Abstract
Background
Starting in 2008, the Central African Republic (CAR) experienced an unprecedented number of reported yellow fever (YF) cases. A risk assessment of YF virus (YFV) activity was conducted to estimate potential disease risk and vaccine needs.
Methods
A multistage cluster sampling design was used to sample humans, non-human primates, and mosquitoes in distinct ecologic zones. Humans and non-human primates were tested for YFV-specific antibodies; mosquitoes were tested for YFV RNA.
Results
Overall, 13.3% (125/938) of humans were found to have naturally-acquired YFV antibodies. Antibody levels were higher in zones in the southern and south central regions of CAR. All sampled non-human primates (n=56) were known YFV reservoirs; one tested positive for YFV antibodies. Several known YF vectors were identified including Aedes africanus, Ae. aegypti, Ae. luteocephalus, and Ae. simpsoni. Several more urban locations were found to have elevated Breateau and Container indices for Ae. aegypti.
Conclusions
A country-wide assessment of YF risk found YFV to be endemic in CAR. The potential for future YF cases and outbreaks, however, varied by ecologic zone. Improved vaccination coverage through mass campaign and childhood immunization was recommended to mitigate the YF risk.
Keywords: Aedes mosquitos, Central African Republic, Enzyme-linked immunosorbent assay, Reverse transcriptase polymerase chain reaction, Yellow fever, Yellow fever virus
Introduction
Yellow fever (YF) is a mosquito-borne disease caused by YF virus (YFV). The majority of YFV infections in humans are asymptomatic. Clinical disease varies from a mild, undifferentiated febrile illness to severe disease with jaundice or hemorrhagic manifestations.1 The case fatality ratio of severe disease is 20–50%. Because no specific treatment exists for YF, prevention through personal protective measures or vaccination is critical to lower disease risk and mortality.
YFV is endemic in tropical areas of Africa and South-Central America, with approximately 90% of cases coming from Africa.2 In Africa, 84 000–170 000 cases of severe yellow fever disease and 29 000–60 000 related deaths are estimated to occur annually.2 The incidence of disease and outbreaks are highest in West Africa. In contrast, disease activity in East Africa is more limited, with sporadic outbreaks occurring at intervals of several decades.3 It is likely that countries in central Africa, such as the Democratic Republic of Congo and the Central African Republic (CAR), have an intermediate risk of YF disease but data to substantiate this are limited.
The first laboratory-confirmed case of YF in CAR occurred in 1938.4 Over the next 60 years, only 11 additional cases were confirmed.4–6 Mass vaccination campaigns that started in the 1940s in all regions of CAR were halted in 1961.5 In 1989, YF vaccine was added to the Expanded Programme on Immunization (EPI) for infants 9 months to 1 year of age in CAR. By 2006, a reported 90% of infants received YF vaccine.7
Despite this EPI coverage, in 2008 through early 2009, six laboratory-confirmed cases of YF were documented in four distinct areas of the country; only one of these cases reported being vaccinated previously.8 This unprecedented number of YF cases over a short period of time prompted the CAR government to request to participate in the ongoing Yellow Fever Initiative in order to implement a mass vaccination campaign.9 However, it was unknown whether the cases resulted from increased disease activity or increased recognition due to improved surveillance started in 2005. WHO assembled a team of experts to assist the country in performing a comprehensive assessment of YFVactivity and risk of outbreaks in CAR.
Materials and methods
Study design and selection of sample sites
We used a multistage cluster sampling design for humans, non-human primates, and mosquitoes in distinct ecologic zones within the country. Ecologic zones were determined based on the length of the dry season, annual rainfall, and associated vegetation (all factors likely to affect vectors and reservoirs for YFV).10,11 Polygons were drawn around each zone and two random points were selected within each zone using a random point generator in ArcGIS (Redlands, CA, USA) (Figure 1A). Using the latitude and longitude of each point, specific urban centers or towns (moderately or densely populated areas) and neighboring villages (rural areas) in closest proximity to the point were identified using Google Earth™ (Figure 1B). Due to security concerns, sampling could not take place in Zone 5 and the initial location of the Zone 4B site had to be moved westward to the closest area where it was safe to survey. In addition, due to its unique urban environment, the capital of Bangui in Zone 2 was sampled separately.
Figure 1.
Ecologic zone and sampling site selection for yellow fever risk assessment in the Central African Republic. (A) Polygons and selected points by ecologic zone; Zone 1: Dense evergreen forest with >1600 mm of rainfall/year and 2 month dry season; Zone 2: Dense semi-deciduous rainforest with 1300–1600 mm of rainfall/year and 3.5 month dry season; Zone 3: Mixed grassland and deciduous forest with 1200–1500 mm of rainfall/year and 4 month dry season; Zone 4: Mixed grassland, shrubland, and deciduous forest with 900–1400 mm of rainfall/year and 5–7 month dry season; Zone 5: Shrub and cropland with 500–800 mm of rainfall/year and 9 month dry season. Source: Rainfall and dry season data obtained from Geoffroy and Cordellier10; Landcover data from Global Land Cover Network (http://www.glcn.org/index_en.jsp). (B) Random points in Google Earth™. Using the latitude and longitude of each randomly selected points, the following specific town and neighboring villages were sampled: Zone 1A: Salo and Ngola; Zone 1B: Bayanga and Babongo; Zone 2A: Boganangone and Boguera; Zone 2B: Gambo and Mabo; Zone 3A: Sibut and Galafondo; Zone 3B: Bangassou and Balifondo; Zone 4A: Kaga Bandoro and Ndenga; and Zone 4B: Mbres and Koukouruo. Bangui was sampled separately given its unique urban nature. This figure is available in black and white in print and in color at Transactions online.
