Abstract
This review focuses on conjunctival goblet cells and their essential function in the maintenance of eye health. The main function of goblet cells is to produce and secrete mucins that lubricate the ocular surface. An excess or a defect in those mucins leads to several alterations that makes goblet cells central players in maintaining the proper mucin balance and ensuring the correct function of ocular surface tissues. A typical pathology that occurs with mucous deficiency is dry eye disease, whereas the classical example of mucous hyperproduction is allergic conjunctivitis. In this review we analyze how goblet cell number and function can be altered in these diseases and in contact lens wearers. We found that most published studies focused exclusively on goblet cell number. However, recent advances have demonstrated that, along with mucin secretion, goblet cells are also able to secrete cytokines and respond to them. We describe the effect of different cytokines on goblet cell proliferation and secretion. We conclude that it is important to further explore the effect of contact lens wear and cytokines on conjunctival goblet cell function.
Keywords: goblet cell, contact lens, cytokines, dry eye, giant papillary conjunctivitis
Goblet cells are highly specialized epithelial cells, present in mucosal tissues along the body. The main function of these cells is to produce and secrete mucins, which hydrate and lubricate mucosal surfaces.1 Under non-pathologic conditions in the eye, goblet cells are confined to the conjunctival epithelium.
Along with the corneal epithelium and the tear film, the conjunctival epithelium is part of the ocular surface.2 Both corneal and conjunctival epithelial cells produce different mucins, but the main mucin-secreting cells are conjunctival goblet cells.
Mucins are highly glycosylated glycoproteins, consisting on a protein core and multiple side chains.3 In the human, up to 20 mucin genes have been identified. Mucins are classified into two different types: membrane-spanning mucins and secretory mucins.4 One of the most studied mucins is MUC5AC, a large gel-forming secretory mucin found in conjunctiva. MUC5AC or a similar mucin MUC5B are located in airway mucosa and the gastrointestinal tract.5 Conjunctival goblet cells are the only cells secreting MUC5AC onto the ocular surface, and for that reason, MUC5AC is one of the best markers for goblet cell identification.
In this review we analyzed what techniques have been developed to identify goblet cells and analyze their changes in different conditions. We focused on dry eye and allergic conjunctivitis due to the high prevalence of these diseases. In addition, we reviewed literature studying goblet cell variations in contact lens wearers, with special attention to patients suffering giant papillary conjunctivitis (GPC). Unfortunately, conjunctival goblet cells have not been studied as much as goblet cells in other mucosal tissues. For that reason, we summarized the effect that several cytokines have on either conjunctival or non-conjunctival goblet cells. Cytokines are immunomodulatory agents that are altered in immune diseases. Altered levels of these molecules in ocular diseases and in contact lens wearers have been broadly studied. For that reason, we aimed at drawing a global picture of the main effects exerted by cytokines in goblet cells. Bearing all this in mind, and knowing which cytokines are altered in which conditions, we hope this review will guide further research.
IDENTIFICATION OF GOBLET CELLS
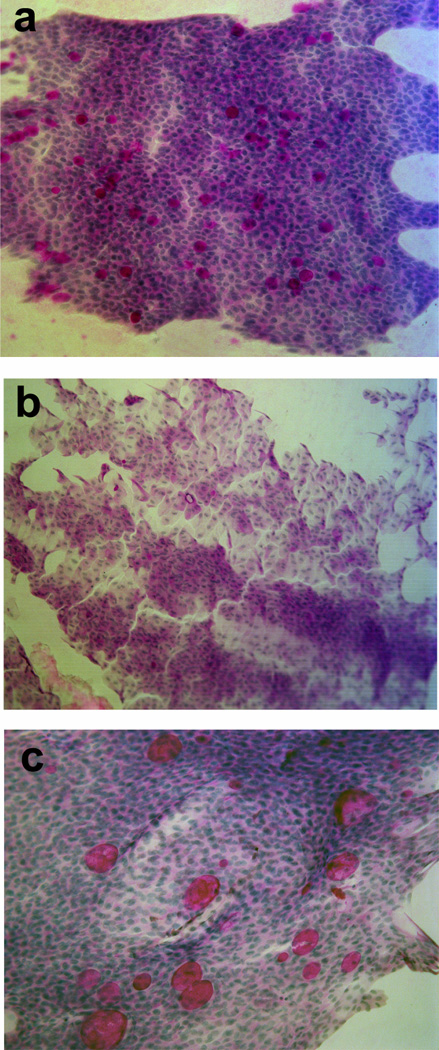
Due to the impact of goblet cell alteration in ocular surface diseases, several techniques to identify these cells have been developed. Goblet cells are usually identified in biopsies or cytologies (mainly, conjunctival impression cytology6) using a classical chemical staining that interacts with mucopolysaccharides, namely periodic acid-Schiff (PAS) staining (Figure 1a). With this method, filled goblet cells are identified, but since the staining is based in the reaction with their mucous content, it is not possible to detect those cells that have already released their products.
Figure 1.
Goblet cell identification by several techniques. a) PAS staining in a human conjunctiva. b) Goblet cells identified by cytokeratin 7 (red) immunostaining and HPA lectin binding (green). c) In vivo laser scanning confocal microscopy image showing possible goblet cells, identified by its rounded shape and brightness.
