Abstract
Chronic kidney disease (CKD) is a major risk factor for end-stage renal disease, cardiovascular disease and premature death. Here we estimated the global prevalence and absolute burden of CKD in 2010 by pooling data from population-based studies. We searched MEDLINE (January 1990 to December 2014), International Society of Nephrology Global Outreach Program funded projects, and bibliographies of retrieved articles and selected 33 studies reporting gender- and age-specific prevalence of CKD in representative population samples. The age standardized global prevalence of CKD stages 1–5 in adults aged 20 and older was 10.4% in men (95% confidence interval 9.3–11.9%) and 11.8% in women (11.2–12.6%). This consisted of 8.6% men (7.3–9.8%) and 9.6% women (7.7–11.1%) in high-income countries, and 10.6% men (9.4–13.1%) and 12.5% women (11.8–14.0%) in low- and middle-income countries. The total number of adults with CKD was 225.7 million (205.7–257.4 million) men and 271.8 million (258.0–293.7 million) women. This consisted of 48.3 million (42.3–53.3 million) men and 61.7 million (50.4–69.9 million) women in high-income countries, and 177.4 million (159.2–215.9 million) men and 210.1 million (200.8–231.7 million) women in low- and middle-income countries. Thus, CKD is an important global-health challenge, especially in low- and middle-income countries. National and international efforts for prevention, detection, and treatment of CKD are needed to reduce its morbidity and mortality worldwide.
Keywords: chronic kidney disease, proteinuria
Introduction
Chronic kidney disease (CKD) is a major global health burden due to its high prevalence and associated risk of end-stage renal disease (ESRD), cardiovascular disease (CVD), and premature death (1–3). The Global Burden of Disease Study 2013 estimated 956,200 deaths worldwide were directly attributable to CKD in 2013, which represents a 134.6% increase from 1990 (4). In addition, CKD was ranked as the 19th highest cause of years of life lost in 2013 (4). This number of deaths and years of life lost has almost certainly underestimated the disease burden of CKD, as it probably only captures deaths due to ESRD. It is well documented that CVD causes most of the deaths in patients with CKD (2,3). Worldwide, an estimated 1.9 million ESRD patients were on renal replacement therapy in 2010 (5). Medical costs for the treatment of CKD and ESRD are enormous and represent an immense financial burden to families and society as a whole. For example, overall US Medicare expenditures for CKD and renal replacement therapy in 2010 were 41.0 and 32.9 billion US dollars, respectively, accounting for 24% of the total Medicare budget (6).
Diabetes and hypertension are the leading causes of CKD in all high-income countries and many low- and middle-income countries (1). The global epidemic of diabetes and hypertension could lead to a worldwide increase in prevalence and in the number of persons with CKD and its complications without effective interventions (7,8). Although the prevalence of CKD has been reported in individual countries, global estimates of CKD prevalence and absolute burden are not available. Accurate estimates of the worldwide prevalence of this condition are essential as a source of primary information and for rational planning of health services. Quantifying the global burden of CKD will allow public-health policy-makers around the world to assign sufficient priority and resources to its prevention and treatment. We aimed to estimate the global prevalence and absolute burden of CKD in 2010 by pooling data from population-based studies worldwide.
Results
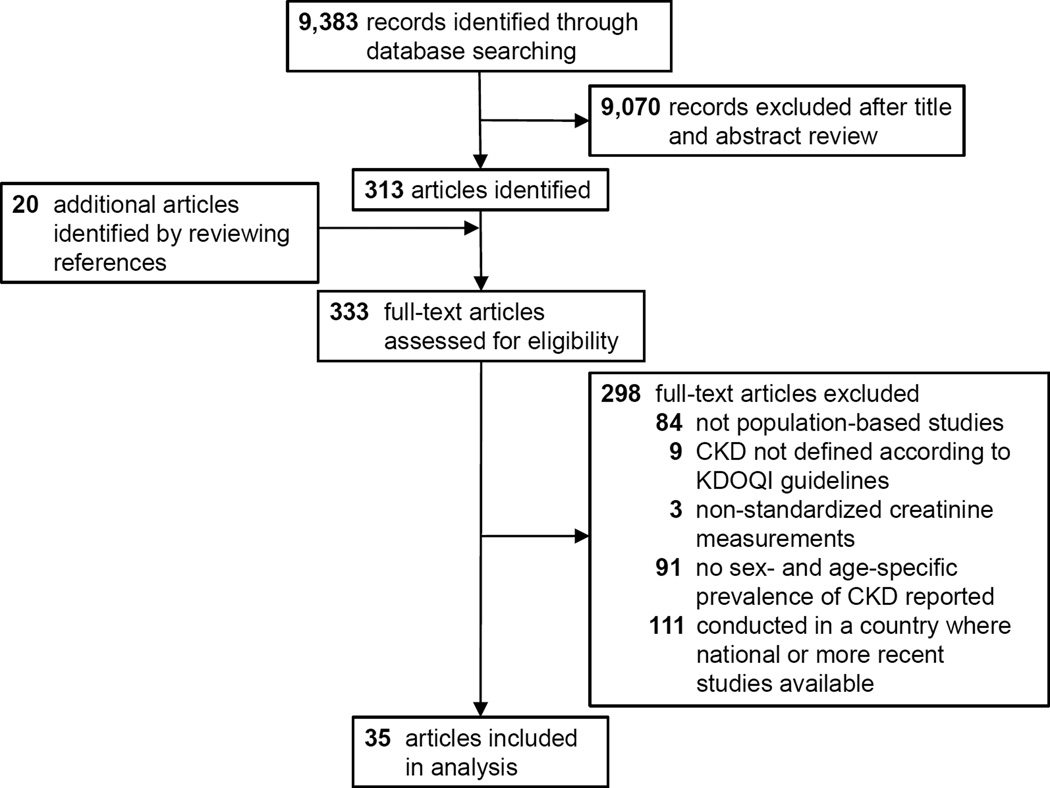
A total of 35 reports from 33 studies conducted in 32 countries, which represent 48.6% of the global population ≥20 years old, were included in the analysis (Figure 1) (9–43). Among the included studies, 16 were conducted in nationally representative samples and the rest were in multisite or regional samples (Table 1). All included studies were published between 2006 and 2013. The crude prevalence of CKD stages 1–5 varied from 4.5% in South Korea to 25.7% in El Salvador in men, and from 4.1% in Saudi Arabia to 16.0% in Singapore in women; stages 3–5 varied from 1.3% in China to 15.4% in Nepal in men and from 1.7% in Singapore to 21.3% in Nepal in women.
Figure 1.
Study selection process
Table 1.
