Abstract
Aims
To identify the Candida species that cause vulvovaginal candidiasis and determine their antifungal susceptibility patterns.
Study Design
This was a cross-sectional study.
Place and Duration of Study
The study was conducted at the antenatal clinic of Mbarara Regional Referral Hospital in Mbarara Municipality, between December 2012 and February 2013.
Methods
High vaginal swabs from 456 pregnant women were subjected to microscopy and culture on Sabouraud Dextrose Agar. Candida isolates were identified by the germ tube and Analytical profile index (API® Candida) tests. Susceptibility to fluconazole, itraconazole and voriconazole was determined by the Etest strips and for clotrimazole and nystatin by the disc diffusion method on Mueller Hinton agar supplemented with 2%w/v glucose and 0.5μg/ml methylene blue dye.
Results
Of the 456 High vaginal swabs cultured, 207 grew Candida species. Species distribution was as follows: C. albicans (78.95%), C. glabrata (14.35%), C. krusei (3.35%), C. tropicalis (1.44%), C. famata (0.96%), C. parapsilosis (0.48%) and C. lusitaniae (0.48%). Resistance to nystatin was only observed in 0.61% of C.albicans. Resistance to clotrimazole was observed in 50%, 36.67% and 0.61% of C. famata, C. glabrata and C. albicans respectively. C. krusei showed a high resistance of 71.43% to fluconazole. C. glabrata, C. krusei, C. famata and C. lusitaniae exhibited 100% resistance to itraconazole. Resistance to voriconazole of less than 11% was exhibited by only C. albicans and C. glabrata.
Conclusion
C.albicans was susceptible to most antifungal agents tested except itraconazole and voriconazole. All isolates were susceptible to nystatin except less than 1% of Candida albicans. Non-albicans demonstrated resistance to some drugs especially itraconazole. We recommend use of Nystatin for empirical management of vulvovaginal candidiasis among pregnant women.
Keywords: Antifungal, susceptible, resistant, vulvovaginal candidiasis, Candida species
1. INTRODUCTION
Vulvovaginal candidiasis (VVC) is an opportunistic fungal infection of the female lower genital tract caused by Candida species [1]. Mucosal candidiasis, especially vulvovaginal candidiasis, is the most common fungal disease in normal healthy women [2,3] On the other hand pregnant women are the most predisposed to severe vulvovaginal candidiasis as a result of lowered immunity [4–6] and this severity may involve among others chorioamnionitis which eventually leads to preterm labour [7,8]. According to available data, preterm birth is the leading cause of neonatal mortality worldwide [9]. Candida albicans is a dimorph and an endogenous commensal implicated as an etiologic agent in most cases of the female lower reproductive tract infections [2,10]. Other non-albicans species such as C. glabrata, C. parapsilosis and C. krusei although rare compared to C. albicans, may also cause serious opportunistic infections and exhibit more resistance to a number of antifungal agents.
Although there are many effective antifungal drugs both topical and oral that are used for treating candidal vulvovaginitis, reduced susceptibility of some vaginal yeasts isolates to some antifungal agents has been reported [11] in Costa Rica involving yeasts isolated from high vaginal swabs.
Understanding of the antifungal susceptibility patterns of pathogenic fungi is key in guiding appropriate therapy selection for mycoses [12–15]. Antifungal susceptibility testing may also provide an estimation of antifungal effectiveness, ensuring good treatment outcome, monitoring of development of drug resistance and therapeutic potential of unproven compounds [12–14].
In Uganda data on the species and susceptibility of vulvovaginal yeasts to antifungal drugs is limited. Routine species typing and in vitro antifungal susceptibility testing of Candida isolates prior to therapy has not been given its appropriate attention to determine the occurrence of resistant genotypes and consequently, there is no data about prevalent Candida species and their antifungal susceptibility patterns in this region. Therefore, the aim of this study was to perform speciation of Candida isolates responsible for causing VVC and determine their susceptibility patterns to the commonly used antifungal drugs in Mbarara, Uganda.
