Abstract
Objective
To investigate the relationship between awareness of memory loss and psychological well-being in a non-clinically depressed sample of participants with mild-moderate Alzheimer’s disease.
Method
Study participants (n=104) enrolled through Columbia University Medical Center and the University of Pennsylvania completed clinical and cognitive assessments. Participants were rated with regard to their degree of awareness of memory deficits and completed questionnaires relating to their psychological well-being, including mood and quality of life (QOL). Mediating models were used to establish the relationship between awareness, depression, and QOL, as well as to examine potential mediators of awareness and depression including psychological distress, objective memory deficits, and negative self-ratings.
Results
There was a direct association between awareness of memory deficits and depressed mood, but not awareness and QOL. However, there was an indirect association between awareness and QOL through depression. Neither psychological distress, memory deficits, nor negative self-ratings mediated the relationship between awareness and depression.
Conclusions
Awareness is associated with depressed mood in non-clinically depressed participants with mild to moderate AD. However, depressed mood does not appear to reflect the direct psychological reaction to awareness of memory loss. Moreover, awareness has only an indirect association with QOL via depressed mood. These results suggest that preserved awareness does not have a direct negative impact on overall psychological well-being in AD.
Keywords: Awareness, Depression, Quality of Life
INTRODUCTION
Disordered awareness of memory loss is a common feature of Alzheimer’s disease (AD) dementia, even at its earliest stages (1,2). Disordered awareness makes patients less likely to comply with treatment (3), increases caregiver burden (2,4), and impairs decision making capacity (3,5). Consequently, interventions to improve awareness of cognitive and functional deficits may have potential value. However, preserved awareness may negatively affect key patient reported outcomes related to psychological well-being, potentially increasing depressive symptoms and reducing perceived quality of life (QOL). This study sought to investigate the relationship between awareness of memory loss and psychological well-being.
There is a strong link between depression and reduced QOL in dementia (6-12), and both have been associated with preserved awareness. (13-22) However, it is unknown whether memory awareness influences these aspects of well-being, well-being influences memory complaints, or even if they occur simultaneously and independently. (23) Researchers and clinicians need to know the answer to these questions to inform strategies for managing and enhancing key patient reported outcomes in the lives of persons with AD. This will be increasingly important as disease modifying treatments are being developed that, if effective, will keep individuals at mild stages of dementia for longer periods of time.
The first goal of this study was therefore to outline the pathways between awareness, depression, and QOL. Redundant metrics of these constructs may obscure the underlying relationships. For example, QOL ratings generally address patient perception of a variety of facets of life including mood (24). Moreover, measures of depression often query a range of cognitive complaints. Distinguishing the measurements of QOL, depression, and awareness in a single mediation model within a large sample of individuals with AD may help clarify the extent and nature of their associations.
The second goal of this study was to examine three specific models by which awareness may be associated with depressed mood. The first model posits that awareness of cognitive decline results in psychological distress surrounding specific cognitive failures, which then leads to more general feelings of sadness and hopelessness (13,14,16). The second model posits that individuals with more depressive symptoms experience more severe memory problems and thus report such problems to a greater degree (25-27). Finally, the third model posits that depressed mood leads to a higher endorsement of complaints in general (cognitive or otherwise), subsequently resulting in increased memory complaints (regardless of the true level of awareness). We evaluate the applicability of these models as well as the overall association between awareness, depressed mood, and QOL in a non-clinically depressed sample of individuals with mild to moderate AD.
METHODS
Participants
104 individuals with Probable AD were recruited through Columbia University Medical Center Department of Neurology (n=49) and the University of Pennsylvania Memory Center (n=55). Diagnoses of AD were made according to the Neurologic Disorders and Stroke - Alzheimer’s Disease and Related Disorders Association (NINDS-ADRDA) criteria. All participants were reimbursed for participation. Patients with Probable AD and a score ≥17 or greater on the Mini-Mental State Examination (28) were included. Individuals with a significant history of or ongoing major psychiatric condition were excluded from the study, as were individuals with history of head injury, stroke, and other neurologic illnesses that might impact cognition and/or the presentation of AD.
Procedures
All studies were approved by the Institutional Review Board at both medical centers and all individuals provided informed consent prior to participation.
Measures
Each measure was selected and modified as described below to eliminate redundancy in the assessment of each construct. All scales demonstrate good reliability across multiple measurements (See Supplemental Digital Content 1, available online).
Awareness
Clinical Rating of Awareness (CRA)
Test sessions began with a brief interview in which examiners asked participants to discuss their current memory abilities. A score from 1 to 4 was assigned using a modified version of the Anosognosia Rating Scale (29) with 4 = Full Awareness (Spontaneous complaint or ready admission of memory loss along with the recognition that the loss is abnormal for age); 3 = Moderate Awareness (Spontaneous admission of memory loss; however, loss is discussed in the context of “normal” age related changes. No discussion of diagnosis); 2 = Shallow Awareness (Inconsistent or transient recognition of memory loss, or uncertainty regarding memory loss. Patients may acknowledge inconsequential memory loss); and 1 = No Awareness (Denial of any memory impairment). Responses were audio recorded and scored before completing the remainder of the battery. Scores were finalized after a consensus meeting with an additional rater following the visit.
