Abstract
We report the first example of intermolecular [4+2] annulation of cyclobutylanilines with alkynes enabled by visible light photocatalysis. Monocyclic and bicyclic cyclobutylanilines successfully undergo the annulation with terminal and internal alkynes to generate a wide variety of amine-substituted cyclohexenes including new hydrindan and decalin derivatives with good to excellent diastereoselectivity. The reaction is overall redox neutral with perfect atom economy.
Keywords: Photocatalysis, Iridium catalyst, N-aryl cyclobutyl amines, Small ring system, Cyclohexenes, Green Chemistry
Graphical Abstract
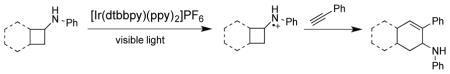
[4+2] Annulation under photoredox catalysis: A new way to construct cyclohexene derivatives
We have developed a mild protocol using visible light photocatalysis to synthesize structurally diverse amine-substituted cyclohexenes. The procedure employs 2 mol% [Ir(dtbbpy)(ppy)2](PF6), two 18 W LED lights, and is performed under degassed conditions. Readily prepared monocyclic and bicyclic cyclobutylanilines are converted to a variety of amine-substituted cyclohexenes via a cascade initialized by visible light.
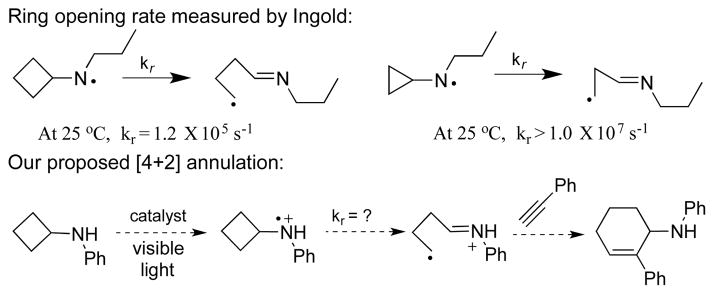
Release of ring strain has often been exploited as a driving force by organic chemists to promote cleavage of typically unreactive bonds such as carbon-carbon bonds.[1] This strategy has been successfully applied to ring opening of cyclopropanes, which leads to the wide use of cyclopropanes in organic synthesis.[2] We recently reported visible light promoted [3+2] annulation reactions of cyclopropylanilines with various pi bonds.[3] In these reactions, the cleavage of cyclopropyl rings is promoted by photooxidation of the parent amine to the corresponding amine radical cation. Since the oxidation potentials of cyclopropylaniline and cyclobutylaniline were found to be similar,[4] we were intrigued by the possibility of using cyclobutylanilines in the annulation reactions in a similar manner. We were further encouraged by the fact that cyclobutane’s strain energy is almost identical to that of cyclopropane.[5,6]
Still, ring opening of cyclopropanes is generally much faster than that of cyclobutanes. Newcomb reported that the rate constant for ring opening of a cyclobutylcarbinyl radical was 1.5 x 103 s−1 at 20 °C compared to 7 x 107 s−1 at 20 °C for ring opening of a cyclopropylcarbinyl radical.[7] Ingold reported the rate constant for ring opening of the cyclobutyl-n-propylaminyl radical to be 1.2 x 105 s−1 at 25 °C while the rate constant was estimated to be greater than 107 s−1 at 25 °C for ring opening of the cyclopropyl-n-propylaminyl radical (Scheme 1).[8] The proposed ring opening of the cyclobutylaniline radical cation was expected to be faster than the neutral aminyl radical,[9] which lent further credence to the proposed annulation reaction. Surprisingly, to the best of our knowledge, there have been no reports of using cyclobutylanilines in the tandem ring opening and annulation sequence to construct six-membered carbocycles. This is in contrast to the increasing use of other types of cyclobutanes in the tandem sequence.[10] Successful ring opening of these cyclobutanes usually relies on the decoration of the cyclobutyl ring with donor-acceptors[11] or ketone/hydroxyl functionality.[12] Herein we describe the successful development of the [4+2] annulation of cyclobutylanilines with alkynes enabled by visible light photoredox catalysis, which greatly broadens the use of cyclobutanes as a four-carbon synthon in organic synthesis. We hope that this method will add to the growing pool of synthetic methods based on photocatalysis.[13]
Scheme 1.