2. MATERIALS AND METHODS
2.1 Study Area
The study was conducted at the antenatal clinic (ANC) of Mbarara Regional Referral Hospital in Mbarara Municipality. Mbarara Regional Referral Hospital is also a teaching Hospital for the Medical School of Mbarara University of Science and Technology. At the antenatal clinic the daily attendance ranges between 40 and 60 pregnant women.
The High vaginal swabs (HVS) were processed from the Microbiology Laboratory at Mbarara University of Science and Technology where clinical specimens from the Hospital are processed.
2.1.1 Study population
The study subjects were pregnant women who had presented to the Mbarara Regional Referral Hospital antenatal clinic for ante-natal care.
2.1.2 Inclusion criteria
The consented pregnant women of age 18years and above who presented at the antenatal clinic for antenatal care were included in the study. Those aged below 18years were also included and considered emancipated minors.
2.1.3 Exclusion criteria
Pregnant women who did not consent and those who reported a history of vaginal bleeding were excluded from the study.
2.1.4 Sampling procedure
Pregnant women at the ANC were selected and recruited into the study by convenience sampling after a health talk about the study had been given by the principal investigator or the nurse on duty.
2.1.5 Consent and counseling
A written consent was sought from those pregnant women who satisfied the inclusion criteria and this was followed by filling in the questionnaire, and counseling for specimen collection. The study subject was then sent to a private room for specimen collection. The results were kept confidential under key and lock.
2.1.6 Questionnaire
This tool was used to capture demographic data and predicting factors for vulvovaginal candidiasis.
2.1.7 Collection of high vaginal swabs
Two High vaginal swabs were taken one after the other by inserting a sterile vaginal speculum into the vagina, and then a sterile cotton wool swab (Copan Italia S.p.A Italy) was inserted into the posterior vaginal fornix and rotated gently before withdrawing. The swab was inserted back into the tube from which it was taken. The tube containing the swab was labelled with the patients study number, initials and date.
2.2 Examination of High Vaginal Swabs
One swab was examined by wet preparation method and Gram stain to give the preliminary results to the clinician for patient management. A few drops of sterile normal saline were added to one of the swabs in the tube and shaken to dislodge materials from the swab into the saline. The swab was withdrawn and a sterile inoculating loop was used to transfer and spread a small amount of the deposit onto a clean dry slide to make a smear. The smear was allowed to air-dry, stained by Gram’s method and then examined under the microscope for yeast cells using the oil immersion objective lens.
A wet film was prepared by placing a drop of the saline deposit from the tube onto a clean dry glass slide then covered with a cover slip and examined under the microscope for budding yeast cells and pseudohyphae first using the 10X objective before switching to 40X objective lens to confirm the presence or absence of yeasts.
2.2.1 Culture and isolation of yeast cells
The second swab was streaked onto a plate of Sabouraud dextrose agar (SDA) with chloramphenicol (Biolab Hungary) and incubated at 37°C for 48 hours. Pure yeast colonies were isolated and a Gram stain done to confirm they were yeast cells.
2.3 Preservation of Yeast Cells
By use of a sterile wire loop, the yeast colonies were scraped from the SDA plate and suspended in1ml of 16.8%v/v glycerol then stored in a −80°C deep freezer till needed for identification and susceptibility testing.
2.4 Subculture and Species Identification
Subculture of preserved yeasts was done on SDA free from chloramphenicol (Mast Group Ltd. Merseyside, UK.) medium, and inoculated plates were incubated at 37°C for 18 to 24 hours. Identification was then carried out by the germ tube test and API analysis.