Depressive Symptoms
Geriatric Depression Scale (GDS) (30)
The GDS is a self-report tool that has been validated as an appropriate measure in patients with mild-moderate AD (31). Participants were prompted to endorse those items they have experienced in the past week (e.g. Do you often feel helpless?). For the purposes of this study, we created a 23-item anhedonic sub-score (GDSa) to eliminate redundancy between depression and awareness scores. Specifically, we removed 4 items on the GDS that assess cognitive complaints (e.g. Do you feel you have more problems with memory than most?). Moreover, we conservatively removed 3 additional items related to concern for future well-being (e.g., Do you frequently worry about the future?) to ensure that these were not related to awareness of a neurodegenerative process.
Quality of Life
Quality of Life in Alzheimer’s Disease (QOL-AD) Scale (10)
QOL-AD is a 13-item measure of overall QOL that was specifically developed for assessing patients with dementia. It has been shown to be a reliable and valid measure for assessing subjective QOL (32). Each of 13 areas was rated using a 4-point scale from poor to excellent. The primary dependent variable was the single item on the questionnaire pertaining to the participant’s life as a whole. This item was selected to: 1) reduce redundancy across measures as some of the items in the scale could be argued to assess our other constructs of interest, mood and awareness; and 2) account for the fact that individuals might rate certain specific aspects of their life negatively despite having an overall positive perception of their life as a whole, and vice versa.
Distress
Global Distress Index (GDI)
Distress surrounding specific cognitive difficulties was measured as a potential mediator of the association between awareness and depressive symptoms more generally. The global distress index (GDI) derives from the Cognitive Difficulties Scale (CDS (33)), a 39-item questionnaire that asks subjects to reflect on “everyday inefficiencies, lapses of attention or memory and related functions that people notice about themselves.” The questionnaire uses a five-point Likert scale from 0 (never) to 4 (very often). The GDI is paired with the CDS to assess the participant’s distress in relation to each of the 39 cognitive difficulties queried. Distress is rated on a five-point Likert scale from 0 (not at all) to 4 (very much), with high scores indicating severe distress regarding the cognitive difficulty. The measure was given to both the participant and to a reliable informant to complete about the patient. An adjusted GDI score was used in analyses to account for the total number of reported cognitive difficulties such that distress scores were not necessarily higher for those who endorsed a greater number of cognitive difficulties (Total GDI / Number of cognitive difficulties reported = Adjusted GDI). Adjusted GDI was included as a mediator in Models 2a and 2b to examine the extent to which depression reflects the psychological reaction to cognitive failures.
Memory
Philadelphia Repeatable Verbal Learning Test (PVLT) (34)
The PVLT is a list-learning task in which participants are required to learn 9 words over the course of five trials. The primary dependent variable was delayed recall after approximately 30 minutes. Memory was included as a mediator in Model 3 which examined the extent to which depression lowers memory, thereby heightening memory complaints (i.e., awareness).
General Self Ratings
General Self-Ratings
Using a brief rating scale (35), participants were asked to judge themselves in comparison to others their age on four abilities (walking, using hands and fingers, sitting still and quietly, and controlling emotions). Ratings were based on a 5 point Likert scale from 1 (very impaired) to 5 (excellent). Participants’ ratings of these abilities (not necessarily expected to be reduced in AD) were included in Model 4 to examine the extent to which negative self-assessment in general may mediate the association between depressive symptoms and awareness.
Data Analysis
Total scores were prorated from the mean score for the GDSa, GDI and CDS if a participant was missing <20% of individual items (<6 on GDSa and <7 on CDS/GDI). 15 subjects and 10 informants received an imputed total score on one of the measures. The totals for these subjects were computed from the average of the remaining items. One way analyses of variance (ANOVAs) and chi-square tests were used to evaluate differences in variables across site. Pearson correlation coefficients were used to examine correlations between demographic variables and the main variables of interest.
Mediation Analyses
We tested four mediation models using path-analytic approaches developed by Hayes (2013) (36). For each mediation model, we estimated the total, direct, and indirect effects of a predictor on the outcome variable through the selected mediator. See Supplemental Digital Content 2, available online, for a detailed description of the mediation analyses and the calculation of effect sizes using the kappa-squared statistic.
RESULTS
Descriptive demographic and clinical information are presented in Tables 1 and 2. The only demographic variable that correlated with awareness was age (r (102)= −0.27, p=0.01). There were no associations between demographic variables and depression or QOL. No variable differed by site. Inclusion of age as a covariate in the mediation models did not change results, and therefore unadjusted results are presented.
Table 1. Demographic Information and Awareness Levels.
| Variable | Mean (SD) or%(N) |
|---|---|
| Age | 77.55 (8.03) |
| Education | 15.38 (2.84) |
| Female | 65.4% (68) |
| Caucasian | 92.3% (96) |
| African American | 7.7% (8) |
| Non-Hispanic Ethnicity | 98.1% (102) |
| Awareness Levels | |
| Full awareness | 21.2% (22) |
| Moderate awareness | 35.6% (37) |
| Shallow awareness | 33.7% (35) |
| No awareness | 9.6% (10) |
Table 2. Cognition, Mood, and QOL.
