Abstract
We examined whether navigation is impacted by experience in two species of nonhuman primates. Five chimpanzees (Pan troglodytes) and seven capuchin monkeys (Cebus apella) navigated a cursor, using a joystick, through two-dimensional mazes presented on a computer monitor. Subjects completed 192 mazes, each one time. Each maze contained one to five choices, and in up to three of these choices, the correct path required moving the cursor away from the Euclidean direction toward the goal. Some subjects completed these mazes in a random order (Random group); others in a fixed order by ascending number of choices and ascending number of turns away from goal (Ordered group). Chimpanzees in both groups performed equivalently, demonstrated fewer errors and a higher rate of self-correcting errors with increasing experience at solving the mazes, and made significantly fewer errors than capuchin monkeys. Capuchins were more sensitive to the mode of presentation than chimpanzees: Monkeys in the Ordered group made fewer errors than monkeys in the Random group. However, capuchins’ performance across testing changed little, and they remained particularly susceptible to making errors when the correct path required moving away from the goal. Thus, these two species responded differently to the same spatial challenges and same learning contexts. The findings indicate that chimpanzees have a strong advantage in this task compared to capuchins, no matter how the task is presented. We suggest that differences between the species in the dynamic organization of attention and motor processes contribute to their differences in performance on this task, and predict similar differences in other tasks requiring, as this one does, sustained attention to a dynamic visual display and self-produced movements variably towards and away from a goal.
Keywords: Planning, learning, species differences, spatial problem solving, memory, attention, vigilance
Introduction
Spatial navigation involves planning when an individual takes into account current location, goal location, and alternative routes to get to the goal. According to Rogoff et al. (1993), skillful planning incorporates “planning in advance of action or during action according to the circumstances, flexibly anticipating constraints and opportunities and adapting to circumstances.” (p. 354). What goes into planning in this context, and can nonhuman primates learn to do it skillfully? Two-dimensional navigation tasks in which the actor moves a visible cursor towards a goal are useful for studying flexible alteration of navigation in changing circumstances, and these tasks are experimentally convenient to present. Computerized two-dimensional layouts can be varied in systematic ways to present numerous paths, and the visual displays are small enough that the actor can perceive the full layout as he or she moves the cursor through it. The movement of the cursor can be tracked automatically. Analysis of the movements of the cursor can illuminate the actor’s use of available features of the layout to guide action.
We studied spatial planning in the sense of flexible behavior in changing circumstances by presenting two-dimensional alley mazes to individuals of two species of nonhuman primates: capuchin monkeys (Cebus apella spp.) and chimpanzees (Pan troglodytes). In previous work, Fragaszy et al. (2003) showed that capuchin monkeys and chimpanzees could use a joystick to navigate computerized mazes containing up to five choice points. Chimpanzees produced fewer errors than capuchins, and in particular they were less likely than capuchins to make errors when the correct alley led away from the goal. Both species corrected their errors before running into the end of an alley on less than half of their errors, but both of them completed mazes without error at rates higher than expected by chance. Thus both species presented evidence of planning, albeit with differences between them.
Fragaszy et al. (2003) interpreted their findings in relation to a hierarchical model of planning containing five levels (see Table 1). This model is roughly patterned after Case’s (1992; Case and Okamoto 1996) relational model of cognitive development. Both species in this first study provided evidence of behavior that met the criteria for Level 2 planning according to our model (making decisions at each choice point on the basis of one property, and monitoring the outcome of each choice), and there was mixed evidence in both species for planning at level 3 (making decisions on the basis of two integrated or prioritized properties). However, comparisons across species and interpretation of changes in performance with experience in Fragaszy et al.’s (2003) study were constrained by two factors: the apes had far more experience at using joysticks than did the capuchins, and we presented the mazes in a specific ordered sequence meant to maximize the subjects’ ability to complete them (i.e., in an order we predicted moved from less to more difficult). Thus we could not clearly isolate the contribution of experience with these mazes to changes in performance in either species. In this report, we present data from capuchins and chimpanzees with equivalent experience using joysticks. The experimental design, with some individuals of each species receiving the mazes in an ordered format (Ordered groups) and others receiving them in a randomized order (Random groups), permits evaluation of the contribution of experience to performance in two ways. The Random presentation allows evaluation of the effects of practice, and contrasting the performance of Random and Ordered groups allows evaluation of the particular benefits of structured practice experience.
Table 1.
Model of planning in a two-dimensional maze task proposed by Fragaszy et al. (2003).
| Level | Planning activity |
|---|---|
| 0 | Absence of planning; movements of the cursor are guided only by encounters with barriers (either the wall or the end of an alley) |
| 1 | Bodily planning: moving the hand in such a way as to make the cursor move in a specific direction (i.e., along a straight line or through a turn), but selections at each choice point are made randomly |
| 2 | One-element planning: making decisions at each choice point on the basis of one property (e.g., directness to the goal); monitoring the outcome one choice at a time (Encompasses “planful” and “forward search” strategies) |
| 3 | Integrated planning: deciding at each choice point on the basis of two (integrated or prioritized) properties (e.g., path continuation first, and goal directedness second) |
| 4 | Sequential integrated planning: implementing a sequence of choices devised in advance and based upon two or more integrated or prioritized properties (e.g., planning backward from the end point to the start point, and subsequently making these choices in the forward direction) |
Chimpanzees and capuchin monkeys, although found on distant branches of the primate lineage, share behavioral qualities that make their comparison interesting (reviewed in Visalberghi and McGrew 1997; compare Fragaszy, Visalberghi and Fedigan 2004b and Boesch and Boesch 2000, Matsuzawa 2001). With reference to cognition, capuchins and chimpanzees share primate-typical abilities to retain locations, actions and events, to learn transitive sequences, to categorize objects, to produce hierarchically structured lists, to learn concepts, and so forth (e.g., reviews in Fragaszy, Visalberghi and Fedigan 2004b and Tomasello and Call 1997). In the realm of spatial skills, both seriate nesting cups (i.e., cups that fit into one another in a fixed order) by an iterative process, rather than strategic assembly (Johnson-Pynn et al. 1999), and both face similar difficulties aligning an object to pass through an aperture or into a grooved base (Fragaszy, Crast and Matsuzawa, 2004; Scott, Fragaszy and Menzel, 2006). These findings suggest the genera share similar abilities to produce specific spatial relations among objects or between objects and surfaces through manual action (Fragaszy and Cummins-Sebree 2005).
However, chimpanzees and capuchins also differ in substantive ways, physically and behaviorally. One of the most obvious differences is size: Capuchins are roughly 1/20th the size of chimpanzees (3 – 4 kg vs. 60 kg or more). In accord with this substantive physical difference, the two genera face widely discrepant predatory risks and locomotor opportunities. Capuchins spend more time in trees than do chimpanzees (compare Hunt 1996 and Wright 2007). By virtue of their smaller body size, capuchins face the risk of aerial predators, whereas chimpanzees do not, and capuchins are susceptible to predation by a wider range of terrestrial predators than chimpanzees. These differences may be associated with differential organization of attention and memory, in line with different needs for monitoring circumstances (both desirable and dangerous) in the immediate environment (an ecological argument predicting species differences in cognitive processes; cf. de Kort et al. 2006, Shettleworth 1998). From an ecological perspective, given the differences in predatory risk, one might expect bouts of focused attention to be shorter in capuchins than in chimpanzees, for example.
In experimental situations, chimpanzees master conceptual reversal problems, wherein the assigned correct stimulus of a pair shifts now and then, so that the new correct choice is the previous incorrect choice (Rumbaugh and Pate 1984). Capuchins can master these also, but they could not master as well as chimpanzees a variation of this problem, in which a new stimulus “stood in” for either the familiar positive or familiar negative stimulus (DeLillo and Visalberghi 1994). The capuchins were prone in this last situation to choose the novel stimulus, no matter whether it replaced the correct or the incorrect stimulus. Thus attraction to a perceptual feature overwhelmed or displaced a conditional evaluation of the problem for them. These findings are reminiscent of the capuchin monkeys’ problems, compared to chimpanzees, with the two-dimensional mazes in the study reported by Fragaszy et al. (2003). In that study, the capuchin monkeys showed a strong tendency throughout the study to move towards the goal even when that path led to a dead-end; chimpanzees did not show this pattern.
Developmental models emphasize the transition through human childhood from simpler forms of planning and problem-solving (remembering the goal; not getting distracted by irrelevant events; Willats 1989, 1999) to more complex forms (e.g., integrating two or more features, shifting behavior after an error, fluidly coordinating sequences of actions (termed “sub-goaling” or “action planning”), and using a strategic process in a greater range of situations; Fischer and Bidell 1998, Case and Okamoto 1996, Cox and Smitsman 2006, Diamond et al. 2002, Klahr 1994, Siegler & Alibali 2004, Spencer, Smith and Thelen et al. 2001). The transitions are attributed to a variety of changes in behavioral organization, depending on one’s theoretical orientation. Information-processing and Neo-Piagetian accounts suggest expanding working memory, better encoding strategies, better control of attentional shifting, improving inhibitory control, improving integration of dual or multiple properties, etc. (Siegler & Alibali 2004; Bjorklund 2004, Barkley 1997, Case 1992).
