Abstract
In Caenorhabditis elegans, homodimeric [kinesin family (KIF) 17, osmotic avoidance abnormal-3 (OSM-3)] and heterotrimeric (KIF3) kinesin-2 motors are required to establish sensory cilia by intraflagellar transport (IFT) where KIF3 and KIF17 cooperate to build the axoneme core and KIF17 builds the distal segments. However, the function of KIF17 in vertebrates is unresolved. We expressed full-length and motorless KIF17 constructs in mouse rod photoreceptors using adeno-associated virus in Xenopus laevis rod photoreceptors using a transgene and in ciliated IMCD3 cells. We found that tagged KIF17 localized along the rod outer segment axoneme when expressed in mouse and X. laevis photoreceptors, whereas KIF3A was restricted to the proximal axoneme. Motorless KIF3A and KIF17 mutants caused photoreceptor degeneration, likely through dominant negative effects on IFT. KIF17 mutant lacking the motor domain translocated to nuclei after exposure of a C-terminal nuclear localization signal. Germ-line deletion of Kif17 in mouse did not affect photoreceptor function. A rod-specific Kif3/Kif17 double knockout mouse demonstrated that KIF17 and KIF3 do not act synergistically and did not prevent rhodopsin trafficking to rod outer segments. In summary, the nematode model of KIF3/KIF17 cooperation apparently does not apply to mouse photoreceptors in which the photosensory cilium is built exclusively by KIF3.—Jiang, L., Tam, B. M., Ying, G., Wu, S., Hauswirth, W. W., Frederick, J. M., Moritz, O. L., Baehr, W. Kinesin family 17 (osmotic avoidance abnormal-3) is dispensable for photoreceptor morphology and function.
Keywords: anterograde intraflagellar transport, kinesin-2 truncation mutants, photoreceptor degeneration, rhodopsin trafficking
The photoreceptor sensory cilium (1) is a modified primary cilium that is much enlarged with hundreds of membranous discs that contain the phototransduction machinery (2). The connecting cilium (CC) of vertebrate photoreceptors connects the biosynthetic inner segment (IS) to the sensory outer segment (OS) (3, 4). The CC is the structural homolog of the transition zone of primary cilia. Synthesis of phototransduction proteins occurs in the IS that contains the principal components of protein biosynthesis, processing, and sorting (5). Proteins destined for the OS traffic through the CC and incorporate into nascent discs by unknown mechanisms (6).
Ciliary membrane proteins are isolated from the plasma membrane by a diffusion barrier located at the CC. Ciliogenesis and maintenance require the active intraflagellar transport (IFT) of building blocks from the cell body across the transition zone to the axonemal tip, a process that requires microtubule-associated molecular motors. An unresolved question is the mechanism for trafficking axonemal building blocks and phototransduction membrane proteins (7, 8). Two IFT canonical molecular motors have been identified: heterotrimeric kinesin-2 [kinesin family (KIF) 3] and homodimeric kinesin-2 (KIF17) (9, 10). We showed previously that KIF3 has no role in trafficking of rhodopsin and other phototransduction membrane proteins but is required during late-stage ciliogenesis after the basal body has docked to the cell cortex; late-stage ciliogenesis stalls in Kif3a−/− photoreceptors, with the consequence that the CC and OS are not formed (11).
Homodimeric KIF17 (OSM-3) is a molecular motor involved in plus-oriented IFT of Caenorhabditis elegans and vertebrates. KIF17 and KIF3 presumably cooperate during ciliogenesis in which KIF3 builds the axoneme core and KIF17 the axoneme distal segments (Fig. 1A) (9, 12). Interaction of KIF17 with KIF3 and the contribution of KIF17 to vertebrate ciliogenesis or membrane protein trafficking are unclear, as studies with KIF17 have produced contradictory results. KIF17 appears largely dispensable for ciliogenesis in zebrafish, as Kif17 homozygous mutant animals are viable and display subtle morphologic defects of olfactory cilia only (13). However, KIF17 appeared to play a role during early photoreceptor development of zebrafish retina (14, 15).
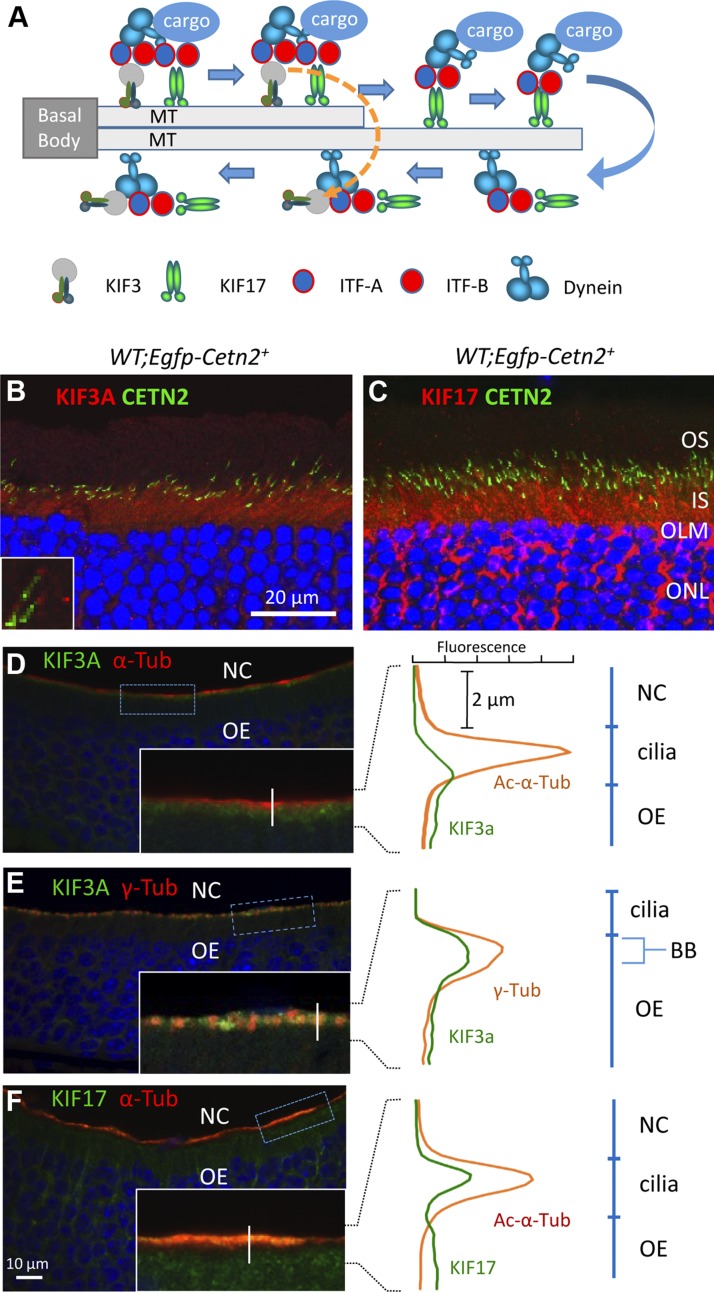
Figure 1.
Endogenous KIF3 and KIF17 in mouse photoreceptors and olfactory sensory neurons (OSNs). A) C. elegans. KIF3 and KIF17 cooperate in anterograde IFT at the proximal axoneme (microtubule, MT doublet) [modified from Inglis et al. (44) and Snow et al. (45)]. Cargo consists of IFT particles, dynein motors, axoneme building blocks, and axoneme-stabilizing factors. KIF3 returns and KIF17 continues to move cargo to tip along axoneme MT singlet. Retrograde trafficking is carried out by a minus-oriented dynein motor DHC1b (46). B and C) Immunohistochemistry of transgenic Egfp-Cetn2+ retina cryosections probed with anti-KIF3A (B, red) and anti-KIF17 (C, red). OLM, outer limiting membrane. D–F) Immunohistochemistry of mouse P14 olfactory epithelia (OE). Sections were probed with antibodies directed against KIF3A (green) and Ac-α-tubulin (red) (D), KIF3A and γ-tubulin (red) (E), and Ac-α-tubulin and anti-KIF17 (green) (F). DAPI (blue) is nuclear marker. D–F) Insets are enlargements of OSN ciliary layer with vertical lines where fluorescence profile plots were created to identify subcellular localizations of KIF3A, KIF17. Fluorescence profile plots of kinesin-2 with different cilium markers and tubulin isoforms are shown (right). NC, nasal cavity; OE, olfactory epithelium, BB, basal body.
We investigated whether KIF17 participates in either building or maintaining the mouse photoreceptor sensory cilium and in phototransduction membrane protein trafficking. We expressed full-length (FL) and motorless (ML) enhanced green fluorescent protein (eGFP)- or mCherry (mC)-tagged truncation mutants in ciliated mammalian cells (IMCD3 and NIH3T3) and mouse and Xenopus laevis photoreceptors to identify the role of KIF17 in IFT. We found that tagged ML mutants of KIF17, as dominant negative inhibitors of KIF3, caused photoreceptor degeneration. A KIF17 mutant lacking motor and neck domains translocated primarily to photoreceptor nuclei, directed by a C-terminal nuclear location signal. Finally, germ-line deletion of KIF17 in mouse revealed that absence of KIF17 has no effect on axoneme structure or photoreceptor function for up to 2 yr, thereby eliminating KIF17 as a participant in rhodopsin trafficking.
MATERIALS AND METHODS
Mice
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained under 12 h cyclic dark/light conditions. Procedures for animal experiments were approved by the University of Utah Institutional Animal Care and Use Committee and conformed to recommendations of the Association for Research in Vision and Ophthalmology.
