Abstract
Peroxisomes are arguably the most biochemically versatile of all eukaryotic organelles. Their metabolic functions vary between different organisms, between different tissue types of the same organism and even between different developmental stages or in response to changed environmental conditions. New functions for peroxisomes are still being discovered and their importance is underscored by the severe phenotypes that can arise as a result of peroxisome dysfunction. The β-oxidation pathway is central to peroxisomal metabolism, but the substrates processed are very diverse, reflecting the diversity of peroxisomes across species. Substrates for β-oxidation enter peroxisomes via ATP-binding cassette (ABC) transporters of subfamily D; (ABCD) and are activated by specific acyl CoA synthetases for further metabolism. Humans have three peroxisomal ABCD family members, which are half transporters that homodimerize and have distinct but partially overlapping substrate specificity; Saccharomyces cerevisiae has two half transporters that heterodimerize and plants have a single peroxisomal ABC transporter that is a fused heterodimer and which appears to be the single entry point into peroxisomes for a very wide variety of β-oxidation substrates. Our studies suggest that the Arabidopsis peroxisomal ABC transporter AtABCD1 accepts acyl CoA substrates, cleaves them before or during transport followed by reactivation by peroxisomal synthetases. We propose that this is a general mechanism to provide specificity to this class of transporters and by which amphipathic compounds are moved across peroxisome membranes.
Keywords: ATP-binding cassette (ABC) transporter, acyl-CoA, asymmetry, β-oxidation, peroxisome, thioesterase
Introduction
Peroxisome functions and the requirement for transport
Peroxisomes are single membrane-delimited organelles found in almost all eukaryotic cells. Originally named for their peroxidative metabolism, it has now become appreciated that they possess multiple, diverse metabolic capabilities and that these vary between organisms and even between cell types or developmental stages of the same organism [1,2] New functions for peroxisomes are still being discovered [3–5]. One conserved function of peroxisomes is β-oxidation, a pathway by which acyl groups are degraded two carbons at a time following activation to the corresponding acyl CoA thioester by a specific acyl CoA synthetase. Many different substrates can be processed by peroxisomal β-oxidation. The degradation of fatty acids by β-oxidation occurs exclusively in the peroxisome in fungi and plants whereas, in mammals, peroxisomes handle degradation of branched, very long chain and dicarboxylic acids which are exported to mitochondria as C-16 units for completion of catabolism [1,2]. Peroxisomal β-oxidation is also important for synthesis of some bioactive molecules such as docosahexaenoic acid in mammals [2] and the plant hormone jasmonic acid (JA) which is synthesized from its precursor 12-oxophytodienoic acid (OPDA) by three rounds of β-oxidation [6].
The metabolic activities of peroxisomes require that many substrates, products and cofactors have to cross the peroxisomal membrane to and from the cytosol. A channel-forming protein, PXMP2 (peroxisomal membrane protein 2), which is present in the peroxisomal membrane, has been shown in vitro to allow transmembrane passage of small solute molecules with a molecular mass below 400 Da [7,8]. Based on this finding, it has been postulated that small molecules below 400 Da can cross the peroxisomal membrane passively via this protein, whereas others suggest a regulated process [9]. Similarly, plant peroxisome membranes possess a pore-forming activity [10]. For bulky solute molecules (>400 Da); however, the peroxisomal membrane is an impermeable barrier and transporter proteins are required [7].
Relatively few peroxisomal transporters have been identified but it is well-established that peroxisome localized members of ATP-binding cassette (ABC) subfamily D mediate uptake of substrates for β-oxidation [10]. The ABCD family also includes the human endoplasmic reticulum (ER)/lysosome localized PMP69 (69kDa peroxisome membrane protein)/ABCD4 which is implicated in cobalamin transport [11] and an Arabidopsis protein AtABCD2 which is in the chloroplast but for which there is little functional information [12]. However, this review will focus on the peroxisomal members of this family.
Yeast ABCD transporters
In S. cerevisiae, two half transporters, termed Pxa1p (peroxisomal ABC transporter1) and Pxa2p (peroxisomal ABC transporter2) are involved in long chain fatty acyl-CoA transport across the peroxisomal membrane [13–16]. Single knockouts pxa1∆ and pxa2∆ cannot utilize oleate (C18:1) as a sole carbon source and display reduced β-oxidation [13,14]. The double mutant pxa1/pxa2∆ does not have an enhanced phenotype in comparison with the single mutants and protein–protein interaction studies demonstrated that the two half size transporters heterodimerize to form a fully functional transporter [15], with the central domain of the C-terminal region in Pxa2p being required for the dimerization process [17].
Non-esterified fatty acids can enter the peroxisome independently of the ABC transporter and are activated by the peroxisomal acyl-CoA synthetase, Faa2p (fatty acid activation protein 2), prior to β-oxidation [14], a process which requires the activity of the peroxisomal ATP carrier, Ant1p (adenine nucleotide transporter1) [18,19]. Interestingly, a peroxisomal ABC transporter homologous to ScPxa1/2p is not essential for the growth of the oleaginous yeast, Yarrowia lipolytica on fatty acids, but growth is impaired when YlPxa1/2p and YlAnt1p are deleted [20].
