasing evidence of the long-term consequences of prenatal or adolescent cannabinoid exposure 173, 174. Though the effects of early life exposure to non-cannabinoid drugs are well studied, the specific contributions of persistent drug-induced disruptions in EC signaling on adult neural function and behavior are not understood. Robust bidirectional interactions between the ECS and sex hormones is now recognized175, but few studies have characterized possible sex differences in the EC influence on reward function, addiction and cognitive processing. There are also substantial limitations in the interpretation and replication of genetic analyses of the EC influence in addiction due to heterogeneity of the populations studied, drug class, polysubstance use and even drug use phenotypes examined. Large-scale future studies across different populations and drug classes will be critical to understand the relative impact and causal nature of ECS-related genetic mutations in the vulnerability to addictive disorders. Filling these gaps of knowledge are critical given the important need for scientific data to help guide current discussions and changes being made in marijuana legalization policies.
Online summary.
Cannabinoid receptors and their endogenous ligands are widely expressed throughout the brain, with particularly strong presence and influence in neuronal circuits such as the mesocorticolimbic pathways highly implicated in reward and addiction.
CB1 receptor signaling influences the motivation for both natural and drug rewards. In comparison to most drugs of abuse CB1 receptors exert only modest influence on psychostimulant intake.
Brain EC levels are increased by most drugs of abuse, though the nature of this effect differs between classes of drugs and across brain regions. The response contingency of drug exposure (volitional vs. response-independent) appears to influence brain EC production, suggesting contributions of both drug-related pharmacological effects and neural activity engaged by active drug-seeking.
Chronic exposure to drugs of abuse generally results in impaired CB1 receptor function, loss of EC-mediated synaptic plasticity in addiction-related neural circuits, and negative affective states that can be ameliorated through pharmacologically enhanced EC tone. The ECS plays a strong role in modulating relapse-like behavior induced by conditioned cues or reward priming, and this is evident for both natural and drug rewards.
Recent investigations of CNR1 and FAAH gene variants generally suggest an association with endophenotypes implicated in addiction susceptibility including reward sensitivity, impulsivity and negative affect. However, confounding factors including restricted sample size, ethnicity and polysubstance use limit interpretational power, and the functional consequences of the variants (causal or linked) are currently unknown.
Acknowledgments
This work was supported by grants from the US National Institutes of Health (NIH) to L.P. (AA020404, AA006420, AA022249 and AA017447) and Y.H. (DA023214, DA030359 and DA033660). This is manuscript number 29049 from The Scripps Research Institute. The authors wish to thank D. Lewis for his help during the preparation of this manuscript.
Glossary of Terms
(note entries with asterisks may be cut as needed)
- Limbic system
A collection of brain structures that includes the amygdala, hippocampus, limbic cortex, limbic midbrain areas and anterior thalamic nuclei, and regulates autonomic and endocrine function and participates in the control of emotion, motivation, long-term memory and olfaction
- Intracranial Self-Stimulation (ICSS)
An operant behavioral paradigm in which subjects elicit a behavioral response (level press, wheel turn) to receive brief electrical pulses into specific regions in the brain reward pathways
- Conditioned Place-Preference (CPP) procedure
Behavioural paradigm to study the rewarding and aversive effects of drugs associated with a specific environment
- Self-administered
Operant self-administration is a behavioral procedure in which experimental subjects learn to elicit an operant response (lever press, nose poke) to receive a drug reward (intravenous infusion, small bolus for oral consumption, delivery of discrete bolus of vapor that is inhaled)
- Discriminative stimulus
a stimulus in a drug discrimination paradigm that the animal has learned to associate with predictable consequence (rewarding or unrewarding) and that increases the elicitation of a specific behavior in
- Non-contingent
Drug delivery that is involuntary (experimenter-administered) or not contingent on the elicitation of a behavioral response by an experimental subject; sometimes referred to as forced administration
- Epigenetic mechanisms
the mechanisms by which functionally relevant changes to the genome occur that do not involve disruptions in the nucleotide sequence of DNA, and include DNA methylation, histone modification and non-coding RNA-associated gene silencing
- Extended amygdala
A grouping of brain regions involved in processing reward perception that includes the central nucleus of the amygdala, sublenticular substantia innominate, nucleus accumbens shell and the bed nucleus of the stria terminalis
- Conditioned reinforcement
The process through which neutral stimuli acquire motivational properties through association with a primary reinforcer
- Stochastic optical reconstruction microscopy (STORM)
A super-resolution imaging technique that uses sequential activation and time-resolved localization of photoswitchable fluorophores to create high resolution images enabling the precise flurophore localization with nanometer resolution
- Synaptic plasticity
The process by which synaptic communication strengthens or weakens as a result of changes in morphology, composition or signal transduction efficiency in response to intrinsic or extrinsic signals
- Haplotype block
A set of DNA variations, or polymorphisms, that tend to be inherited together
- Endophenotype
A term used to separate behavioral symptoms into stable phenotypes with a clear genetic basis, typically applicable to heritable disorders
- Posttranslational histone modification
A covalent modification of proteins that package and order DNA into nucleosomes (e.g. histones). These modifications occur during or after histone biosynthesis
- Cytogenetic Band
Distinct region on the chromosome (visible microscopically after special staining)
Biographies
Prof Loren Parsons
Loren Parsons received his Ph.D. in chemistry from Emory University in Atlanta, Georgia, USA followed by postdoctoral training in behavioral pharmacology and neurochemistry with George Koob and Friedbert Weiss at The Scripps Research Institute (TSRI) in La Jolla, California, USA. He is currently Professor in the Committee on the Neurobiology of Addictive Disorders at TSRI, Director of the TSRI Alcohol Research Center and Director of the Ruth L. Kirchstein Training Program at TSRI. His research focuses on the neurobiological bases of addiction with particular focus on the role of dysregulated endocannabinoid signaling in drug dependence and protracted withdrawal, and the cortical mechanisms underlying dependence-related cognitive impairment. The Parsons lab website is http://www.scripps.edu/parsons/Research.html.
