Abstract
Objective
Leuprorelin acetate (TAP-144-SR) is commonly used worldwide in prostate cancer patients. This study was conducted to assess the non-inferiority of a 6-month depot formulation of TAP-144-SR (TAP-144-SR [6M]) 22.5 mg to a 3-month depot formulation of TAP-144-SR (TAP-144-SR [3M]) 11.25 mg in prostate cancer patients in Japan.
Methods
This was a 48-week Phase III, open-label, parallel-group comparative study. TAP-144-SR (6M) 22.5 mg (6M group) and TAP-144-SR (3M) 11.25 mg (3M group) were administered to 81 and 79 subjects, respectively. The primary endpoint was the rate of serum testosterone suppression to the castrate level (≤100 ng/dl).
Results
Serum testosterone of all subjects excluding one subject in the 3M group was suppressed to the castrate level throughout 48 weeks. The estimated between-group difference (6M group − 3M group) in suppression rate was 1.3% (95% confidence interval: −3.4, 6.8), and its lower confidence interval was more than −10% of the pre-determined allowable limit value to judge the non-inferiority. The prostate-specific antigen concentrations were stable throughout the study in both groups. Progressive disease in the best overall response based on the Response Evaluation Criteria In Solid Tumors was 0.0% for the 6M group and 2.6% for the 3M group. Adverse events occurred in 92.6% in the 6M group and 89.9% in the 3M group. Adverse events leading to discontinuation were reported in 2.5% in the 6M group and 3.8% in the 3M group.
Conclusions
TAP-144-SR (6M) was not inferior to TAP-144-SR (3M) for the suppressive effect on serum testosterone level. TAP-144-SR (6M) was also as well tolerated as TAP-144-SR (3M).
Keywords: leuprorelin, prostate cancer, luteinizing hormone-releasing hormone, testosterone, prostate-specific antigen
Introduction
Prostate cancer was the second most common cancer among new cases of cancer reported in males in 2011 in Japan, and the incidence rate has increased over the years while those of other male cancers have been showing a declining or stable trend (1–3). The incidence rate of prostate cancer is expected to keep increasing due to the prevalence of prostate-specific antigen (PSA) tests and aging, and the number of patients who develop prostate cancer predicted for 2020–24 in Japan is 105 800 per year, which is higher than for any other types of cancer (4).
It is well-known that cell growth in prostate cancer is stimulated by androgens (5). Hormone therapy and orchiectomy providing androgen deprivation have been established as the standard treatments for prostate cancer. The hormone therapy, monotherapy or combined androgen blockade therapy, includes luteinizing hormone-releasing hormone (LH-RH) agonists or an LH-RH antagonist that inhibits the production of testosterone following the suppression of gonadotropin release (6,7).
Leuprorelin acetate is a LH–RH agonist that is commonly used worldwide. Leuprorelin acetate was initially daily administered to patients, but the development of new formulation technology enabled 1-month (USA: 7.5 mg; EU and Japan: 3.75 mg), 3-month (USA: 22.5 mg; EU and Japan: 11.25 mg) and 6-month (USA: 45 mg; EU: 30 mg) depot formulations for prostate cancer, easing the burden on patients and physicians by reducing the number of injections and improving the quality of life of patients. A 6-month (24-week) depot formulation (TAP-144-SR [6M]) with the same formulation as that used in the USA has been developed in Japan (8), and a Phase II study was conducted in Japanese treatment-naive prostate cancer patients. The results showed that the optimal clinical dosage of TAP-144-SR (6M) in Japan was 22.5 mg (9) since the serum testosterone level of all six subjects who received the dosage subcutaneously was suppressed below the castrate level and no new safety signals were observed compared with the safety profile of the approved 1-month and 3-month (TAP-144-SR [3M]) depot formulations of TAP-144-SR. A double-peak pharmacokinetic profile was found in the Phase II study.
A Phase III, open-label, parallel-group comparative study was therefore conducted to assess the non-inferiority of treatment with TAP-144-SR (6M) 22.5 mg compared with TAP-144-SR (3M) 11.25 mg for 48-week suppressive effect on serum testosterone in prostate cancer patients.
Patients and methods
Study design
A Phase III, open-label, parallel-group comparative study of TAP-144-SR (6M) to TAP-144-SR (3M) was conducted to evaluate the hormone dynamics, efficacy, pharmacokinetics and safety of the two formulations in prostate cancer patients who have remained stable on TAP-144-SR (3M) treatment. Subjects who were receiving the marketed product for 3 months (TAP-144-SR [3M]) were randomized to the TAP-144-SR (6M) 22.5 mg arm (6M group) or the TAP-144-SR (3M) 11.25 mg arm (3M group), and received two subcutaneous injections of TAP-144-SR (6M) or four subcutaneous injections of TAP-144-SR (3M) over a period of 48 weeks. The subjects were randomized to one of the two treatment groups, using dynamic allocation with the following factors of randomization: (i) TNM classification (≤ any T any N M0 or any T any N M1); (ii) with or without treatment with nonsteroidal antiandrogen; (iii) with or without radiotherapy or surgical therapy; and (iv) study sites. This study was conducted in accordance with the International Conference on Harmonisation of Good Clinical Practice Guidelines, the principles of the Declaration of Helsinki, and all applicable laws and regulations. The protocol was reviewed and approved by the Institutional Review Boards of all participating study sites. All subjects provided written informed consent for participation before enrollment in the study. The clinical trial registration number is NCT01546623.
