Abstract
Urinary uromodulin (uUMOD) is the most common secreted tubular protein in healthy adults. However, the relationship between uUMOD and clinical outcomes is still unclear. Here we measured uUMOD in 192 participants of the Cardiovascular Health Study with over a 30% decline in estimated glomerular filtration rate (eGFR) over 9 years, 54 with incident end stage renal disease (ESRD), and in a random sub-cohort of 958 participants. The association of uUMOD with eGFR decline was evaluated using logistic regression and with incident ESRD, cardiovascular disease, heart failure and mortality using Cox proportional regression. Mean age was 78 years and median uUMOD was 25.8 μg/mL. In a case-control study evaluating eGFR decline (192 cases and 231 controls), each standard deviation higher uUMOD was associated with a 23% lower odds of eGFR decline (odds ratio 0.77, (95% CI 0.62, 0.96)) and a 10% lower risk of mortality (hazard ratio 0.90, (95% CI 0.83, 0.98)) after adjusting for demographics, eGFR, albumin/creatinine ratio and other risk factors. There was no risk association of uUMOD with ESRD, cardiovascular disease or heart failure after multivariable adjustment. Thus, low uUMOD levels may identify persons at risk of progressive kidney disease and mortality above and beyond established markers of kidney disease, namely eGFR and the albumin/creatinine ratio. Future studies need to confirm these results and evaluate whether uUMOD is a marker of tubular health and/or whether it plays a causal role in preserving kidney function.
Keywords: uromodulin, tamm-horsfall protein, chronic kidney disease, cardiovascular disease, urinary biomarkers
INTRODUCTION
The prevalence of chronic kidney disease (CKD) in persons over the age of 65 years is nearly 44% 1 and older adults with even moderate reductions in kidney function are at increased risk for cardiovascular disease (CVD).2 Current assessment of kidney function and the definition of CKD are limited to measures of estimated glomerular filtration rate (eGFR) and urinary albumin-creatinine ratio (ACR). Tubular health is essential for maintenance of acid-base status, mineral metabolism and hormone production. In addition tubular secretion is the means by which medications are excreted through the kidneys. There are no biomarkers that have been validated as surrogates of tubular health.3,4
Urinary uromodulin (uUMOD), also known as Tamm-Horsfall protein, is a 95-kDa glycoprotein synthesized by the thick ascending limb of the loop of Henle and early distal convoluted tubule. It is the most abundant urinary protein among healthy adults (20–70 mg/day).5 Mutations in the UMOD gene cause congenital hyperuricemic and cystic kidney diseases, lead to kidney failure, and are associated with low uUMOD levels.6,7 Recently, large genome wide association studies (GWAS) have identified that the strongest association with CKD was with common variants in the region of UMOD gene on chromosome 16.8,9 Single nucleotide polymorphisms (SNP) in this region appear to be more strongly associated with maintenance of kidney function in older adults than younger persons.10 As a result there has been a renewed interest in the role of uUMOD in the development and progression of CKD.
To date studies evaluating uUMOD concentrations with CKD prevalence and incidence have not been consistent. Early studies suggested that low uUMOD levels may be associated with reduced kidney function.11,12 In contrast a more recent case-control study reported an association between higher uUMOD levels and incident CKD,13 while a third showed no statistically significant relationship.14 These studies, however, were all relatively small and did not adjust for kidney function measures like ACR.8 The aims of our study were to evaluate the correlates and cross sectional distribution of uUMOD, and to evaluate the associations of uUMOD with kidney function decline, incident ESRD, CVD and mortality independent of eGFR and ACR in community dwelling older adults.
RESULTS
Baseline Correlates of uUMOD levels
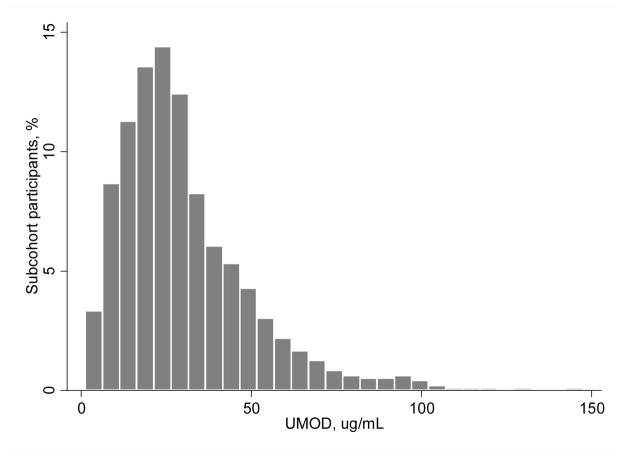
Among the 958 randomly selected participants at baseline (see Figure 1 for participant sampling), median (interquartile range [IQR]) value of uUMOD was 25.8 μg/mL (17.2–38.8) and the distribution was right skewed (Figure 2). The mean age of participants was 78 ± 5 years, 60% were women, and 15% were black. The mean ± SD eGFR was 63 ± 18 ml/min/1.73m2, and median (IQR) urine ACR was 8 (5–20) mg/g. Table 1 shows baseline characteristics by quartiles of uUMOD in the random sub-cohort. Compared to participants with low uUMOD, persons with higher levels were less likely to have diabetes, history of CAD, stroke, and HF, and had lower systolic BP and lower BMI. Persons with higher uUMOD also had higher eGFR, lower urinary ACR and lower CRP. At baseline uUMOD levels were weakly and inversely correlated with ACR (Spearman r=−0.12) and directly correlated with eGFR (Spearman r=0.20) values.
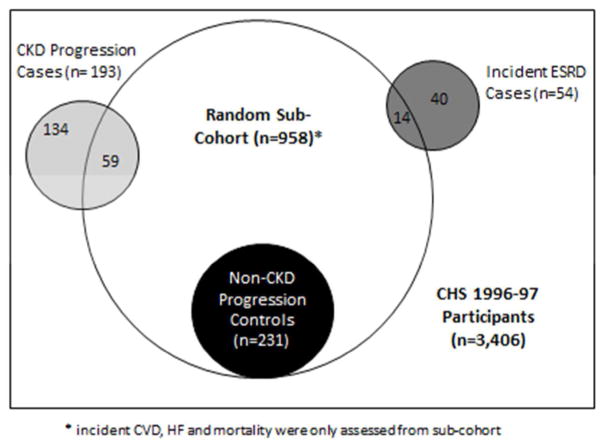
Figure 1.
