Abstract
Succinate dehydrogenase (or Complex II; SDH) is a heterotetrameric protein complex that links the tribarboxylic acid cycle with the electron transport chain. SDH is composed of four nuclear-encoded subunits that must translocate independently to the mitochondria and assemble into a mature protein complex embedded in the inner mitochondrial membrane. Recently, it has become clear that failure to assemble functional SDH complexes can result in cancer and neurodegenerative syndromes. The effort to thoroughly elucidate the SDH assembly pathway has resulted in the discovery of four subunit-specific assembly factors that aid in the maturation of individual subunits and support the assembly of the intact complex. This review will focus on these assembly factors and assess the contribution of each factor to the assembly of SDH. Finally, we propose a model of the SDH assembly pathway that incorporates all extant data.
Keywords: Respiratory chain, succinate dehydrogenase, assembly factors, redox-active cofactors
Electron transport chain complex assembly
Mitochondrial ATP synthesis is dependent on the concerted efforts of the electron transport chain (ETC), which couples the generation of an electrochemical gradient to the oxidation of NADH and FADH2 and the reduction of oxygen to water. The ETC is composed of four multimeric complexes and two mobile electron carriers (coenzyme Q and cytochrome c), all of which are embedded in or associated with the inner mitochondrial membrane (IMM). Electrons derived from the oxidation of NADH by complex I or succinate from the tricarboxylic acid (TCA) cycle by complex II (succinate dehydrogenase; SDH) are passed along the ETC, coupled with the pumping of protons and establishment of the proton gradient across the IMM. In the end, controlled flow of protons down this electrochemical gradient is utilized by Complex V (ATP synthase) to catalyze ATP synthesis.
The assembly of the ETC complexes presents the cell with the problem of coordinating the synthesis and stepwise interactions of individual subunits, transcribed and translated from two distinct genomes in two distinct compartments, into intricate membrane bound complexes. This problem is exacerbated by the nature of the ETC complexes themselves. First, some subunits are embedded in the IMM and, therefore, are hydrophobic and prone to aggregation prior to and during assembly. Furthermore, the complexes contain redox-active cofactors that can perform inappropriate and deleterious reactions when they are not properly secluded within the native complex. As a result, a number of dedicated factors assist the assembly of these complexes by facilitating cofactor insertion, preventing non-productive interactions, and stabilizing assembly intermediates (Fernandez-Vizarra et al., 2009, Diaz et al., 2011). While mammalian ETC complexes I, III and IV contain 44, 11 and 14 subunits, respectively, and include proteins encoded by both mitochondrial and nuclear genomes, Complex II or SDH, is the product of just four nuclear-encoded genes. Despite this somewhat simple quaternary structure it has been made clear recently that SDH, like all ETC complexes, requires assembly factors for its biogenesis.
Succinate dehydrogenase and human disease
The critical role of SDH in mitochondrial metabolism has long been appreciated, however, it has more recently emerged that mutations affecting SDH cause a number of human diseases (Rutter et al., 2010, Hoekstra and Bayley, 2013) (Table1). Interestingly, loss of function mutations in SDH core subunits do not cause a single, common pathology, but rather lead to a variety of disease phenotypes that can be grouped into two categories—cancer and neurodegeneration. With respect to SDH-deficient cancers and other tumor syndromes, mutations in the core subunits are most commonly associated with paraganglioma, pheochromocytoma, renal cell carcinoma (RCC), and WT gastrointestinal stromal tumors (WT-GIST). Paragangliomas are neuro-endocrine tumors that occur in cells of the neural crest and tend to be co-localized with oxygen sensing tissues such as the carotid body, while pheochromocytomas represent a related class of tumors that affect the adrenal gland. These two tumors are most commonly associated with mutations in SDHB, SDHC, and SDHD, however SDHA mutations have recently been implicated in rare cases (Baysal, 2000, Astuti et al., 2001, Peczkowska et al., 2008, Burnichon et al., 2010). Mutations in SDHA, SDHB, and SDHC have also been associated with WT-GIST, a mesenchymal tumor of the digestive tract (Janeway KA, 2011, Pantaleo et al., 2011, Pantaleo et al., 2014). Finally, the link between RCC and SDH dysfunction is supported by the discovery of two families with inherited renal cell tumor syndromes resulting from germline mutations in SDHB (Vanharanta et al., 2004). Taken together, it is clear that normal SDH activity serves to suppress tumors in humans. In addition to the cancers described above, defects in SDH activity also cause a variety of neurodegenerative disorders. In fact, the classical presentation of patients with mutations in SDHA is Leigh Syndrome, an early-onset, progressive neurodegenerative disorder (Bourgeron et al., 1995). SDHA mutations have also been associated with milder forms of atrophy and myopathy (Bourgeron et al., 1995, Horváth et al., 2006). Although mutations in SDHC are rarely, if ever, associated with neurologic disorders, SDHB mutations have been shown to cause infantile leukodystrophy (Alston et al., 2012) and SDHD mutations have recently been identified in patients with progressive encephalomyopathy (Jackson et al., 2014). Therefore SDH activity not only suppresses tumors but also supports normal neurologic development and function. While it is fascinating that mutations in all four subunits of SDH have been found to cause one of the diseases described above, it is perhaps even more interesting that numerous patients present with disease accompanied by a loss of SDH activity, but have no mutations of any of the core subunits (Jain-Ghai et al., 2013). These genetic observations clearly implicate additional auxiliary factors in the maintenance of cellular SDH activity. Furthermore, this supports the notion that a thorough characterization of the SDH assembly pathway will ultimately lead to the discovery of new human disease alleles in the genes that encode SDH assembly factors.
Enzymology and structure of succinate dehydrogenase
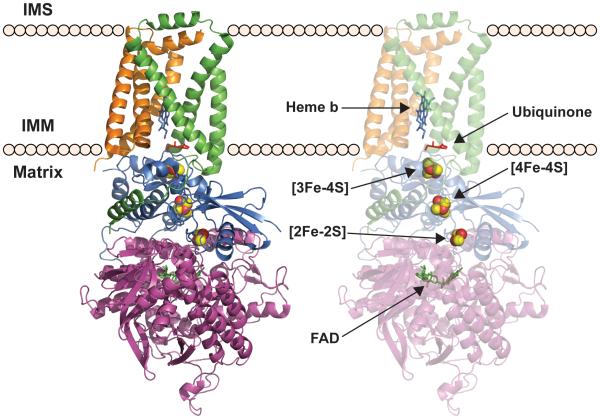
Eukaryotic SDH is a heterotetrameric complex composed of four nuclear-encoded subunits (Sun et al., 2005) (Figure 1). SDH is unique amongst eukaryotic ETC complexes in that it functions as part of both the TCA cycle and the ETC and thus couples two of the primary energy-harvesting pathways within the cell. In addition to this distinction, SDH is the only ETC complex that does not pump protons across the IMM nor contain any proteins encoded by the mitochondrial genome. In the context of the TCA cycle, SDH catalyzes the oxidation of succinate to fumarate and uses the electrons derived from this oxidation to catalyze the reduction of ubiquinione to ubiquinol. These electrons are passed to Complex III and then Complex IV, thereby contributing to the establishment of the electrochemical gradient across the IMM in support of ATP synthesis. The structure of SDH can be characterized as a hydrophilic head that protrudes into the mitochondrial matrix attached to the IMM by a hydrophobic membrane anchor (Yankovskaya et al., 2003, Sun et al., 2005) (Figure 1).
Figure 1. Porcine succinate dehydrogenase (PDB accession number: 1ZOY) embedded in the mitochondrial inner membrane.
SdhA (purple ribbon); SdhB (blue ribbon); SdhC (green ribbon); SdhD (brown ribbon); FAD (green stick); FeS centers, [2Fe-2S], [4Fe-4S], [3Fe-4S] from the bottom (red and yellow sphere); Ubiquinone in the QP site (red stick); Heme b (blue stick)
The membrane anchor domain of SDH consists of Sdh3 (SDHC in mammals) and Sdh4 (SDHD) (Yankovskaya et al., 2003, Sun et al., 2005) and serves as the site of ubiquinone binding to connect this hydrophobic mobile electron carrier to the hydrophilic domain of SDH (Figure 1). The hydrophilic domain represents the catalytic core of SDH and is composed of Sdh1 (SDHA in mammals) and Sdh2 (SDHB), each of which contain the redox active cofactors that facilitate the transfer of electrons from succinate to ubiquinone (Yankovskaya et al., 2003, Sun et al., 2005) (Figure 1). Sdh1 contains a covalently bound FAD cofactor adjacent to the succinate-binding site (Figure 1). Sdh2 harbors the three Fe-S centers that mediate electron transfer from the flavin cofactor to the ubiquinone (Figure 1). The Fe-S clusters of Sdh2, which consist of a 2Fe-2S center adjacent to the FAD site of Sdh1, followed by a 4Fe-4S and finally a 3Fe-4S center proximal to the ubiquinone binding site, serve essentially as a wire used to transfer electrons through the complex (Figure 1). In addition to its important role in the process of electron transfer, Sdh2 also serves as the interface linking the catalytic Sdh1 subunit to the membrane bound Sdh3 and Sdh4 subunits (Yankovskaya et al., 2003, Sun et al., 2005). Interestingly, recent reports indicate that soluble Sdh1-Sdh2 dimers exist in the absence of one or both of the membrane anchors (Kim et al., 2012). This suggests that Sdh1 and Sdh2 are likely to dimerize prior to membrane association, rather than sequentially docking onto the membrane anchor.
The SDH enzymatic reaction begins with the binding of succinate to the open state of Sdh1, which undergoes a conformational change bringing succinate into close proximity with the covalently bound FAD cofactor. Oxidation of succinate is coupled to the two-electron reduction of FAD. Since the Fe-S centers of SDH are single electron carriers, two successive single electron transfer steps are required to re-oxidize FADH2 back to FAD. In the end, the two electrons gained from the oxidation of succinate are used to reduce ubiquinone to ubiquinol, which passes the electrons on to Complex III.
Assembly of SDH
Until recent years the process of SDH assembly remained highly enigmatic and was primarily focused on the core SDH subunits themselves (Cecchini et al., 2002, Lemire and Oyedotun, 2002). Our newfound understanding of this process has been facilitated by the discovery of proteins dedicated to the maturation of individual subunits and the assembly of the holo-complex. Importantly, the discovery of these factors has revealed that SDH assembly is a tightly coordinated process in which the concerted functions of core subunits, dedicated assembly factors, and other ancillary factors must coordinate their various activities to achieve the step-wise assembly of this membrane bound complex. On the basis of knowledge gained in recent years, it is possible to organize the process of SDH assembly into discrete, subunit-specific events. These events, which include cofactor insertion, stabilization of sub-complex assembly intermediates, and prevention of deleterious solvent interactions, result in the maturation of individual subunits and support the complete assembly of SDH. In the subsequent sections we will review the current understanding of the SDH assembly pathway focusing, in particular, on the factors that facilitate this process (Figure 3).