Subjects
Subjects who met the following criteria were enrolled: Japanese patients with histopathologically confirmed prostate cancer; prostate cancer of clinical stages ≥T1b and any NM assessed by TNM classification; Eastern Cooperative Oncology Group performance status ≤2 at screening; PSA level that had not increased ≥25% or ≥2 ng/ml from the nadir at measurement points 4 weeks or longer apart within the screening period of 12 weeks; age ≥20 years and ≤85 years; use of the marketed TAP-144-SR (3M) at screening; previous use of the marketed leuprorelin acetate for 24–96 weeks (excluding the period under neoadjuvant therapy); previous continuous use of the nonsteroidal antiandrogen for ≥12 weeks if taking any; and a serum testosterone level at screening of <100 ng/dl.
Subjects were excluded from the study if they met any of the following criteria: active multiple primary cancers; previous use of chemotherapy for prostate cancer; use or previous use of the marketed leuprorelin acetate as an adjuvant therapy; use or previous use of the marketed TAP-144-SR (3M) for intermittent androgen deprivation therapy; use of the following drugs within 24 weeks prior to the start of the study drug: steroidal antiandrogens or Type II 5α-reductase inhibitors; use of any of the following within 16 weeks prior to the start of the study drug: radiotherapy, prostatectomy or experimental therapy; and a QTcF interval exceeded 460 msec on the 12-lead electrocardiogram (ECG) at screening.
Endpoints
The primary endpoint was the rate of serum testosterone suppression to the castrate level from the start of study drug administration through Week 48. The castrate level in this study was set as ≤100 ng/dl, which has also been used as the castrate level in previous studies of leuprorelin acetate 1-month depot and 3-month depot in Japan (10–12). The primary endpoint was also evaluated at a lower serum testosterone level of 50 ng/dl with reference to the recent castration level (8,13–15). The secondary endpoints included the following: serum testosterone concentrations, serum LH concentrations, serum follicle-stimulating hormone (FSH) concentrations and serum TAP-144 concentrations from the start of study drug administration through Week 48. The efficacy, PSA, soft tissues and bone lesions were evaluated based on the response evaluation criteria in ‘General Rule for Clinical and Pathological Studies on Prostate Cancer’ (4th edition) (16). The soft tissues were evaluated based on the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 (17). Bone lesions were determined using bone scintigraphy. Safety assessments included adverse events (AEs), vital signs, clinical laboratory tests and 12-lead ECG.
Statistical analysis
The full analysis set (FAS) was defined as all subjects who were randomized and received at least one dose of the study drug, and the Hormone level Analysis Set (HAS) was defined as the population of subjects in whom serious protocol deviations were not reported, who satisfied the minimum requirements of the protocol, and in whom primary endpoints were evaluable. The suppression rate of serum testosterone to the castrate level thorough 48 weeks in the FAS was analyzed by calculating the incidence rate by treatment group and the point estimates and two-sided 95% confidence intervals (CIs). The point of estimate and the two-sided 95% CIs of the difference between the treatment groups (i.e. 6M group minus 3M group) was calculated by Newcombe Score Confidence Limits (18). If the lower confidence bound for this percentage was greater than or equal to the allowable limit value (−10%), it was judged that the non-inferiority of TAP-144-SR (6M) to TAP-144-SR (3M) was confirmed. It should be noted that the sensitivity analysis was performed by applying the same analysis method used for the primary analysis in the HAS and in the case of a lower testosterone level (≤50 ng/dl). Summary statistics were obtained for the secondary efficacy endpoints in the FAS.
The required sample size was estimated to be 75 subjects in each treatment group, a total of 150 subjects, from which the condition was set to 10% allowable limit value, with a significance level of 5% on both sides, and ensuring 80% power.
For the safety analysis, AEs and their severity were analyzed by treatment group. AEs were coded by System Organ Class and Preferred Term using the Medical Dictionary for Regulatory Activities, version 16.1, and their severity was graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (19).
