Abstract
Objective
Activation Syndrome (AS) is a side-effect of antidepressants consisting of irritability, mania, self-harm, akathisia, and disinhibition. The current study was conducted to analyze how AS may hinder treatment outcome for multimodal treatment for children and adolescents with Obsessive-Compulsive Disorder.
Methods
Fifty-six children or adolescents were recruited at two treatment sites in a double-blind randomized-controlled trial where participants received Cognitive-Behavioral Therapy and were randomized to slow titration of sertraline, regular titration of sertraline or placebo.
Results
Using a recently developed measure of AS, results suggested that higher average levels of all five AS symptom clusters significantly interfered with treatment response and explained 18% of the variance in obsessive-compulsive symptoms during treatment. Interestingly, only session-to-session increases in irritability resulted in a session-to-session increase in obsessive-compulsive symptoms. The observed results were unchanged with the addition of SSRI dosage as a covariate.
Conclusions
Results provide empirical support for the proposed hypothesis that AS may hinder multimodal treatment outcome for pediatric OCD. These findings suggest that dosage changes due to AS do not explain why those with higher AS had worse multimodal outcome. Other possible mechanisms explaining this observed disruption are proposed, including how AS may interfere with Cognitive-Behavioral Therapy.
Keywords: Selective serotonin reuptake inhibitors, Obsessive-Compulsive Disorder, Children, Cognitive-behavioral therapy, Activation syndrome, Side-effects
1. Introduction
In 2004, the United States Food and Drug Administration (FDA) issued a “Black Box Warning” on antidepressants such as selective serotonin reuptake inhibitors (SSRIs), cautioning that these medications were associated with increased suicidality in children and adolescents (FDA, 2004). According to this FDA report, suicidal thoughts and behaviors were twice as high in children taking an-tidepressants (4%) compared to placebo (2%) in a pooled analysis of clinical trials. Research spurred by the warning has identified several behavioral side-effects of SSRIs beyond suicidality, including anxiety, agitation, panic attacks, insomnia, irritability, aggressiveness, impulsivity, psychomotor restlessness, hypomania, and mania (Murphy et al., 2008; Sinclair et al., 2009).
A variety of terms refer to these symptoms, including jitteriness/anxiety syndrome (Sinclair et al., 2009) or activation syndrome (AS; Murphy et al., 2008), the latter being the title used in this manuscript. Activation Syndrome has been estimated to occur in 4–65% of children taking SSRIs (Sinclair et al., 2009). The variability of this estimate is largely due to an absence of any comprehensive assessment measure of AS. Recently, the Treatment-Emergent Activation and Suicidality Assessment Profile (TEASAP) was developed to remedy this problem, providing a psychometrically validated method to comprehensively assess AS in children and adolescents receiving SSRI treatment (Bussing et al., 2013). The measure identifies five clusters of SSRI-induced side effects, including irritability, akathisia, disinhibition, mania, and self-harm. Utilizing this new measure, clinicians are now better positioned to track AS during SSRI administration and may be more effective at modulating dosage if AS emerges.
Indeed, adverse side-effects from antidepressants have been repeatedly linked to treatment discontinuation (e.g. Goldstein and Goodnick, 1998; Safer and Zito, 2006; Bloch et al., 2010; Shirman et al., 2010). While SSRIs are both safer and more tolerable than older medications like tricylclic antidepressants, meta-analytic findings suggest that up to 12–26% of patients taking SSRIs may drop out due to side-effects (Anderson, 2000; Usala et al., 2008). In addition to being more likely to drop-out, some older research suggest that antidepressant side-effects may hinder pharmacological treatment outcome (e.g. Gorman et al., 1987; Pohl et al., 1988), though these studies were limited by small sample size (Gorman et al., 1987), investigated currently outdated antidepressants (Pohl et al., 1988), and lacked a psychometrically validated measure of these side effects. To the best of the authors' knowledge, no study to date has further investigated how side effects may interfere with pharmacological or multimodal treatment outcome beyond these two older studies. These types of analyses are likely underreported because of methodological complications. Without advanced statistical modeling, it could be difficult to have enough power to analyze the impact of side effects that are waxing and waning throughout treatment. As stated above, a comprehensive measure of AS has only recently been developed as a tool towards this endeavor.
Multimodal treatment, or combined pharmacology-psychotherapy, has been identified as an effective and efficacious treatment for a variety of internalizing disorders (Davidson, 2010; Walkup et al., 2008), including Obsessive-Compulsive Disorder (OCD; Geller & March, 2012; Koran et al., 2007). Considering that Cognitive-Behavioral Therapy with Exposure and Response Prevention (CBT-ERP; Franklin et al., 2015) and SSRIs (Sánchez-meca et al., 2014) each have moderate to large treatment effect sizes for pediatric OCD, it is logical that a multimodal approach would be the first-line treatment regimen for this population (see Jordan et al., 2012 for a review). Combined CBT-ERP with SSRI is currently recommended by numerous organizations, such as the Academy of Child and Adolescent Psychiatry (Geller & March, 2012). Due to its frequency of use and the high doses of SSRIs required to treat pediatric OCD (Bloch et al., 2010), pediatric OCD would be a logical population to investigate how AS may impact multimodal treatment outcome.
This study examined the extent to which symptoms of AS hinder outcome during multimodal treatment of pediatric OCD. It was hypothesized that higher average AS symptoms would be associated with less symptom reduction during treatment (i.e., moderate treatment response). Additionally, session-to-session increases in AS symptom severity was expected to predict session-to-session increases in obsessive-compulsive symptom severity. If expected results are observed, increased monitoring and faster dose adjustment when AS is noted could enhance multimodal treatment outcomes.