Population Sampling from within the Cardiovascular Health Study
Rectangle: All CHS participants at 1996–1997 visit
Large circle: Random sub-cohort
Black circle: Participants included as controls in the case-control study for the CKD progression outcome.
Light grey area: Participants included as cases in the case-control study for the CKD progression
Dark Grey area: Participants included in the case-cohort study for the incident ESRD outcome.
Figure 2.
Distribution of urine uromodulin levels in 958 community-living elderly participants in the Cardiovascular Health Study
None.
Table 1.
Baseline participant characteristics by quartiles urine uromodulin
| Characteristic | Urine uromodulin (μg/mL) | |||
|---|---|---|---|---|
| Quartile Range | <=17.25 | >17.25–25.88 | >25.88–38.86 | >38.86 |
| N (958) | 240 | 239 | 240 | 239 |
| Demographics | ||||
| Age, years | 78.6 ± 5.2 | 78.1 ± 4.7 | 78.4 ± 4.8 | 77.3 ± 4.2 |
| Male | 39.6 | 35.1 | 42.5 | 40.6 |
| Blacks | 15.0 | 18.8 | 12.1 | 15.1 |
| Site | ||||
| Wake Forest | 22.5 | 22.6 | 25.8 | 22.2 |
| UC Davis | 28.3 | 31.8 | 27.9 | 27.2 |
| Johns Hopkins | 20.0 | 20.9 | 22.1 | 23.4 |
| Univ. of Pittsburgh | 29.2 | 24.7 | 24.2 | 27.2 |
| Lifestyle Factors | ||||
| Smoking status | ||||
| Never | 45.8 | 48.5 | 48.8 | 45.6 |
| Former | 45.8 | 45.6 | 42.1 | 47.7 |
| Current | 8.3 | 5.9 | 9.2 | 6.7 |
| Pack years (current & former) | 33.1 ± 32.6 | 27.6 ± 24.8 | 27.3 ± 25.6 | 28.8 ± 24.4 |
| Alcohol consumption | ||||
| None | 63.4 | 59.8 | 56.3 | 51.7 |
| <7 drinks/week | 25.2 | 28.9 | 30.0 | 34.7 |
| ≥7 drinks/week | 11.3 | 11.3 | 13.8 | 13.6% |
| Cardiovascular Risk Factors | ||||
| Diabetes | 22.5 | 10.9 | 10.9 | 11.3 |
| History of MI | 16.3 | 10.9 | 10.4 | 12.1 |
| History of Stroke | 8.8 | 7.9 | 7.1 | 3.3 |
| History of HF | 13.3 | 7.5 | 9.6 | 5.4 |
| CKD (eGFR<60ml/min) | 54.4 | 39.7 | 41.7 | 29.3 |
| Systolic BP (mm Hg) | 141.3 ± 21.6 | 136.1 ± 20.5 | 134.3 ± 21.0 | 135.7 ± 19.7 |
| BMI (kg/m2) | 27.0 ± 5.2 | 27.0 ± 4.6 | 26.7 ± 4.7 | 26.8 ± 4.3 |
| Laboratory measures | ||||
| Fasting glucose (mg/dL) | 115.7 ± 50.4 | 100.9 ± 28.9 | 99.1 ± 24.2 | 99.5 ± 24.5 |
| Total Cholesterol (mg/dL) | 200.6 ± 42.2 | 201.8 ± 37.3 | 200.3 ± 40.1 | 203.1 ± 35.7 |
| CRP (mg/L)* | 5.4 ± 8.7 | 4.5 ± 7.5 | 4.5 ± 9.6 | 4.3 ± 7.0 |
| eGFR (ml/min/1.73m2) | 57.2 ± 20.1 | 64.5 ± 17.8 | 63.5 ± 17.0 | 68.8 ± 16.7 |
| Urine ACR (mg/g)* | 11.1 (5.4,34.4) | 8.6 (4.7,20.0) | 7.4 (4.6,13.8) | 7.5 (4.7,18.1) |
| Medication use | ||||
| Antihypertensive | 65.8 | 56.1 | 55.0 | 44.8 |
| Lipid lowering | 10.4 | 12.1 | 12.9 | 12.6 |
All values represented as % or mean ±SD except those marked with * which are median (inter quartile range). Abbreviations: MI-myocardial infarction, HF-heart failure, CKD- chronic kidney disease, Systolic BP-systolic blood pressure, BMI- body mass index, CRP- C reactive protein, eGFR-estimated glomerular filtration rate, ACR- albumin-creatinine ratio
uUMOD and progressive decline in eGFR
Supplemental Table 1 shows a comparison of baseline characteristics between persons who had ≥30% decline in eGFR and those who did not. There were 192 participants with progressive decline of eGFR during the follow up period. Table 2 shows the association of uUMOD with 30% decline in eGFR. Each SD (19.7 μg/mL) higher uUMOD was associated with a 26% lower risk of progressive eGFR decline in demographic adjusted models and this association was only modestly attenuated after adjusting for baseline eGFR, ACR, CVD and CKD risk factors (OR= 0.77, 95% CI 0.62, 0.96). Participants in the highest quartile of uUMOD (>38.8 μg/mL) had 40% lower risk of progressive GFR decline compared to those in the lowest quartile but this was not statistically significant in fully adjusted analyses (OR= 0.59, 95% CI 0.32, 1.09).
Table 2.
Association of uUMOD with ≥30% decline in eGFR
| uUMOD μg/mL | # of events | Demographic adjusted* OR (95% CI) | Plus eGFR and urine ACR** OR (95% CI) | Plus CVD risk factors† OR (95% CI) |
|---|---|---|---|---|
| Per SD increase (19.7 μg/mL) | 192 | 0.74 (0.61, 0.91) | 0.73 (0.59, 0.91) | 0.77 (0.62, 0.96) |
| <=17.25 | 44 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| >17.25–25.88 | 49 | 0.84 (0.46, 1.51) | 0.89 (0.49, 1.61) | 1.07 (0.57, 2.01) |
| >25.88–38.86 | 52 | 0.77 (0.43, 1.36) | 0.82 (0.46, 1.47) | 1.13 (0.60, 2.12) |
| >38.86 | 47 | 0.51 (0.29, 0.91) | 0.51 (0.29, 0.92) | 0.59 (0.32, 1.09) |
Adjusted for age, gender, race, education, and clinic site.
