Abstract
Objective:
To report 14 patients with immune-mediated relapsing symptoms post–herpes simplex encephalitis (HSE) and to compare the clinical and immunologic features of the teenage and adult group with those of young children.
Methods:
Prospective observational study of patients diagnosed between June 2013 and February 2015. Immunologic techniques have been reported previously.
Results:
Among the teenage and adult group (8 patients, median age 40 years, range 13–69; 5 male), 3 had an acute symptom presentation suggesting a viral relapse, and 5 a presentation contiguous with HSE suggesting a recrudescence of previous deficits. Seven patients developed severe psychiatric/behavioral symptoms disrupting all social interactions, and one refractory status epilepticus. Blepharospasm occurred in one patient. Five patients had CSF antibodies against NMDA receptor (NMDAR) and 3 against unknown neuronal cell surface proteins. In 5/6 patients, the brain MRI showed new areas of contrast enhancement that decreased after immunotherapy and clinical improvement. Immunotherapy was useful in 7/7 patients, sometimes with impressive recoveries, returning to their baseline HSE residual deficits. Compared with the 6 younger children (median age 13 months, range 6–20, all with NMDAR antibodies), the teenagers and adults were less likely to develop choreoathetosis (0/8 vs 6/6, p < 0.01) and decreased level of consciousness (2/8 vs 6/6, p < 0.01) and had longer delays in diagnosis and treatment (interval relapse/antibody testing 85 days, range 17–296, vs 4 days, range 0–33, p = 0.037).
Conclusion:
In teenagers and adults, the immune-mediated relapsing syndrome post-HSE is different from that known in young children as choreoathetosis post-HSE and is underrecognized. Prompt diagnosis is important because immunotherapy can be highly effective.
Herpes simplex virus (HSV) encephalitis (HSE) is a frequent cause of severe, potentially fatal encephalitis among children and adults worldwide. The disease usually follows a monophasic course but 12%–27% of the patients develop relapsing neurologic symptoms a few weeks after the CSF viral studies become negative and the treatment with acyclovir has been discontinued.1–3 Most of these patients are children who develop an encephalopathy with abnormal movements named choreoathetosis post-HSE or relapsing symptoms post-HSE.4 The hypothesis that the disorder is immune-mediated has received strong support by the recent discovery that many of these patients develop immunoglobulin G (IgG) antibodies against the GluN1 subunit of the NMDA receptor (NMDAR)5–11 and sometimes to other known7 or unknown synaptic proteins.6 This clinical complication is less well-known in adults and teenagers, suggesting a lower frequency in these age groups or a different and less recognizable syndrome. Over the last 21 months, we have prospectively identified 14 new patients with relapsing symptoms post-HSE, 8 of them adults or teenagers. In the current study, we show that the clinical picture of these patients is indeed different from that of young children with choreoathetosis, leading to delays in diagnosis and treatment. Prompt recognition of this disorder is important because immunotherapy is effective in reducing the burden of the immune-mediated deficits and improving the quality of life of patients and families.
METHODS
From June 2013 until February 2015, serum and CSF of 14 patients with nonviral relapsing symptoms post-HSE were prospectively studied at the Institute of Biomedical Research August Pi i Sunyer (IDIBAPS), Hospital Clinic, University of Barcelona. Five of the patients (patients 3, 5, 8–10) were examined as part of a 2-year multicenter prospective Spanish study in which all patients with HSE are clinically and immunologically followed after being diagnosed with HSE. The other 9 patients were diagnosed either before this multicenter HSE study was initiated (January 1, 2014, patients 1, 4, 12) or at centers that do not participate in the study (2 from Spain, 4 from other countries). In the prospective multicenter study, physicians are blinded to the immunologic findings unless patients develop relapsing symptoms. Of 20 patients with HSE (6 children and 14 adults) enrolled to date, the indicated 5 cases (25%) have developed relapsing neurologic symptoms post-HSE.