2.4.1 Germ tube test
The germ tube test was performed by dispensing 0.5ml of freshly prepared human serum into sterile 1.25ml cryovial. Using a sterile wire loop the serum was inoculated with a yeast colony from the SDA plate and the cover loosely placed to allow an aerobic condition. The cryovial was placed in an incubator at 37°C for 3 hours. Using a Pasteur pipette, a drop of the serum yeast culture was transferred to a glass slide, and covered with a cover glass.
The preparation was examined using the ×10 and ×40 objectives with the condenser diaphragm closed sufficiently to give a good contrast. A search was done for sprouting yeast cells, which were tube like outgrowths from the cells (known as germ tubes).
If the sprouting cells were seen the culture was reported as ‘C. albicans isolated’ and if no sprouting seen reported as ‘yeast other than C. albicans isolated’.
2.4.2 Analytical Profile Index test strip method
All the yeast isolates were identified to species level by Analytical Profile Index strips (API® Candida BioMerieux® SA) according to the manufacturer’s procedure.
An incubation tray was prepared by adding 5mls of distilled water into the honey-combed wells of the tray to create a humid atmosphere then the isolate identification number was recorded on the elongated flap of the tray. An API strip was removed from its individual packaging and put into the tray. A few Yeast colonies of an18 to 24 hour culture were picked from SDA plate using a sterile cotton swab and emulsified into 2mls of sterile 0.85%w/v sodium chloride and agitated using a vortex mixer to disperse the cells. The turbidity of the suspension was measured using a densimat (BioMerieux) and adjusted to a 3.0 McFarland number using sterile 0.85%w/v sodium chloride, and used immediately.
The yeast suspension was mixed well, and dispensed into the tubes of the API strip using a 1000μl pipettor with sterile tips care being taken to avoid air bubbles. The first five tubes of glucose to raffinose and the last tube of urea were covered with a layer of mineral oil immediately after inoculating the strip.
The cover of the tray was put in place and the strip incubated at 37°C in an aerobic condition for 18 – 24 hours.
2.4.3 Reading and Interpretation of the strip
After 18 – 24 hours of incubation the reaction in the strip was read referring to the interpretation table in the package insert and recorded as + or − on the result sheet.
The reactions were coded into a numerical profile on the result sheet. The tests were separated into groups of three and a number 1, 2 or 4 assigned to each one. By adding together the numbers corresponding to positive reactions within each group, a 4-digit numerical profile was obtained.
Identification was performed by looking up the profile in the list of the package insert, and also with the apiweb™ identification software by entering the 4-digit profile manually via the key board into the mini-API analyzer (BioMerieux) that gave a print out of the species identification.
2.5 Antifungal Susceptibility Testing
Antifungal susceptibility testing was done by agar diffusion method. Etest strips (BioMerieux SA) for fluconazole (0.016–256 μg/ml), itraconazole (0.002–32 μg/ml) and voriconazole (0.002–32 μg/ml) were used. For clotrimazole (50μg) and nystatin (100 IU) antifungal discs (Bio-Rad Laboratories Pvt Ltd. France) were used. Mueller-Hinton Agar (Mast Group Ltd., Merseyside UK.) supplemented with 2%w/v glucose and 0.5μg/ml methylene blue dye was used as recommended by CLSI document M44A [16].
2.5.1 Epsilometer and Disc diffusion method
A yeast colony of 18 to 24 hour incubation culture was picked from the SDA plate using a sterile cotton swab and emulsified into 2mls of 0.85%w/v sterile sodium chloride and agitated on the vortex mixer to achieve a homogeneous suspension.
The turbidity of the suspension was measured using a densimat (BioMerieux) and adjusted to a 0.5 McFarland number using sterile saline.
A sterile cotton swab was moistened in the adjusted inoculum suspension then excess moisture was removed by rolling the swab on the inside of the tube above fluid level.
The surface of Mueller Hinton-glucose agar plate was streaked in 4 different directions (at 90 degree angles) to cover the entire surface. The plate was left to dry at 35°C until no droplets of moisture were on the agar surface. Using a pair of flame sterilized forceps an Etest strip or disc of the antifungal agent was applied onto the surface of the inoculated agar plate and pressed lightly to ensure complete contact with agar.
