Abstract
Background & Aims
Isoniazid is a leading cause of liver injury but it is not clear how many cases are reported or how many clinicians adhere to American Thoracic Society (ATS) guidelines. We collected data on cases of isoniazid hepatotoxicity and assessed adherence to ATS guidelines and reports to the Center for Disease Control’s (CDC) isoniazid severe adverse events program.
Methods
We analyzed Drug Induced Liver Injury Network (DILIN) cases considered definite, highly likely, or probable for isoniazid injury from 2004 through 2013. We assessed the delays in isoniazid discontinuance according to ATS criteria and hepatotoxicity severity by Severity Index Score. We checked reporting to the CDC by matching cases based on age, latency, indication, reporting period, and comorbidities.
Results
Isoniazid was the second most commonly reported agent in the DILIN, with 69 cases; 60 met inclusion criteria. The median age of cases was 49 y (range 4–68 y), 70% were female, 97% had latent tuberculosis, and 62% were hospitalized. Patients took a median of 9 days to stop taking isoniazid (range 0–99 days). Thirty-three of cases (55%) continued taking isoniazid for more than 7 days after the ATS stopping criteria were met. Twenty-four cases (40%) continued isoniazid for more than 14 days after meeting stopping criteria. A delay in stopping was associated with more severe injury (P<.05). Of 13 patients who died or underwent liver transplantation, 9 (70%) continued taking isoniazid for >7 days after meeting stopping criteria. Only 1/25 cases of isoniazid hepatotoxicity eligible for reporting to the CDC were reported.
Conclusions
Poor adherence to ATS guidelines is common in cases of hepatotoxicity and is associated with more severe outcomes including hospitalization, death, and liver transplantation. Isoniazid continues to be a leading cause of DILI in the US, and its hepatotoxicity is significantly under-reported.
Keywords: adverse reaction, antibiotics, drug induced liver injury, hepatotoxicity, tuberculosis
INTRODUCTION
Hepatotoxicity from isoniazid (INH) was reported shortly after the drug’s introduction in the 1950’s.1–4 Elevation of serum ALT greater than 5 times ULN occurs in 3–5% of patients, and severe injury in less than 1%.5 The American Thoracic Society (ATS) recommends monitoring serum alanine aminotransferase (ALT) only in patients with pre-existing liver disorders or other risks for hepatotoxicity.6 Otherwise patients are instructed to stop their INH if they develop nausea, abdominal pain, jaundice or unexplained fatigue.6 If the serum ALT level is greater than 3 times upper limit of normal (ULN), INH should be stopped. If there are no symptoms, INH should be stopped only if the ALT is greater than 5 times ULN.
Though studies suggest a low incidence of severe hepatotoxicity7,8 under reporting is suspected. The Drug Induced Liver Injury Network (DILIN) is a multi-center study funded by the NIH to create a large registry of well-characterized cases of idiosyncratic DILI for clinical and translational studies. Amongst INH hepatotoxicity cases, we noted poor adherence to stopping guidelines with substantial delays in drug discontinuance. We quantified these delays and tested for association with severity of hepatotoxicity. The DILIN was initiated in 2004, the same year the CDC started a national project to capture severe adverse events (SAE) associated with INH given for latent TB.9 Therefore, we assessed completeness of reporting to the CDC by examining whether our cases appeared in the CDC report.
MATERIALS AND METHODS
Design
The design of the DILIN prospective and retrospective studies have been described.10 Patients suspected of having idiosyncratic drug induced liver injury (DILI) are enrolled within 6 months of liver injury for the prospective study. For the retrospective study, patients can be enrolled up to 10 years after the event for 7 agents one of which is isoniazid. Inclusion criteria include serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) greater than 5 times upper limit of normal (ULN) (or pretreatment baseline if abnormal) on 2 consecutive occasions, or alkaline phosphatase (AP) levels greater than 2 times ULN (or pretreatment baseline if abnormal) on 2 consecutive occasions. Patients with serum bilirubin greater than 2.5 mg/dL or INR greater than 1.5, and any elevation in AST, ALT, or AP, are also eligible. Subjects are followed for at least 6 months. Complete history, physical, past medical history, laboratory values, and imaging data are obtained. Severity of DILI is assessed semi-quantitatively using the DILIN Severity Index Score (SIS) (Table 1).