CSF or serum of all patients were extensively examined for antibodies to cell surface/synaptic proteins, including NMDA, mGluR5, AMPA, GABAB, GABAA, D2 receptors, LGI1, Caspr2, and DPPX, using previously reported techniques that included tissue immunohistochemistry, live cultured neurons, and cell-based assays.12–16 All patients underwent repeat CSF PCR for HSV and MRI of the brain (9 with contrast and 5 without). Clinical information was obtained by the authors or from referring physicians. No data of any patient have been reported previously.
Standard protocol approvals, registrations, and patient consents.
Written informed consent for participating in the study was obtained from all patients or guardians of patients. Studies were approved by the internal review board of Hospital Clinic-IDIBAPS and the ethical standards committee on human experimentation of IDIBAPS.
Statistical analysis.
Comparative analyses between the group of teenagers and adults and the group of children were performed with STATA version 13.1 (StataCorp, College Station, TX), using Fisher exact test, χ2 test, or Mann-Whitney U test when appropriate. The Wilcoxon rank-sum test was used for comparative studies between the CSF obtained during the stage of viral encephalitis and that obtained during the autoimmune relapse.
RESULTS
Eight of the 14 patients with nonviral relapsing neurologic symptoms post-HSE were adults or teenagers (median age 40 years, range 13–69; 5 male) and the other 6 were young children (median age 13 months, range 6–20 months; 3 male). Repeat CSF PCR for HSV was negative in all patients. The 6 young children developed a classical syndrome of choreoathetosis post-HSE in association with IgG antibodies against the GluN1 subunit of the NMDAR and one of them also had antibodies against the GABAAR (figure e-1 on the Neurology® Web site at Neurology.org). In many respects, these children were clinically similar to previously reported cases and are not the focus of this study (see information in table e-1, patients 9–14). In contrast, the 8 patients in the teenage and adult group did not develop choreoathetosis and are the main focus of this report (table 1, patients 1–8, and clinical vignettes in supplemental material).