Plant ABCD transporters
Arabidopsis AtABCD1 is the most studied and best understood plant peroxisomal ABC transporter and was identified and named independently by at least four different groups [CTS (COMATOSE), PXA1 (peroxisomal ABC transporter1), PED3 (peroxisome defective 3) and ACN2 (acetate non utilizing 2)] [21–24]. The CTS gene is expressed throughout the plant and encodes a full-sized transporter with two homologous but distinct halves fused in a heterodimer in the arrangement [TMD1] (the transmembrane domain)–[NBD1] (nucleotide-binding domain)–[TMD2]–[NBD2] (Figure 1). cts Mutants are defective in lipid mobilization, require an exogenous carbon source for seedling establishment and accumulate acyl-CoAs [22], suggesting that CTS mediates the transport of fatty acyl-CoAs into the peroxisome.
Figure 1. Molecular models showing putative inward and outward conformations of Arabidopsis CTS.
Models of CTS are based on the structures of (A) ABCB10 [52], with bound AMP–PCP (β,γ-methylene adenosine 5′-triphosphate) (green spheres) in an inward-facing conformation [2YL4.pdb replaced in database by 4AYT.pdb; the two structures can be superimposed with a RMSD of 0.19 Å (1 Å=0.1 nm)] and (B) Sav1866 [53] open-outward ADP-bound complex in which the two NBDs (blue and purple) are closely packed with two nucleotides (green spheres), sandwiched between them (2HYD.pdb). The NBDs face the cytosol [39], views are from side-on (top image) and bottom-up (bottom image) and an angled view to show the pore, the possible site of substrate release, created in the open conformation. Note the domain swapping where TMD2 (yellow) contacts NBD1 (blue) and TMD1 (green) contacts NBD2 (purple).
CTS has also been implicated in the import of a wide range of other substrates: the JA precursor, OPDA [6,25], acetate [24], the auxin precursors 2,4-dichlorophenoxybutyric acid and indole butyric acid (IBA) [21,23], precursors of ubiquinone synthesis [3] and cinnamic acid which is required for the synthesis of benzoic acid [4]. In Arabidopsis, the CTS (COMATOSE) locus was identified in genetic screens for positive regulators of germination [22]. Subsequent studies demonstrated that OPDA accumulation underlies the germination defect through up-regulation of a transcription factor, ABI5 (ABA insensitive 5) which inhibits seed coat rupture [25,26]. Although the presumptive substrates for CTS appear superficially dissimilar in size and shape, they all share a common feature in having an acyl chain of at least four carbons terminating in a carboxy group that is potentially esterified to a CoA. Taken together, these data indicate that CTS acts as a gateway into the peroxisome for β-oxidation, influencing a wide range of metabolic and signalling processes.
In contrast with dicotyledonous plants, cereals contain two ABCD1 homologues arising from a gene duplication that occurred prior to the divergence of the Gramineae [27]. Functional studies in barley (Hordeum vulgare) suggest these two genes are in the process of undergoing neofunctionalization. Whereas both HvABCD1 and 2 are involved in fatty acid and IBA metabolism, only HvABCD2 could complement the Arabidopsis cts germination phenotype, pointing to a role for the latter in OPDA metabolism [28]. ABCD1 proteins are also implicated in control of seed size in Arabidopsis, barley and tomato, since mutants in all these species have reduced seed weight, but the biochemical basis for this is currently unclear [28,29].
Human ABCD transporters
In mammalian cells, peroxisomes are involved in a number of important metabolic pathways, including the α- and β-oxidation of fatty acids and the biosynthesis of ether phospholipids and bile acids [2]. Mammalian peroxisomes contain three members of the ABC family: ABCD1 [adrenoleukodystrophy protein (ALDP); 30], ABCD2 [adrenoleukodystrophy-related protein (ALDR); 31] and ABCD3 [70 kDa peroxisomal membrane protein (PMP70); 32]. All three proteins are half-ABC transporters and need to dimerize to constitute a full, active transporter.
Expression of the human (Hs) peroxisomal ABC transporters in yeast followed by functional characteriszation has shown that the ABCD transporters have distinct but overlapping specificities for different acyl-CoA esters. Most hydrophobic C24:0-CoA and C26:0-CoA esters are preferentially transported by HsABCD1, whereas C22:0-CoA, C22:6-CoA and C24:6-CoA are preferentially transported by HsABCD2. Substrates such as long-chain unsaturated acyl-CoAs, 2-methyl branched-chain acyl-CoAs including pristanoyl-CoA and long-chain dicarboxylic CoA esters are preferentially transported by HsABCD3. Although an active heterodimer of ABCD1 and ABCD2 has been reported [33], these yeast studies also demonstrated that HsABCD1, HsABCD2 and HsABCD3 can function as active homodimers [34].
