Abstract
Wound healing in mammals is a fibrotic process. The mechanisms driving fibrotic (as opposed to regenerative) repair are poorly understood. Herein we report that therapeutic Wnt inhibition with topical application of small-molecule Wnt inhibitors can reduce fibrosis and promote regenerative cutaneous wound repair. In the naturally stented model of ear punch injury, we found that Wnt/β-catenin pathway is activated most notably in the dermis of the wound bed early (d 2) after injury and subsides to baseline levels by d10. Topical application of either of 2 mechanistically distinct small-molecule Wnt pathway inhibitors (a tankyrase inhibitor, XAV-939, and the U.S. Food and Drug Administration–approved casein kinase activator, pyrvinium) in C57Bl/6J mice resulted in significantly increased rates of wound closure (72.3 ± 14.7% with XAV-939; and 52.1 ± 20.9% with pyrvinium) compared with contralateral controls (38.1 ± 23.0 and 40.4.±16.7%, respectively). Histologically, Wnt inhibition reduced fibrosis as measured by α-smooth muscle actin positive myofibroblasts and collagen type I α1 synthesis. Wnt inhibition also restored skin architecture including adnexal structures in ear wounds and dermal–epidermal junction with rete pegs in excisional wounds. Additionally, in ear punch injury Wnt inhibitor treatment enabled regeneration of auricular cartilage. Our study shows that pharmacologic Wnt inhibition holds therapeutic utility for regenerative repair of cutaneous wounds.—Bastakoty, D., Saraswati, S., Cates, J., Lee, E., Nanney, L. B., Young, P. P. Inhibition of Wnt/β-catenin pathway promotes regenerative repair of cutaneous and cartilage injury.
Keywords: wound healing, scar, skin, hair follicle
Acute wounds related to trauma, surgery, or burns are a major medical problem, driving approximately 11 million emergency room visits every year in the United States (1). Repair of these wounds leads to persistent scarring causing long-term pain, discomfort, or disability. Because mammalian skin does not possess an ability to regenerate following cutaneous injury, wounds often heal with a residual fibrotic scar devoid of adnexal structures (2). Therapeutic interventions to effectively direct wound healing toward regenerative rather than the default fibrotic mode are lacking.
Regenerative repair of cutaneous wounds has been observed in some healer strains or species of mice such as MRL/MpJ (3–5) or the African spiny mice, Acomys (5). There is a poor understanding of the mechanisms driving this enhanced regeneration, although certain signals such as p21 signaling (6) or processes such as blastema formation (5, 6) or inflammation (7) have been attributed to enhanced regeneration.
Our goal in this study was to identify signaling pathways that are mediators of regeneration and that can be modulated therapeutically to achieve regenerative repair with minimal scarring. Based on previous studies from our group (8, 9) and others (10–13) of the role of Wnt/β-catenin pathway in multiple adult injury models, we focused our attention on the Wnt signaling pathway. We have shown that that the mesenchymal stem cells derived from the super healer MRL/MpJ mice have elevated expression of the secreted Wnt inhibitor secreted frizzled receptor protein 2 (sFRP2) and that overexpression of sFRP2 in mesenchymal stem cells derived from C57Bl/6J mice enhances their regenerative potential in cell therapy for cutaneous and cardiac injury (8). Subsequent studies using small-molecule Wnt inhibitor recapitulated these findings in both injury models (9, 14). There is extensive literature suggesting a role for the Wnt/β-catenin pathway in promoting fibrosis in multiple injury models (12, 13, 15) including cutaneous injury (16, 17). Mutations in humans resulting in activation of the Wnt/β-catenin pathway cause fibromatoses that arise from overproliferation of fibroblasts (18, 19).
However, in cutaneous injury, Wnt pathway activity is linked with regeneration (20), particularly regeneration of hair follicles (21–23). Most of these studies are based on genetic models of Wnt pathway driven by epidermal or hair follicle-specific promoters (24, 25) and present a folliculocentric story. Because dermal signals are important components of the wound healing response (26), these genetic models may not provide a complete picture of the effects of Wnt signaling in cutaneous wound healing. Indeed, the studies that have employed conditional β-catenin stabilization spanning both the dermis and the epidermis have reported that Wnt/β-catenin signals promote fibrosis and increase in wound size (15, 27). We sought to reconcile these contrasting observations regarding the role of dermal or epidermal Wnt/β-catenin signals in the context of wound therapy using small-molecule Wnt antagonists.
We utilized 2 distinct wound models for our study—full-thickness excisional wound on the backs of C57Bl/6J mice, and the through-and-through ear punch injury model. The full-thickness injury model, which is a widely used model of cutaneous wound in mice, was used for investigating the effect of Wnt inhibitor treatment on regenerative vs. scarred repair. However, this model presents limitations of extensive wound contraction and rapid closure observed in mice but not recapitulated in human wounds. Hence, we utilized the naturally stented model of ear punch injury to minimize the effect of wound contraction and additionally to allow more accurate quantification of wound closure and investigation of regeneration of complex subdermal structures such as cartilage.
MATERIALS AND METHODS
Antibodies
The following antibodies were used: β-catenin (1:200; BD Pharmingen, San Diego, CA, USA); β-galactosidase (1:100, Ab616; Abcam Inc., Cambridge, MA, USA); cytokeratin (Krt)15 (1:100, Ab52816; AbCam); Krt17 (1:1000, Ab53707; AbCam); Sox9 (1:1000, AB5535; EMD Millipore, Billerica, MA, USA); proliferating cell nuclear antigen (1:100, SC-56; Santa Cruz Biotechnology, Santa Cruz, CA, USA); α-SMA (1:1000, A2547; Sigma-Aldrich, MO, USA); collagen type IIC1 (1:100; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA); collagen type X (1:100; Developmental Studies Hybridoma Bank).
Wnt modulators
The small-molecule Wnt inhibitors (CK1α activators) pyrvinium (Pyr) and VU-WS113 (C-113), as well as the nonfunctional analog of the 2 drugs, VU-WS211 (211), were generous gifts from Dr. Ethan Lee (Vanderbilt University, Nashville, TN, USA) (28). XAV-939 (29), a small-molecule stabilizer of axin2 was purchased from Selleck Chemicals (S1180; Houston, TX, USA). LiCl (203637; Sigma-Aldrich) at 100 mM in PBS was used for Wnt activation.
Animals
All procedures were carried out in accordance with Vanderbilt Institutional Animal Care and Use Committee. C57Bl/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained by PPY. Tcf optimal promoter β-galactosidase (TOPGAL) (30) mice were a generous gift from Dr. Antonis Hatzopoulos (Vanderbilt University).
Mouse ear punch model
C57Bl/6J mice (at least 3 mo of age) were anesthetized with 3–5% isoflurane in O2 administered using Table Top Laboratory Animal Anesthesia System (VetEquip Inc., Pleasanton, CA, USA). A 2 mm biopsy punch wound was made in the center of the cartilaginous region of each ear using a disposable biopsy punch (Acuderm Inc., Fort Lauderdale, FL, USA) as described previously (31). The ears were treated topically with 5 µl/ear of drug (1 µM Pyr, 10 µM C-113, 5 µM XAV-939, or 100 mM LiCl) or control solution (1 µM 211, DMSO or PBS, respectively) every day. The ears were imaged at 30 d using a Nikon (Tokyo, Japan) Coolpix 8700 digital camera. For quantification of wound closure, ears were excised after the mice were sacrificed, placed on microscope slides (Denville Scientific, South Plainfield, NJ, USA) and imaged using Nikon Coolpix 8700 affixed to Micromaster Inverted microscope (Thermo Fisher Scientific, Waltham, MA, USA). Wound closure in the images was quantified by measuring the surface are of the hole using ImageJ [U.S. National Institutes of Health (NIH), Bethesda, MD, USA] and calculating percent difference compared with original ear punch area. For longitudinal study of Wnt activation, mice were sacrificed 2, 3, 4, 5, or 10 d after injury. The ears were either paraffin embedded for histology or used for RNA isolation as described in the following sections.