Prof Yasmin Hurd
Yasmin Hurd obtained her Doctor of Medical Science degree (PhD) at the Karolinska Institute, Stockholm, Sweden and conducted her post-doctoral training at the National Institute of Mental Health, Bethesda, Maryland, USA. She is currently Professor of Psychiatry, Neuroscience and Pharmacology & Experimental Therapeutics at the Icahn School of medicine at Mount Sinai in New York where she is also the Ward-Coleman Chair of Translational Neuroscience and Director of the Center for Addictive Disorders. Her laboratory investigates the developmental and cross-generational effects of cannabinoids as well as epigenetic mechanisms and genetic factors contributing to addiction risk. The Hurd lab website is http://neuroscience.mssm.edu/hurd.
Footnotes
Competing interests statement.
The authors have no competing financial interest to declare.
Cited References
- 1.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Dow-Edwards D, Keller E, Hurd YL. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience. 2003;118:681–94. doi: 10.1016/s0306-4522(03)00020-4. References 1 and 2 demonstrated the anatomical expression of CB receptors in the human fetal and adult brains that are important targets for EC and exogenous cannabinoid ligands. [DOI] [PubMed] [Google Scholar]
- 3.Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–79. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–35. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–32. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iemolo A, et al. CRF-CRF1 receptor system in the central and basolateral nuclei of the amygdala differentially mediates excessive eating of palatable food. Neuropsychopharmacology. 2013;38:2456–66. doi: 10.1038/npp.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herkenham M, et al. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–83. doi: 10.1523/JNEUROSCI.11-02-00563.1991. This paper was one of the first to visualize cannabinoid receptors in the rodent brain that emphasized that it was one of the most abundent G-protein-coupled receptor in brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidhpura N, Parsons LH. Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. Neuropharmacology. 2011;61:1070–87. doi: 10.1016/j.neuropharm.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panagis G, Mackey B, Vlachou S. Cannabinoid Regulation of Brain Reward Processing with an Emphasis on the Role of CB1 Receptors: A Step Back into the Future. Front Psychiatry. 2014;5:92. doi: 10.3389/fpsyt.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–19. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- 13.Melis M, Pistis M. Hub and switches: endocannabinoid signalling in midbrain dopamine neurons. Philos Trans R Soc Lond B Biol Sci. 2012;367:3276–85. doi: 10.1098/rstb.2011.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–90. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Klein C, Hill MN, Chang SC, Hillard CJ, Gorzalka BB. Circulating endocannabinoid concentrations and sexual arousal in women. J Sex Med. 2012;9:1588–601. doi: 10.1111/j.1743-6109.2012.02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–9. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fattore L, et al. Cannabinoids and reward: interactions with the opioid system. Crit Rev Neurobiol. 2004;16:147–58. doi: 10.1615/critrevneurobiol.v16.i12.160. [DOI] [PubMed] [Google Scholar]
- 18.Di Marzo V, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–5. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 19.Mechoulam R, Hanus LO, Pertwee R, Howlett AC. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci. 2014;15:757–64. doi: 10.1038/nrn3811. [DOI] [PubMed] [Google Scholar]
- 20.Vlachou S, Panagis G. Regulation of brain reward by the endocannabinoid system: a critical review of behavioral studies in animals. Curr Pharm Des. 2014;20:2072–88. doi: 10.2174/13816128113199990433. [DOI] [PubMed] [Google Scholar]
- 21.Panlilio LV, Justinova Z, Goldberg SR. Inhibition of FAAH and activation of PPAR: new approaches to the treatment of cognitive dysfunction and drug addiction. Pharmacol Ther. 2013;138:84–102. doi: 10.1016/j.pharmthera.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiley JL, et al. Endocannabinoid contribution to Delta(9)-tetrahydrocannabinol discrimination in rodents. Eur J Pharmacol. 2014;737:97–105. doi: 10.1016/j.ejphar.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long JZ, et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A. 2009;106:20270–5. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Justinova Z, et al. Effects of Fatty Acid Amide Hydrolase (FAAH) Inhibitors in Non-Human Primate Models of Nicotine Reward and Relapse. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Ther. 2011;132:215–41. doi: 10.1016/j.pharmthera.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–15. doi: 10.1038/sj.npp.1301127. This study was among the first to demonstrate that adolescent 9-THC exposure results in enhanced mu opioid receptor signaling in the nucleus accumbens that contributes to increased heroin self-administration. [DOI] [PubMed] [Google Scholar]
- 27.Cheer JF, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–5. doi: 10.1523/JNEUROSCI.4152-06.2007. This study was one of the first to demonstrate a common CB1R-dopamine interaction contributing to the dopaminergic effects produced by abused substances with distinct pharmacological properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez-Jaimes L, Parsons LH. Regional influence of CB1 receptor signaling on ethanol self-administration by rats. The Open Neuropsychopharmacology. 2009;2:77–85. doi: 10.2174/1876523800902020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simonnet A, Cador M, Caille S. Nicotine reinforcement is reduced by cannabinoid CB1 receptor blockade in the ventral tegmental area. Addict Biol. 2013;18:930–6. doi: 10.1111/j.1369-1600.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 30.Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–702. doi: 10.1523/JNEUROSCI.4403-06.2007. This study provided the first in vivo evidence that voluntary self-administration of non-cannabinoid drugs produces drug-specific and dose-reliant alterations in extracellular AEA and 2-AG levels in the rat nucleus accumbens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Souza MS, Markou A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict Sci Clin Pract. 2011;6:4–16. [PMC free article] [PubMed] [Google Scholar]