Table 1.
DILIN Severity Index Score
| 1 | Mild | Patient has elevations in serum aminotransferase or alkaline phosphatase levels but total bilirubin is < 2.5 mg/dL and there is no coagulopathy (INR <1.5). |
| 2 | Moderate | Patient has elevations in serum aminotransferase or alkaline phosphatase levels and total bilirubin is ≥ 2.5 mg/dL or there is coagulopathy (INR ≥1.5) without hyperbilirubinemia. |
| 3 | Moderate-Hospitalized | Patient has elevations in serum aminotransferase or alkaline phosphatase levels and total bilirubin is ≥ 2.5 mg/dL or INR >= 1.5 and the patient is hospitalized (or a pre-existing hospitalization is prolonged) because of the drug-induced liver injury. |
| 4 | Severe | Patient has elevations in serum aminotransferase or alkaline phosphatase levels and total bilirubin is ≥ 2.5 mg/dL and there is at least one of the following:
|
| 5 | Fatal | Patient dies or undergoes liver transplantation for drug-induced liver injury |
Diagnosis of DILI (causality assessment)
DILIN uses a consensus expert opinion method of causality assessment previously described.10 Each case is evaluated by 3 DILIN hepatologists. Each independently assigns a causality score representing percent likelihood of attribution (1 = definite or > 95% likelihood, 2 = highly likely or 75–95%, 3 = probable or 50–74%, 4 = possible or 25–49%, and 5 = unlikely or < 25%). Consensus is reached by e-mail and conference call.
Participants
We identified INH cases enrolled by April 15, 2013. Analysis was limited to cases that were probable, highly likely or definite INH hepatotoxicity. Probable cases were included only if no other agent was implicated.
Data and Outcomes
Demographic, history and laboratory data were analyzed with special attention to onset of symptoms, onset of elevated liver enzymes, and drug stop date. Patient data included race, ethnicity, and education level. Primary language, country of birth, English speaking ability, literacy and recollection of instructions given were not recorded. The pattern of injury was categorized as hepatocellular, cholestatic, or mixed based upon the R-value: R-value = (serum ALT value/serum ALT ULN) ÷ (serum AP value/serum AP ULN). R-values greater than 5 are considered hepatocellular, less than 2 cholestatic, and 2–5 mixed. Standard descriptive statistics were used. Correlations between Severity Index Score, delay in stopping INH and clinical variables were performed using Chi-square, Fisher’s Exact, Median test, Mann-Whitney and Spearman’s rho correlation coefficient where appropriate.
Matching cases to the CDC SAE reporting program
The CDC reporting program published findings from 2004 through 2008.9 Therefore, we examined the 25 of our cases that were treated for latent TB and had occurrence of hepatotoxity from January 1, 2004 through December 31, 2008. We looked for these 25 cases in the CDC report by matching age (+/− 3 years), comorbidities, latency to injury (+/− 1 month), delay in stopping (+/−1 week), and outcome.
Role of Funding Source and Institutional Board Review (IRB)
The DILIN Network is structured as a U01 cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Separate IRB approvals were maintained at each DILIN center throughout the time period of this study.
RESULTS
Subjects
Among 1091 patients in the DILIN by April 15, 2013, INH was listed as a potential cause in 69 (6.5%), and 60 of these met our current study inclusion criteria. INH was second only to amoxicillin-clavulanate as a causative medication. Of these 60 patients, 42 (70%) were women (Table 2). The median age was 49 years (range 4–68). Almost all (97%) were prescribed INH for latent TB infection. Four patients were under 18 (range 4–17). All were given INH for latent infection. Jaundice, nausea and abdominal pain were frequent presenting symptoms. Seven (12%) had chronic hepatitis C, but there were no other liver disorders identified.
Table 2.