Characteristics of studies
| Country, Year | Study sample |
Sample size |
Female, % |
Age, years |
Albuminuria or proteinuria measurement |
eGFR equation |
Creatinine measurement |
Prevalence of CKD stages 1–5, %* |
Prevalence of CKD stages 3–5, %* |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Methods | Men | Women | Men | Women | |||||||
| High-income countries | |||||||||||
| Australia (9), 2010 | national | 11,247 | 55.1 | ≥25 | - | CKD-EPI | modified Jaffé method | - | - | 4.9 (3.9, 5.9) | 6.6 (4.2, 9.0) |
| Canada (10), 2013 | national | 3,589 | 52.7 | 18–79 | spot urine ACR | CKD-EPI | enzymatic method | 12.4 (10.8, 14.0) | 12.7 (11.2, 14.2) | 2.8 (1.8, 2.8) | 3.4 (1.9, 4.9) |
| Chile (11), 2012 | national | 4,785 | 60.0 | ≥15 | - | MDRD | kinetic Jaffé method | - | - | 2.3 (1.1, 3.5) | 3.0 (1.9, 4.1) |
| Finland (12), 2012 | national | 4,228 | 54.3 | 25–74 | - | CKD-EPI | enzymatic method | - | - | 1.9 (1.3, 2.5) | 3.1 (2.4, 3.8) |
| France (13), 2012 | regional | 4,727 | 49.5 | 35–74 | - | MDRD | kinetic Jaffé method | - | - | 6.4 (5.4, 7.4) | - |
| Germany (14), 2009 | regional | 9,806 | 55.0 | 50–74 | - | MDRD | kinetic Jaffé method | - | - | 14.5 (13.5, 15.5) | 19.8 (18.7, 20.8) |
| Italy (15), 2006 | regional | 4,574 | 54.5 | 18–95 | - | MDRD | kinetic Jaffé method | - | - | 6.6 (2.4, 10.8) | 6.2 (2.4, 10.0) |
| Italy (16), 2010 | regional | 3,629 | 52.2 | ≥40 | spot urine ACR | CKD-EPI | kinetic Jaffé method | 13.2 (11.6, 14.8) | 12.2 (10.7, 13.7) | 6.5 (5.3, 7.7) | 6.9 (5.8, 8.0) |
| Japan (17,18), 2009 | multi-site | 574,024 | 58.1 | ≥20 | urine dipstick test | Japanese equation | enzymatic method | 8.6 (8.5, 8.7) | 7.3 (7.2, 7.4) | 5.8 (5.7, 5.9) | 5.4 (5.3, 5.5) |
| South Korea (19), 2011 | national | 15,975 | 57.5 | ≥20 | urine dipstick test | MDRD | kinetic Jaffé method | 4.5 (4.0, 5.0) | 6.3 (5.8, 6.8) | 2.6 (2.2, 3.0) | 4.6 (4.2, 5.0) |
| Norway (20,21), 2006 | regional | 65,181 | 53.2 | ≥20 | - | MDRD | kinetic Jaffé method | - | - | 3.6 (3.4, 3.8) | 5.7 (5.4, 5.9) |
| Portugal (22), 2011 | national | 5,167 | 59.8 | 20–79 | - | MDRD | kinetic Jaffé method | - | - | 3.7 (2.8, 4.6) | 7.8 (6.8, 8.8) |
| Saudi Arabia (23), 2010 | regional | 491 | 50.1 | ≥18 | urine dipstick test | MDRD | kinetic Jaffé method | 7.3 (4.0, 10.7) | 4.1 (1.5, 6.6) | - | - |
| Singapore (24), 2011 | national | 4,337 | 51.6 | 18–69 | - | MDRD | kinetic Jaffé method | - | - | 2.8 (2.1, 3.5) | 1.7 (1.2, 2.2) |
| Singapore (25), 2010 | national | 4,499 | 51.9 | 24–95 | spot urine ACR | MDRD | enzymatic method | 17.4 (15.8, 19.0) | 16.0 (14.5, 17.5) | - | - |
| Spain (26), 2010 | national | 2,746 | 58.2 | ≥20 | spot urine ACR | MDRD | NR | 8.6 (7.0, 10.2) | 9.7 (8.2, 11.2) | 5.9 (4.5, 7.2) | 7.7 (6.4, 9.0) |
| Switzerland (27), 2006 | national | 6,317 | 50.9 | ≥28 | - | MDRD | kinetic Jaffé method | - | - | 4.5 (3.8, 5.3) | 16.7 (15.4, 18.0) |
| United Kingdom (28), 2011 | national | 5,988 | 54.8 | ≥16 | spot urine ACR | MDRD | enzymatic method | 13.0 (11.7, 14.3) | 13.0 (11.8, 14.2) | 6.0 (5.1, 6.9) | 7.0 (6.1, 7.9) |
| United States (29), 2012 | national | 15,886 | 50.9 | ≥20 | spot urine ACR | CKD-EPI | kinetic Jaffé method | 10.6 (10.0, 11.3) | 13.0 (12.7, 14.2) | 3.7 (3.2, 4.2) | 5.4 (4.9, 5.9) |
| Uruguay (30), 2013 | regional | 1,584 | 58.8 | 35–74 | - | CKD-EPI | kinetic Jaffé method | - | - | 2.7 (1.7, 3.7) | 3.7 (2.6, 4.7) |
| Low-and middle-income countries | |||||||||||
| Argentina (30), 2013 | multi-site | 3,990 | 60.2 | 35–74 | - | CKD-EPI | kinetic Jaffé method | - | - | 2.0 (1.4, 2.7) | 1.7 (1.2, 2.2) |
| Argentina (31), 2009 | regional | 1,016 | 71.1 | 15–75 | - | MDRD | kinetic Jaffé method | - | - | 3.6 (1.5, 5.7) | 8.6 (6.6, 10.6) |
| Bolivia (32), 2012 | multi-site | 3,436 | 63.8 | ≥18 | - | MDRD | kinetic Jaffé method | - | - | 4.5 (3.3, 5.7) | 2.6 (1.9, 3.3) |