The plates were incubated at 35°C for 24 to 48 hours or until sufficient growth had occurred. Plates were read as early as possible after 24 hours incubation and results recorded.
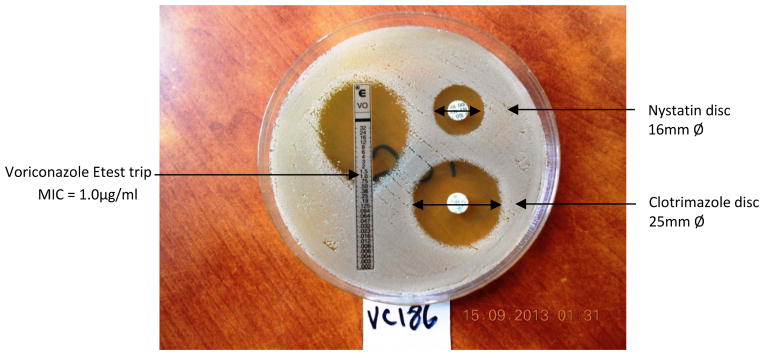
The Minimum Inhibitory Concentration (MIC) value was read at the point where the inhibition ellipse intersected the strip (Fig. 1). Etest strips results were interpreted according to the manufacturer’s interpretation criteria as susceptible, susceptible dose dependent (S-DD) or resistant with MIC values as indicated in Table 1. For antifungal discs the diameters of zones of inhibition were measured in millimeters using a ruler. The results were interpreted according to the manufacturer’s interpretation criteria as sensitive, intermediate or resistant as indicated in Table 2 below.
Fig. 1.
Zones of inhibition
Table 1.
Interpretation criteria MIC μg/ml for the three Etest strips tested (CLSI 2009)
| Antifungal MIC μg/ml | Code | Interpretation criteria MIC μg/ml
|
||
|---|---|---|---|---|
| Susceptible S≤ | Susceptible dose dependent S-DD | Resistant R≥ | ||
| Fluconazole (0.016–256) | FL | 8 | 16–32 | 64 |
| Itraconazole (0.002–32) | IT | 0.12 | 0.25–0.5 | 1 |
| Voriconazole (0.002–32) | VO | 1 | 2 | 4 |
Reference: Etest® Table 1 summary of Etest® performance, interpretive criteria and Quality Control ranges 16029 B- 2011/01
Table 2.
Interpretation criteria of diameters of zones of inhibition for the discs
| Antifungal drug | Diameter of the zone of inhibition (mm) | MIC (μg/ml) | Interpretation |
|---|---|---|---|
| Nystatin (100 IU) | = 10 | - | Resistant |
| > 10 | Sensitive | ||
| Clotrimazole (50μg) | = 20 | = 1.56 | Sensitive |
| 20 – 10 | 1.56 – 6.4 | Intermediate | |
| = 10 | = 6.4 | Resistant |
Reference: Bio rad Laboratories Pvt Ltd. 3, boulevard Raymond Poincaré 92430 Marnes-la-Coquette France
2.6 Data Analysis
Data was entered into excel and imported into STATA 11.0 for analysis. The baseline characteristics of participants were summarized using the mean, median or proportion as appropriate. Proportions were generated to demonstrate the distribution of Candida species. The susceptibility patterns were generated using proportions for each drug type per Candida species isolated.
2.7 Quality Control/Assurance
New Zealand Reference Culture Collection Medical Section (NZRM) control strains were used. NZRM 3394 (ATCC 90028) Candida albicans and NZRM 4072T (ATCC 22019) Candida parapsilosis were cultured along with the clinical Candida isolates. These strains were used for the validation of identification and susceptibility performance tests. Epsilometer strips were stored between −18°C and −20°C and used as per the manufacturer’s recommendation. The Clotrimazole and Nystatin antifungal discs were stored between +2°C and +8°C.