2. Method
2.1. Participants
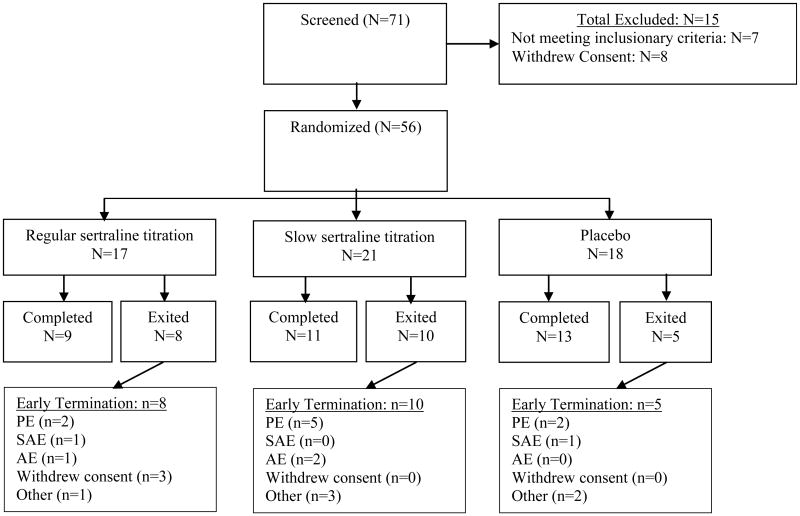
This study consisted of 56 child-parent dyads (M = 11.70 years old; S.D. = 3.30 years; range 7–17 years old) recruited to enroll in a double-blind randomized controlled trial (RCT) to assess pediatric OCD treatment (Storch et al., 2013).1 The sample was 39% female (N = 22) and 96% Caucasian (N = 54). Families were included if the child or adolescent presented with a primary OCD diagnosis, with symptoms present for at least six months and at least of moderate severity. Notable excluding comorbid diagnoses included pervasive developmental disorders, intellectual disability, psychosis, bipolar disorder, substance abuse or dependence within the past 6 months, seizure disorder or degenerative neurological disease. Suicidal intent or history of a suicide attempt in the last year also excluded participants. Participants on a stable stimulant treatment for attention-deficit hyperactivity disorder (ADHD) could continue with their medication regimen, while no other psychoactive medications were allowed during the duration of the study. Three (5%) participants were on a stimulant medication during the study trial. Of the 56 participants, 31 (54%) met criteria for any anxiety disorder, 16 (28.6%) for major depressive disorder (MDD), 12 (21%) for tic disorder, 10(18%) met diagnostic criteria for ADHD, and 8 (14%) had other diagnoses (e.g., oppositional defiant disorder) at baseline. A study flow chart is displayed in Fig. 1 and baseline characteristics for each arm of the RCT can be observed in Table 1.
Fig. 1.
Study flow chart that summarizes recruitment study completion statistics. Reasons for early termination include pharmacy error (PE), suicidal adverse events (SAE), non-suicidal adverse events (AE), and other reasons (e.g., time demand). For more information on the PE, please see the footnote in the discussion section.
Table 1.
Demographic and baseline clinical characteristics of the treatment arms.
| RegSert (n = 17) | SloSert (n = 21) | Placebo (n = 18) | |
|---|---|---|---|
| Gender N (% Female) | 47.10 | 42.90 | 33.30 |
| Age, M (SD) | 11.75 (2.75) | 11.43 (3.41) | 12.17 (3.66) |
| CY-BOCS total | 23.35 (4.62) | 26.10 (5.58) | 25.00 (4.22) |
| Psychiatric comorbidity, N (%) | |||
| Internalizing | 9 (52.94%) | 8 (38.10%) | 12 (66.67%) |
| Externalizing | 5 (29.41%) | 3 (14.29%) | 6 (33.29%) |
| Tic Disorder | 3 (17.65%) | 2 (9.52%) | 7 (38.89%) |
Note. CY-BOCS = Children's Yale-Brown Obsessive–Compulsive Scale; RegSert = Regular Sertraline titration; SloSert = Slow Sertraline titration.
2.2. Procedure
Prior to data collection, the study was approved by the Institutional Review Boards at two large southeastern universities where recruitment occurred. After parents and children provided consent and assent, families were screened for eligibility. The Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997) was used to determine diagnostic status except for OCD and MDD. The Children's Yale-Brown Obsessive-Compulsive Scale was utilized to confirm OCD diagnostic status and that symptom severity was at least of moderate severity (score ≥ 16), as well as for a continuous measure of obsessive-compulsive symptoms. Major Depressive Disorder status was determined by the Children's Depressive Rating Scale-Revised. Once eligibility was determined, patients were enrolled for 17 weeks of multimodal psychopharmacological and psychotherapeutic treatment at OCD-specialty clinics within academic medical centers at each institution. An unblinded study nurse randomly assigned participants to one of three medication groups at screening: regular sertraline titration (RegSert), slow sertraline titration (SlowSert), or placebo in a parallel design (1:1:1 ratio). All participants, investigators, and clinicians were blinded to treatment condition.
The titration schedule for those randomized to the RegSert arm used a flexible upward titration from 25 mg/day to 200 mg/day over 9 weeks. The titration schedule for those in the SloSert arm utilized a slower titration schedule relative to the RegSert arm. Youth remained on 25 mg/day for the first two weeks, 50 mg/day from weeks 3–4, 75 mg/day for weeks 5–6,100 mg/day for week 7, 150 mg/day for week 8, and 200 mg/day for week 9 until the end of the study. Thus, subjects in SloSert did not reach the therapeutic dose defined as a balance of efficacy and tolerability of up to 150 mg/day until week 8 relative to week 4 for RegSert. Across all study arms, dosage increases were delayed or dosages reduced for poor tolerability or clinically significant adverse effects (e.g., those producing distress and dysfunction for which the clinician and the patient or parent believed dosage reduction was indicated). Each participant met on a weekly basis with a board-certified child and adolescent psychiatrist to manage pharmacological treatment. These sessions lasted 30 min each and excluded CBT elements like ERP or cognitive restructuring.