Dynamic systems theory conceptualizes the same developmental changes in thinking as reflecting changing attractors, changing intrinsic dynamics, changing forces, and changing coupling among the interacting elements of the behavioral system (Thelen and Smith 1994; Smith and Thelen 2003). For example, Spencer et al. (2001) interpret the classic “A not B” error (in which the child reaches incorrectly to a place where an object had been retrieved several times before, rather than to the new correct location) in dynamic systems terms. In their words, the A not B error reflects “general processes that produce goal-directed actions to remembered locations” (p. 1327), including visual, attentional, motor, short-term and longer-term memory processes. In other words, there is no single factor that is responsible for the children’s behavior in this task – it is multiply determined, and the factors that promote making the A not B error in young children promote the error in many contexts and in people of all ages (Schutte et al. 2003). Indeed, adult humans show similar memory biases as do 2 year olds in search tasks after delays of 5 to 20 seconds (Spencer and Hund 2003). This theoretical perspective suggests that planning, memory, and problem-solving will be multiply determined in other species, as in humans, and that these processes might undergo microdevelopmental changes initiated by experience. If problem-solving reflects the coordinated outcome of multiple cognitive processes, each differentially responsive to experience and to immediate circumstances, we might expect that the same kinds of experience could have different effects on problem-solving in different species.
We pursued the roles of experience, immediate circumstance, and species on nonhuman primates’ spatial problem-solving in the course of navigating two-dimensional mazes. The primary aim of the study reported here was to evaluate the role of practice on navigation in capuchins and chimpanzees. To achieve this aim, we presented two-dimensional mazes in random order with respect to the presumed level of difficulty to a group of subjects of each species. This design permits a more direct evaluation of the role of practice on performance than we could achieve in a previous study (Fragaszy et al. 2003), in which experience and changing features of the mazes were confounded. A second aim was to evaluate whether the manner in which the mazes are presented impacts the micro-development of skill in navigation. Thus, we compared performance over the course of testing in a group of each species encountering the mazes in an ordered sequence, from simpler to more difficult, and a group encountering the mazes in a random sequence. A third aim was to replicate the comparison of chimpanzees and capuchins, particularly to determine if the species difference evident in the Fragaszy et al. (2003) study reflected different degrees of experience with using a joystick between the chimpanzees (more experienced) and capuchins (less experienced), or alternatively reflected consistent differences between these species in how they approach two-dimensional navigation problems. Therefore, in the study reported here we examine performance on mazes by chimpanzees that, like the capuchins, were relatively naïve to the use of joysticks.
Methods
Subjects and housing
Five adult chimpanzees (Pan troglodytes), one female and four males (all 12 years old), and seven adult male capuchin monkeys (Cebus apella, 9 – 17 years) participated in this study. The chimpanzees were housed in small groups at the Yerkes National Primate Research Center of Emory University. The capuchins were housed in pairs at the University of Georgia. None of the subjects were food-deprived during the course of testing. The chimpanzees were tested in their home cage, in an area physically separated from group-mates. The capuchins were transported in pairs to a room adjacent to their housing area for testing, and tested in individual cages.
The subjects learned to use the joystick with the self-paced training series described by Richardson et al. (1990) and Leighty and Fragaszy (2003). This was the first experimental task any of the subjects performed with a joystick interacting with computer-presented displays. They participated in this experiment immediately following mastery of the joystick, before experiencing any other testing with the joystick. None of the subjects had completed any other maze task.
Test apparatus
The mazes were presented to subjects on a color computer monitor (46 wide × 28 cm high). Subjects manipulated a joystick (5 cm below the screen, capuchins, and 60 cm below the screen, chimpanzees) in order to move a cursor (a white cross) on the monitor from the start of the maze to the goal (a blue circle). The mazes appeared as black pathways (approximately 2.5 cm in width) against a white background. The cursor could be moved within these pathways with the joystick in a linear path and at a constant speed. When the animal deflected the joystick, the cursor moved 1 pixel distance every 0.015 seconds. An animal could reverse the directional movement of the cursor at any location in the maze.
Mazes
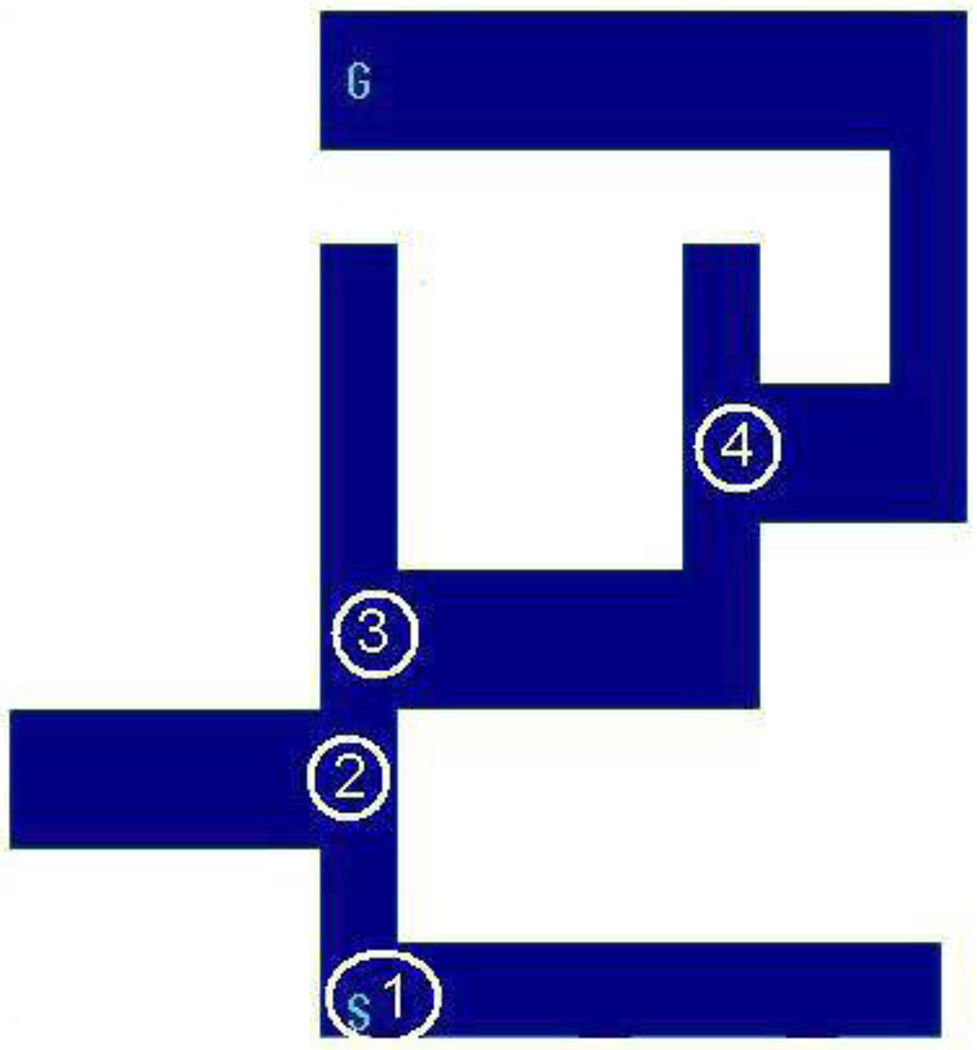
One hundred ninety-two mazes were presented to each subject (with minor exceptions noted below). Each maze represented a novel organization of pathways, choice points, and start and end points. All turns in the pathways were 90 degree angles. A “choice point” was defined as a “T” in the alley. If the cursor appeared in a path, rather than at the end of an alley, at the outset, the first move was also considered a choice. Each of the choices was binary in nature. The mazes were designed so that the start and end points appeared equally often in each of the four quadrants of the screen. The mazes were grouped into sets (or “libraries”) of 16 (see Table 2). The first library was used for training purposes and was composed of simple “L” shaped detour problems that involved no choice points. These problems functioned solely to familiarize subjects with the task of moving the cursor through a pathway toward a goal. The mazes within the 12 maze libraries that were used for testing varied in terms of the number of choices and “Non-obvious” choices (NOCs) they contained. The number of choices within a maze varied between one and five, while the number of NOCs varied between zero and three. Non-obvious choices were those that appeared to require traveling a longer distance to the goal or a greater angle away from the goal than the other choice at that choice point. In these cases, the incorrect path serves as a perceptual “lure” in that it appears to be the shortest route to the goal although it is actually a dead-end. Choices that were not NOC’s are referred to as Obvious choices. Figure 1 illustrates a maze containing four choices, two of which (#’s 3 and 4) are NOC’s. In the entire set of mazes, 288 (54.5%) of the 528 choices were classified as Non-obvious.
Table 2.
Characteristics of maze libraries.
| Library | No. of choice points | Non-obvious choices |
|---|---|---|
| 0 (training) | 0 | 0 |
| 1 | 1 | 0 |
| 2 | 2 | 0 |
| 3 | 3 | 0 |
| 4 | 1 | 1 |
| 5 | 2 | 1 |
| 6 | 3 | 1 |
| 7 | 2 | 2 |
| 8 | 3 | 2 |
| 9 | 4 | 2 |
| 10 | 3 | 3 |
| 11 | 4 | 3 |
| 12 | 5 | 3 |
Figure 1.
Sample maze from Library 9 (four choices, two Non-obvious choices). The start point, where the cursor appears, is marked S; the end point, or goal, is marked G. Choice points are marked with circles, and the sequential order of choices is indicated by numbers 1 to 4. In this maze, the start position occurs at a choice point. Choices 1 and 2 are Obvious choices; Choices 3 and 4 are Non-Obvious choices. Because the cursor is placed along a path, rather than at an edge, at the initiation of action in the maze, the actor must decide in what direction to move (to the right, or up). Thus, Choice 1 is a Forced choice. Choices 2, 3, and 4 are Facultative; the actor can choose to continue in the same direction or to move in a new direction.