DNA constructs
FL and ML kinesin-2 (KIF17 and KIF3A) coding sequences were amplified by PCR from a mouse retina cDNA library and cloned into pmCherry-C1 (Clontech Laboratories, Mountain View, CA, USA). Five mC and myc double-tagged kinesin-2 expression constructs, mC-KIF3A (1–701 aa), mC-KIF3A-ML (Δ351 aa, 352–701), mC-KIF17 (1–1039 aa), mC-KIF17-ML1 (Δ319 aa, 320–1039 aa), and mC-KIF17-ML2 (Δ809 aa, 810–1039 aa), were generated to express kinesin-2 proteins in cultured cell lines and to serve as templates for other kinesin-2 constructs. Primers were as follows: mC-KIF3A: myc-Kif3aFL_EX1F, 5′-GGAATTCTAGAGCCACCATGGAGCAGAAGCTCATCTCAGAAGAAGACCTCATGCCGATCAATAAGTCG, mC-KIF3A-ML: myc-Kif3a-ML_ EX1F, 5′-GGAATTCTAGAATGGAGCAGAAGCTCATCTCAGAAGAAGACCTCATGAATGAGGACCCAAAGGATGCTCTG, and sharing reverse primer: Kif3a_EcoR1R, 5′-GGAATTCTTATCACTGAAGTAAAGAATCAATTACGGTCTCAGG. mC-KIF17: myc-Kif17FL_Sac2F, 5′-TCCCCGCGGCCACCATGGAGCAGAAGCTCATCTCAGAAGAAGACCTCATGGCTTCGGAGTCAGTGAAAGTGG, mC-KIF17-ML1: myc-Kif17-720_Sac2F, 5′-TCCCCGCGGCCACCATGGAGCAGAAGCTCATCTCAGAAGAAGACCTCATGACCCTCAGTACGCTGCGCTATG, mC-KIF17-ML2: myc-Kif17-230_Sac2F, 5′-TCCCCGCGGCCACCATGGAGCAGAAGCTCATCTCAGAAGAAGACCTCATGTATGACTCTATCCAGGAGGAAGTGAGG, and sharing reverse primer: Kif17_Xba1R2, 5′-GTCTAGAAGCAGATAGCAGCAGGCAGCTGGATTATCACAGAGGCTCACCACCGAAGC. KIF17-ML1 and KIF17-ML2 coding sequences were subcloned into pEGFP-C1 (Clontech Laboratories) using conventional restriction sites, and 2 constructs were generated expressing eGFP-tagged transgenic eGFP (eG)-KIF-ML1 and eG-KIF17-ML2. Nuclear localization signal (NLS) mutants of mC-mKIF17-ML1 and mC-mKIF17-ML2 were generated by replacing each predicted NLS with multiple alanine residues by a bridge PCR approach. Primers used for mutagenesis were as follows: NLS1A: Kif17_772Ala8F, 5′-GCTGCTGCAGCAGCTGCTGCTGCGTACGCAGATGAGCGCAAGAAG, and Kif17_772Ala8R, 5′-CGCAGCAGCAGCTGCTGCAGCAGCCCTCAGGTCCTTGTTTTTAGCCTG. NLS2A: Kif17_886Ala6F, 5′-GCTGCTGCAGCAGCTGCGTCGAGCTGGGATGAGGATAATG, and Kif17_886Ala6R, 5′-CGCAGCTGCTGCAGCAGCCAGGTTGCTATAGTTACAGTCTCTGC, NLS3A: Kif17_1024Ala4F, 5′-TCTCCAAAGCCGCTGCTGCTGCAAGCAAAAACAGCTTCGGTGG, and Kif17_1024Ala4R, 5′-CTGTTTTTGCTTGCAGCAGCAGCGGCTTTGGAGAAGGGGATGTC.
To generate the adeno-associated virus (AAV) expressing mC-tagged kinesin-2 proteins, FL and ML kinesin-2 coding sequences were subcloned into the AAV shuttle vector, pAAV-hRK-mC, modified from pAAV-hRK-zsGreen (the kind gift of Dr. Tiansen Li, National Eye Institute), in which a human rhodopsin kinase promoter drives a downstream transgene expressed exclusively in mouse photoreceptors. To produce transgenic X. laevis expressing green fluorescent protein (GFP)-tagged kinesin-2 proteins, we subcloned the kinesin-2 coding sequences into XOP0.8-eGFP-C1 (16), which contains an 800 bp X. laevis rod opsin promoter to express transgenes in X. laevis rods exclusively. All constructs were verified by DNA sequencing.
Antibodies
Antibodies included those directed against the following: KIF17 (WB 1:500, ICC 1:200, ab11261; Abcam, Cambridge, MA, USA), KIF3A (WB 1:500, ICC 1:200, K3513; Sigma-Aldrich, St. Louis, MO, USA), acetylated (Ac)-α-tubulin (1:1000, clone 6-11B-1; Sigma-Aldrich), myc (1:1000, clone 9E10; Sigma-Aldrich), mC (1:1000, 632543; Clontech Laboratories), GFP (1:1000, MAB2510; EMD Millipore, Billerica, MA, USA), adenylate cyclase III (ACIII, 1:1000, C-20; Santa Cruz Biotechnology, Santa Cruz, CA, USA), cyclic nucleotide-gated channel [cyclic nucleotide-gated channel subunit A2 (CNGA2), 1:300, APC-045; Alomone Labs, Jerusalem, Israel], rhodopsin (1:1000, 1D4; Dr. Robert Molday, University of British Columbia), rootletin (Root6, 1:2000; Dr. Jun Yang, University of Utah), and lamin-B1 (1:500; Dr. Katie Ullman, University of Utah). The anti-KIF17 polyclonal antibody (ab11261; Abcam) is directed against aa 589 to 606 of mouse KIF17. The epitope is present in KIF17-ML1 but not KIF17-ML2.
AAV5 preparation and mouse subretinal injection
AAV5 particles expressing mC-tagged kinesin-2 proteins were generated by packaging 5 different pAAV-hRK-mC-Kif17 and Kif3a plasmids into AAV5 particles via the 2-plasmid cotransfection method (17). The AAV5 particles were titered and resuspended in balanced salt solution (Alcon Laboratories, Fort Worth, TX, USA) containing 0.014% Tween-20 at a concentration of ∼1.0 × 1012 virus particles per milliliter for mouse subretinal injection. One microliter of AAV5 particles carrying mC–kinesin-2 expression cassette was injected into the subretinal space as previously described (18). Injections were performed into the left eye, leaving the right eye as an untreated control.
Generation of kinesin-2 transgenic X. laevis
Transgenic X. laevis tadpoles were generated as described elsewhere (19, 20). Briefly, purified NotI linearized expression plasmids were incubated with permeabilized sperm for 5 min. The plasmid DNA–sperm mixture was then treated with X. laevis egg extract and NotI for 10 min and injected into unfertilized eggs. Approximately 24 h after fertilization, embryos were transferred to 0.1× Ringer solution containing 18 µg/ml G418 to select for transgenic animals (21). Embryos were housed in 4 L tanks in an 18°C incubator on a 12:12 light cycle, and tadpoles were fed ground frog brittle (Nasco, Ft. Atkinson, WI, USA) when they reached swimming stages. At 14 d after fertilization, corresponding to developmental stage 48, normally developed X. laevis were killed and fixed in 4% paraformaldehyde (PFA) buffered with 0.1 M sodium phosphate, pH 7.4.
Generation of Kif17 knockout mice
The Kif17 targeting construct consists of a floxed exon 4 of mouse Kif17 gene in tandem with a FRT-flanked Neo cassette in intron 4 and a 5′-loxP site in intron 3. The FRT-flanked Neo cassette is self-removed after ES cell selection as it contains a testis-specific promoter driven Flp-recombinase gene expressed in the male germ line (unpublished data) (22). Heterozygous Kif17flox/+ mice were produced by breeding chimeras with C57/BL6 mice, and germ-line Kif17 knockout mice were produced by crossing Kif17flox/+ with EIIaCre mice expressing Cre ubiquitously. Cre-mediated recombination is predicted to delete the floxed exon 4 of Kif17, resulting in a truncated and unstable KIF17 peptide. Kif17 floxed and null alleles were detected by PCR genotyping using 3 DNA oligonucleotides: Kif17p1: 5′-AGTAGGATCCATGGAGTCTGC, Kif17p2: 5′-CCTAAAGGCTCTGTGGGACA, and Kif17p3: 5′-ACGGTATCAAAGCCTCATGG. To verify deletion of Kif17 exon 4 at the mRNA level, reverse transcription (RT)-PCR was performed with Kif17rtF: 5′-TTCTTAGTGCGGGCTTCCTA and Kif17rtR: 5′-CTTGATGTTCTTGGCCCTGT.
Cell cultures and transfection
HEK293 and NIH3T3 cell lines were grown in DMEM, and the IMCD3 cell line was grown in DMEM–F12 medium, all supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. Cells were transfected with lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. For immunofluorescence, cells were seeded on a sterilized circle coverslip at the bottom of each well of 12-well plates 1 d before transfection and serum starved for 48 to 60 h after transfection to induce ciliogenesis.
Coimmunoprecipitation and immunoblot
Transfected cells were resuspended and sonicated in lysis buffer (50 mM TrisHCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, 1 mM DTT, protease inhibitors) 24 h after cotransfection with mC-KIF3A and eG-KIF17. The cell lysates were then centrifuged at 20,000 g for 20 min at 4°C to remove insoluble material. For coimmunoprecipitation, supernatants were incubated with anti-mC antibody on a rotator for 2 h at 4°C. G-Sepharose beads were added and incubated for additional 45 min. Beads were pelleted by centrifugation at 3000 g for 1 min and washed 3 times in lysis buffer. Proteins were eluted from beads by boiling in 2× Laemmli buffer and analyzed by immunoblotting with anti-GFP antibody.
Mouse retina lysates were generated by brief sonication in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 1% SDS, 50 mM Tris pH 8.0). The supernatant of each lysate was separated by 10% SDS-PAGE and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Membranes were blotted and incubated with primary antibodies followed by horseradish peroxidase–conjugated secondary antibody. Phosphorescence (ECL system; NEN Life Science, PerkinElmer, Waltham, MA, USA) was used to visualize signal on X-ray film.
Immunofluorescence and confocal microscopy of transfected cells and mouse retinas
Transfected IMCD3 and NIH3T3 cells on coverslips were fixed with 4% PFA in PHEM buffer (60 mM PIPES, 27 mM HEPES, 10 mM EGTA, 8 mM MgSO4, pH 7.0) and permeabilized with 1% Triton X-100 in PBS each for 10 min at room temperature before immunostaining.
Mouse eyes and olfactory tissues were immersion fixed with 4% PFA in 0.1 M PBS, pH 7.4, for 2 h (eyes) or 3 to 8 h (olfactory tissues) on ice. After removal of eyeball anterior segments, and decalcification of olfactory tissues in 10% EDTA, pH 7.3, tissues were equilibrated sequentially with 15 and 30% sucrose in 0.1 M PBS for cryoprotection, followed by embedding and sectioning (12 µm thick sections).
For immunolabeling, transfected cells on coverslips and mouse sections were incubated in each primary antibody at 4°C overnight after blocking with 10% donkey serum, then washed 3 times with PBS. Then they were incubated in fluorescence-conjugated secondary antibodies for 1 to 2 h at room temperature, washed 3 times, and mounted with Vectashield medium (Vector Laboratories, Burlingame, CA, USA). DAPI nucleic acid stain (1:50,000, Invitrogen) was added to the secondary antibody solution; images were acquired using a FluoView 1000 confocal microscope with ×60 and ×100 objective lenses (Olympus, Tokyo, Japan).
Fluorescence intensity profile plots were created in ImageJ software (National Institutes of Health, Bethesda, MD, USA) by drawing lines across measurement structures on the transfected cell or mouse olfactory epithelium images for each channel, then exported to generate Excel graphs. Photoreceptor OS lengths of the kinesin-2–transfected retinas were measured in 5 mice of each type by drawing lines parallel to rod outer segments (ROSs) near the optic nerve.