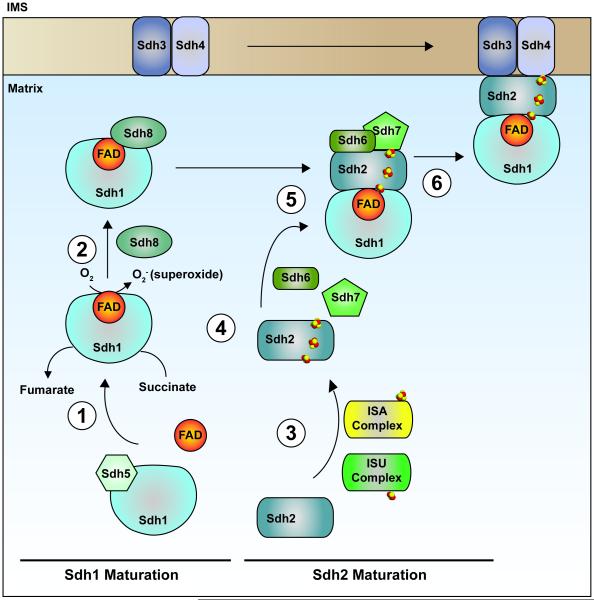
Figure 3. Model of the SDH assembly pathway.
Each SDH core subunit is translated in the cytosol and must be subsequently translocated to the mitochondria. Upon mitochondrial import, apo-Sdh1 is rapidly bound by the subunit-specific chaperone, Sdh5, forming a dimeric complex that supports covalent attachment of the FAD cofactor (1). Following covalent flavinylation, the Sdh1-Sdh5 dimer disintegrates resulting in a pool of flavinylated Sdh1 that is unbound by any other core subunits. This leads to the formation of a complex comprised of Sdh1 and Sdh8, another subunit-specific chaperone (2). The formation of this complex supports the formation of the subsequent Sdh1-Sdh2 soluble dimer and also prevents the spurious production of superoxide by flavinylated Sdh1. Meanwhile, apo-Sdh2 must also mature into a complex-competent subunit. This process involves the insertion of 3 Fe-S clusters generated by the ISU and ISA complexes. (3). Following maturation of Sdh2, it interacts with Sdh6 and Sdh7, which serve to protect exposed Fe-S clusters during the assembly process (4) and further associates with a mature Sdh1 subunit forming a heterotetrameric assembly intermediate (5). Finally, the Sdh1-Sdh2 hydrophillic head docks to the IMM via interactions with the Sdh3-Sdh4 membrane anchor domain, which may or may not preassemble at the IMM (6). In the end, the concerted efforts of core subunits, dedicated assembly factors, and other ancillary factors facilitate the stepwise assembly of SDH.
Sdh1—The catalytic subunit
Architecture of Sdh1
Sdh1 catalyzes the oxidation of succinate to fumarate. The crystal structure of porcine SDH has revealed that mammalian SDHA assumes a Rossmann-type fold with four distinct domains—an FAD binding domain (residues 52-267 and 355-439; human sequence), a capping domain (residues 268-354), a helical domain (residues 440-537), and a C-terminal domain (residues 548-616). The structure indicates the presence of a covalent bond between FAD and His99 (His90 is yeast) with the FAD being further coordinated by a number of additional residues, which form a well-ordered hydrogen bonding network (Sun et al., 2005) (Figure 1). In addition to the native SDH, a co-crystal structure of SDH bound to a competitive inhibitor, 3-nitropropionic acid (NPA) was also determined and facilitated the mapping of the putative succinate binding site, which, as expected, is directly adjacent to the FAD. While the precise mechanism for succinate oxidation by Sdh1 has not yet been elucidated, these studies have provided valuable information as to the residues involved in this reaction. The structure demonstrates that the nitryl group of NPA interacts with the FMN group of FAD as well as the main chain of Glu261 and the side chains of Thr260 and His248 while the NPA carboxyl group is anchored by the amide of Glu246, the guanidinium group of Arg403, and the imidazole of His359 (Sun et al., 2005).
Biosynthesis and delivery of FAD
FAD is an essential cofactor of Sdh1 and the flavinylation of Sdh1 is dependent on adequate FAD levels in the mitochondrial matrix. Therefore, the synthesis and maintenance of free FAD pools in this compartment plays a vital role in the maturation of Sdh1 (Kim and Winge, 2013). In yeast, the biosynthesis of FAD and its delivery to mitochondria is dependent on the activities of three proteins—riboflavin kinase (Fmn1), FAD synthetase (Fad1), and a putative mitochondrial FAD transporter (Flx1). FAD is derived from dietary riboflavin (vitamin B2) and its conversion requires the activities of two ATP-dependent enzymes, Fmn1 and Fad1. Fmn1, the riboflavin kinase, phosphorylates the tricyclic isoalloxazine ring yielding flavin mononucleotide (FMN) (Santos et al., 2000). Although a number of enzymes within the cell use FMN as a cofactor, the majority of FMN is subsequently adenylated and converted to FAD by the FAD synthetase, Fad1(Wu et al., 1995).
Flx1 belongs to the eukaryotic superfamily of IMM carriers and has been described as a putative IMM FAD carrier, but the published literature disagrees as to the exact molecular function of Flx1 (Tzagoloff et al., 1996, Bafunno et al., 2004). At any rate, cells lacking Flx1 exhibit low matrix FAD concentrations and reduced activity of two matrix flavo-proteins, SDH and lipoamide dehydrogenase, due to a defect in flavinylation of these two enzymes (Tzagoloff et al., 1996, Kim et al., 2012). So, while there is no known direct link between Flx1 and Sdh1, it is clear that Flx1 plays an important role in Sdh1 cofactor insertion as Sdh1 flavinylation is severely compromised in flx1Δ cells. This decrease in flavinylation also leads to the destabilization of Sdh1. Interestingly, the flx1Δ defect in Sdh1 flavinylation can be suppressed by the overexpression of Fad1 or Sdh5, a dedicated SDH assembly factor that will be discussed in significant detail in the subsequent section (Hao et al., 2009).
It is important to note that in mammalian cells, it isn't clear whether an Flx1 ortholog is required for SDH maturation. The mammalian ortholog of the FAD1 gene, FLAD1, yields two transcripts encoding two isoforms of FAD synthetase, one of which is cytosolic and the other has a mitochondrial targeting motif (Torchetti et al., 2010). Thus, mammalian cells might synthesize FAD within the mitochondrial matrix perhaps making any IMM carrier dispensable.
Sdh1 cofactor insertion
The first known step in the maturation of Sdh1, which is imported into the mitochondrial matrix as an apo-protein, is the insertion and covalent attachment of its FAD cofactor (Robinson et al., 1994). Interestingly, succinate appears to be an important allosteric effector of this process as it and several other TCA cycle intermediates stimulate flavinylation significantly in vitro (Brandsch and Bichler, 1989). Unlike some bacterial Sdh1 orthologs, eukaryotic Sdh1 is not competent to become flavinylated in the absence of other proteins, which is typically interpreted to mean that the flavinylation of Sdh1 is not autocatalytic (Robinson and Lemire, 1996, Kounosu, 2014). As a result, simply maintaining matrix FAD pools is not sufficient for Sdh1 flavinylation. Early studies focused on this process revealed that there likely exists a protein in the mitochondrial matrix required for flavinylation of Sdh1 as the degree to which Sdh1 can be flavinylated in vitro is proportional to the concentration of isolated matrix fraction used in the assay (Robinson and Lemire, 1996). This insight was subsequently validated by the discovery of Sdh5.
Yeast Sdh5 was discovered in the course of studying a collection of uncharacterized mitochondrial proteins with a high degree of conservation throughout eukaryotes (Hao et al., 2009). Deletion of SDH5 in yeast prevented respiratory dependent growth and caused a dramatic reduction in oxygen consumption—two phenotypes indicative of a strong respiratory deficiency. In an attempt to better understand this phenotype, an unbiased tandem affinity purification was performed and it identified Sdh1 as the sole binding partner of Sdh5. This result, consistent with a clear respiratory deficiency, strongly implicated Sdh5 in the maintenance of SDH activity. This connection with SDH was further strengthened by the observation that yeast cells lacking Sdh5 exhibited a complete loss of SDH activity and, in this regard, essentially mirrored an sdh1Δ deletion strain. Despite these phenotypic similarities, however, Sdh1 protein was still clearly detectable, albeit at reduced levels, in an sdh5Δ mutant strain and, interestingly, the SDH complex remained intact (Hao et al., 2009, Kim et al., 2012).
Based on the evidence above, it is clear that Sdh5 is required for maintaining SDH activity and that this function is mediated through a direct physical interaction with Sdh1. These data raised the possibility that the primary defect in sdh5Δ cells might be failure to covalently flavinylate Sdh1, resulting in a catalytically dead subunit. Indeed, direct interrogation of Sdh1 flavinylation revealed a complete failure to form the covalent bond between Sdh1 His90 and the FAD cofactor. This finding is consistent with related studies that demonstrate flavinylation-deficient Sdh1 His90Ser mutants assemble into a catalytically inactive SDH complex as do sdh5Δ mutants (Robinson et al., 1994, Hao et al., 2009).
It is clear that Sdh5 is required for Sdh1 flavinylation but through what mechanism? The first important clues resulted from further interrogation of the nature of the Sdh1-Sdh5 interaction. BN-PAGE experiments demonstrated that Sdh5 is not a member of the SDH holo-complex but rather migrates to an approximate molecular weight of 90 kDa, consistent with an Sdh1-Sdh5 dimer. Further investigation of this interaction revealed that the formation of this complex is important in maintaining the stability of both proteins. The steady state levels of Sdh1 are ~50% reduced in an sdh5Δ mutant strain. Conversely, deletion of SDH1 causes a near complete destabilization of Sdh5, a result that strongly implicates Sdh5 as a dedicated factor whose sole purpose is to act on Sdh1. While Sdh1 protein is required to maintain the stability of Sdh5, this relationship is not dependent on Sdh1 being competent for flavinylation as substitution of Sdh1 His90 with a Ser residue does not lead to destabilization of Sdh5. It is important to note that only a minor fraction of Sdh1 in the matrix is in association with Sdh5 at any given time. Furthermore, deletion of SDH2 causes the steady state level of Sdh5 to increase, most likely due to an increase in the fraction of Sdh1 that is bound to Sdh5. Because this complex exists independent of all other SDH subunits and accumulates in strains that prevent SDH assembly, it is clear that the actions of Sdh5 and the process of flavinylation are early steps in the SDH assembly pathway (Hao et al., 2009, Kim et al., 2012).