Choice points were further classified as “forced” or “facultative”. A forced choice occurred when the subject was forced to turn onto one of the two paths, or if at the start of the trial, the cursor appears along the path, and the subject was forced to move in one of two directions. Facultative choices occurred when the subject had the option to continue on the same path past an intersection or to turn onto a new path. The maze illustrated in Figure 1 contains one forced choice (# 1) and three facultative choices (#’s 2 – 4).
Procedure
Ordered Presentation
Three chimpanzees and three capuchin monkeys were presented with the maze libraries in numerical sequence (i.e., library 2 followed by library 3). The mazes within each library were presented to subjects in the same pre-determined order. Subjects advanced to the next maze in the sequence after having reached the goal, or after two minutes had elapsed. All trials were video-taped for future play-back and scoring purposes for the capuchins in the Ordered group. The data set for the capuchins in the Ordered group used in the analyses reported here is the same data set used for the analyses reported in Fragaszy et al. 2003.
Random Presentation
Two chimpanzees and four capuchins were exposed to a random presentation of the mazes. These subjects completed 16 maze sets each composed of 12 mazes per set that were members of the ordered libraries (see Table 2). One maze from each of the libraries as defined in Table 2 was assigned to each of the random maze sets. Each set therefore contained a mixture of mazes representing the range of choices (1–5) and Non-obvious choices (0–3). The mazes within the set appeared in a different random order for each subject (although the mazes comprising the set remained the same). Each subject was presented with the random maze sets in a different (random) order. Mazes from a set that were not completed in a single testing session were presented at the beginning of the next testing session. This procedure was necessary only with capuchin monkeys; the chimpanzees completed each maze the first time it was presented. Subjects were given five minutes to complete each maze. If a subject did not complete a maze in this amount of time, the next maze in the series was presented. All mazes that were not solved in their initial presentation were re-presented to the subject up to two additional times prior to the subject advancing to the next set of 16 mazes. Note that each subject completed each maze just once with the following exception. Inadvertently, 6 mazes (1 or 2 from libraries 5, 9, 12 and 13) were omitted from the random test series for the monkeys, and in their places another maze or mazes from the same libraries were presented. Thus these monkeys each encountered 186 different mazes, 6 of them twice, so they completed 192 mazes total.
Subjects in all groups heard a tone upon completion of a maze and also received a small food reward (single fruit-flavored pellet, 20 gm, or small piece of nut or fruit sized appropriately for each species; e.g., quarter peanut for capuchin, slice of apple for chimpanzee), dispensed automatically or delivered by hand. Experimenters delivered high-value food rewards by hand to capuchins in the Random group, which faced considerable challenges to complete a maze, so as to maximize motivation (which frequently flagged in these subjects). Subjects could see the experimenter, but the experimenter was not positioned to see the subject’s monitor. Subjects were tested once per day. Chimpanzees in the Ordered group completed 3 libraries per day (48 mazes total). Chimpanzees in the Random group also completed 48 mazes per day (4 sets, 12 mazes per set). Capuchins in the Ordered group completed one library per session, with occasional (two or three times per subject) continuation of one library into a second session. This testing took place over a seven-week period. Two capuchins in the Random group (SO and LE) completed the series in 16 sessions, but the other two (MI and NI) in the Random group required far more sessions (23 and 41 sessions).
Scoring
Performance was assessed by scoring each subject’s first choice at each choice point as it navigated through the maze. Scoring was completed manually by means of slow-motion play back, either of video of the monitor, or of a digital file of the cursor’s movements, pixel by pixel (using software developed by C. & E. Menzel). We scored if the first choice was correct or an error. Several forms of error were distinguished. An “Overshoot” error occurred when the monkey continued an existing path of movement 2.5 cm past the correct alley. A “Wrong Turn” error occurred when the cursor was moved 2.5 cm down an incorrect alley. Errors resulted in one of two outcomes: “Dead-end” or “Self-correct”. When the cursor made contact with the end of an incorrect alley, the error resulted in a Dead-end. In a Self-correct, the monkey moved the cursor away from the end of an incorrect alley before reaching it.
Analysis
The data set is constituted by 6108 choices (3537 by capuchins, 2571 by chimpanzees; see Table 3). The full set of 192 mazes presented 528 choices. The number of choices contributed by each subject is presented in Table 4. Although all the chimpanzees completed all the mazes, some data for three chimpanzees were lost in processing, so each chimpanzee contributed between 492 and 528 choices (93 – 100% of possible choices). Some data were missing for three capuchins as well, due to non-completion of mazes, as detailed below. Each capuchin contributed between 440 and 528 choices (83 – 100% of possible choices). Therefore, we chose analytical methods that are not sensitive to unequal numbers of cases. To examine our predictions concerning the effect of presentation mode (Ordered or Random), of sequence (experience), and of the structure of the choice points (Obvious vs. Non-obvious) within and between chimpanzees and capuchin monkeys, we used three separate stepwise logistic regression models, a form of generalized linear models, using SAS (SAS Inc.). These models assume a response variable (Error – correct or error) which follows a binomial (i.e. binary or 0–1) distribution. We examined the fixed effects of the eight variables of interest listed below on the frequency of errors in each case. We used Wald’s chi square statistic to evaluate the probability of each observed distribution. These models included the variables Genus (Pan or Cebus), Library (1–12), Presentation (Ordered or Random), Non-obvious Choice (Yes or No), Total Number of Choice Points (1–5), Category of Choice Point (Forced or Facultative), Type of Error (Wrong Turn or Overshoot), and the Type of Correction once an error was committed (Self Correct or Dead End). Separately, Z-tests for proportions were utilized to test for dependence between the types of corrections (dead end and self correct) and the types of errors (wrong turn and overshoot). These dependencies were investigated for each species separately.
Table 3.
Distribution of choices and errors across species and testing groups.
| Species | Total Choices |
Group | Choices per Group |
Correct choices |
Errors | Errors/Choices |
|---|---|---|---|---|---|---|
| Chimpanzees | 2571 | Ordered (n = 3) |
1576 | 1293 | 283 | 0.18 |
| Random (n = 2) |
995 | 806 | 189 | 0.19 | ||
| Sum | 2571 | 2099 | 472 | 0.183 | ||
| Capuchins | 3537 | Ordered (n = 3) |
1425 | 845 | 580 | 0.41 |
| Random (n = 4) |
2112 | 965 | 1147 | 0.54 | ||
| Sum | 3537 | 1810 | 1727 | 0.488 | ||
| TOTAL | 6108 | 6108 | 3909 | 2199 |
Table 4.
Individuals’ total choices, errors, and errors as a function of the layout of the choice (Non-obvious or Other) and (in parentheses) percentage of choices at that kind of choice point that were errors, rounded to the nearest whole percent.
| Species/ Group | Individual | Total choices (% of 528 total choices) |
Total Errors (%) |
Obvious choices |
Errors on Obvious choices (% of Obvious choices) |
Non- obvious choices |
Errors on Non- obvious choices (% of Non- obvious choices) |
|---|---|---|---|---|---|---|---|
| PAN/Ordered | DA | 520 (98) |
1081 (21) |
232 | 411 (18) | 288 | 671 (23) |
| KA | 528 (100) |
591 (11) |
240 | 241 (10) | 288 | 351 (12) | |
| LM | 528 (100) |
1161 (22) |
240 | 441 (18) | 288 | 721 (25) | |
| Sum, (%) | 1568 | 283, (18) |
712 | 109, (15) | 864 | 174, (20) | |
| PAN/ Random | JA | 492 (93) |
1041 (21) |
227 | 411 (18) | 265 | 631 (24) |
| SC | 503 (95) |
851 (17) |
231 | 241 (10) | 272 | 611 (22) | |
| Sum, (%) | 995 | 189, (19) |
458 | 65 (14) | 537 | 124, (23) | |
| CEBUS/Ordered | JO | 481 (91) |
1601 (33) |
205 | 301 (15) | 276 | 130 (47) |
| XA | 440 (83) |
230 (52) |
205 | 671 (33) | 235 | 1652 (70) | |
| XE | 504 (95) |
1901 (38) |
227 | 601 (26) | 277 | 130 (47) | |
| Sum, (%) | 1425 | 580, (41) |
637 | 157, (25) | 788 | 425, (54) | |
| CEBUS/Random | MI | 528 (100) |
2972 (56) |
240 | 102 (42) | 288 | 1952 (68) |
| SO | 528 (100) |
2331 (44) |
240 | 721 (30) | 288 | 1612 (56) |
|
| NI | 528 (100) |
3232 (61) |
240 | 114 (48) | 288 | 2092 (73) | |
| LE | 528 (100) |
2942 (56) |
242 | 110 (45) | 286 | 1842 (64) | |
| Sum, (%) | 2112 | 1147, (54) |
962 | 398, (41) | 1150 | 749, (65) |
Notes
significantly fewer errors than expected where chance = 50% errors (Chi square > 3.481, df = 1, p < 0.05).
significantly more errors than expected where chance = 50% errors (Chi square > 3.481, df = 1, p < 0.05).