Confocal immunolocalization of X. laevis retinas
Transgenic X. laevis eyes were excised and immediately immersion fixed with 4% PFA in 0.1 M PBS, pH 7.4, at 4°C for more than 1 d. Fixed eyes were infiltrated with 20% sucrose for 2 h, embedded in Optimal Cutting Temperature compound, and cut into 12 µm thick cryosections. Sections were labeled overnight with 1:200 dilution of anti-acetylated tubulin monoclonal antibody (Sigma-Aldrich), followed by 1:750 dilution of Cy5-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and counterstained with Alexa Fluor 555–conjugated wheat germ agglutinin (WGA) (Life Technologies, Carlsbad, CA, USA) and Hoechst 33342 (Sigma-Aldrich). Labeled sections were mounted with MOWIOL 4-88 reagent (EMD Millipore). Sections were imaged using a Zeiss LSM 510 confocal microscope and a ×40 (Zeiss C-apochromat, NA 1.2) objective lens (Carl Zeiss GmbH, Jena, Germany). Postmicroscopy image processing was performed using Adobe Photoshop (Adobe Systems, San Jose, CA, USA). Brightness and contrast from native GFP fluorescence was adjusted linearly (i.e., no γ setting change) to preserve the relative intensities from different parts of the cell. However, fluorescent signals derived from WGA and Hoechst 33342 staining were adjusted to best represent retina architecture.
Electroretinography
Full-field electroretinography (ERG) was performed on Kif17−/−, Kif3a/Kif17 double knockout (dKO) and heterozygous control mice by using a UTAS E-3000 system (LKC Technologies, Gaithersburg, MD, USA) as described elsewhere (11).
Olfactory behavior
Olfactory function of 3-mo-old Kif17 knockout, heterozygous, and wild-type mice was examined with a behavior test (23). Test mice in individual cages were deprived of food for 16 h. A piece of cheese was buried under the bedding, and the time it took for each mouse to locate the cheese was measured. Ten mice were tested for each group.
Statistical analysis
Photoreceptor OS lengths and food finding latencies were analyzed by the Student’s t test. Two-way ANOVA was used to analyze scotopic a-wave and photopic b-wave amplitudes. Statistical significance level was set at P = 0.01.
RESULTS
KIF17 and KIF3 in mouse rod photoreceptors and olfactory sensory neurons
Anterograde IFT motor subunits (KIF17, KIF3A, and KIF3B) are highly conserved and widely expressed in the animal kingdom. In mouse retina, KIF3A (Fig. 1B) and KIF17 (Fig. 1C) were observed abundantly in photoreceptor IS and the outer nuclear layer (ONL). We used centrin 2 (CETN2), a centriole and CC marker, to delineate ciliary localization of KIF3A and KIF17 in photoreceptors. In transgenic mice expressing GFP-CETN2, KIF3A and KIF17 localized at the proximal OS axoneme (Fig. 1B, inset, C). In olfactory epithelia, KIF3A was located below the olfactory sensory cilium layer (Fig. 1D) and colocalized with the centriole marker γ-tubulin at dendritic knobs, a compartment corresponding to the IS (Fig. 1E). In contrast, KIF17 colocalized with Ac-α-tubulin at the ciliary layer housing the olfactory cascade, which corresponds to the rod OS (Fig. 1F).
Expression of FL vs. ML KIF3A and KIF17 in mouse and X. laevis photoreceptors
Surprisingly, KIF3A and KIF17 were undetectable by antibody labeling in the photoreceptor OS. We therefore followed expressions of tagged FL KIF3A and KIF17 in vivo (mouse and X. laevis) and in vitro (ciliated IMCD3 cells) to establish their subcellular localizations as active motors. KIF3A (Fig. 2A) and KIF17 (Fig. 2B) polypeptides share a similar protein domain structure, composed of an N-terminal motor segment, a central coiled-coil stalk for dimerization, and a C-terminal cargo-binding domain. Without cargo binding, KIF3 and KIF17 are present at the cytoplasm as an inactive form, while they move along the microtubule axoneme as an active form once cargo is loaded to the C-terminal domain (Fig. 1A). KIF17 is inactivated by autoinhibition via a head-to-tail conformation (24, 25). FL constructs, packaged into AAV5 particles, were injected subretinally to achieve overexpression in mouse retina. Transgenic viral expressions of mC-tagged FL KIF3A, and KIF17 under the control of the human rhodopsin kinase (GRK1) promoter showed strong signal at the photoreceptor IS and ONL (Fig. 2C, E). Only mC-KIF17 fluorescence was found as a string of particles along the OS axoneme (Fig. 2E, inset), suggesting that KIF17 participates in photoreceptor IFT, either as active motor or as cargo, at the distal part of the microtubule axoneme as observed in C. elegans (Fig. 1A). Most KIF3 and KIF17 present in the IS appears to be inactive.
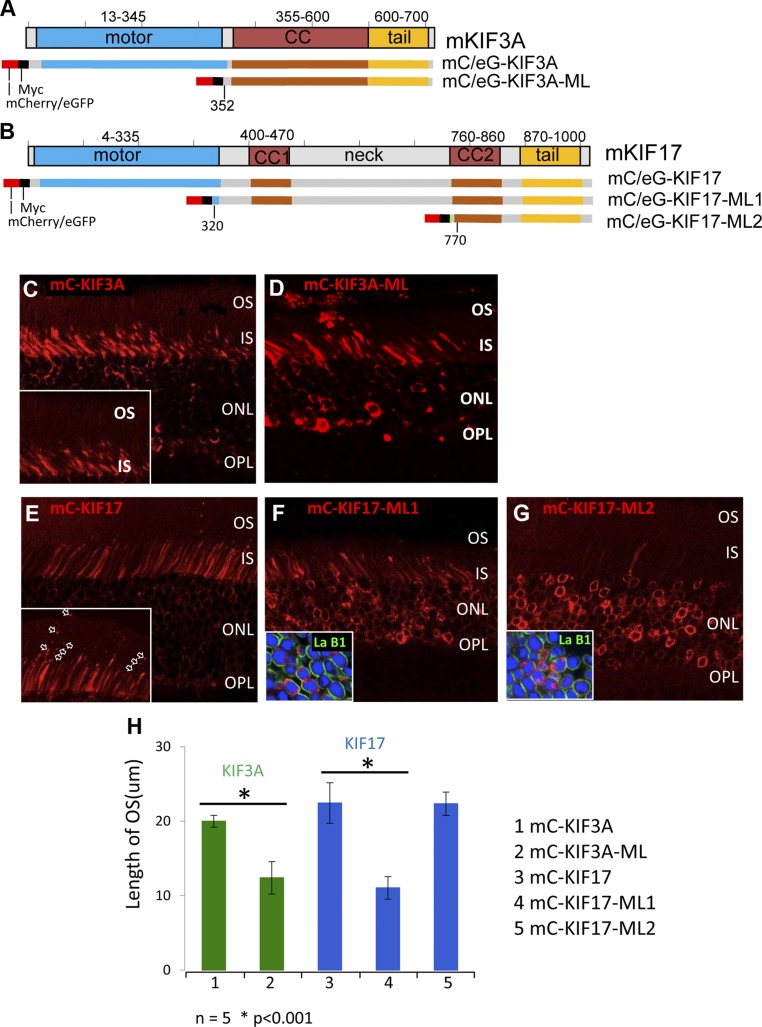
Figure 2.
FL and ML kinesin-2 polypeptides expressed in mouse photoreceptors. A and B) Schematic representation of mouse KIF3A (A) and KIF17 (B) domain structures. Motor (blue), coiled-coil (CC; red), and tail (yellow) domains are indicated. KIF3A and KIF17 FL and ML constructs carry N-terminal fluorescent (mC or eGFP) and myc tags. C and D) Expressions of mC- and myc-tagged FL KIF3A [mC-KIF3A (C)] and ML KIF3A [mC-KIF3A-ML (D)]. Note that in 1 mo AAV-transduced retina, mC-KIF3A was absent in OS (C, inset). E) mC-KIF17 is expressed in photoreceptor IS and ONL; note that mC-KIF17 particles line up along OS axoneme (E, inset, arrows). F and G) Motorless KIF17 mutant mC-KIF17-ML1 localizes at IS and ONL (F); mC-KIF17-ML2 accumulates almost exclusively within ONL nuclei (G). Nuclear envelopes are defined by lamin B1 labeling (F, G, insets, green). H) Quantitation of photoreceptor OS lengths at 1 mo as index of degeneration: mC-KIF3A (lane 1), mC-KIF3A-ML (lane 2), mC-KIF17 (lane 3), mC-KIF17-ML1 (lane 4) and mC-KIF17-ML2 (lane 5) (n = 5, P < 0.01).
Motorless KIF3A and KIF17 proteins are predicted to exert dominant-negative effects on kinesin-2–dependent IFT, as they are unable to transport cargo into cilia. To test whether KIF3 and KIF17 participate in photoreceptor IFT, we generated ML constructs expressing mC-KIF3A-ML (ΔAA 2–352), mC-KIF17-ML1 (ΔAA 2–320), and mC-KIF17-ML2 (ΔAA 2–810) (Fig. 2A, B) in which either the N-terminal motor domain alone or the N-terminal motor including the coil-coiled domains was deleted. At 1 mo after AAV transduction, mC-KIF3A-ML (Fig. 2D) localized mostly in the photoreceptor IS and ONL, but the photoreceptor OS were significantly shortened and ONL thickness was reduced relative to photoreceptors expressing mC-KIF3A (Fig. 2C). mC-KIF17-ML1 containing the entire dimerization domain was present mostly in the IS, was cytotoxic, and caused retinal degeneration (Fig. 2F, H). The N-terminal deletion mutant mC-KIF17-ML2 was less toxic and translocated into nuclei (Fig. 2G, inset). Photoreceptor degeneration in mouse retinas expressing KIF3A-ML or KIF17-ML1 suggests that KIF3 and KIF17 are both involved in photoreceptor IFT. The N-terminal deletion mutant mC-KIF17-ML2 was less toxic, possibly because of its predominant nuclear translocation (Fig. 2G, inset).