| China (33), 2012 | national | 47,204 | 57.3 | ≥18 | spot urine ACR | Chinese modified MDRD | kinetic Jaffé method | 8.7 (8.4, 9.1) | 12.9 (12.4, 13.3) | 1.3 (1.1, 1.5) | 2.2 (2.0, 2.4) |
| Congo (34), 2009 | regional | 503 | 59.0 | 20–79 | 24-h proteinuria | MDRD | kinetic Jaffé method | 12.8 (8.2, 17.4) | 14.7 (9.6, 19.7) | - | - |
| El Salvador (35), 2011 | regional | 775 | 55.7 | ≥18 | urine dipstick test | MDRD | enzymatic method | 25.7 (20.9, 30.5) | 11.8 (8.6, 15.0) | - | - |
| Iran (36), 2009 | national | 16,354 | 51.0 | 15–79 | spot urine ACR | MDRD | modified Jaffé method | 14.1 (13.3, 14.9) | 11.3 (10.6, 12.0) | 9.1 (8.5, 9.8) | 7.5 (6.9, 8.1) |
| Moldova (37), 2012 | multi-site | 1,025 | 71.9 | ≥18 | - | MDRD | kinetic Jaffé method | - | - | 5.2 (2.5, 7.9) | 11.0 (8.7, 13.3) |
| Nepal (32), 2012 | regional | 20,811 | 61.4 | ≥18 | - | MDRD | kinetic Jaffé method | - | - | 15.3 (14.5, 16.1) | 21.3 (20.6, 22.0) |
| Nigeria (38), 2013 | regional | 1,941 | 63.4 | 25–64 | urine dipstick test | CKD-EPI | kinetic Jaffé method | 11.0 (8.7, 13.3) | 10.6 (8.8, 12.4) | 9.5 (7.3, 11.7) | 10.0 (8.3, 11.7) |
| Pakistan (39), 2013 | regional | 2,873 | 52.0 | ≥40 | spot urine ACR | CKD-EPI with correction for South Asians | kinetic Jaffé method | 11.6 (9.9, 13.3) | 13.3 (11.6, 15.0) | 5.2 (3.9, 6.5) | 5.5 (4.3, 6.7) |
| Romania (40), 2012 | regional | 60,969 | 55.4 | ≥18 | - | CKD-EPI | NR | - | - | 4.9 (4.6, 5.1) | 9.3 (9.0, 9.6) |
| South Africa (41), 2013 | regional | 1,202 | 75.3 | 16–95 | - | CKD-EPI | enzymatic method | - | - | 5.7 (3.1, 8.3) | 8.0 (6.2, 9.8) |
| Thailand (42), 2009 | national | 3,117 | 50.0 | ≥15 | - | MDRD with adjustment for Asian ethnicity | modified Jaffé method | - | - | 5.5 (4.2, 6.8) | 12.3 (10.8, 13.8) |
| Turkey (43), 2011 | national | 10,748 | 55.7 | ≥18 | spot urine ACR | MDRD | kinetic Jaffé method | 12.8 (11.8, 13.8) | 18.4 (17.2, 19.6) | 3.0 (2.5, 3.5) | 5.7 (5.1, 6.3) |
Abbreviations: ACR, urine albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; NR, not reported.
When standard errors were not reported, they were estimated as the square root of ([prevalence of CKD × (1-prevalence of CKD)]/sample size).
The age-standardized global prevalence of CKD stages 1–5 among adults aged ≥20 years in 2010 was 10.4% in men (95% CI 9.3 to 11.9%) and 11.8% in women (11.2 to 12.6%). The age-standardized prevalence was 8.6% in men (7.3 to 9.8%) and 9.6% in women (7.7 to 11.1%) in high-income countries, and 10.6% in men (9.4 to 13.1%) and 12.5% in women (11.8 to 14.0%) in low- and middle-income countries (Table 2). The age-standardized global prevalence of CKD stages 3–5 among adults aged ≥20 years in 2010 was 4.7% in men (95% CI 3.4 to 6.7%) and 5.8% in women (4.4 to 8.1%). The age-standardized prevalence was 4.3% in men (3.5 to 5.2%) and 5.7% in women (4.4 to 7.6%) in high-income countries, and 4.6% in men (3.1 to 7.7%) and 5.6% in women (3.9 to 9.2%) in low- and middle-income countries.
Table 2.
Age-specific and age-standardized prevalence estimates and absolute numbers of men and women with chronic kidney disease in high-income and low- and middle-income countries
| High-income countries | Low-and middle-income countries | |||||||
|---|---|---|---|---|---|---|---|---|
| Age, years | CKD stages 1–5 | CKD stages 3–5 | CKD stages 1–5 | CKD stages 3–5 | ||||
| Men | Women | Men | Women | Men | Women | Men | Women | |
| Prevalence % (95% CIs) | ||||||||
| 20–29 | 3.7 (2.7, 5.1) | 5.3 (3.8, 6.3) | 0.7 (0.3, 1.4) | 0.9 (0.4, 1.6) | 7.3 (6.4, 9.0) | 6.6 (6.2, 7.3) | 3.0 (1.7, 5.4) | 2.0 (1.4, 3.3) |
| 30–39 | 5.0 (4.0, 6.0) | 5.9 (4.4, 6.9) | 1.3 (0.7, 2.1) | 1.6 (0.9, 2.6) | 8.1 (6.8, 10.3) | 9.0 (8.6, 9.7) | 3.1 (1.9, 5.0) | 3.1 (2.1, 5.1) |
| 40–49 | 6.8 (5.5, 8.2) | 7.7 (5.9, 9.0) | 2.1 (1.4, 3.1) | 3.2 (2.0, 4.8) | 10.2 (9.0, 12.6) | 11.5 (11.0, 12.7) | 3.0 (1.8, 6.2) | 4.0 (2.5, 7.7) |
| 50–59 | 10.2 (8.6, 12.2) | 11.1 (8.6, 13.8) | 4.6 (3.2, 6.7) | 7.4 (5.2, 10.2) | 12.0 (10.4, 15.1) | 15.7 (14.7, 17.8) | 4.9 (3.5, 8.1) | 6.7 (4.5, 11.7) |