- 32.Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shippenberg TS, Elmer GI. The neurobiology of opiate reinforcement. Crit Rev Neurobiol. 1998;12:267–303. doi: 10.1615/critrevneurobiol.v12.i4.10. [DOI] [PubMed] [Google Scholar]
- 34.Caille S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–13. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- 35.Vlachou S, Nomikos GG, Panagis G. WIN 55,212-2 decreases the reinforcing actions of cocaine through CB1 cannabinoid receptor stimulation. Behav Brain Res. 2003;141:215–22. doi: 10.1016/s0166-4328(02)00370-4. [DOI] [PubMed] [Google Scholar]
- 36.Fattore L, Martellotta MC, Cossu G, Mascia MS, Fratta W. CB1 cannabinoid receptor agonist WIN 55,212-2 decreases intravenous cocaine self-administration in rats. Behav Brain Res. 1999;104:141–6. doi: 10.1016/s0166-4328(99)00059-5. [DOI] [PubMed] [Google Scholar]
- 37.Oliere S, Joliette-Riopel A, Potvin S, Jutras-Aswad D. Modulation of the endocannabinoid system: vulnerability factor and new treatment target for stimulant addiction. Front Psychiatry. 2013;4:109. doi: 10.3389/fpsyt.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xi ZX, et al. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–6. doi: 10.1038/nn.2874. This study provided the first evidence that drug reward can be modulated by changes in brain CB2 receptor activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang HY, et al. Species Differences in Cannabinoid Receptor 2 and Receptor Responses to Cocaine Self-Administration in Mice and Rats. Neuropsychopharmacology. 2014;40:1037–51. doi: 10.1038/npp.2014.297. This study characterized important species differences in the splicing and expression of CB2R genes and receptor structures relevant to different effects noted for CB2R-selective ligands in mice and rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ignatowska-Jankowska BM, Muldoon PP, Lichtman AH, Damaj MI. The cannabinoid CB2 receptor is necessary for nicotine-conditioned place preference, but not other behavioral effects of nicotine in mice. Psychopharmacology (Berl) 2013;229:591–601. doi: 10.1007/s00213-013-3117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarrete F, et al. Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacology. 2013;38:2515–24. doi: 10.1038/npp.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adamczyk P, et al. The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res. 2012;1444:45–54. doi: 10.1016/j.brainres.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 43.Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self administration and reinstatement of nicotine seeking. PLoS One. 2012;7:e29900. doi: 10.1371/journal.pone.0029900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol. 2012;46:185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceccarini J, Casteels C, Koole M, Bormans G, Van Laere K. Transient changes in the endocannabinoid system after acute and chronic ethanol exposure and abstinence in the rat: a combined PET and microdialysis study. Eur J Nucl Med Mol Imaging. 2013;40:1582–94. doi: 10.1007/s00259-013-2456-1. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez-Jaimes L, Stouffer DG, Parsons LH. Chronic ethanol treatment potentiates ethanol-induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J Neurochem. 2009;111:37–48. doi: 10.1111/j.1471-4159.2009.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malinen H, Hyytia P. Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2008;32:1976–83. doi: 10.1111/j.1530-0277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez S, et al. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- 49.Buczynski MW, Polis IY, Parsons LH. The volitional nature of nicotine exposure alters anandamide and oleoylethanolamide levels in the ventral tegmental area. Neuropsychopharmacology. 2013;38:574–84. doi: 10.1038/npp.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vigano D, et al. Chronic morphine modulates the contents of the endocannabinoid, 2-arachidonoyl glycerol, in rat brain. Neuropsychopharmacology. 2003;28:1160–7. doi: 10.1038/sj.npp.1300117. [DOI] [PubMed] [Google Scholar]
- 51.Vigano D, et al. Changes in endocannabinoid levels in a rat model of behavioural sensitization to morphine. Eur J Neurosci. 2004;20:1849–57. doi: 10.1111/j.1460-9568.2004.03645.x. [DOI] [PubMed] [Google Scholar]
- 52.Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–8. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- 53.Bystrowska B, Smaga I, Frankowska M, Filip M. Changes in endocannabinoid and N-acylethanolamine levels in rat brain structures following cocaine self-administration and extinction training. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:1–10. doi: 10.1016/j.pnpbp.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Rivera P, et al. Cocaine self-administration differentially modulates the expression of endogenous cannabinoid system-related proteins in the hippocampus of Lewis vs. Fischer 344 rats. Int J Neuropsychopharmacol. 2013;16:1277–93. doi: 10.1017/S1461145712001186. [DOI] [PubMed] [Google Scholar]
- 55.Palomino A, et al. Effects of acute versus repeated cocaine exposure on the expression of endocannabinoid signaling-related proteins in the mouse cerebellum. Front Integr Neurosci. 2014;8:22. doi: 10.3389/fnint.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gulyas AI, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–58. doi: 10.1111/j.1460-9568.2004.03428.x. This was one of the first studies to characterize the celluar location of the EC metabolic enzymes thereby emphasizing potentially distinct influences of AEA and 2-AG processing on synaptic function. [DOI] [PubMed] [Google Scholar]