Further insights into the role of Sdh5 in Sdh1 flavinylation came from structural characterization of yeast Sdh5 by NMR (Eletsky et al., 2012). No FAD was detected in purified samples of Sdh5, nor did addition of FAD to purified Sdh5 cause any perturbation in Sdh5 chemical shifts, suggesting that Sdh5 does not act as a delivery vehicle that brings FAD to apo-Sdh1 (Eletsky et al., 2012). The NMR studies revealed that the Sdh5 core assumes a compact 5 α-helical bundle and revealed a concentrated patch of conserved residues on the surface of these α-helices, which is theorized to be the Sdh1 binding site (Eletsky et al., 2012)
While it is not yet possible to define the precise mechanism by which Sdh5 supports Sdh1 flavinylation, there is sufficient data to hypothesize as to the role of Sdh5 in this process. Biochemical studies have demonstrated that Sdh5 does not directly bind FAD, thus it seems unlikely that Sdh5 plays a role in physically delivering FAD to Sdh1. While the Sdh1 binding site on Sdh5 has been defined in the above NMR-based study, the region of Sdh1 that binds Sdh5 remains unknown. This information would provide insight into the role of Sdh5 in Sdh1 flavinylation, but for now we are forced to theorize as to such details. It seems likely that Sdh5 binds adjacent to the FAD binding site on apo-Sdh1, which could serve one or more of many possible roles. First, it could facilitate the noncovalent insertion of FAD into the pocket, which would enable subsequent autocatalytic covalent flavinylation. Second, Sdh5 could act as a chaperone for unflavinylated apo-Sdh1, thus supporting flavinylation by stabilizing apo-Sdh1 in a flavinylation-competent conformation. Finally, it is possible that Sdh5 might participate directly in the covalent flavinylation reaction by providing catalytic residues to this active site. Regardless of the precise mechanism, it is clear that Sdh5 specifically binds to apo-Sdh1 and is required for its covalent flavinylation (Figure 2).
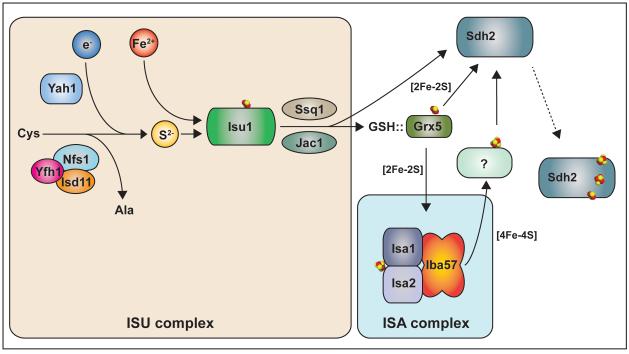
Figure 2. Iron-sulfur cluster synthesis and delivery to Sdh2.
The tan box depicts the de novo synthesis of 2Fe-2S cluster within the ISU complex, in which Isu1 is the scaffold protein. Isu1 interacts with Nfs1 (cysteine desulfurase) and Yah1 (ferredoxin) and receive sulfide ion (S2−) for the synthesis. It is not clear how ferrous ion (Fe2+) is delivered to the ISU complex. The preformed 2Fe-2S cluster is transferred to GSH-bound Grx5 from Isu1 as Ssq1 (Hsp70) and Jac1 (DnaJ protein) are recruited to the ISU complex. The GSH-Grx5 delivers 2Fe-2s clusters to the ISA complex for the subsequent 4Fe-4S cluster synthesis depicted in the blue box. However, it is elusive whether the GSH-Grx5 also is required for 2Fe-2S cluster delivery to the final recipient protein, in this case, Sdh2, or not. It is also unknown whether the preformed 4Fe-4S clusters are directly delivered to the Sdh2 or another factor is in need of this delivery step. All arrows with solid lines indicate transfer of components of Fe-S clusters or pre-formed Fe-S clusters.
Sdh8 is a chaperone for flavinylated Sdh1
Following Sdh5-dependent covalent flavinylation, Sdh1 is destined to form a soluble dimer with Sdh2, however there remains a population of Sdh1 that exists in an unbound state free from any other core subunits in the mitochondrial matrix. It is likely that Sdh1 is present in excess to other core SDH subunits within the mitochondrial matrix as deletion of Sdh1 destabilizes the remaining core subunits, Sdh2, Sdh3, and Sdh4, while Sdh1 protein levels are maintained, albeit at a reduced level, upon deletion of any of the other core subunits. This free, flavinylated Sdh1 appears to be maintained in a soluble, assembly-competent state by a newly discovered, subunit-specific chaperone, Sdh8 (Figure 3).
SDH8 is conserved throughout eukaryotes and encodes a small protein that is localized to the mitochondrial matrix (Van Vranken et al., 2014). Yeast cells lacking Sdh8 exhibit slow growth on non-fermentable carbon sources but are still respiratory competent. The first observation that suggested a role for Sdh8 in SDH assembly or activity was its metabolomics phenotype. Yeast cells and Drosophila mutants lacking Sdh8 (or its ortholog) exhibit a significant but subtle block in the TCA cycle centered at SDH (i.e. an accumulation of succinate and a depletion of fumarate and malate, the two TCA cycle intermediates downstream of SDH). It is important to note that the magnitude of succinate accumulation is much less than a mutant lacking one of the four core subunits or Sdh5, which exhibits a complete loss of SDH activity. Consistent with this finding, sdh8Δ mutant yeast maintain approximately 40% of wild type SDH activity and abundance of SDH holo-complexes. Interestingly, deletion of the Drosophila ortholog, dSdhaf4, causes a much more severe phenotype. These mutants accumulate significantly more succinate and have a ~90% decrease in SDH activity, while assembled SDH complexes are nearly undetectable. Thus, while Sdh8 clearly supports SDH biogenesis, it does not appear to be absolutely required for assembling functional SDH complexes as some level of SDH activity is maintained in organisms lacking Sdh8 or its orthologs (Van Vranken et al., 2014).
While metabolomics clearly implicated Sdh8 in enabling SDH activity, the first evidence as to its particular function came when Sdh1 was discovered to be its primary binding partner. Further interrogation of the specificity of this interaction revealed that Sdh8 bound to Sdh1 independent of any other SDH core subunits and importantly, Sdh5. Additionally, it was determined that the Sdh1-Sdh8 interaction depends on covalent flavinylation of Sdh1, as Sdh8 fails to interact with Sdh1 in an sdh5Δ background and also fails to interact with two covalent flavinylation-deficient Sdh1 mutants (H90S and H90A). BN-PAGE analysis further confirmed that Sdh8 is not associated with the SDH holo-complex, but forms a stable subcomplex with Sdh1, which hyper-accumulates in sdh2Δ and sdh4Δ backgrounds. Consistent with this finding, Sdh8 steady state protein levels also increase in these conditions, presumably in order to occupy the augmented pool of unbound and flavinylated Sdh1. Furthermore, deletion of Sdh1 causes significant destabilization of Sdh8, indicating that the stability of Sdh8 depends upon its ability to form a complex with Sdh1. Taken together these data indicate that Sdh8 is a subunit-specific chaperone that occupies flavinylated Sdh1 prior to the formation of the Sdh1-Sdh2 soluble dimer (Van Vranken et al., 2014) (Figure 3).
Why exactly does unbound Sdh1 require such a chaperone? The first evidence came from assessing the steady state levels of SDH core subunits in the sdh8Δ mutant. While there was little or no destabilization of Sdh1, the primary binding partner of Sdh8, there was a marked decrease in the steady state abundance of Sdh2. This was accompanied by, and probably a result of, a decrease in the Sdh1-Sdh2 soluble dimer as assessed by co-immunoprecipitation. Although this result is somewhat difficult to interpret, it suggests that the interaction between Sdh1 and Sdh8 facilitates the formation of the Sdh1-Sdh2 dimer by maintaining Sdh1 in a state that is competent for Sdh2 binding. Therefore, in the absence of Sdh8, Sdh1 does not interact as efficiently with Sdh2, which is unstable on its own. Interestingly, studies focused on Drosophila dSdhaf4 demonstrate that this assembly factor is required for maintaining the stability of SdhA. Therefore, it is possible that in higher eukaryotes Sdh8 orthologs support the formation of the hydrophilic head by maintaining subunit stability. Overall, this data suggests that the chaperone activity of Sdh8 promotes SDH assembly by stabilizing Sdh1 and maintaining it in an assembly-competent state prior to interaction with Sdh2 (Van Vranken et al., 2014).
In addition to promoting formation of the Sdh1-Sdh2 dimer, Sdh8 might also function to prevent potentially deleterious interactions of free and flavinylated Sdh1 with the solvent. Indeed, studies focused on SDH have demonstrated that impaired electron transport at the level of the FAD can generate significant quantities of superoxide (Messner and Imlay, 2002, Yankovskaya et al., 2003, Ishii et al., 2005). Furthermore, previous studies in mammalian cells demonstrate that silencing of SDHB (Sdh2 ortholog), but not SDHA (Sdh1 ortholog) causes an increase in ROS levels (Guzy et al., 2008, Ishii et al., 2005). This increase in oxidative stress mediated by Sdh1 is thought to be dependent on the ability of the exposed FAD cofactor to interact with the surrounding solvent. In a phenomenon referred to as auto-oxidation, free Sdh1 oxidizes succinate to fumarate independent of the SDH holo-complex. This oxidation causes a reduction of the FAD cofactor, which then reduces molecular oxygen to form superoxide (Messner and Imlay, 2002). If Sdh8 functions to prevent Sdh1 auto-oxidation then overexpression of Sdh1 should be toxic to sdh8Δ mutant cells. In fact, Sdh1 overexpression proved toxic to both WT and sdh8Δ mutant cells, however, cells lacking Sdh8 were particularly sensitive to this stress. Furthermore, overexpression of Sdh8 was capable of partially rescuing the toxicity associated with overexpressed Sdh1. Additional studies demonstrated that sdh8Δ mutant cells exhibited higher levels of oxidative stress and that the growth phenotype associated with sdh8Δ mutant cells was predominantly the result of oxidative stress rather than respiratory deficiency. Indeed, while overexpression of YAP1, a transcription factor involved in mediating oxidative stress, is capable of rescuing the sdh8Δ growth phenotype it fails to have any impact on the SDH deficiency of these cells (Van Vranken et al., 2014). Therefore, Sdh8 might have two important functions related to Sdh1. It appears to facilitate assembly with Sdh2 and may also function to seclude the FAD cofactor from the solvent, thus preventing spurious oxidation (Van Vranken et al., 2014).
Sdh2—The electron wire
Architecture of Sdh2
Sdh2 (SDHB in mammals) is the Fe-S cluster-containing subunit of SDH. The crystal structure of porcine heart SDH demonstrates that SDHB contains two distinct domains (Sun et al., 2005) (Figure 1). The N-terminal domain (residues 37-142; human sequence) consists of a single small α-helix and a five-strand β-sheet. This domain harbors the FAD-proximal 2Fe-2S center, which is ligated by Cys93, Cys98, Cys101 and Cys113. The C-terminal domain (residues 142-280) consists of six α-helices and harbors the 4Fe-4S and 3Fe-4S centers, which are coordinated by Cys186, Cys189, Cys192 and Cys253 and Cys196, Cys243 and Cys249, respectively. These three Fe-S centers essentially act as a wire that carries electrons from FAD to ubiquinone. SDHB also serves to connect the catalytic subunit, SDHA, with the hydrophobic anchor domain. Both the N-terminal domain and the C-terminal domain mediate the interaction with SDHA. Meanwhile, the C-terminal domain is mainly responsible for the interaction with the hydrophobic anchor domain. Importantly, SDHB contributes several residues to the ubiquinone-binding site, which otherwise is mediated by the hydrophobic anchor domain. In this way, the terminal 3Fe-4S center is poised adjacent to the ubiquinone to enable the final electron transfer (Sun et al., 2005).