Dermal full-thickness injury model
C57Bl/6J mice (at least 3 mo of age) were anesthetized with 3–5% isoflurane in O2 administered using Table Top Laboratory Animal Anesthesia System (VetEquip Incorporated). The backs of mice were shaved and disinfected by applying betadine followed by 70% ethanol wipe 3 times. An excision (1 × 1 cm) was made on the back of the mice using scalpel, and skin was removed by using surgical scissors to separate the skin from the underlying muscle. The wound was covered with tegaderm after topical application of 1 µM Pyr or 10 µM 211 (control). The treatments were continued daily for 2 wk following injury. At 30 d, the healed tissue was excised and embedded in paraffin for histology.
Histology and morphometry
Excised ears or skin were fixed for 24 h in 10% buffered formalin, cut longitudinally across the injury and embedded in paraffin blocks. Slides (5 µm) were stained with hematoxylin and eosin (H&E) and Trichrome blue by the Vanderbilt Translational Pathology Shared Resource and imaged using an Olympus DP71 microscope camera (Olympus America, Center Valley, PA, USA). Number of regenerated hair follicles originating beyond the wound margin (defined as the leading edge of healthy pre-existing auricular cartilage) was quantified using ×20 images of H&E-stained slides. For picrosirius red staining, nuclei were stained with Weigert’s hematoxylin, followed by picrosirius red stain overnight. Excess stain was washed off in acidified water; slides were then dehydrated and mounted in resinous medium. Toluidine blue staining was performed as previously described (31). In brief, deparaffinized slides were covered in 0.5% Toluidine blue solution prepared in 0.1 M Na CH3CO2− buffer (pH 2.5) for 2 h at room temperature. The slides were then rinsed in water, subjected to 2 quick dips each in 95% ethanol and xylene, and mounted with Permount (Thermo Fisher Scientific). For Safranin-O and Fast Green staining, deparaffinized and hydrated slides were stained with Wiegert’s iron hematoxylin working solution for 10 min, washed in running tap water, stained with fast green for 5 min, rinsed for 15 s with 1% acetic acid solution, stained with 0.1% Safranin-O solution for 5 min, dehydrated and cleared with 95 and 100% ethanol followed by xylene. Images of sections were taken at ×20–40 magnification using an Olympus DP71 microscope camera. Cartilage regeneration was quantified using ImageJ to measure the distance from the wound margin to the leading edge of newly laid down cartilage matrix (stained purple with toluidine blue). Collagen fiber directional variance (as an indicator of scarring) was quantified using OrientationJ plug-in for ImageJ as described previously (32, 33). In brief, the fiber alignment directional variance was calculated from the local angle distribution in Trichrome blue stained ×20 images at each pixel using coherency-weighted alignment.
For immunofluorescence staining, slides were deparaffinized and hydrated through xylene and ethanol steps. Heat-mediated antigen retrieval was performed by boiling in citrate buffer (pH 6). Following appropriate blocking and primary (overnight) and secondary antibody incubation steps, the slides were counterstained with Hoechst dye 33342 (H21492; Invitrogen, Carlsbad, CA, USA) and mounted with Slowfade Gold (S36936; Life Technologies, Grand Island, NY, USA). Images were taken at ×10, ×20, or ×40 magnification using Axio Imager2 microscope (Carl Zeiss, Thornwood, NY, USA) and CoolSNAP HQ CCD camera (Photometrics, Tuscon, AZ, USA), and quantified using ImageJ.
RNA isolation and Wnt PCR Array
Two holes per ear were made in cartilaginous region of mouse ears using 2 mm biopsy punch. At 2, 5, or 10 d after injury, 1 mm of tissue surrounding the holes was excised and RNA was isolated using RNeasy Mini Kit (Qiagen, Germantown, MD, USA). First-strand DNA synthesis was performed with 1 µg RNA using iScript cDNA synthesis kit (170-8890; Bio-Rad, Hercules, CA, USA). Gene expression of 84 Wnt pathway genes was determined using the Mouse WNT Signaling Pathway RT2 Profiler PCR Array (PAMM-043Z; SABioscience, Valencia, CA, USA). To verify the expression of select genes, quantitative real-time PCR (qRT-PCR) was performed in triplicate for each sample with iCycler (Bio-Rad) and fluorescence detection (SsoFast EvaGreen; 172-5200; Bio-Rad). Each reaction was normalized against 18S. Primer sequences are as shown in Table 1.
TABLE 1.
List of qRT-PCR primers
| Gene | Forward | Reverse | Product size (bp) |
|---|---|---|---|
| cMyc | ATCGTCGTGGCTGTCGGGGT | TGCCCGCGATCAGCTCTCCT | 92 |
| CyclinD1 | CTGGTGTTTGGAAGTAGGAA | CTGGTGTTTGGAAGTAGGAA | 89 |
| Dkk1 | CGAAGTTGAGGTTCCGCAGTCC | CGAAGTTGGGTTCCGCAGTCC | 92 |
| MMP-7 | AGGAAGCTGGAGATGTGAGC | TCTGCATTTCCTTGAGGTTG | 126 |
| sFRP2 | ATGGAAACCCTTTGTAAAAATGACT | TCTTGCTCTTTGTCTCCAGGATGAT | 102 |
| Wnt3a | GCACCACCGTCAGCAACAGC | CAGGAGCGTGTCACTGCGAAAG | 121 |
| Col1a1 | GCCAGATGGGTCCCCGAGGT | GGGGGTCCAGCAGCACCAAC | 106 |
| Col 3 | GAAAAAACCCTGCTCGGAATT | GGATCAACCCAGTATTCTCCACTCT | 78 |
| α-SMA | CAGGCATGGATGGCATCAATCAC | ACTCTAGCTGTGAAGTCAGTGTCG | 154 |
| IL-6 | CCGGAGAGGAGACTTCACAG | TCCACGATTTCCCAGAGAAC | 102 |
Statistical analysis
The statistical significance between experimental and control groups were determined by Student’s t test (unpaired analysis unless otherwise noted in the figure legends), using GraphPad Prism (San Diego, CA, USA). P < 0.05 was considered statistically significant in 2-tailed hypothesis tests.
RESULTS
Wnt/β-catenin pathway is activated temporally in the injury region after ear punch
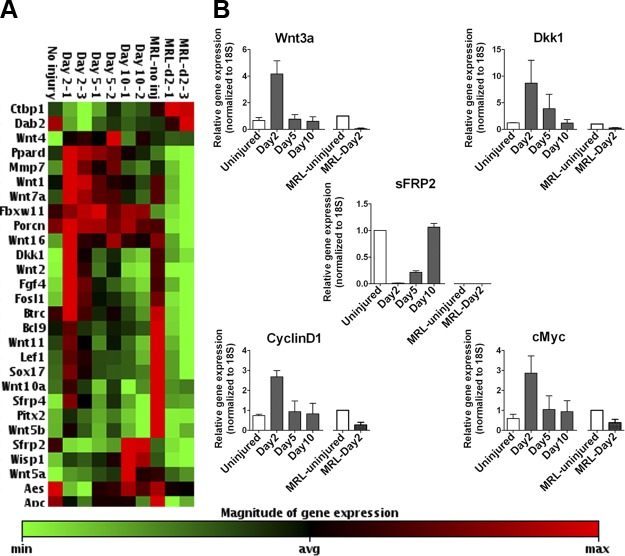
Wnt pathway activation is reported in full-thickness excisional wounds (15). We tested Wnt pathway activity in through-by-through wounds made with a 2 mm biopsy punch in the central cartilaginous region of mouse ear pinnae. We used Wnt pathway RT-PCR array (SA Biosciences, Valencia, CA) to test the expression of Wnt/β-catenin pathway-related genes in the wound bed (shown in Supplemental Fig. 1A) after injury and found that the pathway is activated in the wound tissue between d 2 and 5 after injury in C57Bl/6J mice (Fig. 1A). qRT-PCR validation for Wnt ligand (Wnt3a), Wnt/β-catenin direct target genes (Dkk1, CyclinD1, and cMyc) and secreted Wnt inhibitor (sFRP2) corroborated the PCR array data (Fig. 1B). In contrast, the superhealer MRL mice, that are reported to completely close full-thickness ear punch wounds (4), showed a significant decrease in expression of Wnt pathway agonists and target genes at d 2 after injury compared with uninjured control as indicated by PCR array (Fig. 1A) and qRT-PCR (Fig. 1B). This was in agreement with our previous report that increased expression of the secreted Wnt inhibitor sFRP2 contributes to the enhanced reparative potential of mesenchymal stem cells derived from MRL mice (8). To our knowledge, a link between low Wnt pathway activity and regenerative repair in the MRL mice has not been reported (34).