All began weekly CBT-ERP at week 4 and continued throughout treatment. CBT-ERP followed the March and Mulle manual (March and Mulle, 1998) used in the POTS trial (POTS, 2004). Sessions 1–4 consisted of psychoeducation, individualized cognitive restructuring, and an initial relatively easy exposure. Sessions 5–14 focused on exposure and response prevention based on the patient's fear hierarchy, which was established in sessions 1–4. Daily homework was assigned between sessions, which consisted of similar exposures to those conducted during sessions. The aims of the current study are a secondary analysis of the RCT data (citation blinded for review).
2.3. Measures
2.3.1. Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997)
The K-SADS-PL is a parent-child interview that assesses both current and lifetime psychopathology in children and adolescents and has strong psychometrics (Kaufman et al., 1997).
2.3.2. Children's Depressive Rating Scale-Revised (CDRS-R; Poznanski and Mokros, 1996)
The CDRS-R is a semi-structured, clinician-administered interview that assesses depressive symptom severity in 17 items with good psychometric properties (Mayes et al., 2010; Poznanski and Mokros, 1996). A consistent and blinded rater administered the CDRS-R at the baseline visit, as well as the end of weeks 1–9,13 and 17.
2.3.3. Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS; Scahill et al., 1997)
The CY-BOCS is the gold-standard measure of obsessive-compulsive severity in youth and has demonstrated strong psychometric properties (Freeman et al., 2011; Storch et al., 2004). The CY-BOCS was administered by a consistent, blinded rater at baseline and the end of weeks 1–9,13, and 17, and was the primary outcome measure of this study.
2.3.4. Treatment-Emergent Activation and Suicidality Assessment Profile (TEASAP; Bussing et al., 2013)
The TEASAP was created in response to the absence of a measurement tool that comprehensively assesses AS. The parent-report measure consists of 38 items scored on a 4-point Likert scale that identify the following symptom clusters that characterize AS: irritability (9 items), akathisia/hyperkinesis/somatic anxiety (6 items; referred to as “akathisia”), disinhibition/impulsivity (7 items; referred to as disinhibition), mania (10 items; referred to as mania), and self-injury/suicidality/harm to others (6 items; referred to as “self-injury”). The TEASAP these five subscales, as well as a total score, that all have demonstrated sound psychometric properties including construct validity, internal consistency, test-retest stability, convergent/divergent validity and sensitivity to change in AS symptoms (Bussing et al., 2013). The TEASAP was completed by the parent at every visit for the 17-week duration of this study.
2.4. Data analysis
For a preliminary descriptive analysis, continuous AS total scores were split into three categories representing low AS, average AS, and high AS. These groups were created by identifying those who fell one standard deviation below, one standard deviation above, and within one standard deviation of average total AS observed in the sample. Average change in obsessive-compulsive symptoms was then calculated for each of the three groups. A graphical depiction of treatment response per AS group was also created.
This study utilized multi-level modeling (MLM), in concordance with the model building procedures outlined by Singer and Willett (Singer and Willett, 2003), to test the study hypotheses. Specifically, each model was nested upon the null model and any previously added models. In this stepwise fashion, each model was retained only if it resulted in a significant reduction in the −2 Log Likelihood (−2LL) and a reduction in the Akaike Information Criterion (AIC). In addition, this study utilized Pseudo R2 to provide an effect size estimate and orthagonalized the TEASAP subscale predictors from other subscales and depressive symptom severity to reduce mul-ticolinearity (Kreft and De Leeuw, 1998).
While no power analyses are available for MLM, a consistent “rule of thumb” is a sample size of 30 participants with at least 5 repeated measurements (Maas and Hox, 2005; Snijders and Bosker, 1999). Thus, this study is well powered with a sample of 56 and 11 or more repeated measures for each study variable. MLM is the most appropriate statistical method to investigate the study aims due to its ability to control for multiple confounders, effectively model change over time, address missing data, and test moderation with lower type 1 error and higher statistical power compared to other statistical techniques (Kahn and Schneider, 2013; Mackinnon et al., 2007; Tasca and Gallop, 2009).
Selective Serotonin Reuptake Inhibitor related side effects are consistently reported, at least to a small degree, in placebo treatment groups (e.g., Beasley et al., 1991; March et al., 2004; Riddle et al., 2001; Yeragani et al., 1992). As depicted in Fig. 2, minimal AS symptoms were indeed observed in the placebo group during treatment while the other two arms had substantially higher average AS. Thus, in order to further improve our measurement of pure treatment-emergent AS symptoms, two steps were taken for the MLM analysis. First, all continuous AS scores from each weekly measurement were baseline-centered. In this way, only AS symptoms that arose during treatment would be analyzed in the models described below and any baseline comorbid symptoms that were also captured by the TEASAP would be excluded (e.g., attention-deficit hyperactivity symptoms). Furthermore, a continuous measure of clinician-rated depressive symptoms assessed throughout treatment was included as a covariate to control for depressive symptomology that shares phenomenology with some aspects of AS.
Fig. 2.
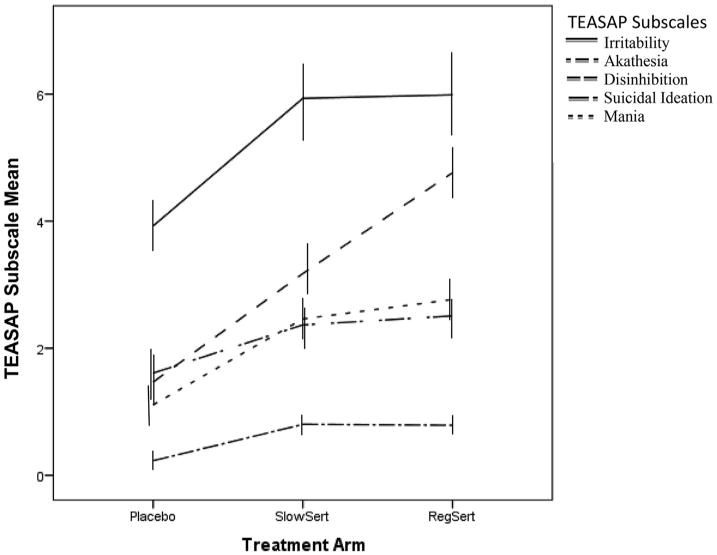
This figure displays average levels of activation syndrome symptoms in each treatment arm over the 18 weeks of treatment (i.e., mean across treatment), as measured by the Treatment-Emergent Activation and Suicidality Assessment Profile (TEASAP). Scores show that, in general, the regular sertraline titration (RegSert), slow sertraline titration (SlowSert) arms experienced notably more activation syndrome symptoms than the placebo arm. Raw means are displayed, therefore means presented are biased by the number of items in the scale.