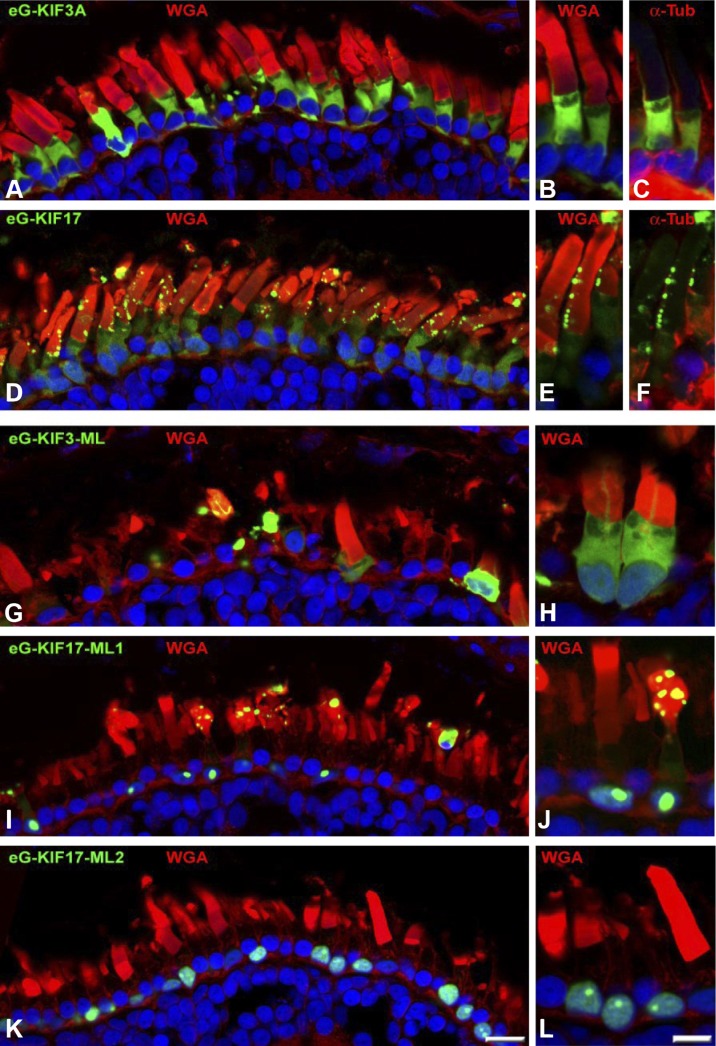
To explore the localization of kinesin-2 subunits in nonmammals, we expressed eG-tagged KIF3A (Fig. 3A–C) and KIF17 (Fig. 3D–F) in X. laevis rods under the control of the X. laevis rod opsin promoter. eG-KIF3A exhibited a cytoplasmic distribution including the axoneme (Fig. 3A–C) but excluding the nucleus whereas eG-KIF17 localized perinuclearly, diffusely in the IS, and in the OS as strings of discrete eGFP puncta along the ciliary axoneme (Fig. 3D–F). Expression of eGFP-tagged constructs (ML eG-KIF3A-ML, eG-KIF17-ML1, and eG-KIF17-ML2) was also analyzed in transgenic X. laevis (Fig. 3G–L). eG-KIF3A-ML (Fig. 3G, H) exhibited a distribution similar to that of eG-KIF3A but with additional diffuse GFP signal in nuclei. Both eG-KIF17-ML1 (Fig. 3I, J) and eG-KIF17-ML2 (Fig. 3K, L) translocated efficiently into nuclei of X. laevis photoreceptors. eG-KIF17-ML2 (and to a lesser extent eG-KIF17-ML1) was sequestered into a nuclear subcompartment that may correspond to the nucleolus or nuclear detention center (26) (Fig. 3J, L). Apparent differences in nuclear localizations of KIF17 ML mutants in X. laevis and mouse (translocation into the nucleus vs. translocation to the nuclear envelope) may be attributed to different nuclear architectures of mouse and X. laevis rod photoreceptors (27). Surprisingly, eG-KIF17-ML1 also localized to the OS axoneme in puncta similar to eG-KIF17, although with lower frequency (Fig. 3I, J). In contrast, eG-KIF17-ML2 did not traffic to the OS. Photoreceptor degeneration was observed in X. laevis retinas expressing eG-KIF3A-ML (Fig. 3G, H), eG-KIF17-ML1 (Fig. 3I), and eG-KIF17-ML2 (Fig. 3K). Thus, in 2 vertebrate model organisms (mouse and X. laevis), kinesin-2 ML mutants KIF3A-ML and KIF17-ML1 caused photoreceptor degeneration, probably by a dominant negative effect on IFT.
Figure 3.
FL and ML mutant kinesin-2 proteins expressed in X. laevis rods. A–C) eGFP-tagged FL KIF3A (eG-KIF3A) expressed in transgenic X. laevis rods. View of photoreceptors expressing eG-KIF3A, counterstained with WGA (A, B); 2 rods enlarged (B, C) and counterstained with anti–α-tubulin (C). D–F) eGFP-tagged FL KIF17 (eG-KIF17) expressed in transgenic X. laevis rods. eG-KIF17 was present at OS as particle strings along microtubule-containing axoneme (D). Two rods, enlarged and counterstained with WGA (E) and α-tubulin (F). G, H) eG-KIF3A-ML expression causes severe retinal degeneration in X. laevis. eG-KIF3A-ML distributed in IS and severely disrupted OS as bulky dots. I–L) eGFP-tagged eG-KIF17-ML1 (I, J) and eG-KIF17-ML2 (K, L) cause photoreceptor degeneration. Photoreceptor membranes and nuclei were visualized by Alexa Fluor 555–conjugated WGA and DAPI, respectively.
FL and ML KIF17 traffic to the distal ends of IMCD3 and NIH3T3 cilia
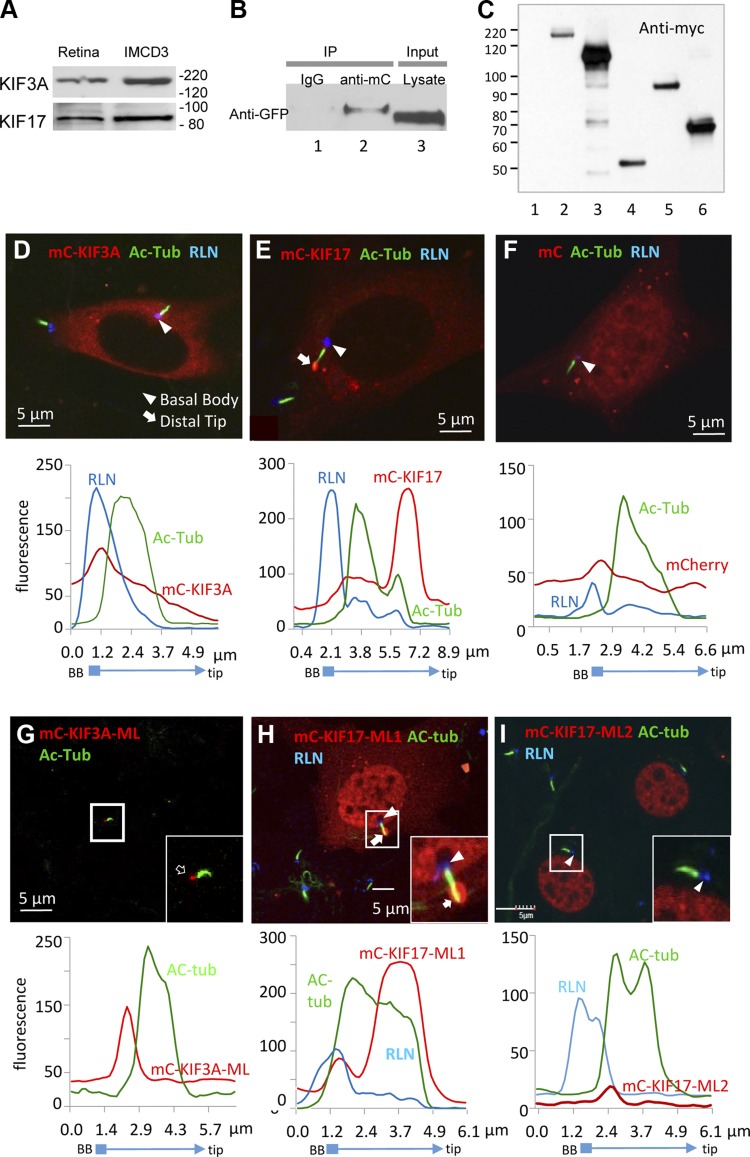
To study how KIF3A-ML and KIF17-ML1, but not KIF17-ML2, interfered with IFT, we expressed these mutants in ciliated IMCD3 (inner medullary collecting duct) cells. KIF3A and KIF17 are present in mouse retina as well as in IMCD3 cells (Fig. 4A). To verify interaction between KIF3 and KIF17 in IMCD3 cells, we cotransfected eG-KIF17 and mC-KIF3A and observed that eGFP-KIF17 coimmunoprecipitated with mC-KIF3A (Fig. 4B, lane 2), as observed in mouse retina (28), presumably by formation of KIF17/KIF3 IFT complexes. Expression of FL and truncated kinesin-2 polypeptides double-tagged with mC and anti-myc in mammalian cells was confirmed by immunoblot with anti-myc antibody (Fig. 4C). FL mC-KIF3A and mC-KIF17 distributed diffusely in the cytoplasm, presumably in their inactive forms (Fig. 4D, E). mC-KIF3A localized at basal body periphery, while FL mC-KIF17 accumulated at the distal tip of the ciliary axoneme (Fig. 4E, arrow), suggesting that the ciliary localizations of active KIF3A and KIF17 (at proximal and distal microtubule axoneme, respectively) in IMCD3 cells are similar to those observed in photoreceptors. As a control, soluble mC diffused throughout transfected IMCD3 cells, including nuclei, but was absent in primary cilia (Fig. 4F). In contrast to mC-KIF3A (Fig. 4D), we observed very few cells expressing low levels of mC-KIF3A-ML after 2 to 3 d of serum starvation (Fig. 4G), suggesting severe cytotoxicity of the ML mutant. Expressed mC-KIF3A-ML localized to the basal body in the very few surviving cells (Fig. 4G, arrow) and was absent from the cytoplasm. Motorless mC-KIF17-ML1 and mC-KIF17-ML2 both translocated to nuclei (Fig. 4H, I). FL mC-KIF17 and mC-KIF17-ML1, but not mC-KIF17-ML2, also distributed in the cytoplasm and trafficked to the ciliary tip (Fig. 4E, H, inset). The results suggest that ML mC-KIF17-ML1 may be transported to the ciliary distal tips as cargo of endogenous KIF3 via formation of IFT complexes.
Figure 4.