| 60–69 | 16.0 (13.6, 18.1) | 15.6 (12.6, 18.9) | 9.4 (7.8, 11.6) | 12.2 (9.6, 15.8) | 16.3 (14.7, 20.0) | 21.3 (19.6, 24.9) | 9.7 (6.1, 15.6) | 13.1 (9.5, 19.7) |
| ≥70 | 28.1 (23.4, 33.0) | 28.9 (22.7, 34.3) | 22.7 (20.0, 26.4) | 28.5 (26.1, 31.5) | 20.6 (19.4, 24.1) | 28.4 (26.3, 32.7) | 11.8 (9.0, 17.6) | 17.3 (12.6, 27.2) |
| Total | 10.1 (8.8, 11.1) | 12.1 (9.9, 13.7) | 5.4 (4.6, 6.5) | 8.6 (6.9, 10.7) | 10.2 (9.1, 12.4) | 12.1 (11.6, 13.3) | 4.3 (2.9, 7.1) | 5.3 (3.8, 8.2) |
| Age-standardized | 8.6 (7.3, 9.8) | 9.6 (7.7, 11.1) | 4.3 (3.5, 5.2) | 5.7 (4.4, 7.6) | 10.6 (9.4, 13.1) | 12.5 (11.8, 14.0) | 4.6 (3.1, 7.7) | 5.6 (3.9, 9.2) |
| Absolute Numbers in Thousands (95% CIs) | ||||||||
| 20–29 | 3,453 (2,485, 4,718) | 4,647 (3,307, 5,500) | 694 (288, 1,275) | 800 (307, 1,570) | 37,121 (32,209, 45,651) | 32,054 (30,213, 35,932) | 14,998 (8,471, 27,644) | 9,863 (6,746, 16,328) |
| 30–39 | 4,699 (3,802, 5,684) | 5,298 (4,000, 6,235) | 1,221 (659, 1,957) | 1,440 (712, 2,514) | 33,274 (27,740, 42,285) | 35,920 (34,256, 38,727) | 12,499 (7,803, 20,591) | 12,265 (8,185, 20,402) |
| 40–49 | 6,326 (5,158, 7,691) | 7,120 (5,519, 8,386) | 2,004 (1,290, 2,934) | 2,990 (1,731, 4,700) | 35,322 (31,190, 43,673) | 39,012 (37,165, 43,201) | 10,253 (6,328, 21,335) | 13,495 (8,579, 26,111) |
| 50–59 | 8,531 (7,149, 10,157) | 9,752 (7,541, 12,136) | 3,874 (2,625, 5,549) | 6,485 (4,218, 9,418) | 29,618 (25,640, 37,287) | 38,337 (35,959, 43,651) | 12,167 (8,612, 19,988) | 16,355 (10,900, 28,611) |
| 60–69 | 9,579 (8,155, 10,849) | 10,425 (8,411, 12,690) | 5,608 (4,694, 6,963) | 8,291 (6,329, 11,264) | 22,434 (20,338, 27,637) | 31,136 (28,731, 36,396) | 13,420 (8,430, 21,497) | 19,232 (13,883, 28,848) |
| ≥70 | 15,699 (13,066, 18,416) | 24,426 (19,150, 29,003) | 12,690 (11,136, 14,745) | 24,065 (21,769, 27,161) | 19,606 (18,459, 22,925) | 33,637 (31,150, 38,706) | 11,176 (8,541, 16,673) | 20,509 (14,912, 32,240) |
| Total | 48,285 (42,349, 53,284) | 61,669 (50,394, 69,929) | 26,091 (22,014, 31,078) | 44,071 (35,296, 54,831) | 177,375 (159,157, 215,924) | 210,096 (200,787, 231,704) | 74,513 (50,324, 123,460) | 91,719 (66,697, 143,121) |
The estimated total number of adults with any stage of CKD in 2010 was 225.7 million (205.7 to 257.4 million) men and 271.8 million (258.0 to 293.7 million) women worldwide. The estimated total number of adults with any stage of CKD was 48.3 million (42.3 to 53.3 million) men and 61.7 million (50.4 to 69.9 million) women in high-income countries, and 177.4 million (162.9 to 209.0 million) men and 210.1 million (200.8 to 231.7 million) women in low- and middle-income countries (Table 2). The estimated total number of adults with CKD stages 3–5 in 2010 was 100.6 million (72.0 to 146.4 million) men and 135.8 million (102.7 to 193.9 million) women worldwide. The estimated total number of adults with CKD stages 3–5 was 26.1 million (22.0 to 31.1 million) men and 44.1 million (35.3 to 54.8 million) women in high-income countries, and 74.5 million (50.3 to 123.5 million) men and 91.7 million (66.7 to 143.1 million) women in low- and middle-income countries.
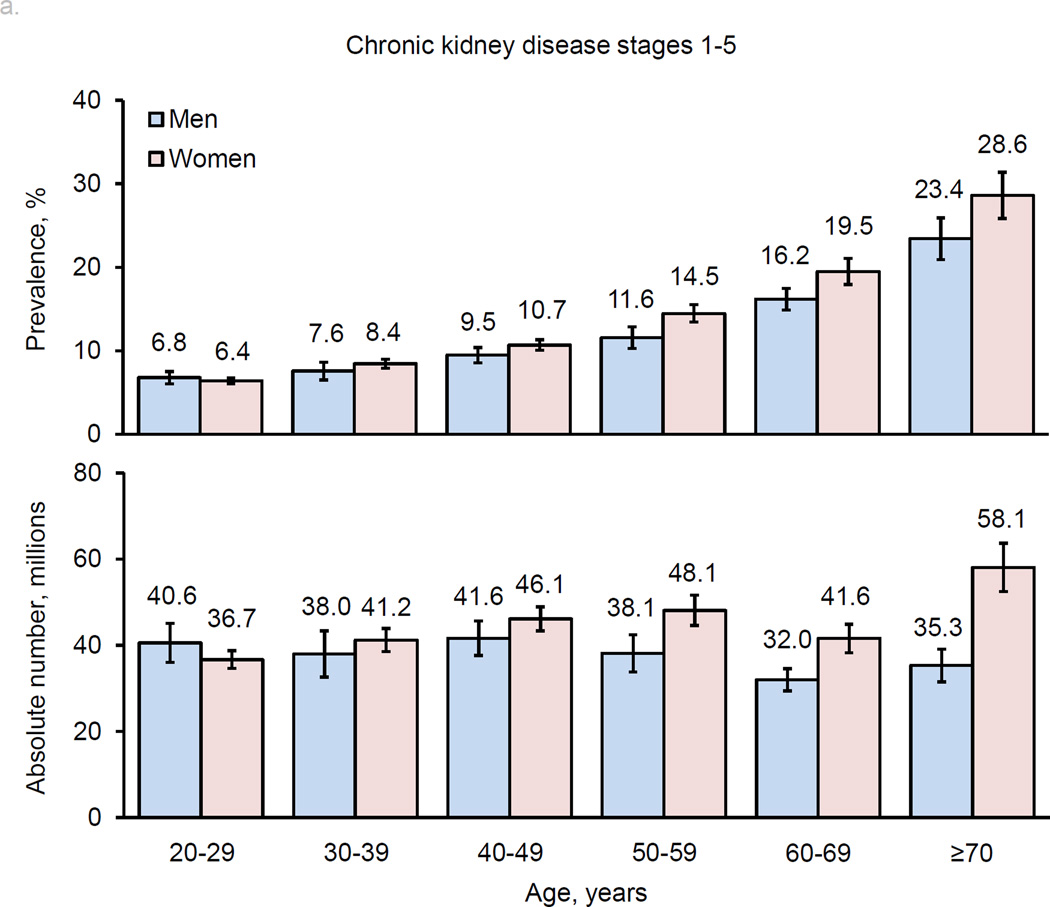
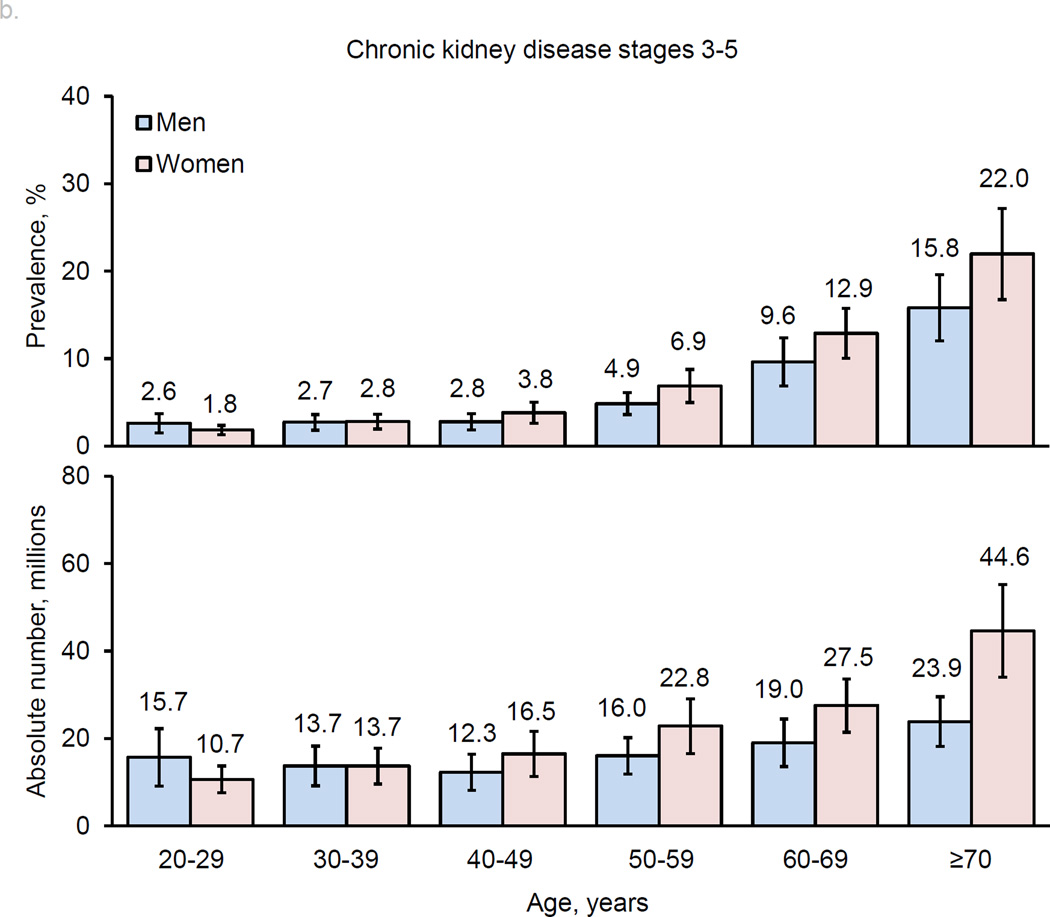
The prevalence of CKD (both stages 1–5 and stages 3–5) increased with age (Figure 2). Overall, men and women had similar prevalences of CKD in younger age groups. Subsequently, women had higher prevalences than men in middle-age groups, and this sex-difference became even greater in older age groups, especially for stages 3–5. The pattern of age-related increase in CKD prevalence in men and women was consistent in high-income and low- and middle-income countries (Table 2).
Figure 2.
Age-specific prevalence estimates and absolute numbers of men and women with chronic kidney disease worldwide: a. stages 1–5; b. stages 3–5.
