Abstract
In most organisms, ABC transporters constitute one of the largest families of membrane proteins. In humans, their functions are diverse and underpin numerous key physiological processes, as well as being causative factors in a number of clinically relevant pathologies. Advances in our understanding of these diseases have come about through combinations of genetic and protein biochemical investigations of these transporters and the power of in vitro and in vivo investigations is helping to develop genotype–phenotype understanding. However, the importance of ABC transporter research goes far beyond human biology; microbial ABC transporters are of great interest in terms of understanding virulence and drug resistance and industrial biotechnology researchers are exploring the potential of prokaryotic ABC exporters to increase the capacity of synthetic biology systems. Plant ABC transporters play important roles in transport of hormones, xenobiotics, metals and secondary metabolites, pathogen responses and numerous aspects of development, all of which are important in the global food security area. For 3 days in Chester, this Biochemical Society Focused Meeting brought together researchers with diverse experimental approaches and with different fundamental questions, all of which are linked by the commonality of ABC transporters.
Keywords: ATP-binding cassette (ABC) transporter, disease, multi-drug resistance, protein structure, transport mechanism
A brief history of ABC transporters
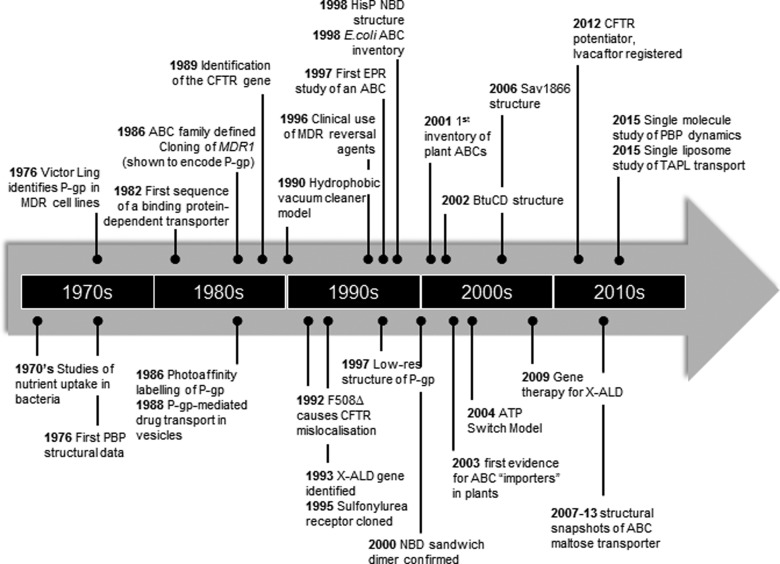
The ATP-binding cassette (ABC) transporter field emerged from studies on nutrient uptake in bacteria in the 1970’s with the biochemical characterization of substrate-binding protein (SBP)-dependent transport systems energized directly by hydrolysis of ATP [1]. The early 1980’s saw the cloning of several genes encoding such transporters, spearheaded by the histidine permease of Salmonella typhimurium and maltose permease of Escherichia coli [2,3]. In parallel, medical researchers were tracking down the gene encoding permeability-glycoprotein/P-glycoprotein (P-gp), a large glycosylated membrane protein associated with multi-drug resistance in mammalian cells [4]. P-gp was eventually cloned in 1985 [5] and with increasing availability of cDNA sequences, it became apparent that both the mammalian and the bacterial transporters contained highly conserved nt-binding motifs (the Walker A and B sequences) [6], hinting at a common evolutionary origin. In 1986, it was recognized that these ATP-binding subunits defined a large superfamily of transport proteins [7–10] although the term ABC transporter was not actually coined until 1990 [11], by which time it was became apparent that ABC transporters were not only ubiquitous but also involved in diverse biochemical and physiological processes. A timeline of key events in the development of ABC transporter research is given in Figure 1 and a vivid account of the trials and tribulations of characterizing one of the prototypical ABC transporters (P-gp) is presented by Callaghan [12].
Figure 1. Timeline showing selected key discoveries and events in ABC research.