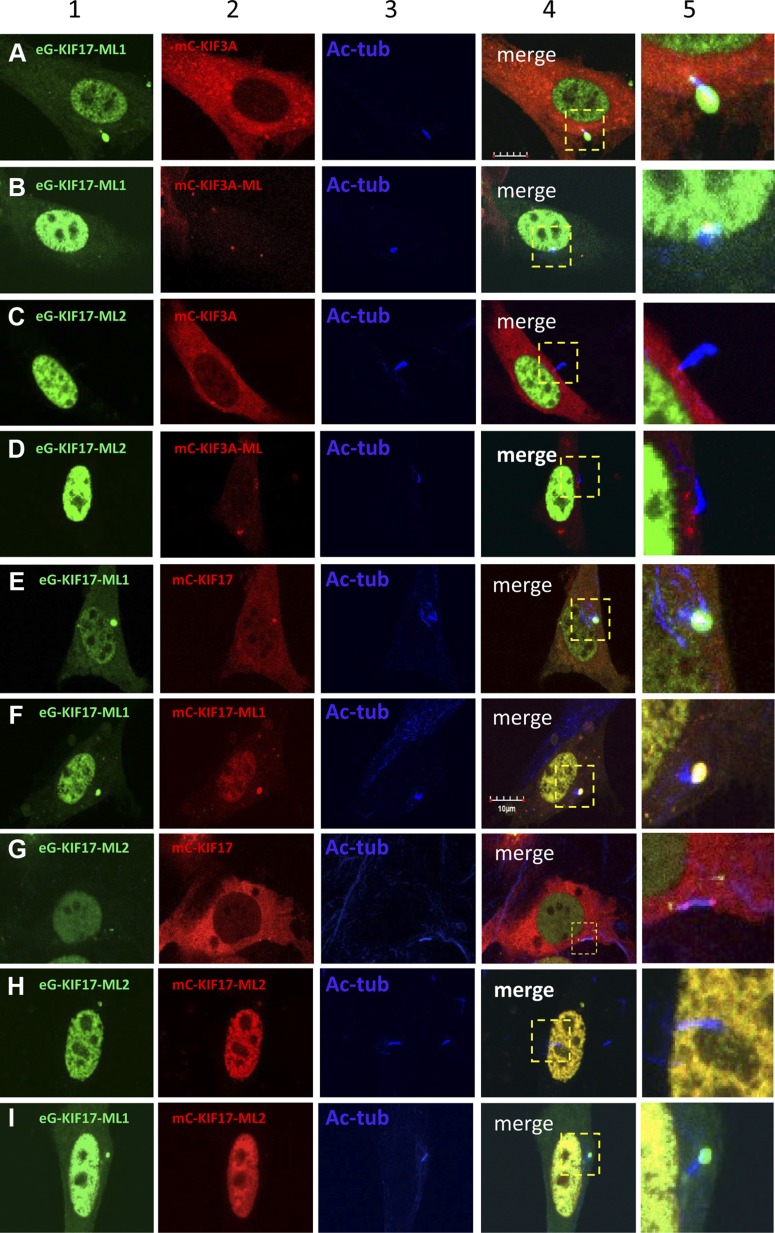
FL and ML kinesin-2 proteins expressed in mammalian cells. A) Immunoblot of mouse retina and IMCD3 cell lysates. B) Immunoprecipitation with nonspecific IgG (lane 1, negative control) and anti-mC antibodies (lane 2, 1:200). Lane 3 is cell lysate input. Immunoblot was probed with anti-GFP (1:1000) antibody. C) Immunoblot of HEK293 cell lysates transfected with FL and ML kinesin-2 constructs. Polypeptides were visualized 48 h after transfection with anti-myc antibody. Lane 1, nontransfected control; lane 2, mC-KIF17; lane 3, mC-KIF17-ML1; lane 4, mC-KIF17-ML2; lane 5, mC-KIF3A; lane 6, mC-KIF3A-ML. D–I) Expression of FL (D, E) and ML (G–I) KIF3A and KIF17 constructs in ciliated IMCD3 cells, as indicated. mC-transfected cells served as control (F). Ciliary localization of transfected kinesin-2 proteins in representative double-transfected cells shown in (D–I) were examined by fluorescence profile plots, shown underneath each panel. Primary cilia and basal bodies (BB) are labeled with anti–Ac-α-tubulin (green) and anti-rootletin (RLN) (blue) antibodies, respectively.
KIF3 traffics KIF17-ML1 to the distal axoneme
To test whether KIF17-ML1 is transported to the cilium distal tip cooperatively with KIF3, we transfected eG-KIF17-ML1 into IMCD3 cells with either mC-KIF3A or its ML mutant, mC-KIF3A-ML. mC-KIF3A forms heterotrimers with native KIF3B and KAP and serves as a KIF3 tracer. Motorless eG-KIF17-ML1 colocalized with mC-KIF3A at the ciliary distal tip (Fig. 5A, columns 4 and 5), providing evidence that eG-KIF17-ML1 traffics with KIF3. eG-KIF17-ML1 and mC-KIF3A also colocalized to a lesser extent at the ciliary basal body and the cytoplasm, but not in the nucleus or cytoplasm (Fig. 5A), indicating that translocation to the nucleus is KIF3A-independent. In IMCD3 cells coexpressing eG-KIF17-ML1 and mC-KIF3A-ML, primary cilia were shortened or absent, suggesting dominant negative interference of KIF3A-ML with IFT, as seen in vertebrates (Fig. 2). Doubly ML eG-KIF17-ML1 and mC-KIF3A-ML colocalized at the basal body periphery and did not traffic (Fig. 5B). eG-KIF17-ML2 lacking CC1 and neck domains (unable to form dimers) cotransfected with mC-KIF3A or mC-KIF3A-ML accumulated exclusively in nuclei unable to interact with either mC-KIF3A (Fig. 5C) or mC-KIF3A-ML (Fig. 5D). The results suggest that wild-type KIF3, but not its ML mutant, is able to transport ML KIF17-ML1 as cargo through IFT complexes.
Figure 5.
Trafficking of FL and ML KIF3A and KIF17 polypeptides in ciliated IMCD3 cells. A and B) Cotransfection of eGFP-tagged ML eG-KIF17-ML1 with mC-KIF3A (A) and mC-KIF3A-ML (B) in ciliated IMCD3 cells. Scale bar in (A), 10 μm. C and D) Cotransfection of eGFP-tagged ML eG-KIF17-ML2 with mC-KIF3A (C) and mC-KIF3A-ML (D). E and F) Cotransfection of eG-KIF17-ML1 with FL mC-KIF17 (E) and mC-KIF17-ML1 (F). G and H) Cotransfection of eG-KIF17-ML2 with FL mC-KIF17 (G) and mC-KIF17-ML2 (H). I) Cotransfection of eG-KIF17-ML1 with mC-KIF17-ML2. Column 1, expression of eGFP-tagged ML KIF17 mutants (green); column 2, expression of mC-tagged KIF3A or KIF17 FL and ML peptides (red); column 3, cilia identified with anti–Ac-α-tubulin antibody; column 4, multichannel merged micrographs focusing on cilia; column 5, enlargement of field in column 4.
We next tested whether ML KIF17 mutants KIF17-ML1 and KIF17-ML2 are able to form dimers by transfecting with FL KIF17 into IMCD3 cells. Expressed eG-KIF17-ML1 (Fig. 5E) colocalized with mC-KIF17 at ciliary tips and partially in the cytoplasm, but eG-KIF17-ML2 (Fig. 5G) was absent from cilia and accumulated in nuclei. As controls, eG-KIF17-ML1 colocalized with mC-KIF17-ML1 (Fig. 5F) at ciliary tips and nuclei, and eG-KIF17-ML2 colocalized with mC-KIF17-ML2 in nuclei (Fig. 5H). Finally, eG-KIF17-ML1 and mC-KIF17-ML2 did not colocalize in the cytoplasm of transfected cells (no dimer formation) (Fig. 5I). The results demonstrate that KIF17-ML1, but not KIF17-ML2, was able to form heterodimers with KIF17, suggesting dimerization is critical for movement of ML KIF17 protein into cilia via KIF3-dependent IFT.
Abolishing nuclear translocation of KIF17 ML mutants
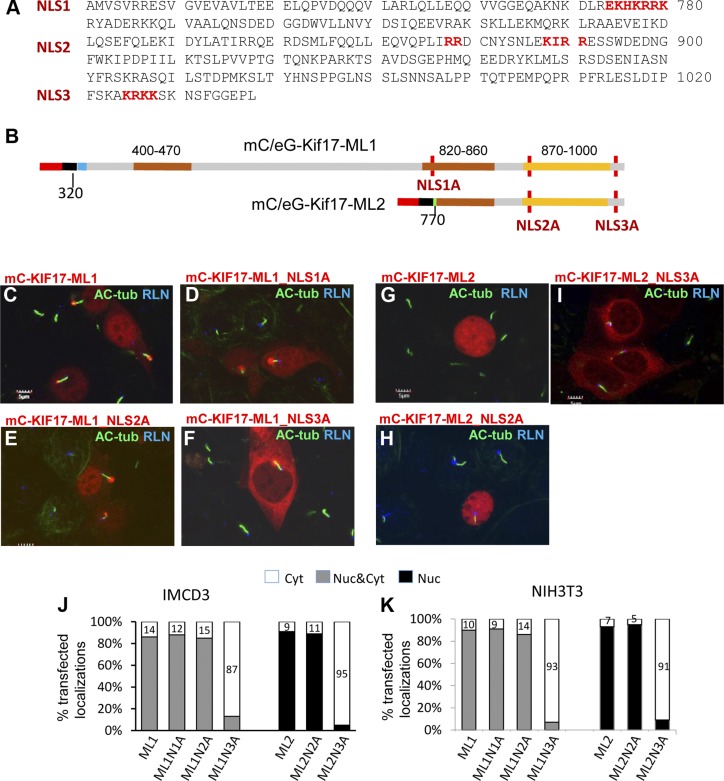
To identify and abolish the nuclear translocation of ML KIF17 mutants, amino acids of the 3 predicted NLS (aa 774–780, 888–891, and 1025–1028) were replaced sequentially with alanines (Fig. 6A). We generated 3 NLS mutants (NLS1A, NLS2A, and NLS3A) for mC-KIF17-ML1 and -2 (NLS2-3A) for mC-KIF17-ML2 (Fig. 6B). Only NLS3A mutants, mC-KIF17-ML1_NLS3A (Fig. 6F) and mC-KIF17-ML2_NLS3A (Fig. 6I) localized predominantly in the cytoplasm, whereas NLS1 (Fig. 6D) and NLS2 (Fig. 6E, H) mutants translocated into nuclei despite mutations. Subcellular localization analyses of 200 transfected IMCD3 cells expressing mC-KIF17-ML1 and its NLS mutants revealed that nuclear translocation decreased to 13% in the mC-KIF17-ML1_NLS3A-expressed cells, compared with 86, 88, or 85% in the mC-KIF17-ML1-, NLS1A-, or NLS2A-expressed cells, respectively (Fig. 6J, gray columns). Similarly, nuclear localization was reduced to only 5% in the mC-KIF17-ML2_NLS3A-transfected cells but continued to translocate to nuclei at 91% efficiency in the mC-KIF17-ML2 and at 89% efficiency in the NLS2A-expressed cells (Fig. 6J, right columns). The result was duplicated in NIH3T3 cells (Fig. 6K). We found that mC-KIF17-ML2, rendered cytoplasmic by mutation of its NLS, was still unable to traffic into cilia, suggesting that interruption of KIF17-ML2 with IFT is due to inability to dimerize. Importantly, dimerization-competent KIF17-ML1 trafficked to cilia even when NLS3 was mutated. Thus, NLS3 (aa 1025–1028) at the distal C terminus is the KIF17 NLS.