Goblet cells release their products in an apocrine manner. When they receive the appropriate stimuli, they secrete all the contents of their secretory granules at once. As a consequence, those cells that have secreted their mucins are completely empty and, remain invisible to the PAS staining. The same problem remains if MUC5AC or lectins such as UEA-1 or HPA are used to identify goblet cells. In 1997, Krenzer and Freddo described that cytokeratin (CK) 7 stained the cell body of goblet cells,7 allowing the identification of all goblet cells, regardless whether they had already secreted their contents or not. Thus, it is possible to identify the total number of goblet cells, filled and empty, using co-immunolocalization with CK7 and HPA or MUC5AC8 (Figure 1b).
Another technique to identify goblet cells is in vivo laser scanning confocal microscopy.9 This technique allows evaluation of tissue structure in vivo identification of goblet cells10 (Figure 1c). The main advantage compared to conjunctival impression cytology, is that confocal microscopy is not invasive at all, whereas cytology is a minimally invasive method. Furthermore, cytologies or biopsies need histological processing that can introduce artifacts, what can invalidate the study or diagnosis; whereas in vivo confocal microscopy does not.
IN VITRO MODELS TO STUDY CONJUNCTIVAL GOBLET CELL FUNCTION
A generally accepted theory some decades ago was that the only function of goblet cells was to secrete mucins and that those mucins acted only in lubricating the ocular surface. Recent studies have demonstrated that goblet cells have more functions, and that they also produce more substances apart from mucins. Cytokine secretion has been described and an immunomodulatory function of conjunctival goblet cells has been identified based on their ability to modulate dendritic cell phenotype.11 Moreover, it has recently been proven that intestinal goblet cells play a role in presenting food antigens to dendritic cells12 which opens a new field of potential treatments against inflammatory bowel disease or celiac disease. Thus, we now know that goblet cells are much more than just mucin-secretory cells and that their mucins have more important functions than simply lubrication.
In fact, the ocular surface depends in part on the levels of mucins present in the tear film to keep its integrity, and in turn, this surface depends largely on goblet cell number and their rate of production and secretion of mucins. These mucins protect the ocular surface against desiccation, but also against pathogen access. Thus, conjunctival goblet cells are one of the first lines of defense of the ocular surface and the entire eye.13
Recent discoveries of unexpected functions of goblet cells makes it imperative to study further the physiology of these cells in both health and disease. However, goblet cells are slow-cycling cells, so it is difficult to culture and expand them in vitro. For that reason, few in vitro studies were performed until 10 years ago. In 2001, Shatos et al. published a method to culture primary goblet cells from rat conjunctiva.14 Later, in 2003, this technique was developed for human cells.15 Since then, the number of reported in vitro studies using goblet cell cultures has experienced a significant increase.
The lack of human tissue sometimes makes it difficult to advance the study of goblet cell pathophysiology. However, parallel studies using both rat and human cultured goblet cells have demonstrated that rat cells are a good model for the human ones.16,17 The main difference between rat and human goblet cells is distribution within the conjunctiva. Human goblet cells usually occur as single cells, mainly in the external layers of the epithelium, whereas rat cells are often associated in clusters. Regarding signaling pathways and cellular functions, results in the species are similar.
ROLE OF GOBLET CELLS IN OCULAR SURFACE DISEASE
As previously mentioned, goblet cells are altered in several diseases (Figure 2). While their specific role in pathologies affecting the gastrointestinal tract or the airway mucosa has been widely studied, the study of their function in ocular surface diseases is at an earlier stage.
Figure 2.
Conjunctival impression cytologies (CIC) obtained from different patients and stained with PAS. a) CIC from a patient with no alterations in conjunctiva. Goblet cells can be identified by the PAS staining, and are distributed along all the cytology. b) CIC from a patient with dry eye disease. No goblet cells were found in the CIC. c) CIC from a patient with allergic conjunctivitis showing goblet cell hyperplasia.
Ocular surface diseases are often associated with inflammation.18,19 Several cell types are involved in the inflammatory reaction. An important cellular participant is the T helper (Th) cell. Depending on the pattern of signals these cells receive, different types of Th cells develop, the most studied being Th1, Th2, Th17, and regulatory cells (Treg).20,21 Each Th cell subtype produces a specific profile of molecules that modulate the immune response. Cytokines are one of these immunomodulatory molecules, and are also classified as Th1, Th2, or Th17 cytokines, among others. The predominant Th response pattern varies between diseases.
From the wide range of pathologies affecting the ocular surface, the role of goblet cells has been most extensively studied in two main conditions dry eye and ocular allergy. These two diseases have been classically associated with different Th responses. Dry eye is a predominantly Th1-mediated disease22 and allergic diseases are typically Th2 inflammatory responses.23
Dry Eye Disease
Dry eye affects millions of people worldwide.24,25 In this inflammatory disease the lacrimal functional unit is altered.26 Increased levels of several cytokines, such as IFN-γ, TNF-α, or IL-6, along with goblet cell loss have been reported in this disease (Figure 2b). In fact, the lack of goblet cells has been the topic of numerous studies on dry eye.27–31
Ocular Allergic Diseases
Allergic diseases of the cornea and conjunctiva are typically associated with mucus hypersecretion. Higher goblet cell densities and/or hyperplasia are usually found in this type of disease (Figure 2c). Allergy has been classically associated with the presence of Th2 cytokines. They are not the only type of cytokines involved in these diseases, but Th2 cytokines, such as IL-4 or IL-13, are especially important in allergy. There are four types of allergic diseases affecting the eye, namely allergic conjunctivitis (seasonal or perennial), vernal keratoconjunctivitis (VKC), atopic keratoconjunctivitis (AKC), and giant papillary conjunctivitis (GPC).32 The inclusion of GPC in this group of allergic diseases is controversial because the pathophysiological features are quite different from the other diseases. For that reason, and because of its special relation with contact lenses, we will comment separately on this disease.