Figure 1.
Wnt signaling is activated in cutaneous wounds after injury. A) Heat map generated from gene expression array of Wnt pathway-associated genes. B) qRT-PCR validation of change in expression of Wnt agonists (Wnt3a), and Wnt direct transcriptional targets (Dkk1, CyclinD1, and c-Myc), and Wnt inhibitor (sFRP2). MRL-uninjured: n = 1; all other groups: n ≥ 3 per group.
Wnt/β-catenin pathway activation in response to wounding occurs primarily in the dermis
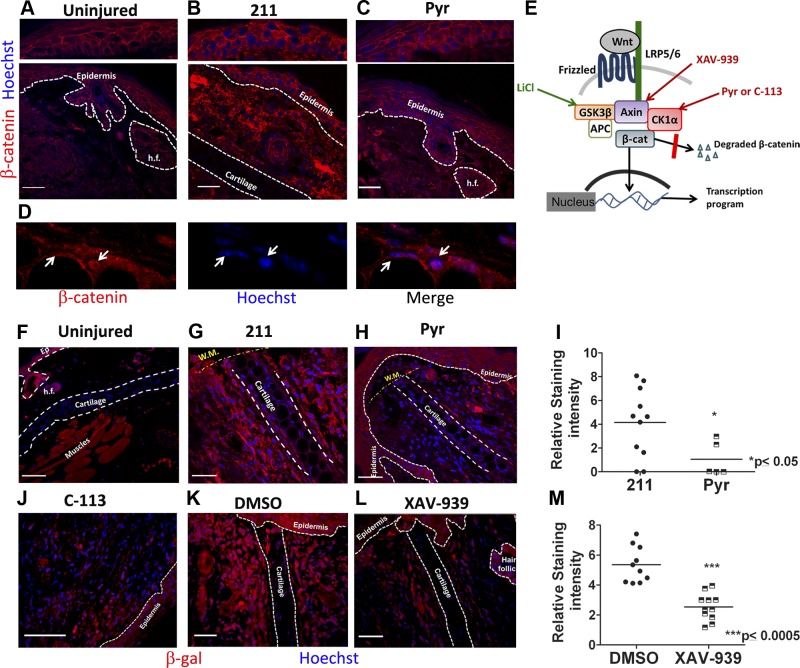
To investigate the nature of Wnt activation observed after injury, we performed immunostaining in the wound bed tissue (schematic in Supplemental Fig. 1B–G) for β-catenin in C57Bl/6J mice and for β-galactosidase protein in the Wnt reporter, TOPGAL mice. We found detectable Wnt pathway activation compared with uninjured control, specifically in the wound dermis, with both β-catenin (Fig. 2A, B; nuclear localization highlighted in Fig. 2D and Supplemental Fig. 3E, F) and β-galactosidase immunostaining (Fig. 2F, G, and K and Supplemental Fig. 3A, B). High-magnification imaging of β-galactosidase-stained sections indicated that perichondrocytes flanking the cartilage near the wound margin, and elongated fibroblast-like cells were among the Wnt-responsive dermal cells early after injury (Supplemental Fig. 3D). The baseline Wnt activity in the epidermis did not appear to change significantly in response to injury as detectable by immunofluorescence for β-catenin (Fig. 2A, B) and β-galactosidase (Supplemental Fig. 3A, B). Consistently, daily topical treatment with distinct Wnt inhibitors (Fig. 2E) caused a reduction in staining intensity of β-catenin (Fig. 2B, C) and total β-galactosidase (in TOPGAL mice) (Fig. 2G–M and Supplemental Fig. 3B, C) proteins, most notably in the dermis.
Figure 2.
Wnt pathway activation and inhibition occurs specifically in the dermis of wound bed. A) Immunostaining for β-catenin (red) on uninjured (A), 211-treated (B), and Pyr-treated (C) tissue sections showing Wnt activation in the dermis (B) compared with uninjured control (A), and inhibition by Pyr treatment (C). Top panels represent relatively undetectable change in epidermal β-catenin levels in response to injury and to treatment. D) High-magnification image of injured dermal tissue highlighting nuclear localization of β-catenin (white arrows). E) Schematic of the Wnt/β-catenin pathway showing target molecules activated or stabilized by Wnt agonist (LiCl) or antagonists (Pyr, C-113 and XAV-939) used in the study. F–L) β-galactosidase immunostaining in TOPGAL mice that express LacZ gene under the Wnt-responsive TCF promoter showing dermal (and not epidermal) Wnt activation in 211 (G), and DMSO-treated (K) sections compared with uninjured (F). Treatment with Wnt inhibitors Pyr (H), C-113 (J), and XAV-939 (L) reduced dermal β-galactosidase staining intensity; nuclei are counterstained blue with Hoechst dye. I, M) Quantification of β-gal protein expression, measured as integrated pixel density in ×20 images by ImageJ. n ≥ 3 mice per group. Scale bars, 50 µm.
Wnt inhibition and not off-target effects promotes closure of ear punch wound
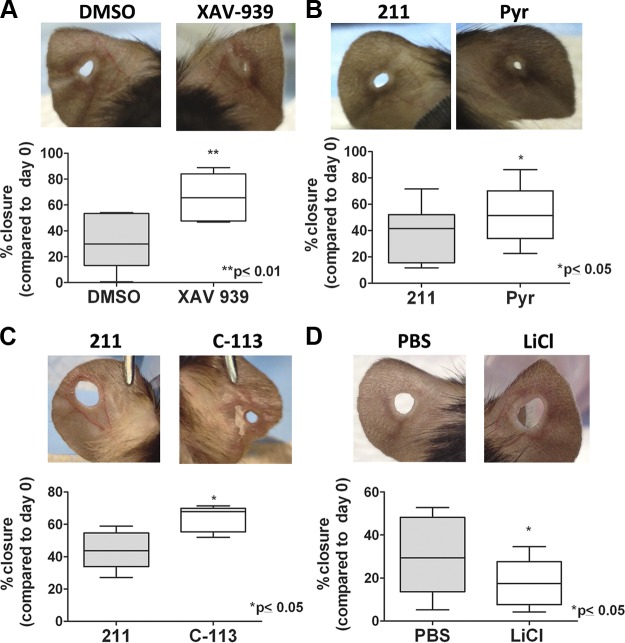
To investigate the effect of Wnt pathway on wound healing, we treated ear punch wounds topically with small-molecule Wnt inhibitors every day after injury for 30 d. To minimize “off-target” effects, we used well-characterized (28, 29) small molecules that are structurally distinct and that inhibit the Wnt/β-catenin pathway through distinct mechanisms of action (Fig. 2E). XAV-939 is a commercially available tankyrase inhibitor, which stabilizes axin (29). Pyr and its structural analog, VU-WS113 (C-113), activate casein kinase1α with different potencies (28). Despite having 2 distinct structures and modes of action (tankyrase inhibition vs. casein kinase1 activation), all 3 Wnt inhibitors promoted wound closure, reduced fibrosis, and enhanced regeneration (as will be discussed in subsequent sections) indicating that their effect was a result of Wnt pathway inhibition and not off-target effects.
At d 30 postinjury, ear punch wounds treated with Wnt inhibitors displayed a significantly greater degree of closure (XAV-939: 72.3 ± 14.7%; Pyr: 52.1 ± 20.9%; and C-113: 63.7 ± 8.1%) than their respective contralateral controls [DMSO: 38.1 ± 23.0%; and VU-WS211 (referred to as 211 hereafter), an inactive analog of Pyr: 40.4 ± 16.7%] (Fig. 3A–C). Conversely, treatment with lithium chloride, which activates the Wnt pathway (35), inhibited wound closure compared with vehicle (PBS)-treated wounds (LiCl: 13.3 ± 6.3%; PBS: 25.1 ± 13.8%; Fig. 3D). We did not see enhanced wound closure when the treatment was started 7 d after injury, or alternatively, started at day 0 and continued only until 7 d after injury indicating a need for treatment during the full 30 d period (Supplemental Fig. 5).