Results
Subject demographics
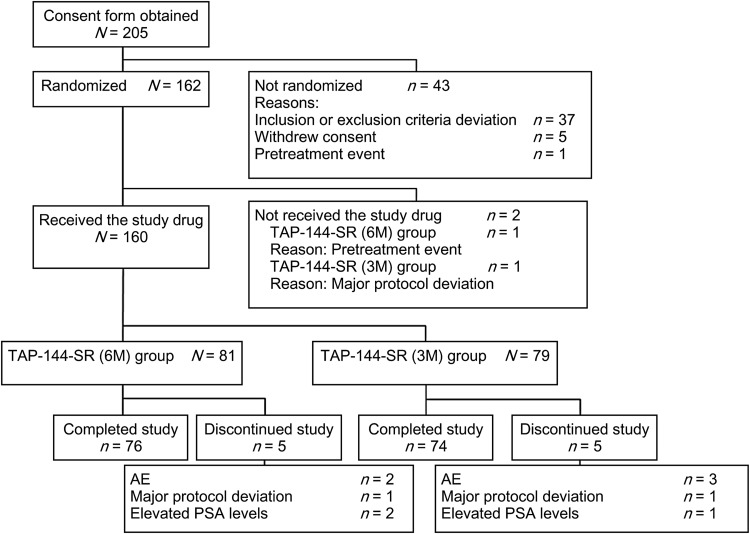
A total of 205 patients were screened, and 160 of the 162 randomized subjects received the study drugs with a breakdown of 81 subjects in the 6M group and 79 subjects in the 3M group (Fig. 1). Among the subjects who received the study drugs, 76 subjects in the 6M group and 74 subjects in the 3M group completed the study. Five subjects in each treatment group discontinued the study for the following reasons: AEs (two subjects in the 6M group and three subjects in the 3M group); major protocol deviations (one subject each in both groups); elevated PSA levels (two subjects in the 6M group and one subject in the 3M group).
Figure 1.
Subjects' disposition. AE, adverse event; PSA, prostate-specific antigen.
The subjects' baseline characteristics are summarized in Table 1. The administration period of marketed leuprorelin acetate before the start of the study drug was similar between the two treatment groups. No major differences were also observed in other subject demographics between the two treatment groups.
Table 1.
Subjects' baseline characteristics
| TAP-144-SR (6M) (N = 82) |
TAP-144-SR (3M) (N = 80) |
Total (N = 162) |
|
|---|---|---|---|
| Age (years) (mean ± SD) | 73.3 ± 6.3 | 72.6 ± 6.5 | 73.0 ± 6.4 |
| BMI (kg/m2) (mean ± SD) | 23.6 ± 2.7 | 24.3 ± 2.8 | 24.0 ± 2.8 |
| Gleason score, n (%) | |||
| 5 | 2 (2.4) | 2 (2.5) | 4 (2.5) |
| 6 | 8 (9.8) | 7 (8.8) | 15 (9.3) |
| 7 | 35 (42.7) | 29 (36.3) | 64 (39.5) |
| 8 | 14 (17.1) | 21 (26.3) | 35 (21.6) |
| 9 | 20 (24.4) | 21 (26.3) | 41 (25.3) |
| 10 | 3 (3.7) | 0 | 3 (1.9) |
| Stage of TNM classification, n (%) | |||
| T | |||
| T1 | 17 (20.7) | 16 (20.3) | 33 (20.5) |
| T2 | 32 (39.0) | 24 (30.4) | 56 (34.8) |
| T3 | 29 (35.4) | 33 (41.8) | 62 (38.5) |
| T4 | 4 (4.9) | 6 (7.6) | 10 (6.2) |
| N | |||
| N0 | 69 (84.1) | 62 (78.5) | 131 (81.4) |
| N1 | 13 (15.9) | 17 (21.5) | 30 (18.6) |
| M | |||
| M0 | 63 (76.8) | 62 (78.5) | 125 (77.6) |
| M1 | 19 (23.2) | 17 (21.5) | 36 (22.4) |
| Serum levels | |||
| N | 81 | 79 | – |
| Testosterone (ng/dl) (mean ± SD) | 9.1 ± 8.5 | 13.3 ± 43.5 | – |
| PSA (ng/ml) (mean ± SD) | 0.9 ± 3.7 | 0.7 ± 3.0 | – |
| Administration period of marketed leuprorelin acetate before the start of the study drug (weeks) (mean ± SD) | 46.3 ± 20.1 | 46.6 ± 20.9 | 46.5 ± 20.4 |
| ECOG PS, n (%) | |||
| 0 | 73 (90.1) | 71 (89.9) | 144 (90.0) |
| 1 | 7 (8.6) | 7 (8.9) | 14 (8.8) |
| 2 | 1 (1.2) | 1 (1.3) | 2 (1.3) |
BMI, body mass index; PSA, prostate-specific antigen; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Hormone dynamics
Testosterone suppression rate
Serum testosterone of all subjects excluding one subject in the 3M group was suppressed to castrate level (≤100 ng/dl) throughout the 48 weeks in the FAS (Table 2). The estimated between-group difference in the suppression rate was 1.3% (two-sided 95% CI: −3.4, 6.8). The lower CI was more than −10% of the pre-determined allowable limit value. Therefore, non-inferiority of TAP-144-SR (6M) to TAP-144-SR (3M) was confirmed for the suppressive effect on serum testosterone level. One subject who did not maintain the castrate level in the 3M group did not meet the inclusion criterion. The serum testosterone level in this subject was 388 ng/dl before treatment with the study drug, 267 ng/dl after 1 week and 55 ng/dl after 2 weeks of treatment. For the sensitivity analysis, the same analysis that was applied to the primary analysis was utilized in the HAS as the secondary analysis. The results in both analysis sets were similar. In the case of the lower serum testosterone level (≤50 ng/dl), the suppression rate of serum testosterone throughout the 48 weeks (FAS) in the 6M group was 98.8% (80/81 subjects) and that in the 3M group was 98.7% (78/79 subjects). The estimated between-group difference in the suppression rate was 0.0% (two-sided 95% CI: −5.5, 5.7).