Adjusted for demographic variable plus baseline eGFR and urine ACR.
Adjusted for demographic variables, eGFR, urine ACR, smoking status, pack-years, BMI, diabetes, systolic blood pressure, antihypertensive medication use, lipid lowering medications use, total cholesterol, and CRP.
We identified 54 incident ESRD cases during 9.5 years of follow up (Table 3). Due to the relatively few ESRD cases in quartiles 2–4, these were collapsed and compared to quartile 1. In demographic adjusted models participants with uUMOD levels in quartiles 2–4 had 80% lower risk of incidence ESRD. After adjusting for eGFR, ACR and CVD risk factors, persons in quartiles 2–4 had 16% lower risk of ESRD but this association failed to reach statistical significance.
Table 3.
Association of uUMOD with incident ESRD
| uUMOD μg/mL | # of events | Incidence Per 1000 person-years | Demographic adjusted* HR (95% CI) | Plus eGFR and urine ACR** HR (95% CI) | Plus CVD risk factors† HR (95% CI) |
|---|---|---|---|---|---|
| <=17.25 | 32 | 16.9 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| >17.25 | 22 | 3.3 | 0.19 (0.11, 0.32) | 0.52 (0.26, 1.04) | 0.84 (0.31, 2.26) |
Adjusted for age, gender, race, education, and clinic site.
Adjusted for demographic variable plus baseline eGFR and urine ACR.
Adjusted for demographic variables, eGFR, urine ACR, smoking status, pack-years, BMI, diabetes, systolic blood pressure, antihypertensive medication use, lipid lowering medications use, total cholesterol, and CRP.
uUMOD and cardiovascular events and mortality
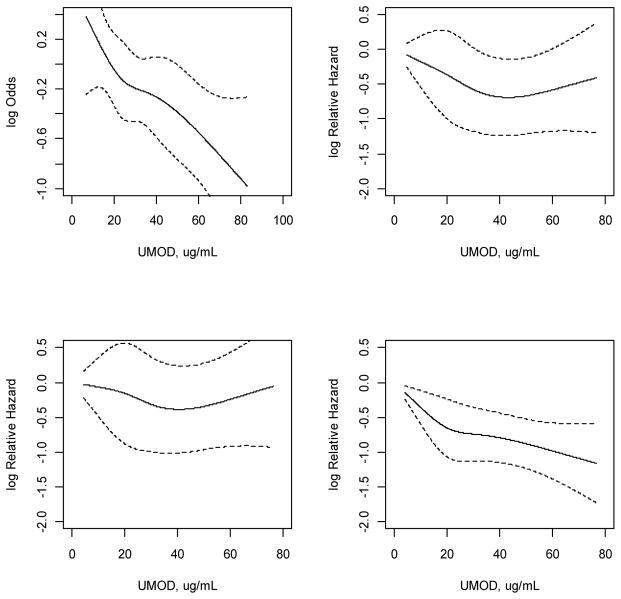
There were 289 incident CVD events during the follow up period. The association of uUMOD and incident CVD appeared curvilinear (Figure 3). The fourth quartile of uUMOD was associated with a 21% decreased risk of incident CVD events. However, this association was not statistically significant in multivariable analysis (Table 4). There were 260 incident HF events and no association was noted between uUMOD and incident HF. There were 694 deaths in the sub-cohort during follow up (Table 4). Each SD increment in uUMOD was associated with a 10% decrease in mortality in adjusted analyses. Compared to the first quartile, the fourth quartile of uUMOD had a 30% lower risk of death (HR 0.69, 95% CI 0.55, 0.87) in multivariable analysis.
Figure 3.
Spline regression plots of urinary uromodulin and clinical outcomes.
Left to right and top to bottom: Progressive GFR decline, incident CVD, HF and mortality. Each model was fitted using a restricted cubic spline function for uUMOD. In each plot, the solid line represents the point estimate and the dotted lines represent 95% confidence intervals. Observations in the highest 2.5% of the distribution were excluded to minimize the influence of extreme values.
Table 4.
Association of uUMOD with cardiovascular outcomes and mortality
| uUMOD μg/mL | # of events | Incidence per 1000 person-years | Demographic adjusted* HR (95% CI) | Plus eGFR and urine ACR** HR (95% CI) | Plus CVD risk factors† HR (95% CI) |
|---|---|---|---|---|---|
| Incident CVD | |||||
| <=17.25 | 75 | 51.4 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| >17.25–25.88 | 71 | 39.7 | 0.79 (0.57, 1.09) | 0.83 (0.59, 1.16) | 0.90 (0.64, 1.27) |
| >25.88–38.86 | 72 | 40.2 | 0.76 (0.55, 1.05) | 0.80 (0.57, 1.11) | 0.84 (0.60, 1.18) |
| >38.86 | 71 | 34.8 | 0.68 (0.49, 0.95) | 0.72 (0.51, 1.00) | 0.79 (0.56, 1.12) |
| Incident Heart Failure | |||||
| <=17.25 | 68 | 40.9 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| >17.25–25.88 | 60 | 30.2 | 0.76 (0.54, 1.08) | 0.84 (0.59, 1.19) | 0.95 (0.66, 1.36) |
| >25.88–38.86 | 56 | 29.0 | 0.69 (0.49, 0.99) | 0.77 (0.54, 1.11) | 0.87 (0.60, 1.26) |
| >38.86 | 76 | 33.8 | 0.89 (0.64, 1.25) | 1.00 (0.71, 1.41) | 1.13 (0.80, 1.60) |
| Mortality | |||||
| Per SD increase (19.7 μg/mL) | 694 | 0.84 (0.77, 0.91) | 0.88 (0.81, 0.96) | 0.90 (0.83, 0.98) | |
| <=17.25 | 195 | 97.5 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| >17.25–25.88 | 171 | 72.7 | 0.74 (0.60, 0.91) | 0.81 (0.66, 1.01) | 0.89 (0.72, 1.10) |
| >25.88–38.86 | 178 | 79.2 | 0.74 (0.60, 0.91) | 0.81 (0.66, 1.00) | 0.84 (0.68, 1.04) |
| >38.86 | 150 | 56.8 | 0.56 (0.45, 0.70) | 0.64 (0.51, 0.80) | 0.69 (0.55, 0.87) |
Adjusted for age, gender, race, education, and clinic site.