In general, age-specific prevalences of CKD were higher in low- and middle-income countries compared to high-income countries, except in those aged ≥70 years, whose prevalence was higher in high-income countries for both men and women. The absolute numbers of men and women with CKD were much greater in low- and middle-income countries compared to high-income countries. Approximately 78.6% of men and 77.3% of women with CKD stage 1–5 and 74.1% of men and 67.5% of women with CKD stage 3–5 were living in low- and middle-income countries.
Discussion
Our analysis indicated that over 497 million adults aged 20 years and older in the world had CKD stages 1–5 in 2010. Of them, 236 million had moderate or severe decreases in kidney function, i.e., CKD stages 3–5. The majority of individuals with CKD were living in low- and middle-income countries. The age-adjusted prevalences of CKD were higher in low- and middle-income countries compared to high-income countries. Our results also suggest that women had a higher prevalence of CKD and that prevalences increased with age consistently in high-income and low- and middle-income countries. However, the estimates in our analysis are limited by available data from published studies, including the use of only a single creatinine measurement to calculate estimated glomerular filtration rate (eGFR). It has been suggested that creatinine-based equations might systematically underestimate the true GFR, particularly among individuals with higher GFR levels (≥60 ml/min per 1.73 m2) (44). In addition, the majority of population-based surveys did not follow patients for 3 months to confirm CKD. Eriksen and Ingebretsen reported that among 6,863 individuals in the municipality of Tromsø with an initial eGFR of 30–59 ml/min/1.73 m2, 2,175 (31.7%) had subsequent estimates ≥60 ml/min/1.73 m2 within 3 months (45). It is uncertain whether these findings are applicable to other populations with different races/ethnicities, geographic regions, and diets. Nevertheless, these data indicate that a single measure of eGFR may substantially overestimate the prevalence of CKD due to false positives. Furthermore, albuminuria or proteinuria was not measured in several studies (9,11–15,20–22,24,27,30–32,37,40–42).
The high prevalence of CKD worldwide has led to an increased prevalence of ESRD and mortality from CVD (1–3). It was estimated that more than 1.9 million ESRD patients were on renal replacement therapy in 2010 (5). ESRD causes reduced life expectancy and poor quality of life in patients and presents an enormous financial burden to families and society as a whole (1,6). CVD is the leading cause of death in the world, and ischemic heart disease and stroke collectively killed 12.9 million people in 2010, which represents one in four deaths worldwide (4). Both reduced GFR and albuminuria or proteinuria are associated with increased risk of ESRD, CVD and premature death (2,3). Therefore, the prevention, detection, and treatment of CKD should be an effective approach for reducing ESRD, CVD, and total mortality in the world.
Our study reported a high prevalence of CKD in the elderly, especially for CKD stages 3–5. This high prevalence of CKD is mirrored by an increased number of elderly on kidney dialysis (46). The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) or the Kidney Disease: Improving Global Outcomes (KDIGO) clinical guidelines were used to define CKD in studies included in this analysis (47,48). The appropriateness of eGFR <60 ml/min/1/73 m2 as the cut-point for CKD in the elderly is debatable since older age is associated with a physiological decline in GFR, and moderately reduced GFR might have less risk predictive value for ESRD, CVD, and total mortality in the elderly (44,49). Therefore, use of a non-age-specific threshold for GFR to define CKD is most likely to substantially overestimate CKD prevalence in older age groups. Further studies are warranted to define an appropriate GFR threshold for CKD in the elderly.