Characteristics of INH Liver Injury Patients (n = 60)
| Age in yrs., median (min., max.) | 49 (4, 68) |
| Gender | 42 women (70%) |
| Body Mass Index, kg/m2, mean (S.D.) | 27.1 (5.5) |
| Race (self-report) | |
| White | 34 (57%) |
| Black/African American | 13 (22%) |
| Other* | 13 (22%) |
| Ethnicity = Latino | 15 (25%) |
| Indication for INH use | |
| Latent TB | 56 (97%) |
| Exposure to TB infected person | 1 (2%) |
| Active TB | 1 (2%) |
| Concurrent alcohol use^ | 22 (37%) |
| Concurrent liver disease | |
| Hepatitis C, chronic | 7 (12%) |
| Hepatitis B | 0 (0%) |
| Non-alcoholic fatty liver disease | 0 (0%) |
| Cirrhosis | 0 (0%) |
| Latencies, mean days (S.D.) | |
| INH start to symptoms | 85.7 (56.1) |
| INH start to DILI onset|| | 103.4 (59.5) |
| Signs and symptoms | |
| Jaundice | 42 (70%) |
| Nausea | 32 (53%) |
| Fever | 9 (15%) |
| Abdominal pain | 27 (45%) |
| Rash | 8 (13%) |
| Pruritus | 17 (18%) |
| DILIN Causality scores | |
| 1: Definite | 29 (48%) |
| 2: Highly likely | 23 (38%) |
| 3: Probable (single agent cases) | 8 (13%) |
| Peak liver biochemistries (mean, std.dev.) | |
| ALT (IU/L) | 1384 (864) |
| AST (IU/L) | 1467 (1137) |
| AP (IU/L) | 279 (159) |
| Bilirubin (mg/dL) | 12 (11.6) |
| INR | 2 (4.3) |
Pacific islander, Asia subcontinent, East Asia, unstated/unknown
At least one alcohol beverage in last 12 months
ALT >5x ULN or AP >2x ULN on two consecutive tests
Diagnostic/Causality Scoring
Nine patients were excluded because of low likelihood of INH hepatotoxicity. In two, INH was the only agent implicated, but each patient scored only a 4 (possible). The other 7 involved other medications along with INH and the INH scored only a 3 (probable) or 4 (possible). Of the 60 meeting inclusion criteria, 18 had enrolled into retrospective study and 42 into the prospective study. Twenty-nine (48%) were considered definite (score 1) and 23 (38%) highly likely (score 2), and 8 (13%) probable (score 3) with only INH involved. In 5 patients, six other agents (rifampin, anakinra, Hydroxycut®, fenofibrate, topiramate, leflunomide) were considered as potentially causal. All these competing agents were considered less likely than INH to be causal, and INH scored either a 2 (highly likely) or 1 (definite) in each of these multi-drug cases.
Retrospective vs. Prospective Cases
There were no statistically significant differences between the 18 retrospectively and the 42 prospectively enrolled patients in terms of age, gender, peak liver enzymes, peak bilirubin, or time from INH start to symptoms. Retrospective patients tended to be more severe with 6 (33%) undergoing liver transplantation and/or dying compared to only 7 (17%) for the prospective patients, p = 0.09.
Pattern of Injury
Patterns of liver injury at presentation were predominantly hepatocellular with 55 (92%) of patients having an R-value > 5. Four patients (7%) had a mixed pattern (R-value 2–5). No patients had a cholestatic pattern (R-value < 2). One patient did not have an alkaline phosphatase drawn at presentation so no R-value could be calculated, but the ALT was 2232 U/L. The mean days from INH initiation to elevated ALT was 97 (standard deviation [S.D.] 64). Mean days to an ALT level 5 times the ULN was 103 (S.D. 59). The mean time from DILI onset to peak ALT was short (6.6 days, S.D. 9). Decline in ALT to 50% or less of peak value took a mean of 38 days (S.D. 66.7) but the data were skewed with median being only 13 days. It took a mean of 200 days (S.D. 348) for the ALT to fall from peak to normal.