Over a 2 week period at the end of the dry season and beginning of the rainy season in late March 2009, multidisciplinary teams were sent to each location to sample humans, non-human primates and mosquitoes. Each team consisted of an entomologist, an epidemiologist, a virologist/laboratory technician, a veterinarian or veterinary technician, and a local guide.
The assessment was reviewed and either approved or deemed to be part of a public health response by the ethics committees of the Ministry of Health, CAR, Institut Pasteur, Bangui, World Health Organization, Geneva, and Centers for Disease Control and Prevention, USA.
Mosquito sampling
Larval and adult mosquito sampling was conducted at each of the randomly selected sites, as previously described.12 Sample sizes were estimated according to random sampling (cluster analysis). Sampling of mosquitoes was conducted simultaneously with the human study and covered all households visited by the survey team. If the sample size for the number of households required for the larval survey was greater than the number needed for the human epidemiologic study, the surrounding houses were randomly selected to complete the sample.
Mosquito classification
Larvae and pupae collected in the field were reared for at least 4–6 days to obtain emerging adults. Mosquito species were identified in the adult stage using a binocular stereomicroscope. Adults were pooled according to their geographic origin, sex, and species (maximum: 10 mosquitoes/pool) then stored at − 80°C or in liquid nitrogen until testing could be performed.
Non-human primate sampling
Due to the safety and ethics considerations of trapping and bleeding live animals, convenience sampling of non-human primates was performed at the randomly selected sites. Animals were obtained at wild game markets or from hunters. Cardiac puncture was performed on freshly killed animals. Blood samples were stored in ice boxes and the serum was separated within 24 hours.
Human sampling
The population and estimated YF vaccination coverage of each zone was used to calculate the target sample size for each zone. A design effect of two was used to account for clustering and oversampling by 15% to account for non-responders (people who were unavailable or refused). The sample size per each zone was divided in half and equally allocated to two randomly selected points within the zone (i.e., A and B). The sample number for each point was proportionally allocated to the selected town and neighboring village based on the population of those locations.
At each location, information was obtained from local officials on the approximate number of households and average number of people per household in order to calculate the number of households to sample. A random number table then was utilized to identify the households to be visited. If no occupants were home despite repeat attempts to visit the household, the household was not replaced.
All residents aged ≥9 months of randomly selected households were invited to participate. The study objectives were explained and consent was obtained from adults or from the parents/guardians for minors. Information was collected on demographics (age and sex), YF vaccination status (year and presence of vaccination cards) and febrile illness in the last month. Up to 5 mL of blood was obtained in serum separator tubes from each participant. All samples were stored in ice boxes and serum was separated within 24 hours.
Archived human serum sampling
To determine if there had been a change in YFV circulation in CAR, a subset of serum samples collected and retained from a nationwide HIV seroprevalence study conducted in 2006 were selected at random for testing. The multiple indicator cluster survey included females aged 15–49 years and males aged 15–59 years.13 All identifiers were removed, except the location from which the sample originated. Since these samples were collected for other reasons, data on YF vaccination status were not available.
Laboratory testing
All serum specimens were tested for YFV-specific IgM and IgG antibodies using ELISA.14 Samples testing positive for YFV antibodies by ELISA were assessed by plaque reduction neutralization at 90% cutoff (PRNT) for YFV. To verify the specificity of the antibodies to YFV, the samples were also assessed by PRNT for West Nile virus (WNV), another flavivirus known to circulate in CAR, that could cause false-positive YF IgM or IgG ELISA results.6,15 Subjects with YFV PRNT titers ≥10 were defined as seropositive and those with PRNT titers ≥20 were considered to be seroprotected against YFV infection.16,17 A sample was considered to be YFV antibody confirmed if the YFV titer was 4-fold higher than WNV titer or the sample was only positive for YFV antibodies by PRNT. A sample was considered to be flavivirus equivocal if YFV titers were <4-fold different than WNV titers.
Mosquitoes were tested for YFV RNA by grinding a maximum of 10 mosquitoes from the same species together, centrifuging and collecting the supernatant. YFV real time reverse transcriptase-polymerase chain reaction (rRT-PCR) was performed on extracted RNA.18
Definitions and data analysis
For the purpose of the human serosurvey, a vaccinated person was defined as a person who reported receiving YF vaccine at any time in the past or did not know if they had been vaccinated. The proportion of participants with naturally-acquired YFV infection was calculated as the number of unvaccinated participants with YFV antibody confirmed results divided by the total number of unvaccinated participants. Vaccinated participants were not included in the proportion of naturally-acquired YFV infection as they were assumed to be immune and unable to be naturally infected by YFV. The proportion of protected participants was determined by taking the number of participants who had confirmed YFV antibodies at seroprotective levels (PRNT≥20) or reported history of YF vaccination, divided by the total number of sampled participants.