Table 2.
Rate of suppression of serum testosterone to castrate level (≤100 ng/dl)
| TAP-144-SR (6M) (N = 81) |
TAP-144-SR (3M) (N = 79) |
|
|---|---|---|
| Rate of suppression of serum testosterone (%) (95% CI) | 100.0 (95.5, 100.0) | 98.7 (93.1, 100.0) |
| TAP-144-SR (6M) − TAP-144-SR (3M) (95% CI) | 1.3 (−3.4, 6.8) | |
Changes in the hormone levels
The serum testosterone level before treatment was maintained throughout the study in both treatment groups. The median serum testosterone concentration in the 6M group and the 3M group, respectively, was 8.0 and 7.0 ng/dl before treatment, 7.0 and 6.0 ng/dl after 24 weeks of treatment and 9.0 and 6.0 ng/dl after 48 weeks of treatment (Fig. 2A).
Figure 2.
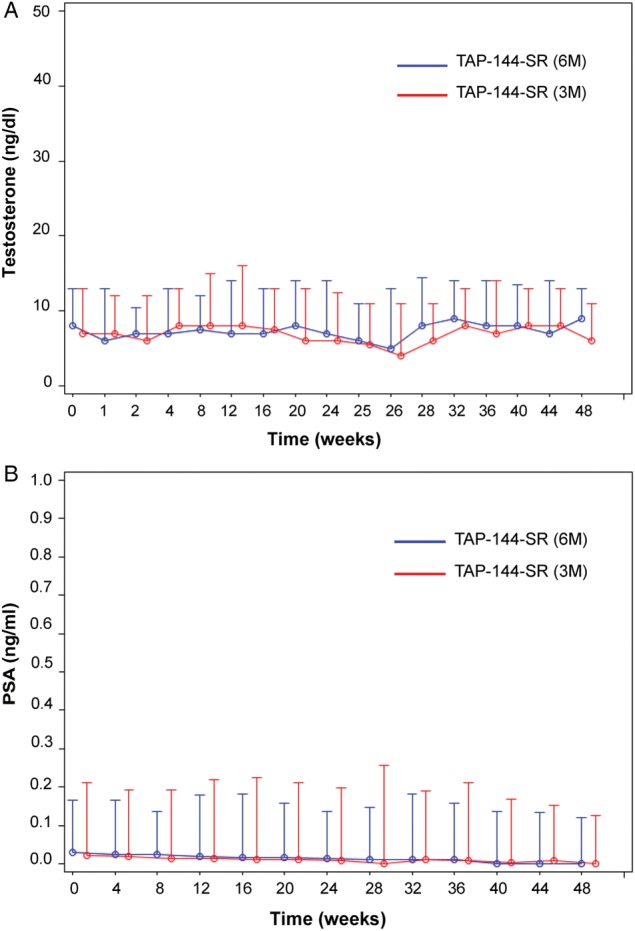
The median serum testosterone (A) and PSA (B) levels during the treatment period (48 weeks). Data are presented as the median and the 75th percentile.
There were no noteworthy changes in the LH and FSH levels throughout the study, compared with those before treatment with the study drugs. No significant differences were found in the LH and FSH levels between the two treatment groups.
Efficacy analysis
PSA concentrations
The changes in the serum PSA before treatment with the study drug throughout the study in both treatment groups were similar. The PSA concentrations were stable throughout the study in both treatment groups. The median serum PSA concentration in the 6M group and the 3M group, respectively, was 0.0300 and 0.0220 ng/ml before treatment, 0.0130 and 0.0095 ng/ml after 24 weeks of treatment and 0.0000 and 0.0000 ng/ml after 48 weeks of treatment (Fig. 2B).
There were six subjects (7.5%) in the 6M group and five subjects (6.5%) in the 3M group who had disease progression.