Figure 6.
Identification of KIF17 NLS. A) C-terminal mouse KIF17 sequence showing 3 predicted NLS (NLS1–3, red). B) Schematic of NLS mutants of ML KIF17 peptides. Amino acids at positions 777–788 (NLS1A), 887–893 (NLS2A), and 1025–1028 (NLS3A) were replaced by alanines, with approximate locations in mC-KIF17-ML1 and mC-KIF17-ML2 peptides indicated (red vertical lines). C–F) Expression of mC-KIF17-ML1 and its 3 NLS mutants in IMCD3 cells. Primary cilia and basal bodies are labeled with anti–Ac-Tub (green) and anti-rootletin (blue) antibodies, respectively. Only mC-KIF17-ML1_NLS3A (F) was excluded from nuclei and localized in cytoplasm exclusively. Notably, mC-KIF17-ML1 and its 3 NLS mutants localized at distal tips of IMCD3 cell cilia. G–I) Expression of mC-KIF17ML2 and its 2 NLS mutants. Only mC-KIF17-ML2_NLS3A (I) was absent from nuclei and was instead distributed in cytoplasm. J and K) Statistical analyses of subcellular localizations of transfected KIF17ML1, KIF17ML2, and their NLS mutants expressed in IMCD3 (J) and NIH3T3 cells (K). Bars represent cytoplasmic (white), whole cell (gray), and nuclear (black) localizations, respectively.
Photoreceptor and olfactory IFT proceed in absence of KIF17
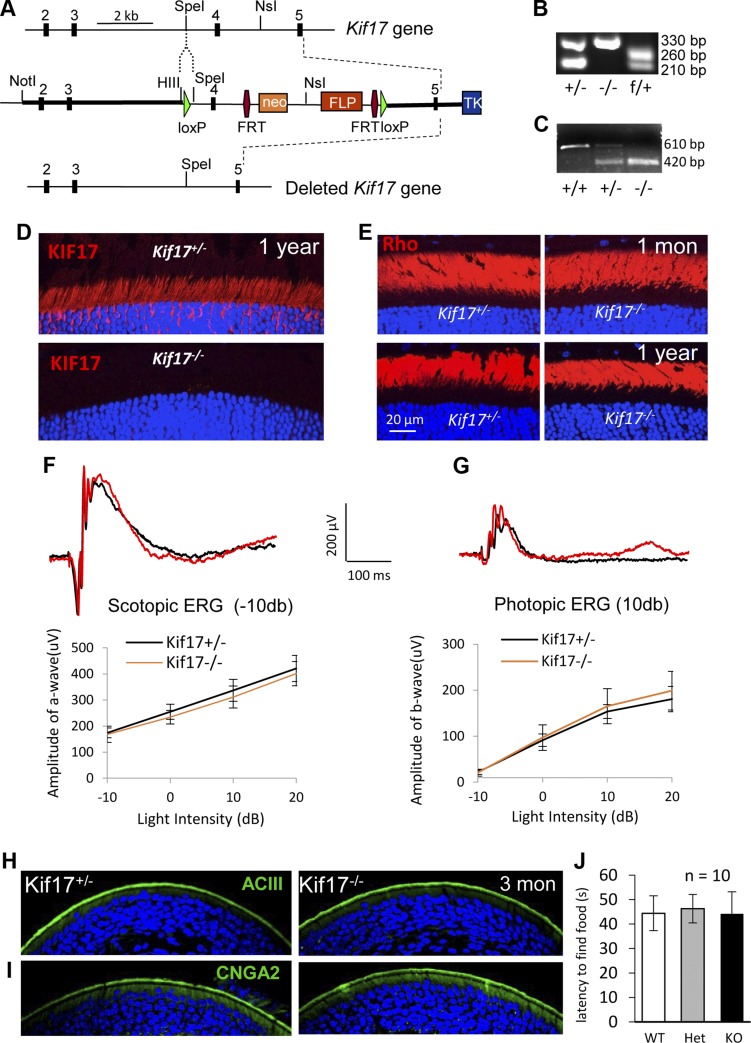
Experiments with vertebrate photoreceptors and ciliated IMCD3 cell indicated that KIF17 is present in cilia and participates in KIF3-driven IFT. What is unclear, however, is whether KIF17 is essential for ciliary protein trafficking. We therefore generated a germ-line Kif17−/− mouse to test whether KIF17 is involved in photoreceptor IFT in the context of ciliogenesis or rhodopsin trafficking. In the Kif17 null allele, we deleted exon 4 of the Kif17 gene using a Cre/loxP gene targeting approach. In the targeted allele, KIF17 was truncated after its motor domain (Fig. 7A). Genotyping and RT-PCR confirmed deletion of exon 4 in the null allele (Fig. 7B, C). Immunohistochemistry with anti-KIF17 antibodies verified absence of KIF17 in Kif17−/− mouse retina (Fig. 7D). Knockout mice were healthy and fertile, and they were indistinguishable from wild-type mice. Kif17−/− retina morphology was normal at all ages analyzed (1, 3, 6, and 12 mo; data not shown), and rhodopsin trafficked to the Kif17−/− ROS at 1 mo and 1 yr of age with no evidence of mislocalization (Fig. 7E). Scotopic a-wave and photopic b-wave ERG amplitudes of the knockout mouse were not significantly different from those of controls (Fig. 7F, G and Table 1).
Figure 7.
Germ-line deletion of Kif17 in mouse. A) Schematic of mouse Kif17 gene, targeting construct, and null allele. Green triangles, lox P sites; red hexagons, FRT sites flanking Neo and FLP cassettes. Relevant restriction sites are indicated. B) PCR genotyping of heterozygous (Kif17+/− and Kif17f/+) and knockout (Kif17−/−) mice. Amplicons of 330, 260, and 210 bp correspond to null, floxed, and WT alleles, respectively. C) RT-PCR analysis of Kif17 mRNA from WT (Kif17+/+), heterozygous (Kif17+/−), and knockout (Kif17−/−) mouse retinas. Amplicons of 610 and 420 bp represent mRNA products of Kif17 WT and null alleles, respectively. D) Immunolocalization of KIF17 (red) in Kif17+/− and Kif17−/− mouse photoreceptors at 1 yr of age. Kif17 is expressed at IS and ONL of Kif17+/− retina (top) but is absent in Kif17−/− retina (bottom). E) Rhodopsin (red) immunolocalization in Kif17+/− and Kif17−/− retinas. Rhodopsin targets Kif17−/− ROS (right) at 1 mo and 1 yr of age as in heterozygous controls (left). F and G) Representative scotopic (−10 db) and photopic (10 db) ERG traces from Kif17 knockout and heterozygous mice at 1 yr. Below is quantitative evaluation of scotopic a-wave and photopic b-wave amplitudes at 4 different light intensities (n = 6). H, I) ACIII and CNGA2 localizations in Kif17+/− and Kif17−/− olfactory epithelia. Expressions of ACIII (H, green) and CNGA2 (I, green) at olfactory epithelial apical surfaces were indistinguishable between heterozygous control and 3 mo old Kif17 knockout mice. J) Olfactory function of Kif17+/− and Kif17−/− mice evaluated at 3 mo of age. Olfactory behavior indicated that Kif17−/− mice (black bar) took 45.3 s to find hidden food, vs. heterozygous (gray, 46.3 s, P = 0.76) and WT mice (white, 44.4 s, P = 0.79), respectively; n = 10 mice per genotype.
TABLE 1.
Statistical analysis of ERG amplitudes recorded from Kif17+/− and Kif17−/− mice
| Mouse | Light intensity (dB) |
P | |||
|---|---|---|---|---|---|
| −10 | 0 | 10 | 20 | ||
| a-wave amplitude of scotopic ERG response (μV) | |||||
| Kif17+/− | 174 ± 19 | 255 ± 29 | 337 ± 42 | 421 ± 50 | 0.11* |
| Kif17−/− | 168 ± 31 | 234 ± 26 | 312 ± 42 | 401 ± 46 | |
| b-wave amplitude of photopic ERG response (μV) | |||||
| Kif17+/− | 22 ± 5 | 91 ± 14 | 154 ± 15 | 181 ± 28 | 0.25* |
| Kif17−/− | 21 ± 7 | 97 ± 28 | 165 ± 38 | 199 ± 42 | |
P < 0.01, n = 6.
Previous in vitro experiments suggested that KIF17 is essential for ciliary targeting of olfactory CNGA2 (29). Confocal immunolocalization of CNGA2 and ACIII showed that each targets to the olfactory ciliary layer, as observed in the Kif17+/− mouse (Fig. 7H, I). We examined Kif17−/− olfactory function by a behavioral test that measures the time needed for 16 h unfed mice to find hidden food. No significant difference in the latency of food finding was measured among Kif17−/− (45.3 s, n = 10), Kif17+/− (46.3 s, n = 10), or Kif17+/+ (44.4 s, n = 10) mice (Fig. 7J). Thus, KIF17 is not required for ciliary trafficking of ACIII or CNGA2 in olfactory neurons.
Rhodopsin traffics in absence of KIF3 and KIF17
Our previous studies of rod-specific Kif3a single knockouts (iCre75+;Kif3af/f) showed that rhodopsin still traffics to OS even as photoreceptors degenerate (11). Further, deletion of KIF3A in the adult mouse by tamoxifen induction does not impair rhodopsin and OS trafficking, suggesting KIF17 may compensate for loss of KIF3A. Therefore, to test whether KIF3 and KIF17 function redundantly in rhodopsin transport via IFT, we generated a rod-specific Kif3a and germ-line Kif17 dKO mouse, iCre75+;Kif3af/f;Kif17−/− (roddKO), by crossing the rodKif3a−/− knockout (iCre75+;Kif3af/f) (8) with Kif17−/− germ-line knockout mice (11).