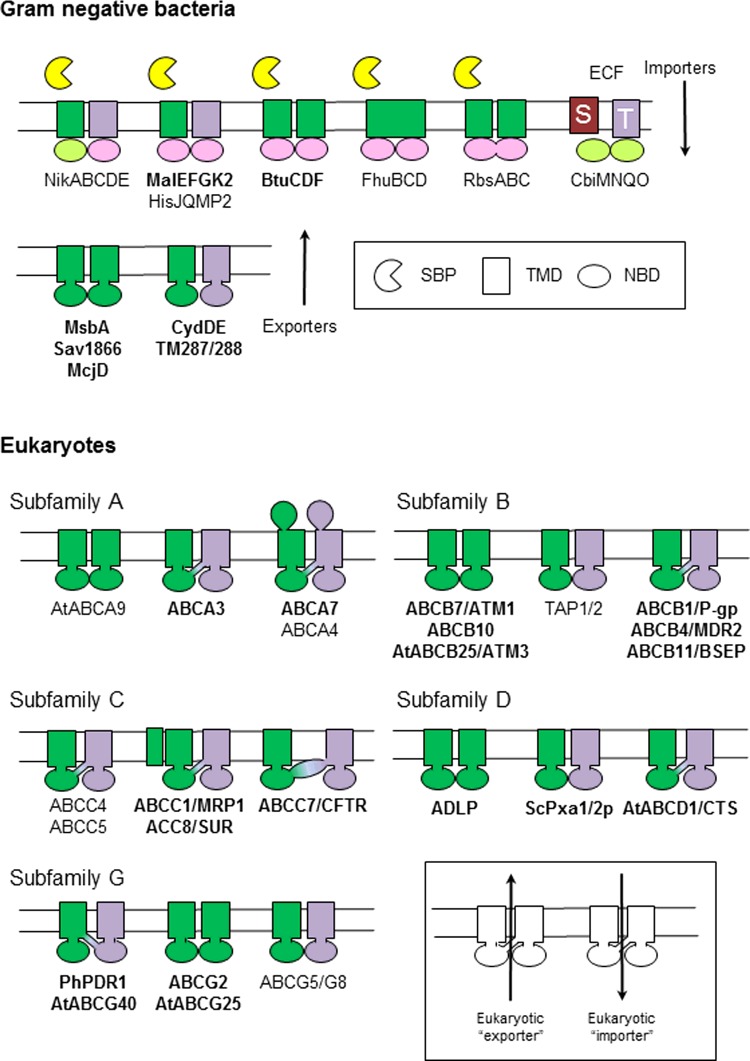
Genome sequencing, complemented by careful studies of individual transporters, has revealed a common architecture with a core ABC transporter comprising two nt-binding domains (NBDs) and two transmembrane domains (TMDs), often with additional domains. These domains are encoded either as individual proteins (typically in prokaryotes) or in varying degrees of fusion, with eukaryotic ABC proteins largely expressed as so-called ‘half-size’ (TMD–NBD or NBD–TMD) or ‘full-size’ (TMD–NBD–TMD–NBD or NBD–TMD–NBD–TMD) transporters (Figure 2) [13]. Domain organization and phylogenetic analysis have defined eight major subfamilies of eukaryotic ABC proteins, two of which (E and F) are not membrane associated and have functions other than transport [14,15]. Intriguingly, the ABC family includes both ‘exporters’ and ‘importers’ [16] (Figure 2). Importers, which typically rely on periplasmic SBPs to deliver substrates to the transporter core, were considered to be restricted to prokaryotes (and, rarely, organelles of endosymbiotic origin) but exporters are common to all kingdoms [15–17]. Previously, the discovery of a group of prokaryotic micronutrient uptake transporters dependent on ABC transporter NBDs but without extracytoplasmic SBPs (the energy coupling factor ABC transporters) has further extended the repertoire of ABC transporter architecture [18].
Figure 2. Domain organization of ABC transporters from different organisms.
In Gram-negative bacteria, importers comprise two TMDs, two NBDs (ATPase subunits) and a periplasmic SBP. In Gram-positive bacteria and archaea which have no outer membrane, SBPs are anchored to the extracellular face of the cytoplasmic membrane, or to the TMDs (not shown). Energy coupling factor (ECF) transporters comprise two ATPase subunits, a transmembrane protein (T) and a substrate-specific integral membrane protein (S). All bacteria and archaea also contain homo- or heterodimeric exporters, in which a TMD is fused with an NBD. Eukaryotic ABC proteins are classified in eight subfamilies (A–H). The organization of core and additional domains is shown for different representative topologies in each subfamily; transporters discussed in this volume are indicated in bold. Not included are soluble subfamilies E and F and transporter subfamily H, which is absent from mammals, plants and fungi. Plant genomes also encode several ‘prokaryotic-like’ ABC transporters, designated subfamily I, which have been acquired via the endosymbiotic origin of mitochondria and plastids [15] (not shown).
Diversity of function: linking physiology with biochemistry
The functions of ABC proteins are too numerous to catalogue in detail here, but it is well established that they transport a huge range of diverse substrates, from simple ions, through polar, amphipathic and hydrophobic organic molecules to peptides, complex lipids and even small proteins. Consequently, ABC transporters are implicated in a wide array of developmental processes [14,15,17,19]. Plants have the largest complement of ABC proteins of all eukaryotes [15,19] and it was fitting that the meeting opened with a session devoted to diversity of function headlined by Enrico Martinoia [20], who described the roles of plant ABC transporters in phytohormone transport. The ABC superfamily expanded dramatically with the conquest of land by plants, associated in part with their sessile nature, multi-cellularity and extensive capacity for synthesis of secondary chemicals. It has long been hypothesized that plant ABC transporters would be involved in the synthesis and deposition of secondary metabolites (from which many important drugs are derived), but an important discovery has been the implication of subfamily G transporters in the transport of several major classes of plant hormones, namely: auxins, cytokinins, abscisic acid and the recently-discovered strigolactones [20]. Intriguingly, Martinoia and colleagues [19,20] drew attention to the increasing number of reports in the literature suggesting that both plant ABCGs and ABCBs can act as importers, challenging the ‘exporters only’ adage for eukaryotic ABC transporters (Figure 2). Much of this evidence is phenomenological and requires confirmation in vitro but perhaps the most convincing example is Arabidopsis ABCB14, which mediates malate uptake when expressed in E.coli and HeLa cells [21]. The ability of plant (and yeast) ABC research to make us rethink paradigms was also highlighted by Alison Baker and colleagues [22,23], who presented a novel mechanism for peroxisomal fatty acyl-CoA import by ABCD proteins, involving substrate cleavage catalysed by a thioesterase activity intrinsic to the transporter, followed by re-esterification by acyl activating enzymes which form a complex with the transporter in the peroxisomal lumen.