Proposed [4+2] annulation of cyclobutylaniline with alkyne.
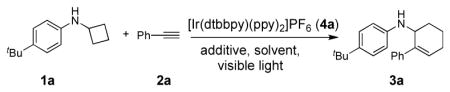
We chose 4-tert-Butyl-N-cyclobutylaniline 1a and phenylacetylene 2a as the standard substrates to optimize the [4+2] annulation. Through extensive screening (see SI), [Ir(dtbbpy)(ppy)2](PF6) 4a in MeOH with two 18 W LEDs was identified as the optimal conditions, providing the desired product 3a in 97% GC yield (90% isolated yield) (Table 1, entry 1). Conducting the experiment without degassing the reaction mixture led to significant decrease in the yield (entry 2, see SI). Two control studies showed that omitting either the photocatalyst or light produced a negligible amount of 3a (entries 3 and 4). The beneficial effect of using two lights could include increasing exposure to light and raising the reaction temperature. An internal temperature of 55 °C was measured with two bulbs while 25 °C was recorded with one bulb. To further probe this phenomenon, we performed a temperature control study in which the test tube was placed in a 55 °C water bath with one LED light (entry 5). Though not as high as using two LED lights, product 3a was detected in 68% yield. The yield improvement, comparing to one LED light at room temperature (entry 6), suggests that higher temperature and photon output are both beneficial to the reaction.
Table 1.
Optimization of the reaction conditions.
| ||||
|---|---|---|---|---|
| Entry[a] | Conditions | t [h] | Conv. of 1a [%][b] | Yield of 3a [%][b] |
| 1 | 4a (2 mol%), MeOH | 12 | 100 | 97 (90)[c] |
| 2 | 4a (2 mol%), MeOH, Air | 16 | 100 | 42 |
| 3 | without 4a, MeOH | 16 | 7 | 3 |
| 4 | 4a (2 mol%), MeOH, light bulb off | 16 | 10 | 7 |
| 5[d] | 4a (2 mol%), MeOH | 12 | 70 | 68 |
| 6[e] | 4a (2 mol%), MeOH | 12 | 29 | 27 |
Conditions: 1a (0.2 mmol, 0.1 M in degassed solvent), 2a (1 mmol), irradiation with two 18 W LED light bulbs at room temperature.
Yield determined by GC analysis using dodecane as an internal standard unless noted.
Isolated yields shown.
One 18 W LED light, reaction tube in a 55 °C water bath.
One 18 W LED light.
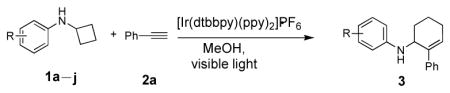
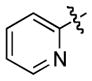
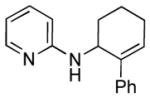
We next examined the scope of cyclobutylaniline derivatives with phenylacetylene 2a as the annulation partner under the optimized conditions (Table 2). Cyclobutylanilines were readily prepared by the Buchwald-Hartwig amination of cyclobutylamine with aryl halides.[14] The annulation reaction generally tolerated both electron-withdrawing (e.g., CF3 and CN) and electron-donating substituents (e.g., OMe, Ph, and alkyl) on the aryl ring. The low yield of 3d (entry 3) was due to the low solubility of 1d in MeOH. Use of a cosolvent, such as DMF, helped solubilize 1d but failed to improve the yield. Steric hindrance was also well tolerated as ortho substituents with various sizes (1e–1g) showed little effect on the reaction. Moreover, it is worth noting that heterocycles can be easily incorporated into the annulation products. The cyclobutylanilines (1i and 1j) substituted by a pyridyl group at the 2- or 3- position underwent the annulation reaction uneventfully, affording the desired product (3i and 3j) in good yields.
Table 2.