- 57.Kim J, Alger BE. Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses. Nat Neurosci. 2010;13:592–600. doi: 10.1038/nn.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther. 2008;326:483–92. doi: 10.1124/jpet.108.138321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muldoon PP, Lichtman AH, Parsons LH, Damaj MI. The role of fatty acid amide hydrolase inhibition in nicotine reward and dependence. Life Sci. 2013;92:458–62. doi: 10.1016/j.lfs.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scherma M, et al. Inhibition of anandamide hydrolysis by cyclohexyl carbamic acid 3’-carbamoyl-3-yl ester (URB597) reverses abuse-related behavioral and neurochemical effects of nicotine in rats. J Pharmacol Exp Ther. 2008;327:482–90. doi: 10.1124/jpet.108.142224. This study provided the first evidence that FAAH inhibition reduces nicotine reward in rats, leading to a series of studies demonstrating that stimulation of PPAR receptors by FAAH substrates including oleoylethanolamide prevents nicotine-induced activation of the mesolimbic dopamine system in rats and non-human primates (these collective studies are reviewed and discussed in reference 61) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melis M, Pistis M. Targeting the interaction between fatty acid ethanolamides and nicotinic receptors: therapeutic perspectives. Pharmacol Res. 2014;86:42–9. doi: 10.1016/j.phrs.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 62.Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–82. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- 63.Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008;104:233–43. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- 64.Hansson AC, et al. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117–26. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- 65.Cippitelli A, et al. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology (Berl) 2008;198:449–60. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- 66.Justinova Z, et al. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–7. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luchicchi A, et al. Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-alpha nuclear receptors. Addict Biol. 2010;15:277–88. doi: 10.1111/j.1369-1600.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mereu M, et al. Cocaine-induced endocannabinoid release modulates behavioral and neurochemical sensitization in mice. Addict Biol. 2013;20:91–103. doi: 10.1111/adb.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scherma M, et al. The anandamide transport inhibitor AM404 reduces the rewarding effects of nicotine and nicotine-induced dopamine elevations in the nucleus accumbens shell in rats. Br J Pharmacol. 2012;165:2539–48. doi: 10.1111/j.1476-5381.2011.01467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gamaleddin I, Guranda M, Goldberg SR, Le Foll B. The selective anandamide transport inhibitor VDM11 attenuates reinstatement of nicotine seeking behaviour, but does not affect nicotine intake. Br J Pharmacol. 2011;164:1652–60. doi: 10.1111/j.1476-5381.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gamaleddin I, et al. AM404 attenuates reinstatement of nicotine seeking induced by nicotine-associated cues and nicotine priming but does not affect nicotine- and food-taking. J Psychopharmacol. 2013;27:564–71. doi: 10.1177/0269881113477710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vlachou S, Stamatopoulou F, Nomikos GG, Panagis G. Enhancement of endocannabinoid neurotransmission through CB1 cannabinoid receptors counteracts the reinforcing and psychostimulant effects of cocaine. Int J Neuropsychopharmacol. 2008;11:905–23. doi: 10.1017/S1461145708008717. [DOI] [PubMed] [Google Scholar]
- 73.Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiatr Clin North Am. 2012;35:495–519. doi: 10.1016/j.psc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76(Pt B):259–68. doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hirvonen J, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–9. doi: 10.1038/mp.2011.82. This study was the first to provide in vivo evidence of regionally selective down-regulation of brain CB1Rs in human cannabis smokers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ceccarini J, et al. [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict Biol. 2015;20:357–67. doi: 10.1111/adb.12116. [DOI] [PubMed] [Google Scholar]
- 77.Breivogel CS, et al. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–59. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- 78.Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:8057–66. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dudok B, et al. Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat Neurosci. 2015;18:75–86. doi: 10.1038/nn.3892. This study employed nano-scale imaging and electrophysiological techniques to demonstrate greater CB1R expression and influence on perisomatically-projecting vs. dendritically-projecting GABA interneurons in the mouse hippocampus, and that persistent deficits in hippocampal LTP following chronic 9-THC exposure results from near complete loss of CB1R at somatic synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Puighermanal E, Busquets-Garcia A, Maldonado R, Ozaita A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philos Trans R Soc Lond B Biol Sci. 2012;367:3254–63. doi: 10.1098/rstb.2011.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Marzo V, et al. Enhancement of anandamide formation in the limbic forebrain and reduction of endocannabinoid contents in the striatum of delta9-tetrahydrocannabinol-tolerant rats. J Neurochem. 2000;74:1627–35. doi: 10.1046/j.1471-4159.2000.0741627.x. [DOI] [PubMed] [Google Scholar]
- 82.Schlosburg JE, et al. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 2009;11:342–52. doi: 10.1208/s12248-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leweke FM, et al. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophr Res. 2007;94:29–36. doi: 10.1016/j.schres.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 84.Morgan CJ, et al. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry. 2013;202:381–2. doi: 10.1192/bjp.bp.112.121178. [DOI] [PubMed] [Google Scholar]
- 85.Muhl D, et al. Increased CB2 mRNA and anandamide in human blood after cessation of cannabis abuse. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:691–5. doi: 10.1007/s00210-014-0984-2. [DOI] [PubMed] [Google Scholar]
- 86.Lutz B. Endocannabinoid signals in the control of emotion. Curr Opin Pharmacol. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Murillo-Rodriguez E, et al. The emerging role of the endocannabinoid system in the sleep-wake cycle modulation. Cent Nerv Syst Agents Med Chem. 2011;11:189–96. doi: 10.2174/187152411798047780. [DOI] [PubMed] [Google Scholar]
- 88.Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–24. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- 89.Gates P, Albertella L, Copeland J. Cannabis withdrawal and sleep: A systematic review of human studies. Subst Abus. 2015 doi: 10.1080/08897077.2015.1023484. [DOI] [PubMed] [Google Scholar]