Vector density was calculated according to WHO standards.19 More specifically, the Breteau index (BI) was calculated for Aedes aegypti as the number of containers with larvae per 100 households inspected. The Container index (CI) was estimated as the percentage of containers with larvae out of the total number of containers inspected. Historically, a BI≥5 or CI≥3% was considered to indicate an increased epidemic risk of YF in an urban setting.20
Categorical variables are presented by frequency distribution and continuous variables as median and range or mean and standard deviation. Statistical analysis was performed using EpiInfo 7 (Atlanta, GA, USA) and SAS 9.3 (Cary, NC, USA) software.
Results
Entomological findings
Aquatic stages
At the 1636 households visited, 4844 containers were inspected and 82 (1.7%) contained larvae (Table 1). Containers used for water storage (e.g., buckets, 5-gallon barrels) were the most commonly inspected (89.8%; 4349/4844) but had the lowest infection rates of Ae. aegypti aquatic stages (0.3%, 11/4349). Unused containers (e.g., tires, bottles) were the second most common container inspected (9.7%; 470/4844) with 12.6% (59/470) infested with Ae. aegypti larvae. Only 25 natural receptacles (e.g., bamboo fences, taro leaves) were inspected but 48% (12/25) were infested. This collection and infestation profile was observed in all zones and Bangui.
Table 1.
Epidemic risk indices for Aedes aegypti in the Central African Republic by ecologic zone and sampling location
| Zone | Localities | No. households visited | HUa | No. containers inspected | No. containers positive | CIb | BIc |
|---|---|---|---|---|---|---|---|
| Bangui | Bangui | 270 | 937 | 724 | 38 | 5.3 | 4.1 |
| District 1 | 10 | 29 | 17 | 8 | 47 | 28 | |
| District 2 | 29 | 119 | 91 | 3 | 3.3 | 2.5 | |
| District 3 | 41 | 138 | 82 | 5 | 6.1 | 3.6 | |
| District 4 | 50 | 185 | 198 | 4 | 2.0 | 2.2 | |
| District 5 | 50 | 183 | 89 | 8 | 9.0 | 4.4 | |
| District 6 | 40 | 132 | 122 | 6 | 4.9 | 4.6 | |
| District 7 | 50 | 151 | 125 | 4 | 3.2 | 2.7 | |
| Mpoko | 16 | 42 | 26 | 0 | 0.0 | 0.0 | |
| 1A | Bayanga | 84 | 200 | 258 | 15 | 5.8 | 7.5 |
| Babongo | 30 | 40 | 170 | 3 | 1.8 | 7.5 | |
| 1B | Salo | 31 | 67 | 76 | 1 | 1.3 | 1.5 |
| Nola | 23 | 49 | 47 | 2 | 4.3 | 4.1 | |
| 2A | Boganangon | 52 | 124 | 68 | 0 | 0.0 | 0.0 |
| Boguera | 90 | 265 | 139 | 1 | 0.7 | 0.4 | |
| 2B | Gambo | 100 | 222 | 350 | 12 | 3.4 | 5.4 |
| Mabo | 23 | 51 | 60 | 0 | 0.0 | 0.0 | |
| 3A | Sibut | 230 | 473 | 852 | 1 | 0.1 | 0.2 |
| Galafondo | 217 | 395 | 701 | 0 | 0.0 | 0.0 | |
| 3B | Bangassou | 110 | 317 | 421 | 4 | 1.0 | 1.3 |
| Balifondo | 12 | 15 | 26 | 0 | 0.0 | 0.0 | |
| 4A | Kagabandaro | 255 | 600 | 727 | 5 | 0.7 | 0.8 |
| Ndenga | 93 | 188 | 199 | 0 | 0.0 | 0.0 |
Zone 4B (Mbres and Koukourou) data were not collected systematically and therefore are not included in this table. However, there were no mosquitoes collected in aquatic and adult stages in these locations suggesting the risk is low.
HU: Habitation unit: a room occupied by at least one individual.
CI: Container index: (Containers positive X 100)/containers inspected.
BI: Breteau index: (Number of positive containers X 100)/number of habitation unit.
The BI and CI varied by locality and were relatively high in the southern part of the country, particularly in Zone 1A, Zone 1B, and Bangui (Table 1). In Bangui, BI were low (range: 2.2–4.6) in most places except in District 1, where the BI was 28. However, CI were above the risk threshold in all communities in Bangui except in District 4. In Zone 1, three of four communities had risk indices above the threshold while in Zone 2 only one of four communities had risk indices above the threshold. For Zones 3 and 4, indices were low in all areas sampled.
Identification of adult mosquitoes emerging from the aquatic stage revealed the presence of Ae. aegypti, usually in association with Ae. albopictus.