Table 1.
Clinical features of adults and teenagers with autoimmune relapsing symptoms post–herpes simplex encephalitis
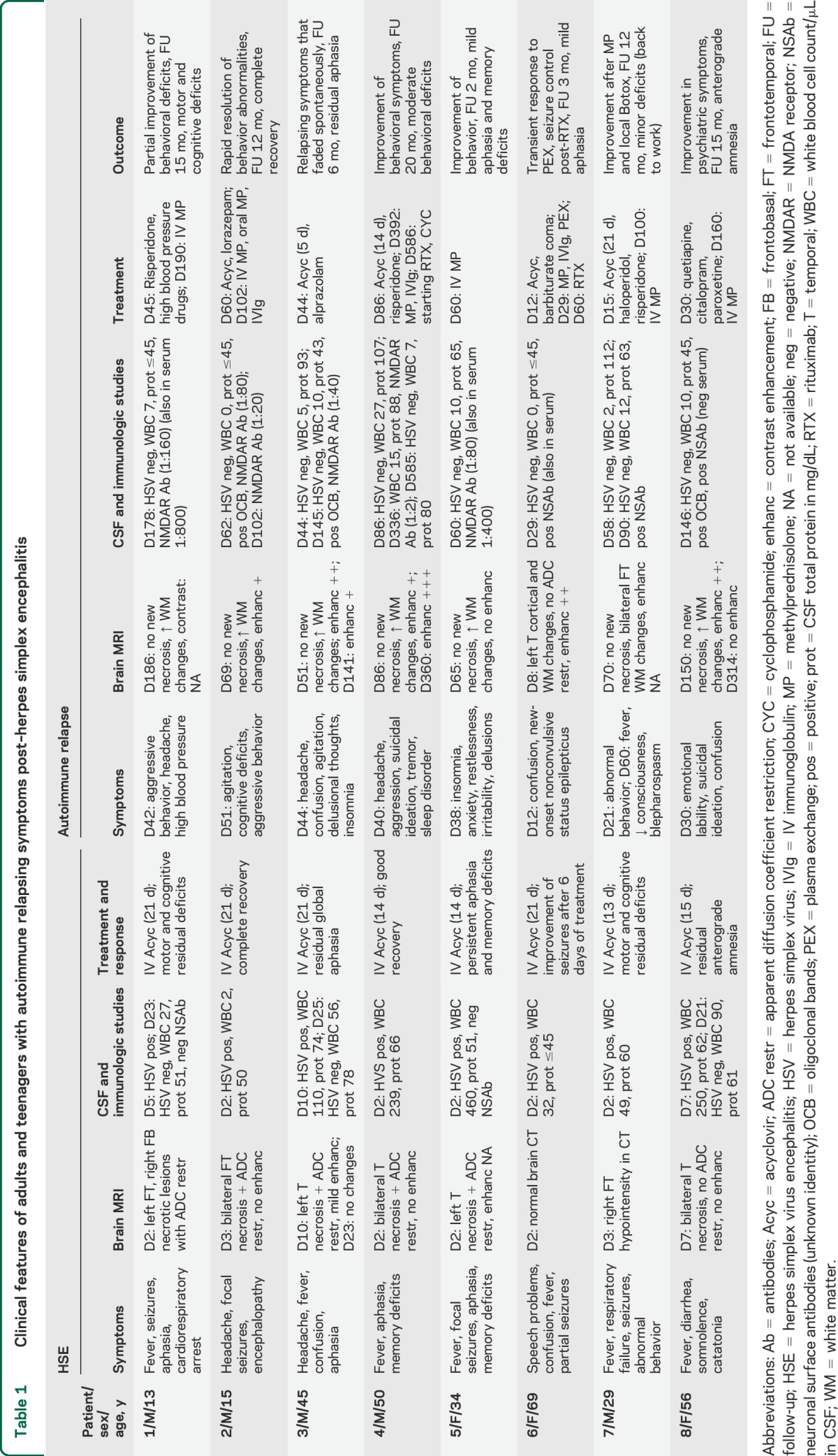
In these 8 patients, the onset of relapsing symptoms started 12–51 days (median 39, interquartile range [IQR] 26–43 days) after onset of HSE. In 3 patients (2–4), symptoms presented acutely, mimicking a viral relapse (biphasic course), and all 3 patients were restarted on acyclovir along with antipsychotic drugs or benzodiazepines while waiting for the results of repeat CSF PCR studies. In the other 5 patients (1, 5–8), the symptoms developed while recovering in rehabilitation centers or at home, or in contiguity with those of HSE, without a clear biphasic stage, and none of the patients was initially considered to have relapsing symptoms. In these patients, the symptoms were initially attributed to a recrudescence of residual viral-related deficits and managed with antipsychotics and antidepressants. The severity and persistence of neuropsychiatric symptoms eventually led to reconsideration of the possibility of an independent complication. Three of these patients (3, 5, and 8) were diagnosed with immune-mediated symptoms when they returned for a routine outpatient visit as part of the prospective study.
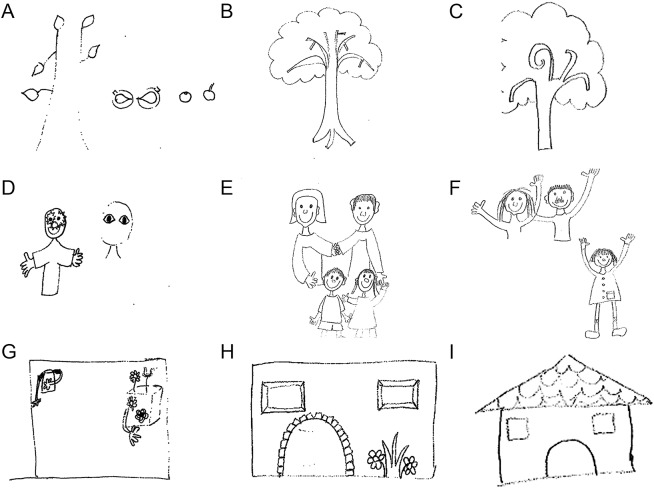
Seven patients presented with acute or subacute change of behavior, agitation, aggression, suicidal ideation, confusion, or delusional thoughts, and one patient with refractory seizures and status epilepticus requiring mechanical ventilation and barbiturate coma (patient 6). An example of the severe alteration of mental functions and imaginary graphic representation is shown in the drawings of patient 8 (figure 1). In 3 patients (patients 1, 3, and 4), the neuropsychiatric manifestations were heralded by intense headache, accompanied in one case by drug-resistant high blood pressure (table 1, and case vignettes in supplemental data). In another patient (patient 7), the change of behavior was followed a few days later by fever, decreased level of consciousness, and severe blepharospasm. Except for this case, no abnormal movements were identified in the other patients.