The overlapping activities of the peroxisomal ABC transporters are also evident from the residual C26:0-CoA β-oxidation activity that is found in fibroblasts of patients with X-linked adrenoleucodystrophy (X-ALD), the most common peroxisomal disorder caused by mutations in the ABCD1 gene [35]. This ABCD1 defect causes impaired peroxisomal β-oxidation of very long-chain fatty acids (VLCFAs, ≥C22:0) and consequently, accumulation of VLCFAs especially in brain and adrenal glands. The residual C26:0-CoA β-oxidation activity is mediated by ABCD3. In addition, the VLCFA β-oxidation activity in fibroblasts from X-ALD patients can be rescued by overexpression of ABCD2 or ABCD3 [35]. Ferdinandusse et al. [5] recently described a new peroxisomal disorder caused by mutations in the ABCD3 gene. A patient with hepatosplenomegaly and severe liver disease had a striking accumulation of bile acid intermediates DHCA (dihydroxycholestanoic acid) and THCA, indicating a role of HsABCD3 in the transport of these compounds. The comparatively high expression level of ABCD3 in organs such as liver, heart, kidney, muscle and brain [35], suggests that HsABCD3 is also (indirectly) involved in fatty acid oxidation as an energy generating process [34]. In line with this, it was shown that overexpression or silencing of ABCD3 in fibroblasts alters palmitate β-oxidation activity accordingly [35,36].
Mechanism of fatty acid transport
Both human and plant ABCD genes can partially rescue the β-oxidation and growth phenotypes of the pxa1/pxa2∆ double mutant, reflecting aspects of conserved function with respect to fatty acid metabolism [28,37–39]. However, human ABCD1 cannot complement the cts plant mutant phenotype and human ABCD2 can only complement the germination defect supporting the notion that the plant transporters have a broader substrate specificity [40]. The precise nature of the molecular species recognized by the transporters and the mechanism of transport have been controversial. Accumulating evidence supports the notion that ABCD proteins transport the CoA esters of fatty acids across the peroxisomal membrane [13,22,34,37–39,41]. Owing to their fragile nature, transport experiments with isolated peroxisomes are challenging; however, RNAi mediated suppression of Trypansoma brucei ABCD transporter Gat1 (glycosomal ABC transporter1) reduced incorporation of 14C-C18:1-CoA into purified glycosomes [41] and isolated human fibroblast peroxisomes could oxidize C26:0-CoA [42]. Two models have been described for acyl-CoA transport: in the first model, esterified fatty acids are delivered directly to the peroxisomal matrix [42], whereas in the other model acyl-CoA is hydrolysed during transport and re-esterified in the peroxisomal lumen by an acyl-CoA synthetase [43–45].
Interestingly, HsABCD1 and HsABCD2 could only complement pxa1/pxa2∆ for β-oxidation of C22:0 and C24:0 if the peroxisomal long chain fatty acid peroxisomal synthase Faa2p was present [43]. When expressed in yeast, Arabidopsis CTS showed ATPase activity that could be stimulated by fatty acid acyl CoA (FA-CoA) derivatives and the ability to complement the yeast pxa1pxa2Δ mutant for growth on oleate as a sole carbon source [27,39]. CTS was also dependent upon the presence in the peroxisome of Faa2p or the equivalent Arabidopsis long chain acyl CoA synthetases (LACS)6 and/or LACS7 for functional complementation of the pxa1/pxa2/faa2∆ mutant [45], which is consistent with the in planta requirement for LACS6 and 7 for fatty acid breakdown during Arabidopsis seedling establishment [44]. Overall, the requirement for a peroxisomal activation step suggested that the CoA moiety is cleaved off during the transport cycle (Figure 2), which was confirmed in experiments using isotopic labelling of yeast cells with 18O, although this study could not reveal the origin of the thioesterase activity [43]. Evidence that it is the ABCD transporter itself came from experiments with membranes from insect cells expressing CTS. These showed ATP stimulated thioesterase activity that was much decreased in membranes from cells expressing a non-functional mutant. This is strongly suggestive of a thioesterase activity intrinsic to the ABC transporter, although there is no domain with homology to either α/β hydrolases or hot-dog fold thioesterases [45]. Whereas a soluble Arabidopsis α/β hydrolase, CGI-58 has been proposed to stimulate the activity of CTS in planta, cgi-58 mutants do not exhibit defects in germination or seed oil mobilization [46].