Giant Papillary Conjunctivitis
GPC is an adverse ocular reaction to contact lenses, and can occur with both soft and rigid contact lenses.33,34 Although it is predominantly associated with contact lenses, additional etiologies such as sutures, corneal foreign bodies, or prostheses have been reported.33,34 Common symptoms usually reported by GPC patients are decrease comfortable CL wearing time, excessive lens movement, foreign body sensation, and blurred vision.
The inclusion of GPC in the group of ocular surface allergy is controversial because this disease is a non-IgE mediated hypersensivity.35 The incorrect inclusion of GPC as an allergic disease was also pointed out by the mechanical theory that suggested an irritative and mechanical etiology for GPC, rather than an allergic etiology.36,37 This hypothesis is also supported by reported cases due to sutures or foreign bodies. For these reasons, GPC is now included in the group of non-allergic hypersensivity disorders.38 However, for many years, GPC was considered an allergic disease. This mistake was probably due to the clinical symptoms that are similar to those observed in allergic diseases: itching, tearing, mucous hyperproduction, and an increase of symptoms during spring pollen season.39 In addition, higher levels of IL-4 and IL-13 have also been found in this pathology. GPC is characterized by papillae on the upper tarsal conjunctiva. There is also a significantly greater number of inflammatory cells in the conjunctiva of these patients, especially in the epithelium.34
In summary, GPC is an ocular surface disease, presumably induced by contact lens wear, with mucous hyperproduction as one of its main symptoms. As we previously explained, the main mucous-producers cells in the ocular surface are goblet cells, so GPC links contact lens with goblet cell function.
CONTACT LENS WEAR AND GOBLET CELL FUNCTION
The influence that contact lenses have on the ocular surface mucous system started to be explored in the 1980s. The Allansmith group published several studies regarding non-goblet epithelial cells in human conjunctiva, and concluded that there were more secretory vesicles in non-goblet epithelial cells in contact lens users.40–42
An extensive review about contact lens wear and goblet cells was published in 2011 by Doughty.43 In that review, the author highlighted the contradictory results found in the literature regarding the effect of contact lens wear on conjunctival goblet cells. The majority of authors found a large decrease in goblet cell numbers in contact lens users,44,45 several others described the opposite,46,47 and two studies found no differences in goblet cell density.48,49 As Doughty explains in his review, the reason for this variability could be inconsistency in the methodologies used.
More recently, the Tear Film and Ocular Surface society (TFOS) International Workshop on Contact Lens Discomfort also summarized the changes found in goblet cell density induced by contact lens wear.50 As well as Doughty, they also remarked on the variations in the methodology as the potential cause for the diversity of results. Another possibility for the diverse results is that the number of goblet cells per unit area varies with location on the conjunctiva.51,52 If goblet cells are not sampled from the same area in each study participant, the results of a study are not valid. In addition, since the contact lens interacts differently with each area of the conjunctiva, if studies use different areas of the conjunctiva they could obtain diverse results. In fact, the TFOS study concluded that the location at which the cytology sample is obtained is likely the main reason of the variations in study results.
However, all the studies described in the previous paragraph focused on the effect of contact lens wear on goblet cell density, but not on other possible influences on goblet cell function. Surprisingly, the authors have not yet explained the reason for changes in goblet cell density. Interestingly, several recent studies have shown the effect of a variety of cytokines on goblet cells, and changes in cytokine levels (mainly increased or decreased) have been reported in contact lens wearers.53–57 Research investigating the effect of contact lens wear on goblet cell function is critically needed.
CYTOKINES AND CONJUNCTIVAL GOBLET CELL FUNCTION
Cytokines are immunomodulatory agents that are secreted by different types of cells. Every cell, except the red blood cell, can produce and respond to cytokines.58 In addition there is a wide range of cytokines, with different biochemical characteristics and biologic functions. However, all of them play an important role in cell signaling. As regulators of the immune response cytokines can exert pro-inflammatory actions, anti-inflammatory actions, or both. These compounds can be classified into different subtypes, depending on the type of T helper (Th) cell that produces them.20 The main subtypes are: Th1, Th2, and Th17.59
Th1-derived cytokines
Interferon gamma (IFN-γ)
IFN-γ is a key cytokine coordinating immune defense.60 It is probably the most studied cytokine in relation to conjunctival goblet cell function. The main reason for this focus is the clear implication of IFN-γ in dry eye disease, as well as the extensive interest in understanding this quality of life-threatening condition. Increased levels of IFN-γ in tears of dry eye patients have been reported by several authors.61,62
IFN-γ binds to its receptor IFN-γ-R to trigger a cell signaling cascade.60,63 The presence of IFN-γRs in goblet cells has been confirmed in C57/BL6 mice,64,65 and we have recently described it in cultured rat and human goblet cells (manuscript under review). The main signaling pathway used by IFN-γ is the JAK-STAT cascade. However, some other alternative pathways have been described.66
IFN-γ has an apoptotic effect on goblet cells.30,64,65 This would explain the loss of goblet cells typically found in dry eye patients. In addition to finding a decrease in goblet cell proliferation by IFN-γ, we have also demonstrated that the secretion of the remaining cells is also altered in the presence of IFN-γ when rat cells were investigated. We showed that this cytokine by itself induced an increase in [Ca2+]i, which relates to high molecular weight glycoconjugate secretion. In addition to increasing [Ca2+]i, IFN-γ blocked the effect of a cholinergic agonist on inducing goblet cell secretion in rat goblet cells.67 Similar results were obtained in cultured mouse goblet cells.65
Tumor Necrosis Factor (TNF) alpha
TNF-α is another Th1 cytokine that is also involved in dry eye disease. This cytokine is upregulated in conjunctival epithelial cells of patients with Sjögren’s syndrome-associated aqueous-deficient dry eye, but not in those with non-Sjögren’s syndrome.68 Due to its central role in the inflammatory state of dry eye disease, TNF-α blockers have been proposed as a treatment for dry eye disease.69
The effect of TNF-α on conjunctival goblet cells has not been extensively explored, but it has been shown that TNF-α inhibits MUC5AC secretion induced by cholinergic agonists and increases goblet cell apoptosis.65 These effects are similar to the ones exert by IFN-γ.