Figure 1. Drawings by patient 8 at presentation of relapsing symptoms post–herpes simplex virus encephalitis and after immunotherapy.
Drawings by patient 8 at the time of relapsing symptoms (tree, family, and house, A, D, and G), 3 weeks after immunotherapy (B, E, H), and at a 6 months follow-up (C, F, I). At presentation of relapsing symptoms, the patient had severe anterograde amnesia, confusion, disorganized thoughts, and disorientation to place, time, and person. After immunotherapy, her symptoms resolved except for amnesia and temporal orientation.
The median time between onset of relapsing symptoms and CSF routine and viral studies was 30 days (IQR 14–81, range 0–136), and for antibody testing 85 days (IQR 37–126, range 17–296). The PCR for HSV was negative in all 8 patients. Five patients had pleocytosis (median 10 leukocytes, IQR 7–10, range 5–27) and 4 had increased protein concentration (median 100 mg/dL, IQR 79–110). The level of pleocytosis was substantially lower than that previously found during the viral encephalitis (median 80 leukocytes, IQR 30–245, range 2–460, p < 0.01). Patients 1–5 had IgG antibodies against the GluN1 subunit of the NMDAR (all 5 in CSF, 2 also in serum; figure 2 and figure e-2); patients 6–8 had antibodies against unknown neuronal antigens (all 3 in CSF, 1 also in serum). Archived CSF and serum obtained at the time of the HSE were available in 4 patients (patients 1, 3, 5, and 7) and all were negative for NMDAR or other autoantibodies (figure e-2).
Figure 2. Demonstration of brain autoantibodies in a patient with autoimmune relapse post–herpes simplex virus encephalitis.
Consecutive sections of rat brain immunostained with CSF of a participant without NMDA receptor (NMDAR) antibodies (A, negative control), a patient with classical anti-NMDAR encephalitis (B, positive control), and the CSF of patient 9 by the time of herpes simplex virus encephalitis (C) and on day 19 when relapsing neurologic symptoms due to autoimmune encephalitis occurred (D). The CSF of patient 9 shows a pattern of antibody reactivity typical of NMDAR but superimposed with diffuse background staining (compare B with D), likely representing disruption of the blood–brain barrier or additional antibodies against other autoantigens (targets unknown). A similar background staining was noted in the CSF of the other patients; this dirty background usually clears up during CSF follow-up studies and eventually disappears (e.g., the reactivity becomes clear and indistinguishable from that seen in B; data not shown). In B and D, the presence of NMDAR antibodies was confirmed with cell-based assay (not shown). Bar = 500 μm.
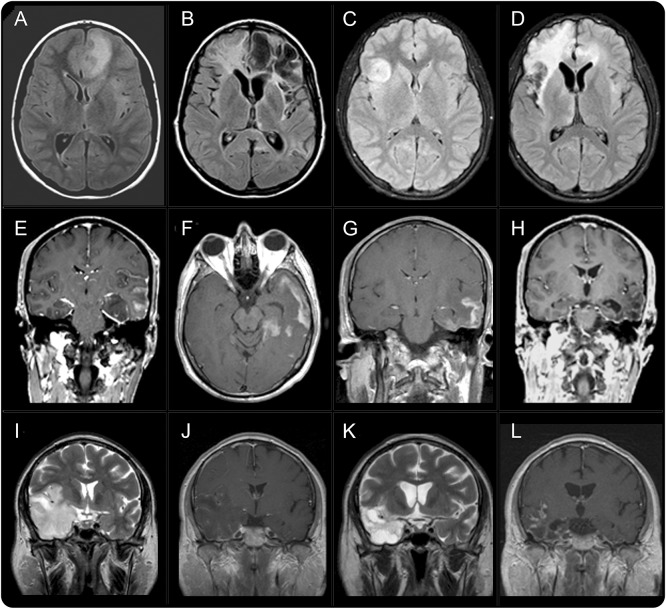
All 8 patients underwent brain MRI; 6 had prior MRI studies obtained by the time of HSE, and the other 2 had CT scans (patients 6 and 7). In the 6 patients with repeat MRI, this showed mild to moderate interval progression of T2/fluid-attenuated inversion recovery (FLAIR) abnormalities compared with that obtained during HSE (figure 3, A–D). Gadolinium was used in 6 of the 8 patients at symptom relapse, showing in 5 contrast enhancement (4 intense, 1 mild) in the same areas with T2/FLAIR abnormalities (figure 3, F, G, and L). In 4 cases, the findings could be compared with those of the MRI obtained during HSE, which showed absence or mild enhancement. Several additional follow-up MRIs were obtained in 3 patients; 2 of them showed dramatic reduction or absence of contrast enhancement after clinical improvement (patients 3 and 8, figure 3H), and the third patient, who had not received immunotherapy and continued with severe deficits, showed persistent contrast enhancement 1 year after onset of relapsing symptoms (patient 4, figure 3L).