Our study also documented that women had a higher prevalence of CKD than men, and the gender difference was more pronounced in older age groups. These findings are consistent with large, nationally-representative studies from the United States, Finland, and China (12,29,33). Data from the US National Health and Nutrition Examination Survey (NHANES) showed that the higher prevalence of CKD in women compared to men was consistent when eGFR was calculated based on the Modification of Diet in Renal Disease (MDRD) equation, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine-based equation, and CKD-EPI cystatin C-based equation (6). However, the prevalence and incidence of ESRD are significantly higher in men than in women (6). One possible explanation for this discrepancy is a faster progression of CKD to ESRD in men compared to women (45).
Our findings indicated that CKD affected many more individuals in low- and middle-income countries than in high-income countries. A higher prevalence of CKD and a much larger population resulted in a considerably greater absolute burden of CKD in low- and middle-income countries. In fact, more than three quarters of patients with CKD were living in low- and middle-income countries in 2010. Lifestyle changes due to rapid urbanization and globalization have led to the increased prevalence of CKD risk factors in low- and middle-income countries, while limited healthcare resources result in extremely low treatment and control rates of CKD risk factors in those countries (7,8). Our study results call for global collaborative efforts to combat CKD in low- and middle-income countries. The World Health Organization Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020 mentions kidney disease as a condition associated with two of its main diseases of interest, CVD and diabetes, but does not include CKD as a main priority (50). The results reported here suggest a more explicit global focus on CKD is needed.
The worldwide epidemics of diabetes and hypertension have likely played a major role in the increased global burden of CKD (1,7,8). In addition, changes in lifestyle risk factors, environmental exposures, and the aging of the population have all likely contributed to the increased burden of CKD (1). These risk factors are also associated with an increased risk of ESRD, CVD, and total mortality in patients with CKD. Despite having an estimated prevalence similar to diabetes in many countries, much less attention and resources have been allocated to CKD surveillance, prevention, and management compared with many other chronic diseases (51). To reduce the global burden of CKD and related morbidities and mortalities, a comprehensive approach is needed that focuses on several inter-related risks to health, including hypertension, high LDL cholesterol, tobacco use, obesity, physical inactivity, poor diet, and diabetes (1,47,48).
In conclusion, our findings indicate that CKD is an important global-health challenge, especially in low- and middle-income countries. National and international efforts on the prevention, detection, and treatment of CKD are needed to reduce its morbidities and mortalities worldwide.
Methods
Literature search, study selection, and data extraction were conducted independently and in duplicate by at least two investigators using a standardized protocol and data-collection form. Discrepancies were resolved by consensus.
Data Sources and Searches
We searched MEDLINE using key words “prevalence”, “cross-sectional study”, “survey”, “renal insufficiency”, “renal disease”, “chronic kidney disease”, “kidney function”, “glomerular filtration rate”, “albuminuria” and “proteinuria”. The search was restricted to studies in humans published from January 1, 1990, to December 31, 2014. A manual search was performed using references cited in reviews and original research articles. In addition, we searched projects awarded by the International Society of Nephrology Global Outreach Program from 2005, when the first Clinical Research Program projects were funded, to 2014. Publications in other languages were translated into English.
Study Selection
Eligibility criteria for inclusion were: (1) population-based original studies in which prevalence of CKD (or data to calculate it) was reported; (2) sex- and age-specific prevalence of CKD was provided; (3) CKD was defined according to the NKF KDOQI or KDIGO guidelines (47,48) and (4) serum creatinine values were standardized. The NKF KDOQI guidelines were selected because most nationally representative studies reporting CKD prevalence use these guidelines to define CKD.
If a national study was available for a country, we used its data. If not, we used data from the largest and most recent multisite or regional study. If data from multiple regional studies within a country were available (15,16,30,31), they were pooled to provide national estimates by weighting by age- and sex-specific sample size. If the most recent prevalence estimates were not published and if study datasets were available, age- and gender-specific prevalence estimates were calculated from the datasets. We calculated age- and sex-specific prevalence of CKD stages 1–5 and stages 3–5 in the US population using data from NHANES 2007–2012 (29) and CKD stages 3–5 in Argentina and Uruguay using data from the Study on the Detection and Follow-up of Cardiovascular Disease and Risk Factors in the Southern Cone of Latin America (CESCAS) (30).
Data Extraction and Imputation
Information on the measurements of albuminuria, proteinuria, and eGFR was extracted. If eGFR from multiple equations was reported, we preferred the CKD-EPI equation over the MDRD equation. Likewise, we preferred urinary albumin excretion ≥30 mg/day (or albumin-to-creatinine ratio ≥30 mg/g) over urinary protein excretion ≥300 mg/day or a positive dipstick test of 1+ or greater to define kidney damage if multiple markers were reported. CKD stages 1–5 was defined as kidney damage or eGFR <60 mL/min/1.73 m2, while CKD stages 3–5 was defined as eGFR <60 mL/min/1.73 m2 (47).
Countries were grouped into high-income countries and low- and middle-income countries using the World Bank classification system updated in July 2013 (52). High-income countries are defined as those with a gross national income (GNI) of $12,616 or more, and low-and middle-income countries are those with a GNI less than $12,616 (52). For countries without prevalence estimates meeting our inclusion criteria, we applied data from the country with the closest geographic proximity within the same geographical region defined using the World Bank classification system (52). If more than one country with data neighbored a country with no data, the country with the most similar GNI was used.
Not all studies provided prevalence data for the full age range under consideration (from 20–29 years to ≥70 years). Incomplete age-specific prevalence was estimated using logistic regression to model the relationship between age and prevalence of CKD for each sex in high-income countries and low- and middle-income countries, separately. Median age and prevalence of CKD within each reported age group from available studies were used to create the predictive models. Goodness of fit was tested by Cragg and Uhler's pseudo R-square, and all models were well fit in high-income countries (R2 between 0.55 and 0.84) and had poorer fit in low- and middle-income countries (R2 between 0.27 and 0.59). The prevalence and mean age in men and women from each study with missing age-specific prevalence were used to calibrate the predictive equation before it was used to estimate the age-specific prevalence for that country. If studies did not provide age-specific data in the 10-year increments of interest (i.e., 20–29, 30–39, etc), we averaged the prevalence from the two closest 10-year age groups weighting by age-specific population size from the country to estimate the prevalence of CKD for the age ranges of interest.
For countries that reported only the prevalence of CKD stages 3–5, logistic regression was used to predict the age-specific prevalence of stages 1–5 in men and women for high-income countries and low- and middle-income countries, separately. Eight high-income countries (48 age-specific prevalence estimates) and five low-income countries (30 age-specific prevalence estimates) reported both prevalence of CKD stages 1–5 and stages 3–5 in men and women. These data were used to generate the predictive models for CKD stages 1–5 prevalence estimates from the age-specific prevalence of CKD stages 3–5. For studies that reported only the prevalence of CKD stages 1–5, the same method was used to predict stages 3–5 from stages 1–5. These models all had good fit assessed by Cragg and Uhler's pseudo R-square (R2 between 0.56 and 0.89).