[4+2] Annulation of phenylacetylene (2a) with monocyclic cyclobutylanilines.
| ||||
|---|---|---|---|---|
| Entry[a] | Substrate | Product | t [h] | Yield[%][b] |
| 1 | 1b, R = H |
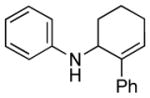 3b |
12 | 76 |
| 2 | 1c, R = 4-CF3 |
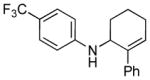 3c |
24 | 79 |
| 3 | 1d, R = 4-OMe |
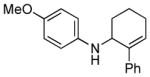 3d |
18 | 27 |
| 4 | 1e, R = 2-CN |
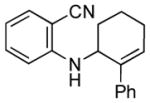 3e |
20 | 84 |
| 5 | 1f, R = 2-phenyl |
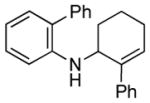 3f |
20 | 78 |
| 6 | 1g, R = 2-iPr |
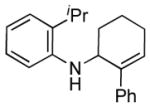 3g |
24 | 73 |
| 7 |
1h
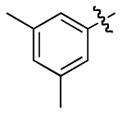
|
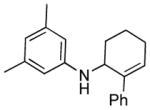 3h |
12 | 87 |
| 8 |
1i
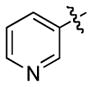
|
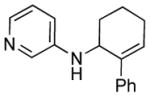 3i |
14 | 76 |
| 9 |
1j
|
 3j |
18 | 83 |
Reaction condition: substrate (0.2 mmol, 0.1 M in degassed MeOH), 2a (1 mmol), 4a (2 mol%), irradiation with two 18 W LED light bulbs.
Yield of the isolated product.
Encouraged by the success in the initial scope studies, we turned our attention to the generality of alkynes (Table 3). The reactivity pattern displayed by alkynes in the annulation reaction generally resembles that in the intermolecular addition of non-polar nucleophilic alkyl radicals to alkynes.[15] Terminal alkynes substituted with a functional group, capable of stabilizing the incipient vinyl radical, were found to be viable annulation partners (vide infra). The functional group included a list of quite diverse groups such as naphthyl (2b), 1,3-benzodioxole (2c), methyl ester (2d), and thiophene (2e). The annulation of these alkynes with several cyclobutylanilines showed complete regioselectivity, affording a range of six-membered carbocycles in good yields. A notable side reaction occurred when less hindered cyclobutylanilines were used in conjunction with methyl propiolate 2d. 1,4–Addition of cyclobutylaniline 1k to 2d completely suppressed the desired [4+2] annulation reaction.[16] An ortho substituent on the N-aryl ring (e.g., 1f) was needed to inhibit the side reaction. Typically, internal alkynes are less reactive than terminal alkynes in intermolecular addition of carbon radicals to alkynes due to steric hindrance.[15] Internal alkynes were unsuccessful in the annulation with cyclopropylanilines.[3b,3c] Surprisingly, divergent reactivity emerged between cyclopropylanilines and cyclobutylanilines with respect to this class of alkynes. Several internal alkynes (2f–h) successfully underwent the annulation reaction with cyclobutylanilines in complete regiocontrol. A limitation of utilizing internal alkynes is that at least one of the two substituents must be capable of stabilizing the vinyl radical. The regiochemistry of the annulation products (5f–h) were assigned based on 2D NMR spectroscopy. The annulation product (5h) was further confirmed by X-ray crystallography (See SI).[17] The regioselectivity can be rationalized based on the substituent’s ability to stabilize the incipient vinyl radical.
Table 3.
Scope of alkynes in the [4+2] annulation.
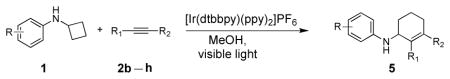
| |||||
|---|---|---|---|---|---|
| Entry[a] | Substrate | Alkyne | Product | t [h] | Yield[%][b] |
| 1 | 1a |
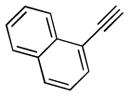 2b |
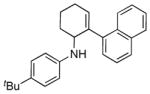 5a |
16 | 71 |
| 2[c] | 1a |
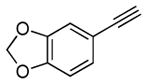 2c |
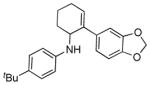 5b |
16 | 57 |
| 3 | 1j | 2b |
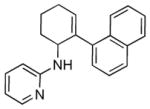 5c |
16 | 72 |
| 4 | 1f |
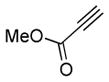 2d |
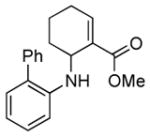 5d |
14 | 42 |
| 5 | 1a |
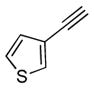 2e |
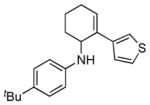 5e |
12 | 66 |
| 6 |
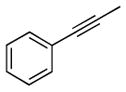 2f |
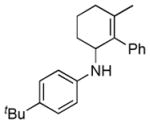 5f |
14 | 61 | |
| 7 | 1h |
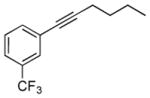 2g |
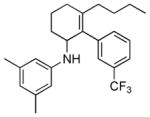 5g |
24 | 42 |
| 8 | 1c |
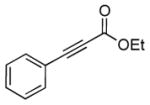 2h |
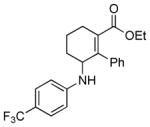 5h |
12 | 92 |
Reaction condition: substrate (0.2 mmol, 0.1 M in degassed MeOH), 2b–2h (0.6 mmol), 4a (2 mol%), irradiation with two 18 W LED light bulbs.