| Measure | N | Range | Mean (SD) |
|---|---|---|---|
| MMSE | 104 | 17-30 | 24.19 (2.64) |
| Geriatric Depression Scale (GDS) | 104 | 0-21 | 4.90 (4.40) |
| GDS anhedonia sub-score | 104 | 0-18 | 3.06 (3.38) |
| Patient Cognitive Difficulties Scale | 90 | 39-138 | 74.64 (20.11) |
| Patient Global Distress Index (GDI) | 90 | 0-104 | 25.70 (23.65) |
| Adjusted Patient GDI | 90 | 0-3.07 | 1.03 (0.74) |
| Informant Cognitive Difficulties Scale | 86 | 43-165 | 109.51 (25.37) |
| Informant Global Distress Index (GDI) | 86 | 0-117 | 43.65 (25.71) |
| Adjusted Informant GDI | 86 | 0-3.20 | 1.39 (0.70) |
| Quality of Life – Total Score | 103 | 25-51 | 41.27 (5.10) |
| Quality of Life – “How would you describe your life as a whole?” |
2-4 | 3.28 (0.62) |
Mediation Analyses
Table 3 and Figures 1-4 contain the unstandardized regression coefficients and 95% CIs for all analyses described below. Direct effects and indirect effects are referred to as DE and IE respectively in the text below.
Table 3. Results of Mediation Models.
| Predictor | Mediator | Outcome | Path A | Path B | Path C’ (Direct Effect) |
Indirect Effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | t(df) | β | SE | t(df) | β | SE | t(df) | β | SE | 95% CI | ||||
| 1 | Aware | Depression | QOL | .86* | .36 | 2.37(101) | − 08*** |
.02 | −4.82(100) | −.01 | .06 | −.11(100) | −.07a | .04 | −.16, −.01 |
| 2a | Aware | PR Distress | Depression | .08 | .09 | .94(88) | 1.01* | .46 | 2.18(87) | 1.06** | .39 | 2.73(87) | .08 | .11 | −.07, .41 |
| 2b | Aware | IR Distress | Depression | .28*** | .08 | 3.41(84) | .24 | .55 | .44(83) | 1.06* | .41 | 2.42(83) | .07 | .16 | −.27, .37 |
| 3 | Depression | Memory | Awareness | .04 | .05 | .83(95) | .10* | .05 | 2.01(94) | .07** | .03 | 2.79(94) | .004 | .007 | −.005, .02 |
| 4 | Depression | Self-Ratings | Awareness | −.27*** | .07 | −3.84(102) | .01 | .03 | .30(101) | .07* | .03 | 2.34(101) | −.003 | .01 | −.02, 02 |
Note. Path A represents the unstandardized coefficient from regressing the predictor on the mediator; Path B refers to the effect of the mediator on the outcome after controlling for the effect of the predictor; and Path C’ refers to the direct effect of the predictor on the outcome after controlling for the effect of the mediator. The indirect effect reflects the product of Path A and Path B, and a significant indirect effect indicates some mediating effect. PR = Patient Rated; IR = Informant Rated;
p< .05;
p<.01,
p<.001;
aSignificant at least at p < .05; statistical software did not distinguish p-values < .05.
Figure 1.
Unstandardized regression coefficients for (a) the effect of Awareness of Memory Loss on Depression, t(101) = 2.37, β = .86, p = .02; (b) the effect of Depression on Quality of Life after controlling for Awareness, t(100) = −4.82, β= −.08, p<.001; and (c’) the effect of Awareness of Memory Loss on Quality of Life after controlling for Depression, t(100) =−.11, β −.007, p=.91. Indirect effect represents the product of a*b, bootstrap CI = −.16, −.01. aSignificant at least at p < .05; statistical software did not distinguish p-values < .05.
Figure 4.
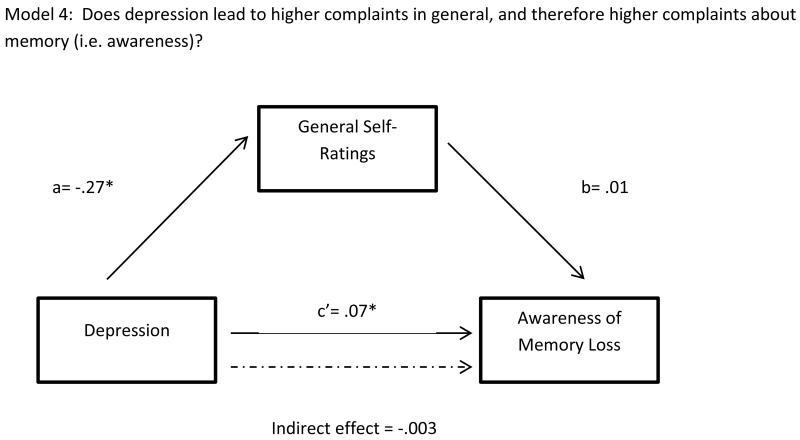
Unstandardized regression coefficients for (a) the effect of Depression on General Self-Ratings, t(102)= −3.84, β = −.27, p <.001; (b) the effect of General Self-Ratings on Awareness of Memory Loss, after controlling for Depression, t(101)=.30, β = .01, p=.76; and (c’) the effect of Depression on Awareness after controlling for General Self-Ratings, t(101) =2.34, β = .07, p=.02. Indirect effect represents the product of a*b, bootstrap CI=−.02, .02.
aSignificant at least at p < .05; statistical software did not distinguish p-values < .05.