Data Synthesis and Analysis
Sex- and age-specific prevalences of CKD for each country were applied to the WHO sex- and age-specific population counts in 2010 to estimate the number of people with CKD in the country for each sex- and age-group (53). The total number of persons with CKD in high-income countries and low- and middle-income countries was summed separately to provide an estimate of the total number of persons with CKD for each income category by sex- and age-groups, and the number from each income category was added to obtain the worldwide count. The sex- and age-specific prevalences of CKD for 2010 in high-income countries and low- and middle-income countries were calculated by dividing the total number of people with CKD in each income category by the number of people in that income category by sex- and age-groups. Worldwide prevalence was estimated by dividing the total number of persons with CKD by the total adult world population. The prevalences of CKD in high-income countries and low- and middle-income countries were standardized by age to the 2010 world population separately for each sex and overall using the direct method. Confidence intervals for all prevalence estimates were calculated using bootstrap methods (54). For each prevalence estimate, we chose N studies, where N is the number of countries used to estimate that prevalence estimate, with replacement and calculated a mean prevalence. Then we repeated this process 10,000 times and choose the values at 2.5% and 97.5% of the distribution as our bootstrap 95% confidence intervals. Confidence intervals for all absolute burden estimates were calculated by multiplying the bootstrap 95% confidence intervals by the population for that prevalence estimate to get the 95% confidence intervals for the number of people with CKD. All analyses were done using SAS (version 9.3), R (version 3.1.2), and Microsoft Excel software.
Acknowledgments
The authors would like to acknowledge Mss. Xiaoling Ye and Caroline E. Stamatakis for their help in extracting data, Ms. Katherine Obst for her editorial assistance, and Mr. Yun Zhu for his help with the bootstrapping analysis. The authors would also like to acknowledge Drs. Luxia Zhang, Haiyan Wang, and Jinwei Wang for providing age-specific prevalence data from China; Dr. Giuseppe Remuzzi for providing age-specific prevalence data from Moldova and information on creatinine measurement methods in Moldova, Bolivia and Nepal; Dr. Sarah L. White for providing age-specific prevalence data from Australia; Dr. Ifeoma I. Ulasi for providing age-specific prevalence data from Nigeria; Dr. Dorothea Nitsch for providing age-specific prevalence data from Switzerland; Drs. Tazeen H. Jafar and Saleem Jessani for providing information on creatinine measurement methods and age-specific prevalence data from Pakistan; Dr. Leopoldo G. Ardiles for providing age-specific prevalence data from Chile; and Drs. Rajiv T. Erasmus and Andre P. Kengne for providing age-specific prevalence data from South Africa.
Financial Support: National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number U01DK060963 and the Fogarty International Center of the National Institutes of Health under Award Number D43TW009107
Footnotes
Disclosure
The authors have no interests to disclose.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 Dec 17; doi: 10.1016/S0140-6736(14)61682-2. pii: S0140-6736(14)61682-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand S, Bitton A, Gaziano T. The gap between estimated incidence of end-stage renal disease and use of therapy. PLoS One. 2013;8:e72860. doi: 10.1371/journal.pone.0072860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. U.S. Renal Data System, USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. [Google Scholar]
- 7.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 8.Danaei G, Finucane MM, Lin JK, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 9.White SL, Polkinghorne KR, Atkins RC, et al. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55:660–670. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Arora P, Vasa P, Brenner D, et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ. 2013;185:E417–E423. doi: 10.1503/cmaj.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Health Survey in Chile 2009–2010. [accessed April 17, 2014]; http://epiminsalcl/estudios-y-encuestas-poblacionales/encuestas-poblacionales/encuesta-nacional-de-salud/resultados-ens/ [Google Scholar]
- 12.Juutilainen A, Kastarinen H, Antikainen R, et al. Trends in estimated kidney function: the FINRISK surveys. Eur J Epidemiol. 2012;27:305–313. doi: 10.1007/s10654-012-9652-3. [DOI] [PubMed] [Google Scholar]
- 13.Bongard V1, Dallongeville J, Arveiler D, et al. Assessment and characteristics of chronic renal insufficiency in France. Ann Cardiol Angeiol (Paris) 2012;61:239–244. doi: 10.1016/j.ancard.2012.03.003. (in French). [DOI] [PubMed] [Google Scholar]
- 14.Zhang QL, Koenig W, Raum E, et al. Epidemiology of chronic kidney disease: results from a population of older adults in Germany. Prev Med. 2009;48:122–127. doi: 10.1016/j.ypmed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Cirillo M, Laurenzi M, Mancini M, et al. Low glomerular filtration in the population: prevalence, associated disorders, and awareness. Kidney Int. 2006;70:800–806. doi: 10.1038/sj.ki.5001641. [DOI] [PubMed] [Google Scholar]
- 16.Gambaro G, Yabarek T, Graziani MS, et al. Prevalence of CKD in northeastern Italy: results of the INCIPE study and comparison with NHANES. Clin J Am Soc Nephrol. 2010;5:1946–1953. doi: 10.2215/CJN.02400310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai E, Horio M, Watanabe T, et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13:621–630. doi: 10.1007/s10157-009-0199-x. [DOI] [PubMed] [Google Scholar]
- 18.Horio M, Imai E, Yasuda Y, et al. Modification of the CKD Epidemiology Collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32–38. doi: 10.1053/j.ajkd.2010.02.344. [DOI] [PubMed] [Google Scholar]
- 19.Kang HT, Lee J, Linton JA, et al. Trends in the prevalence of chronic kidney disease in Korean adults: the Korean National Health and Nutrition Examination Survey for 1998 to 2009. Nephrol Dial Transplant. 2013;28:927–936. doi: 10.1093/ndt/gfs535. [DOI] [PubMed] [Google Scholar]
- 20.Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17:2275–2284. doi: 10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 21.Hallan SI, Dahl K, Oien CM, et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ. 2006;333:1047. doi: 10.1136/bmj.39001.657755.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinhas J, Gardete-Correia L, Boavida JM, et al. Prevalence of chronic kidney disease and associated risk factors, and risk of end-stage renal disease: data from the PREVADIAB study. Nephron Clin Pract. 2011;119:c35–c40. doi: 10.1159/000324218. [DOI] [PubMed] [Google Scholar]
- 23.Alsuwaida AO, Farag YMK, Al Sayyari AA, et al. Epidemiology of chronic kidney disease in the Kingdom of Saudi Arabia (SEEK-Saudi Investigators) – a pilot study. Saudi J Kidney Dis Transplant. 2010;21:1066–1072. [PubMed] [Google Scholar]
- 24.National Health Survey in Singapore 2010. [accessed April 17, 2014]; http://www.moh.gov.sg/content/moh_web/home/Publications/Reports/2011/national_health_survey2010.html.