Severity of Injury
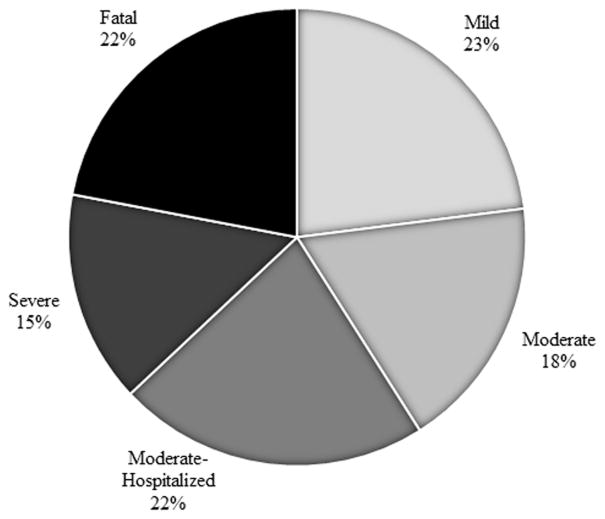
Distribution of DILIN Severity Index Scores is shown in Figure 1. Twelve patients required liver transplant and one of these ultimately died. Another died without transplant. Thirty-seven patients (62%) required hospitalization and 14 (23%) developed encephalopathy. Mean peak ALT, bilirubin and INR were 1385 IU/L (S.D. 864), 12.7 mg/dL (S.D. 11.6) and 2.6 (S.D. 4.3) respectively.
Figure 1.
Distribution of 60 INH hepatotoxicity patients by Severity Index Scores (SIS). (SIS 1 = Mild, SIS 2 = Moderate, SIS 3 = Moderate-Hospitalized, SIS 4 = Severe, SIS 5 = Fatal).
Delay in Discontinuance of INH and Severity of Injury
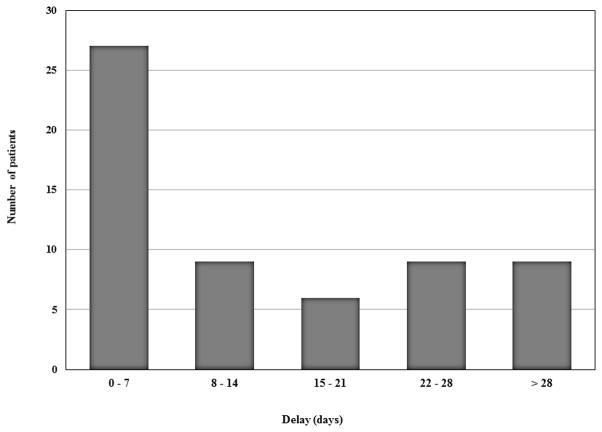
Median delay between meeting ATS stopping criteria and discontinuance was 9 days, with skewing toward longer delays (mean 17.9, S.D. 23.8, range 0 – 99 days). Only 27 (45%) patients stopped within 7 days of meeting criteria, and 24 (40%) kept taking their INH for more than 14 days. (Figure 2) Nine patients (15%) took the INH for > 28 days beyond reaching stopping criteria, and 6 of these kept taking the INH for >60 days. The delays primarily occurred in patients who developed symptoms but did not hold the INH until much later when the patient sought medical attention. In contrast, the median delay after elevated ALT criteria being met was 0 days with a mean of 3.2 days (S.D. 13.2, range 0–99 days). However the delays even after ALT criteria being documented were also skewed with some remarkable continuances of INH for 8, 10, 18, 20 and 99 days. Based on symptoms alone, the median and mean delays were 14 and 20 days.
Figure 2.
Distribution of delay in stopping INH after stopping rules of the American Thoracic Society guidelines had been met. (n = 60)
Longer delays in stopping INH correlated with higher DILIN Severity Index Score (SIS) (p = 0.05, Spearman’s rho). One remarkable patient developed hepatic tolerance where serum ALT reached >1000 U/L but the provider allowed the INH to continue for more than 3 months. The ALT fell while still on INH and there was only mild injury (SIS 1). If this patient is excluded as a rare outlier, correlation between delay and SIS was even stronger (p = 0.02). Of the 27 patients who stopped INH within 7 days of meeting stopping criteria, 6 (22%) had a severe or fatal reaction (SIS 4 or 5), and of the 33 who continued the INH for more than a week, 16 (49%) had a severe or fatal course. Conversely, 5 (36%) of the 14 patients with mild injury (SIS 1) compared to 16 (73%) of the 22 with severe or fatal injury (SIS = 4–5) continued the INH for more than a week after meeting stopping criteria. Higher BMI also correlated with increased SIS (p=0.02). Older age, race, ethnicity, education level and gender did not correlate with severity.