Anti-tumor effects
The best overall response based on the RECIST showed that most subjects were evaluated as Not Evaluable, (68/79 [86.1%] in the 6M group, and 65/76 [85.5%] in the 3M group). The rate of subjects with Progressive Disease in the 6M group was 0.0% (0/79 subjects) and that in the 3M group was 2.6% (2/76 subjects). In the 6M group and the 3M group, 13.9% of subjects (11/79 subjects) and 11.8% of subjects (9/76 subjects), respectively, had Stable Disease. No subjects were evaluated as Complete Response or Partial Response.
One subject in each treatment group had the newly observed bone lesions at two or more sites (6M group, 1.3% of subjects [1/78 subjects] and 3M group, 1.3% of subjects [1/75 subjects]) after 48 weeks of treatment with the study drugs.
Pharmacokinetics analysis
The profile of serum TAP-144 concentration after the initial administration of TAP-144-SR (6M) was similar to that after the second administration. The profile of serum TAP-144 concentration after the repeated administration of TAP-144-SR (3M) was also similar to that after the initial administration. No obvious accumulation was observed either with TAP-144-SR (6M) or TAP-144-SR (3M).
Safety analysis
AEs occurred in 75 subjects (92.6%) in the 6M group and 71 subjects (89.9%) in the 3M group. Although the most common AE was nasopharyngitis, most AEs were unrelated to the study drug (Table 3). Injection site reactions including induration, erythema and pain frequently occurred in both treatment groups. Most AEs were of Grade 1 or 2 in severity. AEs of ≥Grade 3 were reported in 11/81 subjects (13.6%) in the 6M group and 13/79 subjects (16.5%) in the 3M group. The serious AEs (SAEs) were reported in 10 subjects (12.3%) in the 6M group and 8 subjects (10.1%) in the 3M group. SAEs related to the study drug included one pulmonary infarction and one cerebral infarction in the 6M group and one peripheral arterial occlusive disease in the 3M group. There was one death of SAEs, which was unrelated to the study drug, in the 6M group. This death was due to metastatic gastric cancer with multiple liver metastases and it had been considered that the subject had gastric cancer when the subject participated in the study. AEs leading to discontinuation were reported in two subjects (2.5%) in the 6M group and three subjects (3.8%) in the 3M group.
Table 3.
Incidence of adverse events (AEs) (≥5% in any groups)
| Preferred Term | TAP-144-SR (6M) (N = 81) |
TAP-144-SR (3M) (N = 79) |
||
|---|---|---|---|---|
| Subjects with any AEs | 75 | (92.6) | 71 | (89.9) |
| Nasopharyngitis | 18 | (22.2) | 25 | (31.6) |
| Injection site induration | 14 | (17.3) | 11 | (13.9) |
| Injection site erythema | 12 | (14.8) | 6 | (7.6) |
| Blood creatine phosphokinase increased | 5 | (6.2) | 7 | (8.9) |
| Injection site pain | 6 | (7.4) | 5 | (6.3) |
| Arthralgia | 5 | (6.2) | 5 | (6.3) |
| Back pain | 2 | (2.5) | 8 | (10.1) |
| Hot flush | 5 | (6.2) | 5 | (6.3) |
| Fall | 6 | (7.4) | 3 | (3.8) |
| Constipation | 3 | (3.7) | 5 | (6.3) |
| DM | 5 | (6.2) | 3 | (3.8) |
| Hypertension | 5 | (6.2) | 3 | (3.8) |
| Insomnia | 3 | (3.7) | 5 | (6.3) |
| Contusion | 6 | (7.4) | 1 | (1.3) |
| Dental caries | 6 | (7.4) | 1 | (1.3) |
| Musculoskeletal stiffness | 2 | (2.5) | 4 | (5.1) |
| Weight increased | 1 | (1.2) | 5 | (6.3) |
| Inguinal hernia | 1 | (1.2) | 4 | (5.1) |
Values represent n (%).
Vital signs, clinical laboratory tests and electrocardiogram
The change in the diastolic/systolic blood pressure (mean ± SD) after 48 weeks of treatment with the study drug was −2.1 ± 10.4/−0.4 ± 16.6 mmHg in the 6M group and −1.6 ± 8.9/−3.9 ± 14.9 mmHg in the 3M group. The blood pressure levels in both groups were stable throughout the treatment period.
The changes in the glucose levels (mean ± SD) after 48 weeks of treatment with the study drug in the 6M group and the 3M group were −3.1 ± 23.0 and 2.5 ± 22.5 mg/dl, respectively. The differences in the changes of all other clinical laboratory tests between treatment groups were also unremarkable.