3. Results
3.1. Dosage information
Average dosage for the SloSert arm was 76 mg/day (51 mg/day), while the average dosage for the RegSert arm was 148 mg/day (52 mg/day). In terms of the highest dosage reached, 3% of the sample reached 25 mg/day, 17% reached 50 mg/day, 6% reached 75 mg/day, 22% reached 100 mg/day, 22% reached 150 mg/day, and 31% reached 200 mg/day. AS symptoms appeared at the highest levels during the transition from 0 mg/day to 25 mg/day and 50 mg/ day to 75 mg/day. Specifically, average TEASAP scores were 21 (SD = 16) at 25 mg/day, 16 (SD = 14) at 50 mg/day, 20 (SD = 16) at 75 mg/day, 18 (SD = 13) at 100 mg/day, 15 (SD = 13) at 150 mg/day, and 8 (SD = 8) at 200 mg/day.
3.2. Categorical analysis-treatment response based on activation syndrome groups
Preliminary analysis of pre-post change scores in obsessive-compulsive severity indicated that 40% of the sample had at least a 50% reduction in obsessive-compulsive symptoms during treatment. In order to descriptively describe outcome based on AS, three categories representing low, average, and high AS were created. Results indicated that 74% of low AS (58% placebo, 25% SlowSert, 17% RegSert), 37% of average AS (28% placebo, 41% SlowSert, 31% RegSert), and 5% of the high AS group (8% placebo, 46% SlowSert, 46% RegSert) experienced at least a 50% reduction in symptoms (see Fig. 3).
Fig. 3.
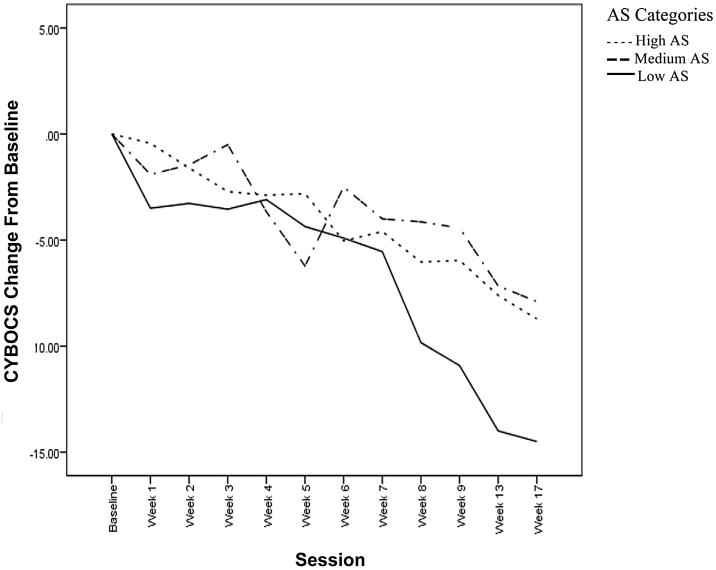
This figure displays average change in obsessive-compulsive symptom severity from the point of randomization, as measured by the gold-standard Children's Yale-Brown Obsessive-Compulsive Scale (CYBOCS). Scores are broken up into a low activation syndrome group (Low AS), medium activation syndrome group (Medium AS), and high activation syndrome group (High AS). These groups were created based on average activation symptoms endorsed throughout treatment.
3.3. Multilevel analysis- covariate outcomes
To quantitatively test our study hypotheses using MLM, 5 models were tested as a predictor of obsessive-compulsive symptoms: linear time (Model A), age and gender (B), treatment site and treatment arm (C), depressive symptoms (D), linear time X sub-scales of AS (E). Model E had Fixed Effects (group level; how average activation moderates average treatment outcome) and Random Effects predictors (within subject level; impact of session-to-session changes in activation on session-to-session changes in obsessive-compulsive severity). Data for all analyses can be observed in Table 2. In terms of the covariate models A-D, age and gender did not significantly predict obsessive-compulsive symptoms but met inclusionary criteria (i.e., significant reduction in −2LL and reduction in AIC). Site location and treatment arm did not predict obsessive-compulsive symptoms and did not meet inclusionary criteria and thus were dropped from all subsequent analyses. Longer treatment (−.62, p < .001) and less depressive symptoms (.21, p < .001) predicted lower average obsessive-compulsive symptoms.
Table 2.
Multilevel model for Activation Syndrome predicting multimodal treatment outcome.