Iron-sulfur cluster biogenesis
The aforementioned Fe-S clusters are key cofactors required for electron transfer from FAD to the QP site. Fe-S clusters are preformed in the mitochondrial matrix on a scaffold complex (ISU) consisting of four proteins, Nfs1, Isd11, Yfh1 and Isu1 (or Isu2; yeast nomenclature) (Schmucker et al., 2011, Lill et al., 2012, Tsai and Barondeau, 2010). The sulfide ions necessary for cluster biogenesis are provided by the Nfs1 cysteine desulfurase, along with its effector proteins Isd11 and Yfh1 (Pandey et al., 2012, Pandey et al., 2013) and the Yah1 ferredoxin reductant (Sheftel et al., 2010). Fe(II) and sulfide ions form 2Fe-2S clusters on the Isu1 (or Isu2) scaffold proteins prior to transfer to client proteins. The preformed cluster is transferred to the monothiol Grx5, an Fe-S shuttle protein, through the binding of the DnaJ protein Jac1 to Isu1, which dissociates the ISU complex (Majewska et al., 2013) and the recruitment the Hsp70 enzyme Ssq1. The cluster is released from Isu1 by the ATPase activity of Ssq1 (Ciesielski et al., 2012, Majewska et al., 2013, Uzarska et al., 2013). Grx5, which is pre-associated with Ssq1, transiently binds the 2Fe-2S cluster together with glutathione (GSH) for subsequent transfer steps (Johansson et al., 2011, Uzarska et al., 2013, Banci et al., 2014). The human ortholog of Jac1 was shown to also bind Fe-S client proteins, so Grx5-mediated cluster transfer may occur in pre-bound complexes with client proteins (Maio et al., 2014). Clusters consisting of 4Fe-4S and perhaps 3Fe-4S stoichiometries are matured on a downstream ISA scaffold complex consisting of Isa1, Isa2 and Iba57 (Sheftel et al., 2012, Gelling et al., 2008, Muhlenhoff et al., 2011) and perhaps Nfu1 (Navarro-Sastre et al., 2011, Cameron et al., 2011, Lill et al., 2012). Nfu1 is likely a targeting factor to Fe-S client proteins in bacteria (Py et al., 2012). Yeast depleted of ISU components or the Fe-S targeting factors Grx5, ISA components and Nfu1 are impaired in SDH activity (Jensen and Culotta, 2000, Rodriguez-Manzaneque et al., 2002, Muhlenhoff et al., 2011). Mutations in human orthologs of Isd11, Isu1, Iba57 or Nfu1 (Crooks et al., 2012, Hall et al., 1993, Lim et al., 2013, Navarro-Sastre et al., 2011, Cameron et al., 2011, Ferrer-Cortes et al., 2013, Ajit Bolar et al., 2013) or RNAi depletion of Isa1, or Isa2 lead to compromised SDH function (Sheftel et al., 2012) (Figure 2).
Recently, mutations in a novel mitochondrial protein BolA3 were shown to result in defects in similar respiratory complexes and 2-oxoacid dehydrogenases as mutations in Nfu1 (Cameron et al., 2011, Baker et al., 2014), suggesting that BolA3 may likewise function in late stages of mitochondrial Fe-S biogenesis or transfer. BolA proteins typically function with glutaredoxins (Li and Outten, 2012), therefore, one prediction is that BolA3 has a role in conjunction with Grx5.
Sdh2 cofactor insertion
The architecture of Sdh2 raises several questions regarding cofactor insertion and maturation. Analogous to Sdh1, which receives its FAD cofactor within the mitochondrial matrix, Sdh2 receives its three Fe-S clusters in the matrix after import (Figure 3). Based on the Fe-S biogenesis pathway outlined above, one prediction is that the 2Fe-2S center in the N-terminal domain of Sdh2 is received from the Grx5:GSH complex, whereas the clusters in the C-terminal domain may be populated by the late-stage targeting factors ISA and Nfu1. Recently, Maio and colleagues reported that the mammalian Jac1 ortholog HSC20, the Ssq1 ortholog HSPA9 and the Isu1 scaffold, ISCU, form a complex with SDHB (Maio et al., 2014). This SDHB assembly intermediate was visualized on BN-PAGE after co-immunoprecipitation of SDHB. SDH activity appeared to be attenuated upon depletion of HSC20 or HSPA9 using siRNA knock down. Therefore, the study suggested that cluster transfer to SDHB occurs within this complex. In vitro synthesized SDHB is readily imported into isolated mitochondria. Co-immunoprecipitation of the imported SDHB at different time points revealed that the interaction of SDHA with SDHB comes later than the formation of the HSC20/HSPA9/ISCU/SDHB complex, indicating that cluster transfer may precede binding of SDHB to SDHA. However, the ISA components and Nfu1 failed to be co-adsorbed with the HSC20 pulldown. These results may imply that only the 2Fe-2S center is transferred within the HSC20/HSPA9/ISCU/SDHB complex and a subsequent transfer step mediates insertion of the C-terminal SDHB clusters. This process could be either a zip-up from 2Fe-2S through 3Fe-4S or a zip-down from 3Fe-4S through 2Fe-2S. Alternatively, SDHB may remain associated with HSC20 during the insertion of the 4Fe-4S and 3Fe-4S centers, although the targeting factors may not stably associate with the HSC20/HSPA9/ISCU/SDHB complex (Maio et al., 2014).
It should be noted that reduced Cys thiolates are required for Fe-S clusters to be assembled into Sdh2 as oxidized Cys ligands cannot coordinate Fe-S clusters. However, it is unknown whether cells depend on the thioredoxin/thioredoxin reductase, glutathione/glutathione reductase or peroxiredoxin to ensure the presence of thiolates for Fe-S coordination. It is also intriguing how meta-stable Sdh2 intermediates are protected specially from ROS after Fe-S cluster insertion. It has been well known that Fe-S clusters are extremely susceptible for damage upon expose to oxygen (Imlay, 2006). Since 2Fe-2S and 3Fe-4S clusters are exposed to solvent in Sdh2 prior to association with Sdh1 and the membrane anchor domain, it is possible that specialized chaperones are required to sequester these Fe-S clusters from oxidative stressors present in the solvent.
In the following section, we discuss two recently reported SDH assembly factors required for the maturation of Sdh2.
Sdh6 and Sdh7 are chaperone required for Sdh2 maturation
Sdh6 (SDHAF1 in human) is the first SDH assembly factor reported. Mutations in human SDHAF1 compromise the stable assembly of SDH, which leads to SDH deficiency associated with infantile leukoencephalophaty (Ghezzi et al., 2009, Ohlenbusch et al., 2012). Sdh6 is a small mitochondrial matrix protein that belongs to the LYR motif protein family, which is defined by the presence of a short LX(L/A)YRXX(L/I)(R/K) motif. Previous studies showed that the functions of several other LYR motif proteins are related to Fe-S cluster metabolism. Ghezzi and colleagues reported that yeast or human cells lacking wild-type Sdh6 (or its ortholog) exhibited significantly reduced SDH activity, accompanied by attenuation of SDH assembly (Ghezzi et al., 2009). They suggested the possibility that Sdh6 is an assembly factor required for the Fe-S containing subunit, Sdh2, based on the fact that Sdh6 is an LYR motif protein. While prescient, this hypothesis remained unproven as the biochemical data supporting it were not yet available. We recently reported that Sdh6 is indeed an assembly factor required for stable Sdh2 maturation during SDH assembly (Na et al., 2014). In yeast cells lacking Sdh6, Sdh2 steady-state levels were significantly diminished, but Sdh1 steady-state levels and covalent flavinylation remained unaffected. Moreover, Sdh6 appeared to accumulate in sdh3Δ or sdh4Δ cells lacking the SDH membrane anchor domain where an Sdh1/Sdh2 subcomplex accumulated. Furthermore, immunoprecipitation of Sdh6 in mitochondrial lysates from cells lacking Sdh4 resulted in co-precipitation of Sdh1 and Sdh2, but immunoprecipitation of Sdh6 in WT or sdh2Δ mutants failed to yield Sdh1 or Sdh2. Thus, these results suggested that Sdh6 interacts with an Sdh1/Sdh2 complex and the interface for the interaction resides within Sdh2 (Figure 3). Indeed, Sdh6 overexpression in sdh1Δ mutants, in which Sdh2 is extremely labile, increased Sdh2 accumulation, thus corroborating the postulate that Sdh6 acts on Sdh2 during SDH assembly.
Why does Sdh2 require the function of Sdh6 during the process of SDH assembly? One possibility is that Sdh6 is a chaperone stabilizing Sdh2 prior to Sdh1 association. However, Sdh2 overexpression failed to restore SDH activity in sdh6Δ mutants. Thus, Sdh6 likely exerts its function on Sdh2 maturation rather simply maintaining an apo-Sdh2 pool. Unbiased high-copy genetic suppressor screening provided a hint regarding Sdh6 function (Na et al., 2014). Yap1, a transcription factor that increases expression of a repertoire of genes required for oxidative stress tolerance was recovered from this screen. Yap1 overexpression enhanced the respiratory growth of sdh6Δ mutants and restored SDH activity. Conversely, artificially increased superoxide generation in the presence of paraquat severely impaired the respiratory growth of sdh6Δ cells. Moreover, Sdh2 protein levels under this condition were dramatically decreased. Given that ROS levels were not increased in sdh6Δ mutants, this exacerbated phenotype with paraquat suggests that Sdh6 is important for protecting Sdh2 from ROS-induced damage.
In the meantime, a genetic interaction between Sdh6 and Acn9 (renamed Sdh7), another protein in the LYR motif protein family, was observed. Sdh7 is also a small mitochondrial matrix protein, required for normal respiratory growth. Overexpression of Sdh6 in sdh7Δ mutants slightly rescued the respiratory growth defect although overexpression of Sdh7 in sdh6Δ mutants failed to do so. Metabolite profiling suggested an SDH deficiency in sdh7Δ mutant cells as succinate accumulated in the mutants. BN-PAGE analysis and SDH activity assay confirmed SDH deficiency with reduced SDH complex levels in sdh7Δ mutants. Biochemical analysis of sdh7Δ mutants suggested that Sdh7 function is similar to that of Sdh6 (Na et al., 2014). Among the SDH structural subunits, the steady-state levels of Sdh2 were the most impaired in sdh7Δ mutants. Sdh7 exhibited increased abundance and interaction with the Sdh1/Sdh2 subcomplex in cells lacking the SDH membrane anchor. In addition, Yap1 overexpression restored SDH activity and paraquat supplement markedly attenuated Sdh2 steady-state levels in sdh7Δ cells. However, Sdh2 overexpression failed to restore SDH activity in sdh7Δ cells. Thus, these results suggested that Sdh7 is another assembly factor for protecting Sdh2 from ROS damage. Deletion of the Drosophila melanogaster SDH7 ortholog, dSdhaf3, caused a dramatic SDH deficiency with muscular and neuronal defects that are reminiscent of neurodegeneration observed in humans with mutations in SDHAF1 (Na et al., 2014).