The changes in the Fridericia's correction of QT (QTcF) interval from prior to the administration of the study drug through 48 weeks of the treatment period remained almost the same in both treatment groups. The QTcF interval (mean ± SD) before treatment with TAP-144-SR (6M) was 429.0 ± 15.8 msec and the change in the QTcF interval after 48 weeks of treatment was −0.9 ± 12.2 msec. The QTcF interval (mean ± SD) before treatment with TAP-144-SR (3M) was 436.2 ± 15.6 msec and the change in the QTcF interval after 48 weeks of treatment was −4.6 ± 11.6 msec.
Discussion
The primary endpoint of this study was to verify the non-inferiority of TAP-144-SR (6M) compared with TAP-144-SR (3M) for the suppressive effect on serum testosterone. Since the lower CI of the between-group difference in the suppression rate was more than −10% of the pre-determined allowable limit value, the non-inferiority of TAP-144-SR (6M) to TAP-144-SR (3M) was confirmed for the suppressive effect on the serum testosterone level. The results of disease progression, as assessed by the PSA concentrations and anti-tumor effects in both treatment groups, were similar. Therefore, there were no noteworthy differences between TAP-144-SR (6M) and TAP-144-SR (3M) in the overall efficacy.
There is general consensus that it is critical to suppress testosterone level to the castrate level for the treatment of prostate cancer (6). In this study in which the suppression of testosterone was investigated in patients receiving hormone therapy, it is shown that the testosterone levels were suppressed to the castrate level of ≤100 ng/dl throughout the 48 weeks in both treatment groups, excluding one subject in the 3M group whose testosterone level was not suppressed at the time of study initiation (inclusion criterion deviation). When testosterone suppression was assessed at the lower level of ≤50 ng/dl in reference to the recent castration level (8,13–15), 1 subject in the 6M group had a testosterone level exceeding 50 ng/dl in addition to the above-mentioned subject in the 3M group. This subject in the 6M group had a transient increase in testosterone (53 ng/dl) after 8 weeks of treatment with the study drug. In the previous Phase II study in hormone therapy naive patients, it has been shown that no subjects had a testosterone level exceeding 50 ng/dl after 4 weeks in the 22.5 mg subcutaneous injection group (9). Taking into account the present and previous results, the 22.5 mg of TAP-144-SR (6M) reliably suppresses serum testosterone in prostate cancer patients.
The PSA concentrations were stable throughout the study and there were no differences in disease progression assessed by the PSA values between the two groups. The results of best overall response assessed by RECIST indicated Stable Disease in all evaluable subjects, excluding two subjects in the 3M group who had Progressive Disease. Therefore, the effects of TAP-144-SR (6M) were comparable to those of TAP-144-SR (3M).
The incidence rate of AEs was 92.6% in the 6M group and 89.9% in the 3M group (Table 3). The AEs reported in the 6M group were similar to those observed in the 3M group, and injection site reactions were frequent in both treatment groups. Injection site reactions have been reported in studies of degarelix, LH-RH antagonists, as well as leuprorelin acetate (8,13,20). In this study, a series of injection site reactions were reported in 30.9% of subjects in the 6M group and 22.8% of subjects in the 3M group, and the injection volume of both was 1 ml per injection. The injection site reaction in the 6M group was slightly greater than in the 3M group; however, none of the injection site reactions in the 6M group were severe. One event of injection site erythema was Grade 2 but the other events were all Grade 1 in the 6M group. One event of injection site ulcer in one subject was reported as Grade 3, one event of injection site induration and injection site erythema, respectively, in two different subjects were reported as Grade 2 and the others events were all Grade 1 in the 3M group. There were no injection site reactions that resulted in discontinuation of the study in the 6M group, but two subjects discontinued the study due to injection site reactions in the 3M group. In a study using degarelix, injection site reactions were reported in 46.3% (13), of which the initial injection volume was 3 ml per injection and the volume of the following maintenance injections was 4 ml per injection. Considering that both studies were conducted using subcutaneous injections, the frequency of injection site reactions might have been associated with the injection volume, because the study of degarelix, in which a higher volume than that of TAP-144-SR (6M) or TAP-144-SR (3M) in this study was used, showed a higher frequency of injection site reactions.
In the previous Phase II study, Grade 3 hypertension was reported in three out of six patients in the 22.5 mg intramuscular injection group (9). In this study with subcutaneous injections, hypertension was reported in five subjects (6.2%; Grade 3 in one subject and Grade 2 in four subjects) in the 6M group and in three subjects (3.8%; Grade 2 in all the subjects) in the 3M group, but no subjects discontinued the study due to hypertension. The results also showed that the changes in the mean systolic and diastolic blood pressure in the 6M group throughout the treatment period were slightly decreased, but no remarkable changes were observed. Since the same trend was observed in the 3M group, these results suggest that the effects of TAP-144-SR (6M) on the blood pressure are similar to those found with TAP-144-SR (3M).