| UMM | Model A | Model B | Model C | Model D | Model E | |
|---|---|---|---|---|---|---|
| Fixed Effects | ||||||
| Intercept | 20.94*** | 24.84*** | 23.17*** | 24.57*** | 19.18*** | 19.54*** |
| A. Linear | −.62*** | −.62*** | −.62*** | −.57*** | −.54*** | |
| B. Age | .133 | .08 | −.06 | .01 | ||
| B. Gender | .245 | .42 | .57 | .70 | ||
| C. Site | −2.22 | Dropped | Dropped | |||
| C. Treatment Arm | −.52 | Dropped | Dropped | |||
| D. Depression | .21*** | .16*** | ||||
| E. Irritability | .36** | |||||
| E. Akathesia | .47* | |||||
| E. Disinhibition | .67** | |||||
| E. SI | .63 | |||||
| E. Mania | .15 | |||||
| Random Effects | ||||||
| Residual | 29.30*** | 19.03*** | 19.11*** | 19.10*** | 16.95*** | 13.85*** |
| F. Irritability | .11* | |||||
| F. Akathesia | .09 | |||||
| F. Disinhibition | .10 | |||||
| F. SI | DNC | |||||
| F. Mania | DNC | |||||
| Fit Statistics | ||||||
| −2LL | 3999.81 | 3749.10 | 3732.90 | 3729.96 | 3351.95 | 3302.94 |
| AIC | 4005.81 | 3757.10 | 3744.90 | 3743.96 | 3365.95 | 3332.94 |
| Δ Fixed R2 | – | .00% | 6.70% | .00% | 21.94% | .00% |
| Δ Random R2 | – | 35.07% | .00% | .00% | .00% | 18.29% |
Note.
p < .05,
p < .01,
p < .001.
SI = Suicidal Ideation; DNC = Did Not Converge; Dropped = inclusionary criteria was not met and the variable was dropped from all subsequent analyses. The pattern of statistical and clinical significance for Model E was not altered when SSRI dosage was added as a covariate.
3.4. Activation and treatment outcome
After controlling for linear time and the covariates discussed above, Model E was run to investigate how each aspect of AS impacts treatment outcome and how the session-to-session changes in each aspect of AS predicts session-to-session changes in obsessive-compulsive symptoms. This model resulted in a −2LL reduction from 3351.95 to 3302.94 (χ2 (6, N = 50) = 49.01, p<.001)) and a decrease in the AIC. The predictors of Model E explained 18% of the variance in obsessive-compulsive severity. Notably, higher average irritability, akathisia, and disinhibition all significantly hindered average treatment outcome while mania and suicidal ideation did not moderate outcome. A session-to-session increase in irritability significantly predicted a similar increase in obsessive-compulsive severity, while similar but non-significant associations were observed for akathisia and disinhibition. Variability in session-to-session changes in suicidal ideation and mania could not be modeled because of a lack of variability.
The MLM analysis described above was repeated with SSRI dosage entered as an additional covariate. Higher average doses of sertraline significantly predicted lower average obsessive-compulsive severity (−.13, p < .01), accounting for an additional 16% of the variance in obsessive-compulsive symptoms. Notably, irritability, akathisia, and disinhibition remained moderators of multimodal treatment outcome and session-to-session changes in irritability continued to predict session-to-session changes in obsessive-compulsive severity. The statistical and clinical significance of these findings were not notably changed by the addition of SSRI dose as a covariate.
4. Discussion
This study aimed to provide a novel exploration of the impact of SSRI related side effects on the effectiveness of multimodal treatment of pediatric OCD. It was hypothesized that the symptoms associated with AS may slow multimodal treatment gains. Both descriptive (see Fig. 3) and analytical (see Table 2) evidence of hindered multimodal outcome was observed. Higher average scores on three out of the five AS domains (irritability, akathisia, and disinhibition) significantly predicted less multimodal treatment response for pediatric OCD. However, irritability was the only AS domain that showed evidence of immediately interfering with treatment outcome upon onset. Specifically, session-to-session increases in irritability were significantly associated with session-to-session increases in obsessive-compulsive severity. These results add to the literature on the negative effect of SSRI related side-effects on clinical outcomes such as drop-out and reduced pharmacological response (Bloch et al., 2010; Goldstein and Goodnick, 1998; Gorman et al., 1987; Safer and Zito, 2006; Pohl et al., 1988; Shirman et al., 2010).
Mechanisms driving the observed results are unknown. Multimodal response could be lower due to the negative effect of AS on the behavioral therapeutic process, such as if the AS interferes with therapeutic alliance or patient motivation to engage in exposures (Keeley et al., 2008), hindered amygdala-dependent learning (Burghardt et al., 2013), or inability to resist compulsive urges due to disinhibition (Taylor et al., 2007). Alternatively, these AS symptoms could also interfere with the effectiveness of CBT by more indirect routes, such as causing sleep disturbances (Alfano and Kim, 2011; Riddle et al., 1990) or lowering treatment expectancy (Lewin et al., 2011).
Yet another possibility is that AS does not interfere with the effectiveness of behavioral treatment but instead disrupts pharmacological treatment. Patients with OCD usually require higher SSRI doses than those with other internalizing disorders, but higher doses are also linked to higher treatment discontinuation due to increased side-effects (Bloch et al., 2010). Thus, when intolerance is observed, SSRI dosage is immediately decreased or higher doses are delayed. In this study, controlling for SSRI dosage did not notably change how AS moderated treatment outcome, suggesting that dosage titration due to intolerance was not the reason why those with higher average AS had worse outcome. More research is needed to understand how AS may impact multimodal treatment outcome for youth with OCD. Perhaps higher AS increases the likelihood of non-adherence or parent's willingness to increase dosage levels (Cooper et al., 2007).
Regardless, AS appears to limit multimodal outcome for youth with OCD and may partially explain why multimodal treatment for OCD is not consistently more advantageous than CBT-ERP alone (Foa et al., 2005; Ivarsson et al., 2015; Storch et al., 2013). Research seeking to better understand how AS impacts multimodal treatment outcome is needed, especially in other pediatric populations beyond OCD where multimodal treatment is common (e.g., Davidson et al., 2010; Walkup et al., 2008). Some have mentioned that starting multimodal treatments simultaneously versus sequentially may result in worse outcomes (Petersen, 2006); our results were observed despite the recommended sequential initiation of treatment.