It is clear that Sdh6 and Sdh7 support the assembly of SDH under normal physiological conditions, although their deficiencies do not result in total ablation of SDH biogenesis. This might be due to a theoretical bypass pathway that can substitute for Sdh6 and Sdh7 functions. Otherwise, the importance of these two assembly factors is limited with passive roles for Sdh2 maturation under certain circumstances. Our study shows that iron salt supplementation enhances the respiratory growth of sdh6Δ cells and sdh7Δ cells. It has been shown that ROS-inactivated aconitase under aerobic conditions regains activity following iron salt supplementation (Kennedy et al., 1983). The active site of aconitase harbors a 4Fe-4S cluster, which loses one Fe atom upon exposure to superoxide, resulting in inactivation. Increased concentration of iron salts facilitates re-insertion of Fe(II) ion back into the damaged 3Fe-4S center, leading to reactivation of aconitase. Therefore, it is possible that Sdh6 and Sdh7 are involved in shielding the aforementioned solvent-exposed Fe-S centers from ROS until an Sdh1/Sdh2 complex is fully assembled with Sdh3 and Sdh4. A recent study on Fe-S cluster insertion to SDHB has revealed that SdhB (Sdh2) can be expressed as two separate domains in vivo and SDHAF1 (Sdh6) interacts with the C-terminal domain of SdhB, but not with the N-terminal domain (Maio et al., 2014). The C-terminal domain harbors 4Fe-4S and 3Fe-4S centers (Sun et al., 2005). Therefore, it is possible that Sdh6 is required for protecting the solvent-exposed 3Fe-4S center from ROS damage. However, there is no data suggesting how Sdh7 would exert its protection on Sdh2.
The origin of the damaging ROS is unclear, but a likely source is either the 2-oxoacid dehydrogenases including 2-oxoglutarate dehydrogenase and pyruvate dehydrogenase or the respiratory complexes (Quinlan et al., 2014). However, it is also possible that ROS generated intrinsically within an Sdh1/Sdh2 subcomplex might play a role. Like flavinylated monomeric Sdh1, an Sdh1/Sdh2 subcomplex is also competent to catalyze succinate oxidation (Nihei et al., 2001, Lemire and Oyedotun, 2002). It is expected that the electrons extracted from succinate oxidation can travel to Fe-S centers. Once the electrons reach the solvent-exposed surface via Fe-S centers, the electrons can react with oxygen to generate local ROS that can damage Fe-S centers in the vicinity. Therefore, one alternative role of Sdh6 and Sdh7 might be that they interact with an Sdh1/Sdh2 complex to inhibit the electron transfer to Fe-S centers within the subcomplex.
It has also been suggested that Sdh6 may be directly involved in the process for Fe-S insertion into Sdh2 based on the observation that the SDH assembly factor SDHAF1 (Sdh6) was recovered in the immunoadsorption of HSC20 (Maio et al., 2014). It remains unclear, however, whether SDHAF1 is a dedicated Fe-S targeting factor for SDHB since no additional evidence is available to show that Sdh6 has an active role in Fe-S cluster formation.
Sdh3 and Sdh4 – The hydrophobic anchor
Architecture of the hydrophobic anchor
The SDH membrane anchor consists of an Sdh3-Sdh4 heterodimer and an intercalated heme b moiety. The structure of mammalian SDH demonstrates that SDHC contains four total helices while SDHD contains five (Sun et al., 2005) (Figure 1). The N-terminal helix of SDHD is localized to the mitochondrial matrix and interacts with SDHB to promote membrane localization of the hydrophilic dimer. Each subunit contributes two transmembrane helices to the formation of a four-helix bundle, which comprises the core of the membrane anchor, while the remaining transmembrane helix from each subunit flanks the core. The remaining helices from each subunit protrude from the membrane and arrange in antiparallel fashion in the IMS essentially capping the transmembrane core. In addition to the helical arrangement of this domain, the structure reveals that SDHC and SDHD coordinate a heme b cofactor at the interface of the core four-helix bundle, which interacts with the porphyrin ring. Each subunit contributes a conserved histidine residue that coordinates the heme iron while two arginine residues, one from SDHC and one from SDHD, as well as another histidine residue from SDHC interact with the heme propionate groups (Sun et al., 2005).
The membrane anchor domain contains the site of ubiquinone binding and reduction, which ultimately facilitates electron transfer from succinate to subsequent ETC complexes (Sun et al., 2005). In fact, this domain contains two ubiquinone binding sites that are distinguished by their disparate affinities for ubiquinone (Oyedotun and Lemire, 2001, Silkin et al., 2007). The high affinity site (QP-proximal) lies on the matrix-proximal side of the IMM and is the dominant ubiquinone binding site (Figure 1). The QP site is composed of residues from SDHC, SDHD, and SDHB including the terminal 3Fe-4S cluster of SDHB (Sun et al., 2005). The second binding site (QD-distal) lies closer to the IMS side of the IMM and is a lower affinity site. This site is composed entirely of residues from SDHD (Sun et al., 2005). Ubiquinone reduction occurs in two stepwise single electron transfers, in contrast to the two-electron reduction of FAD. Importantly, the QP site stabilizes the partially reduced semiquinone intermediate and facilitates full reduction of ubiquinone to ubiquinol, thereby preventing the generation of reactive oxygen species (ROS) (Yankovskaya et al., 2003).
Assembly of the hydrophobic anchor
With respect to assembly of the hydrophobic anchor, there remain more questions than answers. Sdh3 and Sdh4 are translated in the cytosol and imported to mitochondria through the TOM complex. They may then be transferred to the TIM23 complex in the IMM and laterally released to the final destination like other α-helical transmembrane IMM proteins (Dudek et al., 2013). Unfortunately, membrane insertion represents the bulk of our knowledge regarding assembly of this dimer and thus many questions remain. Do Sdh3 and Sdh4 folding and subsequent dimer formation require a chaperone? How is the Sdh3-Sdh4 assembly intermediate stabilized prior to formation of the holo-complex? Although little is known about this process it is intriguing that stable Sdh3/Sdh4 dimerization requires the hydrophilic domain as deletion of either Sdh1 or Sdh2 causes near complete loss of both Sdh3 and Sdh4 (Kim et al., 2012, Na et al., 2014). Thus, it appears that biogenesis of the hydrophobic anchor is in some manner connected to the rest of the assembly process.
Perhaps the most intriguing question regarding the hydrophobic anchor relates to the heme b cofactor. In fact, it is unclear whether heme b is actually required for the electron transfer from FAD to ubiquinone in the QP site. The heme b lies further away from the 3Fe-4S (13.3 Å) than the QP site does (7.6 Å) (Yankovskaya et al., 2003, Sun et al., 2005). Moreover, the redox potential of heme b (−185 mV) is much lower than the 3Fe-4S (+ 60 mV) in SDH (Hägerhäll, 1997). Therefore, these two barriers would make the electron transfer from 3Fe-4S to heme b thermodynamically unfavorable compared to the direct electron transfer to ubiquinone (+113 mV). In fact, SDH complexes lacking heme b (from yeast cells expressing Sdh3 H106A and Sdh4 C78A heme-ligand mutants) appeared to be able to catalyze succinate-dependent quinone reduction (Oyedotun et al., 2007). It should be noted, however, that the heme b is important for the structural integrity of the membrane anchor domain in mammalian cells. SDH and SDHD steady-state levels were decreased in cells expressing SDHC His-ligand mutants (H127A or H127Y) in the absence of wild-type SDHC (Lemarie et al., 2011).
Regardless of its precise function, the heme b cofactor is present in SDH across all species, suggesting its importance in either electron transfer or complex stability. However, it is not yet known how Sdh3 and Sdh4 are assembled with heme b in the IMM. Does an assembly factor deliver and/or insert the heme b into a pre-existing Sdh3/Sdh4 dimer? It has been shown that several assembly factors, including Coa1, Coa2 and Shy1, are required for proper heme insertion into the Complex IV subunit Cox1 in the IMM (Mashkevich et al., 1997, Pierrel et al., 2007, Pierrel et al., 2008, Atkinson et al., 2010). Therefore, one might expect that an assembly factor is required for SDH hemylation. Overall, studies addressing the assembly of this domain represent an important next step in understanding SDH biogenesis.
Conclusion
Prior to the discovery of the assembly factors reviewed herein, it was difficult to fully appreciate the complexity of the SDH assembly pathway. This is perhaps best illustrated by the fact that at least four, and quite possible more, dedicated assembly factors are required for the maturation of the two soluble subunits alone. This does not even consider the process of assembling the Sdh3-Sdh4 hydrophobic domain. In the end, the assembly of SDH does not happen spontaneously, but is rather the result of a highly intricate and stepwise process facilitated by the concerted efforts of a number of accessory assembly factors.
These discoveries have dramatically increased our understanding of the assembly process and allow us to propose a more complete model of the SDH assembly pathway (Hao et al., 2009, Ghezzi et al., 2009, Van Vranken et al., 2014, Na et al., 2014) (Figure 3). Following cytosolic translation Sdh1 and Sdh2 are imported into the mitochondrial matrix as apo-proteins. Each of these subunits must subsequently mature in a process that is facilitated by subunit-specific assembly factors. Upon import, Sdh1 must be flavinylated and is bound by Sdh5, which enables this process. Although the precise mechanism remains poorly defined, Sdh5 most likely maintains apo-Sdh1 in a conformation that facilitates insertion and covalent binding of FAD. Once Sdh1 has been covalently flavinylated, Sdh5 is released and holo-Sdh1 binds another subunit-specific chaperone, Sdh8. Sdh8 appears to serve dual roles in the process of assembly. First, Sdh8 prevents the generation of superoxide by Sdh1 through limiting the spurious reduction of oxygen. In addition to this, Sdh8 also appears to facilitate the formation of the Sdh1-Sdh2 soluble dimer. Like Sdh1, apo-Sdh2 must also mature prior to complex formation. The initial step in the maturation of this subunit is the insertion of the three Fe-S clusters generated by the ISU and ISA complexes. At this point both Sdh1 and Sdh2 have matured into holo-proteins and can proceed through the assembly process. Sdh2 comes into association with two additional chaperones, Sdh6 and Sdh7 as it forms a soluble complex with Sdh1, thereby displacing Sdh8 and generating a heterotetrameric assembly intermediate. This intermediate, which is facilitated by Sdh6 and Sdh7, serves to protect surface-exposed Fe-S clusters and possibly prevents the spurious generation of superoxide by the redox active Sdh1-Sdh2 dimer. Finally, the Sdh1-Sdh2 dimer is bound by the Sdh3-Sdh4 hydrophobic domain, which may or may not be pre-assembled in the IMM, bringing the hydrophilic head in close association with the IMM and forming the active holo-complex (Figure 3).