Much information about the roles of ABC transporters has been deduced using loss of function mutants isolated in genetic screens [20] and the pressing need for complementary biochemical studies of plant ABC transporters was outlined by François Lefèvre, using ABC sub-class G as an exemplar [24]. Some plant ABC transporters have been identified in multiple genetic screens and have been ascribed several different biochemical functions, based on their apparently disparate physiological roles. How do you go about characterizing a transporter when the identity of the transport substrate (or substrates) is not immediately obvious? An interesting approach to this question was provided by Janneke Balk and colleagues [25], who used a LC–MS metabolomic approach [26] to identify glutathione polysulfides as likely physiological substrates for Arabidopsis ABCB25/ABC transporter of mitochondria 3 (ATM3), a mitochondrial ABC transporter involved in iron-sulfur cluster and molybdenum cofactor assembly. Importantly, the physiological relevance of the transport studies was verified by follow-up genetic experiments [25,27]. Also exploring the functions of ABC transporters in redox processes, Mark Shepherd [28] proposed a role for the CydDC complex of E. coli in redox sensing and NO tolerance. CydDC exports cysteine and glutathione to the periplasm but is also thought to bind haem on the periplasmic surface which stimulates reductant export and may interact with gaseous signalling molecules, suggesting a novel virulence mechanism for bacteria.
ABC transporters in health and disease: from bench to bedside and back again
As in plants and microbes, the importance of human ABC proteins and the diversity of their physiological roles are underscored by the consequences of their dysfunction. To date, over 20 ABC proteins, representing all sub-families have been associated with human disease (Table 1) and several others play clinically important roles in drug metabolism and resistance.
Table 1. ABC transporters associated with human health and disease.
References cited are from relevant papers in this Special Issue.
| ABC transporter | Disease | References |
|---|---|---|
| ABCA1 | Tangier disease and familial high density lipoprotein (HDL) deficiency; atherosclerosis; Alzheimer's disease | [38] |
| ABCA3 | Neonatal surfactant deficiency and pulmonary fibrosis; congenital cataract | [37] |
| ABCA4 | Stargardt macular degeneration | |
| ABCA7 | Alzheimer's disease | [38] |
| ABCA12 | Harlequin and lamellar ichthyosis | |
| ABCB1/transporter associated with antigen processing (Tap)2; ABCB2/Tap1 | Immune deficiency; arthritis risk | |
| ABCB4/MDR2 | PFIC3; other types of cholestasis | [39] |
| ABCB7 | Sideroblastic amaemia and ataxia | [26] |
| ABCB11/bile salt export pump (BSEP) | PFIC2; intrahepatic cholestasis of pregnancy; neonatal respiratory distress syndrome | [39] |
| ABCC2/MRP2 | Dubin–Johnson syndrome | |
| ABCC5/MRP5 | Inherited hypertrichosis | |
| ABCC6/MRP6 | Pseudoxanthoma elasticum | |
| ABCC7/CFTR | CF | [30,31] |
| ABCC8/SUR1 | Diabetes | [35] |
| ABCC9/SUR2 | Diabetes | [35] |
| ABCD1/adrenoleukodystrophy protein (ALDP) | X-linked adrenoleukodystrophy | [23,45] |
| X-linked adrenomyeloneuropathy | ||
| ABCD3/peroxisome membrane protein (PMP70) | Hepatosplenomegaly; liver disease | [23,45] |
| ABCD4/PMP69 | Inborn error of vitamin B12 metabolism | [23] |
| ABCG2/breast cancer resistance protein (BCRP) | Gout and hyperuricaemia | |
| ABCG5; ABCG8 | Sitosterolemia; coronary heart disease; gallstone disease | |
| ABCB1/P-gp; ABCC1/MRP1, ABCG2/BCRP | Multi-drug resistance | [12,44,45] |
| ABCC2-6 | Drug transport |
It is now over 25 years since the causative gene in cystic fibrosis (CF) was identified as ABCC7 [also known as the CF transmembrane conductance regulator (CFTR)] [29] and a huge wealth of information has been obtained for this atypical ABC protein which is not in fact a primary active transporter but an ATP-gated chloride channel. David Sheppard emphasized the importance of understanding how different mutations affect CFTR function for the development of chemical correctors and discusses together with Pollock et al. [30,31] in this volume how species differences can be exploited to understand CFTR structure and function. To date, over 2000 mutations have been associated with CF [30], some of which affect key phosphorylation sites of CFTR [32]. Bibek Aryal [33] presented novel examples of ABC transporter regulation by phosphorylation and in this special issue, draws intriguing parallels between phosphorylation of the CFTR regulatory domain and phosphorylation of the linker region in plant ABCB transporters. The sulfonylurea receptors (SUR1/ABCC8; SUR2/ABCC9) are also atypical ABC proteins; by forming a hetero-octameric complex with potassium channel Kir6.2 subunits they produce the ATP-sensitive potassium channel whose activity is critical for insulin secretion [34,35]. Heidi de Wet [35] explained the complex mechanism of channel inhibition by sulfonylureas using elegant electrophysiological approaches and examined implications for treatment of neonatal diabetes.