- 25.Sabanayagam C, Lim SC, Wong TY, et al. Ethnic disparities in prevalence and impact of risk factors of chronic kidney disease. Nephrol Dial Transplant. 2010;25:2564–2570. doi: 10.1093/ndt/gfq084. [DOI] [PubMed] [Google Scholar]
- 26.Otero A, de Francisco A, Gayoso P, et al. Prevalence of chronic renal disease in Spain: results of the EPIRCE study. Nefrologia. 2010;30:78–86. doi: 10.3265/Nefrologia.pre2009.Dic.5732. (in Spanish). [DOI] [PubMed] [Google Scholar]
- 27.Nitsch D, Felber Dietrich D, von Eckardstein A, et al. Prevalence of renal impairment and its association with cardiovascular risk factors in a general population: results of the Swiss SAPALDIA study. Nephrol Dial Transplant. 2006;21:935–944. doi: 10.1093/ndt/gfk021. [DOI] [PubMed] [Google Scholar]
- 28.Health Survey for England 2010. [accessed April 17, 2014]; http://www.hscic.gov.uk/catalogue/PUB03023/heal-surv-eng-2010-resp-heal-ch8-kidn.pdf.
- 29.National Health and Nutrition Examination Survey 2007–2008, 2009–2010, 2011–2012. [accessed April 17, 2014]; http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 30.Rubinstein AL, Irazola VE, Poggio R, et al. Detection and follow-up of cardiovascular disease and risk factors in the Southern Cone of Latin America: the CESCAS I study. BMJ Open. 2011;1:e000126. doi: 10.1136/bmjopen-2011-000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salazar MR, Carbajal HA, Marillet AG, et al. Glomerular filtration rate, cardiovascular risk factors and insulin resistance. Medicina (B Aires) 2009;69:541–546. (in Spanish). [PubMed] [Google Scholar]
- 32.Cravedi P, Sharma SK, Bravo RF, et al. Preventing renal and cardiovascular risk by renal function assessment: insights from a cross-sectional study in low-income countries and the USA. BMJ Open. 2012;2:e001357. doi: 10.1136/bmjopen-2012-001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 34.Sumaili EK, Krzesinski JM, Zinga CV, et al. Prevalence of chronic kidney disease in Kinshasa: results of a pilot study from the Democratic Republic of Congo. Nephrol Dial Transplant. 2009;24:117–122. doi: 10.1093/ndt/gfn469. [DOI] [PubMed] [Google Scholar]
- 35.Orantes CM, Herrera R, Almaguer M, et al. Chronic kidney disease and associated risk factors in the Bajo Lempa region of El Salvador: Nefrolempa Study, 2009. MEDICC Review. 2011;13:14–22. doi: 10.37757/MR2011V13.N4.5. [DOI] [PubMed] [Google Scholar]
- 36.Safarinejad MR. The epidemiology of adult chronic kidney disease in a population-based study in Iran: prevalence and associated risk factors. J Nephrol. 2009;22:99–108. [PubMed] [Google Scholar]
- 37.Codreanu I, Sali V, Gaibu S, et al. Prevalence of hypertension and diabetes and coexistence of chronic kidney disease and cardiovascular risk in the population of the Republic of Moldova. Int J Hypertens. 2012;2012:951734. doi: 10.1155/2012/951734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulasi II, Ijoma CK, Onodugo OD, et al. Towards prevention of chronic kidney disease in Nigeria: a community-based study in Southeast Nigeria. Kidney Int Supplements. 2013;3:S195–S201. [Google Scholar]
- 39.Jessani S, Bux R, Jafar TH. Prevalence, determinants, and management of chronic kidney disease in Karachi, Pakistan – a community based cross-sectional study. BMC Nephrology. 2014;15:90. doi: 10.1186/1471-2369-15-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cepoi V, Onofriescu M, Segall L, et al. The prevalence of chronic kidney disease in the general population in Romania: a study on 60,000 persons. Int Urol Nephrol. 2012;44:213–220. doi: 10.1007/s11255-011-9923-z. [DOI] [PubMed] [Google Scholar]
- 41.Matsha TE, Yako YY, Rensburg MA, et al. Chronic kidney diseases in mixed ancestry South African populations: prevalence, determinants and concordance between kidney function estimators. BMC Nephrol. 2013;14:75. doi: 10.1186/1471-2369-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ong-Ajyooth L, Vareesangthip K, Khonputsa P, et al. Prevalence of chronic kidney disease in Thai adults: a national health survey. BMC Nephrol. 2009;10:35. doi: 10.1186/1471-2369-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Süleymanlar G, Utaş C, Arinsoy T, et al. A population-based survey of chronic renal disease in Turkey-the CREDIT study. Nephrol Dial Transplant. 2011;26:1862–1871. doi: 10.1093/ndt/gfq656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glassock RJ. Estimated glomerular filtration rate: time for a performance review? Kidney Int. 2009;75:1001–1003. doi: 10.1038/ki.2009.38. [DOI] [PubMed] [Google Scholar]
- 45.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69:375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 46.Tonelli M, Riella M. Chronic kidney disease and the ageing population. Lancet. 2014;383:1278–1279. doi: 10.1016/S0140-6736(14)60155-0. [DOI] [PubMed] [Google Scholar]
- 47.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2) Suppl 1:S1–S266. [PubMed] [Google Scholar]
- 48.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 49.Glassock RJ, Winearls C. The global burden of chronic kidney disease: how valid are the estimates? Nephron Clin Pract. 2008;110:c39–c47. doi: 10.1159/000151244. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization. Global Action Plan for the Prevention and Control of NCDs 2013 to 2020. [accessed October 31, 2014]; http://www.who.int/nmh/events/ncd_action_plan/en/
- 51.Zoccali C, Kramer A, Jager KJ. Epidemiology of CKD in Europe: an uncertain scenario. Nephrol Dial Transplant. 2010;25:1731–1733. doi: 10.1093/ndt/gfq250. [DOI] [PubMed] [Google Scholar]
- 52.The World Bank. Country and Lending Groups. [accessed April 17, 2014]; http://data.worldbank.org/about/country-classifications/country-and-lending-groups. [Google Scholar]
- 53.World population prospects, the 2012 revision. [accessed April 17, 2014]; http://esa.un.org/unpd/wpp/index.htm. [Google Scholar]
- 54.Efron B. Bootstrap methods: another look at the jackknife. Ann Statist. 1979;7:1–26. [Google Scholar]