- 90.Serrano A, et al. Differential effects of single versus repeated alcohol withdrawal on the expression of endocannabinoid system-related genes in the rat amygdala. Alcohol Clin Exp Res. 2012;36:984–94. doi: 10.1111/j.1530-0277.2011.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mitrirattanakul S, et al. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res. 2007;31:855–67. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 92.Vinod KY, et al. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J Psychiatr Res. 2010;44:591–7. doi: 10.1016/j.jpsychires.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirvonen J, et al. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry. 2013;18:916–21. doi: 10.1038/mp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ceccarini J, et al. Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J Neurosci. 2014;34:2822–31. doi: 10.1523/JNEUROSCI.0849-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Neumeister A, et al. Positron emission tomography shows elevated cannabinoid CB1 receptor binding in men with alcohol dependence. Alcohol Clin Exp Res. 2012;36:2104–9. doi: 10.1111/j.1530-0277.2012.01815.x. This study was among the first to demonstrate altered brain CB1R availability in human alcoholics. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 96.Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–25. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Werling LL, Reed SC, Wade D, Izenwasser S. Chronic nicotine alters cannabinoid-mediated locomotor activity and receptor density in periadolescent but not adult male rats. Int J Dev Neurosci. 2009;27:263–9. doi: 10.1016/j.ijdevneu.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marco EM, et al. Subchronic nicotine exposure in adolescence induces long-term effects on hippocampal and striatal cannabinoid-CB1 and mu-opioid receptors in rats. Eur J Pharmacol. 2007;557:37–43. doi: 10.1016/j.ejphar.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 99.Rubino T, Tizzoni L, Vigano D, Massi P, Parolaro D. Modulation of rat brain cannabinoid receptors after chronic morphine treatment. Neuroreport. 1997;8:3219–23. doi: 10.1097/00001756-199710200-00007. [DOI] [PubMed] [Google Scholar]
- 100.Cichewicz DL, Haller VL, Welch SP. Changes in opioid and cannabinoid receptor protein following short-term combination treatment with delta(9)-tetrahydrocannabinol and morphine. J Pharmacol Exp Ther. 2001;297:121–7. [PubMed] [Google Scholar]
- 101.Adamczyk P, et al. Long-lasting increase in [(3)H]CP55,940 binding to CB1 receptors following cocaine self-administration and its withdrawal in rats. Brain Res. 2012;1451:34–43. doi: 10.1016/j.brainres.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 102.Pavon FJ, et al. Evaluation of plasma-free endocannabinoids and their congeners in abstinent cocaine addicts seeking outpatient treatment: impact of psychiatric co-morbidity. Addict Biol. 2013;18:955–69. doi: 10.1111/adb.12107. [DOI] [PubMed] [Google Scholar]
- 103.Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008:435–77. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- 104.Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adermark L, Jonsson S, Ericson M, Soderpalm B. Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology. 2011;61:1160–5. doi: 10.1016/j.neuropharm.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 106.Clarke RB, Adermark L. Acute ethanol treatment prevents endocannabinoid-mediated long-lasting disinhibition of striatal output. Neuropharmacology. 2010;58:799–805. doi: 10.1016/j.neuropharm.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 107.DePoy L, et al. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci U S A. 2013;110:14783–8. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fourgeaud L, et al. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–45. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008;28:1385–97. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–31. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grueter BA, et al. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–9. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McElligott ZA, Winder DG. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1329–35. doi: 10.1016/j.pnpbp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci. 2003;23:4815–20. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mato S, et al. A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci. 2004;7:585–6. doi: 10.1038/nn1251. References 113 and 114 were among the first to demonstrate that 9-THC exposure disrupts synaptic plasticity of nucleus accumbens neurons in rodents. [DOI] [PubMed] [Google Scholar]
- 115.Reisiger AR, et al. Nicotine self-administration induces CB1-dependent LTP in the bed nucleus of the stria terminalis. J Neurosci. 2014;34:4285–92. doi: 10.1523/JNEUROSCI.3149-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morena M, Patel S, Bains JS, Hill MN. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ruehle S, Rey AA, Remmers F, Lutz B. The endocannabinoid system in anxiety, fear memory and habituation. J Psychopharmacol. 2012;26:23–39. doi: 10.1177/0269881111408958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bura SA, Burokas A, Martin-Garcia E, Maldonado R. Effects of chronic nicotine on food intake and anxiety-like behaviour in CB(1) knockout mice. Eur Neuropsychopharmacol. 2010;20:369–78. doi: 10.1016/j.euroneuro.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 120.Cippitelli A, et al. Endocannabinoid regulation of acute and protracted nicotine withdrawal: effect of FAAH inhibition. PLoS One. 2011;6:e28142. doi: 10.1371/journal.pone.0028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mannucci C, et al. Interactions between endocannabinoid and serotonergic systems in mood disorders caused by nicotine withdrawal. Nicotine Tob Res. 2011;13:239–47. doi: 10.1093/ntr/ntq242. [DOI] [PubMed] [Google Scholar]
- 122.McKinzie DL, et al. Acoustic startle and fear-potentiated startle in alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 2000;65:691–6. doi: 10.1016/s0091-3057(99)00252-x. [DOI] [PubMed] [Google Scholar]