Model 1: Awareness, Depression and QOL (Figure 1)
Awareness of memory loss significantly predicted depressive symptoms (path A), t(101)=6.53, p=.02, and depressive symptoms predicted QOL (path B), t(100)= −4.82, p<.001. There was no DE of awareness on QOL (path C’), t(100)= −.11, p=.91. However, there was a significant IE of awareness on QOL through depression, IE = .07, bootstrap CI = −.16, −.01, with a medium-large effect size, kappa-squared =.11. To test the specificity of this model (i.e., the extent to which significant results are maintained when the predictor and outcome variables are reversed), we entered depression as the predictor and awareness as the mediator in an alternative model. While depression did have a DE on QOL, t(100) = −4.82, β=-0.08, p<.001, the relationship was not mediated by awareness (IE = −.0004, bootstrap CI = −.01, .01, kappa-squared=.003).
Models 2-4: Mediators of the Relationship between Awareness and Depression (Figures 2-4)
Figure 2.
Top Panel (2A) shows unstandardized regression coefficients for (a) the effect of Awareness of Memory Loss on Patient-Reported Distress over Cognitive Failures, t(88) = .937, β =.08, p=.35; (b) the effect of Patient-Reported Distress on Depression after controlling for Awareness, t(87) = 2.18, β =1.01, p =.03, and (c’) the direct of effect of Awareness of Memory Loss on Depression after controlling for the effect of Patient-Reported Distress over Cognitive Failures, t(87) =2.73, β =1.06, p=.008. Indirect effect represents the product of a*b, bootstrap CI = −.07, .41. Bottom Panel (2B) shows unstandardized regression coefficients for (a), the effect of Awareness of Memory Loss on Patient’s Distress over Cognitive Failures as reported by caregiver, t(84) =3.41, β = .28, p < .001; (b) the effect of Caregiver-Reported Patient Distress on Depression after controlling for Awareness, t(83) =.44, β = .24, p = .66; and (c’) the direct effect of Awareness on Depression after controlling for Caregiver-Reported Patient Distress, t(83) =2.42, β =1.06, p= .02. Indirect effect represents the product of a*b, bootstrap CI = −.27, .37.
aSignificant at least at p < .05; statistical software did not distinguish p-values < .05.
Model 2 (Distress Over Cognitive Failures)
Awareness of memory loss did not predict patient-reported distress over cognitive failures (path A), t(88)=.30, p=.35, although patient distress did predict depression scores (path B), t(87)=2.18, p=.03. The DE of awareness on depression scores remained significant, t(87)=2.73, p=.008, but the effect was not mediated by patient distress over cognitive failures, IE = .08, kappa-squared=0.02. Model 2B: Patient’s awareness of memory loss predicted the informant’s ratings of the patient’s distress over cognitive failures (path A), t(84)=3.40, p=.001. However, the caregiver’s reports about the patient’s distress did not predict depressive symptoms (path B), t(83)=.44, p=.66. The direct path between awareness and depression remained significant when informant-rated distress was included as a mediator, t(83)=2.42, p=.02, but the mediating effect was non-significant, IE= 0.07, kappa-squared=.02.
Model 3 (Memory)
Depression did not predict memory performance, path A, t(95)=.83, p=.41. However, there was a significant positive relationship between memory performance and awareness (path B), t(94)=2.01, p=.05, such that those with higher memories also had better awareness of memory loss. The DE of depression on awareness (path c’) was also significant, t(94)=2.79, p =.006, but was not mediated by memory, IE=.004, kappa-squared=.01.
Model 4 (Negative Self-Ratings)
Depressive symptoms predicted general (non-cognitive) self-ratings, path A, t(102)=3.8, p<.001, however, negative self-ratings did not predict memory awareness, path B, t(101)=.30, p=.76. The direct path between depressive symptoms and awareness was significant, t(101)=2.34, p=.02, but negative self-ratings did not exert a mediating effect, IE= −.003, kappa-squared=.01.
DISCUSSION
Among patients with AD, disordered awareness of memory loss is a prevalent yet incompletely understood symptom. While evidence suggests that disordered awareness is detrimental for decision-making capacity and therefore threatens patients’ autonomy and independence (3) intact awareness has been associated with higher depression and lower QOL. However, the link between awareness and psychological well-being has been inconsistent, and its basis not understood. Using mediation models, we investigated the pathways between awareness and key patient reported outcomes of psychological well-being.
Among participants with mild to moderate AD, we confirmed the well-established link between depression and QOL (9,22,37,38), and demonstrated a direct association between awareness and depression. The latter finding, consistent with a number of earlier investigations (13-21) but not all (2,29,39-46), is compelling because we used non-overlapping measures to assess these constructs, ensuring that the association was not driven by redundancy in the assessment of each construct (e.g., cognitive complaints in the context of the mood assessment). Consideration of the discrepancies across earlier studies reveals two key variables that likely influence whether an association is seen between awareness and depression. First, the severity of depression appears to be a driving factor, with no associations reported between awareness and depression in the context of major depressive disorder (29,39,41,47). This could reflect the fact that MDD is likely associated with a number of genetic, biological and or environmental variables that wash out more subtle associations between mood and awareness.