Figure 3.
Wnt inhibitor treatment promotes closure of ear punch wounds. Images of 2 mm ear punch holes treated topically for 30 d with (A) small-molecule Wnt inhibitor XAV-939 (right panel) or contralateral control treated with vehicle only (DMSO; left panel); (B) small-molecule Wnt inhibitor Pyr (right panel) or a nonfunctional analog, compound 211 (left panel); (C) small-molecule Wnt inhibitor, analog of Pyr (C-113; right panel) or 211 (left panel); and (D) Wnt activator LiCl (right panel) or vehicle (PBS; left panel). The histograms underneath the panels represent quantification of percent closure of ear punches at d 30. Bars represent means ± sd. *P ≤ 0.05 and **P ≤ 0.01 were calculated using paired Student's t test with (A) n = 10, (B) n = 6 per group, (C, D) n = 5 per group.
Wnt/β-catenin pathway inhibition promotes regenerative repair and reduces fibrosis
An essential aspect of regenerative skin repair is controlling fibrosis and recapitulating the properties of normal skin. We investigated the effect of Wnt inhibitor treatment on this aspect of repair using both full-thickness excisional wound model on backs of mice and ear punch injury model.
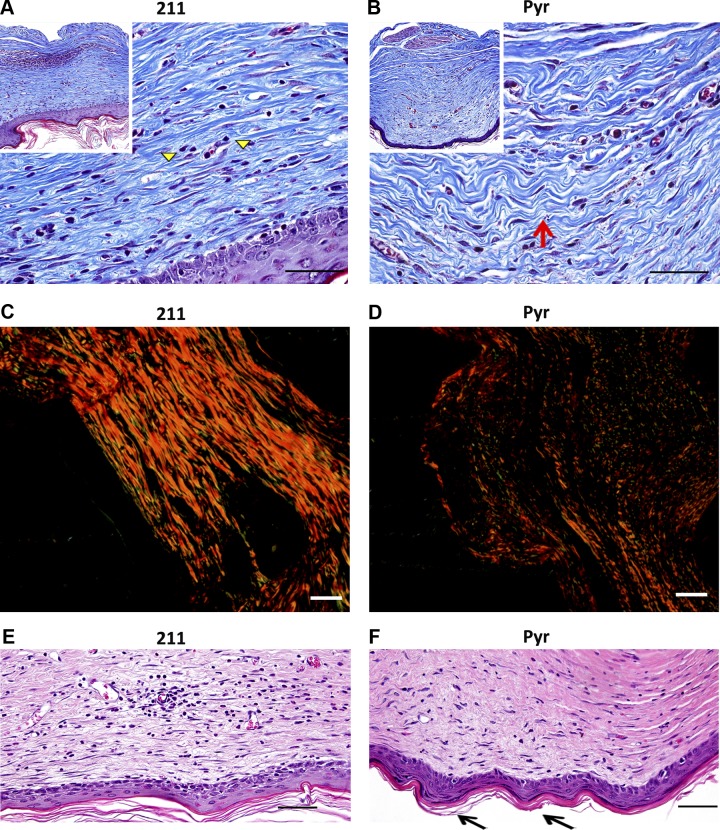
In 1 × 1 cm full-thickness wounds on the back of C57Bl/6J mice, daily topical treatment with Pyr promoted resolution of scar and restoration of reticular collagen lattice closer in arrangement to normal skin compared with 211 control as detected by Gomori trichrome blue staining (Fig. 4A, B) and by picrosirius red staining observed under circularly polarized light (Fig. 4C, D). Dermal–epidermal junction with rete pegs and dermal papillae, indicative of a more regenerative healing (36), were also observed in Pyr- treated skin and not in 211-treated skin (Fig. 4E, F).
Figure 4.
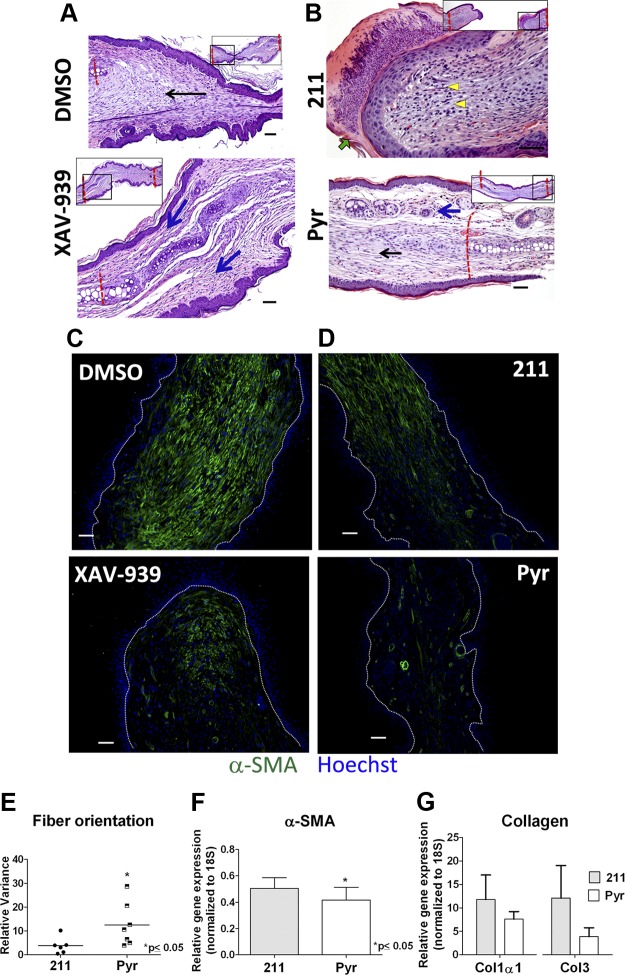
Wnt inhibition promotes scar resolution and rete ridges formation in healing skin. A, B) Gomori trichrome stained sections of skin from full-thickness excisional wound on backs of mice showing more linear (scarlike) extracellular matrix in (A) 211-treated sections (marked by yellow arrowheads), and more reticular collagen and organized extracellular matrix in (B) Pyr-treated sections (marked by red arrow). C, D) Picrosirius red-stained sections imaged with circularly polarized light showing difference in fiber thickness and alignment between 211-treated (C) and Pyr-treated (D) tissue. E–F) H&E-stained sections show increased rete ridge formation (black arrow) in Pyr-treated (F) compared with 211-treated (E) sections. A, B) Insets show low-magnification images for orientation. Scale bars, 50 µm.
Histologic analysis of ear punch wound also indicated more regenerative repair with Wnt inhibitor treatment than control (Fig. 5A, B). At d 30, contralateral control (DMSO or 211-treated) wounds were composed of immature granulation tissue or disorganized extracellular matrix, characteristic of scar tissue (Fig. 5A, B, top panels, and Supplemental Fig. 2A). In contrast, wounds treated with Wnt inhibitors showed evidence of improved healing, with organized (more reticular) extracellular matrix and areas of regenerated tissue that closely resembled normal, uninjured cutaneous tissue (Fig. 5A, B, bottom panels, Supplemental Fig. 2A, and quantification of variance in collagen fiber alignment: Fig. 5E). As expected, Wnt activation by LiCl treatment did not reduce scarring at d 30 (Supplemental Fig. 2B). To understand the cellular and molecular basis of this phenotype, we examined the major drivers of fibrosis. We found that Wnt inhibitor treatment resulted in reduced presence of α smooth muscle actin (α-SMA) positive myofibroblasts in the healing wound (Fig. 5C, D), which corresponded with lower mRNA expression of α-SMA (Fig. 5F), collagen type I α1, and collagen type III (Fig. 5G) in Wnt inhibitor-treated tissue. Because inflammation at early stages of repair is considered an important determinant of subsequent fibrosis (37), we tested the effect of Wnt inhibition on inflammation. Immunohistochemistry with F4/80 showed no difference in macrophage content in the wound at 48 h and 4 d postwounding (Supplemental Fig. 4A–C) in Pyr- and 211-treated ears. qRT-PCR for IL-6 gene expression at 2 d postinjury confirmed this finding (Supplemental Fig. 4D), indicating that the antifibrotic effect Wnt inhibition is likely not mediated by a reduction in inflammation.