Results
After commenting on the overall success of each species at completing the mazes, we present the results in terms of four major themes. First we evaluate the impact of experience with the task in each species. Next we consider the effect of the two modes of presenting the mazes (ordered vs. random) in each species. Third, we consider the impact of the nature of the choices (Non-obvious vs. other) on the probability of making an error. Finally we look at rates of self-correcting errors as a function of species, presentation mode, and nature of the choice where the error was made.
Overall success
Individuals of both species completed a great majority of the mazes presented. In the case of chimpanzees, each individual completed each maze at its first presentation. In the case of the capuchins, the three monkeys receiving the mazes in an ordered series did not finish 9 to 21 mazes on the first presentation; these were re-presented once. These monkeys provided from 440 to 504 choices each. The capuchins receiving the mazes in the random order required 16, 23, 40, and 67 re-presentations of mazes, and these monkeys each contributed the full 528 choices to the data set.
Table 3 presents the distribution of correct choices and errors across species and groups. Overall, chimpanzees made proportionally fewer errors than capuchins (18.3% vs. 48.8%) (Z = 28.22; p = 0.0001). Table 4 presents the number of errors each subject made. Chimpanzees made errors on 11% to 22% of all choices; capuchins, on 33 to 61% of all choices. Random movements at each choice point would produce 50% correct choices; by this index, all the chimpanzeees and three capuchins (JO, XE, SO) made significantly fewer errors than expected by chance; one capuchin’s error scores did not differ from chance (XA) and three capuchins MI, NI, LE) made significantly more errors than expected by chance.
Effects of experience
Examining performance in five blocks of 20% of mazes completed, we found that the four monkeys in the Random group showed no trend toward improvement. In contrast, both chimpanzees in the Random group displayed consistent improvement across blocks of mazes completed, starting in Block 1 with 69 – 72% correct choices and ending Block 5 with 92 and 93% correct choices. A logistic analysis of these data indicated a significant interaction between Species and Block, (χ2 (1, N=3031) = 37.84, p = 0.0001).
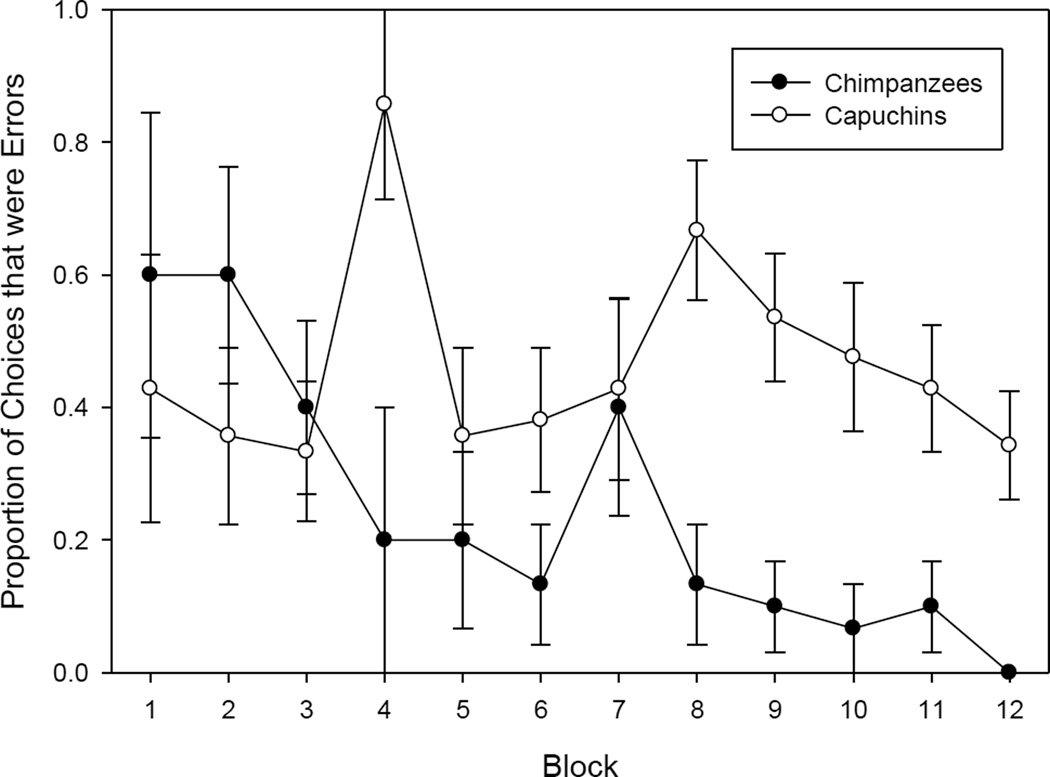
To examine the role of experience in the full sample of subjects, a logistic regression model was used to evaluate the extent to which performance, assessed as % correct choice, improved as individuals completed more mazes in both Ordered and Random groups of both species. This analysis used the first maze completed in each library for the Ordered group, as presented in chronological order: thus, a total of 12 mazes. To match each of these 12 mazes with mazes from the Random group, the selection process required matching both the number of mazes previously completed and the library of each maze. Thus, the first maze from Library 1 presented to the Ordered group was matched with the first maze from Library 1 presented to the Random group (i.e. the maze in the Random group’s Set 1 from Library 1). Next, the first maze from Library 2 presented to the Ordered group (which occurred after the 16 Library 1 mazes) was matched with the first maze from Library 2 presented to the Random group after 16 mazes (i.e. after discarding the first 16 mazes presented to the Random group, the next maze presented from Library 2 was selected as the match). Third, the first maze from Library 3 presented to the Ordered group (which occurred after the 32 Library 1 & 2 mazes) was matched with the first maze from Library 3 presented to the Random group after 32 mazes; and so on. Again, each subject contributed 12 mazes to the analysis. Overall, performance changed with experience at the task (χ2(1, N=395) = 31.44, p = 0.0001). The magnitude of improvement was larger for chimps than capuchins (χ2(1, N=395) = 18.81, p =0.0001; see Figure 2). In the case of chimpanzees, performance improved with experience; the slope of the line was −.0363. In the case of capuchins, for the data subset presented in Figure 2, no net improvement is evident. Chimpanzees averaged 60% errors on the first two chronological test Blocks (where Block is defined as 16 completed mazes, equivalent to 1 library for the ordered group) and capuchins, 39%. On the last two test Blocks, chimpanzees averaged 5% errors, but capuchins’ average percentage of choices that were errors was unchanged (39%). Recall that in Blocks 1, 2, and 3 all correct choices are towards the goal (Obvious choices). Non-obvious choices first appear in Block 4, and capuchins made errors on a very high proportion of choices in Block 4.
Figure 2.
Proportion of choices that were Errors across blocks (where one block is 16 consecutive mazes). This figure uses a subset of the data (one maze per subject per time point) (see text for explanation). Bars indicate the standard error of the mean (SEM). Note that in this subset of the data, in Blocks 1 – 3, all correct choices are Obvious. Non-obvious choices first appear in Block 4 in this data set. Capuchins had particular difficulty with these kinds of choices when they initially appeared.
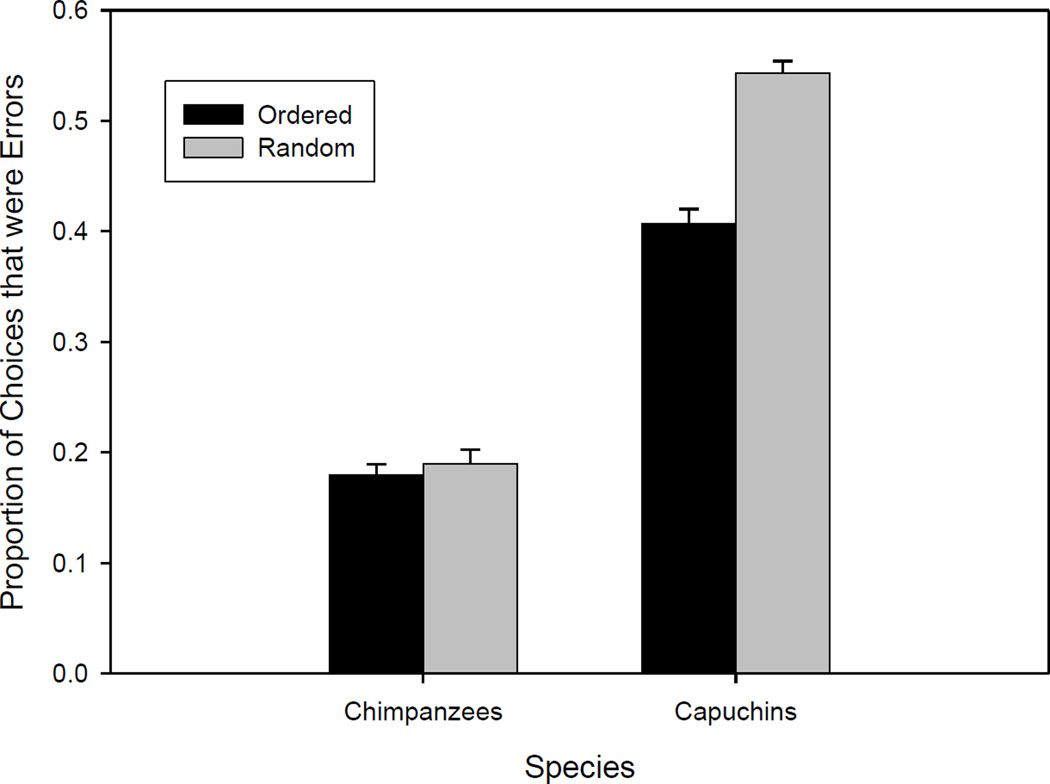
Effects of presentation mode on the production of errors
Overall, individuals receiving the mazes in the Ordered mode made proportionally fewer errors than individuals receiving the mazes in the Random mode (Z = 12.19, p = 0.0001; see Table 3). However, the effect of presentation mode was greater for capuchins than for chimpanzees (see Figure 3). When data from the two species were analyzed separately, for chimpanzees, there was no effect of presentation mode on the commission of errors (χ2 (1, N=2571) = 0.44; p = .51); for capuchins, there was an effect (χ2 (1, N=3461) = 47.34, p = 0.0001). Parsing errors by type of choice point, all the chimpanzees made significantly fewer errors than expected by chance for both Obvious and Non-obvious choice points (see Table 4). Four capuchins made fewer errors than expected by chance on Obvious choices, and the other three performed at chance levels. On Non-obvious choice points, no capuchin made fewer errors than expected by chance and five made more errors than expected by chance (one in the Ordered group and all four in the Random group). For these analyses, chi square tests (with 1 degree of freedom) were used to evaluate deviation from chance.