A recurring theme of the meeting was the need for combinations of techniques to understand ABC transporter function/mis-function and role in physiology/disease, particularly for rare disorders and diseases with complex aetiologies. The involvement of ABCG2 in pre-disposition to gout is familiar to many in the ABC transporter community [36], but here we heard about studies implicating two members of the ABCA subfamily in disease. Andrea Masotti [37] outlined the challenges in diagnosing a spectrum of rare disorders of the surfactant system linked to mutations in the gene encoding the lung specific phospholipid transporter ABCA3 and Brett Garner [38] described how ABCA7 was identified as a genetic risk factor in late-onset Alzheimer's disease, presenting recent work with mouse and cell-based disease models to understand the function of ABCA7 in this context. The multi-disciplinary theme was continued by John Schuetz and Kenny Linton [39] who described work characterizing ABC transporters in liver disease. Owing to a combination of biochemistry, physiology, genetics and clinical science, the roles of three transporters: lipid floppase, ABCB4, bile salt export pump, ABCB11 and the P-type ATPase, ATP8B1 in progressive familial intrahepatic cholestasis (PFIC) are now well understood [39]. Animal models, particularly mouse models, have been extremely instructive in elucidating the functions of human ABC transporters but there is often a marked inconsistency between human and mouse pathologies, for example in CF and X-linked adrenoleukodystrophy [30,40]. In the case of cholestatic disease, this has proved informative, since transporter function could be studied in abcb11 knockout mice without the development of secondary pathology but this disparity also emphasizes the importance and utility of studying the biochemistry of recombinant human ABCs [39].
In addition to roles associated with endogenous substrates, several ABC transporters are known to show relatively limited substrate specificity. This multi-drug transport capability is well established for ABCB1, ABCC1 and ABCG2 (and new drugs must be tested as transport substrates of these three promiscuous pumps) [41]. Two clinical correlates of multi-drug transport were explored: the first was the role ABC transporters play in drug metabolism. Andrew Owen [42,43] described mathematical approaches in pharmacokinetics (PK), contrasting population PK (starting with clinical data and fitting a model) with physiological PK (predicting clinical outcome from models of transport data). Such PK models can already be used to simulate different treatment regimens and predict how they might work for patients with different transporter SNPs (single-nt polymorphisms) [42,43]. Secondly, multi-drug transporters are partially responsible for cancer drug resistance, which causes tens of thousands of cancer deaths each year. The possibility of specifically targeting cancer stem cells expressing multi-drug pumps (e.g. ABCB1) was described by Beth Coyle [44]. By repurposing a hypertension drug, Vardenafil, inhibition of ABCB1-mediated drug resistance was achieved in cell lines established from difficult to treat children's brain tumours. Intriguingly, Vardenafil may not only overcome one particular challenge to brain tumour chemotherapy since it crosses the blood–brain barrier, but its inhibition of ABCB1 may also have a collateral benefit in reducing dissemination and invasion of drug resistant tumour cells [44]. Although ABCB1, C1 and G2 are the three usual suspects in multi-drug resistance, other ABC transporters also play roles in drug metabolism and beyond in cancer biology [41]. In this context, Viktor Hlaváč [45] presented emerging evidence for differential regulation of sub-class D ABC transporters associated with both positive and negative outcomes in diverse cancers.
Structure and mechanism: an ideal pas de deux?
Visualizing the structure of ABC transporters at atomic resolution has become something of a holy grail in the field but an understanding of their dynamics is equally important [46]. Ironically, the first ABC-related structure of a soluble SBP was published 10 years before the ABC transporter family was defined [47]. Although many crystal structures have followed and SBPs are the best understood components of ABC transporters, they still hold a few surprises. Gavin Thomas [48] elegantly elucidated the molecular basis of substrate specificity in previously uncharacterized E. coli SBPs. Liganded crystals not only identified unexpected substrates but also revealed how the specificity can be changed dramatically by a single alteration in a residue distant to the binding site. Thorben Cordes followed with a fascinating presentation of mechanistic detail derived from single-molecule FRET studies of SBPs, challenging the classical ‘Venus Flytrap’ model [49,50].