Second, those studies that focus on the psychological and affective experience of depression (e.g. sad affect, depressed mood, dysthymia) find a positive association between awareness and depressed mood (13,14,16-19,48). In contrast, most studies finding no association either employ scales that include somatic symptoms (e.g., sleep, appetite, etc.) with questionable validity in older adults (12) or employ a broad measure of psychiatric symptoms with only a few items specific to depressed mood. As an example, Troisi and colleagues (49) divided the Hamilton Depression Rating Scale scores into intrapsychic and somatic subscales. Although somatic symptoms were unrelated to insight, psychological symptoms of depression were more severe among patients with preserved awareness.
Interestingly, we found no direct path between awareness and QOL, consistent with some (6,9,11,22) but not all (8,50) previous studies in mild AD. Discrepancies in earlier studies might be explained by a novel finding in the current study, which is that there was an indirect path between awareness and QOL through depressive symptoms, and the effect size of this association was medium to large. Thus, while memory awareness did not negatively influence peoples’ perceptions of their “life as a whole”, there was a relationship between the two constructs through depressive symptoms. Thus it appears that depression, not awareness, is the key driver of QOL. This confirms the idea that efforts to alleviate depressive symptoms may bolster QOL in early AD (6,9,22), and suggests that being aware of one’s symptoms in early AD does not lead directly to negative perceptions of one’s quality of life.
The second aim of the current study was to delve more deeply into the association between awareness and depressive symptoms by investigating the mechanisms that may underlie this relationship. Surprisingly, none of the hypothesized mechanisms were supported by mediation models. The first model posited that individuals’ awareness surrounding cognitive decline could contribute to feelings of sadness and hopelessness (13,16). Although there was an association between self-reported distress surrounding cognitive failures and depressed mood, we found no evidence for an association between self-reported distress levels and awareness. The second model posited that patients with depressive symptoms experience higher degrees of memory impairment, and thus estimate their memory ability as worse than those with few to no depressive symptoms. However, not only were depressive symptoms unrelated to memory performance, better memory was associated with higher awareness. The third model investigated whether endorsing complaints (memory or otherwise) is a symptom of depressed mood. This model, in line with Schema Theory and the notion of depressive realism (23), posits that depressed patients experience negative beliefs regarding various aspects of their own self. Thus, AD patients with depressive symptoms may have negative estimations of themselves in general, whether in reference to cognitive or non-cognitive abilities, and not necessarily intact awareness. This theory has previously been proposed as a potential explanation for higher memory awareness in AD patients with clinical depression (51). As such, higher awareness scores would be related to the psychological construct of ‘complaining behavior’ (25) rather than genuine awareness of impairment. However, the final mediation model revealed that while those with higher depressive symptoms reported greater impairment in non-cognitive domains, individuals judged to be more aware of memory problems did not make more negative self-ratings in general. While limited power to detect mediating effects in these models may be a consideration with a sample size of 104, the very small to small effect size of all indirect effects casts doubt on the applicability of these models even with a larger sample.
Given that none of the three hypotheses explored were supported by mediation models, it is possible that current results at least partially reflect the specifics of our study sample and operational definitions of the constructs measured. It is also possible that other unmeasured factors account for the association between awareness and depressive symptoms. For example, existing work across a number of different clinical populations has pointed to a link between left hemisphere compromise and increased levels of depression (52,53), and a separate link between right hemisphere compromise and lower levels of awareness (16,29,49,54,55). It is thus plausible that an early and disproportionate burden of right hemisphere pathology in AD could have clinical manifestations including lower levels of both awareness and depressive symptoms, whereas a disproportionate burden of left hemisphere pathology could underlie increased levels of both. It has also been posited that reduced awareness and depression occur independently, but may converge to generate an “affective anosognosia” (49) such that patients who are unaware of memory impairment may also be unaware of depressive symptoms, particularly in the later stages of the disease.
Conclusions
We examined the extent to which and mechanisms by which awareness of memory symptoms in mild to moderate AD is associated with reduced psychological well-being. Findings support the association between memory awareness and depressed mood, but the data did not provide evidence that distress surrounding cognitive failures has a direct effect on mood. Moreover, preserved awareness only appears to co-occur with poor perceptions of QOL in the context of depressed mood. Taken together, results suggest that efforts to improve awareness for the sake of enhancing decision making capacities, treatment compliance, and patient – family relationships would not sacrifice patients’ psychological well-being. Rather, a combined effort to preserve awareness and manage depressive symptoms in early AD may have the overall effect of enhancing a number of important clinical and practical outcomes related to autonomy and QOL.
Limitations
A potential limitation of the current study was the cross-sectional nature of the mediation analyses. Such analyses have been shown to significantly underestimate or overestimate mediation parameters as compared to those generated by longitudinal data, and the direction of bias cannot be determined based on the cross-sectional results (56). Certainly, longitudinal studies examining the development of depressive symptoms in relation awareness of memory loss are needed to more fully understand the causal pathways and complex association between these constructs. However, the selection of time points for such a study would not be trivial. Both awareness and mood are variables that can fluctuate within individuals, and may fluctuate frequently in between time points included in a longitudinal study. In this sense, the contemporaneous examination of these variables at least ensures that their co-occurrence is detected. The role of a given variable as a mediator in the current context is thus evaluated with regard to its expected relationship with the “predictor” and “outcome” at this single point in time. Future studies should also attempt to comprehensively examine the constructs of awareness and psychological well-being in a single model throughout the full spectrum of depressive symptoms as well as memory functioning. The current study may also have been limited by relatively low levels of depression in our participants who endorsed an average of 3/23 items on the GDSa. As such, we may have limited our ability to identify mediating variables. However, we did find an association between depressed mood and awareness suggesting that there was sufficient range to detect associations. Finally, variable levels of self-awareness in individuals with dementia raise questions regarding the validity and reliability of self-report. The decision to use self-reported measures of mood was driven by a desire to understand the patient’s psychological experience, rather than the informant’s perspective of the patient’s experience. Another option would have been to use the informant’s report of mood or QOL, however, subtle symptoms of mood are not easily observable and it is not necessarily the case that participants would share this information with informants. Moreover, reliability estimates for the scales included suggest that patients are largely stable in their reports over time.