It has been reported that androgen deprivation therapy increases insulin resistance and the risk of diabetes mellitus (DM) (8). DM was observed in six subjects (7.4%, Grade 2 in all subjects; five subjects coded DM and one subject coded Type 2 DM) in the 6M group and three subjects (3.8%, Grade 2 in all subjects) in the 3M group, but none of these subjects discontinued the study due to DM. The glucose levels in the 6M group and the 3M group hardly changed throughout the study. These results indicate that there are no significant differences between TAP-144-SR (6M) and TAP-144-SR (3M) concerning the risk of aggravation or the onset of DM and an increase in insulin resistance.
It has been shown that the QTcF interval was prolonged for longer than 10 msec after 24 weeks of treatment with TAP-144-SR (6M) in treatment-naive prostate cancer patients in the previous Phase II study (9). Similar prolongation with leuprolide acetate was reported in another study, and the results showed that the change in the QTcF interval from baseline was 14.0 msec after 12 months in the leuprolide 7.5 mg group (14,21). However, in this study, the QTcF interval did not fluctuated much throughout the 48 weeks of the treatment period in subjects receiving hormone therapy in both treatment groups. Therefore, reduced testosterone, rather than a direct pharmacological effect of leuprolide acetate, could have caused the prolongation of the QTcF interval because prolongation of the QTcF interval was not observed in subjects in whom the serum testosterone had already been suppressed to the castrate level. This hypothesis is supported by the fact that the QTcF prolongation with degarelix was also reported in hormonal therapy-naive prostate cancer patients, and the change was 13.0 msec after 12 months (14,21), and some reports that indicate that the changes in the testosterone level would be related with prolongation of the QTcF interval (21–23).
TAP-144-SR (6M) can reduce the frequency of injections, and could thus reduce the burden on patients and medical staff. However, the intervals between visits might be much longer, and medical practitioners and staff need to keep reminding patients of the next appointment to visit the hospital.
The pharmacokinetic profile of TAP-144-SR (6M) in the Phase II study exhibited a double peak (9), which was different from that of TAP-144-SR (3M), indicating that TAP-144-SR (6M) was sustainably released for 24 weeks. In this study, there were no significant differences in efficacy and safety between TAP-144-SR (6M) and TAP-144-SR (3M) in prostate cancer patients, and no safety concerns were observed. TAP-144-SR (6M) was therefore confirmed to have excellent usability for the treatment of prostate cancer.
Conclusion
In conclusion, our results indicate that TAP-144-SR (6M) is not inferior to TAP-144-SR (3M) for the suppressive effect on serum testosterone level. The efficacy of TAP-144-SR (6M) was comparable to that of TAP-144-SR (3M), and TAP-144-SR (6M) was as well tolerated as TAP-144-SR (3M). Since TAP-144-SR (6M) can reduce the frequency of injections and the burden on patients and medical staff, and provide an option as a new administration method in the clinical setting, it is expected to become widely applied to patients depending on the patients' medical condition and life cycle.
Funding
This work was supported by Takeda Pharmaceutical Company Limited. Funding to pay the Open Access publication charges for this article was provided by Takeda Pharmaceutical Company Limited.
Conflict of interest statement
Tsukasa Fujimoto, Nobuyoshi Takabayashi and Kentarou Kudou are employees of Takeda Pharmaceutical Company Limited. Kazuhiro Suzuki and Mikio Namiki are paid by Takeda Pharmaceutical Company Limited for research grant and honoraria. Hideyuki Akaza is paid by Takeda Pharmaceutical Company Limited, Astellas Pharma Inc. and Johnson & Johnson K.K.
Acknowledgements
We are grateful to all the patients, investigators and clinical research coordinators who participated in this study. The study centers were as follows: Hokkaido Memorial Hospital of Urology, Hokkaido Cancer Center, Tohoku University Hospital, Gunma University Hospital, Ageo Central General Hospital, Saitama Cancer Center, Chiba Cancer Center, Keio University Hospital, Teikyo University Hospital, Yokohama City University Medical Center, Yokohama City University Hospital, Japan Labour Health and Welfare Organization Yokohama Rosai Hospital, Niigata Cancer Center Hospital, Niigata City General Hospital, Kanazawa University Hospital, Shizuoka General Hospital, Nagoya University Hospital, Osaka University Hospital, Osaka City University Hospital, Osaka Medical Center for Cancer and Cardiovascular Diseases, Kyushu University Hospital, Hara Sanshin Hospital and National Hospital Organization Kyushu Cancer Center. Editorial assistance in the preparation of this manuscript was provided by WysiWyg Co., Ltd., and financial support by Takeda Pharmaceutical Company Ltd.; the authors retained editorial control over the content.