3. RESULTS
3.1 Identity and Proportions of Candida species
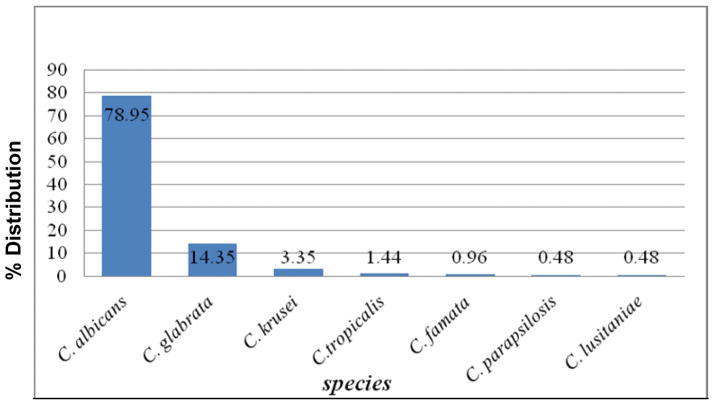
Of the 456 High vaginal swabs cultured 207 grew Candida giving a prevalence of 45.4%. Two high virginal swabs yielded two different species of Candida giving a total of 209 Candida species. C. albicans 165 (78.95%) was predominant followed by C. glabrata 30 (14.35%), C. krusei 7 (3.35%), C. tropicalis 3 (1.44%), C. famata 2 (0.96%), C. parapsilosis 1(0.48%) and C. lusitaniae 1 (0.48%) as shown in Fig. 2 below. The overall rate of non albicans isolates was 21.05%.
Fig. 2.
Distribution of Candida species
3.2 Antifungal Susceptibility Patterns of Isolated Candida species
Antifungal susceptibility testing was done using fluconazole, itraconazole, voriconazole, nystatin and clotrimazole.
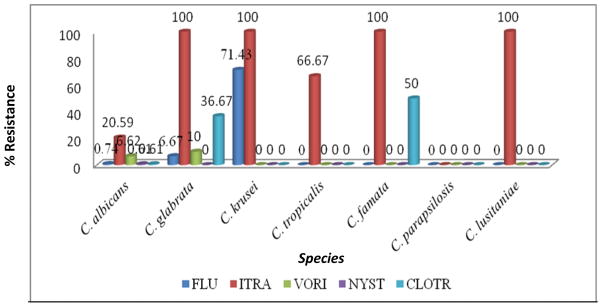
C. krusei showed a high resistance of 71.43% to fluconazole. C. glabrata, C. krusei, C. famata and C. lusitaniae exhibited 100% resistance to itraconazole. Resistance to voriconazole and nystatin was minimal. Resistance of all species to nystatin was below 1%. C. glabrata and C. famata showed a 36.67% and 50% resistance to clotrimazole respectively. C. albicans exhibited resistance of 20.59% to itraconazole and 6.62% to voriconazole as shown in Fig. 3 below.
Fig. 3.
Percentage antifungal resistance of Candida species
4. DISCUSSION
4.1 Species Identification and Distribution
Vulvovaginal candidiasis is a common female genital problem that affects women especially of child bearing age and pregnant women [4]. Pregnant women are more susceptible to both vaginal colonization and infection by yeasts as a result of high concentration of oestrogen hormone during pregnancy which provides a suitable environment for the growth of Candida [17]. In this study the center of attention was pregnant women with the broad objective to identify the Candida species that cause vulvovaginal candidiasis and determine their antifungal susceptibility patterns.