Yield of the isolated product.
Mixed solvent of MeOH and CH3NO2 (1:1).
Bicyclo[4.3.0]nonane (hydrindan) and bicyclo[4.4.0]decane (decalin) are two common structural motifs in small organic molecules. Yet, only a handful of methods such as the Diels-Alder reaction[18] and the Robinson annulation[19] are available for their preparation. Hence, these structures are ideal targets to test the scope of the [4+2] annulation reaction (Table 4). The requisite starting materials, cis-fused 5,4-membered (6a–d) and 6,4-membered (6e and 6f) bicycles, were readily accessible in four steps from commercially available cyclopentene and cyclohexene, respectively.[20,21] Under the optimized conditions, a pair of diastereomeric 5-4 membered bicycles (6a and 6b), which differ in the stereochemistry at C6, underwent the annulation with phenylacetylene 2a to provide 7a as the major product in similar yields and almost identical drs (entries 1 and 2). High diastereoselectivity was achieved (>10:1) with 7a being trans-fused. This data is consistent with regioselective ring opening of 6a or 6b at the C5-C6 bond, which leads to formation of the identical distonic radical iminium ion[22] and subsequent loss of the stereochemical integrality of the C6 stereocenter. The observed regioselective ring opening was probably driven by the formation of a more stable secondary carbon radical versus a primary radical. Incorporation of a strong electron-withdrawing group (e.g., CF3) (entry 3) into the N-aryl ring showed little effect on both the yield and diastereoselectivity. Internal alkyne 2h successfully participated in the annulation reaction, affording the annulation products 7c and 7c′ in excellent yield albeit lower diastereoselectivity when compared to terminal alkynes (entries 1–3). The annulation of 6,4-membered bicycles (6e and 6f) with terminal alkynes (2a and 2c) furnished cis-fused decalin derivatives (7e, 7e′ and 7f, 7f′) in excellent yields, although a decrease in diastereoselectivity was observed in comparison to 5,4-membered bicycles (entries 6 and 7). Two cis-fused decalin derivatives were obtained along with a third diastereomer whose relative configuration was unidentified in both examples (6e and 6f).[23] The structure and stereochemistry of the annulation products were assigned by 2D NMR spectroscopy. X-ray crystallographic analysis of 7d was also obtained to further support our assignments.[24]
Table 4.
[4+2] Annulation of alkynes with bicyclic cyclobutylanilines.