Th2-derived cytokines
Th2 cells are characterized by the production of IL-4, IL-5, and IL-13.70 These cytokines have a critical role in allergic diseases affecting the mucosa, such as asthma or allergic conjunctivitis.
Interleukin 4
The presence of IL-4 is essential for a naïve Th cell to become a Th2 cell. IL-4 is necessary for Th2 lymphocyte differentiation and it is later secreted by these Th2 cells.71 This pleiotropic cytokine binds to IL-4 receptors to activate signal transduction, mainly through Jak1 and Jak2 kinases. We have demonstrated the presence of IL-4 receptor in cultured human goblet cells.72
The effect of IL-4 on goblet cells has been studied in the airway epithelium.73 These authors showed that IL-4 induced the differentiation of epithelial cells into mucous-producing goblet cells, and that after IL-4 exposure there was an increase in glycoconjugate secretion. These experiments were performed in vitro with a human pulmonary mucoepidermoid cell line and in vivo with BALB/c mice. We showed results consistent with those of Dabbagh K. et al in rat cultured conjunctival goblet cells.67 We found that IL-4 increased conjunctival goblet cell proliferation by 1.94 fold over basal. In addition, intracellular [Ca2+] was also increased by IL-4 exposure. Intracellular [Ca2+] measurement after different stimuli is especially relevant in goblet cells, since it is directly related with mucin secretion.74,75
Interleukin 13
IL-13 is, along with IFN-γ, the most extensively studied cytokine in relation to conjunctival goblet cells. IL-13 is a small glycoprotein with a broad spectrum of actions, such as promotion of eosinophil migration, upregulation of adhesion molecules, and goblet cell differentiation and mucous hypersecretion. IL-13 induces mucin synthesis and hyperplasia in airway goblet cells.76 The role of this cytokine in asthma is well known, where it is a critical mediator in the pathology of this disease.77 In fact, a vaccine that neutralized endogenous IL-13 has been proposed as a therapy to attenuate allergic inflammation in asthma.78
In the eye, it has been demonstrated that IL-13 promotes conjunctival goblet cell differentiation and proliferation.65,79 These authors confirmed the expression of IL-13 receptor in mouse goblet cells, and we have done the same in human cultured goblet cells.72
Interleukin 5
IL-5 is an important cytokine in allergic processes. Its main role is to enhance eosinophilic accumulation and activation during allergen-induced inflammation.80 Although it has been extensively studied in allergy, where it is found elevated, the interaction of IL-5 with goblet cells is not clear. Lee et al. stated that IL-5 expression in the lung epithelium of mice leads to several pulmonary changes, including goblet cell hyperplasia.81 However, it is unclear if this hyperplasia is produced directly by IL-5, or if it is eosinophils-mediated.82,83
Th17-derived cytokines
The Th17 cell subtype was described in 2005.59,84–86 These cells act as regulators of the immune response and produce IL-17, IL-6, IL-21, or IL-22, among other cytokines.87
Interleukin 17
The prototypic member of the IL-17 family is IL-17A, a cytokine with high homology among mouse, rat, and human.88 It has been associated with ocular inflammatory diseases, such as uveitis, scleritis, and dry eye syndrome.89
IL-17 along with IL-6 is able to increase MUC5AC levels in primary human tracheobronquial epithelial cells.90 Regarding the conjunctiva, Contreras-Ruiz et al. have recently shown an increase in proliferation of cultured goblet cells when cells were exposed to IL-17A.65
Interleukin 6
IL-6 is another well studied Th17-type cytokine. IL-6 acts as an activator of the immune system, and is elevated in most inflammatory states.91 This cytokine is secreted mainly by monocytes/macrophages and epithelial cells, including conjunctival epithelial cells.92,93 Several authors have determined levels of IL-6 in tears.57,94,95 Boehm et al. described a 2.1 fold increase of IL-6 levels in dry eye patients compared to healthy controls. Others have studied expression levels of this cytokine on conjunctival epithelial cells.92,96 However, in most of these studies, goblet cells have not been analyzed.
Other cytokines
Recently, interest in the study of IL-22 and IL-33 in mucosal pathologies has increased.97–99 Turner et al. suggested a key role for IL-22 in intestinal goblet cells, showing IL-22-induced mucin secretion and goblet cell activation.99 Luzina et al. reported that full-length IL-33 isoforms induced goblet cell hyperplasia in lungs97 and Tanabe et al. showed that IL-33 acts directly on goblet cells stimulating IL-8 secretion, what may maintain mucus hypersecretion in asthma patients.98 However, the effect of these cytokines on conjunctival goblet cells is still unexplored. The similarity previously found in goblet cells among different mucosa suggests that IL-33 would probably play an important role in the ocular surface that still needs to be discovered.