Figure 3.
Proportion of choices that were Errors, comparing groups receiving the mazes in ordered or random series. Bars indicate the standard error of the mean (SEM).
Self-correction of errors
Chimpanzees self-corrected the direction of cursor movement after moving the cursor into a dead-end alley more often than capuchins (Mean = 62%, chimpanzees, and 28%, capuchins; Z = 13.50; p = 0.0001; see Table 5). Logistic analysis revealed a significant interaction between genus and mode of presentation (Ordered or Random) for this variable (χ2 (1, N=2123) = 12.46, p = 0.0001). The capuchins in the Ordered group self-corrected more often than capuchins in the Random group (42% of errors vs. 21% of errors, Ordered and Random groups, respectively). There was no difference in this measure for chimpanzees in the two groups (65% and 61%, Ordered and Random groups, respectively).
Table 5.
Frequency of self-correcting an error before striking the end of the alley across species and testing groups.
| Species | Total Errors |
Group | Errors per Groups |
Self- corrected Errors |
Errors that were not self- corrected |
% Errors that were self-corrected |
|---|---|---|---|---|---|---|
| Chimpanzees | 472 | Ordered | 283 | 184 | 99 | 65 |
| Random | 189 | 116 | 73 | 61 | ||
| Total | 472 | 300 | 172 | 63.6 | ||
| Capuchins | 1727 | Ordered | 580 | 244 | 336 | 42 |
| Random | 1147 | 239 | 908 | 21 | ||
| Total | 1727 | 483 | 1244 | 28.0 |
Effect of experience on Self Correct
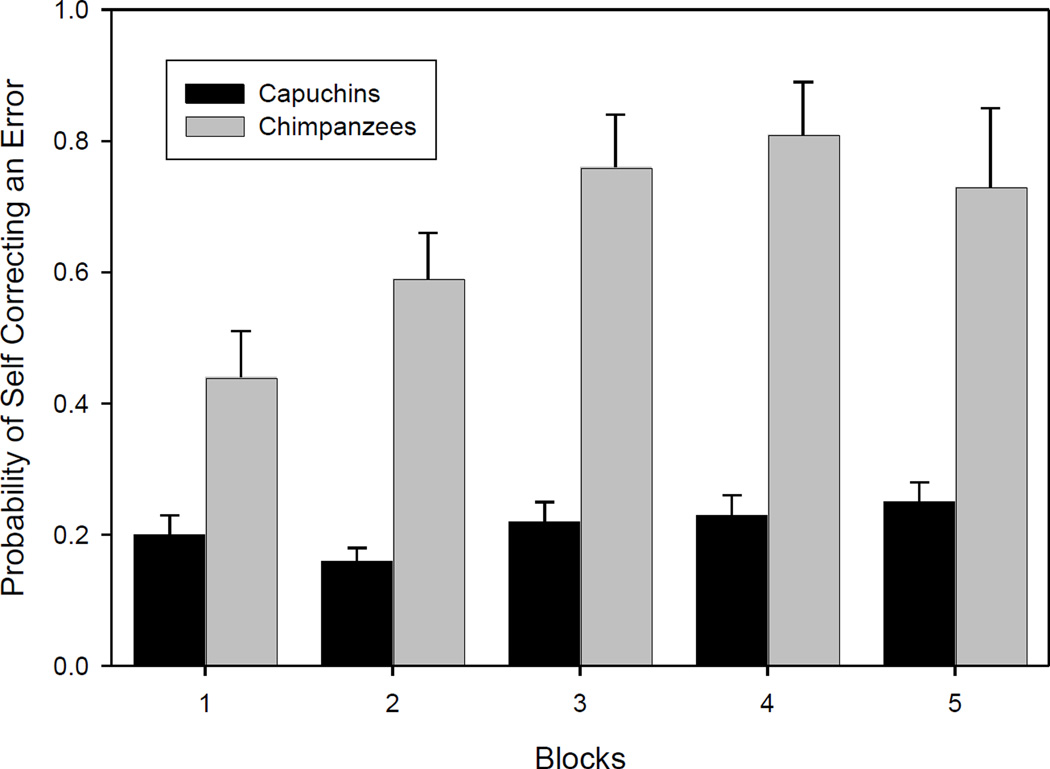
The proportion of errors that chimpanzees and monkeys in the Ordered groups self-corrected shifted moderately over testing (χ2 1, N= 428, p = 0.074). Looking at performance on the mazes in five consecutive Blocks (each Block composed of 20% of mazes), chimpanzees in the Ordered group self-corrected on 64, 57, 57, 80 and 75% of errors. Comparable figures for the capuchins are 51%, 44, 37, 44, and 46%, and 45%. In contrast to their counterparts in the Ordered group, the chimpanzees in the Random group self-corrected a substantially greater proportion of their errors as testing progressed, from 44% of errors in the first 20% Block of trials to 81% and 73% on their final two Blocks. Capuchins in the Random group initially self-corrected a mere 20% of their errors in Block 1, but they too improved on this measure, albeit far less than the chimpanzees: They self-corrected 24% of their errors in Block 5. The pattern produced a significant interaction effect between block and species (χ2 (1, N=1336) = 6.77, p = 0.01; see Figure 4). A separate logistic regression revealed that capuchins in the Random group displayed, though not significantly, higher rates of self-correcting errors across 5 Blocks (χ2 (1, N=1147) = 3.12, p = 0.08).
Figure 4.
Probability of correcting an Error by monkeys and apes in the Random groups, plotted by Block of 20% of mazes completed. Bars indicate the standard error of the mean (SEM).
Effects of the layout of the choice
Non-obvious choices degraded choice performance in both species (χ2 (1, N=6032) = 6.14, p = 0.02) compared to other choices, although the effect was larger for capuchins than chimpanzees (interaction between choice type and genus: χ2 (1, N = 6032) = 27.51, p = 0.0001). There was also an interaction between choice, species, and presentation format (χ2 (1, N = 6032) = 4.51, p = 0.04). Capuchins in the Random group encountered greater difficulty successfully maneuvering mazes with Non-obvious choices than capuchins in the Ordered group or chimpanzees in either group (see Table 4).
The layout of the choice in relation to the goal (as illustrated in Figure 1) also impacted the probability that a monkey or ape would self-correct its path if it made an error (see Table 6). If an error was made on a Non-obvious choice then the probability of that error being self corrected was less than if the error occurred on an Obvious choice (chimpanzees, 60% vs. 70%; capuchins 24% vs. 36%; χ2 (1, N=2199) = 29.78, p = 0.0001). Once an error was committed on a Non-obvious choice, the probability of self correcting was larger for chimpanzees than capuchins (60%, chimpanzees vs. 24%, capuchins; χ2 (1, N=2199) = 184.27, p = 0.0001). There was no interaction between species and type of error (overshoot or wrong turn) on the probability that the subject would correct an error before striking the end of the alley.
Table 6.
Frequency of self-correcting an Error before striking the end of the alley at Non-obvious choice points vs. Obvious choice points across species.
| Species | Total Errors | Type of choice points |
Errors | Self-Corrected (% Errors) |
|---|---|---|---|---|
| Chimpanzees | 472 | Obvious | 174 | Yes 121 (70%) |
| No 53 | ||||
| Non-obvious | 298 | Yes179 (60%) |
||
| No 119 | ||||
| Self-Corrected/ Total errors |
300/472 (64%) |
|||
| Capuchins | 1727 | Obvious | 555 | Yes 200 (36%) |
| No 355 | ||||
| Non-obvious | 1172 | Yes 283 (24%) |
||
| No 889 | ||||
| Self-Corrected/ Total errors |
483/1727 (28%) |
We also investigated the relation between errors and the structure of the choice as facultative or forced. As in every analysis of errors chimpanzees made proportionally far fewer errors than capuchins on Forced choices (22%, vs. 49%), and on Facultative choices (16%, chimps; 47%, capuchins). Apparently the facultative or forced structure of a choice point did not affect its difficulty, for either species.
Discussion
Our primary aim in this study was to evaluate the role of experience in navigation through two-dimensional mazes in two species of nonhuman primates, tufted capuchins and common chimpanzees. Additionally, we evaluated the impact of two different ways of presenting the mazes (in order of progressively more choices and more Non-obvious choices, in which the correct path led away from the goal, or in a random order), and the impact of the layout of the choices (that is, as continuations or as turns) within a maze. As it turns out, the effect of species overshadowed virtually all other features of the experiment. The differences between the species in the way they chose a path at successive choice points provide a glimpse of a profound cognitive difference between them that, we suggest, likely impacts how these two species approach any problem requiring on-line monitoring and adjustment of movement through space.