Data on the prevalence of Ugandan Candida species causing vulvovaginitis remains scarce since cultures are rarely performed. Our study showed that C. albicans was the most prevalent species (79%). The overall prevalence of non-albicans species was 21% with C. glabrata the most predominant. This dominancy agrees well with findings of 20% from another study [18], but differs from some other two studies [19,20] who found lower values of 17% and 11% respectively
The findings in our study are similar to those of the study in Kenya [21] which showed that of the Candida species isolated, C.albicans were the most common (73.7%). Among the non albicans species, C. glabrata (13%), C. famata (5%), C. krusei (3%) and C. parapsilosis (1%) were the species isolated.
Furthermore, our study findings are also similar to those of the study in Brazil [22] about species distribution from pregnant women, where, C. albicans were 83 (92.3%), C. krusei 3 (3.3%), C. glabrata 2 (2.2%), C. parapsilosis 1 (1.1%), and 1 (1.1%) as C.tropicalis. When we look at this species distribution in our study and other similar studies, it is worth noting that C. albicans is the most prevalent compared with other species, the reason probably being that it is a normal flora that takes advantage of risk factors such as pregnancy, antibiotic therapy, uncontrolled diabetes mellitus, immunosupression due to HIV and others.
Other than C. albicans, species such as Candida glabrata and Candida tropicalis are less commonly implicated etiologic agents of vaginal infections and have a propensity to be more resistant to treatment. On the other hand, our study findings differ from those of a recent study in India [23] where only five Candida species were isolated and identified. Their study results further showed a lower proportion of Candida albicans (35.5%), followed by higher incidence of non-albicans species of C. tropicalis (26.4%), C. glabrata (20.6%), C. krusei (15.7%), and C. dubliniensis (1.6%).
This difference shows an increase in frequency of non-albicans species particularly, C. glabrata, C. krusei, and C. tropicalis as potential causes of vulvovaginal candidiasis. We contemplate this increasing detection of non albicans species is probably related to the widespread and inappropriate use of antimycotic drugs most especially obtained over the counter.
4.2 Antifungal Susceptibility Patterns
Antifungal susceptibility testing in our study revealed that most of the Candida albicans isolates tested were susceptible to fluconazole, voriconazole, nystatin and clotrimazole. All isolates were susceptible to nystatin with only an exception of less than 1% of C. albicans. This finding is in agreement with a similar study carried out in Argentina [17].
In this study the resistance rate of Candida albicans to itraconazole was slightly low whereas the susceptible dose dependent was high. In relation to fluconazole, a similar study [24] C. albicans showed a very high susceptibility (96%) to fluconazole and MIC values equal to or higher than 64μg/ml were also detected. These percentages are similar to those obtained in our study. As expected, higher azole MICs were found among non albicans Candida species. A larger proportion of the C. glabrata isolates in this study was not susceptible to fluconazole, itraconazole and clotrimazole and showed the highest susceptible dose dependent rate to fluconazole and absolute resistance rate to itraconazole, followed by clotrimazole resistance and intermediate rate of 60% to the later drug. Similarly, a larger proportion of C. krusei was not susceptible to fluconazole and itraconazole and showed very high resistance rates to fluconazole and itraconazole but this is not a surprise because C. krusei is intrinsically recognized to be resistant to azoles. C. tropicalis isolates showed high resistance to itraconazole and intermediate to clotrimazole. C. famata and C. lusitaniae showed absolute resistance to itraconazole. C. famata showed a 50% intermediate and resistance to clotrimazole. This resistance to itraconazole was high probably because the isolates were few and therefore it may not be worth concluding generally that these species are absolutely resistant to itraconazole. C. parapsilosis was susceptible to all other drugs except with an absolute susceptible dose dependent to itraconazole indicating that itraconazole may not be appropriate for the treatment of C. parapsilosis infection. These in-vitro results from this study therefore support the use of alternative antimycotic agents when treating vulvovaginal candidiasis caused by non albicans species especially C. glabrata and C. krusei. This study had limited resources that led to the use of a few antifungal agents for susceptibility testing.