SUMMARY
Goblet cells have been studied in several diseases due to their mucin-secreting capacity. It is known that the number of these cells is lower in dry eye diseases, and that higher goblet cell densities are found in allergic diseases. The cytokine balances in these two diseases is also opposite. While a Th1 profile predominates in dry eye disease with special involvement of the Th1-cytokine IFN-γ, a Th2 profile predominates in allergic diseases that are associated with mucous hyperproduction. Recent findings on the effect of cytokines on goblet cells have shown that Th1 cytokines have pro-apoptotic effects on goblet cells, whereas Th2 cytokines promote goblet cell proliferation. A summary of the effect that cytokines have on conjunctival goblet cells is shown in Table 1.
TABLE 1.
Summary of cytokines effect on conjunctival goblet cells.
| Cytokine | Effect | Specie | Authors |
|---|---|---|---|
| IFN-γ | ↑ apoptosis | Mouse | Zhang et al. (2011, 2014)64, 30; Contreras-Ruiz et al. (2013)65 |
| ↓ proliferation | Rat | García-Posadas et al. (ARVO 2013)67 | |
| ↑ secretion | Rat | García-Posadas et al. (ARVO 2013)67 | |
| TNF-α | ↑ apoptosis | Mouse | Contreras-Ruiz et al. (2013)65 |
| IL-4 | ↑ proliferation | Rat; Human | García-Posadas et al. (ARVO 2013, 2014)67, 72 |
| ↑ secretion | Rat; Human | García-Posadas et al. (ARVO 2013, 2014)67, 72 | |
| IL-13 | ↑ proliferation | Mouse | De Paiva et al. (2011)79; Contreras-Ruiz et al. (2013)65 |
| ↑ GC differentiation | Mouse | De Paiva et al. (2011)79 | |
| IL-17 | ↑ proliferation | Mouse | Contreras-Ruiz et al. (2013)65 |
| IL-6 | ↑ proliferation | Mouse | Contreras-Ruiz et al. (2013)65 |
Interestingly, several authors have studied goblet cell density in contact lens wearers. Most of studies have found a decrease in goblet cell number in these patients. However, the biological processes that lead to this decrease are not fully understood.
CONCLUSIONS
Recent studies about the influence of cytokines on goblet cells open a new field of research. It is important to observe how different types of Th cytokines seem to have opposite effects on conjunctival goblet cells, especially when comparing Th1 versus Th2-derived cytokines.
Goblet cells are becoming increasingly more important in the study of all types of pathologies affecting the ocular surface, from dry eye to allergies. It is surprising that, until now, most clinical studies regarding goblet cells were only focused on goblet cell number, but not on goblet cell function. The latest advances in this area are revealing previously unknown functions of these important cells and we know now that it is not only an issue of numbers.
Figure 3.
Schematic illustrating the effect of cytokines on goblet cells in dry eye disease and ocular allergy.
ACKNOWLEDGEMENTS
Authors thank José Carlos López and Nieves Fernández, from the IOBA Ocular Pathology Laboratory, for providing CIC pictures, and Inmaculada Pérez for her help in the obtaining of in vivo laser scanning confocal microscopy images.
Financial support: FPI Scholarship Program BES-2011-046381 and CICYT Grant MAT2010-20452-C03-01(Ministry of Science and Innovation, Spain), NIH R01 EY019470.
REFERENCES
- 1.Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49. doi: 10.1016/s0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- 2.Gipson IK. The ocular surface: The challenge to enable and protect vision: The friedenwald lecture. Invest Ophthalmol Vis Sci. 2007;48(10):4390, 4391–4398. doi: 10.1167/iovs.07-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dartt DA. Duane's foundations of clinical ophthalmology 2. Philadelphia: Lippincott Williams & Wilkins; 2006. The conjunctiva - structure and function. [Google Scholar]
- 4.Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008;8(5):477–483. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodges RR, Dartt DA. Tear film mucins: Front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp Eye Res. 2013;117:62–78. doi: 10.1016/j.exer.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calonge M, Diebold Y, Saez V, et al. Impression cytology of the ocular surface: A review. Exp Eye Res. 2004;78(3):457–472. doi: 10.1016/j.exer.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Krenzer KL, Freddo TF. Cytokeratin expression in normal human bulbar conjunctiva obtained by impression cytology. Invest Ophthalmol Vis Sci. 1997;38(1):142–152. [PubMed] [Google Scholar]
- 8.Moore JE, Vasey GT, Dartt DA, et al. Effect of tear hyperosmolarity and signs of clinical ocular surface pathology upon conjunctival goblet cell function in the human ocular surface. Invest Ophthalmol Vis Sci. 2011;52(9):6174–6180. doi: 10.1167/iovs.10-7022. [DOI] [PubMed] [Google Scholar]
- 9.Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26(4):398–436. doi: 10.1016/j.preteyeres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Mastropasqua L, Agnifili L, Fasanella V, et al. Conjunctival goblet cells density and preservative-free tafluprost therapy for glaucoma: An in vivo confocal microscopy and impression cytology study. Acta Ophthalmol. 2013;91(5):e397–e405. doi: 10.1111/aos.12131. [DOI] [PubMed] [Google Scholar]