Figure 5.
Wnt inhibitor treatment promotes regenerative repair of ear punch wounds. Representative images of XAV-939- or DMSO-treated tissue (A), and Pyr- or 211-treated (B) tissue showing difference in regenerative repair. XAV-939- and Pyr-treated sections show scar resolution (black arrow in B) and regenerated tissue that closely resembles healthy skin (blue arrows in A and B). The 211-treated section shows organizing granulation tissue with higher cellularity (yellow arrowheads) and scale crust (green arrow) at 30 d. DMSO-treated tissue shows an abundance of scar (black arrow) without regenerating tissue. Insets show lower-magnification images of the tissue for orientation (black box indicates magnified regions). C–D) Immunostaining for α-SMA on (C) DMSO- and XAV-939 and (D) 211- and Pyr-treated tissue at 20 d postwounding showing fewer α-SMA+ myofibroblasts (green fluorescence) in Wnt inhibitor-treated tissue (C and D, lower panels). Nuclei are counterstained with Hoechst; white dashed lines demarcate dermal–epidermal border. E) Directional variance of collagen fiber alignment calculated from ×20 images of trichrome blue-stained sections. Relative mRNA expression of (F) α-SMA (n = 5 per group; paired t test), and (G) collagen type I α1 (Col1α1) and type III in wound bed tissue (n = 3 per group). Scale bars, 50 µm.
Wnt inhibition promotes cartilage regeneration in wounded ears
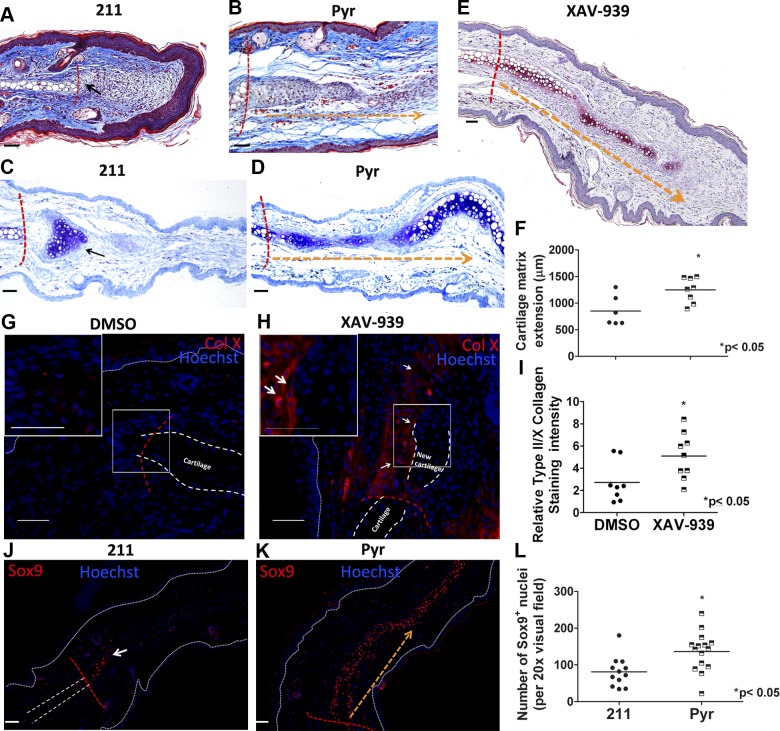
An important component of regenerative repair in the ear punch injury model is cartilage regeneration, which is essential for complete restoration of tissue function at this site. Only a few “superhealer” strains or species of mice are capable of cartilage regeneration after ear punch injury (5, 38). The mechanism driving this regeneration is unknown in these models. We noted that Wnt inhibition resulted in increased chondrogenesis extending from the uninjured cartilage at the edge of the wound compared with control wounds (Fig. 6A, B). Toluidine blue (Fig. 6C, D, and F) and safranin-O (Fig. 6E) staining for chondroid matrix proteoglycans also showed that Wnt inhibition promotes the formation of cartilaginous matrix in the healing punch wound. Furthermore, immunostaining for type II and X collagen showed greater deposition in the leading edge of existing cartilage in ears treated with Wnt inhibitors compared with contralateral controls (Fig. 6G–I). The transcription factor Sox9 is essential for chondrocyte differentiation (39). We observed significantly greater numbers of Sox9-positive nuclei extending from the uninjured cartilage at the wound margin in tissue treated with Wnt inhibitors compared with their respective controls (Fig. 6J–L), suggesting that Wnt inhibition promotes chondrogenesis in this model of cutaneous injury.
Figure 6.
Topical treatment with Wnt inhibitors stimulates cartilage regeneration. Trichrome (A, B) and toluidine blue-stained (C, D) sections of tissues treated with 211 or Pyr. A, C) 211-treated sections show very limited extension of cartilage matrix (black arrow). In contrast, Pyr-treated tissue (B, D) shows extension of new cartilage matrix (indicated by orange dashed arrow) beyond wound margin (red dashed lines). Toluidine blue staining of new cartilage matrix is quantified in F. E) Safranin-O staining of proteoglycan deposition (indicated by orange dashed arrow) beyond wound margin (red dashed line) in XAV-939-treated tissue. G, H) type X collagen (ColX)-stained sections show increased ColX deposition at the leading edge of injured cartilage in XAV-939-treated (H) tissue than DMSO-treated (G) tissue. I) Quantification of ColX and ColII immunostaining. J, K) Sox9+ chondrocytes (red fluorescence) extending from the leading edge of existing cartilage (red dashed line) indicates more chondrogenesis in (K) Pyr-treated wound compared with 211-treated (J) tissue. Dotted white lines mark epidermal layer, and dashed white lines in G marks existing cartilage. Nuclei are counterstained blue with Hoechst dye. L) Quantification of Sox9+ nuclei. F) n ≥ 5, (I) n ≥ 8, and (L) n ≥ 4 mice per group. Scale bars, 50 µm.
Topical treatment with Wnt inhibitors does not impede hair follicle neogenesis
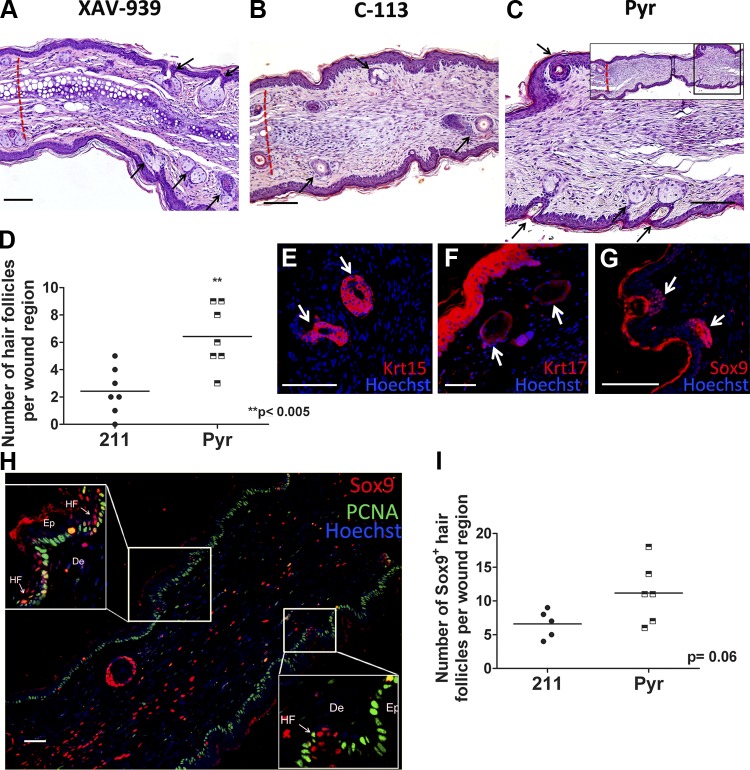
Our analysis of the dermal and subdermal layers indicated a more regenerative and less fibrotic healing by treatment with Wnt inhibitors. However, given the large body of literature suggesting an essential role for epidermal Wnt signaling in hair follicle neogenesis in injured skin (21–23), we were surprised to note that topical Wnt inhibitor treatment of ear punch wounds did not inhibit hair follicle regrowth in regenerating wound tissue. In contrast, XAV-939 (Fig. 7A), C-113 (Fig. 7B), or Pyr (Fig. 7C, D) treatment resulted in a significantly increased number of hair follicles in the regenerated tissue. Immunostaining for the hair follicle bulge stem cell marker cytokeratin 15 (40) (Fig. 7E), and the epithelial placode marker cytokeratin 17 (21, 41) (Fig. 7F) confirmed the presence of mature and nascent hair follicles. To further confirm that these follicles were nascent follicles arising from regenerated tissue, we stained for Sox9+ epithelial placode cells, which mark the beginning of hair follicle formation (42). More Sox9+ placode cell clusters were detected in Wnt inhibitor treated wounds compared with respective controls (Fig. 7G–I). Although studies using epidermis-specific promoters to modulate Wnt signals (21–23) have shown that epidermal Wnt activation is an important requirement for hair follicle neogenesis, it is possible that in our model, dermal Wnt inhibitory signals promote regeneration without disrupting follicle regeneration mediated by Wnt in the epidermis. This is also evident by our immunofluorescence data (Fig. 2B, C and Supplemental Fig. 3B, C) where we did not observe a detectable reduction in Wnt signals in the epidermis as measured by β-catenin and β-galactosidase immunofluorescence intensity by treatment with our Wnt inhibitors.