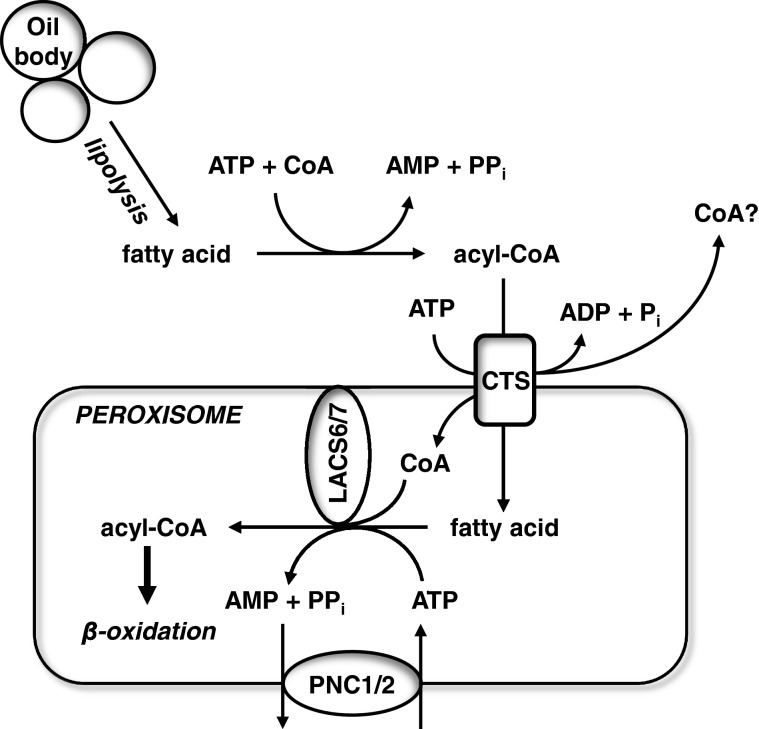
Figure 2. Proposed transport mechanism for CTS.
Fatty acids are released from oil body stores of triacylglycerol by lipolysis. Cytosolic or microsomal acyl CoA synthetases activate non-esterified fatty acids to the corresponding acyl-CoA esters. CTS accepts acyl-CoAs; once bound, the intrinsic thioesterase activity of the transporter releases fatty acids which may be flip-flopped in the membrane and released into the peroxisome, where they are re-esterified by the activity of LACS6 and 7. A pool of LACS6/7 protein is physically associated with CTS on the lumenal side of the membrane [45] and ATP for the activation reaction is provided by peroxisomal adenine nucleotide carriers PNC1/2. The CoA moiety is thought to be imported into the peroxisome via the ABC transporter or alternatively may be released into the cytosol.
At first sight, cleavage and reactivation of the acyl CoA appears energetically wasteful, since the equivalent of two molecules of ATP are used per activation reaction. However such a mechanism could provide a solution to the problem of how diverse substrates are accepted if the CoA moiety is an important determinant for recognition and cleavage potentially enables separate permeation pathways for the polar (CoA) and hydrophobic (fatty acid) moieties of β-oxidation substrates. Where CoA is released and whether and if so how it enters peroxisomes remains to be determined. At 750 kDa, it is too large to utilize the general pore; so, some means of transport must be necessary to maintain the supply of CoA for β-oxidation. Furthermore, different substrates require different intraperoxisomal synthetases for activation, potentially lending further specificity. Nevertheless why mammals have three ABCD proteins with distinct but overlapping specificity whereas most plants and yeast have a single one with apparently broad substrate specificity is unclear.
Symmetry and asymmetry in ABCD transporters
A curious feature of the ABCD family is that it contains both homodimeric and heterodimeric transporters which mediate similar transport functions and apparently share the same unusual mechanism. This has implications not only for ATP binding and hydrolysis but also for binding and translocation of the transported substrates. It is well established that the NBDs of ABC transporters form a sandwich dimer with two composite ATP-binding sites comprising the Walker A, Walker B and H-loops of one NBD and the signature motif of the other [47]; Figures 1 and 3. By definition, these sites are identical in homodimeric transporters such as ALDP but they differ in heterodimeric peroxisomal transporters found in yeast and plants. Based on sequence comparisons, Pxa1/2p and CTS have one consensus and one degenerate site and analysis of an allelic series of cts mutants demonstrated that the NBDs of CTS are not functionally equivalent (Figure 3B) [48]. Asymmetry appears to have evolved several times independently in the ABC protein superfamily, although the functional consequences of substitutions in conserved motifs have not been investigated in all cases [49]. In CFTR (cystic fibrosis transconductance regulator) and multidrug resistance protein1 (MRP1), nucleotide hydrolysis is rapid at the consensus site whereas nucleotide is bound but turned over slowly at the degenerate site [50,51]. Numerous mutagenesis studies have shown that an efficient ATP hydrolysis cycle at a single consensus site is sufficient to provide all the necessary conformational changes for substrate transport [49].