In general, the contribution of subunit-specific assembly factors to the process of SDH assembly can be organized into three distinct functions. First, they mediate the insertion of essential cofactors. All ETC complexes utilize cofactors to perform the redox chemistry necessary to oxidize and reduce substrates and transfer electrons. Therefore the maturation of individual subunits and subsequent assembly of active complexes is dependent on the post-translational insertion of essential redox-active cofactors. This is highlighted by the fact that eukaryotic genomes have maintained a specific Sdh1/SDHA flavinylation factor, which is absolutely required for the covalent attachment of FAD to this subunit. It remains to be determined whether Sdh6 or Sdh7 also has an active role in the insertion of one or more of the Fe-S clusters in Sdh2.
Second, assembly factors act as subunit-specific chaperones that stabilize individual subunits and assembly intermediates. The process of SDH, and more generally, ETC assembly, relies on the step-wise assembly of potentially dozens of individual subunits translated from two different genomes to form a single intricately constructed complex. Because the structures of individual subunits are optimized to exist and function in the context of fully assembled complexes, it is not surprising that individual subunits and sub-complexes require chaperones to maintain stability during assembly. In terms of SDH assembly, this has been validated by discovery of specific factors that mediate the stability of both individual subunits and multimeric assembly intermediates. Indeed, work in Drosophila has demonstrated that the fly ortholog of Sdh8 is required for stabilization of holo-SdhA. Furthermore, the requirement for stabilizing holo-assembly intermediates is more manifested in the case of assembly intermediates containing oxidatively labile cofactors such as Fe-S centers. Sdh6 and Sdh7 appear to specifically bind and stabilize the Sdh1-Sdh2 soluble dimer prior to membrane association via Sdh3/Sdh4.
Third, assembly factors serve to prevent spurious and potentially deleterious interactions between individual redox-active subunits and the surrounding solvent. As a result of the unique chemistry enabled by their cofactors, individual ETC complex subunits are potentially toxic when not secluded within a fully assembled complex. Indeed, the cell contains numerous protein complexes that need to be assembled in an organized fashion, however, dedicated assembly factors are much more common for those complexes in which individual subunits contain redox-active cofactors. This is highlighted by the role of Sdh8 as a chaperone for covalently flavinylated Sdh1. In isolation, flavinylated Sdh1 is capable of oxidizing succinate, which results in the spurious generation of superoxide upon reduction of molecular oxygen. By occupying free Sdh1, Sdh8 serves to minimize these deleterious chemical reactions, thus protecting the matrix from ROS. Sdh6 and Sdh7, which specifically bind the potentially redox-active Sdh1-Sdh2 dimer, could potentially mediate similar protection as Sdh8.
In addition to providing valuable insights into the SDH assembly pathway the recent discovery of SDH assembly factors has also facilitated a greater understanding of SDH-deficient pathologies (Table 1). The literature has many reports of patients with SDH deficiencies, however, it is clear that only a subset of these cases can be explained by mutations in the genes encoding the four core subunits. With the discovery of a number of proteins intimately involved in the SDH assembly pathway, this disparity may now start to be resolved. Indeed, it is now clear that loss of function mutations in SDH assembly factors are capable of causing the same pathologies as the core subunits themselves. Analogous to mutations in SDHA, mutations in SDHAF1, the human ortholog of SDH6, were discovered as a cause of leukoencephalopathy, a neurodegenerative disorder similar to Leigh Syndrome (Ghezzi et al., 2009). Furthermore, mutations in SDHAF2, the human ortholog of SDH5, were shown to be the causative lesion in at least two families with familial paraganglioma syndrome, mirroring the physiological consequences of core subunit mutations (Hao et al., 2009). Currently there are no published reports describing human mutations in SDHAF3 (SDH7) or SDHAF4 (SDH8), however, these genes have only recently been implicated in the SDH assembly pathway. We suspect that, in time, mutations in these genes will ultimately be discovered in patients with SDH-deficient pathologies, thus further clarifying the connection between succinate dehydrogenase and human disease.
Table 1.
SDH associated genes and clinical phenotypes
| Yeast gene | Human ortholog | Disease |
|---|---|---|
| SDH1 | SDHA | Leigh Syndrome Paraganglioma/Pheochromocytoma WT-GIST |
| SDH2 | SDHB | Paraganglioma/Pheochromocytoma Renal Cell Carcinoma WT-GIST Infantile Leukodystrophy |
| SDH3 | SDHC | Paraganglioma/Pheochromocytoma Renal Cell Carcinoma WT-GIST |
| SDH4 | SDHD | Paraganglioma/Pheochromocytoma WT-GIST Progressive Encephalomyopathy |
| SDH5 | SDHAF2 | Paraganglioma/Pheochromocytoma |
| SDH6 | SDHAF1 | Infantile Leukodystrophy |
| SDH7 | SDHAF3 | N/A |
| SDH8 | SDHAF4 | N/A |
Footnotes
Declaration of Interest
The authors report no declaration of interest.
Literature Cited
Uncategorized References
- AJIT BOLAR N, VANLANDER AV, WILBRECHT C, VAN DER AA N, SMET J, DE PAEPE B, VANDEWEYER G, KOOY F, EYSKENS F, DE LATTER E, DELANGHE G, GOVAERT P, LEROY JG, LOEYS B, LILL R, VAN LAER L, VAN COSTER R. Mutation of the iron-sulfur cluster assembly gene IBA57 causes severe myopathy and encephalopathy. Hum Mol Genet. 2013;22:2590–602. doi: 10.1093/hmg/ddt107. [DOI] [PubMed] [Google Scholar]
- ALSTON CL, DAVISON JE, MELONI F, VAN DER WESTHUIZEN FH, HE L, HORNIG-DO H-T, PEET AC, GISSEN P, GOFFRINI P, FERRERO I, WASSMER E, MCFARLAND R, TAYLOR RW. Recessive germline SDHA and SDHB mutations causing leukodystrophy and isolated mitochondrial complex II deficiency. J Med Gen. 2012;49:569–577. doi: 10.1136/jmedgenet-2012-101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASTUTI D, LATIF F, DALLOL A, DAHIA PLM, DOUGLAS F, GEORGE E, SKÖLDBERG F, HUSEBYE ES, ENG C, MAHER ER. Gene Mutations in the Succinate Dehydrogenase Subunit SDHB Cause Susceptibility to Familial Pheochromocytoma and to Familial Paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATKINSON A, KHALIMONCHUK O, SMITH P, SABIC H, EIDE D, WINGE DR. Mzm1 Influences a Labile Pool of Mitochondrial Zinc Important for Respiratory Function. J Biol Chem. 2010;285:19450–19459. doi: 10.1074/jbc.M110.109793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAFUNNO V, GIANCASPERO TA, BRIZIO C, BUFANO D, PASSARELLA S, BOLES E, BARILE M. Riboflavin Uptake and FAD Synthesis in Saccharomyces cerevisiae Mitochondria: INVOLVEMENT OF THE Flx1p CARRIER IN FAD EXPORT. Journal of Biological Chemistry. 2004;279:95–102. doi: 10.1074/jbc.M308230200. [DOI] [PubMed] [Google Scholar]
- BAKER PR, 2ND, FRIEDERICH MW, SWANSON MA, SHAIKH T, BHATTACHARYA K, SCHARER GH, AICHER J, CREADON-SWINDELL G, GEIGER E, MACLEAN KN, LEE WT, DESHPANDE C, FRECKMANN ML, SHIH LY, WASSERSTEIN M, RASMUSSEN MB, LUND AM, PROCOPIS P, CAMERON JM, ROBINSON BH, BROWN GK, BROWN RM, COMPTON AG, DIECKMANN CL, COLLARD R, COUGHLIN CR, 2ND, SPECTOR E, WEMPE MF, VAN HOVE JL. Variant non ketotic hyperglycinemia is caused by mutations in LIAS, BOLA3 and the novel gene GLRX5. Brain : a journal of neurology. 2014;137:366–79. doi: 10.1093/brain/awt328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANCI L, BRANCACCIO D, CIOFI-BAFFONI S, DEL CONTE R, GADEPALLI R, MIKOLAJCZYK M, NERI S, PICCIOLI M, WINKELMANN J. [2Fe-2S] cluster transfer in iron-sulfur protein biogenesis. Proc Natl Acad Sci U S A. 2014;111:6203–8. doi: 10.1073/pnas.1400102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYSAL BE. Mutations in SDHD, a Mitochondrial Complex II Gene, in Hereditary Paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- BOURGERON T, RUSTIN P, CHRETIEN D, BIRCH-MACHIN M, BOURGEOIS M, VIEGAS-PEQUIGNOT E, MUNNICH A, ROTIG A. Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat Genet. 1995;11:144–9. doi: 10.1038/ng1095-144. [DOI] [PubMed] [Google Scholar]
- BRANDSCH R, BICHLER V. Covalent cofactor binding to flavoenzymes requires specific effectors. Eur J Biochem. 1989;182:125–8. doi: 10.1111/j.1432-1033.1989.tb14808.x. [DOI] [PubMed] [Google Scholar]
- BURNICHON N, BRIERE JJ, LIBE R, VESCOVO L, RIVIERE J, TISSIER F, JOUANNO E, JEUNEMAITRE X, BENIT P, TZAGOLOFF A, RUSTIN P, BERTHERAT J, FAVIER J, GIMENEZ-ROQUEPLO AP. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–20. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMERON JM, JANER A, LEVANDOVSKIY V, MACKAY N, ROUAULT TA, TONG WH, OGILVIE I, SHOUBRIDGE EA, ROBINSON BH. Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes. Am J Hum Genet. 2011;89:486–95. doi: 10.1016/j.ajhg.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CECCHINI G, SCHRODER I, GUNSALUS RP, MAKLASHINA E. Succinate dehydrogenase and fumarate reductase from Escherichia coli. Biochim Biophys Acta. 2002;1553:140–57. doi: 10.1016/s0005-2728(01)00238-9. [DOI] [PubMed] [Google Scholar]