This study is not without limitations. The TEASAP has strong psychometric properties, including convergent validity with clinician-rated AS and divergent validity with comorbid diagnoses such as Attention-Deficit Hyperactivity Disorder and Major Depressive Disorder (Bussing et al., 2013). That being said, parent-report of activation may be somewhat confounded by comorbid symptoms outside of AS. Therefore, in this study a baseline-centered measure of AS was used for all analyses to control for variability in AS due to comorbid symptoms and, furthermore, depressive symptoms throughout treatment were controlled for in all analyses. Also, the parent-report TEASAP measure has used in this study been shown to be comparable to blinded clinician-rated AS (Bussing et al., 2013). A second limitation of this study is drop-out that may limit generalizability.2 However, advanced statistical modeling was well-powered and is well suited to handle missing data of this nature. Of note, a small number of patients with suicidal intent or recent histories of attempted suicide were excluded from this study, which could also limit generalizability. Finally, a longer observation period may have allowed for SSRIs to reach their full treatment potential. Nevertheless, the study period passed the three-month recommendation for SSRIs (Kellner, 2010) and was a longer study period than previous multimodal pediatric trials (POTS, 2004).
The results of this study have notable clinical implications; maximizing multimodal treatment outcome for youth with OCD may lie in careful monitoring of AS. Further development of subjective (i.e., TEASAP; Bussing et al., 2013) and objective (i.e., actigraphy watches; Bussing et al., 2014) measurement of AS may allow clinicians to better assess and address AS symptoms as they emerge. For example, the TEASAP and actigraphy watches appear to be able to capture session-to-session increases in irritability (Bussing et al., 2013, 2014), which this study linked to immediate interference with treatment outcome. This is especially promising considering the recent advancements in the integration of actigraphy measurement in mobile phones and wearable technology. If AS emerges, immediate SSRI dose adjustment may suffice to increase multimodal outcomes; however, in those children or adolescents who experience notable AS at lower doses, alternative pharmacological treatment may be warranted if the patient is not responding to CBT-ERP alone (Arumugham and Reddy, 2013). Future research utilizing combined objective and subjective measures of AS in a larger pediatric sample should aim to replicate this study's findings, while also investigating the potential mechanisms that may explain how AS hinders multimodal treatment outcome. More broadly, numerous key questions remain unanswered regarding SSRI related side effects that, when answered, will help inform clinicians on how to limit these side effects from occurring during pharmacological treatment in children and adolescents (Sinclair et al., 2009).
Acknowledgments
This research was supported by grant 5UO1 MH078594 from the NIMH (ClinicalTrials.gov Identifier: NCT00382291). Pfizer provided sertraline and matching placebo at no cost. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors thank Jeannette Reid and Johanna Meyer, all staff members who contributed to data collection, the families for their participation, and Drs. Ayesha Lall, Jane Mutch and Omar Rahman for their contribution to the study interventions.
Funding source: During the design of this study, the NIMH advised on the various aspects of the study methodology. The NIMH monitored the progress of the study throughout data collection, consulting when needed. The NIMH played no role in the analysis and interpretation, writing of the report, or the decision to submit for publication. Pfizer was annually notified about the progress of the study but was not involved in the design or implementation of any part of the study.
Dr. Regina Bussing has received research support from Pfizer Inc. and Otsuka Pharmaceuticals and payment from Pfizer Inc. and Shire Pharmaceuticals for consultation. Dr. Tanya Murphy discloses research support from the AstraZeneca Neuroscience iMED, Otsuka Pharmaceuticals, Shire Pharmaceuticals, Roche Pharmaceuticals, Sunovion Pharmaceuticals Inc., and Pfizer Inc. Finally, Dr. Eric Storch has received grant funding in the last 2 years from the National Institutes of Health, Centers for Disease Control, Agency for Healthcare Research and Quality, International OCD Foundation, and Ortho-McNeil Scientific Affairs. He receives textbook honorarium from Springer publishers, American Psychological Association, Wiley, Inc. and Lawrence Erlbaum. He is a consultant for Prophase, Inc., Rogers Memorial Hospital, and CroNos, Inc., and is on the Speaker's Bureau and Scientific Advisory Board for the International OCD Foundation.
Footnotes
The sample size of our study is identical to the Storch et al. (2013) study, although those impacted by the pharmacy error (see footnote in discussion section below) were completely excluded from the Storch et al. (2013) final sample used for analyses.
During the conduct of the study, a pharmacy-related medication error occurred, which affected 9 of 56 initially randomized study participants and resulted in a temporary study suspension. Per DSMB recommendations, these 9 participants were dropped from the study at the point of exposure to the pharmacological error. Participant data up to the point of exposure was retained. Those who were exposed to the pharmacological error did not differ in any study variable from the rest of the sample.
Contributors: Mr. Reid contributed to data collection and management of the Randomized Controlled Trial, helped manage the grant funding, conceptualized and designed the study analyses, led analyses, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript.
Dr. McNamara led treatment for one of the study sites, conceptualized and designed the study analyses, supervised the analyses, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript.
Dr. Storch conceptualized and designed the Randomized Controlled Trial, acquired and managed grant funding, oversaw data collection and management, contributed to the conceptualization and design of the study analyses, reviewed and revised the manuscript, and approved the final manuscript.
Mr. Guzick conceptualized and designed the study analyses, contributed to the analyses, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript.
Dr. Murphy conceptualized and designed the study, acquired and managed grant funding, oversaw data collection and management, contributed to the conceptualization and design of the study analyses, reviewed and revised the manuscript, and approved the final manuscript.
Dr. Geffken contributed to the conceptualization and design of the study analyses, reviewed and revised the manuscript, and approved the final manuscript.
Dr. Bussing conceptualized and designed the Randomized Controlled Trial, acquired and managed grant funding, oversaw data collection and management, contributed to the conceptualization and design of the study analyses, reviewed and revised the manuscript, and approved the final manuscript.
Conflicts of interest: All other authors of this paper have no conflicts of interest to report.
Uncited reference: Antony et al., 2007, In-albon and Schneider, 2007, Tolin et al., 2005, Watson and Rees, 2008, Weisz et al., 2006
Contributor Information
Adam M. Reid, Email: reidam@phhp.ufl.edu.
Joseph P.H. McNamara, Email: jpm2@ufl.edu.