Although important 3D insights have been obtained from EM and single particle analysis of full-length membrane transporters [51,52], high-resolution ABC crystal structures have been eagerly awaited. Membrane proteins are notoriously challenging for structural studies, the first task being to produce pure, preferably active protein; an appreciation of the magnitude of this undertaking is evident when considering the huge worldwide effort that has gone into characterizing P-gp [12] and the difficulties in heterologous expression of many plant ABC transporters [24]. In this context, Naomi Pollock [31] outlined different approaches to preparing recombinant CFTR for structural studies, emphasizing the importance of testing orthologues from different species and optimization of detergent solubilization [31]. Perhaps unsurprisingly, most structures obtained to date are of prokaryotic ABC transporters which are generally easier to express and purify in quantities amenable to crystallization, reviewed here by Kostas Beis [53], who presented a synthesis of structural and biophysical data for the anti-bacterial peptide transporter, McjD at the meeting.
Liz Carpenter [54] highlighted a different problem frequently faced by structural biologists that the proteins which crystallize most readily are not necessarily those for which functional information is available. ABCB10 was the first human ABC transporter to produce diffracting crystals in a structural genomics project but its precise function remains frustratingly obscure [25,54]. Although high resolution crystal structures of orphan transporters are helpful for generating homology models and can give insights into different conformational states, their utility is greatly enhanced when the substrates are known and where complementary biochemical or biophysical data are available.
An armoury of complementary approaches is now available to probe ABC transporter structure and mechanism and these can be particularly powerful when combined. For example, van Veen discussed the use of cysteine-reactive fluorescent reagents to investigate the catalytic cycle of MsbA in the framework of crystal structures [55] and Fraser Macmillan [46,56] used P-gp to explain how EPR spectroscopy can be employed in combination with site-directed spin labelling to measure distances and probe accessibility in ABC transporters. Where structures are available, MD simulations, together with homology modelling, offer the possibility to describe conformational dynamics, albeit over short time scales. Laura Domicevica and Philip Biggin [57] presented an analysis of P-gp drug binding using homology modelling with MD and Thomas Stockner [46] discuss here how EPR and MD can be combined to investigate the conformational flexibility of ABC transporters, comparing dynamic data for MsbA and P-gp with mechanistic predictions from crystal structures in different conformations. A promising complementary technique was introduced by Bavro [58]; X-ray radiolytic footprinting combined MS (XF–MS) though not yet applied to ABC transporters, offers valuable insights into the dynamics of membrane proteins; in particular XF–MS permits identification of structural waters and conformational changes in proteins and is applicable to protein complexes, which will interest many members of the ABC transport community.
Conclusions and perspectives: whither ABCs?
In 40 years, ABC transporter research has evolved into a diverse, dynamic field with activity focused on both basic and translational issues. The meeting brought home how these extremes can inform and complement each other and how biochemistry and genetics are still very much at the heart of ABC transporter research. Much remains still to be discovered, encapsulated by two major questions:
1) What do all those transporters do? In medicine, GWAS (genome-wide association studies) and exome sequencing are likely to reveal ABC involvement in even more medical conditions, particularly rare diseases, whereas in yeast, bacteria and plants, forward genetic and chemical screens are predicted to uncover new functions for ABC transporters. Identifying substrates for these ‘orphan’ transporters will remain a major challenge as it has been for the g-protein coupled receptor community.
2) How do they all work? Emerging techniques have much to offer the field, including new methods for purification and reconstitution [59], single molecule and single liposome approaches [50,60,61], oxidative footprinting [58] and subnanometer cryoelectron microscopy [62]. Although we remain tantalized by the prospect of a series of high-resolution liganded stuctures in states comprising the entire catalytic cycle, with accompanying biophysical data, we should remain sanguine and appreciate that a single unifying mechanistic model of transport may not apply to this diverse family of intriguing proteins.
Acknowledgments
We thank all speakers, poster presenters, other participants and sponsors of the ATP-binding cassette proteins: from mechanism to organism Biochemical Society Focused Meeting.
Abbreviations
- ABC
ATP-binding cassette
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- GWAS
genome-wide association study
- NBD
nt-binding domain
- P-gp
permeability glycoprotein
- PFIC
progressive familial intrahepatic cholestasis
- PK
pharmacokinetics
- SBP
substrate-binding protein
- SUR
sulfonylurea receptor
- TMD
transmembrane domain
- XF–MS
X-ray radiolytic footprinting with mass spectroscopy
Footnotes
ATP binding cassette transporters: from mechanism to organism: Held at University of Chester, U.K., 16–18 April 2015.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council [grant numbers BB/L001691/1 (to F.L.T.)]. ABC research in IDK's lab is funded by BBSRC Doctoral Training Programme studentships.