- 123.Barrenha GD, Chester JA. Genetic correlation between innate alcohol preference and fear-potentiated startle in selected mouse lines. Alcohol Clin Exp Res. 2007;31:1081–8. doi: 10.1111/j.1530-0277.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 124.Powers MS, Barrenha GD, Mlinac NS, Barker EL, Chester JA. Effects of the novel endocannabinoid uptake inhibitor, LY2183240, on fear-potentiated startle and alcohol-seeking behaviors in mice selectively bred for high alcohol preference. Psychopharmacology (Berl) 2010;212:571–83. doi: 10.1007/s00213-010-1997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci. 2013;34:637–44. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marsicano G, Lafenetre P. Roles of the endocannabinoid system in learning and memory. Curr Top Behav Neurosci. 2009;1:201–30. doi: 10.1007/978-3-540-88955-7_8. [DOI] [PubMed] [Google Scholar]
- 127.Carter BL, Tiffany ST. Cue-reactivity and the future of addiction research. Addiction. 1999;94:349–51. [PubMed] [Google Scholar]
- 128.De Vries TJ, Schoffelmeer AN. Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci. 2005;26:420–6. doi: 10.1016/j.tips.2005.06.002. This review details seminal studies by the authors and others demonstrating that CB1R blockade attenuates relapse-like behavior in rats, thus paving the way for numerous studies demonstrating a potent influence of CB1R signaling on relapse-like behavior induced both by drug exposure and drug-paired conditioned cues across multiple classes of abused drugs. [DOI] [PubMed] [Google Scholar]
- 129.Fattore L, et al. An endocannabinoid mechanism in relapse to drug seeking: a review of animal studies and clinical perspectives. Brain Res Rev. 2007;53:1–16. doi: 10.1016/j.brainresrev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 130.Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33:2483–93. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- 131.Kodas E, Cohen C, Louis C, Griebel G. Cortico-limbic circuitry for conditioned nicotine-seeking behavior in rats involves endocannabinoid signaling. Psychopharmacology (Berl) 2007;194:161–71. doi: 10.1007/s00213-007-0813-0. [DOI] [PubMed] [Google Scholar]
- 132.De Vries TJ, de Vries W, Janssen MC, Schoffelmeer AN. Suppression of conditioned nicotine and sucrose seeking by the cannabinoid-1 receptor antagonist SR141716A. Behav Brain Res. 2005;161:164–8. doi: 10.1016/j.bbr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 133.Ward SJ, Rosenberg M, Dykstra LA, Walker EA. The CB1 antagonist rimonabant (SR141716) blocks cue-induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug Alcohol Depend. 2009;105:248–55. doi: 10.1016/j.drugalcdep.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xi ZX, et al. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–6. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–6. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- 136.van der Stelt M, et al. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci. 2006;63:1410–24. doi: 10.1007/s00018-006-6037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cippitelli A, et al. The anandamide transport inhibitor AM404 reduces ethanol self-administration. Eur J Neurosci. 2007;26:476–86. doi: 10.1111/j.1460-9568.2007.05665.x. [DOI] [PubMed] [Google Scholar]
- 138.Mangieri RA, Hong KI, Piomelli D, Sinha R. An endocannabinoid signal associated with desire for alcohol is suppressed in recently abstinent alcoholics. Psychopharmacology (Berl) 2009;205:63–72. doi: 10.1007/s00213-009-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–67. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 140.Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–73. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- 141.Marsicano G, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. This paper provided the first evidence for a strong influence of CB1Rs in the amygdala in extinction learning related to fear, and this has led to critical insight into EC mechanisms related to emotional pathologies including anxiety disorders and PTSD. [DOI] [PubMed] [Google Scholar]
- 142.Lutz B. The endocannabinoid system and extinction learning. Mol Neurobiol. 2007;36:92–101. doi: 10.1007/s12035-007-8004-x. [DOI] [PubMed] [Google Scholar]
- 143.Kaplan GB, Heinrichs SC, Carey RJ. Treatment of addiction and anxiety using extinction approaches: neural mechanisms and their treatment implications. Pharmacol Biochem Behav. 2011;97:619–25. doi: 10.1016/j.pbb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 144.Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57:261–9. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- 145.Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–81. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 146.Hillard CJ, Weinlander KM, Stuhr KL. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. 2012;204:207–29. doi: 10.1016/j.neuroscience.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lopez-Moreno JA, Echeverry-Alzate V, Buhler KM. The genetic basis of the endocannabinoid system and drug addiction in humans. J Psychopharmacol. 2012;26:133–43. doi: 10.1177/0269881111416689. [DOI] [PubMed] [Google Scholar]
- 148.Zhang PW, et al. Human cannabinoid receptor 1: 5’ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol Psychiatry. 2004;9:916–31. doi: 10.1038/sj.mp.4001560. This paper provided detailed structural characterization of the human CNR1 gene, thereby providing information on splice variants and novel SNPs as well as evidence of haplotype-specific association with Cnr1 mRNA expression and substance abuse phenotype. [DOI] [PubMed] [Google Scholar]
- 149.Schroth GP, Chou PJ, Ho PS. Mapping Z-DNA in the human genome. Computer-aided mapping reveals a nonrandom distribution of potential Z-DNA-forming sequences in human genes. J Biol Chem. 1992;267:11846–55. [PubMed] [Google Scholar]
- 150.Comings DE, et al. Cannabinoid receptor gene (CNR1): association with i.v. drug use. Mol Psychiatry. 1997;2:161–8. doi: 10.1038/sj.mp.4000247. [DOI] [PubMed] [Google Scholar]
- 151.Ballon N, et al. (AAT)n repeat in the cannabinoid receptor gene (CNR1): association with cocaine addiction in an African-Caribbean population. Pharmacogenomics J. 2006;6:126–30. doi: 10.1038/sj.tpj.6500352. [DOI] [PubMed] [Google Scholar]
- 152.Covault J, Gelernter J, Kranzler H. Association study of cannabinoid receptor gene (CNR1) alleles and drug dependence. Mol Psychiatry. 2001;6:501–2. doi: 10.1038/sj.mp.4000925. [DOI] [PubMed] [Google Scholar]
- 153.Benyamina A, Kebir O, Blecha L, Reynaud M, Krebs MO. CNR1 gene polymorphisms in addictive disorders: a systematic review and a meta-analysis. Addict Biol. 2011;16:1–6. doi: 10.1111/j.1369-1600.2009.00198.x. [DOI] [PubMed] [Google Scholar]