Adjusted for demographic variable plus baseline eGFR and urine ACR.
Adjusted for demographic variables, eGFR, urine ACR, smoking status, pack-years, BMI, diabetes, systolic blood pressure, antihypertensive medication use, lipid lowering medications use, total cholesterol, and CRP.
Each 1-SD increment in uUMOD was associated with 26% lower odds of the composite renal outcome of 30% eGFR decline or ESRD (OR=0.74; 95% CI: 0.59–0.93), with those in the highest quartile having nearly 50% lower odds of the renal outcome compared to those in the lowest quartile (OR 0.53, 95% CI 0.29, 0.98; Supplementary Table 2). When a composite non-renal outcome of incident CVD and mortality was considered, there was no significant association with per-SD increment in uUMOD (HR 0.94, 95% CI 0.86, 1.03), although the highest quartile of uUMOD was significantly associated with a 25% lower risk compared to the lowest quartile (HR=0.75; 95%CI: 0.59–0.95; Supplementary Table 3). There was no evidence of interaction between uUMOD and CKD status or uUMOD and diabetes for any of the clinical outcomes tested (p for interaction >0.20 for all analyses). Sensitivity analysis adjusting for urine creatinine to account for urine tonicity did not alter our results (data not shown).
DISCUSSION
Traditionally, clinical assessment of kidney function has been restricted to the glomerular axis using serum creatinine, eGFR and more recently ACR.15 Although, abnormalities in tubular function presenting as acidosis, vascular calcification, and anemia are risk factors for CVD,3,4 there are no validated urinary measures of tubular health and relatively little is known about the relationship between urinary measures of tubular health and clinical outcomes. In this large community dwelling population of older adults, we demonstrate that higher levels of uUMOD are associated with lower risk of progression of kidney disease and lower risk of mortality in multivariable analyses that included adjustment for eGFR and ACR. Although the association between higher uUMOD and lower risk of ESRD failed to reach significance after adjusting for other covariates, its directionality was consistent with the association of higher uUMOD with eGFR decline and the composite renal outcome. To our knowledge these findings are novel. We propose that uUMOD may be a marker of tubular health and may provide prognostic information independent of eGFR and ACR.
Uromodulin, the most common urinary protein in healthy adults, forms a gel on the surface of the thick ascending limb of the loop of Henle preventing water permeability in this segment.16 It is also thought to bind pathogenic bacteria, prevent stone formation by impairing aggregation of calcium oxalate crystals and assist in excretion of uric acid.16,17,18,19 A number of congenital and hereditary kidney diseases including medullary cystic kidney disease type 2, familial juvenile hyperuricemic nephropathy and glomerulocystic kidney disease involve mutations of the UMOD gene.7 These uromodulin associated kidney diseases (UAKD) present with hyperuricemia, gout and progressive kidney failure.20 Levels of uUMOD are significantly decreased in patients with UAKD due to aberrant protein folding and trafficking through the endoplasmic reticulum.21 In the general population, initial small studies have suggested that uUMOD excretion increases from birth till adulthood and remains stable until age 60 after which there is a decline.22,23 Our study represents the first report to describe the distribution of uUMOD in a large population of community dwelling older adults in addition to evaluating its longitudinal association with progressive eGFR decline, incident ESRD, CVD events, HF and mortality.
The use of GWAS has led to a renewed interest in the role of uUMOD in maintenance of kidney health. The presence of two single SNPs in the promoter region of the UMOD gene (C allele of rs4293393 and T allele of rs12917707) have been associated with lower levels of uUMOD and a reduced risk of progressive kidney disease.9,13 However, these findings are counter to cross sectional studies which demonstrated lower excretion of uUMOD in the setting of decreased GFR and tubular injury.11,12,24 In recent years, there has been significant variation in results of studies evaluating the associations between uUMOD and progressive kidney function decline in specific patient populations including renal transplant recipients,25,26 persons with diabetes27 or IgA nephropathy28 and case-control studies of patients at risk for CKD 8,14,29 Some studies reported that persons with higher levels of uUMOD have an increased risk of progressive kidney disease,8,25,27 others have noted that higher levels are associated with lower risk of kidney disease,28,29 one study reported a bimodal association, 26 while one reported no association of uUMOD with progressive kidney disease.14 Differences in results could be due to a number of reasons. First, there is significant heterogeneity in the populations studied including presence or absence of CKD and if present, the cause of CKD. For example, it is possible that higher levels of uUMOD may be associated with decreased likelihood of progression in those without CKD (or in healthy adults as noted in the current study) but once disease is established the relationships change. Second, different assays were used to measure UMOD across the studies, 8,14 and although we used a commercial uUMOD assay which has been utilized previously in studies of kidney transplant recipients and type 1 diabetics,26,27 the exact reasons for differences in results remain unclear.
The underlying mechanism linking uUMOD and progressive kidney function decline remains unclear. In vitro and in vivo studies suggest that uUMOD may be involved in modulation of immune response to tubular injury.30–32 There are also data to support both a pro-inflamatory29 and anti-inflammatory effect of uUMOD in the tubular interstitium,33 and several authors have hypothesized that the association of uUMOD with outcomes may depend on the timing of the injury, the stage of CKD as well as the population being studied and its associated comorbid conditions.5,16,34 Data indicates that some amount of uromodulin is released from the basolateral surface of the tubular cells through basolateral exocytosis and by back leakage into the interstitium.5 While the function of this interstitial and serum uromodulin is unclear, a recent study of 289 healthy individuals over the age of 60 years, demonstrated that serum uromodulin levels were lower at lower values of eGFR.35 The authors concluded that as uromodulin is secreted exclusively by tubular cells, lower serum uromodulin serum may reflect a reduction in number or function of these cells in CKD. The directionality of the association is consistent with our results of uUMOD and eGFR in cross-sectional and longitudinal analysis.