References
- 1.Berger E.A., Heppel L.A. Different mechanisms of energy coupling in the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J. Biol. Chem. 1974;249:7747–7755. [PubMed] [Google Scholar]
- 2.Higgins C.F., Haag P.D., Nikaido K., Ardeshir F., Garcia G., Ames G. Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature. 1982;298:723–727. doi: 10.1038/298723a0. [check] [DOI] [PubMed] [Google Scholar]
- 3.Gilson E., Higgins C.F., Hofnung M., Ames G.F.-L., Nikaido H. Extensive homology between membrane-associated components of histidine and maltose transport systems of Salmonella typhimurium and Escherichia coli. J. Biol Chem. 1982;257:9915–9918. [PubMed] [Google Scholar]
- 4.Juliano R.L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 5.Riordan J.R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature. 1985;316:817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- 6.Walker J.E., Saraste M., Runswick M.J., Gay N.J. Distantly related sequences in the a- and b-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide-binding fold. EMBO J. 1982;1:945–95. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlach J.H., Endicott J.A., Juranka P.F., Henderson G., Sarangi F., Deuchars K.L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986;324:485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- 8.Higgins C.F., Hiles I.D., Salmond G.P.C., Gill D.R., Downie J.A., Evans I.J., Holland I.B., Gray L., Buckel S.D., Bell A.W., et al. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986;323:448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- 9.Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986;47:371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- 10.Chen C.J., Chin J.E., Ueda K., Clark D.P., Pastan I., Gottesman M.M., Roninson I.B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 11.Hyde S.C., Emsley P., Hartshorn M.J., Mimmack M.M., Gileadi U., Pearce S.R., Gallgaher M.P., Gill D.R., Hubbard R.E., Higgins C.F. Structural and functional relationships of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- 12.Callaghan R. Providing a molecular mechanism for P-glycoprotein: why would I bother? Biochem. Soc. Trans. 2015;43:995–1002. doi: 10.1042/BST20150131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biemans-Oldehinkel E., Doeven M.K., Poolman B. ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett. 2006;580:1023–1035. doi: 10.1016/j.febslet.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 14.Dean M., Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 15.Verrier P.J., Bird D., Burla B., Dassa E., Forestier C., Geisler M., Klein M., Lee Y., Martinoia E., et al. Plant ABC proteins- a unified nomenclature and updated inventory. Trends Plant. Sci. 2008;13:151–159. doi: 10.1016/j.tplants.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Saurin W., Hofnung M., Dassa E. Getting in or out: early segregation between importers and exporters in the evolution of ATP-binding cassette (ABC) transporters. J. Mol. Evol. 1999;48:22–41. doi: 10.1007/PL00006442. [DOI] [PubMed] [Google Scholar]
- 17.Davidson A.L., Dassa E., Orelle C., Chen J. Structure, function and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 2008;72:317–364. doi: 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eitinger T., Rodionov D.A., Grote M., Schneider E. Canonical and ECF-type ATP-binding cassette importers in prokaryotes: diversity in modular organization and cellular functions. FEMS Microbiol. Rev. 2011;35:3–67. doi: 10.1111/j.1574-6976.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- 19.Kang J., Park J., Choi H., Burla B., Kretzschmar T., Lee Y., Martinoia E. Plant ABC transporters. Arabidopsis Book. 2011;9 doi: 10.1199/tab.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borghi L., Kang J., Kp D., Lee Y., Martinoia E. The role of ABCG-type ABC transporters in phytohormone transport. Biochem. Soc. Trans. 2015;43:924–930. doi: 10.1042/BST20150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M., Choi Y., Burla B., Kim Y.Y., Jeon B., Maeshima M., Yoo J.Y., Martinoia E., Lee Y. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat. Cell Biol. 2008;10:1217–1223. doi: 10.1038/ncb1782. [DOI] [PubMed] [Google Scholar]
- 22.Lousa C, van Roermund C.W., Postis V.L.G., Dietrich D., Kerr I.D., Wanders R.J.A., Baldwin S.A., Baker A., Theodoulou F.L. Intrinsic acyl-CoA thioesterase activity of a peroxisomal ABC transporter is required for transport and metabolism of fatty acids. Proc. Natl. Acad. Sci. U.S.A. 2013;110:1279–1284. doi: 10.1073/pnas.1218034110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker A., Carrier D. J., Schaedler T., Waterham H.R., van Roermund C.W., Theodoulou F.L. Peroxisomal ABC transporters: functions and mechanism. Biochem. Soc. Trans. 2015;43:959–965. doi: 10.1042/BST20150127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefèvre F., Bijot A., Boutry M. Plant ABC transporters: time for biochemistry? Trans. 2015;43:931–936. doi: 10.1042/BST20150108. [DOI] [PubMed] [Google Scholar]