Figure 3. MRI findings in patients with relapsing symptoms post–herpes simplex encephalitis.
Axial fluid-attenuated inversion recovery sequences of patients 1 (A, B) and 2 (C, D) during herpes simplex virus encephalitis (HSE) (A, C) and during relapsing symptoms due to autoimmune encephalitis (B, D). In both cases, there is an interval change due to areas of encephalomalacia, brain atrophy, and white matter changes. Panels E–H correspond to T1 sequences with contrast from patient 3 obtained during HSE (E), a few weeks later during relapsing symptoms due to autoimmune encephalitis (F, G), and after symptom improvement (H). Note that the areas of contrast enhancement during autoimmune encephalitis resolved after symptom improvement. Panels I–L correspond to patient 4 during HSE (I, T2; J, T1 with contrast) and 1 year later (K, T2; L, T1 with contrast). In this patient, the relapsing symptoms post-HSE were not recognized as autoimmune encephalitis for 1 year; during this year, he did not receive immunotherapy and had persistent symptoms and contrast enhancement in MRI.
Before immunotherapy, 6 patients (patients 1, 2, 4, 6–8) received acyclovir, antipsychotics, or antidepressants, and all continued to deteriorate: 5 developed drug-resistant psychiatric symptoms (one of them, patient 7, progressing to coma), and 1 developed refractory status epilepticus needing barbiturate coma. Only one patient (patient 3) improved without immunotherapy (described below).
The median time between onset of relapsing symptoms and immunotherapy was 79 days (IQR 22–148, range 17–352 days). This included steroids in 4 patients, steroids and IV immunoglobulin (IVIg) in 1, and steroids, IVIg, and plasma exchange in the other 2 patients. In all 7 cases, a substantial improvement was noted after the immunotherapy was started. At last follow-up (median 12 months, IQR 4.5–15, range 2–20 months), 2 patients had full or near complete recovery (patients 2 and 6), and the other 5 had substantial improvement of the neuropsychiatric abnormalities or seizures, returning to their baseline residual deficits of HSE (table 1 and supplemental case vignettes). The patient with refractory status epilepticus improved transiently after the first plasma exchange but because of recurrent electrographic seizures he was started on IVIg and rituximab. This treatment resulted in complete seizure control and improved level of consciousness, but he was left with HSE-related residual aphasia and critical illness neuropathy.
The patient who was not treated with immunotherapy (patient 3, table 1 and case vignettes in supplemental data) was tested for antibodies 3 months after the neurologic relapse as part of the prospective multicenter study of patients with HSE. This patient on day 40 post-HSE developed agitation, delusional thoughts, and insomnia that improved with alprazolam. By the time he was found to have NMDAR antibodies in CSF (serum negative), his symptoms had substantially improved and the contrast enhancement in the MRI had decreased.