Chronic hepatitis C (CHC) was present in 7 (12%) (Table 2). These cases tended to have fewer severe or fatal DILIs compared to non-CHC cases, but the difference did not reach statistical significance [1 of 7 vs. 19 of 53, p = 0.20]. However, the median delay in stopping INH was statistically shorter for those with CHC compared to non-CHC cases (0 vs. 12 days, p = 0.03). Only 2 of the CHC cases stopped based on elevated liver enzymes alone, while 2 stopped when both symptoms and elevated enzymes were discovered on the same day and 3 stopped for only symptoms with elevated enzymes found several days later. The one fatal case with CHC had cirrhosis and hepatocellular carcinoma already.
There was no correlation between delay and age, gender, race, ethnicity, education level, or indication for INH therapy (latent versus active TB infection). Of the 4 children, a 17 year old delayed stopping her INH by 15 days and required transplant. The other 3 had mild (SIS =1) or moderate (SIS = 2) injuries with delays in stopping of 0 to 29 days.
Matching Cases in the CDC Reporting Program
The CDC report obtained clinical details on only 10 of their 17 cases reported from 2004 through 2008.9 Of the 25 DILIN cases that should have been reported to the CDC program during this period, only 1 appeared to be among the 10 CDC cases with detailed clinical history based on matched age, duration of therapy, delay in stopping INH, comorbidities (depression) and outcome (transplanted). For this one case, the matching of data was nearly perfect. The other 9 CDC cases differed widely in age, outcome, latency and/or comorbidities from the other 24 DILIN cases, making them very unlikely to be any of our cases.
DISCUSSION
Approximately 300,000 people in the U.S. are treated for latent TB each year and another 10,000 are treated for active infection.11,12 American Thoracic Society (ATS) guidelines to prevent liver injury rely heavily on patient self-identification of symptoms.6 Patients are instructed to stop taking INH for symptoms of hepatitis and seek medical attention. Our data suggest that poor adherence to these guidelines is common in patients with INH hepatotoxicity and leads to more severe injury. Of those that died or needed transplantation, 70% had continued the INH for a week or more after meeting stopping criteria. Therefore many of the severe injuries may have been avoided. Our data also raises concerns for under reporting and under estimation of risk.
The prolonged use of INH in our 60 patients was substantial with over half taking the INH more than 1 week beyond stopping criteria and 40% more than 2 weeks (Figure 2). Almost all (97%) of the patients in our study were treated for latent TB and one fifth required liver transplantation and/or died. The vast majority of delays occurred because patients ignored symptoms (even jaundice), but occasionally providers also failed to recognize symptoms or elevated transaminases. Four patients continued INH for over a week after serum ALT exceeded stopping levels. Delay correlated with injury severity including need for hospitalization and fatality.
Surveillance by self-identification of symptoms is appealing because patients are empowered to stop the INH without waiting to see a provider. However inadequate patient instruction or comprehension may be undermining effectiveness for a small but important subset of patients. Such delay in stopping INH has been reported in two smaller case series over the last 20 years.9,13 Clear stopping criteria were published in 2006,6 but our data suggest delays continue to cause severe hepatotoxicity. There is no lower age limit for latent TB treatment.14 Thus children deserve special consideration because they may not identify symptoms as readily as adults. One child in our study needed transplantation after continuing INH for 15 days beyond stopping criteria.
The 7 patients with concurrent chronic hepatitis C (CHC) tended to have less severe injury and significantly less delay in stopping. Adherence to guidelines which includes monitoring of liver biochemistries seems better in those with chronic liver disease. In fact, the median delay in discontinuance for the CHC patients was 0 days. DILI was detected by symptoms as often as by lab abnormalities, suggesting that these patients were more vigilant of their symptoms.