- 25.Krumpochova P., Sapthu S., Brouwers J.F., de Haas M., de Vos R., Borst P., Wetering K. Transportomics: screening for substrates of ABC transporters in body fluids using vesicular transport assays. FASEB J. 2012;26:738–747. doi: 10.1096/fj.11-195743. [DOI] [PubMed] [Google Scholar]
- 26.Schaedler T.A., Faust A., Shintre C.A., Carpenter E.P., Srinivasan V., van Veen H.W., Balk J. Structures and functions of mitochondrial ABC transporters. Biochem. Soc. Trans. 2015;43:943–951. doi: 10.1042/BST20150118. [DOI] [PubMed] [Google Scholar]
- 27.Schaedler T.A., Thornton J.D., Kruse I., Schwarzländer M., Meyer A.J., van Veen H.W., Balk J. A conserved mitochondrial ATP-binding cassette transporter exports glutathione polysulfide for cytosolic metal cofactor assembly. J. Biol. Chem. 2014;289:23264–23274. doi: 10.1074/jbc.M114.553438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepherd M. The CydDC ABC transporter of Escherichia coli: new roles for a reductant efflux pump. Biochem. Soc. Trans. 2015;43:908–912. doi: 10.1042/BST20150098. [DOI] [PubMed] [Google Scholar]
- 29.Riordan J.R., Rommens J.M., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J.-L., et al. Identification of the cystic fibrosis gene: cloning and characterisation of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 30.Bose S.J., Scott-Ward T.S., Cai Z., Sheppard D.N. Exploiting species differences to understand the CFTR Cl− channel. Biochem. Soc. Trans. 2015;43:975–982. doi: 10.1042/BST20150129. [DOI] [PubMed] [Google Scholar]
- 31.Pollock N.L., Rimington T.L., Ford R.C. Characterising diverse orthologues of the cystic fibrosis transmembrane conductance regulator protein for structural studies. Biochem. Soc. Trans. 2015;43:894–900. doi: 10.1042/BST20150081. [DOI] [PubMed] [Google Scholar]
- 32.Cohen P. The role of phosphorylation in human health and disease. Eur. J. Biochem. 2001;268:5001–5010. doi: 10.1046/j.0014-2956.2001.02473.x. [DOI] [PubMed] [Google Scholar]
- 33.Aryal B., Laurent C., Geisler M. Learning from each other: ABC transporter regulation by protein phosphorylation in plant and mammalian systems. Biochem. Soc. Trans. 2015;43:966–974. doi: 10.1042/BST20150128. [DOI] [PubMed] [Google Scholar]
- 34.Aguilar-Bryan L., Nichols C.G., Wechsler S.W., Clement J.P., Boyd A.E., Gonzalez G., Herrera-Sosa H., Nguy K., Bryan J., Nelson D.A. The beta cell high affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 35.de Wet H., Proks P. Molecular action of sulfonylureas on KATP channels: a real partnership between drugs and nucleotides. Biochem. Soc. Trans. 2015;43:901–907. doi: 10.1042/BST20150096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodward O.M., Köttgen A., Coresh J., Boerwinkle E., Guggino W.B., Köttgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. U.S.A. 2009;106:10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peca D., Cutrera R., Masotti A., Boldrini R., Danhaive D. ABCA3, a key player in the neonatal respiratory transition and genetic disorders of the surfactant system. Biochem. Soc. Trans. 2015;43:913–919. doi: 10.1042/BST20150100. [DOI] [PubMed] [Google Scholar]
- 38.Li H., Karl T., Garner B. Understanding the function of ABCA7 in Alzheimer's disease. Biochem. Soc. Trans. 2015;43:920–923. doi: 10.1042/BST20150105. [DOI] [PubMed] [Google Scholar]
- 39.Linton K.J. Lipid flopping in the liver. Biochem. Soc. Trans. 2015;43:1003–1010. doi: 10.1042/BST20150132. [DOI] [PubMed] [Google Scholar]
- 40.Kemp S., Theodoulou F.L., Wanders R.J.A. Mammalian peroxisomal ABC transporters: from endogenous substrates to pathology and clinical significance. Br. J. Pharmacol. 2011;164:1753–1766. doi: 10.1111/j.1476-5381.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui H., Zhang A.J., Chen M., Liu J.J. ABC transporter inhibitors in reversing multidrug resistance to chemotherapy. Curr. Drug Targets. 2015 doi: 10.2174/1389450116666150330113506. in the press. [DOI] [PubMed] [Google Scholar]
- 42.Schipani A., Back D., Owen A., Davies G., Khoo S., Siccardi M. Use of in vitro to in vivo extrapolation to predict the optimal strategy for patients switching from efavirenz to maraviroc or nevirapine. Clin. Pharmacokinet. 2015;54:107–116. doi: 10.1007/s40262-014-0184-8. [DOI] [PubMed] [Google Scholar]
- 43.Schipani A., Siccardi M., D'Avolio A., Baietto L., Simiele M., Bonora S., Novoa S., Cuenca L., Soriano V., Chierakul N., et al. Population pharmacokinetic modeling of the association between 63396C->T pregnane X receptor polymorphism and unboosted atazanavir clearance. Antimicrob. Agents Chemother. 2010;54:5242–5250. doi: 10.1128/AAC.00781-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coyle B., Kessler M., Sabnis D., Kerr I.D. ABCB1 in children's brain tumours. Biochem. Soc. Trans. 2015;43:1018–1022. doi: 10.1042/BST20150137. [DOI] [PubMed] [Google Scholar]