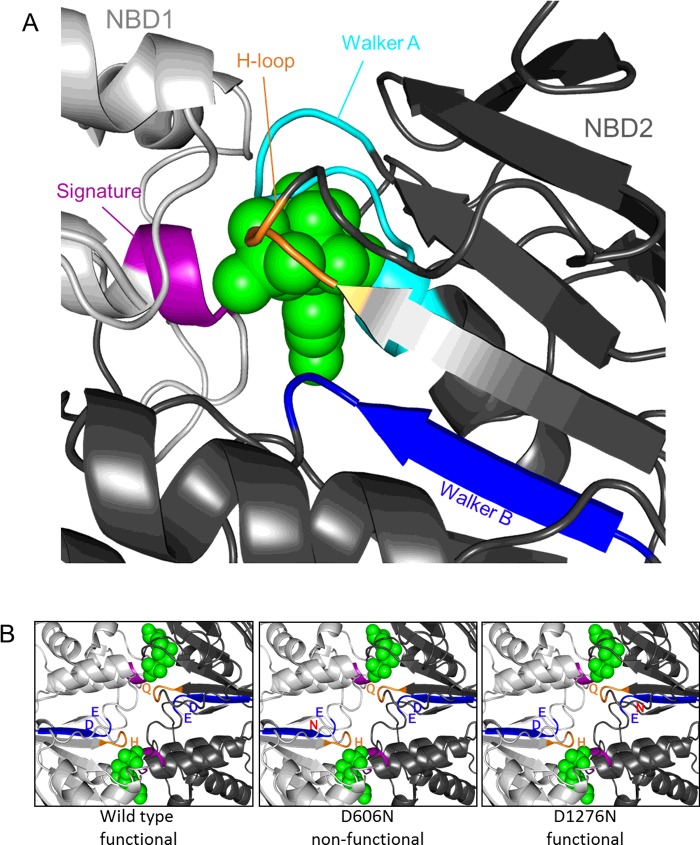
Figure 3. Molecular model showing asymmetry in the two nucleotide binding sites of CTS.
(A) Nucleotide binding between the Walker A (cyan), Walker B (blue) and H-loops (orange) motifs of NBD2 (dark grey) and the signature motif (purple) of NBD1 (light grey). (B) In the wild-type protein, the upper site is formed from the signature motif of NBD1 and the Walker motifs and H loop of NBD2 and the lower site is formed from the Walker motifs and H loop of NBD1 and the signature motif of NBD2. In the upper site, the conserved H of the H loop is replaced by Q leading to a degenerate site whereas the lower site has all the consensus amino acids. Mutation of the Walker B aspartate (D606N) in NBD1 leads to two degenerate sites and loss of function whereas the equivalent mutation in NBD2 (D1276N) still retains one consensus site and is functional [48].
CTS and Pxa1/2p also exhibit sequence asymmetry in TMDs (Figure 1) which potentially influences binding and translocation of substrates. This may underpin the broader substrate specificity of the heterodimeric transporters although the substrate-binding sites of ABCDs remain to be identified. Intriguingly, both homodimeric and heterodimeric ABCD transporters appear to share a thioesterase-dependent transport mechanism [43,45], so it will be interesting to determine whether there is one or two thioesterase sites in symmetrical transporters such as ALDP.
Concluding remarks
In recent years, much progress has been made in understanding the physiological functions and biochemical roles of peroxisomal ABC transporters, with the emergence of exciting, novel mechanistic insights. Future challenges include elucidating the transport mechanism in further detail and relating this to the as yet elusive structure of ABC subfamily D proteins.
Acknowledgments
We thank Jocelyn Baldwin for the creation of the models presented in this article. We apologize to authors whose work could not be cited for reasons of space.
Abbreviations
- ABC
ATP-binding cassette
- ABCD
ABC transporter of subfamily D
- ABI5
ABA insensitive 5
- ACN2
acetate non utilizing 2
- ALDP
adrenoleucodystrophy protein
- AMPPCP
β,γ-methyleneadenosine 5′-triphosphate
- Ant1p
adenine nucleotide transporter1
- CFTR
cystic fibrosis transconductance regulator
- CTS
comatose
- Faa2p
fatty acid activation protein 2
- FA-CoA
fatty acid acyl CoA
- Gat1
glycosomal ABC transporter1
- IBA
indole butyric acid
- JA
jasmonic acid
- LACS
long chain acyl-CoA synthetases
- MRP1
multidrug resistance protein1
- NBD
nt-binding domain
- OPDA
12-oxophytodienoic acid
- PED3
peroxisome defective 3
- PMP69
69kDa peroxisome membrane protein
- PNC
peroxisome nucleotide carrier
- PXA1
peroxisomal ABC transporter1
- PXMP2
peroxisomal membrane protein 2
- TMD
transmembrane domain
- VLCFA
very long-chain fatty acid
- X-ALD
X-linked adrenoleucodystrophy
Footnotes
ATP binding cassette transporters: from mechanism to organism: Held at University of Chester, U.K., 16–18 April 2015.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council [grant numbers BB/L001691/1 and BB/L001012/1 (to F.L.T. and A.B.)].