Treating latent TB has been a marked success.8,15 Deaths due to TB have fallen since 1988 to an all-time low of 0.2/100,000 in 2009.16 However, INH remains the most common cause of idiosyncratic DILI leading to liver transplantation nationwide.17 It is the second most common agent implicated in the DILIN despite under reporting. In 2004, the CDC began a national reporting project of SAE’s associated with latent TB therapy.9 From 2004 through 2008, only 17 cases of INH hepatotoxicity were reported. Twenty-five of our 60 case should have been recorded with the CDC, but only 1 was reported. The CDC report had clinical details on only 10 of 17 cases. Even if all 7 remaining CDC cases lacking clinical details were in the DILIN cohort, then, at most, 8 of 25 (32%) of our cases may have been reported. Moreover, the 8 DILIN sites represent only 9% of U.S. liver transplant centers. Non-transplant centers probably care for many more unreported INH hepatotoxicity patients that do not need or qualify for transplantation. Thus under-reporting of INH hepatotoxicity is assuredly substantial. DILI, in general, may be more common than previously estimated according to pharmacoepidemiologic results from Iceland where data on prescriptions and hepatotoxicity are robust.18 A meaningful incidence for INH was not available in this study because INH is uncommonly prescribed in Iceland.
Our study is subject to recall bias by patients reporting symptom start dates. However, this bias would tend to underestimate delay in stopping because of regrets or embarrassment about ignoring symptoms. Reporting bias toward more severe cases is another limitation. We do not capture mild injury cases where guidelines were followed. Therefore, the minority of significant hepatotoxicity cases is small compared to the large number who are safely treated. However, these cases of hepatotoxicity must not be summarily dismissed as an unavoidable cost to treating the overall latent TB population either. Many of the life threatening injures in our registry may have been easily prevented with better provider-to-patient education.
We found no correlation between delay in stopping and age, gender, race, ethnicity or education level. However, we did not collect data on health care providers, quality of patient instruction, patient primary language, health care literacy, or cultural barriers. These data are critical to assessing compliance especially in the latent TB population. The clinician must strike a difficult balance between convincing asymptomatic patients to take the INH, while also warning them of hepatotoxicity risk. A complete behavioral model addressing compliance is beyond the scope of our data, but the DILIN draws attention to this group of harmed patients who otherwise may continue to be overlooked.
For now, there are measures to improve compliance that should be considered. There are no data on directly observed therapy (DOT) and hepatotoxicity avoidance, but piggybacking a review of systems onto DOT might be cost effective. DOT of INH for 3–9 months is already a recommended option for latent TB.12 Unfortunately we did not record DOT information. Finger-stick technology for point-of-care ALT measurement could also be effective.19 Over half the U.S. population own smartphones, so automated texting to remind patients of stopping rules may be feasible.20,21 Such interventions could result in substantial cost savings by avoiding hospitalizations, intensive care, and transplants.
Our study also highlights the limitations symptoms and ALT monitoring. Of the 13 fatal or transplanted patients, 4 (31%) stopped INH quickly (within 7 days of meeting criteria). Conversely, two patients continued INH for more than 3 weeks yet had mild injury. One had remarkable adaptation with the ALT peaking over 1000 U/L only to fall despite continuing the INH. Such adaptation to INH injury has been described, but is quite rare with such severe ALT elevation.22–25 In 1976, Mitchell and colleagues concluded that ALT alone could not predict serious liver injury with complete accuracy.25 A better diagnostic test is overdue.
Current monitoring guidelines are successful for the vast majority of patients treated for latent TB and there is no arguing the public health success of treatment programs. However our registry draws attention to an important minority of latent TB patients who are harmed by suboptimal adherence and prolonged drug exposure. Underreporting is common, so the true incidence of such cases is significantly larger than currently captured. Failures to avoid such severe iatrogenic injury are particularly difficult to countenance when the latent TB patient was otherwise healthy prior to therapy and simply needed better education, reminders or monitoring.