Figure 7.
Wnt inhibition does not affect hair follicle neogenesis in newly regenerated tissue. H&E-stained sections of ear wound treated withXAV-939 (A), C-113 (B), or Pyr (C) showed newly regenerating hair follicles (black arrows). Dashed red lines represent wound margin. D) Graphical representation of number of new hair follicles arising in the regenerated tissue with 211 or Pyr treatment. Anti-cytokertain-15 (red) (E), anti-cytokeratin-17 (red) (F), and anti-Sox9 (red) (G)-stained sections of XAV-939-treated tissue highlights new hair follicles arising in the regenerated tissue. H) Sox9 (red)-stained section of Pyr-treated tissue showing positive staining of placode cells (magnified in the insets) in very early stages of hair follicle formation; proliferating cell nuclear antigen (green) marks proliferating basal cells of epidermis; nuclei in E–H are counterstained blue with Hoechst dye. HF, hair follicle; Ep, epidermis; D, dermis. I) Number of early hair placodes stained with Sox9. D, I) n ≥ 5 per group. Scale bars, 50 µm.
DISCUSSION
In this study, we demonstrated a cutaneous wound healing response characterized by reduced fibrosis and regeneration of auricular cartilage and skin adnexa in response to inhibition of Wnt/β-catenin signaling. Given the conflicting literature supporting the need for epidermal Wnt activation in hair follicle regeneration (21–23) and dermal Wnt activity in promoting fibrosis (16, 17), our study highlights the potential of therapeutic Wnt inhibition in balancing both the aspects of wound repair.
Topical treatment with 3 Wnt inhibitors with 2 distinct mechanisms of action reduced β-catenin and β-galactosidase (in TOPGAL mice) protein levels most notably in the dermis. Unlike the epidermis, which exhibited high, continuous baseline Wnt activation, Wnt activity in the dermis was more dynamic—relatively quiescent in uninjured skin and activated shortly after injury, similar to previously published reports in skin injury (15). This dynamic aspect of dermal Wnt activity may make it more susceptible to therapeutic inhibition. Similar phenomenon has been reported by de la Roche et al. (43) where transiently Wnt-stimulated cells responded robustly to tankyrase inhibition XAV-939, although long-term overstimulation with Wnt rendered the cells resistant to the Wnt inhibitory effect of the drug.
In the dermis, we noted that Wnt inhibition promoted granulation tissue resolution, promoting deposition of more organized extracellular matrix with reticular fiber alignment, in both the excisional wound model and ear punch injury model. In the excisional wound, Wnt inhibition promoted formation of rete-pegs and dermal papillae indicating better restoration of dermal–epidermal junction. Wnt inhibitor treatment in ear injury also reduced myofibroblast number and modestly reduced gene expression of α-SMA and collagen types I and III, all major indicators of fibrosis (44). IL-6 gene expression and macrophage immunohistochemistry showed no significant difference in immune activation between Wnt inhibitor and vehicle-treated tissue, suggesting that the effect of Wnt inhibition on fibrosis was not mediated by modulating inflammation. Our findings are supported by other studies demonstrating the role of Wnt/β-catenin signals in promoting fibrosis in various wound models (12, 13, 16, 45). Within the wound milieu, Wnt pathway activity is associated with proliferative and migratory phenotype of fibroblasts (15), as well as with fibroblast differentiation into myofibroblasts (16), which can potentially contribute to fibrosis.
The role of Wnt pathway in folliculogenesis has been extensively studied. During mammalian development, the Wnt pathway is necessary for hair follicle initiation (24, 46), and stabilization of β-catenin in the epidermis leads to ectopic de novo hair morphogenesis (47). Previous studies indicate that Wnt pathway activity is necessary for initiation of new hair follicle formation in the wounded epidermis (21–23). However, most of these studies focus on folliculogenesis, and do not report an overall regenerative repair of cutaneous wound. Hence our results may be explained by the possibility that the molecular signals driving folliculogenesis in a regenerating wound may be distinct from the signals (such as Wnt) transiently activated specifically in the epidermis during the initial phase of hair follicle neogenesis. Indeed, hair follicle maintenance in normal skin is thought to be driven by “short-range” Wnt activation signals that are surrounded and blunted by “long-range” Wnt inhibition signals (48).
The Wnt inhibitors allowed us to target the dermis, which may also explain our results that are in direct contrast to the studies of follicle neogenesis based mostly on epithelial or follicle-specific promoter-driven models of Wnt modulation (21–23). In agreement with our data, previous studies in which β-catenin was stabilized both in the dermis and the epidermis (15, 27) showed that Wnt activation promoted fibrosis and increase in wound size. Given the strong evidence that Wnt inhibits regenerative repair in lung (12), kidney (13), heart (49), and even skin (50), it is important to examine folliculogenesis in the context of regenerative skin repair. Our findings may also be attributed to the fact that pharmacological Wnt inhibition can be titrated to achieve optimal therapeutic outcome, whereas genetic models of complete Wnt inhibition (e.g., through β-catenin inactivating mutation) are more likely to have a deleterious effect given the importance of the pathway in skin homeostasis. Indeed, we saw reduction in cytoplasmic β-catenin staining in the dermis, but did not notice an effect on β-catenin levels or localization in the epidermis of Wnt inhibitor-treated tissues compared with controls. Moreover, our data suggest a negative role of Wnt signaling in the regeneration of cartilage. Although Wnt signaling has not been implicated directly in the degradation of auricular cartilage, the Wnt/β-catenin pathway is known to play an inhibitory role in cartilage development (51), and promote hypertrophic chondrocyte maturation during endochondral ossification by suppressing chondrogenic gene expression program (52). Wnt signaling is also thought to promote cartilage degradation in osteoarthritis and rheumatoid arthritis by promoting chondrocyte dedifferentiation and degradation of the cartilage extracellular matrix (53). Finally, β-catenin-mediated transcription has been shown to down-regulate Sox9, an important mediator of chondrocyte differentiation from mesenchymal cells (52).
Cartilage regeneration may also offer an explanation for our observation that treatment throughout the 30 d period was required for optimal ear punch closure, although a spike in Wnt activity was observed early after injury. Because treatment for 2 wk was sufficient to induce a reduction in scarring in the excisional wound model, it is likely that in the ear punch injury model, the need for 30 d treatment is tied closely to the requirement for cartilage regeneration to enable wound closure in this model. Although with RT-PCR, we see Wnt pathway activity reduce to baseline levels by 10 d, it is possible that Wnt pathway remains active in the leading edge of the injured cartilage—undetectable by RT-PCR of the whole tissue—and that quenching of the pathway with inhibitors promotes regeneration of the cartilage, driving closure of the wound. Studies that explore the role of Wnt inhibition on cartilage regeneration in this and other injury models may help test this hypothesis.