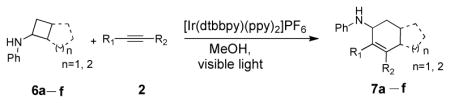
| |||||
|---|---|---|---|---|---|
| Entry[a] | Substrate | Alkyne | Product | Yield[%][b] d.r.[c] | |
| major | minor | ||||
| 1 |
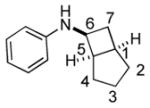 6a |
2a |
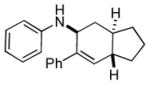 7a |
n.a. | 95 |
| 13:1 | |||||
| 2 |
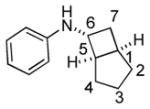 6b |
2a |
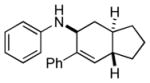 7a |
n.a. | 97 |
| 11:1 | |||||
| 3 |
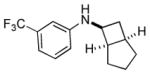 6c |
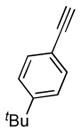 2i |
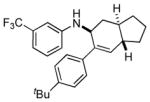 7b |
n.a. | 89 |
| 20:1 | |||||
| 4 |
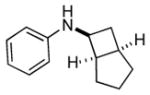 6a |
2h |
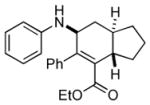 7c |
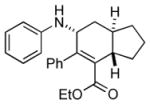 7c′ |
98 |
| 4:1 | |||||
| 5 |
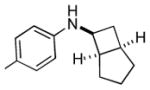 6d |
2h |
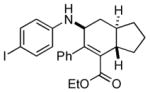 7d |
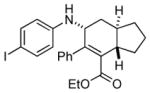 7d′ |
89 |
| 4:1 | |||||
| 6 |
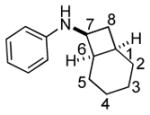 6e |
2a |
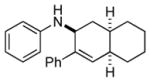 7e |
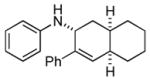 7e′ |
94 |
| 6:1 | |||||
| 7 |
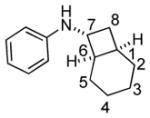 6f |
2c |
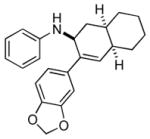 7f |
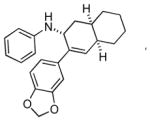 7f′ |
92 |
| 4:1 | |||||
Reaction condition: substrate (0.2 mmol, 0.1 M in degassed MeOH), 2a, 2c, 2h and 2i (0.6 mmol), 4a (2 mol%), irradiation with two 18 W LED light bulbs for 12 h.
Combined yields of the two isomers after column chromatography.
Determined by 1H NMR analysis of the crude product.
A catalytic cycle similar to the [3+2] annulation is proposed for the [4+2] annulation (See SI).[3b,3c] The oxidation peak potential of 1a was measured to be 0.8 V vs. SCE, which is more positive than the reduction potential of the photoexcited 4a (Ir3+*/Ir2+: 0.66 V vs. SCE). Although thermodynamically unfavorable, such SET processes have been reported.[25] Stern-Volmer quenching studies revealed that cyclobutylaniline 1a quenches the photoexcited 4a while alkynes 2a and 2h showed little quenching (see SI).
In conclusion, we have accomplished the first example of cleaving C-C bonds of cyclobutylanilines enabled by visible light photoredox catalysis. Monocyclic and bicyclic cyclobutylanilines undergo the [4+2] annulation with terminal and internal alkynes to produce amine-substituted six-membered carbocycles. Good to excellent diastereoselectvity is observed for the latter class of the compounds, yielding new hydrindan and decalin derivatives. Finally, the approach we have developed to overcome cyclobutylanilines’ less propensity for ring cleavage can be potentially applied to open rings larger than three- and four-membered with suitable built-in ring strain.
Supplementary Material
Acknowledgments
We thank the University of Arkansas, the Arkansas Bioscience Institute, the National Institutes of Health (NIH) under Grant Number P30 GM103450 from the National Institute of General Medical Sciences, NSF Career Award under Award Number CHE-1255539 for generous support of this research.
References
- 1.a) Trost BM. Top Curr Chem. 1986;133:3–82. [Google Scholar]; b) Rybtchinski B, Milstein D. Angew Chem. 1999;111:918–932. doi: 10.1002/(SICI)1521-3773(19990401)38:7<870::AID-ANIE870>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1999;38:870–883. doi: 10.1002/(SICI)1521-3773(19990401)38:7<870::AID-ANIE870>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; c) Walczak MAA, Krainz T, Wipf P. Acc Chem Res. 2015 doi: 10.1021/ar500437h. [DOI] [PubMed] [Google Scholar]
- 2.For reviews on the use of cyclopropane in organic synthesis: Reissig HU, Zimmer R. Chem Rev. 2003;103:1151–1196. doi: 10.1021/cr010016n.Yu M, Pagenkopf ML. Tetrahedron. 2005;61:321–347.Carson CA, Kerr MA. Chem Soc Rev. 2009;38:3051–3060.2. doi: 10.1039/b901245c.Tang P, Qin Y. Synthesis. 2012;44:2969–2984.