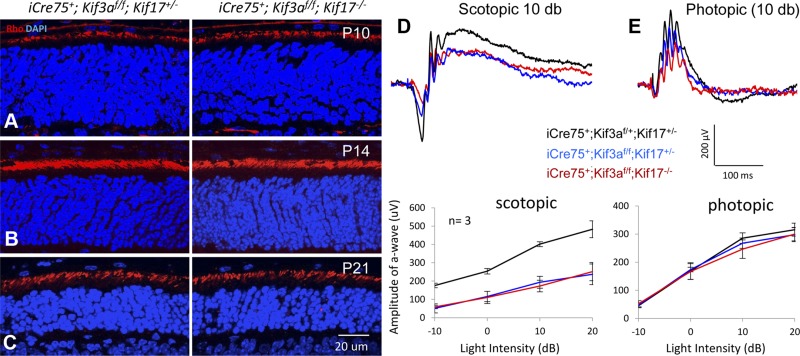
Immunohistochemistry revealed normal rhodopsin transport to ROS of roddKO mice at P10, P14, and P21, comparable to rodKif3a−/−;Kif17+/− controls (Fig. 8A–C). Scotopic and photopic ERGs of P21 roddKO mice were not significantly different from control measurements (Fig. 8D, E and Table 2), taking into account that dKO rods degenerate at P12 owing to the absence of KIF3A. Neither single knockouts nor Kif17/Kif3a dKO cause rhodopsin mistrafficking in mature rod photoreceptors, suggesting that KIF17 and KIF3 do not act synergistically.
Figure 8.
dKO of rod Kif17 and Kif3a. A–C) Rhodopsin localization in iCre75+;Kif3af/f; Kif17+/− (rodKif3a single knockout) and iCre75+;Kif3af/f;Kif17−/− (kinesin-2 roddKO) retinas. Rhodopsin localizes in ROS of rodKif3a control (left) and roddKO (right) photoreceptors at P10 (A), P14 (B), and even P21 (C), when ROS degeneration elicited by Kif3a depletion is severe (8). D and E) Representative scotopic (D) and photopic (E) ERG traces (10 db) from kinesin-2 roddKO (red traces), rodKif3a single KO (blue), and double heterozygous (black) control mice at P21. Below are scotopic a-wave and photopic b-wave amplitudes evaluated at 4 different light intensities from 3 mice of each type. Scotopic ERG responses are significantly decreased in dKO and single knockout, while photopic ERG responses are only slightly reduced as cones survive longer.
TABLE 2.
Statistical analysis of ERG amplitudes recorded from rod-specific Kif3a (iCre75+;Kif3af/f) and germ-line Kif17−/− (dKO) mice
| Mouse | Light intensity (dB) |
P | |||
|---|---|---|---|---|---|
| −10 | 0 | 10 | 20 | ||
| a-wave amplitude of scotopic ERG response (μV) | |||||
| iCre75+;Kif3af/+;Kif17+/− | 175 ± 12 | 254 ± 15 | 402 ± 13 | 483 ± 46 | |
| iCre75+;Kif3af/f;Kif17+/− | 51 ± 25 | 116 ± 28 | 193 ± 33 | 237 ± 56 | |
| iCre75+;Kif3af/f;Kif17−/− | 59 ± 9 | 111 ± 33 | 173 ± 32 | 251 ± 50 | |
| iCre75+;Kif3af/+;Kif17+/− vs. iCre75+;Kif3af/f;Kif17+/− | <0.001* | ||||
| iCre75+;Kif3af/+;Kif17+/− vs. iCre75+;Kif3af/f;Kif17−/− | <0.001* | ||||
| iCre75+;Kif3af/f;Kif17+/− vs. iCre75+;Kif3af/f;Kif17−/− | 0.64 | ||||
| b-wave amplitude of photopic ERG response (μV) | |||||
| iCre75+;Kif3af/+;Kif17+/− | 45 ± 4 | 168 ± 30 | 286 ± 19 | 316 ± 23 | |
| iCre75+;Kif3af/f;Kif17+/− | 47 ± 9 | 174 ± 8 | 268 ± 19 | 298 ± 25 | |
| iCre75+;Kif3af/f;Kif17−/− | 52 ± 11 | 167 ± 28 | 246 ± 33 | 300 ± 24 | |
| iCre75+;Kif3af/+;Kif17+/− vs. iCre75+;Kif3af/f;Kif17+/− | 0.36 | ||||
| iCre75+;Kif3af/+;Kif17+/− vs. iCre75+;Kif3af/f;Kif17−/− | 0.21 | ||||
| iCre75+;Kif3af/f;Kif17+/− vs. iCre75+;Kif3af/f;Kif17−/− | 0.56 | ||||
P < 0.01, n = 3.
DISCUSSION
The goal of this study was to identify the role of KIF17 (OSM-3), a plus-oriented anterograde molecular motor, in mouse and X. laevis photoreceptor IFT. OSM-3, the C. elegans ortholog of KIF17, has a well-defined role in elongation of microtubule singlets in the distal axoneme. Single mutants lacking KIF3 in C. elegans amphid channel cilia still form middle and distal segments of the axoneme, and only KIF3/OSM-3 double mutants lack the entire axoneme (30, 31). This provides clear evidence that OSM-3 participates in anterograde transport of axonemal building blocks and IFT particles in the nematode.
We previously demonstrated that embryonic retina-specific deletions of Kif3A/Ift88 abrogate ciliogenesis and that tamoxifen-induced depletion of Kif3A/Ift88 in adult mice reveal shortened OS by 2 to 3 wk after injection, a time interval over which OS are completely replaced more than once (11). Yet visual pigments traffic normally for more than 2 wk after induction, demonstrating that IFT supported by KIF3A and IFT88 is not required for rhodopsin transport to the ROS. This finding raised the possibility that KIF17 may participate in photoreceptor IFT as a redundant anterograde motor.
The function of KIF17 in zebrafish photoreceptors is unclear. KIF17 coimmunoprecipitates with IFT particles (IFT88, IFT57, and IFT20) (32), and KIF17-GFP accumulates in the OS distal tip (33). Morpholino knockdown of KIF17, truncating KIF17 in the N-terminal motor region, disrupts OS formation and visual pigment targeting (32); however, ciliogenesis proceeds largely normally. In the zebrafish loss-of-function mutant kif17sa0119, in which a stop codon in the coiled-coil stalk results in truncation of the 271 C-terminal amino acids including the cargo-binding domain, ciliogenesis proceeds, and visual pigments traffic normally (13). However, KIF17 can substitute for loss of KIF3 in some tissues (13).
KIF3 and KIF17 polypeptides are each comprised of 3 functional domains: an N-terminal motor domain of ∼330 aa, a central dimerization region containing coil-coiled motifs and a hinge region, and a divergent C-terminal cargo/adaptor binding domain (Fig. 2A, B). The C-terminal region has a ciliary/NLS (34). Binding of cargo is regulated by phosphorylation and interaction with the scaffolding protein Mint1 and other adaptors (35). The highly conserved motor domain consists of an ATP hydrolysis core and a microtubule-binding site, which is essential for kinesin-2 motor trafficking on the microtubule axoneme toward the plus end in the cell periphery. In the absence of cargo, KIF17 is autoinhibited and folds around the central hinge, enabling nonmotor regions to contact the motor domain thereby blocking ATPase activity and ADP release (25, 36). This mechanism resembles the interaction of guanine nucleotide dissociation inhibitors, with small G proteins preventing GTP/GDP exchange to keep the G protein inactive (37). Binding of cargo at the C-terminal region relieves autoinhibition.
Expressions of tagged FL KIF17 and KIF3A showed diffuse presence in the mouse and X. laevis rod IS and IMCD3 cell cytoplasm, presumably in the autoinhibited conformation. KIF3A was present at the proximal axoneme (Figs. 1B and 2C), and KIF17 lined up along mouse (Fig. 2E, inset) and X. laevis (Fig. 3D–F) OS axonemes, as expected from the C. elegans IFT model (Fig. 1A). In IMCD3 cells, mC-KIF3A located to the basal body (Fig. 4D) and mC-KIF17 to the ciliary distal tip, visible as an intensely fluorescing dot (Fig. 4E, arrow). Deletion of either KIF3A or KIF17 motor domains triggered retina degeneration in mouse and X. laevis. Degeneration most likely was caused by dominant negative interference with IFT. The interpretation is that KIF17-ML1 caused photoreceptor cell toxicity by interfering with IFT-mediated ciliogenesis and maintenance. However, KIF17-ML2 lacking the CC1 and neck domains was not transported to the cilia (Figs. 2G and 3K), probably because of its inability to interact with IFT complexes and/or predominant nuclear localization. KIF17-ML2 appears to be less toxic, as it caused only minor retinal degeneration. In KIF3A-ML–expressing X. laevis photoreceptors, the mutant was also observed to localize to cilia (Fig. 3G, H), and it therefore may cause severe photoreceptor degeneration by a dominant negative mechanism such as KIF17-ML1.
In mouse CNS, KIF17 has been reported to transport the NMDA receptor subunit NR2B (38), kainate receptor subunits GluR5 and GluR6 (39), and voltage-gated K+ channel KV4.2 (40) in vesicles from the cell body along microtubules to dendrites. Disruption of KIF17 inhibits NR2B transport, resulting in loss of synaptic NR2B concomitant with hippocampus-dependent memory impairment (41), suggesting that KIF17 motor function has been preserved in dendritic trafficking. Unexpectedly, deletion of KIF17 in photoreceptors had no consequence on rhodopsin trafficking, axoneme structure, or photoreceptor function (Fig. 7). Kif17−/− retina morphology was normal up to 2 yr postnatally, demonstrating that KIF17 is dispensable for photoreceptor development and vision. Furthermore, a rod-specific Kif3a knockout on a Kif17−/− background phenocopied the Kif3a single knockout (Fig. 8), demonstrating that KIF17 and KIF3A do not act synergistically. KIF17, although abundantly expressed in photoreceptors, is not involved in building the distal ciliary axoneme and has no discernible role in photoreceptor function.
Questions remaining are how rhodopsin traffics through the CC, how discs are formed at the proximal OS, and how rhodopsin is incorporated into nascent discs. Our experiments rule out heterotrimeric and homodimeric kinesin-2–dependent IFT for transport of phototransduction components to the OS. It seems unlikely that other kinesins traffic rhodopsin-containing cargo by IFT pathway through the CC, as deletion of IFT88 has no effect on trafficking (11). Mechanisms to be explored in the future include lateral diffusion of rhodopsin through the CC membrane avoiding the ciliary gate, followed by invagination of the proximal OS membrane to form discs. Alternatively, rhodopsin-laden vesicles may diffuse through the CC and enlarge in the proximal OS to form disks (42), which may be amenable to tracking rhodopsin movement with high-resolution microscopy techniques (43).