Acknowledgments
Funding: The DILIN Network is structured as a U01 cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under grants: 2U01-DK065176-06 (Duke), 2U01-DK065201-06 (UNC), 2U01-DK065184-06 (Michigan), 2U01-DK065211-06 (Indiana), 5U01DK065193-04 (UConn), 5U01-DK065238-08 (UCSF/CPMC), 1U01-DK083023-01 (UTSW), 1U01-DK083027-01 (TJH/UPenn), 1U01-DK082992-01 (Mayo), 1U01-DK083020-01 (USC). Additional funding is provided by CTSA grants: UL1 RR025761 (Indiana), UL1 RR025747 (UNC), UL1 RR024134 (UPenn), UL1 RR024986 (Michigan), UL1 RR024982 (UTSW), UL1 RR024150 (Mayo) and in part by the Intramural Research Program of the NIH, National Cancer Institute.
Abbreviations
- ALT
alanine aminotransferase
- AP
Alkaline phosphatase
- AST
aspartate aminotransferase
- ATS
American Thoracic Society
- CDC
Center for Disease Control
- CHC
chronic hepatitis C
- DILI
drug-induced liver injury
- DILIN
Drug-induced Liver Injury Network
- DOT
directly observed therapy
- INH
isoniazid
- NIH
National Institutes of Health
- SAE
severe adverse event
- SIS
Severity Index Score
- S.D
standard deviation
- TB
tuberculosis
- ULN
upper limit of normal
- UNOS
United Network for Organ Sharing
Footnotes
Disclosures: PH Hayashi: No relevant disclosures or conflicts of interest; RJ Fontana: Consultant GSK, Tibotec, Vertex, Gilead; N Chalasani: Consultant Merck, Abbvie, Aegerion, Salix, BMS, Lilly; AS Stolz: No relevant disclosures or conflicts of interest; JA Talwalkar: No relevant disclosures or conflicts of interest; VJ Navarro: No relevant disclosures or conflicts of interest; TJ Davern: No relevant disclosures or conflicts of interest; DE Kleiner: No relevant disclosures or conflicts of interest; J Gu: No relevant disclosures or conflicts of interest; JH Hoofnagle: No relevant disclosures or conflicts of interest.
Writing Assistance: No writing assistance beyond the authors listed.
Author contributions:
Concept and design: PHH, JHH, RFJ, NPC
Acquisition of data: PHH, RJF, NPC, AAS, JAT, VJN, WML, TJD, DEK
Analysis and interpretation: PHH, JG, DEK, JHH, RJF
Drafting of the manuscript: PHH, JHH
Critical revision of the manuscript for important intellectual content: PHH, RJF, NPC, AAS, JAT, VJN, WML, TJD, DEK, JHH
Statistical analysis: JG, PHH
Obtaining funding: PHH, RJF, NPC, AAS, VJN, WML, TJD, JHH
Administrative, technical, material support: JG
Study supervision: JHH
References
- 1.Garibaldi RA, Drusin RE, Ferebee SH, Gregg MB. Isoniazid-associated hepatitis. Report of an outbreak. Am Rev Respir Dis. 1972 Sep;106(3):357–365. doi: 10.1164/arrd.1972.106.3.357. [DOI] [PubMed] [Google Scholar]
- 2.Runyon EH. Preventive Treatment in Tuberculosis: A Statement by the Committee on Therapy, American Thoracic Society. Am Rev Respir Dis. 1965 Feb;91:297–298. doi: 10.1164/arrd.1965.91.2.297. [DOI] [PubMed] [Google Scholar]
- 3.Randolph H, Joseph S. Toxic hepatitis with jaundice occuring in a patient treated with isoniazid. J Am Med Assoc. 1953 May 2;152(1):38–40. doi: 10.1001/jama.1953.63690010014007i. [DOI] [PubMed] [Google Scholar]
- 4.Gellis SN, Murphy RV. Hepatitis following isoniazid. Dis Chest. 1955 Oct;28(4):462–464. doi: 10.1378/chest.28.4.462. [DOI] [PubMed] [Google Scholar]
- 5. [accessed Jun 6, 2013];LiverTox: Isoniazid. http://livertox.nlm.nih.gov/Isoniazid.htm.