- 45.Hlaváč V., Souček P. Role of family D ATP-binding cassette transporters (ABCD) in cancer. Biochem. Soc. Trans. 2015;43:937–942. doi: 10.1042/BST20150114. [DOI] [PubMed] [Google Scholar]
- 46.Stockner T., Mullen A., MacMillan F. Investigating the dynamic nature of the ABC transporters: ABCB1 and MsbA as examples for the potential synergies of MD theory and EPR applications. Biochem. Soc. Trans. 2015;43:1023–1032. doi: 10.1042/BST20150138. [DOI] [PubMed] [Google Scholar]
- 47.Jr G.N., Mahajan V.K., Siu A.K., Quiocho F.A. Structure of L-arabinose-binding protein from Escherichia coli at 5 Å resolution and preliminary results at 3.5 Å. Proc. Natl. Acad. Sci. U.S.A. 1976;73:2186–2190. doi: 10.1073/pnas.73.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maqbool A., Horler R.S.P., Muller A., Wilkinson A.J., Wilson K.S., Thomas G.H. The substrate binding protein in bacterial ABC transporters: dissecting roles in the evolution of substrate specificity. Biochem. Soc. Trans. 2015;43:1011–1017. doi: 10.1042/BST20150135. [DOI] [PubMed] [Google Scholar]
- 49.Husada F., Gouridis G., Vietrov R., Poolman B., Cordes T. Watching conformational states of ABC transporters with single-molecule tools. Biochem. Soc. Trans. 2015;43:1041–1047. doi: 10.1042/BST20150140. [DOI] [PubMed] [Google Scholar]
- 50.Gouridis G., Schuurman-Wolters G.K., Ploetz E., Husada F., Vietrov R., de Boer M., Cordes T., Poolman B. Conformational dynamics in substrate-binding domains influences transport in the ABC importer GlnPQ. Nat. Struct. Mol. Biol. 2015;22:57–64. doi: 10.1038/nsmb.2929. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg M.F., Callaghan R., Ford R.C., Higgins C.F. Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J. Biol. Chem. 1997;272:10685–10694. doi: 10.1074/jbc.272.16.10685. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg M.F., Velarde G., Ford R.C., Martin C., Berridge G., Kerr I.D., Callaghan R., Schmidlin A., Wooding C., Linton K.J., Higgins C.F. Repacking of the transmembrane domains of P-glycoprotein during the transport ATPase cycle. EMBO J. 2001;20:5615–5625. doi: 10.1093/emboj/20.20.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beis K. Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 2015;43:889–893. doi: 10.1042/BST20150047. [DOI] [PubMed] [Google Scholar]
- 54.Shintre C.A., Pike A.C., Li Q., Kim J.I., Barr A.J., Goubin S., Shrestha L., Yang J., Berridge G., Ross J., et al. Structures of ABCB10, a human ATP-binding cassette transporter in apo- and nucleotide-bound states. Proc. Natl. Acad. Sci. U.S.A. 2013;110:9710–9715. doi: 10.1073/pnas.1217042110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doshi R., van Veen H.W. Substrate binding stabilizes a pre-translocation intermediate in the ATP-binding cassette transport protein MsbA. J. Biol. Chem. 2013;288:21638–21647. doi: 10.1074/jbc.M113.485714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Wonderen J.H., McMahon R.M., O'Mara M.L., McDevitt C.A., Thomson A.J., Kerr I.D., MacMillan F., Callaghan R. The central cavity of ABCB1 undergoes alternating access during ATP hydrolysis. FEBS J. 2014;281:2190–2201. doi: 10.1111/febs.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Domicevica L., Biggin P.C. Homology modelling of human P-glycoprotein. Biochem. Soc. Trans. 2015;43:952–958. doi: 10.1042/BST20150125. [DOI] [PubMed] [Google Scholar]
- 58.Bavro V.N., Gupta S., Ralston C. Oxidative footprinting in the study of structure and function of membrane proteins: current state and perspectives. Biochem. Soc. Trans. 2015;43:983–994. doi: 10.1042/BST20150130. [DOI] [PubMed] [Google Scholar]
- 59.Gulati S., Jamshad M., Knowles T.J., Morrison K.A., Downing R., Cant N., Collins R., Koenderink J.B., Ford R.C., Overduin M., et al. Detergent-free purification of ABC (ATP-binding-cassette) transporters. Biochem. J. 2014;461:269–278. doi: 10.1042/BJ20131477. [DOI] [PubMed] [Google Scholar]
- 60.Chen B., Boël G., Hashem Y., Ning W., Fei J., Wang C., Gonzalez R.L., Jr., Hunt J.F., Frank J. EttA regulates translation by binding the ribosomal E site and restricting ribosome-tRNA dynamics. Nat. Struct. Mol. Biol. 2014;21:152–159. doi: 10.1038/nsmb.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zollmann T., Moiset G., Tumulka F., Tampé R., Poolman B., Abele R. Single liposome analysis of peptide translocation by the ABC transporter TAPL. Proc. Natl. Acad. Sci. U.S.A. 2015;112:2046–2051. doi: 10.1073/pnas.1418100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim J., Wu S., Tomasiak T.M., Mergel C., Winter M.B., Stiller S.B., Robles-Colmanares Y., Stroud R.M., Tampé R., Craik C.S., Cheng Y. Subnanometre-resolution electron cryomicroscopy structure of a heterodimeric ABC exporter. Nature. 2015;517:396–400. doi: 10.1038/nature13872. [DOI] [PMC free article] [PubMed] [Google Scholar]