All the subjects in this study, seven capuchins and five chimpanzees, were relatively naïve to the use of joysticks and two-dimensional spatial problems, in contrast to a previous study (Fragaszy et al. 2003) in which the chimpanzee subjects were more experienced than the capuchin subjects. However, the robust difference in performance between capuchins and chimpanzees observed in the first study was replicated in this study. Chimpanzees made less than half as many errors on the mazes as capuchins, and they self-corrected (that is, they moved the cursor out of the incorrect alley before striking the end of the alley) on a larger proportion of their errors than did capuchins. Chimpanzees’ performance was not affected by whether they encountered the mazes in a fixed order or in a random order (perhaps because they were near a ceiling in terms of the number of correct choices they made – more than 80% correct choices, overall) and they made fewer errors as testing progressed. Capuchins, in contrast, made more errors and self-corrected on a smaller proportion of these errors if they received the mazes in random order than if they received them in a fixed order of ascending numbers of choices, and of Non-obvious choices. Thus, for capuchins, practice completing simpler/shorter mazes supported better subsequent performance (fewer errors) on more complicated/longer mazes. Capuchins had particular difficulty with Non-obvious choices, in which the correct choice led away from the goal, and capuchins that encountered the mazes in a random order had greater difficulty with Non-obvious choices than did capuchins that encountered them in a fixed order from fewer to more choices. Capuchins showed no decline over sessions in rates of committing errors; monkeys in the Random group showed a statistically non-significant increase in the rate at which they self-corrected errors as they gained more experience solving mazes.
This study and the previous one (Fragaszy et al. 2003) included a small number of participants (nine chimpanzees and seven capuchins), and thus we must necessarily be cautious about generalizing our results. However, the consistency among the chimpanzees’ performance in the two studies, across quite different age, rearing, social, and testing conditions as well as variable previous experience using a joystick or in other experiments, provides confidence that we have acquired an accurate assessment of chimpanzees’ characteristics in this task (Murnane et al. 2001). For example, four chimpanzees in the first study (Fragaszy et al. 2003) made errors on 14% of choices, overall, compared to 18% by the five chimpanzees in this study on the same mazes. The relative consistency in performance among the chimpanzees as well as capuchins, together with the large differences in performance between the species, provide confidence that we are seeing robust species differences.
Under different circumstances than presented in this study, chimpanzees moving a cursor toward a goal in a computerized navigation task also displayed good mastery of moving away from the goal, and they showed similar errors to those observed in both species in this study. Iversen and Matsuzawa (2001; Experiment 1) presented a series of detour problems on a touchscreen to two chimpanzees. As in this study, the subject’s task was to move a cursor across a monitor to a stationary visible goal, the precise layouts of the problems varied across all trials within testing sessions, and tasks were presented in order of hypothetical difficulty (easier tasks first). One important difference in procedure between our study and Iversen and Matsuzawa’s study is that in the latter, the subjects practiced solving the same mazes to a specified criterion of accuracy (two or fewer trials per session in which the chimpanzee moved the ball directly toward the target, despite a barrier blocking the path). Both chimpanzees often moved the cursor directly into a “barrier” and one subject occasionally did not complete a trial after moving the cursor back and forth between barriers. When presented with 24 new mazes (each presented twice) in the final phase of this experiment, one subject moved the ball in a path directly toward the goal despite a barrier blocking that route on three trials (out of 48 trials), and the other subject did so 11 times (out of 48 trials). Thus in both studies, chimpanzees retain a discernable propensity to move directly toward the goal even though a direct route is blocked by a barrier, and even after considerable practice moving around a barriers. However, this propensity had a minor influence on chimpanzees’ travel paths, and it diminished with practice, whereas for the capuchins it was a distinguishing feature of performance and did not, in the time frame of this study, diminish with practice.
Why were chimpanzees so much better at our task than were capuchins, in both commission of fewer errors and proportionally more frequent self-correction after committing an error? Why did chimpanzees have a declining rate of committing errors and an increasing rate of self-correcting, but capuchins did not, with the exception of a modestly improving rate of self-correction for the monkeys in the Random group? Why did mode of presentation (ordered vs. random) make such a difference for capuchins, and no discernible difference for chimpanzees?
Consider first the problem presented by a Non-obvious choice to an actor navigating our experimental mazes. At each choice point, one path continues and the other is a dead-end. In the case of a “regular” choice, the path that continues also leads towards the goal. In the case of a Non-obvious choice, the path that continues leads away from the goal, and the non-continuing path leads toward the goal. Thus one can conceive of the problem at Non-obvious choices as composed of two elements: (a) recognizing which path ends and/or which path continues and (b) generating movement in a direction away from the goal.
The perceptual challenge, noticing the continuity of the paths, would seem well within the capabilities of capuchins, given the visual perceptual learning that capuchins display in completing other computer-presented visual tasks such as discriminating relative position of a dot above or below a line (Spinozzi, Lubano and Truppa 2004) or the groupings of items in a visual display (Spinozzi, De Lillo and Castelli 2004), and relational ordering of multiple items within sets of items in a touch-screen task (McGonigle et al. 2003). Thus we assume that both species could learn to notice the continuity of the path. The fact that individuals of both species sometimes self-corrected their errors (on 64% of errors, chimpanzees, and 28%, capuchins), suggests that they both did (often) notice continuity of a path, once they began to travel along it.
This brings us to look particularly at the challenge of moving away from the goal. Traveling away from the goal requires replacing a prepotent response (moving to the goal) with a different behavior (moving away from the goal). One can conceive of this challenge as one of stopping an action (inhibition) or as a challenge of selecting and shifting activity (Kenemans, Bekker, Lijffijt, Overtoom, Jonkman, and Verbaten, 2005; Spencer et al. 2001). Chimpanzees made fewer errors than capuchins at Non-obvious choices (21% vs. 61%). The difference between the species was equally evident whether the choice was made at a facultative choice point or a forced choice. This could indicate that chimpanzees looked down the alley to evaluate continuity more consistently than capuchins, and/or that they could move away from the goal more consistently than capuchins. If one considers the rate of self-correcting an error to indicate the propensity of the individual to look ahead, capuchins do so approximately once every three or four choices (28% of errors are self-corrected). Thus they might be expected to make errors on about 67 – 75% of Non-obvious choices. Four of seven capuchins made errors at about this rate (64 – 70%) on Non-obvious choices; the other three made errors at a lesser rate (47 – 56%). Thus most capuchins made errors on Non-obvious choices at the same rate as they continued movement to the end of the alley after making an error.
Chimpanzees looked ahead after making a wrong choice approximately twice every three errors (i.e., they self-corrected 64% of their errors), but they made errors at only one out of four or five (21%) Non-obvious choice points. Thus they apparently looked ahead before they made a choice, and moved away from the goal at Non-obvious choice points, even more effectively than they looked toward the end of the alley after making a (wrong) choice. This pattern could arise if chimpanzees tended to be inattentive to the task more often after they made a choice than at the time they made a choice. In any case, it suggests that the chimpanzees had no particular difficulty in moving away from the goal at Non-obvious choice points. We conclude that even though the species may differ substantively in propensity to look ahead before and after making a choice, the difference between them in propensity to move away from the goal probably contributes to the performance differences between them.
Our findings indicate that both species altered their behavior as a consequence of experience, but not in the same way: Chimpanzees made fewer errors as testing progressed, and some capuchins increasingly self-corrected after making an error. This varied outcome is congruent with the joint propositions that problem-solving reflects the coordinated outcome of multiple cognitive processes, each differentially responsive to experience and to immediate circumstances (Spencer et al. 2001) and that the coordination among these processes varies across species. We need to evaluate performance of these two species (and others) on more targeted problems to distinguish among the many possible explanations for these changes that flow from developmental models (e.g, changes in working memory, encoding strategies, attentional shifting, inhibitory control, integration of dual properties, system organization).
Should we expect capuchins to master this problem at all? The evidence suggests yes: Macaques (Macaca mulatta) mastered a reversed-contingency task that, similar to the Non-obvious choice problem, required the monkeys to make the “opposite” choice rather than the more familiar choice following each reversal event (Murray et al. 2005). Similarly, Japanese macaques, cotton-top tamarins, squirrel monkeys and brown and black lemurs (Saguinus oedipus, Saimiri sciureus, Eulemur fulvus and E. macaco) learned to forego choosing the larger quantity of food (which resulted in their receiving nothing) to choose a smaller quantity of food (which resulted in their receiving the larger quantity). After mastering this problem, most individuals also mastered the reverse-reward problem, in which choosing the smaller quantity delivers the larger quantity and vice versa (Anderson et al. 2000, Silberberg and Fujita 1996, Genty et al 2004, Kralik 2005). Most of the squirrel monkeys succeeded after correction trials and a time-out procedure were instituted; cotton-top tamarins succeeded in this task only following experience with selecting the smaller quantity and eating it (Kralik 2005). Cotton-top tamarins mastered reaching for an object in a transparent box, reaching into the open side of the box rather than striking the front of the box, after experience reaching into the side of similarly positioned opaque boxes (Santos, Ericson and Hauser 1999). In the latter study in particular, a mastery experience provided an alternative action (reach to the side of the box) that the monkeys could use with the transparent box. Capuchin monkeys have mastered one task in which they had to substitute a different behavior for a more probable one: Evans and Westergaard (2006) report that capuchin monkeys held an edible object rather than ate it when they could take the object to a site where it could be used as a tool to gain a higher-quality food. Evans and Westergaard (2006) mention that monkeys with more experience using tools were better able to do this than monkeys with less experience, congruent with the proposal flowing from dynamic systems thinking that experience allows individuals to re-organize behavior to include additional options, or in metaphorical terms, sculpts a new landscape of behavioral probabilities (Thelen and Smith 1994). Santos et al. (1999) and Kralik (2005) similarly comment that the availability of a (practiced) alternative action supported the tamarins’ effective management of shifting from a prepotent behavior to another behavior. In sum, in a variety of tasks and species, practicing alternative behaviors supports microdevelopment of problem-solving skills. This line of reasoning suggests that more practice than was afforded to the capuchins in this study would support improvement in this task. We are pursuing this possibility in further testing.