- 154.Schmidt LG, et al. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65:221–4. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- 155.Ehlers CL, Slutske WS, Lind PA, Wilhelmsen KC. Association between single nucleotide polymorphisms in the cannabinoid receptor gene (CNR1) and impulsivity in southwest California Indians. Twin Res Hum Genet. 2007;10:805–11. doi: 10.1375/twin.10.6.805. [DOI] [PubMed] [Google Scholar]
- 156.Proudnikov D, et al. Association of polymorphisms of the cannabinoid receptor (CNR1) and fatty acid amide hydrolase (FAAH) genes with heroin addiction: impact of long repeats of CNR1. Pharmacogenomics J. 2010;10:232–42. doi: 10.1038/tpj.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li MJ, et al. GWASdb: a database for human genetic variants identified by genome-wide association studies. Nucleic Acids Res. 2012;40:D1047–54. doi: 10.1093/nar/gkr1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van den Wildenberg E, Janssen RG, Hutchison KE, van Breukelen GJ, Wiers RW. Polymorphisms of the dopamine D4 receptor gene (DRD4 VNTR) and cannabinoid CB1 receptor gene (CNR1) are not strongly related to cue-reactivity after alcohol exposure. Addict Biol. 2007;12:210–20. doi: 10.1111/j.1369-1600.2007.00064.x. [DOI] [PubMed] [Google Scholar]
- 159.Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103:1678–86. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–75. doi: 10.1038/npp.2009.200. This study leveraged previous investigations to provide insights on CNR1 and FAAH gene x gene interaction in association with in vivo neural reactivity in the reward system to marijuana-related cues. The work emphasized the additive genetic influence on cue reactivity as an intermediate endophenotype in cannabis abuse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hutchison KE, et al. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Arch Gen Psychiatry. 2008;65:841–50. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Agrawal A, et al. Evidence for association between polymorphisms in the cannabinoid receptor 1 (CNR1) gene and cannabis dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:736–40. doi: 10.1002/ajmg.b.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hopfer CJ, et al. Cannabis receptor haplotype associated with fewer cannabis dependence symptoms in adolescents. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:895–901. doi: 10.1002/ajmg.b.30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hartman CA, et al. The association between cannabinoid receptor 1 gene (CNR1) and cannabis dependence symptoms in adolescents and young adults. Drug Alcohol Depend. 2009;104:11–6. doi: 10.1016/j.drugalcdep.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Herman AI, Kranzler HR, Cubells JF, Gelernter J, Covault J. Association study of the CNR1 gene exon 3 alternative promoter region polymorphisms and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:499–503. doi: 10.1002/ajmg.b.30325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A. 2002;99:8394–9. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–9. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- 168.Tyndale RF, Payne JI, Gerber AL, Sipe JC. The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: studies of drug use and dependence in Caucasians. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:660–6. doi: 10.1002/ajmg.b.30491. [DOI] [PubMed] [Google Scholar]
- 169.Buhler KM, et al. Risky alcohol consumption in young people is associated with the fatty acid amide hydrolase gene polymorphism C385A and affective rating of drug pictures. Mol Genet Genomics. 2014;289:279–89. doi: 10.1007/s00438-013-0809-x. [DOI] [PubMed] [Google Scholar]
- 170.Hariri AR, et al. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–9. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maccarrone M, Guzman M, Mackie K, Doherty P, Harkany T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci. 2014;15:786–801. doi: 10.1038/nrn3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76(Pt B):416–24. doi: 10.1016/j.neuropharm.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Calvigioni D, Hurd YL, Harkany T, Keimpema E. Neuronal substrates and functional consequences of prenatal cannabis exposure. Eur Child Adolesc Psychiatry. 2014;23:931–41. doi: 10.1007/s00787-014-0550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gorzalka BB, Dang SS. Minireview: Endocannabinoids and gonadal hormones: bidirectional interactions in physiology and behavior. Endocrinology. 2012;153:1016–24. doi: 10.1210/en.2011-1643. [DOI] [PubMed] [Google Scholar]
- 176.Stoving RK, et al. Leptin, ghrelin, and endocannabinoids: potential therapeutic targets in anorexia nervosa. J Psychiatr Res. 2009;43:671–9. doi: 10.1016/j.jpsychires.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 177.Monteleone P, et al. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: a pilot study. J Clin Endocrinol Metab. 2012;97:E917–24. doi: 10.1210/jc.2011-3018. [DOI] [PubMed] [Google Scholar]
- 178.Monteleone P, et al. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology. 2005;30:1216–21. doi: 10.1038/sj.npp.1300695. [DOI] [PubMed] [Google Scholar]
- 179.Siegfried Z, et al. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: differences between restricting and binging/purging subtypes. Am J Med Genet B Neuropsychiatr Genet. 2004;125B:126–30. doi: 10.1002/ajmg.b.20089. [DOI] [PubMed] [Google Scholar]
- 180.Muller TD, et al. Lack of association of genetic variants in genes of the endocannabinoid system with anorexia nervosa. Child Adolesc Psychiatry Ment Health. 2008;2:33. doi: 10.1186/1753-2000-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Monteleone P, et al. Association of CNR1 and FAAH endocannabinoid gene polymorphisms with anorexia nervosa and bulimia nervosa: evidence for synergistic effects. Genes Brain Behav. 2009;8:728–32. doi: 10.1111/j.1601-183X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 182.Sipe JC, Waalen J, Gerber A, Beutler E. Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH) Int J Obes (Lond) 2005;29:755–9. doi: 10.1038/sj.ijo.0802954. [DOI] [PubMed] [Google Scholar]