Tanya K. Murphy, Email: tmurphy@health.usf.edu.
Andrew G. Guzick, Email: guzick@ufl.edu.
Eric A. Storch, Email: estorch@health.usf.edu.
Gary R. Geffken, Email: geffken@ufl.edu.
Regina Bussing, Email: rbussing@ufl.edu.
References
- Alfano CA, Kim KL. Objective sleep patterns and severity of symptoms in pediatric obsessive compulsive disorder: a pilot investigation. J Anxiety Disord. 2011;25:835–839. doi: 10.1016/j.janxdis.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000;58:19–36. doi: 10.1016/s0165-0327(99)00092-0. [DOI] [PubMed] [Google Scholar]
- Antony MM, Purdon C, Summerfeldt LJ, editors. Psychological Treatments of Obsessive Compulsive Disorder: Fundamentals and Beyond. American Psychological Association; Washington DC: 2007. pp. 9–29. [Google Scholar]
- Arumugham SS, Reddy JY. Augmentation strategies in obsessive-compulsive disorder. Expert Rev Neurother. 2013;13:187–202. doi: 10.1586/ern.12.160. [DOI] [PubMed] [Google Scholar]
- Beasley CM, Jr, Sayler ME, Bosomworth JC, Wernicke JF. High-dose fluoxetine: efficacy and activating-sedating effects in agitated and retarded depression. J Clin Psychopharmacol. 1991;11:166–174. [PubMed] [Google Scholar]
- Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mole Psychiatry. 2010;15:850–855. doi: 10.1038/mp.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Sigurdsson T, Gorman JM, Mcewen BS, Ledoux JE. Chronic antidepressant treatment impairs the acquisition of fear extinction. Biol Psychiatry. 2013;73:1078–1086. doi: 10.1016/j.biopsych.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussing R, Murphy TK, Storch EA, McNamara JPH, Reid AM, Garvan CW, et al. Psychometric properties of the Treatment-Emergent Activation and Suicidality Assessment Profile (TEASAP) in youth with OCD. Psychiatry Res. 2013;205:253–261. doi: 10.1016/j.psychres.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussing R, Reid AM, McNamara JP, Meyer JM, Guzick AG, Mason DM, et al. A pilot study of actigraphy as an objective measure of SSRI activation symptoms: results from a randomized placebo controlled psychopharmacological treatment study. Psychiatry Res. 2014 doi: 10.1016/j.psychres.2014.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C, Bebbington P, King M, Brugha T, Meltzer H, Bhugra D, Jenkins R. Why people do not take their psychotropic drugs as prescribed: results of the 2000 National Psychiatric Morbidity Survey. Acta Psychiatr Scand. 2007;116:47–53. doi: 10.1111/j.1600-0447.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- Davidson JR. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry. 2010;71:e04. doi: 10.4088/JCP.9058se1c.04gry. [DOI] [PubMed] [Google Scholar]
- Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151–161. doi: 10.1176/appi.ajp.162.1.151. [DOI] [PubMed] [Google Scholar]
- Franklin ME, Kratz HE, Freeman JB, Ivarsson T, Heyman I, Sookman D, et al. Cognitive-behavioral therapy for pediatric obsessive-compulsive disorder: empirical review and clinical recommendations. Psychiatry Res. 2015 doi: 10.1016/j.psychres.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Freeman J, Flessner CA, Garcia A. The Children's Yale-Brown Obsessive Compulsive Scale: reliability and validity for use among 5 to 8 year olds with obsessive-compulsive disorder. J Abnorm Child Psychol. 2011;39:877–883. doi: 10.1007/s10802-011-9494-6. [DOI] [PubMed] [Google Scholar]
- Geller DA, March J. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:98–113. doi: 10.1016/j.jaac.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Goodnick PJ. Selective serotonin reuptake inhibitors in the treatment of affective disorders—III. Tolerability, safety and pharmacoeconomics. J Psychopharmacol. 1998:55–S87. doi: 10.1177/0269881198012003041. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Liebowitz MR, Fyer AJ, Goetz D, Campeas RB, Fyer MR, et al. An open trial of fluoxetine in the treatment of panic attacks. J Clin Psychopharmacol. 1987;7:329–332. [PubMed] [Google Scholar]
- In-albon T, Schneider S. Psychotherapy of childhood anxiety disorders: a meta-analysis. Psychother Psychosom. 2007;76:15–24. doi: 10.1159/000096361. [DOI] [PubMed] [Google Scholar]
- Ivarsson T, Skarphedinsson G, Kornør H, Axelsdottir B, Biedilæ S, Heyman I, et al. The place of and evidence for serotonin reuptake inhibitors (SRIs) for obsessive compulsive disorder (OCD) in children and adolescents: views based on a systematic review and meta-analysis. Psychiatry Res. 2015 doi: 10.1016/j.psychres.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Jordan C, Reid AM, Mariaskin A, Augusto B, Sulkowski ML. First-line treatment for pediatric obsessive-compulsive disorder. J Contemp Psychother. 2012;42:243–248. [Google Scholar]
- Kahn JH, Schneider WJ. It's the destination and it's the journey: using multilevel modeling to assess patterns of change in psychotherapy. J Clin Psychol. 2013;69:543–570. doi: 10.1002/jclp.21964. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keeley ML, Storch EA, Merlo LJ, Geffken GR. Clinical predictors of response to cognitive-behavioral therapy for obsessive-compulsive disorder. Clin Psychol Rev. 2008;28:118–130. doi: 10.1016/j.cpr.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Kellner M. Drug treatment of obsessive-compulsive disorder. Dialogue Clin Neurosci. 2010;12:187. doi: 10.31887/DCNS.2010.12.2/mkellner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB. American Psychiatric Association: practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164:5–53. [PubMed] [Google Scholar]
- Kreft IG, De Leeuw J. Introducing Multilevel Modeling. Sage; London, UK: 1998. [Google Scholar]
- Labeling change request letter for antidepressant medication. [Accessed October 10, 2014];US Food and Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm096352.htm. Published October 15, 2004.