Adult results
There were 1247 adult mosquitoes from 35 species and 6 genera collected.12 Of all mosquitoes collected, 261 (20.9%) were species that are known YFV vectors (i.e., Ae. africanus, Ae. aegypti, Ae. luteocephalus, and Ae. simpsoni) (Table 2). Of the YFV vectors, Ae. aegypti was the most common species (93.1%; 243/261) and collected predominantly in Bangui. Of sylvatic vectors, Ae. africanus was present in Zones 1, 3 and 4, Ae. luteocephalus was found in Zone 1 and 3, while Ae. simpsonsi was found in Zone 1 and Bangui. The number of adult mosquitoes captured was relatively low except for Ae. aegypti in Bangui where there were >3 mosquitoes biting per person per hour (Table 2).
Table 2.
Yellow fever virus vectors collected in adult stages in the Central African Republic by ecologic zone
| Species | Zone 1
|
Zone 2
|
Zone 3
|
Zone 4
|
Bangui
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | D | F | D | F | D | F | D | F | D | |
| Aedes aegypti | 0 | 3 (0.1) | 2 (0.2) | 3 (0.3) | 1 (0.1) | 5 (0.4) | 0 | 0 | 13 (0.4) | 216 (3.1) |
| Ae. africanus | 3 (0.1) | 0 | 0 | 0 | 1 (0.1) | 0 | 1 (0.1) | 0 | 0 | 0 |
| Ae. luteocephalus | 3 (0.1) | 0 | 0 | 0 | 1 (0.1) | 0 | 0 | 0 | 0 | 0 |
| Ae. simpsoni | 0 | 4 (0.7) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (0.1) | 3 (<0.1) |
D: domestic environment; F: forest environment.
Values in parentheses are number of mosquitoes biting per person per hour for each species and zone.
YFV RNA was not detected by rRT-PCR in any of the pooled mosquito samples.
Non-human primate findings
A total of 56 primates from 4 species (Chlorocebus spp., Cercopitheque spp., Cynocephalus spp., and Erythrocebus spp.) were successfully sampled. All four species are known to be competent reservoirs of YFV. Of the 56 primates, 26 (46%) were adults and 31 (55%) were males.
The number of primates sampled varied between zones from a low of 4 (7%) animals in Zone 2 and Bangui to a high of 26 (46%) in Zone 3. Only one (2%) of the 56 sampled primates had con-firmed YFV antibodies. The seropositive animal was <1 year of age and was among the eight animals sampled in Zone 1.
Human serosurvey findings
Of 1620 persons identified to participate in the study, 1300 (80.3%) consented and provided a blood sample. Of the 1300 participants, 571 (43.9%) were male and most (82.5%; 1073/1300) were aged <40 years (Table 3). All zones showed similar proportions for both sex and age.
Table 3.
Sex and age of serosurvey participants in the Central African Republic by ecologic zone
| Zone 1 n=137 n (%) |
Zone 2 n=199 n (%) |
Zone 3 n=396 n (%) |
Zone 4 n=381 n (%) |
Bangui n=187 n (%) |
Total n=1300 n (%) |
|
|---|---|---|---|---|---|---|
| Male sex | 58 (42.3) | 90 (45.2) | 156 (39.4) | 179 (47.0) | 88 (47.1) | 571 (43.9) |
| Age group | ||||||
| <15 years | 51 (37.2) | 81 (40.7) | 190 (48.0) | 141 (37.0) | 72 (38.5) | 535 (41.2) |
| 15–39 years | 59 (43.1) | 72 (36.2) | 138 (34.8) | 180 (47.2) | 89 (47.6) | 538 (41.3) |
| 40–64 years | 26 (19.0) | 36 (18.1) | 53 (13.4) | 52 (13.6) | 20 (10.7) | 187 (14.3) |
| ≥65 years | 1 (0.7) | 10 (5.0) | 15 (3.8) | 8 (2.1) | 6 (3.2) | 40 (3) |
A total of 362 (27.8%) participants were considered vaccinated against YF. Children aged <10 years had the highest vaccine coverage rate and accounted for 47.8% (173/362) of the vaccinated participants (Table 4). Only 15 (4.1%) vaccinated participants had a record of the vaccination (infant card or yellow card).
Table 4.
Proportion of participants vaccinated against yellow fever in the Central African Republic by age group
| Age group in years | n/n | % |
|---|---|---|
| 0–9 | 173/373 | 46.4 |
| 10–19 | 84/347 | 24.2 |
| 20–29 | 31/219 | 14.2 |
| 30–39 | 25/134 | 18.7 |
| 40–49 | 23/101 | 22.8 |
| 50–59 | 13/63 | 21 |
| >60 | 13/63 | 21 |
| Total | 362/1300 | 27.8 |
Of the 938 participants who did not report receiving vaccination, 125 (13.3%) had confirmed YFV infections, 10 (1.1%) had confirmed WNV infections, 8 (0.9%) tested flavivirus equivocal, 2 (0.2%) were YF IgM or IgG positive but lacked sufficient sample volume for PRNTs, and 793 (84.5%) either lacked YF IgM or IgG antibodies (n=739) or did not confirm with PRNT testing (n=54). Of the 125 participants with YFV confirmed results, 7 (5.6%) participants had YFV IgM antibodies, 3 (2.4%) had both YFV IgM and IgG antibodies and 115 (92.0%) had only YFV IgG antibodies. Four of the 10 participants with YFV IgM reported a febrile illness in the preceding month; one in Zone 3 and three in Zone 4.