Supplementary Material
Figure 3.
Unstandardized regression coefficients for (a) the effect of Depression on Memory Performance, t(95)= .83, β = .04, p =0.4; (b) the effect of Memory Performance on Awareness of Memory Loss after controlling for depression, t(94) = 2.01, β =0.1, p= .05; and (c’) the effect of Depression on Awareness of Memory Loss, after controlling for Memory Performance, t(94)= 2.79, β = .07, p=.006. Indirect effect represents the product of a*b, bootstrap CI =−0.005, 0.03.
aSignificant at least at p < .05; statistical software did not distinguish p-values < .05.
Acknowledgments
This study was supported by the Marilyn S. Ware Alzheimer Program and Dr. Cosentino’s Paul B. Beeson Career Development Award in Aging (K23 AG032899) funded jointly by the National Institute on Aging (NIA) and the American Federation of Aging Research. This study was also supported by NIA grants T32 AG000026 and K01AG035061.
Footnotes
A portion of these data was presented at the Annual Meeting of the International Neuropsychological Society, February 2014. There are no conflicts of interest to report.
REFERENCES
- 1.Cosentino S, Stern Y. Metacognitive theory and assessment in dementia: do we recognize our areas of weakness? Journal of the International Neuropsychological Society. 2005;11:910–919. doi: 10.1017/s1355617705050964. [DOI] [PubMed] [Google Scholar]
- 2.DeBettignies BH, Mahurin RK, Pirozzolo FJ. Insight for impairment in independent living skills in Alzheimer’s disease and multi-infarct dementia. Journal of Clinical and Experimental Neuropsychology. 1990;12:355–363. doi: 10.1080/01688639008400980. [DOI] [PubMed] [Google Scholar]
- 3.Karlawish JH, Casarett DJ, James BD, et al. The ability of persons with Alzheimer disease (AD) to make a decision about taking an AD treatment. Neurology. 2005;64:1514–1519. doi: 10.1212/01.WNL.0000160000.01742.9D. [DOI] [PubMed] [Google Scholar]
- 4.Seltzer B, Vasterling JJ, Yoder JA, et al. Awareness of deficit in Alzheimer’s disease: relation to caregiver burden. Gerontologist. 1997;37:20–24. doi: 10.1093/geront/37.1.20. [DOI] [PubMed] [Google Scholar]
- 5.Cosentino S, Metcalfe J, Cary M, et al. Memory Awareness Influences Everyday Decision Making Capacity in Alzheimer’s Disease. International Journal of Alzheimer’s Disease. 2011 doi: 10.4061/2011/483897. Article ID 483897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurt CS, Banerjee S, Tunnard C, et al. Insight, cognition and quality of life in Alzheimer’s disease. Journal of neurology, neurosurgery, and psychiatry. 2010;81:331–336. doi: 10.1136/jnnp.2009.184598. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee S, Samsi K, Petrie CD, et al. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. International journal of geriatric psychiatry. 2009:24. doi: 10.1002/gps.2090. [DOI] [PubMed] [Google Scholar]
- 8.Sousa MF, Santos RL, Arcoverde C, et al. Quality of life in dementia: the role of non-cognitive factors in the ratings of people with dementia and family caregivers. International psychogeriatrics / IPA. 2013;25:1097–1105. doi: 10.1017/S1041610213000410. [DOI] [PubMed] [Google Scholar]
- 9.Vogel A, Mortensen EL, Hasselbalch SG, et al. Patient versus informant reported quality of life in the earliest phases of Alzheimer’s disease. International journal of geriatric psychiatry. 2006;21:1132–1138. doi: 10.1002/gps.1619. [DOI] [PubMed] [Google Scholar]
- 10.Logsdon RG, Gibbons LE, McCurry SM, et al. Quality of life in Alzheimer’s disease: Patient and caregiver reports. Journal of Mental Health and Aging. 1999;5:21–32. [Google Scholar]
- 11.Ready RE, Ott BR, Grace J. Patient versus informant perspectives of Quality of Life in Mild Cognitive Impairment and Alzheimer’s disease. International journal of geriatric psychiatry. 2004;19:256–265. doi: 10.1002/gps.1075. [DOI] [PubMed] [Google Scholar]
- 12.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a Geriatric Depression Screening Scale: A preliminary report. 1982-1983. Journal of Psychiatric Research. 1982:17. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 13.Horning SM, Melrose R, Sultzer D. Insight in Alzheimer’s disease and its relation to psychiatric and behavioral disturbances. International journal of geriatric psychiatry. 2014;29:77–84. doi: 10.1002/gps.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harwood DG, Sultzer DL, Wheatley MV. Impaired insight in Alzheimer disease: association with cognitive deficits, psychiatric symptoms, and behavioral disturbances. Neuropsychiatry, neuropsychology, and behavioral neurology. 2000;13:83–88. [PubMed] [Google Scholar]