- 3.(a) Maity S, Zhu M, Shinabery RS, Zheng N. Angew Chem. 2012;124:226–230. doi: 10.1002/anie.201106162. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:222–226. [Google Scholar]; b) Nguyen TH, Maity S, Zheng N. Beilstein J Org Chem. 2014;10:975–980. doi: 10.3762/bjoc.10.96. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Nguyen TH, Morris SA, Zheng N. Adv Synth Catal. 2014;356:2831–2837. doi: 10.1002/adsc.201400742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The oxidation peak potential of 4-tert-butyl-N-cyclobutylaniline was measured to be +0.8 V vs. SCE in comparison with +0.83 V vs. SCE for N-cyclopropylaniline.
- 5.a) Wiberg KB. In: The Chemistry of Cyclobutanes. Rappoport ZZ, Liebman JF, editors. Wiley; Chichester: 2005. pp. 1–15. [Google Scholar]; b) Khoury PR, Goddard JD, Tam W. Tetrahedron. 2004;60:8103– 8112. [Google Scholar]
- 6.a) Bach RD, Dmitrenko O. J Am Chem Soc. 2004;126:4444–4452. doi: 10.1021/ja036309a. [DOI] [PubMed] [Google Scholar]; b) Wu W, Ma B, Wu JI, Schleyer PvR, Mo Y. Chem Eur J. 2009;15:9730–9736. doi: 10.1002/chem.200900586. [DOI] [PubMed] [Google Scholar]
- 7.a) Musa OM, Horner JH, Shahin H, Newcomb M. J Am Chem Soc. 1996;118:3862–3868. [Google Scholar]; b) Jin J, Newcomb M. J Org Chem. 2008;73:4740–4742. doi: 10.1021/jo800500e. [DOI] [PubMed] [Google Scholar]
- 8.Maeda Y, Ingold KU. J Am Chem Soc. 1980;102:328–331. [Google Scholar]
- 9.Horner JH, Martinez FN, Musa OM, Newcomb M, Shahin HE. J Am Chem Soc. 1995;117:11124–11133. [Google Scholar]
- 10.For selected recent reviews: Namyslo JC, Kaufmann DE. Chem Rev. 2003;103:1485–1537. doi: 10.1021/cr010010y.Seiser T, Saget T, Tran DC, Cramer N. Angew Chem. 2011;123:7884–7896. doi: 10.1002/anie.201101053.Angew Chem Int Ed. 2011;50:7740–7752. doi: 10.1002/anie.201101053.Reissig HU, Zimmer R. Angew Chem. 2015 doi: 10.1002/ange.201501135.Angew Chem Int Ed. 2015 doi: 10.1002/anie.201501135.
- 11.For selected recent examples: Matsuo J, Sasaki S, Tanaka H, Ishibashi H. J Am Chem Soc. 2008;130:11600–11601. doi: 10.1021/ja8045684.Matsuo J, Negishi S, Ishibashi H. Tetrahedron Lett. 2009;50:5831–5833.Kawano M, Kiuchi T, Negishi S, Tanaka H, Hoshikawa T, Matsuo J, Ishibashi H. Angew Chem. 2013;125:940–944. doi: 10.1002/anie.201206734.Angew Chem Int Ed. 2013;52:906–910. doi: 10.1002/anie.201206734.Parsons AT, Johnson JS. J Am Chem Soc. 2009;131:14202–14203. doi: 10.1021/ja906755e.Moustafa MMAR, Stevens AC, Machin BP, Pagenkopf BL. Org Lett. 2010;12:4736–4738. doi: 10.1021/ol102063f.Stevens AC, Palmer C, Pagenkopf BL. Org Lett. 2011;13:1528–1531. doi: 10.1021/ol200220d.Perrotta D, Racine S, Vuilleumier J, de Nanteuil F, Waser J. Org Lett. 2015;17:1030–1033. doi: 10.1021/acs.orglett.5b00149.
- 12.For selected recent examples: Xu T, Dong G. Angew Chem. 2012;124:7685–7689.Angew Chem Int Ed. 2012;51:7567–7571. doi: 10.1002/anie.201202771.Xu T, Ko HM, Savage NA, Dong G. J Am Chem Soc. 2012;134:20005–20008. doi: 10.1021/ja309978c.Chen PH, Xu T, Dong G. Angew Chem. 2014;126:1700–1704.Angew Chem Int Ed. 2014;53:1674–1678. doi: 10.1002/anie.201310100.Murakami M, Ashida S, Matsuda T. J Am Chem Soc. 2005;127:6932–6933. doi: 10.1021/ja050674f.Ishida N, Sawano S, Masuda Y, Murakami M. J Am Chem Soc. 2012;134:17502–17504. doi: 10.1021/ja309013a.Liu L, Ishida N, Murakami M. Angew Chem. 2012;124:2535–2538.Angew Chem Int Ed. 2012;51:2485–2488.Souillart L, Parker E, Cramer N. Angew Chem. 2014;126:3045–3049. doi: 10.1002/anie.201311009.Angew Chem Int Ed. 2014;53:3001–3005. doi: 10.1002/anie.201311009.Souillart L, Cramer N. Chem Sci. 2014;5:837–840.