Based on confirmatory testing, 13.3% (125/938) of survey participants in CAR had naturally-acquired antibodies. The proportion of participants with naturally-acquired antibodies ranged from 3.8% in Zone 3 to 28.5% in Zone 2 (Table 5). Although the proportions with naturally-acquired antibodies increased with increasing age, these differences were not statistically significant (Table 6).
Table 5.
Naturally-acquired yellow fever virus antibodies for the Central African Republic (CAR) by zone and sampling location
| Zone | Localities | n/n | % | 95% CI |
|---|---|---|---|---|
| Zone 1 | 10/70 | 14 | 0–31 | |
| A | Salo | 9/30 | 30 | 0–63 |
| Ngola | 1/9 | 11 | 0–52 | |
| B | Bayanga | 0/25 | 0 | |
| Babongo | 0/16 | 0 | ||
| Zone 2 | 39/137 | 28.4 | 13–44 | |
| A | Baganangone | 2/31 | 6 | 0–24 |
| Boguera | 9/33 | 27 | 3–58 | |
| B | Gambo | 28/72 | 39 | 16–61 |
| Mabo | 0/1 | 0 | ||
| Zone 3 | 10/262 | 3.8 | 0–8 | |
| A | Sibut | 8/155 | 5.2 | 0–16 |
| Galafondo | 0/56 | 0 | ||
| B | Bangassou | 2/91 | 2 | 0–8 |
| Balifondo | 0/0 | 0 | ||
| Zone 4 | 48/357 | 13.4 | 6–21 | |
| A | Kaga Bandoro | 38/199 | 19.1 | 8–30 |
| Ndenga | 5/8 | 63 | 0–100 | |
| B | Mbres | 5/132 | 3.8 | 0–10 |
| Koukourou | 0/18 | 0 | ||
| Bangui | 18/112 | 16.1 | 2–30 | |
| 1e | 1/12 | 8 | 0–40 | |
| 7e | 17/100 | 17.0 | 2–32 | |
| CAR | All areas | 125/938 | 13.3 | 9–18 |
Table 6.
Yellow fever virus naturally-acquired antibodies in the Central African Republic by sex and age
| Age group | Females
|
Males
|
Total
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n/n | % | 95% CI | n/n | % | 95% CI | n/N | % | 95% CI | |
| <15 years | 13/157 | 8.3 | 0–17 | 12/150 | 8.0 | 0–17 | 25/307 | 8.1 | 2–14 |
| 15–39 years | 29/277 | 10.4 | 3–18 | 30/176 | 17.0 | 6–28 | 59/453 | 13.0 | 7–19 |
| 40–64 years | 18/81 | 22.2 | 4–40 | 16/68 | 24 | 3–44 | 34/149 | 22.8 | 9–36 |
| ≥65 years | 5/18 | 27 | 0–69 | 2/11 | 18 | 0–64 | 7/29 | 24 | 0–55 |
| Total | 65/533 | 12.2 | 7–18 | 60/405 | 14.8 | 8–22 | 125/938 | 13.3 | 9–18 |
Of the 1300 survey participants, 484 (37.2%) were likely protected against YFV infection, including 362 who reported receiving YF vaccine and 122 additional people with seroprotective levels of presumed naturally-acquired YFV antibodies. The proportion of participants protected by zone ranged from 18.6% in Zone 4 to 56.2% in Zone 1 (Table 7).
Table 7.
Proportion of participants protected against yellow fever virus in the Central African Republic (CAR) by ecologic zone
| Zones | n/n | % | 95% CI |
|---|---|---|---|
| Zone 1 | 77/137 | 56.2 | 40–73 |
| Zone 2 | 99/199 | 49.7 | 36–64 |
| Zone 3 | 144/396 | 36.4 | 27–46 |
| Zone 4 | 71/381 | 18.6 | 11–26 |
| Bangui | 93/187 | 49.7 | 35–64 |
| CAR | 484/1300 | 37.2 | 32–42 |
Archived human serosurvey results
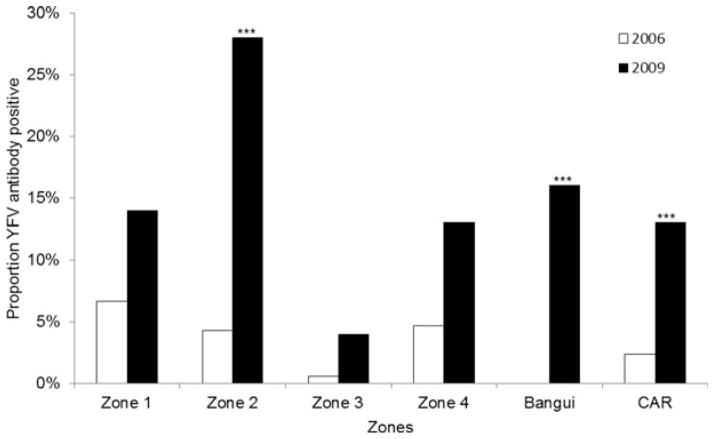
Of the 549 human serum samples tested from the 2006 national HIV survey, 13 (2.4%) had confirmed YFV antibodies. When comparing the 2006 data to the current serosurvey results of those who had naturally-acquired antibodies, all areas had an increase in seropositive rates over the last 3 years (Figure 2). The increase in seropositivity was significant for Zone 2, Bangui, and the country as a whole.