The ability to slow or pace action has been suggested to be an important element allowing selection or guidance of behavior from among alternatives, thereby supporting selection of a behavior different from the “prepotent” one. Four-year-old children asked to give a verbal response counter to their normal experience (to say “night” to a picture of the sun and “day” to a picture of the moon) did better on this task when a delay was inserted by the experimenter between the initial request and the child’s answer (Diamond, Kirkham, and Amso, 2002). Diamond et al. (2002) comment that children needed time to produce the correct answer; they did well when they were forced to take that time before stating their answer. In this study, the speed of the cursor was equivalent for chimpanzees and capuchins, ruling out a direct effect of the tempo of movement on differences between the two species in the probability of making errors. But, this idea could be adopted for analysis within subjects, by altering the cursor speed. Perhaps if monkeys are forced to slow their actions at choice points they will be more likely to look ahead and organize behavior in accord with what they see.
Why did chimpanzees’ performance (in terms of the rate of making errors and self-correcting errors) improve with practice, whereas capuchins’ performance overall improved minimally? We do not have a strong explanation for this difference in susceptibility to experience. Here we suggest two plausible explanations, and a way to compare them. Wulf and Shea (2002), discussing variations in the efficiency of learning simple and complex skills, comment that inducing a focus on the consequences of movement, rather than a focus on the movements themselves, aids learning a “complex” motor skill (one with several degrees of freedom). Perhaps chimpanzees are better able than capuchins to focus attention on the travel of the cursor, the external consequence of their body movement. If this is so, then manipulations of the visual properties of the task that alter the ability to focus on the consequences of movement (e.g., degrading the detail of the cursor’s movements within the maze) should affect chimpanzees’ performance more than capuchins’ performance. Alternatively, perhaps chimpanzees are generally better at shifting attention and behavior over brief time intervals than capuchins, in which case, all alterations of the task which increase difficulty will have less effect on chimpanzees than capuchins.
Finally, why might mode of presentation have impacted capuchins more than chimpanzees? For chimpanzees, it may be that the task was sufficiently easy for both groups that the mode of presentation made no discernible difference. This was certainly not the case for capuchins, however. Many lines of evidence suggest that moving away from the goal presented a greater challenge to the capuchin monkeys than the chimpanzees, so perhaps any aspect of the task that aided mastery of this challenge impacted capuchins more than chimpanzees. Menzel and Menzel (2007) reported on chimpanzees’ behavior in a task where they used a joystick to move a cursor around a barrier presented at various positions on a computer monitor to reach a goal location on the other side of the barrier. Individuals with extensive practice using a joystick produced more direct paths of movement (fewer pixel jumps) than did chimpanzees with little experience using a joystick. Menzel and Menzel’s (2007) findings are congruent with the suggestion made here that experience at navigating varied two-dimensional layouts leads to altered behavior when solving these problems.
Returning to the model of planning presented in Table 1, we suggest that both species mastered planning at Level 2, maintaining a focus on moving to the goal and monitoring the effect of each choice. Chimpanzees clearly mastered planning at Level 3, using continuity of the path to guide their choices. The evidence suggests that capuchins, even if they recognized continuity of the path as an important property, could not routinely use that knowledge to guide action at a choice point. Instead, especially for monkeys in the Random group, their actions at choice points were usually “captured” by the Euclidean spatial relation between the alleys and the goal. In a general sense, the capuchin monkeys seemed less able than chimpanzees to redirect behavior to multiple possibilities. Future work will be directed at probing what circumstances support learning by capuchin monkeys to manage the challenge of moving away from the goal at a Non-obvious choice-point
An ecological perspective suggests at least one reason why these species should differ in their attentional management in ways that would advantage chimpanzees in the maze problems. Solving mazes benefits from focused attention over time to a small visual stimulus. Capuchins, as smaller animals subject to higher risk of predation (compared to chimpanzees), typically interrupt their activity every few seconds to look around themselves, an activity termed “vigilance” (Treves 2000, van Schaik and van Noordwijk 1989, Hirsch 2002, Rose and Fedigan 1995). Capuchins in captivity, where predators are not present, still interrupt ongoing activity to look around themselves every few seconds while working on various experimental problems and in the absence of external disruptions (Vickers, Jeyeraj and Fragaszy, unpublished data). This style of attention is not conducive to learning contingencies that are evident only visually, divorced from kinesthesis. In casual observation, captive chimpanzees are less vigilant than captive capuchins during everyday activity (personal observation). It would be interesting to relate patterns of vigilance in individual monkeys and apes as they navigate through mazes to the accuracy of their choices at particular choice points. It would also be interesting to compare aptitude for traveling away from the goal in another primate species that shares a slower tempo of vigilance with chimpanzees, but is phylogenetically distant from apes. For example, baboons are adept at using a joystick-mediated interface with computerized displays (Fagot, Wasserman and Young 2001). Although data are sparse, what data there are suggest that frequency of vigilance in baboons is similar to that of chimpanzees (Alberts 1994, Treves 2000). We predict that performance on spatial problems of the sort used in this experiment, intermittently requiring travel away from the goal, will track patterns of vigilance across species more closely than it will track phylogeny.
Acknowledgments
We thank James Fuller for developing the original maze presentation software, Emil Menzel for development of the pixel tracking program, and the UGA Instrument Shop for the creation of our testing apparatus. We thank Liz Hirsch, Amy Venable, Monique Dase, Jacqueline Montoya, Dayana Atalah, and Katie Leighty for assistance in testing the monkeys, and Carrie Rosengart, Jinae Lee and Jaxx Reeves for aid in statistical analysis. We thank our anonymous reviewers for careful comments which substantially improved this manuscript. This work was supported by HD06016, HD38051, and MH58855 from the National Institutes of Health (USA) and SBR-9729485 from the National Science Foundation to Georgia State University, and the University of Georgia. This study complied with all laws regulating animal care and use in the United States.
