Abstract
This review summarizes the (patho)-physiological effects of ventilation with high FiO2 (0.8–1.0), with a special focus on the most recent clinical evidence on its use for the management of circulatory shock and during medical emergencies. Hyperoxia is a cornerstone of the acute management of circulatory shock, a concept which is based on compelling experimental evidence that compensating the imbalance between O2 supply and requirements (i.e., the oxygen dept) is crucial for survival, at least after trauma. On the other hand, “oxygen toxicity” due to the increased formation of reactive oxygen species limits its use, because it may cause serious deleterious side effects, especially in conditions of ischemia/reperfusion. While these effects are particularly pronounced during long-term administration, i.e., beyond 12–24 h, several retrospective studies suggest that even hyperoxemia of shorter duration is also associated with increased mortality and morbidity. In fact, albeit the clinical evidence from prospective studies is surprisingly scarce, a recent meta-analysis suggests that hyperoxia is associated with increased mortality at least in patients after cardiac arrest, stroke, and traumatic brain injury. Most of these data, however, originate from heterogenous, observational studies with inconsistent results, and therefore, there is a need for the results from the large scale, randomized, controlled clinical trials on the use of hyperoxia, which can be anticipated within the next 2–3 years. Consequently, until then, “conservative” O2 therapy, i.e., targeting an arterial hemoglobin O2 saturation of 88–95 % as suggested by the guidelines of the ARDS Network and the Surviving Sepsis Campaign, represents the treatment of choice to avoid exposure to both hypoxemia and excess hyperoxemia.
Background
The “double-edged sword” character of molecular oxygen (O2) is well established and has been a matter of debate since its discovery at the end of the eighteenth century. On the one hand, O2 plays a crucial role during adenosine triphosphate (ATP) synthesis [1]. On the other hand, its chemical characteristics lead to strong oxidizing properties, capable of damaging any biological molecule [1], and thereby defining the paradigm of oxygen toxicity. This phenomenon is due to the formation of reactive oxygen species (ROS), its magnitude being directly correlated to the level of the O2 partial pressure [2, 3]. Moreover, during mitochondrial respiration, 1–3 % of O2 consumption leads to ROS formation [3]. Like O2, ROS also exert Janus-headed properties: while being of importance for host defense and signaling cascades, their toxic effects are well known [4].
Circulatory shock is defined as “…an imbalance between O2 supply and requirements…” [5], and consequently, a logical therapeutic strategy is to increase the inspired O2 concentration (FiO2). The recommendation that the administration of oxygen should be started immediately to increase O2 delivery [6] has been known for a long time as a part of the “V” (“ventilate”) component of the “VIP” (“ventilate–infuse–pump”) rule for shock resuscitation [6]. Due to this, supplemental O2 was an integral part of all resuscitation protocols of the recently published Protocolized Care for Early Septic Shock (ProCESS) trial [7]. However, literature data concerning the high-dose administration of O2 are still highly controversial [8–14]. Moreover, hyperoxia (i.e., an increased FiO2) must be distinguished from hyperoxemia (i.e. increased arterial O2 partial pressure): in patients with severe Acute Respiratory Distress Syndrome (ARDS), hyperoxia may be mandatory to avoid hypoxemia with the least mechanical ventilation-induced hemodynamic compromise and/or ventilator-induced damage to the lung possible. This is nicely demonstrated by the results of the ARDSNetwork trial on low tidal volume ventilation: the FiO2 was significantly higher in the group with lower tidal volumes that ultimately had improved survival [15]. Figure 1 [16] summarizes the key pro and con arguments concerning the use of O2 therapy during shock states. Data from prospective, controlled, randomized trials on the use of therapeutic hyperoxia, however, are surprisingly scarce. Consequently, given the possible deleterious side effects of hyperoxia, the current guidelines of the ARDSNetwork and the Surviving Sepsis Campaign recommend using an FiO2 that allows achieving an arterial hemoglobin O2 saturation of 88–95 % at airway plateau pressures and PEEP levels of <30 and 5–20 cmH2O, respectively [15, 17]. The present review will, therefore, discuss the role of ventilation with high FiO2 (0.8–1.0) during circulatory shock, during medical emergencies and in the peri-operative period; the first part will briefly summarize the (patho)-physiological effects of hyperoxia, the second part will review its use in the context of important pathological entities, with a particular focus on the most recent clinical evidence.
Fig. 1.
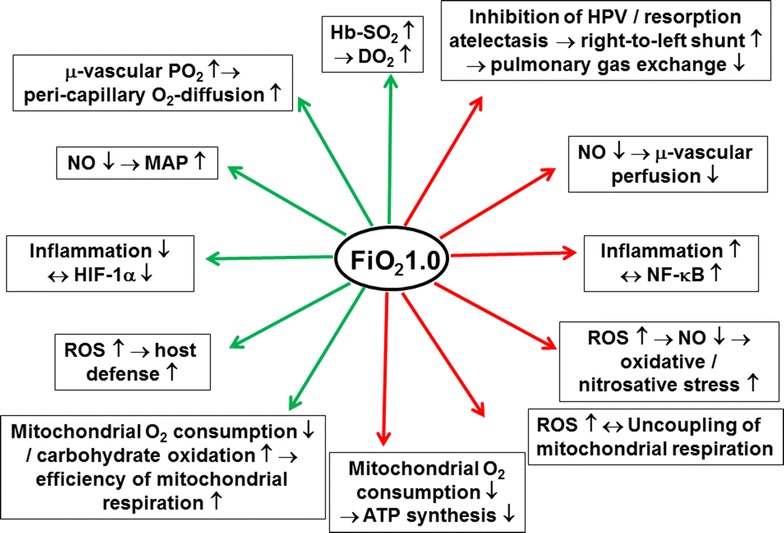
Beneficial (green arrows) and deleterious (red arrows) effects of hyperoxia, i.e., breathing pure oxygen, during circulatory shock and/or in medical emergencies. FiO 2 fraction of inspired oxygen, PO 2 oxygen partial pressure, µ micro, Hb haemoglobin, SO 2 oxygen saturation, DO 2 systemic oxygen transport, HPV hypoxic pulmonary vasoconstriction, MAP mean arterial pressure, SVR systemic vascular resistance, NO: nitric oxide, HIF-1α: hypoxia-inducible factor 1 alpha, NF-κB nuclear transcription factor kappaB, ROS reactive oxygen species, ATP adenosine triphosphate; adapted from Asfar et al. [16] with kind permission from Springer Science and Business Media
(Patho)physiology of hyperoxia: pulmonary, vascular, metabolic, and cerebral effects
Blood O2 content
According to textbook physiology, increasing the FiO2 from 0.21 (i.e., air) to 1.0 (i.e., 100 % O2) will moderately affect total blood O2 content under conditions of normal cardiopulmonary function: at normal pH and temperature, arterial PO2 levels of 90–100 mmHg lead to hemoglobin O2 saturations close to 100 % due to the sigmoid shape of the hemoglobin-O2-dissociation curve. Therefore, pure O2 breathing will only raise the amount of physically dissolved O2, the maximum effect being a five-fold increase, while hardly modifying the amount of O2 bound to hemoglobin. It is self-evident from the afore-mentioned estimate that the effect of pure O2 breathing on total blood O2 content will be the more important the lower the hemoglobin concentration. Therefore, ventilation with 100 % O2 was particularly protective in various models comprising critical hemodilution (reviewed in Calzia et al. [12]): the most impressive evidence in this context are the data reported in the “Live without blood” experiment as early as in 1960 [18]: in pigs subjected to hemodilution to a hematocrit <1–2 % (!), mechanical ventilation with pure O2 allowed preventing the otherwise marked ECG signs of myocardial ischemia, and no sequelae were observed after blood re-transfusion and return to air breathing. Strikingly, however, despite its frequent routine use, so far there are no clinical data on the role of mechanical ventilation with FiO2 = 1.0 during the management hemorrhagic shock, most likely due to ethical constraints. The available pre-clinical data are equivocal: deleterious and beneficial effects as well as no therapeutic efficacy at all were reported, depending on the species used, the severity of shock, and the concomitant use of therapeutic hypothermia [19–25].
No matter the definitive role of pure O2 breathing during situations of critical reductions in blood O2 transport capacity due to blood loss and/or hemodilution, pre-oxygenation, i.e., administration of 100 % O2 prior to induction of anesthesia and/or airway management, is well established to markedly increase the margin of safety: the “safe time of apnea” (i.e., the time until transcutaneous O2 saturation fell below 90 %) was doubled, when the FiO2 was increased from 60 to 100 % [26]. It must be noted, however, that even short-term pure O2 ventilation under these conditions may be associated with formation of atelectasis (see below). In healthy, non-obese patients with American Society of Anesthesiologists physical status I or II, this atelectasis formation was prevented by using an FiO2 of 0.8, but the safe time of apnea was significantly shorter [26]. Unfortunately, there is no ideal FiO2, which allows achieving a maximum “safe time of apnea” with the least formation of atelectasis [27, 28]: the degree of the latter depends on the patients’ age [29], body mass index [30], and underlying chronic pulmonary co-morbidity [31].
Interestingly, this concept of a prolonged margin of safety seems to be valid in coronary artery disease for O2 breathing as a preventive, pre-treatment measure: breathing 15 L/min O2 prevented the recurrence of pacing- [32] and prolonged the time until occurrence of exercise-induced angina [33].