Figure 2.
Proportion of participants with confirmed yellow fever virus (YFV) antibodies in the Central African Republic (CAR) for 2006 and 2009 by ecologic zone. ***p<0.001 (comparison between 2006 and 2009 data for each zone).
Discussion
This study represents the first national-wide evaluation of YFV activity in CAR since the 1970s.4 We found evidence of YFV infections in humans as well as the presence of known vectors and non-human primate hosts in all areas of the country sampled. The risk of YF outbreaks was variable in the different ecologic zones, with higher risk being noted in Zone 1, Zone 2, and Bangui where there is moist savannah bordering forests. This correlates well with the known ecology of the virus with transmission occurring in forested areas or savannahs at the edge of forests (i.e., intermediate transmission) and during times of increased rainfall.21 Human YFV infections appear to have increased in the 3 years prior to the assessment. Zones with a higher seroprevalence of YFV antibodies in 2009 corresponded to locations with cases in 2008 and 2009.8 These data suggest there has been an increase in YFV activity in those areas rather than just improvements in surveillance detecting more cases.
We found 13% of randomly sampled residents had naturally-acquired antibodies against YFV. This seroprevalence is lower than that reported in four previous studies in CAR (range 24–79%).4,5,22,23 However, three of these four studies included persons who likely received YF vaccine during routine vaccination campaigns before 1961. Including people who reported previous YF vaccination, 37% of the participants in our study had confirmed YFV antibodies. Furthermore, the proportion of persons with naturally-acquired YFV antibodies in our study is similar to the results obtained from two studies performed in non-vaccinated Pygmy populations in CAR.15,24 In these studies, 2–11% of the sampled population had YFV antibodies. In addition to variations in vaccine coverage, testing practices (e.g., increasing use of neutralization testing to identify for cross-reactive flavivirus antibodies) and changes in virus circulation over time may have also contributed to the different seroprevalence rates between studies.6
We found the proportion of persons with naturally-acquired YFV antibodies varied by zone and areas within zones. Other studies have also noted marked variation of YF antibody levels in areas sampled in the same region of the country.4,5 Some of this variation is likely due to the small numbers sampled at specific sites. However, the focal nature of YFV transmission, fluctuations in circulating levels of the virus, and different risk behaviors among the population likely also contributed.
We could not sample in the most northern zone (Zone 5) in the country due to civil unrest at the time of the survey. In previous studies, residents in this area had high levels of YFV antibodies and thus our national seroprevalence estimate may be low.4
Previous studies have found an increasing proportion of persons with YFV-specific antibodies with increasing age, likely due to increased cumulative infection over time.4,5,23,25,26 In addition several studies also have documented higher rates of YF disease and infection among males, particularly in South America.1,27 Although there were a slightly higher rate of YFV-specific antibodies in older age groups and males in our study, this difference was not significant.
Compared to previous studies that evaluated mosquito indices in CAR,10,28,29 we found a higher density of Ae. aegypti mosquitoes in Bangui and Zone 1 but decreased risk indices in other geographic zones. Although we identified several sylvatic YFV vectors in CAR, overall numbers and density were low. This was likely impacted by the timing of the study during the dry season and challenges in adequately sampling sylvatic mosquitoes that reside in the forest canopy. In regards to the non-human primate data, the convenience sample prevents us from comparing these data to other studies or inferring the level of YFV antibodies in non-human primates throughout the country. Furthermore, the non-human-primates that were tested came predominantly from wild game markets and it is possible that hunters could have trafficked these animals from areas outside the randomly selected points or zones.
The human, non-human, and mosquito data collected in this assessment suggest future YF outbreaks could occur in CAR, particularly in Zone 1, Zone 2 and Bangui. Factors contributing to potential outbreaks include the: 1) presence of competent mosquitoes; 2) elevated indices of Ae. aegypti; 3) presence of competent primates; 4) presence of YFV naturally-acquired antibodies in humans and primates; 5) increase in YFV seroprevalence in the 3 years before the assessment; and 6) low proportion (37%) of the population that are seroprotected. However, no circulating virus was detected in mosquitoes at the time of sampling (during the dry season) and few participants had YFV IgM antibodies suggesting recent infection.
There are several limitations to our study. The relatively small sample size limits the precision of our estimates and the ability to detect differences between areas and demographic groups. The benefits, cost, and logistics of such country-wide assessments need to be balanced against more extensive sampling at a smaller number of locations to improve the precision and power to detect differences. Cross-neutralization testing was not performed for all potential flaviviruses previously reported to occur in CAR.6,30 Thus, we may have classified someone previously infected by another flavivirus as having naturally-acquired YF antibodies. We lacked accurate country-level population data by region, age, and sex and thus were not able to adjust for differences between the sample population and the population of the country. A conservative approach was taken in the classification of vaccination (included all verbal reports of potential vaccination regardless of the timing of the vaccination and whether YF IgM or IgG antibodies were detected) and thus we might have overestimated vaccination levels and underestimated naturally-acquired YFV antibody levels. For the archived samples, variations in the age of population sampled, sampling methodology, and unknown YF vaccination status likely impacted the comparability of these samples to the current serum samples.