References
- 1.Hu J., Baker A., Bartel B., Linka N., Mullen R.T., Reumann S., Zolman B.K. Plant peroxisomes: biogenesis and function. Plant Cell. 2012;24:2279–2303. doi: 10.1105/tpc.112.096586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wanders R.J.A., Waterham H.R. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 2006;75:295–332. doi: 10.1146/annurev.biochem.74.082803.133329. [DOI] [PubMed] [Google Scholar]
- 3.Block A., Widhalm J.R., Fatihi A., Cahoon R.E., Wamboldt Y., Elowsky C., Mackenzie S.A., Cahoon E.B., Chapple C., Dudareva N., Basset G.J. The origin and biosynthesis of the benzenoid moiety of ubiquinone (coenzyme Q) in Arabidopsis. Plant Cell. 2014;26:1938–1948. doi: 10.1105/tpc.114.125807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bussell J.D., Reichelt M., Wiszniewski A.A.G., Gershenzon J., Smith S.M. Peroxisomal ATP-binding cassette transporter COMATOSE and the multifunctional protein abnormal inflorescence meristem are required for the production of benzoylated metabolites in arabidopsis seeds. Plant Physiol. 2014;164:48–54. doi: 10.1104/pp.113.229807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferdinandusse S., Jimenez-Sanchez G., Koster J., Denis S., Van Roermund C.W., Silva-Zolezzi I., Moser A.B., Visser W.F., Gulluoglu M., Durmaz O., et al. A novel bile acid biosynthesis defect due to a deficiency of peroxisomal ABCD3. Hum. Mol. Genet. 2015;24:361–370. doi: 10.1093/hmg/ddu448. [DOI] [PubMed] [Google Scholar]
- 6.Theodoulou F.L., Job K., Slocombe S.P., Footitt S., Holdsworth M., Baker A., Larson T.R., Graham I.A. Jasmonic acid levels are reduced in comatose ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 2005;137:835–840. doi: 10.1104/pp.105.059352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonenkov V.D., Hiltunen J.K. Peroxisomal membrane permeability and solute transfer. Biochim. Biophys. Acta. 2006;1763:1697. doi: 10.1016/j.bbamcr.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 8.Rokka A., Antonenkov V.D., Soininen R., Immonen H.L., Pirila P.L., Bergmann U., Sormunen R.T., Weckstram M., Benz R., Hiltunen J.K. Pxmp2 is a channel-forming protein in mammalian peroxisomal membrane. PLoS One. 2009;4:e5090. doi: 10.1371/journal.pone.0005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunze M., Hartig A. Permeability of the peroxisomal membrane: lessons from the glyoxylate cycle. Front. Physiol. 2013;4:204. doi: 10.3389/fphys.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linka N., Theodoulou F.L. Metabolite transporters of the plant peroxisomal membrane: known and unknown. Subcell. Biochem. 2013;69:169–194. doi: 10.1007/978-94-007-6889-5. [DOI] [PubMed] [Google Scholar]
- 11.Coelho D., Kim J.C., Miousse I.R., Fung S., du Moulin M., Buers I., Suormala T., Burda P., Frapolli M., Stucki M., et al. Mutations in ABCD4 cause a new inborn error of vitamin B-12 metabolism. Nat. Genet. 2012;44:1152–1155. doi: 10.1038/ng.2386. [DOI] [PubMed] [Google Scholar]
- 12.Hurlock A.K., Roston R.L., Wang K., Benning C. Lipid trafficking in plant cells. Traffic. 2014;15:915–932. doi: 10.1111/tra.12187. [DOI] [PubMed] [Google Scholar]
- 13.Verleur N., Hettema E.H., VanRoermund C.T., Tabak H.F., Wanders R.A. Transport of activated fatty acids by the peroxisomal ATP- binding-cassette transporter Pxa2 in a semi-intact yeast cell system. Eur. J. Biochem. 1997;249:657–661. doi: 10.1111/j.1432-1033.1997.00657.x. [DOI] [PubMed] [Google Scholar]
- 14.Hettema E.H., van Roermund C.W., Distel B., van den Berg M., Vilela C., Rodrigues-Pousada C., Wanders R.J., Tabak H.F. The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. EMBO J. 1996;15:3813–3822. [PMC free article] [PubMed] [Google Scholar]
- 15.Shani N., Valle D. A Saccharomyces cerevisiae homolog of the human adrenoleukodystrophy transporter is a heterodimer of two half ATP-binding cassette transporters. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11901–11906. doi: 10.1073/pnas.93.21.11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swartzman E.E., Viswanathan M.N., Thorner J. The PAL1 gene product is a peroxisomal ATP-binding cassette transporter in the yeast Saccharomyces cerevisiae. J. Cell Biol. 1996;132:549–563. doi: 10.1083/jcb.132.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang C.Y., Chen L.Y., Fu R.H., Chen S.M., Ho M.H., Huang J.M., Hsu C.C., Wang C.C., Chen M.S., Tsai R.T. Involvement of the carboxyl-terminal region of the yeast peroxisomal half ABC transporter Pxa2p in its interaction with Pxa1p and in transporter function. PLoS One. 2014;9:e104892. doi: 10.1371/journal.pone.0104892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Roermund C.W.T., Drissen R., van den Berg M., Ijlst L., Hettema E.H., Tabak H.F., Waterham H.R., Wanders R.J.A. Identification of a peroxisomal ATP carrier required for medium-chain fatty acid beta-oxidation and normal peroxisome proliferation in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001;21:4321–4329. doi: 10.1128/MCB.21.13.4321-4329.