When the clinical features of these 8 teenagers and adults were compared with those of the 6 young children with autoimmune relapses (table e-2), the young children were more likely to have choreoathetosis (6/6 vs 0/8, p < 0.01) and decreased level of consciousness (6/6 vs 2/8, p < 0.01). In addition, 3/6 young children developed refractory seizures and status epilepticus (2 preceding choreoathetosis) while only 1/8 in the teenager and adult group had seizures. MRI with contrast was obtained in 3 young children; 1 showed contrast enhancement and the other 2, who had the study performed after immunotherapy, did not show enhancement.
The interval from onset of HSE to relapsing symptoms was similar in the teenager and adult group (median 39 days, range 12–51) and in the young children group (median 27 days, range 17–40, p = 0.25), but the first group had a longer delay in the recognition of the relapsing symptoms than the young children group (interval onset of symptom relapse/antibody testing 85 days, range 17–296, vs 4 days, range 0–55 in children, p = 0.037). Moreover, all young children were promptly treated with immunotherapy, while one of the adult patients did not receive immunotherapy and the other 7 were treated with substantial delay (interval from relapsing symptoms to immunotherapy in the teenager and adult group 79 days, range 17–352, vs 4 days, range 0–12, in the young children group, p = 0.043).
DISCUSSION
The recent identification of antibodies to NMDAR and other synaptic proteins has provided a proof of principle to the long-held theory that relapsing symptoms post-HSE (or choreoathetosis post-HSE) can be immune-mediated, and has increased awareness for this complication in children and adults.5,6,9 In the current study, we report several novel findings in the age group of adults and teenagers demonstrating that (1) the main clinical manifestations are different from those of young children; (2) the symptom presentation may occur as a relapse of encephalitis (biphasic course), or in contiguity with HSE, suggesting progression or recrudescence of residual deficits after the viral infection; (3) an immune-mediated pathogenesis is often not suspected, or is considered late in the course of the disease, likely explaining substantial delays in immunotherapy; (4) the brain MRI frequently shows contrast enhancement during the autoimmune relapse; (5) in addition to NMDAR, patients may develop antibodies to GABAAR or other, unknown, neuronal cell-surface antigens; and (6) prompt diagnosis and immunotherapy improve symptoms and favorably affect the quality of life of patients and families despite persistence of HSE-related deficits.
The current data confirm that in young children the most characteristic manifestation of the disorder is choreoathetosis,4,6,17 which in some patients may be accompanied or preceded by refractory seizures or status epilepticus and the most common autoantibody is against NMDAR. In contrast, none of the teenagers or adults developed choreoathetosis; in these patients, the NMDAR antibodies also predominated in the antibody repertoire, but some patients had antibodies against unknown neuronal cell surface proteins. The novel finding of GABAAR antibodies in one of the young children with choreoathetosis and status epilepticus and a previous report demonstrating dopamine receptor antibodies7 support the concept that the viral encephalitis triggers an immune response against a wide number of antigens. Further support is provided by the reactivity of the CSF or serum of some patients with live neuronal cultures even when studies with cell-based assays expressing all known surface antigens are negative.6 Given that we have always found that CSF IgG antibodies to GluN1 associate with anti-NMDAR encephalitis18,19 and these antibodies are pathogenic in models of cultured neurons20 and mice,21 we postulate they contribute to patients' symptoms. The pathogenic role of the other antibodies is unclear.
Preliminary data of our ongoing prospective study in which all patients with HSE are clinically and immunologically followed after the viral infection show that 5/20 patients (25%) developed immune-mediated neurologic symptoms, suggesting that this complication might be underrecognized. In teenagers and adults, the problem of syndrome underrecognition is worse than in younger children given that they had substantial longer delays in antibody testing (unless they were part of the prospective study) and initiation of immunotherapy. The 2 main reasons for these delays included the type of syndrome, which in teenagers and adults was less stereotyped (e.g., absence of choreoathetosis), and the initial symptom presentation, which in some patients was not suggestive of a clinical relapse. Indeed, the symptom presentation in most patients of the teenager and adult group was initially attributed to a progression or recrudescence of residual deficits and therefore not suspected to be autoimmune nor viral-induced; the clinical interval change noted in the scheduled visits suggested the autoimmune process. These findings have led to modification of the protocol to include early follow-up visits (e.g., 1 month after hospital discharge).