- CIESIELSKI SJ, SCHILKE BA, OSIPIUK J, BIGELOW L, MULLIGAN R, MAJEWSKA J, JOACHIMIAK A, MARSZALEK J, CRAIG EA, DUTKIEWICZ R. Interaction of J-protein co-chaperone Jac1 with Fe-S scaffold Isu is indispensable in vivo and conserved in evolution. J Mol Biol. 2012;417:1–12. doi: 10.1016/j.jmb.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROOKS DR, JEONG SY, TONG WH, GHOSH MC, OLIVIERRE H, HALLER RG, ROUAULT TA. Tissue specificity of a human mitochondrial disease: differentiation-enhanced mis-splicing of the Fe-S scaffold gene ISCU renders patient cells more sensitive to oxidative stress in ISCU myopathy. J Biol Chem. 2012;287:40119–30. doi: 10.1074/jbc.M112.418889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAZ F, KOTARSKY H, FELLMAN V, MORAES CT. Mitochondrial disorders caused by mutations in respiratory chain assembly factors. Semin Fetal Neonatal Med. 2011;16:197–204. doi: 10.1016/j.siny.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEK J, REHLING P, VAN DER LAAN M. Mitochondrial protein import: Common principles and physiological networks. BBA-Mol Cell Res. 2013;1833:274–285. doi: 10.1016/j.bbamcr.2012.05.028. [DOI] [PubMed] [Google Scholar]
- ELETSKY A, JEONG M-Y, KIM H, LEE H-W, XIAO R, PAGLIARINI DJ, PRESTEGARD JH, WINGE DR, MONTELIONE GT, SZYPERSKI T. Solution NMR Structure of Yeast Succinate Dehydrogenase Flavinylation Factor Sdh5 Reveals a Putative Sdh1 Binding Site. Biochemistry. 2012;51:8475–8477. doi: 10.1021/bi301171u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ-VIZARRA E, TIRANTI V, ZEVIANI M. Assembly of the oxidative phosphorylation system in humans: what we have learned by studying its defects. Biochim Biophys Acta. 2009;1793:200–11. doi: 10.1016/j.bbamcr.2008.05.028. [DOI] [PubMed] [Google Scholar]
- FERRER-CORTES X, FONT A, BUJAN N, NAVARRO-SASTRE A, MATALONGA L, ARRANZ JA, RIUDOR E, DEL TORO M, GARCIA-CAZORLA A, CAMPISTOL J, BRIONES P, RIBES A, TORT F. Protein expression profiles in patients carrying NFU1 mutations. Contribution to the pathophysiology of the disease. J Inherit Metab Dis. 2013;36:841–7. doi: 10.1007/s10545-012-9565-z. [DOI] [PubMed] [Google Scholar]
- GELLING C, DAWES IW, RICHHARDT N, LILL R, MUHLENHOFF U. Mitochondrial Iba57p is required for Fe/S cluster formation on aconitase and activation of radical SAM enzymes. Mol Cell Biol. 2008;28:1851–61. doi: 10.1128/MCB.01963-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHEZZI D, GOFFRINI P, UZIEL G, HORVATH R, KLOPSTOCK T, LOCHMULLER H, D'ADAMO P, GASPARINI P, STROM TM, PROKISCH H, INVERNIZZI F, FERRERO I, ZEVIANI M. SDHAF1, encoding a LYR complex-II specific assembly factor, is mutated in SDH-defective infantile leukoencephalopathy. Nat Genet. 2009;41:654–6. doi: 10.1038/ng.378. [DOI] [PubMed] [Google Scholar]
- GUZY RD, SHARMA B, BELL E, CHANDEL NS, SCHUMACKER PT. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol. 2008;28:718–31. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HÄGERHÄLL C. Succinate:quinone oxidoreductases. variations on a censerved theme. Biochim Biophys Acta. 1997;1320:107–141. doi: 10.1016/s0005-2728(97)00019-4. [DOI] [PubMed] [Google Scholar]
- HALL RE, HENRIKSSON KG, LEWIS SF, HALLER RG, KENNAWAY NG. Mitochondrial myopathy with succinate dehydrogenase and aconitase deficiency. Abnormalities of several iron-sulfur proteins. J Clin Invest. 1993;92:2660–6. doi: 10.1172/JCI116882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAO HX, KHALIMONCHUK O, SCHRADERS M, DEPHOURE N, BAYLEY JP, KUNST H, DEVILEE P, CREMERS CW, SCHIFFMAN JD, BENTZ BG, GYGI SP, WINGE DR, KREMER H, RUTTER J. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–42. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOEKSTRA AS, BAYLEY J-P. The role of complex II in disease. BBA-Bioenergetics. 2013;1827:543–551. doi: 10.1016/j.bbabio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- HORVÁTH R, ABICHT A, HOLINSKI-FEDER E, LANER A, GEMPEL K, PROKISCH H, LOCHMÜLLER H, KLOPSTOCK T, JAKSCH M. Leigh syndrome caused by mutations in the flavoprotein (Fp) subunit of succinate dehydrogenase (SDHA) J Neurol, Neurosurg Psychiatry. 2006;77:74–76. doi: 10.1136/jnnp.2005.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMLAY JA. Iron-sulphur clusters and the problem with oxygen. Mol Microbiol. 2006;59:1073–82. doi: 10.1111/j.1365-2958.2006.05028.x. [DOI] [PubMed] [Google Scholar]
- ISHII T, YASUDA K, AKATSUKA A, HINO O, HARTMAN PS, ISHII N. A Mutation in the SDHC Gene of Complex II Increases Oxidative Stress, Resulting in Apoptosis and Tumorigenesis. Cancer Res. 2005;65:203–209. [PubMed] [Google Scholar]
- JACKSON CB, NUOFFER J-M, HAHN D, PROKISCH H, HABERBERGER B, GAUTSCHI M, HÄBERLI A, GALLATI S, SCHALLER A. Mutations in SDHD lead to autosomal recessive encephalomyopathy and isolated mitochondrial complex II deficiency. Journal of Medical Genetics. 2014;51:170–175. doi: 10.1136/jmedgenet-2013-101932. [DOI] [PubMed] [Google Scholar]
- JAIN-GHAI S, CAMERON JM, AL MAAWALI A, BLASER S, MACKAY N, ROBINSON B, RAIMAN J. Complex II deficiency--a case report and review of the literature. Am J Med Genet A. 2013;161A:285–94. doi: 10.1002/ajmg.a.35714. [DOI] [PubMed] [Google Scholar]
- JANEWAY KA KS, LODISH M, NOSÉ V, RUSTIN P, GAAL J, DAHIA PL, LIEGL B, BALL ER, RAYGADA M, LAI AH, KELLY L, HORNICK JL, NIH PEDIATRIC AND WILD-TYPE GIST CLINIC. O'SULLIVAN M, DE KRIJGER RR, DINJENS WN, DEMETRI GD, ANTONESCU CR, FLETCHER JA, HELMAN L, STRATAKIS CA. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN LT, CULOTTA VC. Role of Saccharomyces cerevisiae ISA1and ISA2 in Iron Homeostasis. Molecular and Cellular Biology. 2000;20:3918–3927. doi: 10.1128/mcb.20.11.3918-3927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHANSSON C, ROOS AK, MONTANO SJ, SENGUPTA R, FILIPPAKOPOULOS P, GUO K, VON DELFT F, HOLMGREN A, OPPERMANN U, KAVANAGH KL. The crystal structure of human GLRX5: iron-sulfur cluster co-ordination, tetrameric assembly and monomer activity. Biochem J. 2011;433:303–11. doi: 10.1042/BJ20101286. [DOI] [PubMed] [Google Scholar]
- KENNEDY MC, EMPTAGE MH, DREYER JL, BEINERT H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983;258:11098–105. [PubMed] [Google Scholar]
- KIM HJ, JEONG MY, NA U, WINGE DR. Flavinylation and assembly of succinate dehydrogenase are dependent on the C-terminal tail of the flavoprotein subunit. J Biol Chem. 2012;287:40670–9. doi: 10.1074/jbc.M112.405704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM HJ, WINGE DR. Emerging concepts in the flavinylation of succinate dehydrogenase. Biochim Biophys Acta. 2013;1827:627–36. doi: 10.1016/j.bbabio.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOUNOSU A. Analysis of covalent flavinylation using thermostable succinate dehydrogenase from Thermus thermophilus and Sulfolobus tokodaii lacking SdhE homologs. FEBS Letters. 2014;588:1058–1063. doi: 10.1016/j.febslet.2014.02.022. [DOI] [PubMed] [Google Scholar]
- LEMARIE A, HUC L, PAZARENTZOS E, MAHUL-MELLIER AL, GRIMM S. Specific disintegration of complex II succinate:ubiquinone oxidoreductase links pH changes to oxidative stress for apoptosis induction. Cell Death Differ. 2011;18:338–49. doi: 10.1038/cdd.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMIRE BD, OYEDOTUN KS. The Saccharomyces cerevisiae mitochondrial succinate:ubiquinone oxidoreductase. Biochim Biophys Acta. 2002;1553:102–16. doi: 10.1016/s0005-2728(01)00229-8. [DOI] [PubMed] [Google Scholar]
- LI H, OUTTEN CE. Monothiol CGFS glutaredoxins and BolA-like proteins: [2Fe-2S] binding partners in iron homeostasis. Biochemistry. 2012;51:4377–89. doi: 10.1021/bi300393z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILL R, HOFFMANN B, MOLIK S, PIERIK AJ, RIETZSCHEL N, STEHLING O, UZARSKA MA, WEBERT H, WILBRECHT C, MUHLENHOFF U. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta. 2012;1823:1491–508. doi: 10.1016/j.bbamcr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- LIM SC, FRIEMEL M, MARUM JE, TUCKER EJ, BRUNO DL, RILEY LG, CHRISTODOULOU J, KIRK EP, BONEH A, DEGENNARO CM, SPRINGER M, MOOTHA VK, ROUAULT TA, LEIMKUHLER S, THORBURN DR, COMPTON AG. Mutations in LYRM4, encoding iron-sulfur cluster biogenesis factor ISD11, cause deficiency of multiple respiratory chain complexes. Hum Mol Genet. 2013;22:4460–73. doi: 10.1093/hmg/ddt295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIO N, SINGH A, UHRIGSHARDT H, SAXENA N, TONG WH, ROUAULT TA. Cochaperone Binding to LYR Motifs Confers Specificity of Iron Sulfur Cluster Delivery. Cell Metab. 2014;19:445–57. doi: 10.1016/j.cmet.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJEWSKA J, CIESIELSKI SJ, SCHILKE B, KOMINEK J, BLENSKA A, DELEWSKI W, SONG JY, MARSZALEK J, CRAIG EA, DUTKIEWICZ R. Binding of the chaperone Jac1 protein and cysteine desulfurase Nfs1 to the iron-sulfur cluster scaffold Isu protein is mutually exclusive. J Biol Chem. 2013;288:29134–42. doi: 10.1074/jbc.M113.503524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASHKEVICH G, REPETTO B, GLERUM DM, JIN C, TZAGOLOFF A. SHY1, the Yeast Homolog of the MammalianSURF-1 Gene, Encodes a Mitochondrial Protein Required for Respiration. Journal of Biological Chemistry. 1997;272:14356–14364. doi: 10.1074/jbc.272.22.14356. [DOI] [PubMed] [Google Scholar]
- MESSNER KR, IMLAY JA. Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J Biol Chem. 2002;277:42563–71. doi: 10.1074/jbc.M204958200. [DOI] [PubMed] [Google Scholar]