In our study we noted that higher uUMOD levels were associated with lower risk of progressive kidney disease and mortality. There are several potential reasons why higher levels may be associated with lower risk. First, higher levels may reflect a higher number of functioning tubules and/or reserve of the tubules, which may in turn limit progression of kidney disease. If so, then uUMOD may mark tubular health and provide prognostic information above and beyond glomerular markers of kidney health (eGFR and ACR). Second, higher levels may reflect better tubular function such as erythropoietin production as well as maintenance of acid base and mineral metabolism homeostasis. In turn, preservation of these tubular functions may decrease mortality. Thirdly, it is possible that higher levels are uUMOD are protective (i.e. a directly causal relationship) through anti-inflammatory properties, binding of bacteria, and preventing crystal formation. The finding that higher levels of uUMOD were correlated with lower ACR and less CVD and kidney disease risk factors at baseline, suggests that not all proteinuria is necessarily harmful. Further studies are needed to determine if these elevated levels of uUMOD truly indicate better ‘tubular health’.
Our study has a number of important limitations which should be considered while interpreting the results. First, the definition of progressive CKD required participants to survive and return for repeat blood testing. In this older cohort many individuals died between visits and others may have been too ill or debilitated to return, which may have introduced informative censoring. This would likely have biased our kidney progression data to the null. Second, while the National Kidney Foundation has recommended that a 30% decline in eGFR is a valid surrogate for kidney failure,36 as with the definition of CKD, the 30% decline outcome would ideally be confirmed with a subsequent eGFR assessment 3 months after the initial blood test. Third, we measured uUMOD in urine specimens that were collected and stored for approximately 16 years before measurement. One study suggests that storage for over 8 months, even at −80C may slightly decrease the values of uUMOD, although numerous freeze-thaw cycles don’t affect the stability of uUMOD stored at this temperature.37 However, decreases in uUMOD levels due to freezing should be non-differential with respect to clinical outcomes. Fourth, participants in CHS who had CKD had only low levels of proteinuria with eGFR Stage 3a. As a result, our results cannot be generalized to those with more severely reduced kidney function or proteinuric nephropathies. However, we noted no interaction of uUMOD with CKD with regard to eGFR decline or mortality in our analysis. Fifth, our study was limited by the fact that we did not include the full cohort, but used a sample instead. Therefore, there is the potential for sampling biases inherent to each of the different study designs. However, to the extent that the random sub-cohort is representative of the full cohort, the various study designs should provide relative risk estimates consistent with that using the full cohort. Nonetheless, these findings will need to be validated in other cohorts. Finally, the observational nature of our study cannot distinguish if uUMOD has a direct causal role in maintaining kidney function or whether it is a marker of overall health.
Despite these limitations our study also has a number of strengths. First, this is the first population level study to evaluate uUMOD levels in a large community living population of older adults. Second, the CHS cohort has a long duration of follow up with accurate ascertainment of risk factors as well as adjudication of CVD and mortality events done by a panel of experts. Finally, we propose that our results reflect a new understanding of how tubular health is associated with adverse outcomes in the elderly. This is important as traditional assessment of the glomerular axis alone may not adequately capture overall kidney health.
In conclusion, higher levels of uUMOD, the most commonly found urinary protein in health adults are associated with lower odds of progressive kidney disease and lower risk of mortality in community-living older adults. This association is independent of eGFR, ACR and traditional CVD risk factors. If confirmed, uUMOD levels may provide a simple, inexpensive and non-invasive method of assessing tubular health and identifying persons at higher risk for progressive kidney disease and mortality.
METHODS
Participants
The Cardiovascular Health Study (CHS) is an observational study of risk factors for CVD among 5888 men and women 65 years or older living in 4 communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pa). Initially 5,201 participants were enrolled in 1989–1990 using Medicare eligibility lists. In 1992–1993, an additional 687 African Americans were recruited. For both enrollment periods, random samples of Medicare eligibility lists were used to recruit participants. All gave informed consent for participation, and study methods were approved by local institutional review boards. A detailed description of the recruitment and examination methods has been published previously.38 The baseline examination included medical history, physical examination, laboratory testing, and assessment for the presence of CVD. Participants were seen for yearly study visits until 1998–1999 and interviewed by telephone between study visits. After 1998–1999 participants were contacted by telephone every 6 months. Using twice yearly participant-reports and Medicare hospitalization records, discharge summaries were requested for all hospitalizations and full medical records were reviewed for all adjudicated outcomes.
Study Design
Figure 1 depicts the sampling design for this study. Among 3,406 individuals who participated and provided blood at the 1996–1997 visit, we excluded individuals with missing serum creatinine (n=1) or ACR (n=92), leaving us with 3,313 participants. Since measuring uUMOD in all 3,313 participants would be prohibitively expensive, we sought to design the most cost-efficient study that would maximize power and allow us to evaluate the association of uUMOD with several different endpoints of interest: ESRD, CVD, HF and mortality (all time-to-event data), and progression of CKD. We therefore planned a case-control study for CKD progression (a binary endpoint requiring participation at the 2005–2006 follow-up CHS examination at which eGFR was measured), a case-cohort design for ESRD, and for CVD, HF and mortality, we took advantage of the large numbers of these events in CHS to use standard prospective cohort time-to-event analysis.
From the 3,313 participants we randomly selected a sub-cohort of 960 individuals, two of whom lacked sufficient urine to measure uUMOD resulting in a sub-cohort of 958 individuals. The random selection of these individuals (i.e. not based on the presence or absence of CKD progression, ESRD, CVD, HF, or death) allowed us to conduct the different analysis listed below.
We defined CKD progression as ≥ 30% decline in eGFR from the 1996–97 visit to the next CHS follow-up visit in 2005–06 where blood was obtained for eGFR measurement.39 Among the 3,406 individuals who participated and provided blood at the 1996–97 visit, 1,001 were alive and provided blood specimens again at the 2005–2006 visit. Among these, 193 had ≥ 30% decline in eGFR and we excluded 1 participant who was missing uUMOD measure. These individuals were identified as cases, and were all selected for urine uUMOD measurement. Of the 958 randomly selected sub-cohort, 289 were alive and provided blood for repeat eGFR measurement at the 2005–06 visit. Among these, 59 had ≥ 30% decline in eGFR and were therefore already in the case sample. The remaining 231 individuals with lesser change in eGFR served as controls for the CKD progression analyses.