The Wnt pathway has been ascribed roles in regulating stemness and differentiation of various stem/progenitor cell types. Work by our group and others have shown a role for Wnt inhibition in maintaining stemness and inhibiting differentiation of mesenchymal stem cells (8, 54, 55). In the heart, the Wnt pathway inhibits proliferation of cardiac progenitor cells in vivo (56), and in skeletal muscle Wnt signaling is associated with differentiation of myogenic progenitors (11). Studies in cutaneous injury suggest similar roles for Wnt/β-catenin pathway activity—specifically, promoting differentiation of epithelial stem cells to follicle lineages during the hair cycle (57, 58). Consistent with this, we have observed in excisional wound model, and using engineered skin substitute in vitro, that Wnt inhibition slows keratinization and stratification of wound epidermis, possibly by inhibiting differentiation and promoting a proliferative phenotype in keratinocytes (data not shown), also in agreement with published report (59). It is known that quiescent stem cells localized in the hair follicle bulge directly contribute to wound repair (60). Hence, Wnt inhibition may promote regenerative repair through its effect on epithelial stem cells. Further studies are necessary to confirm this hypothesis.
The clinical significance of topical application of an agent that effectively promotes regenerative cutaneous repair and inhibits scarring is immense. Scar-free wound healing can significantly improve the quality of life of thousands of burn and trauma survivors. We found regeneration of auricular cartilage with Wnt inhibitor treatment. Further studies may also reveal a role for Wnt inhibition in promoting regeneration of damaged articular cartilage in conditions such as degenerative joint disease in humans. Moreover, given the availability of Pyr, a U.S. Food and Drug Administration-approved drug that can be effectively repurposed for cutaneous Wnt inhibition, and the ongoing therapeutic development of Wnt inhibitors for other indications such as cancer (clinicaltrials.gov) (61), our findings have immediate and broad clinical impact.
Acknowledgments
This work was supported by the U.S. Veterans Affairs Merit Award (to P.P.Y.); a Vanderbilt University Clinical and Translational Science Award from the U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) Grant UL1-RR024975-01 to P.P.Y.; American Heart Association Predoctoral Fellowship 3PRE16080004 to D.B.; and NIH NIGMS Grants R01GM081635 and R01GM103926 to E.L. P.P.Y. and E.L. are listed as inventors on a patent application related to wound healing owned by Vanderbilt University.
Glossary
- 211
VU-WS211
- α-SMA
α smooth muscle actin
- C-113
VU-WS113
- H&E
hematoxylin and eosin
- sFRP2
secreted frizzled receptor protein 2
- TOPGAL
Tcf optimal promoter β-galactosidase
- Pyr
pyrvinium
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Centers for Disease Control and Prevention (2011) National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables. Accessed August 5, 2015 at: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf
- 2.Atala A., Irvine D. J., Moses M., Shaunak S. (2010) Wound healing versus regeneration: role of the tissue environment in regenerative medicine. MRS Bull. 35, 35–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gourevitch D., Clark L., Chen P., Seitz A., Samulewicz S. J., Heber-Katz E. (2003) Matrix metalloproteinase activity correlates with blastema formation in the regenerating MRL mouse ear hole model. Dev. Dyn. 226, 377–387 [DOI] [PubMed] [Google Scholar]
- 4.Clark L. D., Clark R. K., Heber-Katz E. (1998) A new murine model for mammalian wound repair and regeneration. Clin. Immunol. Immunopathol. 88, 35–45 [DOI] [PubMed] [Google Scholar]
- 5.Seifert A. W., Kiama S. G., Seifert M. G., Goheen J. R., Palmer T. M., Maden M. (2012) Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489, 561–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedelbaeva K., Snyder A., Gourevitch D., Clark L., Zhang X. M., Leferovich J., Cheverud J. M., Lieberman P., Heber-Katz E. (2010) Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc. Natl. Acad. Sci. USA 107, 5845–5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gourevitch D., Kossenkov A. V., Zhang Y., Clark L., Chang C., Showe L. C., Heber-Katz E. (2014) Inflammation and its correlates in regenerative wound healing: an alternate perspective. Adv. Wound Care (New Rochelle) 3, 592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfaro M. P., Pagni M., Vincent A., Atkinson J., Hill M. F., Cates J., Davidson J. M., Rottman J., Lee E., Young P. P. (2008) The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc. Natl. Acad. Sci. USA 105, 18366–18371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saraswati S., Alfaro M. P., Thorne C. A., Atkinson J., Lee E., Young P. P. (2010) Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One 5, e15521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He W., Zhang L., Ni A., Zhang Z., Mirotsou M., Mao L., Pratt R. E., Dzau V. J. (2010) Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc. Natl. Acad. Sci. USA 107, 21110–21115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brack A. S., Murphy-Seiler F., Hanifi J., Deka J., Eyckerman S., Keller C., Aguet M., Rando T. A. (2009) BCL9 is an essential component of canonical Wnt signaling that mediates the differentiation of myogenic progenitors during muscle regeneration. Dev. Biol. 335, 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim T. H., Kim S. H., Seo J. Y., Chung H., Kwak H. J., Lee S. K., Yoon H. J., Shin D. H., Park S. S., Sohn J. W. (2011) Blockade of the Wnt/β-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J. Exp. Med. 223, 45–54 [DOI] [PubMed] [Google Scholar]
- 13.He W., Dai C., Li Y., Zeng G., Monga S. P., Liu Y. (2009) Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saraswati S., Deskins D. L., Holt G. E., Young P. P. (2012) Pyrvinium, a potent small molecule Wnt inhibitor, increases engraftment and inhibits lineage commitment of mesenchymal stem cells (MSCs). Wound Repair Regen. 20, 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheon S. S., Cheah A. Y., Turley S., Nadesan P., Poon R., Clevers H., Alman B. A. (2002) beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc. Natl. Acad. Sci. USA 99, 6973–6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akhmetshina A., Palumbo K., Dees C., Bergmann C., Venalis P., Zerr P., Horn A., Kireva T., Beyer C., Zwerina J., Schneider H., Sadowski A., Riener M. O., MacDougald O. A., Distler O., Schett G., Distler J. H. (2012) Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat. Commun. 3, 735–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell S. B., Russell J. D., Trupin K. M., Gayden A. E., Opalenik S. R., Nanney L. B., Broquist A. H., Raju L., Williams S. M. (2010) Epigenetically altered wound healing in keloid fibroblasts. J. Invest. Dermatol. 130, 2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alman B. A., Li C., Pajerski M. E., Diaz-Cano S., Wolfe H. J. (1997) Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am. J. Pathol. 151, 329–334 [PMC free article] [PubMed] [Google Scholar]
- 19.Tejpar S., Nollet F., Li C., Wunder J. S., Michils G., dal Cin P., Van Cutsem E., Bapat B., van Roy F., Cassiman J. J., Alman B. A. (1999) Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene 18, 6615–6620 [DOI] [PubMed] [Google Scholar]
- 20.Whyte J. L., Smith A. A., Liu B., Manzano W. R., Evans N. D., Dhamdhere G. R., Fang M. Y., Chang H. Y., Oro A. E., Helms J. A. (2013) Augmenting endogenous Wnt signaling improves skin wound healing. PLoS One 8, e76883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito M., Yang Z., Andl T., Cui C., Kim N., Millar S. E., Cotsarelis G. (2007) Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 [DOI] [PubMed] [Google Scholar]
- 22.Lowry W. E., Blanpain C., Nowak J. A., Guasch G., Lewis L., Fuchs E. (2005) Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 19, 1596–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gay D., Kwon O., Zhang Z., Spata M., Plikus M. V., Holler P. D., Ito M., Yang Z., Treffeisen E., Kim C. D., Nace A., Zhang X., Baratono S., Wang F., Ornitz D. M., Millar S. E., Cotsarelis G. (2013) Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 19, 916–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andl T., Reddy S. T., Gaddapara T., Millar S. E. (2002) WNT signals are required for the initiation of hair follicle development. Dev. Cell 2, 643–653 [DOI] [PubMed] [Google Scholar]