Pulmonary effects
Pure O2 breathing impairs pulmonary gas exchange as a result of inhibition of hypoxic pulmonary vasoconstriction induced by the rise in alveolar and mixed-venous PO2 [34, 35]. Moreover, as already mentioned-above, within a few minutes, e.g., after only 5 min of apnea and oxygenation during induction of anesthesia [26], pure O2 breathing causes formation of atelectasis with increased intrapulmonary right-to-left shunt. This “adsorption atelectasis” [36, 37] is due to instability of lung regions that are still open but poorly ventilated in relation to perfusion, so-called low ventilation/perfusion-ratio (VA/Q) regions [38], when the inert carrier gas N2 is washed out. In healthy volunteers, breathing 100 % O2 over approx. 25 min under normobaric conditions doubled intra-pulmonary right-to-left shunt, while breathing air at equal inspiratory O2 partial pressure, i.e., in a hyperbaric chamber pressurized to 4.9 atmospheres of ambient pressure, did not affect gas exchange [12]. During induction of anesthesia, this atelectasis formation was prevented at least in part by using CPAP breathing and subsequent face mask ventilation with a PEEP of 6–10 cmH2O [39, 40]. In mechanically ventilated patients with acute lung injury, the degree of hyperoxia-induced adsorption atelectasis could at least be attenuated by using higher PEEP levels: increasing PEEP from 5 to 14 cm H2O completely blunted the fall of the PaO2/FiO2 ratio from 200 to 150 mm Hg induced by increasing the FiO2 from 0.6 to 1.0 [37].
Acute hyperoxia-induced impairment of gas exchange must be discriminated from pulmonary O2 toxicity [41, 42], the so-called Lorrain-Smith effect [43] first described by Lavoisier in 1783 [44]. Pulmonary O2 toxicity may present as severe pulmonary inflammation, ultimately leading to hemorrhagic pulmonary edema, and is referred to excess ROS and reactive nitrogen species (RNS) formation [45, 46]. However, despite the abundant evidence on hyperoxia-induced acute lung injury from studies in experimental animal (for reviews see [45, 47]), so far no biomarkers have been identified in humans that would allow evaluating the degree of ROS and/or RNS formation, and, moreover, thereby avoiding pulmonary O2 toxicity. Consequently, albeit intuitively being a logical therapeutic approach, there are no large scale data on the prevention of pulmonary O2 toxicity by ROS scavengers in humans, similar to the equivocal role of antioxidants in critically ill patients in general [48, 49]. In healthy experimental animals, pulmonary O2 toxicity is a result of either long-term exposure and/or injurious ventilator settings leading to ventilator-induced lung injury (VILI) (for examples, see [50, 51]). In contrast, lung-protective ventilation using low tidal volumes with higher PEEP levels and/or titrated to the thoraco-pulmonary compliance curve [52] over shorter periods had no deleterious effect at all [53], and this was also true during 24 h of lung-protective ventilation at FiO2 1.0 in in large animals [25, 54]. In humans, the duration of pure O2 breathing needed to provoke pulmonary O2 toxicity is unknown [55]: various studies reported that exposure period of 6–25 h were associated with clinical and histological signs of tracheitis and/or alveolitis [45, 56, 57], whereas other authors suggested that “…direct oxygen toxicity only plays a negligible role in regards to perioperative administration..” [58] and that breathing an FiO2 of 0.96–1.0 for 48 h does not produce symptoms of toxicity in most men [45, 59]. The only data available from mechanically ventilated ICU patients originate from mechanical ventilation with FiO2 >0.85–0.9 for >10 days [60, 61], and FiO2 = 1.0 over 14 h to 30 days [62, 63], respectively. Unfortunately, the studies do not report ventilator settings, but, given the publication years (1967–1972), it is unlikely that low tidal volumes and high PEEP levels according to current guidelines were used. Two more recent studies yielded equivocal results: observational data from patients mechanically ventilated for >48 h with “excessive inspired O2” (defined as an “FiO2 >0.5 while maintaining SO2 >92 %” observed in 155 out of 210 patients during a 12-month observation period) showed significantly lower PaO2/FiO2 ratio and higher mean airway pressures at 48 h [64]. In contrast, retrospective analyses of patients after cardiac arrest showed that higher quartiles of the “area under curve of FiO2” were not associated with any effect on gas exchange or lung mechanics during the first 24 h of mechanical ventilation [65]. Nevertheless, in this study the highest quartile of the “area under curve of FiO2” coincided with decreased survival to hospital discharge and worse neurological outcomes. Hence, no threshold value for the duration of hyperoxia exposure leading to pulmonary O2 toxicity is known in mechanically ventilated patients. Most likely, defining such a threshold is per se impossible: it is well known from hyperbaric (patho)physiology that intermittent exposure to hyperoxia with interspersed short periods of air breathing markedly attenuates pulmonary O2 toxicity when compared to an equally long, but continuous exposure [66]. The problem of defining a threshold value for the initiation of pulmonary O2 toxicity was highlighted during the discussion of “Oxygen” during the 50th Respiratory Care Journal Conference held April 13–14, 2012, in San Francisco, CA: “…oxygen toxicity is like Bigfoot: everybody’s heard about it, but nobody’s ever seen it…” [45].
Vascular effects
Hyperoxia decreases cardiac output, on the one hand due to a fall in heart rate caused by increased parasympathetic tone [67], on the other hand due to a rise in systemic vascular resistance [68–70]. The latter may result from decreased ATP release from red blood cells [71] and/or reduced NO bioavailability. Stamler et al. elegantly demonstrated that the hyperoxia-induced increase in tissue and, consequently, venous PO2 levels blocks the release of NO from cystein-binding in the hemoglobin-molecule (S-nitrosothiol) [72]. In addition, increased ROS formation contributes to hyperoxia-induced vasoconstriction: administration of vitamin C (200 mg intra-arterial [69] and 3 g intra-venous [73], respectively), restored forearm [69] and coronary vascular resistance [73]. While varying among the different vascular regions, the degree of the hyperoxia-induced vasoconstriction is particularly pronounced in the cerebral and coronary circulation. Therefore, it was argued that this hyperoxia-related vasoconstriction may impede tissue O2 delivery in patients with sepsis [74] or cardiovascular disease [75], but it is still a matter of debate whether the hyperoxia-induced vasoconstriction is beneficial or deleterious: in fact, 30 min of pure O2 breathing impaired the sublingual microcirculatory perfusion by decreasing the number and density of perfused vessels, while it even increased perfusion heterogeneity [76]. It must be noted, however, that most of the studies available in the literature on hyperoxia-induced systemic or regional vasoconstriction were performed in healthy volunteers or at least under stable hemodynamic conditions, i.e., without imbalance between O2 supply and demand, or, during circulatory shock. In addition, any hyperoxia-related increase in vasomotor tone could possibly allow reducing vasopressor demands required to counteract shock-induced hypotension. Finally, experimental data suggest that pure O2 ventilation may redistribute cardiac output in favor of the kidney and the hepato-splanchnic system and thereby improve visceral organ function [25, 54, 77]. Yet, scarce data are only available on the effects of ventilation with FiO2 = 1.0 on systemic or regional hemodynamics and organ function in patients with circulatory shock. Only one prospective pilot study, including 83 patients admitted to the emergency department with two or more systemic inflammatory response syndrome (SIRS) criteria and a suspected infection, i.e., sepsis, reported no association between in-hospital mortality and hyperoxia (FiO2 between 0.4 and 0.8) [78], but only three patients with septic shock were included in total. Therefore, the results of the prospective, randomized, controlled HYPER2S (NCT01722422) trial (see Table 1) will certainly help to answer this question.
Table 1.
Clinical trials on the effects of hyperoxia in intensive care and emergency medicine
| Study acronym | Trial no. | Patient condition | Intervention | Primary outcome measures | Planned enrolment |
|---|---|---|---|---|---|
| OXYGEN-ICU | NCT01319643 | ICU treatment for 3 days | FiO2 titrated to SpO2 94–98/PaO2 70–100 mmHg vs. SpO2 >97 %/PaO2 100–150 mmHg | Mortality day 30 | Terminated at n = 434 (slow recruitment) |
| HYPER2S | NCT01722422 | Septic shock | FiO2 titrated to SpO2 88–95 % vs. FiO2 = 1.0 over the first 24 h | Mortality day 28 | Terminated at n = 442 |
| AVOID | NCT01272713 | Acute myocardial infarction | Air (unless SpO2 <94 %) vs. 8 L/min O2 during pre-hospital phase, thereafter according to hospital protocol | Infarct size, time course of CK-MB and cTnI | Completed at n = 638 |
| DETO2X-AMI | NCT01787110 | Acute coronary syndrome | Air (unless SpO2 <90 %) vs. 6 L/min O2 over 6–12 h | Mortality at 1 year | 6600 |
| BRAINOXY | NCT01201291 | TBI, GCS ≤8 | FiO2 0.4 vs. 0.7 | GOS/GOSE at 6 months | n un-specified; terminated (slow recruitment) |
| SO2S | ISRCTN52416964 | Stroke, ICH | Air vs. 2 (SpO2 >93 %)/3 L/min overnight vs. 2 (SpO2 >93 %)/3 L/min continuously until day 3 | Modified Rankin scale at day 90 | Completed at n = 8003 |
| REOX | NCT01881243 | Cardiac arrest | Observational study; association between hyperoxia and outcome | Blood isofuranes/-prostanes | 133 |
ICU intensive care unit, FiO 2 fraction of inspired O2 concentration, SpO 2 transcutaneous hemoglobin O2 saturation, PaO 2 arterial O2 partial pressure, CK-MB myocardial creatine kinase, cTnI cardiac troponin I, TBI traumatic brain injury, GCS Glasgow Coma Score, GOS Glasgow Outcome Score, GOSE Extended Glasgow Outcome Score
Metabolic effects
No matter the definitive effect of hyperoxia on vascular tone during circulatory shock, any conclusion on the role of hyperoxia-induced vasoconstriction must be considered in the context of the effects of hyperoxia on metabolic activity. Clearly, in vitro long-term (≥24 h) exposure to hyperoxia was associated with impaired mitochondrial respiratory capacity as a result of partial inhibition of NADH and succinate dehydrogenase, i.e., complex I and II [79, 80], whereas cytochrome c oxidase (complex IV) remained unaffected [79]. Pure O2 breathing also decreased whole body O2 uptake in healthy volunteers [76, 81] as well as in critically ill patients [70, 82], and myocardial O2 consumption in patients with coronary artery disease [83]. Nevertheless, this reduced O2 uptake more likely mirrored decreased O2 demand rather than impaired O2 utilization: There was no deleterious effect on any marker of systemic energy balance [70, 82], and myocardial lactate extraction was even enhanced [83]. Moreover, studies in experimental animals [25, 54] and healthy volunteers [81] showed that hyperoxia increased the respiratory quotient to values close to 1.0, in other words suggesting that hyperoxia shifted energy metabolism to preferential utilization of carbohydrates [25, 54], which is well established to increase the yield of the mitochondrial respiratory chain [84], i.e., the molar ratio of O2 consumption and ATP formation [85]. Similar to the situation during exercise in highlanders [86], this effect might assume particular importance under conditions of limited tissue O2 supply, e.g., hemorrhagic and/or cardiogenic shock.