5. CONCLUSION
In this study the pregnant women presenting with vulvovaginal candidiasis were infected with C. albicans as the predominant etiologic agent. The commonest non Candida albicans species was C. glabrata. Almost all Candida isolates were susceptible to nystatin. Resistance to clotrimazole was exhibited by C. famata, C. glabrata and less than one percent of Candida albicans.
6. RECOMMENDATION
Nystatin, a relatively cheap and readily available antifungal agent is recommended for empirical treatment where laboratory identification and susceptibility tests cannot be done.
Acknowledgments
We thank Mbarara University of Science and Technology for the financial support. This study was supported by MEPI-MESAU Grant Number 5R24TW008886 supported by OGAC, NIH and HRSA. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the supporting offices.
We are also indebted to the study subjects, Epicentre Mbarara Research Centre, the staff of antenatal Clinic Mbarara Regional Referral Hospital and Microbiology department Mbarara University of Science and Technology.
Footnotes
Authors’ contributions
This work was carried out in collaboration between all authors. Author KJM designed the study, supervised and participated in site recruitment, laboratory analysis, data entry and wrote the first draft of the manuscript. Author IH participated in the study design and laboratory quality assurance. Author AD performed data analysis. Author BJ read and reviewed the manuscript. Author KLS participated in the study design. Author BF participated in study design, drafted and critically reviewed the manuscript. All authors read and approved the final manuscript.
ETHICAL APPROVAL
Ethical approval of the study was sought from Microbiology department, Mbarara University of Science and Technology Faculty Research and Ethics committee, Mbarara University of Science and Technology Institutional Review Committee and the Uganda National Council for Science and Technology. Written consent was sought from the study subjects before inclusion into the study.
COMPETING INTERESTS
Authors have declared that no competing interests exist.
References
- 1.Sobel J. Vulvovaginal candidosis. Lancet. 2007;369(9577):1961–71. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 2.Fidel PJ. Host defense against oropharyngeal and vaginal candidiasis: site-specific differences. RevIberoam Micro. 1999;16(1):8–15. [PubMed] [Google Scholar]
- 3.Mohanty S, Xess I, Hasan F, Kapil A, Mittal S, Tolosa JE. Prevalence & susceptibility to fluconazole of Candida species causing vulvovaginitis. India J Med Res. 2007;126(3):216–219. [PubMed] [Google Scholar]
- 4.Monif GR, Baker DA, editors. Infectious diseases in obstetrics and gynecology. 5. New York, NY: Parthenon Press; 2003. pp. 405–21. [Google Scholar]
- 5.Francisca I, Okungbowa I, Omoanghe S. The distribution and frequency of Candida species in the genitourinary tract among symptomatic individuals in Nigerian cities. Rev Iberoam Micol. 2003;20:60–63. [PubMed] [Google Scholar]
- 6.De Leon EM, Jacober SJ, Sobel JD, Foxman B. Prevalence and risk factors for vaginal Candida colonization in women with type 1and type 2diabetes. BMC Infect Dis. 2002;2:1. doi: 10.1186/1471-2334-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiGiulio DB, Romeo R, Amogan HP, Kusanovic JP, Bik EM, et al. Microbial Prevalence, Diversity and Abundance in Amniotic Fluid During Preterm Labor: A Molecular and Culture-Based Investigation. PLoS ONE. 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fumitake I, Tomoharu O, Tadahiro Y, Taisuke M, Koichi I, Kazuhiro I, et al. Premature delivery due to intrauterine Candida infection that caused neonatal congenital cutaneous candidiasis: A case report. J Obstet Gynaecol Res. 2013;39(1):341–343. doi: 10.1111/j.1447-0756.2012.01938.x. [DOI] [PubMed] [Google Scholar]