- MUHLENHOFF U, RICHTER N, PINES O, PIERIK AJ, LILL R. Specialized function of yeast Isa1 and Isa2 proteins in the maturation of mitochondrial [4Fe-4S] proteins. J Biol Chem. 2011;286:41205–16. doi: 10.1074/jbc.M111.296152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NA U, YU W, COX JE, BRICKER DK, BROCKMANN K, RUTTER J, THUMMEL CS, WINGE DR. The LYR Factors SDHAF1 and SDHAF3 Mediate Maturation of the Iron-Sulfur Subunit of Succinate Dehydrogenase. Cell Metabolism. 2014;20:253–266. doi: 10.1016/j.cmet.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAVARRO-SASTRE A, TORT F, STEHLING O, UZARSKA MA, ARRANZ JA, DEL TORO M, LABAYRU MT, LANDA J, FONT A, GARCIA-VILLORIA J, MERINERO B, UGARTE M, GUTIERREZ-SOLANA LG, CAMPISTOL J, GARCIA-CAZORLA A, VAQUERIZO J, RIUDOR E, BRIONES P, ELPELEG O, RIBES A, LILL R. A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am J Hum Genet. 2011;89:656–67. doi: 10.1016/j.ajhg.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIHEI C, NAKAYASHIKI T, NAKAMURA K, INOKUCHI H, GENNIS RB, KOJIMA S, KITA K. Abortive assembly of succinate-ubiquinone reductase (complex II) in a ferrochelatase-deficient mutant of Escherichia coli. Mol Genet Genomics. 2001;265:394–404. doi: 10.1007/s004380100444. [DOI] [PubMed] [Google Scholar]
- OHLENBUSCH A, EDVARDSON S, SKORPEN J, BJORNSTAD A, SAADA A, ELPELEG O, GARTNER J, BROCKMANN K. Leukoencephalopathy with accumulated succinate is indicative of SDHAF1 related complex II deficiency. Orphanet J Rare Dis. 2012;7:69. doi: 10.1186/1750-1172-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OYEDOTUN KS, LEMIRE BD. The Quinone-binding sites of the Saccharomyces cerevisiae succinate-ubiquinone oxidoreductase. J Biol Chem. 2001;276:16936–43. doi: 10.1074/jbc.M100184200. [DOI] [PubMed] [Google Scholar]
- OYEDOTUN KS, SIT CS, LEMIRE BD. The Saccharomyces cerevisiae succinate dehydrogenase does not require heme for ubiquinone reduction. Biochim Biophys Acta. 2007;1767:1436–45. doi: 10.1016/j.bbabio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- PANDEY A, GOLLA R, YOON H, DANCIS A, PAIN D. Persulfide formation on mitochondrial cysteine desulfurase: enzyme activation by a eukaryote-specific interacting protein and Fe-S cluster synthesis. Biochem J. 2012;448:171–87. doi: 10.1042/BJ20120951. [DOI] [PubMed] [Google Scholar]
- PANDEY A, GORDON DM, PAIN J, STEMMLER TL, DANCIS A, PAIN D. Frataxin directly stimulates mitochondrial cysteine desulfurase by exposing substrate-binding sites, and a mutant Fe-S cluster scaffold protein with frataxin-bypassing ability acts similarly. J Biol Chem. 2013;288:36773–86. doi: 10.1074/jbc.M113.525857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANTALEO MA, ASTOLFI A, INDIO V. SDHA loss-of-function mutations in KIT-PDGFRA wild-type gastrointestinal stromal tumors identified by massively parallel sequencing. J Natl Cancer Inst. 2011;103:983–987. doi: 10.1093/jnci/djr130. [DOI] [PubMed] [Google Scholar]
- PANTALEO MA, ASTOLFI A, URBINI M, NANNINI M, PATERINI P, INDIO V, SAPONARA M, FORMICA S, CECCARELLI C, CASADIO R, ROSSI G, BERTOLINI F, SANTINI D, PIRINI MG, FIORENTINO M, BASSO U, BIASCO G. Analysis of all subunits, SDHA, SDHB, SDHC, SDHD, of the succinate dehydrogenase complex in KIT/PDGFRA wild-type GIST. Eur J Hum Genet. 2014;22:32–9. doi: 10.1038/ejhg.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECZKOWSKA M, CASCON A, PREJBISZ A, KUBASZEK A, B CWIKLA J, FURMANEK M, ERLIC Z, ENG C, JANUSZEWICZ A, NEUMANN HPH. Extra-adrenal and adrenal pheochromocytomas associated with a germline SDHC mutation. Nat Clin Pract End Met. 2008;4:111–115. doi: 10.1038/ncpendmet0726. [DOI] [PubMed] [Google Scholar]
- PIERREL F, BESTWICK ML, COBINE PA, KHALIMONCHUK O, CRICCO JA, WINGE DR. Coa1 links the Mss51 post-translational function to Cox1 cofactor insertion in cytochrome c oxidase assembly. 2007 doi: 10.1038/sj.emboj.7601861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERREL F, KHALIMONCHUK O, COBINE PA, BESTWICK M, WINGE DR. Coa2 Is an Assembly Factor for Yeast Cytochrome c Oxidase Biogenesis That Facilitates the Maturation of Cox1. Mol Cell Biol. 2008;28:4927–4939. doi: 10.1128/MCB.00057-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PY B, GEREZ C, ANGELINI S, PLANEL R, VINELLA D, LOISEAU L, TALLA E, BROCHIER-ARMANET C, GARCIA SERRES R, LATOUR JM, OLLAGNIER-DE CHOUDENS S, FONTECAVE M, BARRAS F. Molecular organization, biochemical function, cellular role and evolution of NfuA, an atypical Fe-S carrier. Mol Microbiol. 2012;86:155–71. doi: 10.1111/j.1365-2958.2012.08181.x. [DOI] [PubMed] [Google Scholar]
- QUINLAN CL, GONCALVES RL, HEY-MOGENSEN M, YADAVA N, BUNIK VI, BRAND MD. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. J Biol Chem. 2014;289:8312–25. doi: 10.1074/jbc.M113.545301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON KM, LEMIRE BD. Covalent attachment of FAD to the yeast succinate dehydrogenase flavoprotein requires import into mitochondria, presequence removal, and folding. J. Biol. Chem. 1996;271:4055–4060. doi: 10.1074/jbc.271.8.4055. [DOI] [PubMed] [Google Scholar]
- ROBINSON KM, ROTHERY RA, WEINER JH, LEMIRE BD. The covalent attachment of FAD to the flavoprotein of Saccharomyces cerevisiae succinate dehydrogenase is not necessary for import and assembly into mitochondria. Eur J Biochem. 1994;222:983–90. doi: 10.1111/j.1432-1033.1994.tb18949.x. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-MANZANEQUE MAT, TAMARIT J, BELLÍ G, ROS J, HERRERO E. Grx5 Is a Mitochondrial Glutaredoxin Required for the Activity of Iron/Sulfur Enzymes. Molecular Biology of the Cell. 2002;13:1109–1121. doi: 10.1091/mbc.01-10-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUTTER J, WINGE DR, SCHIFFMAN JD. Succinate dehydrogenase - Assembly, regulation and role in human disease. Mitochondrion. 2010;10:393–401. doi: 10.1016/j.mito.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTOS MAA, JIMÉNEZ A, REVUELTA J. Molecular Characterization of FMN1, the Structural Gene for the Monofunctional Flavokinase of Saccharomyces cerevisiae. Journal of Biological Chemistry. 2000;275:28618–28624. doi: 10.1074/jbc.M004621200. [DOI] [PubMed] [Google Scholar]
- SCHMUCKER S, MARTELLI A, COLIN F, PAGE A, WATTENHOFER-DONZE M, REUTENAUER L, PUCCIO H. Mammalian frataxin: an essential function for cellular viability through an interaction with a preformed ISCU/NFS1/ISD11 iron-sulfur assembly complex. PLoS ONE. 2011;6:e16199. doi: 10.1371/journal.pone.0016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEFTEL AD, STEHLING O, PIERIK AJ, ELSASSER HP, MUHLENHOFF U, WEBERT H, HOBLER A, HANNEMANN F, BERNHARDT R, LILL R. Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc Natl Acad Sci U S A. 2010;107:11775–80. doi: 10.1073/pnas.1004250107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEFTEL AD, WILBRECHT C, STEHLING O, NIGGEMEYER B, ELSASSER HP, MUHLENHOFF U, LILL R. The human mitochondrial ISCA1, ISCA2, and IBA57 proteins are required for [4Fe-4S] protein maturation. Mol Biol Cell. 2012;23:1157–66. doi: 10.1091/mbc.E11-09-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILKIN Y, OYEDOTUN KS, LEMIRE BD. The role of Sdh4p Tyr-89 in ubiquinone reduction by the Saccharomyces cerevisiae succinate dehydrogenase. Biochim Biophys Acta. 2007;1767:143–50. doi: 10.1016/j.bbabio.2006.11.017. [DOI] [PubMed] [Google Scholar]
- SUN F, HUO X, ZHAI Y, WANG A, XU J, SU D, BARTLAM M, RAO Z. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell. 2005;121:1043–57. doi: 10.1016/j.cell.2005.05.025. [DOI] [PubMed] [Google Scholar]
- TORCHETTI EM, BRIZIO C, COLELLA M, GALLUCCIO M, GIANCASPERO TA, INDIVERI C, ROBERTI M, BARILE M. Mitochondrial localization of human FAD synthetase isoform 1. Mitochondrion. 2010;10:263–73. doi: 10.1016/j.mito.2009.12.149. [DOI] [PubMed] [Google Scholar]
- TSAI CL, BARONDEAU DP. Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry. 2010;49:9132–9. doi: 10.1021/bi1013062. [DOI] [PubMed] [Google Scholar]
- TZAGOLOFF A, JANG J, GLERUM DM, WU M. FLX1 Codes for a Carrier Protein Involved in Maintaining a Proper Balance of Flavin Nucleotides in Yeast Mitochondria. Journal of Biological Chemistry. 1996;271:7392–7397. doi: 10.1074/jbc.271.13.7392. [DOI] [PubMed] [Google Scholar]
- UZARSKA MA, DUTKIEWICZ R, FREIBERT SA, LILL R, MUHLENHOFF U. The mitochondrial Hsp70 chaperone Ssq1 facilitates Fe/S cluster transfer from Isu1 to Grx5 by complex formation. Mol Biol Cell. 2013;24:1830–41. doi: 10.1091/mbc.E12-09-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN VRANKEN JG, BRICKER DK, DEPHOURE N, GYGI SP, COX JE, THUMMEL CS, RUTTER J. SDHAF4 Promotes Mitochondrial Succinate Dehydrogenase Activity and Prevents Neurodegeneration. Cell Metab. 2014;20:241–52. doi: 10.1016/j.cmet.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHARANTA S, BUCHTA M, MCWHINNEY SR, VIRTA SK, PEÇZKOWSKA M, MORRISON CD, LEHTONEN R, JANUSZEWICZ A, JÄRVINEN H, JUHOLA M, MECKLIN J-P, PUKKALA E, HERVA R, KIURU M, NUPPONEN NN, AALTONEN LA, NEUMANN HPH, ENG C. Early-Onset Renal Cell Carcinoma as a Novel Extraparaganglial Component of SDHB-Associated Heritable Paraganglioma. The American Journal of Human Genetics. 2004;74:153–159. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU M, REPETTO B, GLERUM DM, TZAGOLOFF A. Cloning and characterization of FAD1, the structural gene for flavin adenine dinucleotide synthetase of Saccharomyces cerevisiae. Molecular and Cellular Biology. 1995;15:264–71. doi: 10.1128/mcb.15.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANKOVSKAYA V, HORSEFIELD R, TORNROTH S, LUNA-CHAVEZ C, MIYOSHI H, LEGER C, BYRNE B, CECCHINI G, IWATA S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–4. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]