We evaluated the association of uUMOD with incident ESRD using a case-cohort design. This design uses a sub-sampling technique in survival data for estimating the relative risk of disease in a cohort study without collecting data from the entire cohort. From the 3,406 participants at the 1996–1997 visit, we identified 54 subsequent ESRD events. Among them, 14 originated within the random sub-cohort of 958 participants and the remaining 40 were outside of the sub-cohort. Urine from the 1996–97 visit was measured for uUMOD in all 54 ESRD cases.
Last, we analyzed the association of uUMOD with risk of incident CVD, HF, and all-cause mortality. Each of these outcomes was common in this elderly cohort. Therefore, we conducted these analyses among the randomly selected sub-cohort using a standard prospective cohort design. For the evaluation of incident CVD events, we excluded 167 persons with prevalent CVD at the 1996–97 visit, resulting in a sample size of 791 individuals for this outcome. Likewise, for the incident HF outcome, we excluded 86 individuals with prevalent HF at the 1996–97 visit, resulting in a sample size of 872 individuals for this outcome.
In aggregate, the random sub-cohort, the CKD progression cases, and the incident ESRD cases provided a study sample of 1,122 individuals who had uUMOD measured.
Exposure Variable
Spot urine specimens were obtained at the time of the 1996–97 study visit and stored at −70° Celsius until thaw and uUMOD was measured at the University of Cincinnati Children’s Hospital Medical Center in 2014. We measured uUMOD by a commercially available ELISA kit (catalog Number M036020, MD Bioproducts, St. Paul, MN) according to the manufacturer’s instructions. The principle of the assay is based on a colorimetric sandwich immunoassay utilizing a polyclonal antibody against human UMOD as the capture antibody and a biotinylated polyclonal antibody against human UMOD as the detection antibody. For this assay, the inter-assay coefficient of variation is 10.5% at a mean concentration of 21.8ng/mL and 12.2% at a mean concentration 95ng/mL. The intra-assay coefficient of variation is 9.2% at a mean concentration of 22.9ng/mL and 7.0% at 103.4 ng/mL. The minimum detectable level was 0.75 ng/mL.
Outcomes
A ≥ 30% decline in eGFR from baseline defined CKD progression cases.39 The eGFR was estimated using an equation that included serum cystatin C concentration, age, sex, and race derived in the CKD-EPI study.40 Cystatin C measurements were made by a Siemens nephelometric assay at both study visits, as previously described.41 We chose cystatin C based equations for assessing eGFR rather than creatinine based equations because the creatinine measurements at the 2005–2006 examination were IDMS standardized while creatinine measurements at the 1996–1997 were not. Therefore, laboratory drift between the two visits may have biased the case selection if defined by a ≥30% decline by eGFR creatinine.
For the ESRD outcome, we merged CHS data with Centers for Medicare and Medicaid Services (CMS) claims data. We used the ESRD eligibility flag for fee for service Medicare, which begins on the first day of the fourth month after dialysis initiation. A prior linkage of CHS data with United States Renal Data System (USRDS) had been conducted through 2003,42 and when compared to the USRDS data, we found that CMS linkage had 70.1% sensitivity (95% CI 59.4, 79.5%) and 99.9% specificity (95% CI 99.8, 99.9%) for ESRD case definition.
Incident CVD was defined as a composite of CVD mortality, myocardial infarction (MI), and stroke.43 We examined HF separately because of previous literature suggesting that measures of kidney function are more strongly associated with HF than MI, and given possible differences in the pathophysiology of each of these outcomes in persons with kidney disease.41,44 Methods of ascertainment for adjudication of CVD, HF, and mortality events have been described previously.43,45–47 All CVD and HF events were adjudicated by the CHS Events Committee. MI was ascertained from hospital records and was indicated by a clinical history of cardiac symptoms, elevated cardiac enzyme concentrations, and serial electrocardiographic changes.45 Cases of possible stroke were adjudicated by a committee of neurologists, neuroradiologists, and internists on the basis of interviews with patients, medical records, and brain imaging studies.46 Incident HF required a physician’s diagnosis of HF, and adjudication by the Events Committee required symptoms, signs, chest radiographic findings, and treatment of heart failure.43,45 Deaths were identified by review of obituaries, medical records, death certificates, the CMS health care-utilization database for hospitalizations and from household contacts; 100% complete follow-up for ascertainment of mortality status was achieved. CVD death was defined as death caused by atherosclerotic coronary heart disease, or cerebrovascular disease.47
Finally, we separately evaluated the associations of uUMOD with a composite renal outcome of ESRD or 30% decline in eGFR using logistic regression and a composite non-renal outcome of incident CVD and mortality using proportional hazards regression.
Covariates
Covariates for the models were selected based on prior knowledge about the factors that could be potential confounders of the associations of UMOD with the endpoints. A series of sequential models were fit to evaluate the effect of adding certain sets of covariates. Traditional CVD and kidney disease risk factors including age, gender, race/ethnicity, body mass index (BMI), physical activity level, smoking, diabetes (defined by use of hypoglycemic agents, fasting plasma glucose >126 mg/dL or non-fasting glucose ≥ 200 mg/dL), systolic blood pressure, total cholesterol level, lipid lowering medications, blood pressure medications and C-reactive protein (CRP) were considered as confounding variables. Analyses were also adjusted for baseline eGFR and ACR.
Statistical analysis
We described the distribution of uUMOD using summary statistics and frequency histograms and compared baseline participant characteristics (demographics, kidney function, CVD risk factors) by uUMOD quartiles among members of the subcohort. We evaluated the correlations of uUMOD, eGFR, and albuminuria using Spearman correlation coefficients.
Prior to fitting models, we evaluated each uUMOD-outcome association for the presence of non-linearity using generalized additive models and restricted cubic splines. If the functional form of the association was approximately linear, we calculated risk estimates for a 1-SD increment in uUMOD. When the relationships appeared non-linear; we did not present a linear estimate for those outcomes and instead included spline plots depicting the non-linear association of uUMOD with each of the clinical outcomes. Risk estimates were also calculated by categories of uUMOD (defined by quartiles of the distribution among the random subcohort) for all outcomes.
For each outcome, we fit a series of sequential models. Initial models were adjusted for age, sex, race, and clinic site. A subsequent model additionally adjusted for baseline eGFR and urine ACR. A final model included CVD and CKD risk factors (smoking status [current, former, never], pack-years, body mass index, diabetes, systolic blood pressure, blood pressure medication use, total cholesterol, lipid lowering medication and CRP).