Cerebral effects
In addition to the above-mentioned pulmonary toxicity, pure O2 breathing may also have toxic effects on the central nervous system, the so-called Paul-Bert effect [87], the most dramatic manifestation being generalized tonic–clonic (grand mal) seizures [11]. This central nervous toxicity, however, requires pure O2 breathing under supra-atmospheric pressures, i.e., during diving and/or in a hyperbaric chamber. Hence, only critically ill patients treated with hyperbaric oxygenation (HBO: pure O2 breathing at supra-atmospheric pressures; e.g., for decompression injury (DCI), gas embolism, carbon monoxide (CO) poisoning, and gas gangrene or necrotizing fasciitis) will present with central nervous O2 toxicity-induced convulsions, which occur within approx. 20–30 min of pure O2 exposure at ambient pressures of three atmospheres. Interestingly, in contrast to the cerebral vasoconstriction normally observed during pure O2 breathing, symptoms are preceded by a paradoxical increase in cerebral blood flow velocity [88] (Fig. 2), which is referred to peroxynitrite (ONOO−) formation resulting from the reaction of NO with the superoxide radical (O2−) [89], and thereby causing a dysregulation of the endogenous NO availability [46, 90].
Fig. 2.
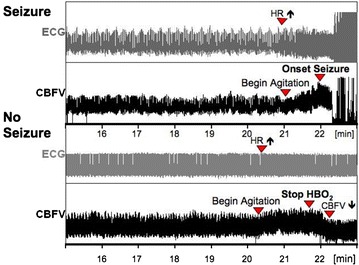
Original recordings of ECG and cerebral blood flow velocity (CBFV) in two volunteers undergoing an HBO exposure-test with pure O2 breathing at three atmospheres of ambient pressure. In the upper panel, HBO-induced seizures were preceded by tachycardia, agitation, and a subsequent marked increase in CBFV. The lower panel shows a volunteer, in whom seizures could be prevented by removing the O2 face mask; CBFV consecutively fell to lower levels comparable to those during the asymptomatic period
Clinical application of hyperoxia
CO intoxication, gas embolism, and DCI
No matter any possible deleterious effects related to enhanced ROS and RNS formation, pure O2 breathing is the therapy of choice during CO intoxication, gas embolism, and DCI. While the beneficial effect of hyperoxia during CO intoxication is related to the competitive replacement of CO in heme moieties, the salutary role of O2 during DCI and/or gas embolism is due to the so-called oxygen window effect.
Recent reports show that approx. 1 ‰ of patients admitted to emergency departments present with occult CO-intoxications [91]. CO has a several-fold higher affinity to heme moieties than O2, and thus it reduces the blood O2 transport capacity by preventing hemoglobin (Hb) O2 saturation. This effect on tissue O2 transport is further aggravated by the leftward-shift of the Hb-O2-dissociation curve, which impairs O2 release from oxy-hemoglobin [92]. Nevertheless, CO toxicity is mainly due to the blockade of complex IV of the mitochondrial respiratory chain (i.e., cytochrome c oxidase) [93]. Ultimately, this inhibition of mitochondrial respiration will result in oxidative and nitrosative stress [94], which also explains that pure O2 breathing is the therapy of choice in patients with CO intoxication: albeit at first glance paradoxical, increasing the PO2 in fact reduces rather than further increases ROS and RNS formation during CO intoxication [94], because high O2 concentrations will restore normal electron transport within the respiratory chain and thereby decrease radical production. The half-life of CO elimination is inversely related to the arterial PO2 [95], and therefore, intuitively, HBO therapy is indicated in patients with CO-intoxication. However, the results of the available RCT are equivocal [96–98], and a recent meta-analysis concluded that normobaric hyperoxia is as efficient as HBO, in part as a result of the CO elimination achieved with normobaric pure O2 breathing during patient transport to an HBO chamber [99].
By definition gas embolism is the—mostly iatrogenic—entry of gas bubbles into the vascular system in general [100], whereas decompression injury (DCI) comprises medical disorders resulting from a decrease in ambient pressure (i.e., decompression) that results in intra- or extra-vascular bubble formation due to excess (i.e., supersaturation) inert gas (in most cases N2) tensions [101]. However, DCI can also cause arterial gas embolism due to introduction of alveolar gas emboli via cardiac shunts and/or pulmonary vessels, but more frequently presents as decompression sickness (DCS), which is caused by excess supersaturation during and after decompression [102]. Treatment is breathing 100 % O2, and, as far as DCI is concerned, in combination with recompression, i.e., HBO [103]. In addition to its ability to improve tissue oxygenation and attenuate inflammation, pure O2 breathing is therapy of choice because it maximizes the inert gas gradient from the tissues to the alveolar gas and thereby accelerates inert gas washout [102]. Moreover, it will enhance bubble resolution due to the increased inert gas diffusion gradient (i.e, the oxygen window) (Fig. 3 [103]).
Fig. 3.
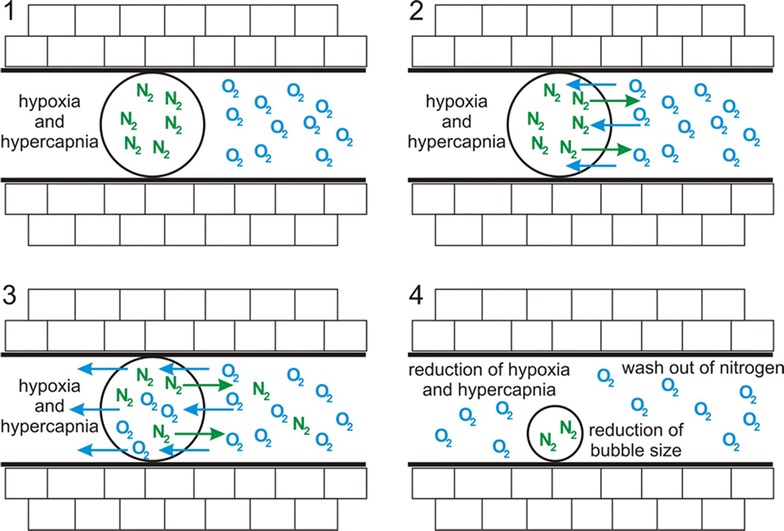
Effect of increased oxygen partial pressure on bubble size. After creation of a concentration gradient (1), oxygen starts to diffuse into the bubble, simultaneously nitrogen diffuses out of the bubble (2). Thereby, oxygen molecules are now capable of passing the bubble with concomitant reduction of nitrogen concentration (3). Finally, the bubble size decreases significantly (4). Adapted from Muth et al. [103] with kind permission from Springer Science and Business Media
Acute coronary syndrome
In 1940, supplemental O2 breathing was described as “…as an efficient method of relieving the intense pain which may accompany acute coronary thrombosis and as an important therapeutic adjunct in the symptomatic control of severe angina pectoris…” [104], and subsequently became a cornerstone of the management of the Acute Coronary Syndrome. However, due to the above-mentioned coronary vasoconstriction [83], which was also more recently demonstrated to be due to NO quenching [105] associated with oxidative and nitrosative stress [73], this approach has been questioned [106, 107], despite data from patients with acute decompensated heart failure showing no effect of the arterial PO2 on all-cause mortality [108]. Therefore, the latest guidelines of the European Resuscitation Council on the initial management of acute coronary syndromes recommend that an “…O2 saturation of 94–98 %, or 88–92 % if the patient is at risk of hypercapnic respiratory failure…” should be achieved, in other words, “…supplementary O2 should be given only to those patients with hypoxaemia, breathlessness or pulmonary congestion…” [109]. Until most recently, the evidence for these guidelines was surprisingly scarce, because over four decades only four clinical trials enrolling a total of just 447 patients were published [110–113]. Moreover, the results of these trials were far from being conclusive: In 17 patients with anterior transmural myocardial infarction (MI), Madias et al. reported reduced ischemic injury as assessed by precordial ST-mapping during 48–80 min of breathing 15 L/min O2; however, this study did not include any control group [110]. In 157 patients with confirmed MI, Rawles et al. found no difference in the incidence of arrhythmias and use of analgesics after 6 L/min O2 over 24 h vs. air breathing; however, mortality in the O2-group tended to be higher without reaching statistical significance (3.9 vs. 11.3 %, p = 0.08) [111]. More recently, in a total of 137 patients using two different protocols for supplemental O2, Ukholkina et al. demonstrated that a FiO2 of 0.3–0.4 until 3 h after interventional myocardial revascularization decreased the number of early post-intervention arrhythmia, which was associated with lower peak values of myocardial creatine kinase activity, and, ultimately, smaller relative area of ischemic damage. However, 37 % of the patients had baseline O2 saturations <94 %, i.e., below the threshold recommended for initiating supplemental O2 administration. Moreover, for reasons unexplained, time to revascularization was longer in the O2-group [112]. Finally, Ranchord et al. found “…no evidence of benefit or harm…” from high-concentration (6 L/min O2 over 6 h) vs. titrated O2 therapy (to achieve O2 saturations of 93–96 %) in 136 patients with initially uncomplicated ST-elevation myocardial infarction [113]. Therefore, as highlighted in recent reviews [106, 107], there is urgent need for large clinical trials assessing whether or not O2 therapy should be used for the management of acute coronary syndrome, and the results of the DETO2X-AMI (NCT01787110) (see Table 1) trial is to answer this question. The most recently completed AVOID (NCT01272713) (see Table 1) trial has partly answered this need: in non-hypoxaemic (transcutaneous hemoglobin O2 saturation >94 %) patients with ST-elevation myocardial infarction (n = 441), high flow face mask O2 (8 L/min) up to 4 h after percutaneous coronary intervention increased myocardial injury, recurrent infarction, major cardiac arrhythmia, and late (6 months) myocardial infarct size. Mortality at hospital discharge did not significantly differ (p = 0.11), but interestingly, was 2.5 fold higher in the normoxia group. At 6 months, however, both overall (hyperoxia: 3.8, normoxia: 5.9 %; p = 0.32) and cardiac (hyperoxia: 2.9, normoxia 4.1 %) mortality were comparable [114].