- 11.Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PloS one. doi: 10.1371/journal.pone.0120284. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDole JR, Wheeler LW, McDonald KG, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483(7389):345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saban DR, Calder V, Kuo CH, et al. New twists to an old story: Novel concepts in the pathogenesis of allergic eye disease. Curr Eye Res. 2013;38(3):317–330. doi: 10.3109/02713683.2012.747617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shatos MA, Rios JD, Tepavcevic V, Kano H, Hodges R, Dartt DA. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest Ophthalmol Vis Sci. 2001;42(7):1455–1464. [PubMed] [Google Scholar]
- 15.Shatos MA, Rios JD, Horikawa Y, et al. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44(6):2477–2486. doi: 10.1167/iovs.02-0550. [DOI] [PubMed] [Google Scholar]
- 16.Horikawa Y, Shatos MA, Hodges RR, et al. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44(6):2535–2544. doi: 10.1167/iovs.02-1117. [DOI] [PubMed] [Google Scholar]
- 17.Rios JD, Ghinelli E, Gu J, Hodges RR, Dartt DA. Role of neurotrophins and neurotrophin receptors in rat conjunctival goblet cell secretion and proliferation. Invest Ophthalmol Vis Sci. 2007;48(4):1543–1551. doi: 10.1167/iovs.06-1226. [DOI] [PubMed] [Google Scholar]
- 18.Ueta M, Kinoshita S. Ocular surface inflammation mediated by innate immunity. Eye Contact Lens. 2010;36(5):269–281. doi: 10.1097/ICL.0b013e3181ee8971. [DOI] [PubMed] [Google Scholar]
- 19.Ueta M, Kinoshita S. Ocular surface inflammation is regulated by innate immunity. Prog Retin Eye Res. 2012;31(6):551–575. doi: 10.1016/j.preteyeres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13(2):139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120(2):247–254. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 22.El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009;50(8):3802–3807. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kool M, Hammad H, Lambrecht BN. Cellular networks controlling Th2 polarization in allergy and immunity. F1000 Biol Rep. 2012;4:6-6. doi: 10.3410/B4-6. Epub 2012 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 25.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: Estimates from the physicians' health studies. Arch Ophthalmol. 2009;127(6):763–768. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78(3):409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Li N, Deng X, Gao Y, Zhang S, He M, Zhao D. Establishment of the mild, moderate and severe dry eye models using three methods in rabbits. BMC Ophthalmol. 2013;13 doi: 10.1186/1471-2415-13-50. 50-2415-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantelli F, Massaro-Giordano M, Macchi I, Lambiase A, Bonini S. The cellular mechanisms of dry eye: From pathogenesis to treatment. J Cell Physiol. 2013;228(12):2253–2256. doi: 10.1002/jcp.24398. [DOI] [PubMed] [Google Scholar]
- 29.Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol. 2013;183(1):35–48. doi: 10.1016/j.ajpath.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, De Paiva CS, Su Z, Volpe EA, Li DQ, Pflugfelder SC. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp Eye Res. 2014;118:117–124. doi: 10.1016/j.exer.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar P, Bhargava R, Kumar M, Ranjan S, Kumar M, Verma P. The correlation of routine tear function tests and conjunctival impression cytology in dry eye syndrome. Korean J Ophthalmol. 2014;28(2):122–129. doi: 10.3341/kjo.2014.28.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono SJ, Abelson MB. Allergic conjunctivitis: Update on pathophysiology and prospects for future treatment. J Allergy Clin Immunol. 2005;115(1):118–122. doi: 10.1016/j.jaci.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 33.Donshik PC. Contact lens chemistry and giant papillary conjunctivitis. Eye Contact Lens. 2003;29(1 Suppl):S37–S39. doi: 10.1097/00140068-200301001-00011. discussion S57-9, S192-4. [DOI] [PubMed] [Google Scholar]
- 34.Elhers WH, Donshik PC. Giant papillary conjunctivitis. Curr Opin Allergy Clin Immunol. 2008;8(5):445–449. doi: 10.1097/ACI.0b013e32830e6af0. [DOI] [PubMed] [Google Scholar]
- 35.Leonardi A. Allergy and allergic mediators in tears. Exp Eye Res. 2013;117:106–117. doi: 10.1016/j.exer.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Friedlaender MH. Some unusual nonallergic causes of giant papillary conjunctivitis. Trans Am Ophthalmol Soc. 1990;88:343–349. discussion 349–51. [PMC free article] [PubMed] [Google Scholar]
- 37.Lin MC, Yeh TN. Mechanical complications induced by silicone hydrogel contact lenses. Eye Contact Lens. 2013;39(1):115–124. doi: 10.1097/ICL.0b013e31827c77fd. [DOI] [PubMed] [Google Scholar]
- 38.Leonardi A, Bogacka E, Fauquert JL, et al. Ocular allergy: Recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy. 2012;67(11):1327–1337. doi: 10.1111/all.12009. [DOI] [PubMed] [Google Scholar]
- 39.Bielory L. Allergic and immunologic disorders of the eye. part II: Ocular allergy. J Allergy Clin Immunol. 2000;106(6):1019–1032. doi: 10.1067/mai.2000.111238. [DOI] [PubMed] [Google Scholar]
- 40.Greiner JV, Kenyon KR, Henriquez AS, Korb DR, Weidman TA, Allansmith MR. Mucus secretory vesicles in conjunctival epithelial cells of wearers of contact lenses. Arch Ophthalmol. 1980;98(10):1843–1846. doi: 10.1001/archopht.1980.01020040695020. [DOI] [PubMed] [Google Scholar]