- Lewin AB, Peris TS, Lindsey bergman R, Mccracken JT, Piacentini J. The role of treatment expectancy in youth receiving exposure-based CBT for obsessive compulsive disorder. Behav Res Ther. 2011;49:536–543. doi: 10.1016/j.brat.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas CJ, Hox JJ. Sufficient sample sizes for multilevel modeling. Methodology. 2005;49:86. [Google Scholar]
- Mackinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Mulle K. OCD in Children and Adolescents: a Cognitive-Behavioral Treatment Manual. Guliford Press; New York: 1998. [Google Scholar]
- March JS, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ. Psychometric properties of the Children's Depression Rating Scale–Revised in adolescents. J Child Adol Psychop. 2010;20:513–516. doi: 10.1089/cap.2010.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Segarra A, Storch EA, Goodman WK. SSRI adverse events: how to monitor and manage. Int Rev Psychiatry. 2008;20:203–208. doi: 10.1080/09540260801889211. [DOI] [PubMed] [Google Scholar]
- Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292:1969–1976. doi: 10.1001/jama.292.16.1969. [DOI] [PubMed] [Google Scholar]
- Petersen TJ. Enhancing the efficacy of antidepressants with psychotherapy. J Psychopharmacol. 2006;20:19–28. doi: 10.1177/1359786806064314. [DOI] [PubMed] [Google Scholar]
- Pohl RB, Yeragani VK, Balon R, Lycaki H. The jitteriness syndrome in panic disorder patients treated with antidepressants. J Clin Psychiatry. 1988;49:100–104. [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB. Children's Depression Rating Scale, Revised (CDRS-R) Western Psychological Services; Los Angeles: 1996. [Google Scholar]
- Riddle MA, King RA, Hardin MT, Scahill L, Ort SI, Chappell P, et al. Behavioral side effects of fluoxetine in children and adolescents. J Child Adol Psychop. 1990;1:193–198. [Google Scholar]
- Riddle MA, Reeve EA, Yaryura-Tobias JA, Yang HM, Claghorn JL, Gaffney G. Fluvoxamine for children and adolescents with obsessive-compulsive disorder: a randomized, controlled, multicenter trial. J Am Acad Child Adolesc Psychiatry. 2001;40:222–229. doi: 10.1097/00004583-200102000-00017. [DOI] [PubMed] [Google Scholar]
- Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1–2):159–169. doi: 10.1089/cap.2006.16.159. [DOI] [PubMed] [Google Scholar]
- Sanchez-meca J, Rosa-alcazar AI, Iniesta-sepúlveda M, Rosa-alcazar A. Differential efficacy of cognitive-behavioral therapy and pharmacological treatments for pediatric obsessive-compulsive disorder: a meta-analysis. J Anxiety Disord. 2014;28:31–44. doi: 10.1016/j.janxdis.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, Mcswiggin-hardin M, Ort SI, King RA, Goodman WK, et al. Children's Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Schirman S, Kronenberg S, Apter A, Brent D, Melhem N, Pick N, et al. Effectiveness and tolerability of citalopram for the treatment of depression and anxiety disorders in children and adolescents: an open-label study. J neural Transm. 2010;117(1):139–145. doi: 10.1007/s00702-009-0330-x. [DOI] [PubMed] [Google Scholar]
- Sinclair LI, Christmas DM, Hood SD, Potokar JP, Robertson A, Isaac A, et al. Antidepressant-induced jitteriness/anxiety syndrome: systematic review. Br J Psychiatry. 2009;194:483–490. doi: 10.1192/bjp.bp.107.048371. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- Snijders T, Bosker R. Multilevel Analysis: an Introduction to Basic and Advanced Multilevel Modeling. Sage; London: 1999. [Google Scholar]
- Storch EA, Murphy TK, Geffken GR, Soto O, Sajid M, Allen P, et al. Psychometric evaluation of the Children's Yale-Brown Obsessive-Compulsive Scale. Psychiatry Res. 2004;129:91–98. doi: 10.1016/j.psychres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Storch EA, Bussing R, Small BJ, Geffken GR, McNamara JP, Rahman O, et al. Randomized, placebo-controlled trial of cognitive-behavioral therapy alone or combined with sertraline in the treatment of pediatric obsessive-compulsive disorder. Behav Res Ther. 2013;51:823–829. doi: 10.1016/j.brat.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasca GA, Gallop R. Multilevel modeling of longitudinal data for psychotherapy researchers: I. The basics. Psychother Res. 2009;19:429–437. doi: 10.1080/10503300802641444. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Abramowitz JS, Diefenbach GJ. Defining response in clinical trials for obsessive-compulsive disorder: a signal detection analysis of the Yale-Brown obsessive compulsive scale. J Clin Psychiat. 2005;66:1549–1557. doi: 10.4088/jcp.v66n1209. [DOI] [PubMed] [Google Scholar]
- Usala T, Clavenna A, Zuddas A, Bonati M. Randomised controlled trials of selective serotonin reuptake inhibitors in treating depression in children and adolescents: a systematic review and meta-analysis. Eur Neurol. 2008;18:62–73. doi: 10.1016/j.euroneuro.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson HJ, Rees CS. Meta-analysis of randomized, controlled treatment trials for pediatric obsessive-compulsive disorder. J Child Psychol Psychiatry. 2008;49:489–498. doi: 10.1111/j.1469-7610.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- Weisz JR, Mccarty CA, Valeri SM. Effects of psychotherapy for depression in children and adolescents: a meta-analysis. Psychol Bull. 2006;132:132–149. doi: 10.1037/0033-2909.132.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeragani VK, Pohl R, Balon R, Ramesh C, Weinberg P. Imipramine-induced jitteriness and decreased serum iron levels. Neuropsychobiology. 1992;25:8–10. doi: 10.1159/000118801. [DOI] [PubMed] [Google Scholar]