Traumatic and ischemic brain injury
From a pathophysiological point of view, any hyperoxia-induced vasoconstriction could theoretically represent an interesting approach in the management of brain injury, inasmuch as it would allow reducing intracranial pressure (ICP) and thereby improving cerebral perfusion pressure without impairment of O2 supply. Clearly, HBO (60 min of mechanical ventilation with pure O2 at 1.5 atmospheres of ambient pressure was shown to efficiently decrease ICP in patients with traumatic brain injury (TBI) [115]. Combining HBO with subsequent normobaric hyperoxia even improved long-term outcome: at 6 months mortality was reduced (9 out of 22 vs. 3 out of 20 patients, p = 0.048), and overall neurological outcome was more favorable as evaluated with the sliding dichotomized Glasgow Outcome Score (8 out of 21 vs. 14 out of 19 patients, p = 0.024) [116]. However, normobaric hyperoxia alone yielded equivocal results with respect to tissue oxygenation and metabolism as assessed by microdialysis [117–122], which was referred to a lacking effect on brain tissue oxygenation in hypo-perfused regions [120] and/or a possibly enhanced hyperoxia-related excitotoxicity [122]. Albeit there is some data available using magnetic resonance imaging (MRI), suggesting that hyperoxia may have a beneficial effect in the peri-lesional penumbra [123], the role of hyperoxia in TBI is still controversially discussed because of the equivocal outcome data [124]: while a uni-variate analysis found a significant association between hyperoxemia (arterial PO2 >100 mmHg) and a decreased risk of 6-month mortality in a retrospective analysis of 1116 patients, the corresponding multi-variate logistic regression adjusted for illness severity did not show any significant relationship [125]. However, Davis and co-workers showed in a large retrospective cohort analysis including 3420 patients that both hypoxemia (PaO2 < 110 mmHg) and extreme hyperoxemia (PaO2 > 487 mmHg) were associated with increased mortality and unfavorable outcome among TBI patients [126]. Moreover, two other retrospective studies analyzing a total of 1759 patients using multi-variate approaches showed that hyperoxemia defined as arterial PO2 >200 or >300 mmHg, respectively, was independently associated with increased mortality [127, 128]. These data are in contrast to another retrospective study, reporting that oxygen partial pressures between 250 and 486 mm Hg were associated with improved all-cause survival in patients with severe TBI [129]. So far, the answer to the question of the use of hyperoxia in TBI is still pending: the BRAINOXY study (NCT01201291), which was to answer this question, was terminated due to slow recruitment.
The currently available data on hyperoxia (with consecutive hyperoxemia) during ischemic brain injury, i.e., stroke and/or intracranial bleeding, is less conflicting: Albeit there is compelling experimental evidence (for review, see [130]) and some encouraging pilot data in patients [131, 132], evidence from large trials suggests that hyperox(em)ia is deleterious. A prospective, single-center observational study in 252 patients showed that hyperoxemia (as defined as a PaO2 >173 mmHg) was associated with delayed cerebral ischemia and, consequently, poor neurological outcome [133]. In addition, a more recent retrospective analysis of 2894 mechanically ventilated patients with ischemic stroke, subarachnoid or intracerebral hemorrhage demonstrated that more pronounced hyperoxemia (arterial PO2 >300 mmHg) significantly increased in-hospital mortality at day 28 [134]. In contrast, a retrospective analysis of 2,643 adults, ventilated for ischemic stroke in ICUs in Australia and New Zealand, showed no apparent relationship between mortality and PaO2 levels during the first 24 h in ICU [135]. Finally, the Normobaric Oxygen Therapy in Acute Ischemic Stroke Trial (NCT00414726), which was to study the effects of high-flow O2 (30–45 L/min for 8 h via facemask) was terminated prematurely after enrolment of 85 of 240 patients due to imbalance in deaths favoring the control arm (hyperoxia: 17 out of 43 patients, room air: 7 out of 42 patients, p = 0.03). The question, however, whether hyperoxia is definitely deleterious, remained unanswered: deaths were not attributed to treatment by the blinded external medical monitor. No matter the impact of high-flow supplemental O2, even low-dose O2 administration (2 L/min either continuously over 72 h or over-night only) only targeted to compensate for mild, in particular nocturnal, hypoxemia (Stroke Oxygen Study, SO2S; ISRCTN52416964) did not improve outcome after ischemic stroke: despite promising pilot data in 289 patients at 1 week and 6 months [136, 137], the complete, full-scale study in 8003 patients did not show any difference in morbidity (disability at day 90 as assessed by the modified Rankin Scale) or mortality (data presented at the XXIII European Stroke Conference, Nice, May 7, 2014).
Cardiac arrest
The pronounced vasoconstrictor effect in the cerebral circulation together with the potential to aggravate oxidative stress during ischemia/reperfusion have prompted investigations on the association between hyperox(em)ia and outcome after cardiopulmonary resuscitation. So far only retrospective analyses are available, except for one randomized controlled single centre trial including 28 patients in total, the results being again fairly equivocal: a multicenter cohort study on 6326 patients concluded that hyperoxemia defined as PaO2 >300 mmHg was associated with higher mortality than normoxemia and even hypoxemia defined as PaO2 <60 mmHg) [138]. A secondary analysis of 4459 patients of this study even yielded a direct linear relationship between PaO2 increments and increased risk of mortality, a PaO2 increment of 100 mmHg being associated with a 24 % higher odds ratio for unfavorable outcome [139]. Another retrospective analysis of 12,108 patients found no association between PaO2 deciles or hyperoxemia defined as PaO2 >309 mmHg and mortality adjusted for illness severity [140]. Other authors analyzing smaller data bases confirmed this latter finding [141, 142]. Clearly, different temperature management (lowest temperature: median 34.9 °C in [140] vs. mean 36 °C in [138]; 33 vs. 6 % of patients <34 °C) in the various countries may have contributed to these divergent findings, albeit a single-center, retrospective analysis of 170 patients treated with therapeutic hypothermia (12–24 h at 32–34 °C core temperature) showed that mortality and poor neurological outcome were more frequent in patients with higher maximum PaO2 values (median 254 vs. 198 mmHg) during the first 24 h after cardiac arrest [143]. This is in line with another retrospective analysis in 213 patients after cardiac arrest, treated with therapeutic hypothermia, demonstrating a U-shaped independent association between the mean PaO2 and poor neurologic outcome at hospital discharge [144]. The sole randomized controlled trial, comparing 14 patients in each group ventilated with either 30 or 100 % oxygen for 1 h after return of spontaneous circulation (ROSC), showed increased levels of neuron specific enolase in the hyperoxic group at 24 h post cardiac arrest [145]. Unfortunately, this study was not powered to analyze outcome parameters. Finally, other smaller studies focusing on the role of arterial PCO2 did not yield any deleterious effect of hyperoxemia per se on neurological outcome [146–148]. Consequently, a recent meta-analysis concluded that hyperoxemia (PaO2 >300 mmHg) “…appears to be correlated with increased in-hospital mortality…”, which, however, “…should be interpreted cautiously because of the significant heterogeneity…of studies analyzed…” [149]. Lately, two more interesting retrospective cohort analyses reported that severe hyperoxia was associated with decreased survival as well as decreased survival and worse neurological outcome, respectively [65, 150]. Nelskylä et al. offer an interesting explanation for the vast majority of retrospective studies being in favor of normoxia: In their retrospective analysis of 119 out of hospital cardiac arrest patients, hyperoxia occurred more frequently in association with out-of-hospital cardiac arrest, longer times to ROSC, and delays to ICU admission, i.e., the patients with the worst prognosis per se [142]. In addition, safe titration of oxygen therapy to achieve a SpO2 of 90–94 % after out-of-hospital cardiac arrest might not be feasible, at least in the pre-hospital period [151]. Taken together, all these studies demonstrate the urgent need for data from prospective, randomized controlled trials, and the ongoing REOX trial (NCT01881243) will at least help to answer this demand.