- 15.Kashiwa Y, Kitabayashi Y, Narumoto J, et al. Anosognosia in Alzheimer’s disease: association with patient characteristics, psychiatric symptoms and cognitive deficits. Psychiatry and clinical neurosciences. 2005;59:697–704. doi: 10.1111/j.1440-1819.2005.01439.x. [DOI] [PubMed] [Google Scholar]
- 16.Sevush S, Leve N. Denial of memory deficit in Alzheimer’s disease. Am J Psychiatry. 1993;150:748–751. doi: 10.1176/ajp.150.5.748. [DOI] [PubMed] [Google Scholar]
- 17.Migliorelli R, Teson A, Sabe L, et al. Anosognosia in Alzheimer’s disease: a study of associated factors. J Neuropsychiatry Clin Neurosci. 1995;7:338–344. doi: 10.1176/jnp.7.3.338. [DOI] [PubMed] [Google Scholar]
- 18.Migliorelli R, Teson A, Sabe L, et al. Prevalence and correlates of dysthymia and major depression among patients with Alzheimer’s disease. The American journal of psychiatry. 1995;152:37–44. doi: 10.1176/ajp.152.1.37. [DOI] [PubMed] [Google Scholar]
- 19.Seltzer B, Vasterling JJ, Hale MA, et al. Unawareness of memory deficit in Alzheimer’s disease: Relation to mood and other disease variables. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1995 Jul;:8. 1995. [Google Scholar]
- 20.Seltzer B, Vasterling JJ, Buswell A. Awareness of deficit in Alzheimer’s disease: Association with psychiatric symptoms and other disease variables. Journal of Clinical Geropsychology. 1995 Jan;:1. 1995. [Google Scholar]
- 21.Starkstein SE, Sabe L, Chemerinski E, et al. Two domains of anosognosia in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1996;61:485–490. doi: 10.1136/jnnp.61.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conde-Sala JL, Rene-Ramirez R, Turro-Garriga O, et al. Clinical differences in patients with Alzheimer’s disease according to the presence or absence of anosognosia: implications for perceived quality of life. J Alzheimers Dis. 2013;33:1105–1116. doi: 10.3233/JAD-2012-121360. [DOI] [PubMed] [Google Scholar]
- 23.Mograbi DC, Morris RG. On the relation among mood, apathy, and anosognosia in Alzheimer’s disease. J Int Neuropsychol Soc. 2014;20:2–7. doi: 10.1017/S1355617713001276. [DOI] [PubMed] [Google Scholar]
- 24.Hoe J, Katona C, Roch B, et al. Use of the QOL-AD for measuring quality of life in people with severe dementia--the LASER-AD study. Age and ageing. 2005;34:130–135. doi: 10.1093/ageing/afi030. [DOI] [PubMed] [Google Scholar]
- 25.Slavin MJ, Brodaty H, Kochan NA, et al. Prevalence and predictors of "subjective cognitive complaints" in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2010;18:701–710. doi: 10.1097/jgp.0b013e3181df49fb. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed S, Mitchell J, Arnold R, et al. Memory complaints in mild cognitive impairment, worried well, and semantic dementia patients. Alzheimer disease and associated disorders. 2008;22:227–235. doi: 10.1097/WAD.0b013e31816bbd27. [DOI] [PubMed] [Google Scholar]
- 27.Bassett SS, Folstein MF. Memory complaint, memory performance, and psychiatric diagnosis: a community study. Journal of geriatric psychiatry and neurology. 1993;6:105–111. doi: 10.1177/089198879300600207. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Reed BR, Jagust WJ, Coulter L. Anosognosia in Alzheimer’s disease: relationships to depression, cognitive function, and cerebral perfusion. Journal of Clinical and Experimental Neuropsychology. 1993;15:231–244. doi: 10.1080/01688639308402560. [DOI] [PubMed] [Google Scholar]
- 30.Yesavage J. The use of self-rating depression scales in the elderly. American Psychological Association; Washington DC: 1986. [Google Scholar]
- 31.Feher Edward P., Larrabee Glenn J., Crook Thomas H. Factors attenuating the validity of the Geriatric Depression Scale in a dementia population. Journal of the American Geriatrics Society. 1992 Sep;40(9):906–909. doi: 10.1111/j.1532-5415.1992.tb01988.x. [DOI] [PubMed] [Google Scholar]
- 32.Logsdon Rebecca G., Gibbons Laura E., McCurry Susan M., Teri Linda. Quality of Life in Alzheimer’s Disease: Patient and Caregiver Reports. Journal of Mental Health and Aging. 1999;5(1) [Google Scholar]
- 33.McNair DMaK RJ. In: Self-assessment of cognitive deficits, in Assessment in Geriatric Psychopharmacology. Crook T, editor. Mark Powley; New Canaan, Connecticut: 1983. pp. 119–136. SFaRB. [Google Scholar]
- 34.Price CC, Garrett KD, Jefferson AL, et al. Leukoaraiosis severity and list-learning in dementia. The Clinical Neuropsychologist. 2009;23:944–961. doi: 10.1080/13854040802681664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deckel AW, Morrison D. Evidence of a neurologically based “denial of illness” in patients with Huntington’s disease. Archives of Clinical Neuropsychology. 1996;11:295–302. [PubMed] [Google Scholar]