Compared with the brain MRIs obtained during HSE (which showed mild or absent contrast enhancement), the MRIs obtained during symptom relapse had intense contrast enhancement that decreased or disappeared after the use of immunotherapy and clinical improvement. This observation has not been previously reported and deserves further study with a larger number of patients in order to assess if contrast-enhancing MRI is a potential biomarker of the autoimmune response.
An important finding of this study is the symptom response to immunotherapy. In addition to the remarkable improvement of the patient shown in figure 1, the clinical response in other patients was similarly impressive despite their residual deficits caused by the viral encephalitis. Before the autoimmune relapse, all patients were collaborative or able to communicate and carry out some activities of daily living according to the expected limitations caused by the areas of viral-induced necrosis (usually affecting short-term memory and language). However, this clinical picture contrasted with that observed during the autoimmune relapse, when most of the patients were agitated, aggressive, not collaborative, some of them with suicidal thoughts, or with seizures or decreased level of consciousness progressing to coma. In all but one patient, who improved with symptomatic treatment, immunotherapy (usually first-line, such as steroids, IVIg, or plasma exchange) restored the clinical picture to the baseline deficits, allowing continuation of rehabilitation or discharge home.
The current findings suggest that patients with HSE should be carefully followed for any symptom relapse, worsening of deficits, or development of behavioral-psychiatric alterations with or without choreoathetosis or abnormal movements. Any of these symptoms should raise concern for a viral relapse or an immune-mediated complication. Determination of CSF and serum neuronal cell surface antibodies (mainly NMDAR) is a relatively new and important aid in the diagnosis of immune-mediated relapses post-HSE, and should be considered in all patients. If NMDAR antibodies are negative and testing for other antibodies is not available, a research laboratory should be contacted for further studies. Meanwhile, if the CSF PCR for HSV is negative, it seems reasonable to start these patients with empiric immunotherapy (e.g., first-line steroids, IVIg, or plasma exchange), and depending on the symptom response and antibody results, more intense therapies such as rituximab considered. The ongoing prospective multicenter study will clarify whether neuronal cell surface antibodies may occur without relapsing symptoms post-HSE, or if there is a titer threshold required for symptom development. The significance of MRI contrast enhancement in the areas previously affected by HSE, and the identity of additional target autoantigens, should be goals of future studies.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and their families for volunteering to participate in this study; and Ibrahim Shahnaz, MD (Aga Khan University Hospital, Karachi, Pakistan), Jan Farida, MD (Aga Khan University Hospital, Karachi, Pakistan), Marc Udina, MD, PhD (Department of Psychiatry, Hospital Clinic, Barcelona, Spain), Xavier Pintor, MD, PhD (Department of Psychiatry, Hospital Clinic, Barcelona, Spain), and Francisco Alonso, MD (Hospital General de Vic, Barcelona), for providing clinical information.
GLOSSARY
- FLAIR
fluid-attenuated inversion recovery
- HSE
herpes simplex virus encephalitis
- HSV
herpes simplex virus
- IDIBAPS
Institute of Biomedical Research August Pi i Sunyer
- IgG
immunoglobulin G
- IQR
interquartile range
- IVIg
IV immunoglobulin
- NMDAR
NMDA receptor
Footnotes
Supplemental data at Neurology.org
Editorial, page 1730
Contributor Information
Collaborators: Spanish Prospective Multicentric Study of Autoimmunity in Herpes Simplex Encephalitis, Esther Aguilar, Sergio Aguilera-Albesa, Izaskun Arratibel, Luis Bataller, Beatriz Bosch, Carlos Cervera, David Conejo-Moreno, Íñigo Corral, Marta Dapena, Domingo Escudero, Jordi Estela, Juan Carlos García-Monco, Verónica González-Álvarez, Robert Güerri, Sara Guillen-Martin, Rogelio López-Cuevas, Maria López, Eduardo López-Laso, Francisco López-Pisón, Virginia Pomar, Luis Manuel Prieto-Tato, Beatriz Martínez-Menéndez, Leticia Martin-Gil, Juan Navarro-Moron, María Pérez-Poyato, Miquel Raspall-Chaure, Maria Rodes, Raquel Sanchez-Valle, Estevo Santamaria, Pere Soler-Palacín, Manuel Toledo, and Miguel Tomás-Vila
AUTHOR CONTRIBUTIONS
Design/conceptualization of the study: T.A., F.G., J.D.; analysis/interpretation of the data: T.A., G.M., V.C.-E., C.E.C., K.R., M.E.E., J.C.P.-C., E.T.-V., I.M., B.M.-C., C.T.-T., S.L., L.G.-G.-S., G.G., I.C.-N., M.R., F.G., J.D.; statistical analysis and figure development: T.A.; drafting/revising the manuscript: T.A., F.G., J.D. All authors give final approval of the version to be published.