Peri-operative hyperoxia
The use of intra-operative (and, in a broader sense, peri-operative) hyperoxia to prevent surgical site infection does not directly refer to the treatment of circulatory shock and medical emergencies, but patho-physiological effects of hyperoxemia also assume importance in this context. The antimicrobial properties of oxygen are due to the bactericidal properties associated with increased ROS production, and were already recognized in the 1980s (“oxygen as an antibiotic” [152]), subsequently prompting several clinical studies which have so far enrolled more than 5000 patients. Recent meta-analyses of these studies concluded that high inspired O2 concentrations values (FiO2 = 0.8 vs. 0.3 as the standard approach) during the peri-operative period reduced the risk of surgical site infection, both after elective and emergency surgery, without leading to major post-operative atelectasis [153, 154]. This protective effect was specifically present in non-obese patients undergoing colo-rectal surgery, one possible component being a better patency of anastomoses [155]. The molecular mechanisms of hyperoxia-related reduction in surgical site infection remain unclear: hyperoxic ventilation was reported to restore the local inflammatory response to normal—rather than leading to potentially deleterious hyper-inflammation—thereby improving the antimicrobial potential of alveolar macrophages [156]. However, other authors found that ex vivo exposure to hyperoxia not only enhanced ROS formation but even decreased the capacity of endotoxin-stimulated leukocytes to release tumor necrosis factor-α [157]. It is noteworthy that despite this short-term (up to 2 weeks within surgery) benefit, high intra-operative FiO2 was associated with higher long-term (>2 years) post-operative mortality. This observation was nearly exclusively due to a higher mortilaty in patients that had undergone cancer surgery [158], and coincided with a significantly shorter cancer-free survival interval [159]. Therefore, and taking into account the trials showing no benefit for surgical site infection after abdominal surgery [160, 161], the most recent Cochrane analysis concluded that “…evidence is insufficient to support the routine use of a high fraction of inspired O2 during anesthesia and surgery….” [162].
What is the optimal PaO2 for ICU survival?
So far, this question remains unanswered as well: a retrospective analysis of arterial PO2 measurements in 36,307 patients during the first 24 h of ICU stay demonstrated a U-shaped relationship of in-hospital mortality, the nadir of the mortality curve (as calculated from the logistic regression with the PaO2 incorporated using a spline function) being at values of 15–20 kPa (110–150 mmHg); mortality sharply increased both at PaO2 values <9 (67 mmHg) and >30 kPa (225 mmHg) [163]. Interestingly, this group of authors recently showed a similar U-shaped relation between arterial PCO2 and PO2, respectively, and hospital mortality after cardiac arrest, the highest probability of survival being associated with a PaO2 values of 180–200 mmHg, i.e., most likely with an FiO2 >0.6 in a substantial number of patients [164]. A more recent study of unadjusted odds ratios for PaO2 deciles in 152,680 patients confirmed the impact of hypoxemia, whereas hyperoxemia even >40 kPa (300 mmHg) had no impact on outcome [165]. Finally, a retrospective cohort study including 83,060 patients after cardiac surgery showed that there was no association between mortality and hyperoxia in the first 24 h in ICU after cardiac surgery [166]. Therefore, two recent meta analyses concluded that hyperoxia may be associated with increased mortality in patients with stroke, TBI, and post cardiac arrest and with poor hospital outcome, respectively [167, 168]. However, due to heterogeneity of the included studies, the authors state that more evidence is needed to provide optimal oxygen targets for critical care physicians. The results of the OXYGEN-ICU (NCT01319643) trial (see Table 1) will certainly contribute to the answer of this question. Interestingly, most ICU clinicians acknowledge the potential adverse effects of prolonged exposure to hyperoxia, however, in actual clinical practice, a large proportion of their patients was exposed to higher arterial oxygen levels than self-reported target ranges [169].
Conclusion
Hyperoxia (i.e., ventilation with a FiO2 = 1.0) is a cornerstone of the acute management of circulatory shock, a concept which is based on compelling experimental evidence that compensating the imbalance between O2 supply and requirements (i.e., the oxygen dept) is crucial for survival, at least after trauma [170, 171]. On the other hand oxygen toxicity due to the increased formation of ROS limits its use, because it may cause serious deleterious side effects, especially in conditions of ischemia/reperfusion. While these effects are particularly pronounced during long-term administration, i.e., beyond 12–24 h, several retrospective studies suggest that even hyperoxemia of shorter duration is also associated with increased mortality and morbidity. In fact, albeit the clinical evidence from prospective studies is surprisingly scarce, a recent meta-analysis suggests that hyperoxia is associated with increased mortality at least in patients after cardiac arrest, stroke and TBI [172]. Most of these data, however, originate from heterogenous, observational studies with inconsistent results, and therefore, there is a need for the results from the large scale, randomized, controlled clinical trials on the use of hyperoxia, which can be anticipated within the next 2–3 years. Consequently, until then, “…conservative…” O2 therapy [140] represents the treatment of choice to avoid exposure to both hypoxemia and excess hyperoxemia.
Authors’ contributions
SH, FB carried out the literature review and helped to draft the manuscript. PR conducted the literature review and drafted the manuscript. PA helped to conduct the literature review and drafted the manuscript. AK reviewed the sub-chapter on “cerebral effects” and provided the material documenting the vascular effect of hyperbaric hyperoxia (Fig. 2). All authors read and approved the final manuscript.
Acknowledgements
Supported by the Deutsche Forschungsgemeinschaft (KFO 200, DFG RA 396/9-2, and the SFB 1149) and the German MoD (Vertragsforschungsvorhaben E/U2AD/CF523/DF556).
Abbreviations
- ARDS
Acute Respiratory Distress Syndrome
- ATP
adenosine triphosphate
- CO
carbon monoxide
- DCI
decompression injury
- DCS
Decompression sickness
- ECG
electrocardiogram
- FiO2
fraction of inspired oxygen
- Hb
hemoglobin
- HBO
hyperbaric oxygenation
- ICP
intracranial pressure
- ICU
intensive care unit
- MI
myocardial infarction
- MRI
magnetic resonance imaging
- N2
nitrogen
- NO
nitric oxide
- O2
oxygen
- PaO2
arterial partial pressure of oxygen
- PCO2
partial pressure of carbon dioxide
- PEEP
Positive end-expiratory pressure
- PO2
partial pressure of oxygen
- RCT
randomized controlled trial
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- ROSC
return of spontaneous circulation
- SIRS
systemic inflammatory response syndrome
- SpO2
peripheral hemoglobin oxygen saturation
- TBI
traumatic brain injury
- VA/Q
ventilation/perfusion ratio
- VILI
ventilator-induced lung injury
Contributor Information
Sebastian Hafner, Email: sebastian.hafner@gmx.de.
François Beloncle, Email: francois.beloncle@chu-angers.fr.
Andreas Koch, Email: a.koch@iem.uni-kiel.de.
Peter Radermacher, Phone: 49 731 500 60214, Email: peter.radermacher@uni-ulm.de.
Pierre Asfar, Email: piasfar@chu-angers.fr.
References
- 1.Leverve XM. To cope with oxygen: a long and still tumultuous story for life. Crit Care Med. 2008;36:637–638. doi: 10.1097/CCM.0B013E31816296AD. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson D, Chance B, Cadenas E, Boveris A. The relation of free radical production to hyperoxia. Ann Rev Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- 3.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magder S. Reactive oxygen species: toxic molecules or spark of life? Crit Care. 2006;10:208. doi: 10.1186/cc3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 6.Weil MH, Shubin H. The, “VIP” approach to the bedside management of shock. JAMA. 1969;207:337–340. doi: 10.1001/jama.1969.03150150049010. [DOI] [PubMed] [Google Scholar]
- 7.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oxer HF. Simply add oxygen: why isn’t oxygen administration taught in all resuscitation training? Resuscitation. 2000;43:163–169. doi: 10.1016/S0300-9572(99)00146-X. [DOI] [PubMed] [Google Scholar]
- 9.Singer M. Give oxygen, get a blood pressure… but don’t overdo it. Hosp Med. 2005;66:73–75. doi: 10.12968/hmed.2005.66.2.17548. [DOI] [PubMed] [Google Scholar]
- 10.Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care. 2007;13:73–78. doi: 10.1097/MCC.0b013e32801162cb. [DOI] [PubMed] [Google Scholar]