- 25.Choi Y. S., Zhang Y., Xu M., Yang Y., Ito M., Peng T., Cui Z., Nagy A., Hadjantonakis A. K., Lang R. A., Cotsarelis G., Andl T., Morrisey E. E., Millar S. E. (2013) Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 13, 720–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner S., Peters K. G., Longaker M. T., Fuller-Pace F., Banda M. J., Williams L. T. (1992) Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc. Natl. Acad. Sci. USA 89, 6896–6900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheon S. S., Wei Q., Gurung A., Youn A., Bright T., Poon R., Whetstone H., Guha A., Alman B. A. (2006) Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J. 20, 692–701 [DOI] [PubMed] [Google Scholar]
- 28.Thorne C. A., Hanson A. J., Schneider J., Tahinci E., Orton D., Cselenyi C. S., Jernigan K. K., Meyers K. C., Hang B. I., Waterson A. G., Kim K., Melancon B., Ghidu V. P., Sulikowski G. A., LaFleur B., Salic A., Lee L. A., Miller D. M. III, Lee E. (2010) Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat. Chem. Biol. 6, 829–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang S.-M. A., Mishina Y. M., Liu S., Cheung A., Stegmeier F., Michaud G. A., Charlat O., Wiellette E., Zhang Y., Wiessner S., Hild M., Shi X., Wilson C. J., Mickanin C., Myer V., Fazal A., Tomlinson R., Serluca F., Shao W., Cheng H., Shultz M., Rau C., Schirle M., Schlegl J., Ghidelli S., Fawell S., Lu C., Curtis D., Kirschner M. W., Lengauer C., Finan P. M., Tallarico J. A., Bouwmeester T., Porter J. A., Bauer A., Cong F. (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 [DOI] [PubMed] [Google Scholar]
- 30.Aisagbonhi O., Rai M., Ryzhov S., Atria N., Feoktistov I., Hatzopoulos A. K. (2011) Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis. Model. Mech. 4, 469–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rai M. F., Hashimoto S., Johnson E. E., Janiszak K. L., Fitzgerald J., Heber-Katz E., Cheverud J. M., Sandell L. J. (2012) Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis Rheum. 64, 2300–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonck E., Feigl G. G., Fasel J., Sage D., Unser M., Rüfenacht D. A., Stergiopulos N. (2009) Effect of aging on elastin functionality in human cerebral arteries. Stroke 40, 2552–2556 [DOI] [PubMed] [Google Scholar]
- 33.Rezakhaniha R., Agianniotis A., Schrauwen J. T., Griffa A., Sage D., Bouten C. V., van de Vosse F. N., Unser M., Stergiopulos N. (2012) Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol. 11, 461–473 [DOI] [PubMed] [Google Scholar]
- 34.Heydemann A. (2012) The super super-healing MRL mouse strain. Front. Biol. (Beijing) 7, 522–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedgepeth C. M., Conrad L. J., Zhang J., Huang H. C., Lee V. M., Klein P. S. (1997) Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 185, 82–91 [DOI] [PubMed] [Google Scholar]
- 36.Nguyen, D. T., Orgill, D. P., and Murphy, G. F. (2009) The pathophysiologic basis of wound healing and cutaneous regeneration. In Biomaterials for Treating Skin Loss (Orgill, D. P., and Blanco, C., eds.), pp. 25–53, Woodhead Publishing, Cambridge, UK [Google Scholar]
- 37.Stramer B. M., Mori R., Martin P. (2007) The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J. Invest. Dermatol. 127, 1009–1017 [DOI] [PubMed] [Google Scholar]
- 38.Heber-Katz E., Leferovich J., Bedelbaeva K., Gourevitch D., Clark L. (2004) The scarless heart and the MRL mouse. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akiyama H., Chaboissier M. C., Martin J. F., Schedl A., de Crombrugghe B. (2002) The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Lyle S., Yang Z., Cotsarelis G. (2003) Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J. Invest. Dermatol. 121, 963–968 [DOI] [PubMed] [Google Scholar]
- 41.McGowan K. M., Coulombe P. A. (2000) Keratin 17 expression in the hard epithelial context of the hair and nail, and its relevance for the pachyonychia congenita phenotype. J. Invest. Dermatol. 114, 1101–1107 [DOI] [PubMed] [Google Scholar]
- 42.Vidal V. P., Chaboissier M. C., Lützkendorf S., Cotsarelis G., Mill P., Hui C. C., Ortonne N., Ortonne J. P., Schedl A. (2005) Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 15, 1340–1351 [DOI] [PubMed] [Google Scholar]
- 43.De la Roche M., Ibrahim A. E., Mieszczanek J., Bienz M. (2014) LEF1 and B9L shield β-catenin from inactivation by Axin, desensitizing colorectal cancer cells to tankyrase inhibitors. Cancer Res. 74, 1495–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wynn T. A., Ramalingam T. R. (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei J., Melichian D., Komura K., Hinchcliff M., Lam A. P., Lafyatis R., Gottardi C. J., MacDougald O. A., Varga J. (2011) Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 63, 1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai S. Y., Sennett R., Rezza A., Clavel C., Grisanti L., Zemla R., Najam S., Rendl M. (2014) Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation. Dev. Biol. 385, 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gat U., DasGupta R., Degenstein L., Fuchs E. (1998) De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 95, 605–614 [DOI] [PubMed] [Google Scholar]
- 48.Lim X., Nusse R. (2013) Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb. Perspect. Biol. 5, 5–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergmann M. W. (2010) WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circ. Res. 107, 1198–1208 [DOI] [PubMed] [Google Scholar]
- 50.Beyer C., Reichert H., Akan H., Mallano T., Schramm A., Dees C., Palumbo-Zerr K., Lin N. Y., Distler A., Gelse K., Varga J., Distler O., Schett G., Distler J. H. (2013) Blockade of canonical Wnt signalling ameliorates experimental dermal fibrosis. Ann. Rheum. Dis. 72, 1255–1258 [DOI] [PubMed] [Google Scholar]
- 51.Rudnicki J. A., Brown A. M. (1997) Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev. Biol. 185, 104–118 [DOI] [PubMed] [Google Scholar]
- 52.Day T. F., Guo X., Garrett-Beal L., Yang Y. (2005) Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 8, 739–750 [DOI] [PubMed] [Google Scholar]
- 53.Hwang S. G., Ryu J. H., Kim I. C., Jho E. H., Jung H. C., Kim K., Kim S. J., Chun J. S. (2004) Wnt-7a causes loss of differentiated phenotype and inhibits apoptosis of articular chondrocytes via different mechanisms. J. Biol. Chem. 279, 26597–26604 [DOI] [PubMed] [Google Scholar]
- 54.Gaur T., Lengner C. J., Hovhannisyan H., Bhat R. A., Bodine P. V., Komm B. S., Javed A., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (2005) Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 280, 33132–33140 [DOI] [PubMed] [Google Scholar]
- 55.Otto T. C., Lane M. D. (2005) Adipose development: from stem cell to adipocyte. Crit. Rev. Biochem. Mol. Biol. 40, 229–242 [DOI] [PubMed] [Google Scholar]
- 56.Oikonomopoulos A., Sereti K. I., Conyers F., Bauer M., Liao A., Guan J., Crapps D., Han J. K., Dong H., Bayomy A. F., Fine G. C., Westerman K., Biechele T. L., Moon R. T., Force T., Liao R. (2011) Wnt signaling exerts an antiproliferative effect on adult cardiac progenitor cells through IGFBP3. Circ. Res. 109, 1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DasGupta R., Fuchs E. (1999) Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 [DOI] [PubMed] [Google Scholar]
- 58.Huelsken J., Vogel R., Erdmann B., Cotsarelis G., Birchmeier W. (2001) beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105, 533–545 [DOI] [PubMed] [Google Scholar]
- 59.Zhu X. J., Liu Y., Dai Z. M., Zhang X., Yang X., Li Y., Qiu M., Fu J., Hsu W., Chen Y., Zhang Z. (2014) BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet. 10, e1004687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito M., Liu Y., Yang Z., Nguyen J., Liang F., Morris R. J., Cotsarelis G. (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11, 1351–1354 [DOI] [PubMed] [Google Scholar]
- 61.Novartis Pharmaceuticals (2014) A Study of Oral LGK974 in Patients with Malignancies Dependent on Wnt Ligands. Available at: https://clinicaltrials.gov/ct2/show/NCT01351103 [Google Scholar]