STUDY FUNDING
Supported in part by Instituto Carlos III (FIS PI12/00611, F.G.; FIS 14/00203, J.D.; and CM14/00081, T.A.), NIH RO1NS077851 (J.D.), Fundació Cellex (J.D.), Mutual Medica, and a grant from the Spanish Society of Pediatric Neurology (T.A.).
DISCLOSURE
T. Armangue, G. Moris, V. Cantarin-Extremera, C. Conde, K. Rostasy, M. Erro, J. Portilla-Cuenca, E. Turon-Vinas, I. Malaga, B. Munoz-Cabello, M. Torres-Torres, S. Llufriu, L. Gonzalez-Gutierrez-Solana, G. Gonzalez, I. Naranjo, M. Rosenfeld, and F. Graus report no disclosures relevant to the manuscript. J. Dalmau has a research grant from Euroimmun and receives royalties from patents for the use of Ma2 and NMDAR as autoantibody tests. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Skoldenberg B, Aurelius E, Hjalmarsson A, et al. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol 2006;253:163–170. [DOI] [PubMed] [Google Scholar]
- 2.Kimura H, Aso K, Kuzushima K, Hanada N, Shibata M, Morishima T. Relapse of herpes simplex encephalitis in children. Pediatrics 1992;89:891–894. [PubMed] [Google Scholar]
- 3.Schleede L, Bueter W, Baumgartner-Sigl S, et al. Pediatric herpes simplex virus encephalitis: a retrospective multicenter experience. J Child Neurol 2013;28:321–331. [DOI] [PubMed] [Google Scholar]
- 4.De Tiège X, Rozenberg F, Des Portes V, et al. Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology 2003;61:241–243. [DOI] [PubMed] [Google Scholar]
- 5.Armangue T, Titulaer MJ, Malaga I, et al. Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr 2013;162:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armangue T, Leypoldt F, Malaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 2014;75:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammad SS, Sinclair K, Pillai S, et al. Herpes simplex encephalitis relapse with chorea is associated with autoantibodies to N-methyl-D-aspartate receptor or dopamine-2 receptor. Mov Disord 2014;29:117–122. [DOI] [PubMed] [Google Scholar]
- 8.Hacohen Y, Deiva K, Pettingill P, et al. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord 2014;29:90–96. [DOI] [PubMed] [Google Scholar]
- 9.Pruss H, Finke C, Holtje M, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 2012;72:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bektas O, Tanyel T, Kocabas BA, Fitoz S, Ince E, Deda G. Anti-N-methyl-D-aspartate receptor encephalitis that developed after herpes encephalitis: a case report and literature review. Neuropediatrics 2014;45:396–401. [DOI] [PubMed] [Google Scholar]
- 11.Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 2013;81:1637–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petit-Pedrol M, Armangue T, Xiaoyu P, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014;13:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dale RC, Merheb V, Pillai S, et al. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain 2012;135:3453–3468. [DOI] [PubMed] [Google Scholar]
- 15.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 2010;30:5866–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Titulaer MJ, Hoftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti-NMDA receptor encephalitis. Ann Neurol 2014;75:411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barthez MA, Billard C, Santini JJ, Ruchoux MM, Grangeponte MC. Relapse of herpes simplex encephalitis. Neuropediatrics 1987;18:3–7. [DOI] [PubMed] [Google Scholar]
- 18.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2014;13:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2014;76:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Planaguma J, Leypoldt F, Mannara F, et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain 2015;138:94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.