- 183.Frieling H, et al. Elevated cannabinoid 1 receptor mRNA is linked to eating disorder related behavior and attitudes in females with eating disorders. Psychoneuroendocrinology. 2009;34:620–4. doi: 10.1016/j.psyneuen.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 184.Gerard N, Pieters G, Goffin K, Bormans G, Van Laere K. Brain type 1 cannabinoid receptor availability in patients with anorexia and bulimia nervosa. Biol Psychiatry. 2011;70:777–84. doi: 10.1016/j.biopsych.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 185.Ishiguro H, et al. A nonsynonymous polymorphism in cannabinoid CB2 receptor gene is associated with eating disorders in humans and food intake is modified in mice by its ligands. Synapse. 2010;64:92–6. doi: 10.1002/syn.20714. [DOI] [PubMed] [Google Scholar]
- 186.Verty AN, McGregor IS, Mallet PE. Paraventricular hypothalamic CB(1) cannabinoid receptors are involved in the feeding stimulatory effects of Delta(9)-tetrahydrocannabinol. Neuropharmacology. 2005;49:1101–9. doi: 10.1016/j.neuropharm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 187.Lewis DY, Brett RR. Activity-based anorexia in C57/BL6 mice: effects of the phytocannabinoid, Delta9-tetrahydrocannabinol (THC) and the anandamide analogue, OMDM-2. Eur Neuropsychopharmacol. 2010;20:622–31. doi: 10.1016/j.euroneuro.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 188.Andries A, Frystyk J, Flyvbjerg A, Stoving RK. Dronabinol in severe, enduring anorexia nervosa: a randomized controlled trial. Int J Eat Disord. 2014;47:18–23. doi: 10.1002/eat.22173. [DOI] [PubMed] [Google Scholar]
- 189.Pataky Z, et al. Efficacy of rimonabant in obese patients with binge eating disorder. Exp Clin Endocrinol Diabetes. 2013;121:20–6. doi: 10.1055/s-0032-1329957. [DOI] [PubMed] [Google Scholar]
- 190.Scherma M, et al. Pharmacological modulation of the endocannabinoid signalling alters binge-type eating behaviour in female rats. Br J Pharmacol. 2013;169:820–33. doi: 10.1111/bph.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cottone P, et al. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009;106:20016–20. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Blasio A, et al. Rimonabant precipitates anxiety in rats withdrawn from palatable food: role of the central amygdala. Neuropsychopharmacology. 2013;38:2498–507. doi: 10.1038/npp.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.D’Addario C, Di Francesco A, Pucci M, Finazzi Agro A, Maccarrone M. Epigenetic mechanisms and endocannabinoid signalling. FEBS J. 2013;280:1905–17. doi: 10.1111/febs.12125. [DOI] [PubMed] [Google Scholar]
- 195.Franklin TB, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–15. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 196.Rotter A, et al. CB1 and CB2 receptor expression and promoter methylation in patients with cannabis dependence. Eur Addict Res. 2013;19:13–20. doi: 10.1159/000338642. This was the first study to report epigenetic differences of the CNR1 gene demonstrating alteration of DNA methylation status (in peripheral blood cells) correlated to CB1R expression. [DOI] [PubMed] [Google Scholar]
- 197.DiNieri JA, et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol Psychiatry. 2011;70:763–9. doi: 10.1016/j.biopsych.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tomasiewicz HC, et al. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol Psychiatry. 2012;72:803–10. doi: 10.1016/j.biopsych.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stringer RL, Laufer BI, Kleiber ML, Singh SM. Reduced expression of brain cannabinoid receptor 1 (Cnr1) is coupled with an increased complementary micro-RNA (miR-26b) in a mouse model of fetal alcohol spectrum disorders. Clin Epigenetics. 2013;5:14. doi: 10.1186/1868-7083-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Matyas F, et al. Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology. 2008;54:95–107. doi: 10.1016/j.neuropharm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Suarez J, et al. Distribution of diacylglycerol lipase alpha, an endocannabinoid synthesizing enzyme, in the rat forebrain. Neuroscience. 2011;192:112–31. doi: 10.1016/j.neuroscience.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 202.Egertova M, Simon GM, Cravatt BF, Elphick MR. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: A new perspective on N-acylethanolamines as neural signaling molecules. J Comp Neurol. 2008;506:604–15. doi: 10.1002/cne.21568. [DOI] [PubMed] [Google Scholar]
- 203.Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–71. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 204.Matyas F, et al. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–61. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 205.Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology. 2012;37:1164–76. doi: 10.1038/npp.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–8. doi: 10.1523/JNEUROSCI.3695-04.2004. This study described the presynaptic and postsynaptic cellular mechanisms that control EC release and regulation of mesolimbic (VTA) DA neuron activity via the retrograde activation of presynaptic CB1Rs. This work set the model of endocannabinoid-dopamine interactions where increased DA neuron burst firing is mediated through EC inhibition of VTA GABA cells (DSI) thus leading to disinhibition of midbrain DA neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kortleven C, Fasano C, Thibault D, Lacaille JC, Trudeau LE. The endocannabinoid 2-arachidonoylglycerol inhibits long-term potentiation of glutamatergic synapses onto ventral tegmental area dopamine neurons in mice. Eur J Neurosci. 2011;33:1751–60. doi: 10.1111/j.1460-9568.2011.07648.x. [DOI] [PubMed] [Google Scholar]
- 208.Marinelli S, et al. N-arachidonoyl-dopamine tunes synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology. 2007;32:298–308. doi: 10.1038/sj.npp.1301118. [DOI] [PubMed] [Google Scholar]
- 209.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Stuber GD, et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–2. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–16. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–25. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Giuffrida A, et al. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–63. doi: 10.1038/7268. This paper provided the first in vivo evidence for interactions between the striatal DA system and EC formation which has substantial implications for a number of pathologies including addiction and movement disorders. [DOI] [PubMed] [Google Scholar]
- 214.Winters BD, et al. Cannabinoid receptor 1-expressing neurons in the nucleus accumbens. Proc Natl Acad Sci U S A. 2012;109:E2717–25. doi: 10.1073/pnas.1206303109. [DOI] [PMC free article] [PubMed] [Google Scholar]