- 36.Hayes A. Introduction to mediation, moderation, and conditional process analysis. The Guilford Press; New York, New York: 2013. [Google Scholar]
- 37.Karttunen K, Karppi P, Hiltunen A, et al. Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer’s disease. International journal of geriatric psychiatry. 2011;26:473–482. doi: 10.1002/gps.2550. [DOI] [PubMed] [Google Scholar]
- 38.Logsdon RG, Gibbons LE, McCurry SM, et al. Assessing quality of life in older adults with cognitive impairment. Psychosomatic medicine. 2002;64:510–519. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Verhey FRJ, Rozendaal N, Ponds RWHM, Jolles J. Dementia, awareness, and depression. International Journal of Geriatric Psychiatry. 1993;8:851–856. [Google Scholar]
- 40.Arkin S, Mahendra N. Insight in Alzheimer’s patients: results of a longitudinal study using three assessment methods. Am J Alzheimers Dis Other Demen. 2001;16:211–224. doi: 10.1177/153331750101600401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez OL, Becker JT, Somsak D, et al. Awareness of cognitive deficits and anosognosia in probable Alzheimer’s disease. European Neurology. 1994;34:277–282. doi: 10.1159/000117056. [DOI] [PubMed] [Google Scholar]
- 42.Ott BR, Lafleche G, Whelihan WM, et al. Impaired awareness of deficits in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10:68–76. doi: 10.1097/00002093-199601020-00003. [DOI] [PubMed] [Google Scholar]
- 43.Michon A, Deweer B, Pillon B, et al. Relation of anosognosia to frontal lobe dysfunction in Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:805–809. doi: 10.1136/jnnp.57.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verhey FR, Ponds RW, Rozendaal N, et al. Depression, insight, and personality changes in Alzheimer’s disease and vascular dementia. Journal of geriatric psychiatry and neurology. 1995;8:23–27. [PubMed] [Google Scholar]
- 45.Starkstein SE, Vazquez S, Migliorelli R, et al. A single-photon emission computed tomographic study of anosognosia in Alzheimer’s disease. Archives of Neurology. 1995;52:415–420. doi: 10.1001/archneur.1995.00540280105024. [DOI] [PubMed] [Google Scholar]
- 46.Feher EP, Mahurin RK, Inbody SB, et al. Anosognosia in Alzheimer’s disease. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1991 Sum;:4. 1991. [Google Scholar]
- 47.Cummings JL, Ross W, Absher J, et al. Depressive symptoms in Alzheimer disease: Assessment and determinants. Alzheimer disease and associated disorders. 1995 Sum;:9. doi: 10.1097/00002093-199509020-00005. 1995. [DOI] [PubMed] [Google Scholar]
- 48.Starkstein SE, Chemerinski E, Sabe L, et al. Prospective longitudinal study of depression and anosognosia in Alzheimer’s disease. Br J Psychiatry. 1997;171:47–52. doi: 10.1192/bjp.171.1.47. [DOI] [PubMed] [Google Scholar]
- 49.Troisi A, Pasini A, Gori G, et al. Clinical predictors of somatic and psychological symptoms of depression in Alzheimer’s disease. International journal of geriatric psychiatry. 1996 Jan;:11. 1996. [Google Scholar]
- 50.Bosboom PR, Alfonso H, Eaton J, et al. Quality of life in Alzheimer’s disease: Different factors associated with complementary ratings by patients and family carers. International Psychogeriatrics. 2012:24. doi: 10.1017/S1041610211002493. [DOI] [PubMed] [Google Scholar]
- 51.Nakaaki S, Murata Y, Sato J, et al. Impact of depression on insight into memory capacity in patients with Alzheimer disease. Alzheimer disease and associated disorders. 2008;22:369–374. doi: 10.1097/WAD.0b013e3181820f58. [DOI] [PubMed] [Google Scholar]
- 52.Robinson RG, Kubos KL, Starr LB, et al. Mood disorders in stroke patients. Importance of location of lesion. Brain : a journal of neurology. 1984;107(Pt 1):81–93. doi: 10.1093/brain/107.1.81. [DOI] [PubMed] [Google Scholar]
- 53.Starkstein SE, Robinson RG, Price TR. Comparison of cortical and subcortical lesions in the production of poststroke mood disorders. Brain : a journal of neurology. 1987;110(Pt 4):1045–1059. doi: 10.1093/brain/110.4.1045. [DOI] [PubMed] [Google Scholar]
- 54.McGlynn SM, Schacter DL. Unawareness of deficits in neuropsychological syndromes. J Clin Exp Neuropsychol. 1989;11:143–205. doi: 10.1080/01688638908400882. [DOI] [PubMed] [Google Scholar]
- 55.Belyi BI. Mental impairment in unilateral frontal tumours: role of the laterality of the lesion. Int J Neurosci. 1987;32:799–810. doi: 10.3109/00207458709043334. [DOI] [PubMed] [Google Scholar]
- 56.Maxwell SE, Cole DA, Mitchell MA. Bias in cross-sectional analysis of longitudinal mediation: Partial and complete mediation under an autoregressive model. Multivariate Behavioral Research. 2011;46(5):816–841. doi: 10.1080/00273171.2011.606716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.