References
- Alberts S. Vigilance in young baboons. Effects of habitat, age, sex and maternal rank on glance rate. Anim Behav. 1994;47:749–755. [Google Scholar]
- Anderson J, Awazu S, Fujita K. Can squirrel monkeys (Saimiri sciureus) learn self-control? A study using food array selection tests and reverse-reward contingency. J Exp Psych: An Beh Proc. 2000;26:87–97. doi: 10.1037//0097-7403.26.1.87. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHS. Psych Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF. Cognitive development and individual differences. Belmont, CA: Wadsworth; 2004. Children’s thinking. [Google Scholar]
- Boesch C, Boesch-Achermann H. The chimpanzees of the Taï Forest: Behavioural ecology and evolution. New York: Oxford Univ Press; 2000. [Google Scholar]
- Boesch C, Boesch H. Mental map in wild chimpanzees: An analysis of hammer transports for nut cracking. Primates. 1984;25:160–170. [Google Scholar]
- Case R. The mind’s staircase. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- Case R, Okamoto Y. The role of central conceptual structures in the development of children's thought. Monographs of the Society for Research in Child Development. 1996;61(1/2) doi: 10.1111/j.1540-5834.1996.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Cox RFA, Smitsman AW. Action planning in young children's tool use. Dev Sci. 2006;9:628–641. doi: 10.1111/j.1467-7687.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- De Kort S, Tebbich S, Dally J, Emery S, Clayton N. The comparative cognition of caching. In: Wasserman E, Zentall T, editors. Comparative cognition: Experimental explorations of animal intelligence. New York: Oxford Univ Press; 2006. pp. 529–552. [Google Scholar]
- De Lillo C, Visalberghi E. Transfer index and mediational learning in tufted capuchins (Cebus apella) Int J Primatol. 1994;15:275–287. [Google Scholar]
- Diamond A, Kirkham N, Amso D. Conditions under which young children can hold two rules in mind and inhibit a prepotent response. Dev Psych. 2002;38:352–362. [PubMed] [Google Scholar]
- Evans TA, Westergaard GC. Self-control and tool-use in tufted capuchin monkeys (Cebus apella) J Comp Psych. 2006;120:163–166. doi: 10.1037/0735-7036.120.2.163. [DOI] [PubMed] [Google Scholar]
- Fagot J, Wasserman E, Young M. Discriminating the relation between relations: The role of entropy in abstract conceptualization by baboons (Papio papio) and humans (Homo sapiens) J Exp Psych: Anim Beh Proc. 2001;27:316–328. [PubMed] [Google Scholar]
- Fischer KW, Bidell T. Dynamic development of psychological structures in action and thought. In: Damon W, Lerner R, editors. Handbook of Child Psychology: Vol. 1. Theoretical models of human development (Fifth edition) New York: John Wiley & Sons; 1998. pp. 467–561. (series ed) (volume ed) [Google Scholar]
- Fragaszy D, Crast J, Matsuzawa T. Intrinsic movements of the hands in chimpanzees and capuchin monkeys. Folia Primatol. 2004;75:83. [Google Scholar]
- Fragaszy D, Johnson-Pynn J, Hirsh E, Brakke K. Strategic navigation of two-dimensional alley mazes: Comparing capuchin monkeys and chimpanzees. Anim Cogn. 2003;6:149–160. doi: 10.1007/s10071-002-0137-8. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Cummins-Sebree SE. Relational spatial reasoning by a nonhuman: the example of capuchin monkeys. Behav Cogn Neurosci Rev. 2005;4:282–306. doi: 10.1177/1534582306286573. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Visalberghi E, Fedigan LM. The complete capuchin: The biology of the genus Cebus. New York: Cambridge Univ Press; 2004b. [Google Scholar]
- Genty E, Palmier C, Roeder J. Learning to suppress responses to the larger of two rewards in two species of lemurs,Eulemur fulvus and E macaco. Anim Behav. 2004;67:925–932. [Google Scholar]
- Hirsch BT. Social monitoring and vigilance behavior in brown capuchin monkeys (Cebus apella) Behav Ecol Sociobiol. 2002;52:458–464. [Google Scholar]
- Hunt KD. The postural feeding hypothesis: An ecological model for the evolution of bipedalism. South African J Sci. 1996;92(2):77–90. [Google Scholar]
- Iversen I, Matsuzawa T. Acquisition of navigation by chimpanzees (Pan troglodytes) in an automated fingermaze task. Anim Cogn. 2001;4:179–192. doi: 10.1007/s100710100101. [DOI] [PubMed] [Google Scholar]
- Johnson-Pynn J, Fragaszy DM, Brakke KE, Hirsh EM, Greenfield PM. Strategies used to combine seriated cups by chimpanzees (Pan troglodytes), bonobos (Pan paniscus), and capuchins (Cebus apella) J Comp Psych. 1999;113:137–148. doi: 10.1037/0735-7036.113.2.137. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Bekker EM, Lijffijt M, Overtoom CCE, Jonkman LM, Verbaten MN. Attention deficit and impulsivity: Selecting, shifting, and stopping. Int J Psychophysio. 2005;58:59–70. doi: 10.1016/j.ijpsycho.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Klahr D. Discovering the present by predicting the future. In: Haith MM, Benson JB, Roberts RJ Jr, Pennington BF, editors. The development of future-oriented processes. Chicago: University of Chicago Press; 1994. pp. 177–218. 1994. [Google Scholar]
- Kralik JD. Inhibitory control and response selection in problem solving: how cotton-top tamarins (Saguinus oedipus) overcome a bias for selecting the larger quantity of food. J Comp Psych. 2005;119:78–89. doi: 10.1037/0735-7036.119.1.78. [DOI] [PubMed] [Google Scholar]
- Leighty KA, Fragaszy DM. Joystick acquisition in tufted capuchins (Cebus apella) Anim Cogn. 2003;6:141–148. doi: 10.1007/s10071-003-0176-9. [DOI] [PubMed] [Google Scholar]
- Matsuzawa T. Primate foundations of human intelligence: A view of tool use in nonhuman primates and fossil hominids. In: Matsuzawa T, editor. Primate origins of human cognition and behavior. Tokyo: Springer; 2001. pp. 3–25. [Google Scholar]
- McGonigle B, Chalmers M, Dickinson A. Concurrent disjoint and reciprocal classification by Cebus apella in seriation tasks: evidence for hierarchical organization. Anim Cogn. 2003;6:185–197. doi: 10.1007/s10071-003-0174-y. [DOI] [PubMed] [Google Scholar]
- Menzel E, Jr, Menzel C. Do primates plan routes? Simple detour problems reconsidered. In: Washburn D, editor. Primate Perspectives on Behavior and Cognition. Washington, DC: American Psychological Association; 2007. pp. 175–206. [Google Scholar]
- Murnane A, Fragaszy D, Hopkins W. Spatial cognition: Experience affects how apes navigate two-dimensional mazes. Am J Primatol. 2001;54:82. [Google Scholar]
- Murray EA, Kralik JD, Wise SP. Learning to inhibit prepotent responses: successful performance by rhesus macaques,Macaca mulatta on the reversed-contingency task. Anim Behav. 2005;69:991–998. [Google Scholar]
- Richardson W, Washburn D, Hopkins W, Savage-Rumbaugh E, Rumbaugh D. The NASA/LRC Computerized test system. Behavioral Research Methods, Instruments & Computers. 1990;22:127–131. doi: 10.3758/bf03203132. [DOI] [PubMed] [Google Scholar]
- Rogoff B, Mistry J, Goncu A, Mosier C. Guided participation in cultural activity by toddlers and caregivers. Monographs of the Society for Research in Child Development. 1993;58:R5. [PubMed] [Google Scholar]
- Rose L, Fedigan L. Vigilance in white-faced capuchins,Cebus capucinus in Costa Rica. An Beh. 1995;49:759–796. [Google Scholar]
- Rumbaugh DM, Pate JL. The evolution of cognition in primates: A comparative perspective. In: Roitblat HL, Bever TG, Terrace HS, editors. Animal Cognition: Proceedings of the Harry N. Frank Guggenheim Conference. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1984. pp. 569–587. [Google Scholar]
- Santos LR, Ericson BN, Hauser MD. Constraints on problem solving and inhibition: Object retrieval in cotton-top tamarins (Saguinus oedipus oedipus) J Comp Psych. 1999;113:186–193. [Google Scholar]
- Schutte AR, Spencer JR, Schoner G. Testing the dynamic field theory: Working memory for locations become more spatially precise over development. Child Dev. 2003;74:1393–1417. doi: 10.1111/1467-8624.00614. [DOI] [PubMed] [Google Scholar]
- Scott N, Fragaszy D, Menzel C. Chimpanzees’ (Pan troglodytes) strategies for managing concurrent, asymmetric spatial relations in an insertion task. Am J Primatol. 2006;68(Suppl 1):118. [Google Scholar]
- Shettleworth S. Cognition, Evolution and Behavior. New York: Oxford University Press; 1998. [Google Scholar]
- Siegler R, Alibali M. Children’s Thinking. 4th ed. Upper Saddle River, NJ: Prentice Hall; 2004. [Google Scholar]
- Silberberg A, Fujita K. Pointing at smaller food amounts in an analogue of Boysen and Berntson’s (1995) procedure. J Exp Anal Beh. 1996;66:143–147. doi: 10.1901/jeab.1996.66-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LB, Thelen E. Development as a dynamic system. Trends Cogn Sci. 2003;7:343–348. doi: 10.1016/s1364-6613(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Spencer JR, Hund AM. Developmental continuity in the processes that underlie spatial recall. Cogn Psych. 2003;47:432–480. doi: 10.1016/s0010-0285(03)00099-9. [DOI] [PubMed] [Google Scholar]
- Spencer JR, Smith LB, Thelen E. Tests of a dynamic systems account of the A-not-B error: The influence of prior experience on the spatial memory abilities of two-year-olds. Child Dev. 2001;72:1327–1346. doi: 10.1111/1467-8624.00351. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, De Lillo C, Castelli S. Detection of grouped and ungrouped parts in visual patterns by tufted capuchin monkeys (Cebus apella) and humans (Homo sapiens) J Comp Psych. 2004a;118:297–308. doi: 10.1037/0735-7036.118.3.297. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, Lubrano G, Truppa V. Categorization of above and below spatial relations by tufted capuchin monkeys (Cebus apella) J Comp Psych. 2004b;118:403–412. doi: 10.1037/0735-7036.118.4.403. [DOI] [PubMed] [Google Scholar]
- Thelen E, Smith LB. A dynamic systems approach to the development of cognition and action. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- Tomasello M, Call J. Primate Cognition. New York: Oxford Univ Press; 1997. [Google Scholar]
- Treves A. Theory and method in studies of vigilance and aggregation. An Beh. 2000;60:711–722. doi: 10.1006/anbe.2000.1528. [DOI] [PubMed] [Google Scholar]
- van Schaik C, van Noordwijk M. The special role of male Cebus monkeys in predation avoidance and its effect on group composition. Behav Ecol Sociobiol. 1989;24:265–276. [Google Scholar]
- Visalberghi E, McGrew WC. Cebus meets Pan. Int J Primatol. 1997;18:677–681. [Google Scholar]
- Willatts P. Development of problem-solving in infancy. In: Slater A, Bremner G, editors. Infant Development. London: Erlbaum; 1989. pp. 143–182. [Google Scholar]
- Willats P. Development of means-end behavior in young infants: Pulling a support to retrieve a distant object. Devel Psych. 1999;35:651–667. doi: 10.1037//0012-1649.35.3.651. [DOI] [PubMed] [Google Scholar]
- Wright K. The relationship between locomotor behavior and limb morphology in brown (Cebus apella) and weeper (Cebus olivaceus) capuchins. Am J of Primatol. 2007;69:1–21. doi: 10.1002/ajp.20391. [DOI] [PubMed] [Google Scholar]
- Wulf G, Shea CH. Principles derived from the study of simple skills do not generalize to complex skill learning. Psychon Bull Rev. 2002;9:185–211. doi: 10.3758/bf03196276. [DOI] [PubMed] [Google Scholar]