- 13.For selected recent reviews: Yoon TP. ACS Catal. 2013;3:895–902. doi: 10.1021/cs400088e.Prier CK, Rankic DA, MacMillan DWC. Chem Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r.Xuan J, Lu LQ, Chen JR, Xiao WJ. Eur J Org Chem. 2013:6755–6770.Douglas JJ, Nguyen JD, Cole KP, Stephenson CRJ. Aldrichimica Acta. 2014;47:15–25.
- 14.For selected reviews: Hartwig JF. Acc Chem Res. 2008;41:1534–1544. doi: 10.1021/ar800098p.Surry DS, Buchwald SL. Chem Sci. 2011;2:27–50. doi: 10.1039/C0SC00331J.
- 15.a) Giese B, Lachhein S. Angew Chem. 1982;94:780–781. [Google Scholar]; Angew Chem Int Ed Engl. 1982;21:768–775. [Google Scholar]; b) Fischer H, Radom L. Angew Chem. 2001;113:1380–1414. doi: 10.1002/1521-3773(20010417)40:8<1340::aid-anie1340>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2001;40:1340–1371. doi: 10.1002/1521-3773(20010417)40:8<1340::aid-anie1340>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; c) Wille U. Chem Rev. 2013;113:813–853. doi: 10.1021/cr100359d. [DOI] [PubMed] [Google Scholar]
-
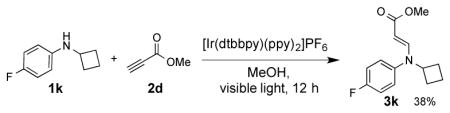
16.Upon irradiation of cyclobutylaniline 1k and 2d in the presence of 4a (2 mol%) under degassed condition, a 1,4- addition reaction product of 1k to 2d was formed with a yield of 38%.
- 17.CCDC 1060321 (5h) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic data Center via www.ccdc.cam.ac.uk/data_request/cif.
- 18.For selected reviews on the Diels-Alder reaction, see: Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew Chem. 2002;114:1703–1706. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z.Angew Chem Int Ed. 2002;41:1668–1698. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z.Fringuelli F, Taticchi A. The Diels-Alder Reaction: Selected Practical Methods. Wiley; Chichester, UK: 2002. Takao K, Munakata R, Tadano K. Chem Rev. 2005;105:4779–4807. doi: 10.1021/cr040632u.
- 19.For selected reviews on the Robinson annulation reaction, see: Gawley RE. Synthesis. 1976;12:777–794.Jung MJ. Tetrahedron. 1976;32:3–31.
- 20.Ghosez L, Montaigne R, Roussel A, Vanlierde H, Mollet P. Tetrahedron. 1971;27:615–633. [Google Scholar]
- 21.McLaughlin M, Palucki M, Davies IW. Org Lett. 2006;8:3307–3310. doi: 10.1021/ol061232r. [DOI] [PubMed] [Google Scholar]
-
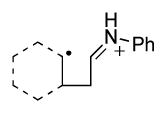
22.Proposed distonic radical iminium ion 8c:
- 23.The third isomer from the annulation of 6e and 2a could not be detected by 1H NMR or GC analysis of the crude product. The diastereomer ratio for the three isomers (7f : 7f′ : the third isomer) from the annulation of 6f and 2c was measured by GC to be 13 : 3 : 1.
- 24.CCDC 1060320 (7d) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic data Center via www.ccdc.cam.ac.uk/data_request/cif.
- 25.a) Yasu Y, Koike T, Akita M. Adv Synth Catal. 2012;354:3414–3420. [Google Scholar]; b) Primer DN, Karakaya I, Tellis JC, Molander GA. J Am Chem Soc. 2015;137:2195–2198. doi: 10.1021/ja512946e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.