Conclusions
The multistage cluster design of the survey allowed for a countrywide YF risk assessment to be conducted in 2 weeks. Although testing took longer to complete, within 1 year of the assessment the country was able to conduct preventive vaccine campaigns that were organized in stages, starting in Zone 1, Zone 2 and Bangui before moving to other areas of the country. In addition, recommendations were made to improve YF surveillance, maintain childhood vaccination rates and educate the public on eliminating mosquito breeding sites around the home. This risk assessment approach has now been used in Cameroon, Kenya, Uganda, Sudan, and Rwanda to evaluate the risk of YF disease and outbreaks.31 There are plans to conduct future YF risk assessments in other East African countries (e.g., Ethiopia, Tanzania and South Sudan). Information gleaned from these assessments will help inform vaccination strategies to prevent disease and spread of the virus within the countries and outside the region.
Acknowledgments
Members of RCA Risk Assessment Team: Augustin Balekouzou, Eddy Patrick Gamba, Virginie Gbatoumba, Dieudonné Guezza, Léon Kobangue, Jean Charles Kounda Gboumbi, Elie Didier Louango, Grégorie Malemoko, Auguste Nangouma, Guy Chantal Opandy, Rock Ouambita-Mabo, Simon Pounguinza, Joseph Sendazo, Jean Bertrand Wata at the Ministry of Health of RCA; Franklin Danague Passi, Barthélémy Gnikoli, Adolphe-Hilaire Gokra, Essène Hamat Mal-Mal, and Abel Ngoutendji at the Ministry of Agriculture of RCA; Ionela Gouandijka-Vasilache, Xavier Konamna, Rémi Laganier, and Benjamin Sélekon at Institut Pasteur-Bangui; Peggy Conjugo, Alexis Kamba, and Mirindi Ruhana at World Health Organization-Bangui. Veronique Millot at WHO-Geneva for her support in the risk assessment planning and with the mission. Drs. Brad Biggerstaff and Marc Fischer for their input on the sampling schema, analysis, and comments on the manuscript. We sincerely appreciate the support of the Global Alliance for Vaccines and Immunisation (GAVI) Alliance for this and other YF risk assessments and vaccine campaigns in the region.
Funding: This work was supported by the World Health Organization.
Footnotes
Authors’ contributions: The authors wish it to be known that, in their opinion, the first two authors and the last two authors were equal with respect to their roles.
JES, MD, KBJ, WP, SY and AAS conceived this study; JES, MD, KBJ, RFL, CM, SY, and AAS designed the study protocol; RFL, SY, JES, MD, KBJ, CM and AAS were responsible for coordination and implementation of the study and collection of data; AAS and MD supervised the laboratory testing and interpretation of results; JES, MD, RFL, and KBJ were primarily responsible for analyzing and interpreting the results; JES, MD, SY, AAS and RFL drafted the manuscript; RFL, KBJ, WP and CM critically revised the manuscript for content. All authors read and approved the final manuscript. JES and SY are guarantors of the paper.
Competing interests: None declared.
Ethical approval: The assessment was reviewed and either approved or deemed to be part of a public health response by the ethics committees of the Ministry of Health, CAR; Institut Pasteur, Bangui; World Health Organization, Geneva; and Centers for Disease Control and Prevention, USA.
References
- 1.Monath TP, Gershman M, Staples JE, Barrett ADT. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6. Philadelphia, PA: Elsevier Saunders; 2013. [Google Scholar]
- 2.World Health Organization. Vaccines and vaccination against yellow fever. WHO position paper – June 2013. Wkly Epidemiol Rec. 2013;88:269–83. [PubMed] [Google Scholar]
- 3.Ellis BR, Barrett AD. The enigma of yellow fever in East Africa. Rev Med Virol. 2008;18:331–46. doi: 10.1002/rmv.584. [DOI] [PubMed] [Google Scholar]
- 4.Digoutte JP. Yellow fever in Central Africa [in French] Cah ORSTOM Ser Ent Med Parasitol. 1972;10:145–54. [Google Scholar]
- 5.Chippaux-Hyppolite C, Chippaux A. Yellow fever antibodies among children in the Central African Republic [in French] Bull World Health Organ. 1966;34:105–11. [PMC free article] [PubMed] [Google Scholar]
- 6.Mathiot CC, Gonzalez JP, Georges AJ. Current problems of arboviruses in central Africa [in French] Bull Soc Pathol Exot Filiales. 1988;81:396–401. [PubMed] [Google Scholar]
- 7.World Health Organization. [accessed 14 May 2014];Reported estimates of immunization coverage time series: Yellow fever vaccine. 2013 http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tscoverageyfv.html.
- 8.World Health Organization. [accessed 15 July 2013];Yellow Fever in the Central African Republic. 2009 http://www.who.int/csr/don/2009_12_01/en/index.html.