Acknowledgments
This work was supported by U.S. National Institutes of Health Grants EY08123, EY019298 (to W.B.), EY014800-039003 (National Eye Institute core grant to the Department of Ophthalmology, University of Utah), and Canadian Institutes of Health Research MOP-64400, as well as the Foundation Fighting Blindness (Toronto, ON, Canada) (to O.M.) and unrestricted grants to the Department of Ophthalmology, University of Utah, from Research to Prevent Blindness (New York, NY, USA). W.B. is a recipient of a Research to Prevent Blindness Senior Investigator and a Nelson Trust Award.
Glossary
- AAV
adeno-associated virus
- Ac
acetylated
- ACIII
adenylate cyclase III
- CC
connecting cilium
- CETN2
centrin 2
- CNGA2
cyclic nucleotide-gated channel subunit A2
- dKO
double knockout
- eG
transgenic enhanced green fluorescent protein
- eGFP
enhanced green fluorescent protein
- ERG
electroretinography
- FL
full-length
- IFT
intraflagellar transport
- IS
inner segment
- KIF
kinesin family
- mC
mCherry
- ML
motorless
- NLS
nuclear localization signal
- ONL
outer nuclear layer
- OS
outer segment
- OSM-3
osmotic avoidance abnormal-3
- PFA
paraformaldehyde
- ROS
rod outer segment
- RT
reverse transcription
- WGA
wheat germ agglutinin
REFERENCES
- 1.Liu Q., Zhang Q., Pierce E. A. (2010) Photoreceptor sensory cilia and inherited retinal degeneration. Adv. Exp. Med. Biol. 664, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilliam J. C., Chang J. T., Sandoval I. M., Zhang Y., Li T., Pittler S. J., Chiu W., Wensel T. G. (2012) Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell 151, 1029–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfrum U., Schmitt A. (2000) Rhodopsin transport in the membrane of the connecting cilium of mammalian photoreceptor cells. Cell Motil. Cytoskeleton 46, 95–107 [DOI] [PubMed] [Google Scholar]
- 4.Besharse J. C., Horst C. J. (1990) The photoreceptor connecting cilium: a model for the transition zone. In Ciliary and Flagellar Membranes (Bloodgood R. A., ed.), pp. 389–417, Springer, New York: [Google Scholar]
- 5.Deretic D., Wang J. (2012) Molecular assemblies that control rhodopsin transport to the cilia. Vision Res. 75, 5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young R. W. (1967) The renewal of photoreceptor cell outer segments. J. Cell Biol. 33, 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marszalek J. R., Liu X., Roberts E. A., Chui D., Marth J. D., Williams D. S., Goldstein L. S. (2000) Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell 102, 175–187 [DOI] [PubMed] [Google Scholar]
- 8.Avasthi P., Watt C. B., Williams D. S., Le Y. Z., Li S., Chen C. K., Marc R. E., Frederick J. M., Baehr W. (2009) Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J. Neurosci. 29, 14287–14298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Signor D., Wedaman K. P., Rose L. S., Scholey J. M. (1999) Two heteromeric kinesin complexes in chemosensory neurons and sensory cilia of Caenorhabditis elegans. Mol. Biol. Cell 10, 345–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholey J. M. (2013) Kinesin-2: a family of heterotrimeric and homodimeric motors with diverse intracellular transport functions. Annu. Rev. Cell Dev. Biol. 29, 443–469 [DOI] [PubMed] [Google Scholar]
- 11.Jiang L., Wei Y., Ronquillo C. C., Marc R. E., Yoder B. K., Frederick J. M., Baehr W. (2015) Heterotrimeric kinesin-2 (KIF3) mediates transition zone and axoneme formation of mouse photoreceptors. J. Biol. Chem. 290, 12765–12778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan X., Ou G., Civelekoglu-Scholey G., Blacque O. E., Endres N. F., Tao L., Mogilner A., Leroux M. R., Vale R. D., Scholey J. M. (2006) Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J. Cell Biol. 174, 1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao C., Omori Y., Brodowska K., Kovach P., Malicki J. (2012) Kinesin-2 family in vertebrate ciliogenesis. Proc. Natl. Acad. Sci. USA 109, 2388–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Insinna C., Luby-Phelps K., Link B. A., Besharse J. C. (2009) Analysis of IFT kinesins in developing zebrafish cone photoreceptor sensory cilia. Methods Cell Biol. 93, 219–234 [DOI] [PubMed] [Google Scholar]
- 15.Malicki J., Besharse J. C. (2012) Kinesin-2 family motors in the unusual photoreceptor cilium. Vision Res. 75, 33–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karan S., Tam B. M., Moritz O. L., Baehr W. (2011) Targeting of mouse guanylate cyclase 1 (Gucy2e) to Xenopus laevis rod outer segments. Vision Res. 51, 2304–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zolotukhin S., Byrne B. J., Mason E., Zolotukhin I., Potter M., Chesnut K., Summerford C., Samulski R. J., Muzyczka N. (1999) Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6, 973–985 [DOI] [PubMed] [Google Scholar]
- 18.Jiang L., Zhang H., Dizhoor A. M., Boye S. E., Hauswirth W. W., Frederick J. M., Baehr W. (2011) Long-term RNA interference gene therapy in a dominant retinitis pigmentosa mouse model. Proc. Natl. Acad. Sci. USA 108, 18476–18481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroll K. L., Amaya E. (1996) Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122, 3173–3183 [DOI] [PubMed] [Google Scholar]
- 20.Tam B. M., Xie G., Oprian D. D., Moritz O. L. (2006) Mislocalized rhodopsin does not require activation to cause retinal degeneration and neurite outgrowth in Xenopus laevis. J. Neurosci. 26, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moritz O. L., Biddle K. E., Tam B. M. (2002) Selection of transgenic Xenopus laevis using antibiotic resistance. Transgenic Res. 11, 315–319 [DOI] [PubMed] [Google Scholar]
- 22.Wu S., Ying G., Wu Q., Capecchi M. R. (2008) A protocol for constructing gene targeting vectors: generating knockout mice for the cadherin family and beyond. Nat. Protoc. 3, 1056–1076 [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi K., Kasahara K., Miyazaki I., Shimizu S., Taniguchi M., Matsuzaki S., Tohyama M., Asanuma M. (2009) Pericentrin, a centrosomal protein related to microcephalic primordial dwarfism, is required for olfactory cilia assembly in mice. FASEB J. 23, 3289–3297 [DOI] [PubMed] [Google Scholar]
- 24.Imanishi M., Endres N. F., Gennerich A., Vale R. D. (2006) Autoinhibition regulates the motility of the C. elegans intraflagellar transport motor OSM-3. J. Cell Biol. 174, 931–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammond J. W., Blasius T. L., Soppina V., Cai D., Verhey K. J. (2010) Autoinhibition of the kinesin-2 motor KIF17 via dual intramolecular mechanisms. J. Cell Biol. 189, 1013–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Audas T. E., Jacob M. D., Lee S. (2012) The nucleolar detention pathway: a cellular strategy for regulating molecular networks. Cell Cycle 11, 2059–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solovei I., Kreysing M., Lanctôt C., Kösem S., Peichl L., Cremer T., Guck J., Joffe B. (2009) Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137, 356–368 [DOI] [PubMed] [Google Scholar]
- 28.Insinna C., Humby M., Sedmak T., Wolfrum U., Besharse J. C. (2009) Different roles for KIF17 and kinesin II in photoreceptor development and maintenance. Dev. Dyn. 238, 2211–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins P. M., Hurd T. W., Zhang L., McEwen D. P., Brown R. L., Margolis B., Verhey K. J., Martens J. R. (2006) Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr. Biol. 16, 1211–1216 [DOI] [PubMed] [Google Scholar]
- 30.Evans J. E., Snow J. J., Gunnarson A. L., Ou G., Stahlberg H., McDonald K. L., Scholey J. M. (2006) Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J. Cell Biol. 172, 663–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholey J. M. (2012) Kinesin-2 motors transport IFT-particles, dyneins and tubulin subunits to the tips of Caenorhabditis elegans sensory cilia: relevance to vision research? Vision Res. 75, 44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Insinna C., Pathak N., Perkins B., Drummond I., Besharse J. C. (2008) The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev. Biol. 316, 160–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bader J. R., Kusik B. W., Besharse J. C. (2012) Analysis of KIF17 distal tip trafficking in zebrafish cone photoreceptors. Vision Res. 75, 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dishinger J. F., Kee H. L., Jenkins P. M., Fan S., Hurd T. W., Hammond J. W., Truong Y. N., Margolis B., Martens J. R., Verhey K. J. (2010) Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat. Cell Biol. 12, 703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillaud L., Wong R., Hirokawa N. (2008) Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat. Cell Biol. 10, 19–29 [DOI] [PubMed] [Google Scholar]
- 36.Verhey K. J., Hammond J. W. (2009) Traffic control: regulation of kinesin motors. Nat. Rev. Mol. Cell Biol. 10, 765–777 [DOI] [PubMed] [Google Scholar]
- 37.DerMardirossian C., Bokoch G. M. (2005) GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 15, 356–363 [DOI] [PubMed] [Google Scholar]
- 38.Setou M., Nakagawa T., Seog D. H., Hirokawa N. (2000) Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 288, 1796–1802 [DOI] [PubMed] [Google Scholar]
- 39.Kayadjanian N., Lee H. S., Piña-Crespo J., Heinemann S. F. (2007) Localization of glutamate receptors to distal dendrites depends on subunit composition and the kinesin motor protein KIF17. Mol. Cell. Neurosci. 34, 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu P. J., Rivera J. F., Arnold D. B. (2006) A role for Kif17 in transport of KV4.2. J. Biol. Chem. 281, 365–373 [DOI] [PubMed] [Google Scholar]
- 41.Yin X., Takei Y., Kido M. A., Hirokawa N. (2011) Molecular motor KIF17 is fundamental for memory and learning via differential support of synaptic NR2A/2B levels. Neuron 70, 310–325 [DOI] [PubMed] [Google Scholar]
- 42.Chuang J. Z., Zhao Y., Sung C. H. (2007) SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell 130, 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye F., Breslow D. K., Koslover E. F., Spakowitz A. J., Nelson W. J., Nachury M. V. (2013) Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. eLife 2, e00654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inglis P. N., Ou G., Leroux M. R., Scholey J. M. (2007) The sensory cilia of Caenorhabditis elegans. In The Wormbook (Kramer, J. M., Oerman, D. G., eds.), pp. 1–22, http://wormbook.org. [DOI] [PMC free article] [PubMed]
- 45.Snow J. J., Ou G., Gunnarson A. L., Walker M. R., Zhou H. M., Brust-Mascher I., Scholey J. M. (2004) Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat. Cell Biol. 6, 1109–1113 [DOI] [PubMed] [Google Scholar]
- 46.Signor D., Wedaman K. P., Orozco J. T., Dwyer N. D., Bargmann C. I., Rose L. S., Scholey J. M. (1999) Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J. Cell Biol. 147, 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]