- 41.Greiner JV, Allansmith MR. Effect of contact lens wear on the conjunctival mucous system. Ophthalmology. 1981;88(8):821–832. doi: 10.1016/s0161-6420(81)34942-2. [DOI] [PubMed] [Google Scholar]
- 42.Greiner JV, Weidman TA, Korb DR, Allansmith MR. Histochemical analysis of secretory vesicles in nongoblet conjunctival epithelial cells. Acta Ophthalmol (Copenh) 1985;63(1):89–92. doi: 10.1111/j.1755-3768.1985.tb05222.x. [DOI] [PubMed] [Google Scholar]
- 43.Doughty MJ. Contact lens wear and the goblet cells of the human conjunctiva-A review. Cont Lens Anterior Eye. 2011;34(4):157–163. doi: 10.1016/j.clae.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Knop E, Brewitt H. Induction of conjunctival epithelial alterations by contact lens wearing. A prospective study. Ger J Ophthalmol. 1992;1(3–4):125–134. [PubMed] [Google Scholar]
- 45.Cakmak SS, Unlu MK, Karaca C, Nergiz Y, Ipek S. Effects of soft contact lenses on conjunctival surface. Eye Contact Lens. 2003;29(4):230–233. doi: 10.1097/01.icl.0000086492.28723.0C. [DOI] [PubMed] [Google Scholar]
- 46.Connor CG, Campbell JB, Steel SA, Burke JH. The effects of daily wear contact lenses on goblet cell density. J Am Optom Assoc. 1994;65(11):792–794. [PubMed] [Google Scholar]
- 47.Lievens CW, Connor CG, Murphy H. Comparing goblet cell densities in patients wearing disposable hydrogel contact lenses versus silicone hydrogel contact lenses in an extended-wear modality. Eye Contact Lens. 2003;29(4):241–244. doi: 10.1097/01.icl.0000090884.26379.7D. [DOI] [PubMed] [Google Scholar]
- 48.Pisella PJ, Malet F, Lejeune S, et al. Ocular surface changes induced by contact lens wear. Cornea. 2001;20(8):820–825. doi: 10.1097/00003226-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Hori Y, Argueso P, Spurr-Michaud S, Gipson IK. Mucins and contact lens wear. Cornea. 2006;25(2):176–181. doi: 10.1097/01.ico.0000177838.38873.2f. [DOI] [PubMed] [Google Scholar]
- 50.Efron N, Jones L, Bron AJ, et al. The TFOS international workshop on contact lens discomfort: Report of the contact lens interactions with the ocular surface and adnexa subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS98–TFOS122. doi: 10.1167/iovs.13-13187. [DOI] [PubMed] [Google Scholar]
- 51.Doughty MJ. Goblet cells of the normal human bulbar conjunctiva and their assessment by impression cytology sampling. Ocul Surf. 2012;10(3):149–169. doi: 10.1016/j.jtos.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Doughty MJ. Sampling area selection for the assessment of goblet cell density from conjunctival impression cytology specimens. Eye Contact Lens. 2012;38(2):122–129. doi: 10.1097/ICL.0b013e3182480eb1. [DOI] [PubMed] [Google Scholar]
- 53.Tan M, Thakur A, Morris C, Willcox MD. Presence of inflammatory mediators in the tears of contact lens wearers and non-contact lens wearers. Aust N Z J Ophthalmol. 1997;25(Suppl 1):S27–S29. doi: 10.1111/j.1442-9071.1997.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 54.Schultz CL, Kunert KS. Interleukin-6 levels in tears of contact lens wearers. J Interferon Cytokine Res. 2000;20(3):309–310. doi: 10.1089/107999000312441. [DOI] [PubMed] [Google Scholar]
- 55.Thakur A, Willcox MD. Contact lens wear alters the production of certain inflammatory mediators in tears. Exp Eye Res. 2000;70(3):255–259. doi: 10.1006/exer.1999.0767. [DOI] [PubMed] [Google Scholar]
- 56.Dogru M, Ward SK, Wakamatsu T, et al. The effects of 2 week senofilcon-A silicone hydrogel contact lens daily wear on tear functions and ocular surface health status. Cont Lens Anterior Eye. 2011;34(2):77–82. doi: 10.1016/j.clae.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Poyraz C, Irkec M, Mocan MC. Elevated tear interleukin-6 and interleukin-8 levels associated with silicone hydrogel and conventional hydrogel contact lens wear. Eye Contact Lens. 2012;38(3):146–149. doi: 10.1097/ICL.0b013e3182482910. [DOI] [PubMed] [Google Scholar]
- 58.Dinarello CA. Historical insights into cytokines. Eur J Immunol. 2007;37(Suppl 1):S34–S45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13(6):668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 61.Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28(9):1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 62.Lee SY, Han SJ, Nam SM, et al. Analysis of tear cytokines and clinical correlations in sjogren syndrome dry eye patients and non-sjogren syndrome dry eye patients. Am J Ophthalmol. 2013;156(2):247.e1–253.e1. doi: 10.1016/j.ajo.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Chen W, De Paiva CS, et al. Interferon-gamma exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Invest Ophthalmol Vis Sci. 2011;52(9):6279–6285. doi: 10.1167/iovs.10-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Contreras-Ruiz L, Ghosh-Mitra A, Shatos MA, Dartt DA, Masli S. Modulation of conjunctival goblet cell function by inflammatory cytokines. Mediators Inflamm. 2013;2013:636812. doi: 10.1155/2013/636812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gough DJ, Levy DE, Johnstone RW, Clarke CJ. IFNgamma signaling-does it mean JAK-STAT? Cytokine Growth Factor Rev. 2008;19(5–6):383–394. doi: 10.1016/j.cytogfr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Posadas L, Li D, Hodges RR, Shatos MA, Diebold Y, Dartt DA. Differential effect of Th1- and Th2-type cytokines on rat conjunctival goblet cell function. Invest. Ophthalmol. Vis. Sci. 2013;54 E-Abstract 972. [Google Scholar]