We evaluated the association of uUMOD with CKD progression using logistic regression. To evaluate the association of uUMOD with incident ESRD we used modified Cox regression to account for the case-cohort approach.48,49 Sub-cohort participants were weighted with the inverse of the sampling fraction. Cases that arose outside the sub-cohort were not weighted before their failure. All cases (irrespective of whether they arose in the sub-cohort or not) were assigned a weight of 1 at the time of failure. Robust variance estimators were computed. We evaluated associations of uUMOD with incident CVD events, incident HF and mortality using a standard prospective cohort study design, among members of the random subcohort. Models were fitted using Cox proportional hazards regression.
For each of the clinical outcomes, we evaluated whether the uUMOD-outcome association was modified by either CKD status (defined as eGFR<60ml/min/1.73m2)12 or by diabetes.50,51 The effect of urine tonicity on uUMOD levels was evaluated in sensitivity analyses by adjusting for urine creatinine. Analyses were conducted using Stata, version 12.1 (STATA Corp., College Station, Texas) with the exception of the spline plots, which were fitted in R version 3.0.2. (www.r-project.org). Estimates with p-values < 0.05 were considered statistically significant for all analyses.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Diabetes Digestive and Kidney Diseases (NIDDK) R01 DK098234 (to Drs. Ix and Shlipak) and from the National Heart, Lung, and Blood Institute (NHLBI) and R01AG 027002 (Drs. Sarnak and Shlipak). The Cardiovascular Health Study was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC-85079 through N01HC-85086, N01HC-35129, N01HC-15103, N01HC-55222, N01HC-75150, N01HC-54133, and N01-HC85239 and grant U01 HL080295 from the NHLBI, with additional contributions from the National Institute of Neurological Disorders and Stroke. Dr. Devarajan was supported by National Institutes of Health grant P50 DK096418. Dr. Garimella is supported by National Institutes of Health training grant 5 T32 DK007777-13. Drs. Biggs, Garimella and Sarnak had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis. A full list of the principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Footnotes
DISCLOSURES
None to disclose
AUTHORSHIP:
Pranav S. Garimella: Conceived study, designed study, interpreted results, drafted manuscript
Mary L. Biggs: Performed statistical analysis, interpreted results, revised manuscript
Ronit Katz: Designed study, interpreted results, approved final manuscript
Joachim H. Ix: Acquired data, revised manuscript, approved final manuscript
Michael R. Bennett: Acquired data, approved final manuscript
Prasad Devarajan: Acquired data, approved final manuscript
Bryan R. Kestenbaum: Revised manuscript, approved final manuscript
David S. Siscovick: Approved final manuscript
Majken K. Jensen: Approved final manuscript
Michael G. Shlipak: Designed study, acquired data, revised manuscript, approved final manuscript
Paulo H. M. Chaves: Revised manuscript, approved final manuscript
Mark J. Sarnak: Conceived study, designed study, acquired data, revised manuscript, approved final manuscript
References
- 1.Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;55(3 Suppl 2):S23–33. doi: 10.1053/j.ajkd.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shastri S, Katz R, Rifkin DE, et al. Kidney function and mortality in octogenarians: Cardiovascular Health Study All Stars. Journal of the American Geriatrics Society. 2012;60(7):1201–7. doi: 10.1111/j.1532-5415.2012.04046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briet M, Bozec E, Laurent S, et al. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69(2):350–7. doi: 10.1038/sj.ki.5000047. [DOI] [PubMed] [Google Scholar]
- 4.Vlagopoulos PT, Tighiouart H, Weiner DE, et al. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2005;16(11):3403–10. doi: 10.1681/ASN.2005030226. [DOI] [PubMed] [Google Scholar]
- 5.Lhotta K. Uromodulin and chronic kidney disease. Kidney Blood Press Res. 2010;33(5):393–8. doi: 10.1159/000320681. [DOI] [PubMed] [Google Scholar]
- 6.Dahan K, Devuyst O, Smaers M, et al. A cluster of mutations in the UMOD gene causes familial juvenile hyperuricemic nephropathy with abnormal expression of uromodulin. Journal of the American Society of Nephrology : JASN. 2003;14(11):2883–93. doi: 10.1097/01.asn.0000092147.83480.b5. [DOI] [PubMed] [Google Scholar]
- 7.Hart TC, Gorry MC, Hart PS, et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39(12):882–92. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kottgen A, Hwang SJ, Larson MG, et al. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. Journal of the American Society of Nephrology : JASN. 2009;21(2):337–44. doi: 10.1681/ASN.2009070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kottgen A, Glazer NL, Dehghan A, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009;41(6):712–7. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gudbjartsson DF, Holm H, Indridason OS, et al. Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet. 2010;6(7):e1001039. doi: 10.1371/journal.pgen.1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty J, Below AA, Solaiman D. Tamm-Horsfall protein in patients with kidney damage and diabetes. Urol Res. 2004;32(2):79–83. doi: 10.1007/s00240-003-0374-6. [DOI] [PubMed] [Google Scholar]
- 12.Lynn KL, Marshall RD. Excretion of Tamm-Horsfall glycoprotein in renal disease. Clinical nephrology. 1984;22(5):253–7. [PubMed] [Google Scholar]
- 13.Kottgen A, Pattaro C, Boger CA, et al. New loci associated with kidney function and chronic kidney disease. Nat Genet. 2010;42(5):376–84. doi: 10.1038/ng.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shlipak MG, Li Y, Fox C, et al. Uromodulin concentrations are not associated with incident CKD among persons with coronary artery disease. BMC Nephrol. 2011;12:2. doi: 10.1186/1471-2369-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int, Suppl. 2013;3(2):1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 16.Vyletal P, Bleyer AJ, Kmoch S. Uromodulin biology and pathophysiology--an update. Kidney Blood Press Res. 2010;33(6):456–75. doi: 10.1159/000321013. [DOI] [PubMed] [Google Scholar]
- 17.Pak J, Pu Y, Zhang ZT, et al. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem. 2001;276(13):9924–30. doi: 10.1074/jbc.M008610200. [DOI] [PubMed] [Google Scholar]