- 6.Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006 Oct 15;174(8):935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 7.Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a public health tuberculosis clinic. JAMA. 1999 Mar 17;281(11):1014–1018. doi: 10.1001/jama.281.11.1014. [DOI] [PubMed] [Google Scholar]
- 8.LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med. 2003 Aug 15;168(4):443–447. doi: 10.1164/rccm.200303-390OC. [DOI] [PubMed] [Google Scholar]
- 9.Severe isoniazid-associated liver injuries among persons being treated for latent tuberculosis infection - United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2010 Mar 5;59(8):224–229. [PubMed] [Google Scholar]
- 10.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32(1):55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterling TR, Bethel J, Goldberg S, Weinfurter P, Yun L, Horsburgh CR. The scope and impact of treatment of latent tuberculosis infection in the United States and Canada. Am J Respir Crit Care Med. 2006 Apr 15;173(8):927–931. doi: 10.1164/rccm.200510-1563OC. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control. [accessed July 27, 2013];Tuberculosis. http://www.cdc.gov/tb/statistics/default.htm.
- 13.Severe isoniazid-associated hepatitis--New York, 1991–1993. MMWR Morb Mortal Wkly Rep. 1993 Jul 23;42(28):545–547. [PubMed] [Google Scholar]
- 14.Gayle HD, Castro KG. Targeted Tuberculin Testing and Treatment of Latent Tuberculosis Infection. MMWR Morb Mortal Wkly Rep. 2000 Jun 9;49(RR-6):1–54. [PubMed] [Google Scholar]
- 15.Nolan CM. Isoniazid for latent tuberculosis infection: approaching 40 and reaching its prime. Am J Respir Crit Care Med. 2003 Aug 15;168(4):412–413. doi: 10.1164/rccm.2306004. [DOI] [PubMed] [Google Scholar]
- 16.American Lung Association. [accessed Nov 12, 2013];Trends in Tuberculosis Morbidity and Mortality. 2013 http://www.lung.org/finding-cures/our-research/trend-reports/TB-Trend-Report.pdf.
- 17.Mindikoglu AL, Magder LS, Regev A. Outcome of liver transplantation for drug-induced acute liver failure in the United States: analysis of the United Network for Organ Sharing database. Liver Transpl. 2009 Jul;15(7):719–729. doi: 10.1002/lt.21692. [DOI] [PubMed] [Google Scholar]
- 18.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of iceland. Gastroenterology. 2013 Jun;144(7):1419–1425. e1413. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Pollock NR, Rolland JP, Kumar S, et al. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci Transl Med. 2012 Sep 19;4(152):152ra129. doi: 10.1126/scitranslmed.3003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith A. Smartphone Ownership 2013. [accessed Oct. 15, 2014];PewRearch Internet Project. 2013 http://www.pewinternet.org/2013/06/05/smartphone-ownership-2013/
- 21.Nglazi MD, Bekker LG, Wood R, Hussey GD, Wiysonge CS. Mobile phone text messaging for promoting adherence to anti-tuberculosis treatment: a systematic review. BMC infectious diseases. 2013;13:566. doi: 10.1186/1471-2334-13-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danielides IC, Constantoulakis M, Daikos GK. Hepatitis on high dose isoniazid: reintroduction of the drug in severe tuberculous meningitis. Am J Gastroenterol. 1983 Jun;78(6):378–380. [PubMed] [Google Scholar]
- 23.Sharma SK, Singla R, Sarda P, et al. Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clin Infect Dis. 2010 Mar 15;50(6):833–839. doi: 10.1086/650576. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell JR, Long MW, Thorgeirsson UP, Jollow DJ. Acetylation rates and monthly liver function tests during one year of isoniazid preventive therapy. Chest. 1975 Aug;68(2):181–190. doi: 10.1378/chest.68.2.181. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell JR, Zimmerman HJ, Ishak KG, et al. Isoniazid liver injury: clinical spectrum, pathology, and probable pathogenesis. Ann Intern Med. 1976 Feb;84(2):181–192. doi: 10.7326/0003-4819-84-2-181. [DOI] [PubMed] [Google Scholar]