- 9.World Health Organization. Update on progress controlling yellow fever in Africa, 2004–2008. Wkly Epidemiol Rec. 2008;83:450–8. [PubMed] [Google Scholar]
- 10.Geoffroy B, Cordellier R. Observation on potential vectors of yellow fever in Central African Republic [in French] Cah ORSTOM, Ser Ent Med et Parasitol. 1972;10:127–44. [Google Scholar]
- 11.Saluzzo JF, Ivanoff B, Languillat G, Georges AJ. Serological survey for arbovirus antibodies in the human and simian populations of the South-East of Gabon (author’s transl) [in French] Bull Soc Pathol Exot Filiales. 1982;75:262–6. [PubMed] [Google Scholar]
- 12.Diallo M, Laganier R, Nangouma A. First record of Ae. albopictus (Skuse 1894), in Central African Republic. Trop Med Int Health. 2010;15:1185–9. doi: 10.1111/j.1365-3156.2010.02594.x. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. [accessed 2 December 2013];HIV/AIDS Epidemiological Surveillance Report for the WHO African Region: 2007 Update. http://www.who.int/hiv/pub/me/afro_epi_sur_2007.pdf.
- 14.Faye O, Diallo M, Dia I, et al. Integrated approach to yellow fever surveillance: pilot study in Senegal in 2003–2004 [in French] Bull Soc Pathol Exot. 2007;100:187–92. [PubMed] [Google Scholar]
- 15.Sureau P, Jaeger G, Pinerd G, et al. Sero-epidemiological survey of arbovirus diseases in the Bi-Aka pygmies of Lobaye, Central African Republic [in French] Bull Soc Pathol Exot Filiales. 1997;70:131–7. [PubMed] [Google Scholar]
- 16.Niedrig M, Lademann M, Emmerich P, Lafrenz M. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop Med Int Health. 1999;4:867–71. doi: 10.1046/j.1365-3156.1999.00496.x. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–27. [PubMed] [Google Scholar]
- 18.Weidmann M, Faye O, Faye O, et al. Improved LNA probe-based assay for the detection of African and South American yellow fever virus strains. J Clin Virol. 2010;48:187–92. doi: 10.1016/j.jcv.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 19.WHO. Dengue: Guidelines for diagnosis, treatment, prevention, and control. Geneva, Switzerland: World Health Organization; [accessed 15 July 2013]. http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf. [Google Scholar]
- 20.Bang YH, Bown DN, Onwubiko AO. Prevalence of larvae of potential yellow fever vectors in domestic water containers in south-east Nigeria. Bull World Health Organ. 1981;59:107–14. [PMC free article] [PubMed] [Google Scholar]
- 21.Germain M, Mouchet J, Cordellier R, et al. Epidemiology of yellow fever in Africa in French] Med Mal Infect. 1978;2:69–77. [Google Scholar]
- 22.Beeuwkes H, Mahaffy AF, Burke AW, Paul JH. Yellow fever protection test surveys in the French Cameroons, French Equitorial Africa, the Beglian Congo, and Angola. Trans R Soc Trop Med Hyg. 1934;28:233–58. [Google Scholar]
- 23.Saluzzo JF, Gonzalez JP, Herve JP, Georges AJ. Serological survey for the prevalence of certain arboviruses in the human population of the south–east area of Central African Republic [in French] Bull Soc Pathol Exot Filiales. 1981;74:490–9. [PubMed] [Google Scholar]
- 24.Nakounne E, Selekon B, Morvan J. Microbiological surveillance: viral hemorrhagic fever in Central African Republic: current serological data in man [in French] Bull Soc Pathol Exot. 2000;93:340–7. [PubMed] [Google Scholar]
- 25.Nasidi A, Monath TP, DeCock K, et al. Urban yellow fever epidemic in western Nigeria, 1987. Trans R Soc Trop Med Hyg. 1989;83:401–6. doi: 10.1016/0035-9203(89)90518-x. [DOI] [PubMed] [Google Scholar]
- 26.Thonnon J, Spiegel A, Diallo M, et al. Yellow fever outbreak in Kaffrine, Senegal 1996: epidemiological and entomological findings. Trop Med Int Health. 1998;3:872–7. doi: 10.1046/j.1365-3156.1998.00317.x. [DOI] [PubMed] [Google Scholar]
- 27.Pan American Health Organization. Present status of yellow fever: memorandum from a PAHO meeting. Bull World Health Organ. 1986;64:511. [PMC free article] [PubMed] [Google Scholar]
- 28.Herve JP, Germain M, Mouchet J, et al. Monitoring Aedes aegypti indices in the Central African Empire, 1972–1976 [in French] Cah ORSTOM Ser Ent Med Parasitol. 1978;16:55–62. [Google Scholar]
- 29.Diemer JM, Herve JP, Geoffroy B, et al. Monitoring Aedes aegypti Indices in the Central African Republic During the Years 1977–1980 [in French] Bangui: Central African Republic: Institut Pasteur; 1982. [Google Scholar]
- 30.Chippaux-Hyppolite C, Chippaux A. Use of microtiter Takatsy for serological study of arboviruses in the Central African Republic [in French] Med Trop (Mars) 1968;28:796–801. [PubMed] [Google Scholar]
- 31.World Health Organization. [accessed 2 December 2013];Global alert and response (GAR): yellow fever. 2010–2013 http://www.who.int/csr/don/archive/disease/yellow_fever/en/