References
- 1.Hinotsu S. An international comparison of epidemiologic factors of prostate cancer. Nihon Rinsho 2011;69(Suppl 5):187–92 (in Japanese). [PubMed] [Google Scholar]
- 2.Katanoda K, Matsuda T, Matsuda A et al. . An updated report of the trends in cancer incidence and mortality in Japan. Jpn J Clin Oncol 2013;43:492–507. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Information Service, National Cancer Center, Japan. Tokyo: Cancer statistics in Japan http://ganjoho.jp/public/statistics/pub/statistics01.html (9 June 2015, date last accessed; in Japanese).
- 4.Sobue T, Katanoda K, Ajiki W, Tsukuma H, Ioka A. White paper on cancer statistics in 2012. Tokyo: Shinoharashinsha Publishers Inc, 2012. (in Japanese). [Google Scholar]
- 5.Kanda T, Jiang X, Yokosuka O. Androgen receptor signaling in hepatocellular carcinoma and pancreatic cancers. World J Gastroenterol 2014;20:9229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Japanese Urological Association. Practice guideline for prostate cancer in 2012. Tokyo: Kanehara & Co., Ltd, 2012. (in Japanese). [Google Scholar]
- 7.Tunn UW, Gruca D, Bacher P. Six-month leuprorelin acetate depot formulations in advanced prostate cancer: a clinical evaluation. Clin Interv Aging 2013;8:457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spitz A, Young JM, Larsen L, Mattia-Goldberg C, Donnelly J, Chwalisz K. Efficacy and safety of leuprolide acetate 6-month depot for suppression of testosterone in patients with prostate cancer. Prostate Cancer Prostatic Dis 2012;15:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komura E, Fujimoto T, Takabayashi N, Okamoto H, Akaza H. A phase II pharmacological study of leuprolide acetate 6-month depot, TAP-144-SR (6M), in treatment-naive patients with prostatic cancer who received a single subcutaneous or intramuscular injection. Gan To Kagaku Ryoho 2014;41:587–93. [PubMed] [Google Scholar]
- 10.Niijima T, Aso Y, Akaza H et al. . Clinical phase I and phase II study on a sustained release formulation of leuprorelin acetate (TAP-144-SR), an LH-RH agonist, in patients with prostatic carcinoma. Collaborative Studies on Prostatic Carcinoma by the Study Group for TAP-144-SR. Hinyokika Kiyo 1990;36:1343–60 (in Japanese). [PubMed] [Google Scholar]
- 11.Koiso K, Yamanaka H, Ito K, Yoshinaka R, Uchida S, Yokokawa K. Clinical effects of a 3-month formulation LH-RH agonist, TAP-144-SR (3M) in prostate cancer patients. Hinyokika Kiyo 2002;48:771–9 (in Japanese). [PubMed] [Google Scholar]
- 12.Koiso K, Akaza H, Naito S et al. . Clinical effects of a 3-month formulation LH-RH agonist, TAP-144-SR (3M) in prostate cancer patients. Hinyokika Kiyo 2002;48:781–95 (in Japanese). [PubMed] [Google Scholar]
- 13.Ozono S, Ueda T, Hoshi S et al. . The efficacy and safety of degarelix, a GnRH antagonist: a 12-month, multicentre, randomized, maintenance dose-finding phase II study in Japanese patients with prostate cancer. Jpn J Clin Oncol 2012;42:477–84. [DOI] [PubMed] [Google Scholar]
- 14.Klotz L, Boccon-Gibod L, Shore ND et al. . The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int 2008;102:1531–8. [DOI] [PubMed] [Google Scholar]
- 15.Translational Research Informatics Center. Kobe: National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 2. 2014. http://202.70.140.4/nccn/guideline/urological/english/prostate.pdf (9 June 2015, date last accessed).
- 16.The Japanese Urological Association, The Japanese Society of Pathology, Japan Radiological Society. General Rule for Clinical and Pathological Studies on Prostate Cancer. 4th edn Tokyo: Kanehara & Co., Ltd., 2010. (in Japanese). [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 18.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med 1998;17:873–90. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Maryland: Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (9 June 2015, date last accessed).
- 20.Tunn UW, Wiedey K. Safety and clinical efficacy of a new 6-month depot formulation of leuprorelin acetate in patients with prostate cancer in Europe. Prostate Cancer Prostatic Dis 2009;12:83–7. [DOI] [PubMed] [Google Scholar]
- 21.Smith MR, Klotz L, Persson BE, Olesen TK, Wilde AA. Cardiovascular safety of degarelix: results from a 12-month, comparative, randomized, open label, parallel group phase III trial in patients with prostate cancer. J Urol 2010;184:2313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charbit B, Christin-Maître S, Démolis JL, Soustre E, Young J, Funck-Brentano C. Effects of testosterone on ventricular repolarization in hypogonadic men. Am J Cardiol 2009;103:887–90. [DOI] [PubMed] [Google Scholar]
- 23.Rautaharju PM, Zhou SH, Wong S et al. . Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol 1992;8:690–5. [PubMed] [Google Scholar]