- 11.Bitterman H. Bench-to-bedside review: oxygen as a drug. Crit Care. 2009;13:205. doi: 10.1186/cc7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calzia E, Asfar P, Hauser B, Matejovic M, Ballestra C, Radermacher P, et al. Hyperoxia may be beneficial. Crit Care Med. 2010;38:S559–S568. doi: 10.1097/CCM.0b013e3181f1fe70. [DOI] [PubMed] [Google Scholar]
- 13.Cornet AD, Kooter AJ, Peters MJ, Smulders YM. The potential harm of oxygen therapy in medical emergencies. Crit Care. 2013;17:313. doi: 10.1186/cc12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjöberg F, Singer M. The medical use of oxygen: a time for critical reappraisal. J Intern Med. 2013;274:505–528. doi: 10.1111/joim.12139. [DOI] [PubMed] [Google Scholar]
- 15.Network The Acute Respiratory Distress Syndrome. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 16.Asfar P, Singer M, Radermacher P. Understanding the benefits and harms of oxygen therapy. Intensive Care Med. 2015;41:1118–1121. doi: 10.1007/s00134-015-3670-z. [DOI] [PubMed] [Google Scholar]
- 17.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boerema I, Meyne NG, Brummelkamp WH, Bouma S, Mensch MH, Kamermans F, et al. Life without blood. Ned Tijdschr Geneeskd. 1960;104:949–954. [PubMed] [Google Scholar]
- 19.Takasu A, Iwamoto S, Ando S, Minagawa Y, Kashiba M, Yamamoto Y, et al. Effects of various concentrations of inhaled oxygen on tissue dysoxia, oxidative stress, and survival in a rat hemorrhagic shock model. Resuscitation. 2009;80:826–831. doi: 10.1016/j.resuscitation.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Blasiole B, Bayr H, Vagni VA, Janesko-Feldman K, Cheikhi A, Wisniewski SR, et al. Effect of hyperoxia on resuscitation of experimental combined traumatic brain injury and hemorrhagic shock in mice. Anesthesiology. 2013;118:649–663. doi: 10.1097/ALN.0b013e318280a42d. [DOI] [PubMed] [Google Scholar]
- 21.Takasu A, Prueckner S, Tisherman SA, Stezoski SW, Stezoski J, Safar P. Effects of increased oxygen breathing in a volume controlled hemorrhagic shock outcome model in rats. Resuscitation. 2000;45:209–220. doi: 10.1016/S0300-9572(00)00183-0. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Stezoski SW, Safar P, Tisherman SA. Hypothermia, but not 100% oxygen breathing, prolongs survival time during lethal uncontrolled hemorrhagic shock in rats. J Trauma. 1998;44:485–491. doi: 10.1097/00005373-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Takasu A, Carrillo P, Stezoski SW, Safar P, Tisherman SA. Mild or moderate hypothermia but not increased oxygen breathing prolongs survival during lethal uncontrolled hemorrhagic shock in rats, with monitoring of visceral dysoxia. Crit Care Med. 1999;27:1557–1564. doi: 10.1097/00003246-199908000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Leonov Y, Safar P, Sterz F, Stezoski SW. Extending the golden hour of hemorrhagic shock tolerance with oxygen plus hypothermia in awake rats. An exploratory study. Resuscitation. 2002;52:193–202. doi: 10.1016/S0300-9572(01)00453-1. [DOI] [PubMed] [Google Scholar]
- 25.Knöller E, Stenzel T, Broeskamp F, Hornung R, Scheuerle A, McCook O, et al. Effects of hyperoxia and mild therapeutic hypothermia during resuscitation from porcine hemorrhagic shock. Crit Care Med. 2016, in press. [DOI] [PubMed]
- 26.Edmark L, Kostova-Aherdan K, Enlund M, Hedenstierna G. Optimal oxygen concentration during induction of general anesthesia. Anesthesiology. 2003;98:28–33. doi: 10.1097/00000542-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna A. Prevention of atelectasis during general anaesthesia. Lancet. 1995;345:1387–1391. doi: 10.1016/S0140-6736(95)92595-3. [DOI] [PubMed] [Google Scholar]
- 28.Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna G. Atelectasis and pulmonary shunting during induction of general anaesthesia–can they be avoided? Acta Anaesthesiol Scand. 1996;40:524–529. doi: 10.1111/j.1399-6576.1996.tb04483.x. [DOI] [PubMed] [Google Scholar]
- 29.Gunnarsson L, Tokics L, Gustavsson H, Hedenstierna G. Influence of age on atelectasis formation and gas exchange impairment during general anaesthesia. Br J Anaesth. 1991;66:423–432. doi: 10.1093/bja/66.4.423. [DOI] [PubMed] [Google Scholar]
- 30.Strandberg A, Tokics L, Brismar B, Lundquist H, Hedenstierna G. Constitutional factors promoting development of atelectasis during anaesthesia. Acta Anaesthesiol Scand. 1987;31:21–24. doi: 10.1111/j.1399-6576.1987.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 31.Gunnarsson L, Tokics L, Lundquist H, Brismar B, Strandberg A, Berg B, et al. Chronic obstructive pulmonary disease and anaesthesia: formation of atelectasis and gas exchange impairment. Eur Respir J. 1991;4:1106–1116. [PubMed] [Google Scholar]
- 32.Horvat M, Yoshida S, Prakash R, Marcus HS, Swan HJ, Ganz W. Effect of oxygen breathing on pacing-induced angina pectoris and other manifestations of coronary insufficiency. Circulation. 1972;45:837–844. doi: 10.1161/01.CIR.45.4.837. [DOI] [PubMed] [Google Scholar]
- 33.Ranchord AM, Perrin K, Weatherall M, Beasley R, Simmonds M. A randomised controlled trial of the effect of high concentration oxygen on myocardial ischaemia during exercise. Int J Cardiol. 2012;160:201–205. doi: 10.1016/j.ijcard.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Domino KB, Wetstein L, Glasser SA, Lindgren L, Marshall C, Harken A, et al. Influence of mixed venous oxygen tension (PvO2) on blood flow to atelectatic lung. Anesthesiology. 1983;59:428–434. doi: 10.1097/00000542-198311000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Sandoval J, Long GR, Skoog C, Wood LD, Oppenheimer L. Independent influence of blood flow rate and mixed venous PO2 on shunt fraction. J Appl Physiol. 1983;55:1128–1133. doi: 10.1152/jappl.1983.55.4.1128. [DOI] [PubMed] [Google Scholar]
- 36.Hedenstierna G. The hidden pulmonary dysfunction in acute lung injury. Intensive Care Med. 2006;32:1933–1934. doi: 10.1007/s00134-006-0383-3. [DOI] [PubMed] [Google Scholar]
- 37.Aboab J, Jonson B, Kouatchet A, Taille S, Niklason L, Brochard L. Effect of inspired oxygen fraction on alveolar derecruitment in acute respiratory distress syndrome. Intensive Care Med. 2006;32:1979–1986. doi: 10.1007/s00134-006-0382-4. [DOI] [PubMed] [Google Scholar]
- 38.Dantzker DR, Wagner PD, West JB. Instability of lung units with low VA/Q ratios during O2 breathing. J Appl Physiol. 1975;38:886–895. [Google Scholar]
- 39.Neumann P, Rothen HU, Berglund JE, Valtysson J, Magnusson A, Hedenstierna G. Positive end-expiratory pressure prevents atelectasis during general anaesthesia even in the presence of a high inspired oxygen concentration. Acta Anaesthesiol Scand. 1999;43:295–301. doi: 10.1034/j.1399-6576.1999.430309.x. [DOI] [PubMed] [Google Scholar]
- 40.Rusca M, Proietti S, Schnyder P, Frascarolo P, Hedenstierna G, Spahn DR, et al. Prevention of atelectasis formation during induction of general anesthesia. Anesth Analg. 2. [DOI] [PubMed]
- 41.Carraway MS, Piantadosi CA. Oxygen toxicity. Respir Care Clin N Am. 1999;5:265–295. [PubMed] [Google Scholar]
- 42.Bornstein A. Ueber Sauerstoffvergiftung. Dtsch Med Wochenschr. 1912;43:1495–1497. doi: 10.1055/s-0029-1189712. [DOI] [Google Scholar]
- 43.Lorrain Smith J. The pathological effects due to increase of oxygen tension in the air breathed. J Physiol (London). 1899;24:19–35. [DOI] [PMC free article] [PubMed]
- 44.Lavoisier AL. Mémoires de Médecine et de Physique Médicale. Société de Médecine Royale: Paris 1783.
- 45.Kallett RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58:123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eynan M, Krinsky N, Biram A, Aieli Y, Arieli R. A comparison of factors involoved in the development of central nervous system and pulmonary oxygen toxicity in the rat. Brains Res. 2014;1574:77–83. doi: 10.1016/j.brainres.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 47.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crimi E, Sica V, Williams-Ignarro S, Zhang H, Slutsky AS, Ignarro LJ, Napoli C. The role of oxidative stress in adult critical care. Free Rad Biol Med. 2006;398–406. [DOI] [PubMed]
- 49.Magder S. Reactive oxygen species: toxic molecules or spark of life? Crit Care. 2006;10:208. doi: 10.1186/cc3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zenri H, Rodriquez-Capote K, McCaig L, Yao LJ, Brackenbury A, Possmayer F, et al. Hyperoxia exposure impairs surfactant function and metabolism. Crit Care Med. 2004;32:1155–1160. doi: 10.1097/01.CCM.0000126264.00551.C8. [DOI] [PubMed] [Google Scholar]
- 51.Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med. 2004;32:2496–2501. doi: 10.1097/01.CCM.0000148231.04642.8D. [DOI] [PubMed] [Google Scholar]
- 52.Wagner K, Gröger M, McCook O, Scheuerle A, Asfar P, Stahl B, et al. Blunt Chest trauma in mice after cigarette smoke-exposure: effects of mechanical ventilation with 100% O2. PLoS One. 2015;10:e0132810. doi: 10.1371/journal.pone.0132810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cannizzaro V, Hantos Z, Sly PD, Zosky GR. Linking lung function and inflammatory responses in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2011;300:L112–L120. doi: 10.1152/ajplung.00158.2010. [DOI] [PubMed] [Google Scholar]