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmieri L., Rottensteiner H., Girzalsky W., Scarcia P., Palmieri F., Erdmann R. Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 2001;20:5049–5059. doi: 10.1093/emboj/20.18.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dulermo R., Gamboa-Melendez H., Ledesma-Amaro R., Thevenieau F., Nicaud J.M. Unraveling fatty acid transport and activation mechanisms in Yarrowia lipolytica. Biochim. Biophys. Acta. 2015;1851:1202–1217. doi: 10.1016/j.bbalip.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi M., Nito K., Takei-Hoshi R., Yagi M., Kondo M., Suenaga A., Yamaya T., Nishimura M. Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid beta-oxidation. Plant Cell Physiol. 2002;43:1–11. doi: 10.1093/pcp/pcf023. [DOI] [PubMed] [Google Scholar]
- 22.Footitt S., Slocombe S.P., Larner V., Kurup S., Wu Y.S., Larson T., Graham I., Baker A., Holdsworth M. Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J. 2002;21:2912–2922. doi: 10.1093/emboj/cdf300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zolman B.K., Silva I.D., Bartel B. The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid beta-oxidation. Plant Physiol. 2001;127:1266–1278. doi: 10.1104/pp.010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooks M.A., Turner J.E., Murphy E.C., Johnston K.A., Burr S., Jaroslawski S. The Arabidopsis ALDP protein homologue COMATOSE is instrumental in peroxisomal acetate metabolism. Biochem. J. 2007;406:399–406. doi: 10.1042/BJ20070258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dave A., Hernandez M.L., He Z., Andriotis V.M.E., Vaistij F.E., Larson T.R., Graham I.A. 12-Oxo-phytodienoic acid accumulation during seed development represses seed germination in arabidopsis. Plant Cell. 2011;23:583–599. doi: 10.1105/tpc.110.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanai M., Nishimura M., Hayashi M. A peroxisomal ABC transporter promotes seed germination by inducing pectin degradation under the control of ABI5. Plant J. 2010;62:936–947. doi: 10.1111/j.1365-313X.2010.04205.x. [DOI] [PubMed] [Google Scholar]
- 27.Nyathi Y., Zhang X., Baldwin J.M., Bernhardt K., Johnson B., Baldwin S.A., Theodoulou F.L., Baker A. Pseudo half-molecules of the ABC transporter, COMATOSE, bind Pex19 and target to peroxisomes independently but are both required for activity. FEBS Lett. 2012;586:2280–2286. doi: 10.1016/j.febslet.2012.05.065. [DOI] [PubMed] [Google Scholar]
- 28.Mendiondo G.M., Medhurst A., van Roermund C.W., Zhang X., Devonshire J., Scholefield D., Fernández J., Axcell B., Ramsay L., Waterham H.R., et al. Barley has two peroxisomal ABC transporters with multiple functions in β-oxidation. J. Exp. Bot. 2014;65:4833–4847. doi: 10.1093/jxb/eru243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orsi C.H., Tanksley S.D. Natural variation in an ABC transporter gene associated with seed size evolution in tomato species. PLos Genet. 2009;5:e1000347. doi: 10.1371/journal.pgen.1000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosser J., Douar A.M., Sarde C.O., Kioschis P., Feil R., Moser H., Poustka A.M., Mandel J.L., Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- 31.Lombard-Platet G., Savary S., Sarde C.O., Mandel J.L., Chimini G. A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1265–1269. doi: 10.1073/pnas.93.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamijo K., Taketani S., Yokota S., Osumi T., Hashimoto T. The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J. Biol. Chem. 1990;265:4534–4540. [PubMed] [Google Scholar]
- 33.Geillon F., Gondcaille C., Charbonnier S., Van Roermund C.W., Lopez T.E., Dias A.M.M., Pais de Barros J.-P., Arnould C., Wanders R.J., Trompier D., Savary S. Structure-function analysis of peroxisomal ATP-binding cassette transporters using chimeric dimers. J. Biol. Chem. 2014;289:24511–24520. doi: 10.1074/jbc.M114.575506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Roermund C.W.T., Ijlst L., Wagemans T., Wanders R.J.A., Waterham H.R. A role for the human peroxisomal half-transporter ABCD3 in the oxidation of dicarboxylic acids. Biochim. Biophys. Acta. 2014;1841:563–568. doi: 10.1016/j.bbalip.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Kemp S., Theodoulou F.L., Wanders R.J.A. Mammalian peroxisomal ABC transporters: from endogenous substrates to pathology and clinical significance. Br. J. Pharmacol. 2011;164:1753–1766. doi: 10.1111/j.1476-5381.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Benedetto R., Denti M.A., Salvati S., Sanchez M., Attorri L., David G., Di Biase A. RNAi-mediated silencing of ABCD3 gene expression in rat C6 glial cells: a model system to study PMP70 function. Neurochem. Int. 2008;52:1106–1113. doi: 10.1016/j.neuint.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 37.van Roermund C.W., Visser W.F., Ijlst L., van Cruchten A., Boek M., Kulik W., Waterham H.R., Wanders R.J. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22:4201–4208. doi: 10.1096/fj.08-110866. [DOI] [PubMed] [Google Scholar]