- 68.Caffery BE, Joyce E, Heynen ML, Ritter R, 3rd, Jones LA, Senchyna M. Quantification of conjunctival TNF-alpha in aqueous-deficient dry eye. Optom Vis Sci. 2014;91(2):156–162. doi: 10.1097/OPX.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 69.Ji YW, Byun YJ, Choi W, et al. Neutralization of ocular surface TNF-alpha reduces ocular surface and lacrimal gland inflammation induced by in vivo dry eye. Invest Ophthalmol Vis Sci. 2013;54(12):7557–7566. doi: 10.1167/iovs.12-11515. [DOI] [PubMed] [Google Scholar]
- 70.Li Z, Zhang Y, Sun B. Current understanding of Th2 cell differentiation and function. Protein Cell. 2011;2(8):604–611. doi: 10.1007/s13238-011-1083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 72.Garcia-Posadas L, Li D, Hodges RR, Shatos MA, Diebold Y, Dartt DA. Human goblet cell function in an in vitro allergic microenvironment. Invest. Ophthalmol. Vis. Sci. 2014;55 E-Abstract 2579. [Google Scholar]
- 73.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol. 1999;162(10):6233–6237. [PubMed] [Google Scholar]
- 74.Dartt DA. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog Retin Eye Res. 2002;21(6):555–576. doi: 10.1016/s1350-9462(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 75.Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004;78(2):173–185. doi: 10.1016/j.exer.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Rogers DF. The airway goblet cell. Int J Biochem Cell Biol. 2003;35(1):1–6. doi: 10.1016/s1357-2725(02)00083-3. [DOI] [PubMed] [Google Scholar]
- 77.Corren J. Role of interleukin-13 in asthma. Curr Allergy Asthma Rep. 2013;13(5):415–420. doi: 10.1007/s11882-013-0373-9. [DOI] [PubMed] [Google Scholar]
- 78.Jing Q, Yin T, Wan Y, et al. Interleukin-13 peptide kinoid vaccination attenuates allergic inflammation in a mouse model of asthma. Int J Mol Med. 2012;30(3):553–560. doi: 10.3892/ijmm.2012.1036. [DOI] [PubMed] [Google Scholar]
- 79.De Paiva CS, Raince JK, McClellan AJ, et al. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011;4(4):397–408. doi: 10.1038/mi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamelmann E, Gelfand EW. IL-5-induced airway eosinophilia--the key to asthma? Immunol Rev. 2001;179:182–191. doi: 10.1034/j.1600-065x.2001.790118.x. [DOI] [PubMed] [Google Scholar]
- 81.Lee JJ, McGarry MP, Farmer SC, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185(12):2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cohn L, Homer RJ, MacLeod H, Mohrs M, Brombacher F, Bottomly K. Th2-induced airway mucus production is dependent on IL-4Ralpha, but not on eosinophils. J Immunol. 1999;162(10):6178–6183. [PubMed] [Google Scholar]
- 83.Burgel PR, Lazarus SC, Tam DC, et al. Human eosinophils induce mucin production in airway epithelial cells via epidermal growth factor receptor activation. J Immunol. 2001;167(10):5948–5954. doi: 10.4049/jimmunol.167.10.5948. [DOI] [PubMed] [Google Scholar]
- 84.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 87.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: Multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133(3):621–631. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 88.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 89.Kang MH, Kim MK, Lee HJ, Lee HI, Wee WR, Lee JH. Interleukin-17 in various ocular surface inflammatory diseases. J Korean Med Sci. 2011;26(7):938–944. doi: 10.3346/jkms.2011.26.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278(19):17036–17043. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 91.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 92.Enriquez-de-Salamanca A, Calder V, Gao J, et al. Cytokine responses by conjunctival epithelial cells: An in vitro model of ocular inflammation. Cytokine. 2008;44(1):160–167. doi: 10.1016/j.cyto.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 93.Garcia-Posadas L, Arranz-Valsero I, Lopez-Garcia A, Soriano-Romani L, Diebold Y. A new human primary epithelial cell culture model to study conjunctival inflammation. Invest Ophthalmol Vis Sci. 2013;54(10):7143–7152. doi: 10.1167/iovs.13-12866. [DOI] [PubMed] [Google Scholar]
- 94.Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–873. [PMC free article] [PubMed] [Google Scholar]
- 95.Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol Vis Sci. 2011;52(10):7725–7730. doi: 10.1167/iovs.11-7266. [DOI] [PubMed] [Google Scholar]
- 96.Turner K, Pflugfelder SC, Ji Z, Feuer WJ, Stern M, Reis BL. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea. 2000;19(4):492–496. doi: 10.1097/00003226-200007000-00018. [DOI] [PubMed] [Google Scholar]
- 97.Luzina IG, Pickering EM, Kopach P, et al. Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion. J Immunol. 2012;189(1):403–410. doi: 10.4049/jimmunol.1200259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tanabe T, Shimokawaji T, Kanoh S, Rubin BK. IL-33 stimulates CXCL8/IL-8 secretion in goblet cells but not normally differentiated airway cells. Clin Exp Allergy. 2014;44(4):540–552. doi: 10.1111/cea.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Turner JE, Stockinger B, Helmby H. IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog. 2013;9(10):e1003698. doi: 10.1371/journal.ppat.1003698. [DOI] [PMC free article] [PubMed] [Google Scholar]