- 54.Barth E, Bassi G, Maybauer DM, Simon F, Groger M, Oter S, et al. Effects of ventilation with 100 % oxygen during early hyperdynamic porcine fecal peritonitis. Crit Care Med. 2008;36:495–503. doi: 10.1097/01.CCM.0B013E318161FC45. [DOI] [PubMed] [Google Scholar]
- 55.Suttner S, Boldt J. Routine use of high inspired oxygen concentration—con. Anasthesiol Intensivmed Notfallmed Schmerzther. 2005;40:354–357. doi: 10.1055/s-2005-861246. [DOI] [PubMed] [Google Scholar]
- 56.Sackner MA, Landa J, Hirsch J, Zapata A. Pulmonary effects of oxygen breathing. A 6-hour study in normal men. Ann Intern Med. 1975;82:40–43. doi: 10.7326/0003-4819-82-1-40. [DOI] [PubMed] [Google Scholar]
- 57.Comroe JH, Dripps RD, Dumke PR, Deming M. Oxygen toxicity: the effect of inhalation of high concentrations of oxygen for twenty-four hours on normal men at sea level and at a simulated altitude of 18,000 feet. JAMA. 1945;128:710–717. doi: 10.1001/jama.1945.02860270012004. [DOI] [Google Scholar]
- 58.Kabon B, Kurz A. Optimal perioperative oxygen administration. Curr Opin Anaesthesiol. 2006;19:11–18. doi: 10.1097/01.aco.0000192775.24774.15. [DOI] [PubMed] [Google Scholar]
- 59.Bean JW. Effects of oxygen at increased pressure. Physiol Rev. 1945;25:1–147. [Google Scholar]
- 60.Nash G, Blennerhassett JB, Pontoppidan H. Pulmonary lesions assocaited with oxygen therapy and artificial ventilation. N Engl J Med. 1967;276:368–374. doi: 10.1056/NEJM196702162760702. [DOI] [PubMed] [Google Scholar]
- 61.Hyde RW, Rawson AJ. Unintentional iatrogenic oxygen pneumonitis—response to therapy. Ann Intern Med. 1969;71:517–531. doi: 10.7326/0003-4819-71-3-517. [DOI] [PubMed] [Google Scholar]
- 62.Gould VE, Tosco R, Wheelis RF, Gould NS, Kapanci Y. Oxygen pneumonitis in man. Ultrastructural observations on the development of alveolar lesions. Lab Invest. 1972;26:499–508. [PubMed] [Google Scholar]
- 63.Kapanci Y, Tosco R, Eggermann J, Gould VE. Oxygen pneumonitis in man. Light- and electron-microscopic morphometric studies. Chest. 1972;62:162–169. doi: 10.1378/chest.62.2.162. [DOI] [PubMed] [Google Scholar]
- 64.Rachmale S, Li G, Wilson G, Malinchoc M, Gajic O. Practice of excessive FIO2 and effects on pulmonary outcomes in mechanically ventilated patients with acute lung injury. Respir Care. 2012;57:1887–1893. doi: 10.4187/respcare.01696. [DOI] [PubMed] [Google Scholar]
- 65.Elmer J, Wang B, Melhem S, Pullalarevu R, Vaghasia N, Buddineni J, et al. Exposure to high concentrations of inspired oxygen does not worsen lung injury after cardiac arrest. Crit Care. 2015;19:105. doi: 10.1186/s13054-015-0824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hendricks PL, Hall DA, Hunter WL, Jr, Haley PJ. Extension of pulmonary O2 tolerance in man at 2 ATA by intermittent O2 exposure. J Appl Physiol. 1977;42:593–599. doi: 10.1152/jappl.1977.42.4.593. [DOI] [PubMed] [Google Scholar]
- 67.Whalen RE, Saltzman HA, Holloway DH, Jr, McIntosh HD, Sieker HO, Brown IW., Jr Cardiovascular and blood gas responses to hyperbaric oxygenation. Am J Cardiol. 1965;15:638–646. doi: 10.1016/0002-9149(65)90350-4. [DOI] [PubMed] [Google Scholar]
- 68.Mak S, Azevedo ER, Liu PP, Newton GE. Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest. 2001;120:467–473. doi: 10.1378/chest.120.2.467. [DOI] [PubMed] [Google Scholar]
- 69.Mak S, Egri Z, Tanna G, Colman R, Newton GE. Vitamin C prevents hyperoxia-mediated vasoconstriction and impairment of endothelium-dependent vasodilation. Am J Physiol Heart Circ Physiol. 2002;282:H2414–H2421. doi: 10.1152/ajpheart.00947.2001. [DOI] [PubMed] [Google Scholar]
- 70.Reinhart K, Bloos F, Konig F, Bredle D, Hannemann L. Reversible decrease of oxygen consumption by hyperoxia. Chest. 1991;99:690–694. doi: 10.1378/chest.99.3.690. [DOI] [PubMed] [Google Scholar]
- 71.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 72.Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 73.McNulty PH, Robertson BJ, Tulli MA, Hess J, Harach LA, Scott S, et al. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. J Appl Physiol. 1985;2007(102):2040–2045. doi: 10.1152/japplphysiol.00595.2006. [DOI] [PubMed] [Google Scholar]
- 74.Rossi P, Tauzin L, Weiss M, Rostain JC, Sainty JM, Boussuges A. Could hyperoxic ventilation impair oxygen delivery in septic patients? Clin Physiol Funct Imaging. 2007;27:180–184. doi: 10.1111/j.1475-097X.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- 75.Farquhar H, Weatherall M, Wijesinghe M, Perrin K, Ranchord A, Simmonds M, et al. Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J. 2009;158:371–377. doi: 10.1016/j.ahj.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 76.Cortés D, Puflea F, Donadello K, Taccone F, Gottin L, Creteur J, et al. Normobaric hyperoxia alters the microcirculation in healthy volunteers. Microvasc Res. 2015;98:23–28. doi: 10.1016/j.mvr.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 77.Bitterman H, Brod V, Weisz G, Kushnir D, Bitterman N. Effects of oxygen on regional hemodynamics in hemorrhagic shock. Am J Physiol. 1996;271:H203–H211. doi: 10.1152/ajpheart.1996.271.1.H203. [DOI] [PubMed] [Google Scholar]
- 78.Stolmeijer R, ter Maaten JC, Zijlstra JG, Ligtenberg JJ. Oxygen therapy for sepsis patients in the emergency department: a little less? Eur J Emerg Med. 2014;21:233–235. doi: 10.1097/MEJ.0b013e328361c6c7. [DOI] [PubMed] [Google Scholar]
- 79.Schoonen WG, Wanamarta AH, van der Klei-van Moorsel JM, Jakobs C, Joenje H. Hyperoxia-induced clonogenic killing of HeLa cells associated with respiratory failure and selective inactivation of Krebs cycle enzymes. Mutat Res. 1990;237:173–81. [DOI] [PubMed]
- 80.Das KC. Hyperoxia decreases glycolytic capacity, glycolytic reserve and oxidative phosphorylation in MLE-12 cells and inhibits complex I and II function, but not complex IV in isolated mouse lung mitochondria. PLoS One. 2013;8:e73358. doi: 10.1371/journal.pone.0073358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lauscher P, Lauscher S, Kertscho H, Habler O, Meier J. Hyperoxia reversibly alters oxygen consumption and metabolism. Scie World J. 2012;2012:410321. doi: 10.1100/2012/410321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reinhart K, Spies CD, Meier-Hellmann A, Bredle DL, Hannemann L, Specht M, et al. N-acetylcysteine preserves oxygen consumption and gastric mucosal pH during hyperoxic ventilation. Am J Respir Crit Care Med. 1995;151:773–779. doi: 10.1164/ajrccm/151.3_Pt_1.773. [DOI] [PubMed] [Google Scholar]
- 83.Ganz W, Donoso R, Marcus H, Swan HJ. Coronary hemodynamics and myocardial oxygen metabolism during oxygen breathing in patients with and without coronary artery disease. Circulation. 1972;45:763–768. doi: 10.1161/01.CIR.45.4.763. [DOI] [PubMed] [Google Scholar]
- 84.Leverve XM. Mitochondrial function and substrate availability. Crit Care Med. 2007;35:S454–S460. doi: 10.1097/01.CCM.0000278044.19217.73. [DOI] [PubMed] [Google Scholar]
- 85.Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1345–H1351. doi: 10.1152/ajpheart.2000.278.4.H1345. [DOI] [PubMed] [Google Scholar]
- 86.Hochachka PW, Stanley C, Matheson GO, McKenzie DC, Allen PS, Parkhouse WS. Metabolic and work efficiencies during exercise in Andean natives. J Appl Physiol. 1985;1991(70):1720–1730. doi: 10.1152/jappl.1991.70.4.1720. [DOI] [PubMed] [Google Scholar]
- 87.La Bert P. Pression Barométrique. Paris: Masson; 1878. [Google Scholar]
- 88.Demchenko IT, Moskvin AN, Krivchenko AI, Piantadosi CA, Allen BW. Nitric oxide-mediated central sympathetic excitation promotes CNS and pulmonary O2 toxicity. J Appl Physiol. 2012;112:1814–1823. doi: 10.1152/japplphysiol.00902.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koch AE, Kähler W, Wegner-Bröse H, Weyer D, Kuhtz-Buschbeck J, Deuschl G, et al. Monitoring of CBFV and time characteristics of oxygen-induced acute CNS toxicity in humans. Eur J Neurol. 2008;15:746–748. doi: 10.1111/j.1468-1331.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 90.Bitterman N, Bitterman H. L-Arginine-NO pathway and CNS oxygen toxicity. J Appl Physiol. 1998;84:1633–1638. doi: 10.1152/jappl.1998.84.5.1633. [DOI] [PubMed] [Google Scholar]
- 91.Roth D, Schreiber W, Herkner H, Havel C. Prevalence of carbon monoxide poisoning in patients presenting to a large emergency department. Int J Clin Pract. 2014;68:1239–1245. doi: 10.1111/ijcp.12432. [DOI] [PubMed] [Google Scholar]
- 92.Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339:1603–1608. doi: 10.1056/NEJM199811263392206. [DOI] [PubMed] [Google Scholar]
- 93.Miro O, Alonso JR, Casademont J, Jarreta D, Urbano-Marquez A, Cardellach F. Oxidative damage on lymphocyte membranes is increased in patients suffering from acute carbon monoxide poisoning. Toxicol Lett. 1999;110:219–223. doi: 10.1016/S0378-4274(99)00161-7. [DOI] [PubMed] [Google Scholar]