- 38.van Roermund C.W., Visser W.F., Ijlst L., Waterham H.R., Wanders R.J. Differential substrate specificities of human ABCD1 and ABCD2 in peroxisomal fatty acid beta-oxidation. Biochim. Biophys. Acta. 2011;1811:148–152. doi: 10.1016/j.bbalip.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Nyathi Y., De Marcos Lousa C., van Roermund C.W., Wanders R.J., Johnson B., Baldwin S.A., Theodoulou F.L., Baker A. The Arabidopsis peroxisomal ABC transporter, comatose, complements the Saccharomyces cerevisiae pxa1 pxa2Delta mutant for metabolism of long-chain fatty acids and exhibits fatty acyl-CoA-stimulated ATPase activity. J. Biol. Chem. 2010;285:29892–29902. doi: 10.1074/jbc.M110.151225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X., De Marcos Lousa C., Schutte-Lensink N., Ofman R., Wanders R.J., Baldwin S.A., Baker A., Kemp S., Theodoulou F.L. Conservation of targeting but divergence in function and quality control of peroxisomal ABC transporters: an analysis using cross-kingdom expression. Biochem. J. 2011;436:547–557. doi: 10.1042/BJ20110249. [DOI] [PubMed] [Google Scholar]
- 41.Igoillo-Esteve M., Mazet M., Deumer G., Wallemacq P., Michels P.A. Glycosomal ABC transporters of Trypanosoma brucei: characterisation of their expression, topology and substrate specificity. Int. J. Parasitol. 2011;41:429–438. doi: 10.1016/j.ijpara.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Wiesinger C., Kunze M., Regelsberger G., Forss-Petter S., Berger J. Impaired very long-chain acyl-coA β-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dysfunction. J. Biol. Chem. 2013;288:19269–19279. doi: 10.1074/jbc.M112.445445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Roermund C.W., Ijlst L., Majczak W., Waterham H.R., Folkerts H., Wanders R.J., Hellingwerf K.J. Peroxisomal fatty acid uptake mechanism in Saccharomyces cerevisiae. J. Biol. Chem. 2012;287:20144–20153. doi: 10.1074/jbc.M111.332833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fulda M., Schnurr J., Abbadi A., Heinz E., Browse J. Peroxisomal Acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell. 2004;16:394–405. doi: 10.1105/tpc.019646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Marcos Lousa C., van Roermund C.W., Postis V.L., Dietrich D., Kerr I.D., Wanders R.J., Baldwin S.A., Baker A., Theodoulou F.L. Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proc. Natl. Acad. Sci. U.S.A. 2013;110:1279–1284. doi: 10.1073/pnas.1218034110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park S., Gidda S.K., James C.N., Horn P.J., Khuu N., Seay D.C., Keereetaweep J., Chapman K.D., Mullen R.T., Dyer J.M. The α/β hydrolase CGI-58 and peroxisomal transport protein PXA1 coregulate lipid homeostasis and signaling in arabidopsis. Plant Cell. 2013;25:1726–1739. doi: 10.1105/tpc.113.111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith P.C., Karpowich N., Millen L., Moody J.E., Rosen J., Thomas P.J., Hunt J.F. ATP Binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol. Cell. 2002;10:139–149. doi: 10.1016/S1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dietrich D., Schmuths H., Lousa C.D.M., Baldwin J.M., Baldwin S.A., Baker A., Theodoulou F.L., Holdsworth M.J. Mutations in the arabidopsis peroxisomal ABC Transporter COMATOSE allow differentiation between multiple functions in planta: insights from an allelic series. Mol. Biol. Cell. 2009;20:530–543. doi: 10.1091/mbc.E08-07-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Procko E., O'Mara M.L., Bennett W.F.D., Tieleman D.P., Gaudet R. The mechanism of ABC transporters: general lessons from structural and functional studies of an antigenic peptide transporter. FASEB J. 2009;23:1287–1302. doi: 10.1096/fj.08-121855. [DOI] [PubMed] [Google Scholar]
- 50.Hou Y.-x., Cui L., Riordan J.R., Chang X.-B. Allosteric interactions between the two non-equivalent nucleotide binding domains of multidrug resistance protein MRP1. J. Biol. Chem. 2000;275:20280–20287. doi: 10.1074/jbc.M001109200. [DOI] [PubMed] [Google Scholar]
- 51.Aleksandrov L., Aleksandrov A.A., Chang X.-B., Riordan J.R. The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J. Biol. Chem. 2002;277:15419–15425. doi: 10.1074/jbc.M111713200. [DOI] [PubMed] [Google Scholar]
- 52.Shintre C.A., Pike A.C.W., Li Q., Kim J.-I., Barr A.J., Goubin S., Shrestha L., Yang J., Berridge G., Ross J., et al. Structures of ABCB10, a human ATP-binding cassette transporter in apo- and nucleotide-bound states. Proc. Natl. Acad. Sci. U.S.A. 2013;110:9710–9715. doi: 10.1073/pnas.1217042110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dawson R.J.P., Locher K.P. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]