- 9.Lawn JE, Causens S, Zupan J. 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 10.Saporiti AM, Gomez D, Levalle S, Galeano M, Davel G, Vivot W, et al. Vaginal candidiasis: Etiology and sensitivity profile to antifungal agents in clinical use. Rev Argent Microbiol. 2001;33(4):217–222. [PubMed] [Google Scholar]
- 11.Gross NT, Arias ML, Moraga M, Baddasarow Y, Jarstrand C. Species distribution and susceptibility to azoles of vaginal yeasts isolated from prostitutes. Infect Dis Obstet Gynecol. 2007:82412. doi: 10.1155/2007/82412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff A. Clinical relevance of antifungal resistance. Infect Dis Clin North Am. 1997;11:929–944. doi: 10.1016/s0891-5520(05)70398-6. [DOI] [PubMed] [Google Scholar]
- 13.Pfaller MA, Yu WL. Antifungal susceptibility testing. New technology and clinical applications. Infect Dis Clin North Am. 2001;15:1227–1261. doi: 10.1016/s0891-5520(05)70192-6. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller MA, Rex JH, Rinaldi MG. Antifungal susceptibility testing: Technical advances and potential clinical applications. Clin Infect Dis. 1997;24:776–784. doi: 10.1093/clinids/24.5.776. [DOI] [PubMed] [Google Scholar]
- 15.Rex JH, Pfaller MA, Walsh TJ, Chaturvedi V, Espinel-Ingroff A, Ghannoum MA, et al. Antifungal susceptibility testing: Practical aspects and current challenges. Clin Microbiol Rev. 2001;14:643–658. doi: 10.1128/CMR.14.4.643-658.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wayne PA. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts. CLSI. 2004:M44-A. [Google Scholar]
- 17.García HM, García SD, Copolillo EF, Cora EM, Barata AD, Vay CA, et al. Prevalence of vaginal candidiasis in pregnant women. Identification of yeasts and susceptibility to antifungal agents. Revista Argentina de Microbiología. 2006;38(1):9–12. [PubMed] [Google Scholar]
- 18.Sandra SR, Rudolph P, Shawn A, Messer, Hollis Richard J, et al. Antifungal Susceptibilities of Candida Species Causing Vulvovaginitis and Epidemiology of Recurrent Cases. J Clin Microbiol. 2005;43(5):2155. doi: 10.1128/JCM.43.5.2155-2162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spinillo A, Capuzzo E, Gulminetti R, Marone P, Colonna L, Piazzi G. Prevalence of and risk factors for fungal vaginitis caused by non-albicans species. Am J Obstet Gynecol. 1997;176:138–141. doi: 10.1016/s0002-9378(97)80026-9. [DOI] [PubMed] [Google Scholar]
- 20.Holland J, Young ML, Lee O, Chen CA. Vulvovaginal carriage of yeasts other than Candida albicans. Sex Transm Infect. 2003;79(249) doi: 10.1136/sti.79.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutua F, Revath G, Machoki JM. Species distribution and antifungal sensitivity patterns of vaginal yeasts. East African Medical Journal. 2010;87(4) doi: 10.4314/eamj.v87i4.62202. [DOI] [PubMed] [Google Scholar]
- 22.Dias LB, et al. Vulvovaginal Candidiasis in Mato Grosso, Brazil. Pregnancy Status, Causative Species and Drugs Tests. Brazilian Journal of Microbiology. 2011;42:1300–1307. doi: 10.1590/S1517-83822011000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deepa B, Subbannayya KP, Sunil Rao, Ra TV. Clinico-mycological profile of vaginal candidiasis in a tertiary care hospital in Kerala. International Journal of Research in Biological Sciences. 2013;3(1):55–59. [Google Scholar]
- 24.Sobel JD, Zervos M, Reed BD, Hooton T, Soper D, Nyirjesy P, et al. Fluconazole Susceptibility of Vaginal Isolates Obtained from Women with Complicated Candida Vaginitis: Clinical Implications. Antimicrob Agents Chemother. 2003;47:34–38. doi: 10.1128/AAC.47.1.34-38.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]