- 18.Glauser A, Hochreiter W, Jaeger P, et al. Determinants of urinary excretion of Tamm-Horsfall protein in non-selected kidney stone formers and healthy subjects. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2000;15(10):1580–7. doi: 10.1093/ndt/15.10.1580. [DOI] [PubMed] [Google Scholar]
- 19.Weichhart T, Zlabinger GJ, Saemann MD. The multiple functions of Tamm-Horsfall protein in human health and disease: a mystery clears up. Wien Klin Wochenschr. 2005;117(9–10):316–22. doi: 10.1007/s00508-005-0353-8. [DOI] [PubMed] [Google Scholar]
- 20.Scolari F, Caridi G, Rampoldi L, et al. Uromodulin storage diseases: clinical aspects and mechanisms. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004;44(6):987–99. doi: 10.1053/j.ajkd.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Bleyer AJ, Hart TC, Shihabi Z, et al. Mutations in the uromodulin gene decrease urinary excretion of Tamm-Horsfall protein. Kidney Int. 2004;66(3):974–7. doi: 10.1111/j.1523-1755.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 22.Ollier-Hartmann MP, Pouget-Abadie C, Bouillie J, et al. Variations of urinary Tamm-Horsfall protein in humans during the first thirty years of life. Nephron. 1984;38(3):163–6. doi: 10.1159/000183300. [DOI] [PubMed] [Google Scholar]
- 23.Dulawa J, Kokot F, Kokot M, et al. Urinary excretion of Tamm-Horsfall protein in normotensive and hypertensive elderly patients. J Hum Hypertens. 1998;12(9):635–7. doi: 10.1038/sj.jhh.1000680. [DOI] [PubMed] [Google Scholar]
- 24.Torffvit O, Agardh CD. Tubular secretion of Tamm-Horsfall protein is decreased in type 1 (insulin-dependent) diabetic patients with diabetic nephropathy. Nephron. 1993;65(2):227–31. doi: 10.1159/000187479. [DOI] [PubMed] [Google Scholar]
- 25.Reznichenko A, Boger CA, Snieder H, et al. UMOD as a susceptibility gene for end-stage renal disease. BMC medical genetics. 2012;13:78. doi: 10.1186/1471-2350-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reznichenko A, van Dijk MC, van der Heide JH, et al. Uromodulin in renal transplant recipients: elevated urinary levels and bimodal association with graft failure. Am J Nephrol. 2011;34(5):445–51. doi: 10.1159/000332231. [DOI] [PubMed] [Google Scholar]
- 27.Schlatzer D, Maahs DM, Chance MR, et al. Novel urinary protein biomarkers predicting the development of microalbuminuria and renal function decline in type 1 diabetes. Diabetes care. 2012;35(3):549–55. doi: 10.2337/dc11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Chen Y, Liu Y, et al. Urinary uromodulin excretion predicts progression of chronic kidney disease resulting from IgA nephropathy. PloS one. 2013;8(8):e71023. doi: 10.1371/journal.pone.0071023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prajczer S, Heidenreich U, Pfaller W, et al. Evidence for a role of uromodulin in chronic kidney disease progression. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25(6):1896–903. doi: 10.1093/ndt/gfp748. [DOI] [PubMed] [Google Scholar]
- 30.Hoyer JR. Tubulointerstitial immune complex nephritis in rats immunized with Tamm-Horsfall protein. Kidney Int. 1980;17(3):284–92. doi: 10.1038/ki.1980.34. [DOI] [PubMed] [Google Scholar]
- 31.Cavallone D, Malagolini N, Serafini-Cessi F. Binding of human neutrophils to cell-surface anchored Tamm-Horsfall glycoprotein in tubulointerstitial nephritis. Kidney Int. 1999;55(5):1787–99. doi: 10.1046/j.1523-1755.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- 32.Muchmore AV, Decker JM. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science. 1985;229(4712):479–81. doi: 10.1126/science.2409603. [DOI] [PubMed] [Google Scholar]
- 33.El-Achkar TM, Wu XR, Rauchman M, et al. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol. 2008;295(2):F534–44. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Achkar TM, Wu XR. Uromodulin in kidney injury: an instigator, bystander, or protector? American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;59(3):452–61. doi: 10.1053/j.ajkd.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risch L, Lhotta K, Meier D, et al. The serum uromodulin level is associated with kidney function. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2014;52(12):1755–61. doi: 10.1515/cclm-2014-0505. [DOI] [PubMed] [Google Scholar]
- 36.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64(6):821–35. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 37.Youhanna S, Weber J, Beaujean V, et al. Determination of uromodulin in human urine: influence of storage and processing. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(1):136–45. doi: 10.1093/ndt/gft345. [DOI] [PubMed] [Google Scholar]
- 38.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 39.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014 doi: 10.1001/jama.2014.6634. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. The New England journal of medicine. 2005;352(20):2049–60. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 42.Dalrymple LS, Katz R, Kestenbaum B, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med. 2011;26(4):379–85. doi: 10.1007/s11606-010-1511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35(6):1628–37. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 44.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Annals of internal medicine. 2005;142(7):497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 45.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 46.Price TR, Psaty B, O’Leary D, et al. Assessment of cerebrovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(5):504–7. doi: 10.1016/1047-2797(93)90105-d. [DOI] [PubMed] [Google Scholar]
- 47.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 48.Barlow WE, Ichikawa L, Rosner D, et al. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–72. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 49.Therneau TM, Li H. Computing the Cox model for case cohort designs. Lifetime Data Anal. 1999;5(2):99–112. doi: 10.1023/a:1009691327335. [DOI] [PubMed] [Google Scholar]
- 50.Torffvit O, Agardh CD, Thulin T. A study of Tamm-Horsfall protein excretion in hypertensive patients and type 1 diabetic patients. Scand J Urol Nephrol. 1999;33(3):187–91. doi: 10.1080/003655999750015970. [DOI] [PubMed] [Google Scholar]
- 51.Zimmerhackl LB, Pfleiderer S, Kinne R, et al. Tamm-Horsfall-Protein excretion as a marker of ascending limb transport indicates early renal tubular damage in diabetes mellitus type I. The Journal of diabetic complications. 1991;5(2–3):112–4. doi: 10.1016/0891-6632(91)90037-p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.