- 94.Stamler JS, Piantadosi CA. O=O NO: it’s CO. J Clin Invest. 1996;97:2165–2166. doi: 10.1172/JCI118656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weaver LK, Howe S, Hopkins R, Chan KJ. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100 % oxygen at atmospheric pressure. Chest. 2000;117:801–808. doi: 10.1378/chest.117.3.801. [DOI] [PubMed] [Google Scholar]
- 96.Annane D, Chadda K, Gajdos P, Jars-Guincestre MC, Chevret S, Raphael JC. Hyperbaric oxygen therapy for acute domestic carbon monoxide poisoning: two randomized controlled trials. Intensive Care Med. 2011;37:486–492. doi: 10.1007/s00134-010-2093-0. [DOI] [PubMed] [Google Scholar]
- 97.Weaver LK, Hopkins RO, Chan KJ, Churchill S, Elliott CG, Clemmer TP, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347:1057–1067. doi: 10.1056/NEJMoa013121. [DOI] [PubMed] [Google Scholar]
- 98.Scheinkestel CD, Bailey M, Myles PS, Jones K, Cooper DJ, Millar IL, et al. Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: a randomised controlled clinical trial. Med J Aust. 1999;170:203–210. doi: 10.5694/j.1326-5377.1999.tb140318.x. [DOI] [PubMed] [Google Scholar]
- 99.Chiew AL, Buckley NA. Carbon monoxide poisoning in the 21st century. Crit Care. 2014;18:221. doi: 10.1186/cc13846. [DOI] [Google Scholar]
- 100.Muth CM, Shank ES. Gas embolism. N Engl J Med. 2000;342:476–482. doi: 10.1056/NEJM200002173420706. [DOI] [PubMed] [Google Scholar]
- 101.Vann RD, Butler FK, Mitchell SJ, Moon RE. Decompression illness. Lancet. 2011;377:153–164. doi: 10.1016/S0140-6736(10)61085-9. [DOI] [PubMed] [Google Scholar]
- 102.Tetzlaff K, Shank ES, Muth CM. Evaluation and management of decompression illness–an intensivist’s perspective. Intensive Care Med. 2003;29:2128–2136. doi: 10.1007/s00134-003-1999-1. [DOI] [PubMed] [Google Scholar]
- 103.Muth CM, Shank ES, Larsen B. Severe diving accidents: physiopathology, symptoms, therapy. Anaesthesist. 2000;49:302–316. doi: 10.1007/s001010050832. [DOI] [PubMed] [Google Scholar]
- 104.Boland EW. Oxygen in high concentrations for relief of pain in coronary thrombosis and severe angina pectoris. JAMA. 1940;114:1512–1514. [Google Scholar]
- 105.McNulty PH, King N, Scott S, Hartman G, McCann J, Kozak M, et al. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;288:H1057–H1062. doi: 10.1152/ajpheart.00625.2004. [DOI] [PubMed] [Google Scholar]
- 106.Kones R. Oxygen therapy for acute myocardial infarction-then and now. A century of uncertainty. Am J Med. 2011;124:1000–1005. doi: 10.1016/j.amjmed.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 107.Shuvy M, Atar D, Gabriel Steg P, Halvorsen S, Jolly S, Yusuf S, et al. Oxygen therapy in acute coronary syndrome: are the benefits worth the risk? Eur Heart J. 2013;34:1630–1635. doi: 10.1093/eurheartj/eht110. [DOI] [PubMed] [Google Scholar]
- 108.Minana G, Nunez J, Banuls P, Sanchis J, Nunez E, Robles R, et al. Prognostic implications of arterial blood gases in acute decompensated heart failure. Eur J Intern Med. 2011;22:489–494. doi: 10.1016/j.ejim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 109.HR, Bossaert LL, Danchin N, Nikolaou NI. European Resuscitation Council Guidelines for Resuscitation 2010 Section 5. Initial management of acute coronary syndromes. Resuscitation. 2010;81:1353–63. [DOI] [PubMed]
- 110.Madias JE, Madias NE, Hood WB., Jr Precordial ST-segment mapping. 2. Effects of oxygen inhalation on ischemic injury in patients with acute myocardial infarction. Circulation. 1976;53:411–417. doi: 10.1161/01.CIR.53.3.411. [DOI] [PubMed] [Google Scholar]
- 111.Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1:1121–1123. doi: 10.1136/bmj.1.6018.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ukholkina GB, Kostyanov IY, Kuchkina NV, Grendo EP, Gofman YB. Oxygen therapy in combination with endovascular reperfusion during the first hours of acute myocardial infarction: clinical and laboratory findings. Int J Interv Cardioangiol. 2005;9:45–51. [Google Scholar]
- 113.Ranchord AM, Argyle R, Beynon R, Perrin K, Sharma V, Weatherall M, et al. High-concentration versus titrated oxygen therapy in ST-elevation myocardial infarction: a pilot randomized controlled trial. Am Heart J. 2012;163:168–175. doi: 10.1016/j.ahj.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 114.Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, et al. Air versus oxygen in ST-segment elevation myocardial infarction. Circulation. 2015;131:2143–2150. doi: 10.1161/CIRCULATIONAHA.114.014494. [DOI] [PubMed] [Google Scholar]
- 115.Rockswold SB, Rockswold GL, Zaun DA, Zhang X, Cerra CE, Bergman TA, et al. A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J Neurosurg. 2010;112:1080–1094. doi: 10.3171/2009.7.JNS09363. [DOI] [PubMed] [Google Scholar]
- 116.Rockswold SB, Rockswold GL, Zaun DA, Liu J. A prospective, randomized Phase II clinical trial to evaluate the effect of combined hyperbaric and normobaric hyperoxia on cerebral metabolism, intracranial pressure, oxygen toxicity, and clinical outcome in severe traumatic brain injury. J Neurosurg. 2013;118:1317–1328. doi: 10.3171/2013.2.JNS121468. [DOI] [PubMed] [Google Scholar]
- 117.Magnoni S, Ghisoni L, Locatelli M, Caimi M, Colombo A, Valeriani V, et al. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: a microdialysis study. J Neurosurg. 2003;98:952–958. doi: 10.3171/jns.2003.98.5.0952. [DOI] [PubMed] [Google Scholar]
- 118.Tolias CM, Reinert M, Seiler R, Gilman C, Scharf A, Bullock MR. Normobaric hyperoxia–induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J Neurosurg. 2004;101:435–444. doi: 10.3171/jns.2004.101.3.0435. [DOI] [PubMed] [Google Scholar]
- 119.Diringer MN, Aiyagari V, Zazulia AR, Videen TO, Powers WJ. Effect of hyperoxia on cerebral metabolic rate for oxygen measured using positron emission tomography in patients with acute severe head injury. J Neurosurg. 2007;106:526–529. doi: 10.3171/jns.2007.106.4.526. [DOI] [PubMed] [Google Scholar]
- 120.Hlatky R, Valadka AB, Gopinath SP, Robertson CS. Brain tissue oxygen tension response to induced hyperoxia reduced in hypoperfused brain. J Neurosurg. 2008;108:53–58. doi: 10.3171/JNS/2008/108/01/0053. [DOI] [PubMed] [Google Scholar]
- 121.Nortje J, Coles JP, Timofeev I, Fryer TD, Aigbirhio FI, Smielewski P, et al. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: preliminary findings. Crit Care Med. 2008;36:273–281. doi: 10.1097/01.CCM.0000292014.60835.15. [DOI] [PubMed] [Google Scholar]
- 122.Quintard H, Patet C, Suys T, Marques-Vidal P, Oddo M. Normobaric hyperoxia is associated with increased cerebral excitotoxicity after severe traumatic brain injury. Neurocrit Care. 2015;22:243–250. doi: 10.1007/s12028-014-0062-0. [DOI] [PubMed] [Google Scholar]
- 123.Veenith TV, Carter EL, Grossac J, Newcombe VF, Outtrim JG, Nallapareddy S, et al. Use of diffusion tensor imaging to assess the impact of normobaric hyperoxia within at-risk pericontusional tissue after traumatic brain injury. J Cereb Blood Flow Metab. 2014;34:1622–1627. doi: 10.1038/jcbfm.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Narotam PK. Eubaric hyperoxia: controversies in the management of acute traumatic brain injury. Crit Care. 2013;17:197. doi: 10.1186/cc13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Raj R, Bendel S, Reinikainen M, Kivisaari R, Siironen J, Lang M, et al. Hyperoxemia and long-term outcome after traumatic brain injury. Crit Care. 2013;17:R177. doi: 10.1186/cc12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Davis DP, Meade W, Sise MJ, Kennedy F, Simon F, Tominaga G, et al. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009;26:2217–2223. doi: 10.1089/neu.2009.0940. [DOI] [PubMed] [Google Scholar]
- 127.Brenner M, Stein D, Hu P, Kufera J, Wooford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147:1042–1046. doi: 10.1001/archsurg.2012.1560. [DOI] [PubMed] [Google Scholar]
- 128.Rincon F, Kang J, Vibbert M, Urtecho J, Athar MK, Jallo J. Significance of arterial hyperoxia and relationship with case fatality in traumatic brain injury: a multicentre cohort study. J Neurol Neurosurg Psychiatry. 2014;85:799–805. doi: 10.1136/jnnp-2013-305505. [DOI] [PubMed] [Google Scholar]
- 129.Asher SR, Curry P, Sharma D, Wang J, O’Keefe GE, Daniel-Johnson J, et al. Survival advantage and PaO2 threshold in severe traumatic brain injury. J Neurosurg Anesthesiol. 2013;25:168–173. doi: 10.1097/ANA.0b013e318283d350. [DOI] [PubMed] [Google Scholar]
- 130.Qi Z, Liu W, Luo Y, Ji X, Liu KJ. Normobaric hyperoxia-based neuroprotective therapies in ischemic stroke. Med Gas Res. 2013;3:2. doi: 10.1186/2045-9912-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, et al. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005;36:797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- 132.Singhal AB, Ratai E, Benner T, Vangel M, Lee V, Koroshetz WJ, et al. Magnetic resonance spectroscopy study of oxygen therapy in ischemic stroke. Stroke. 2007;38:2851–2854. doi: 10.1161/STROKEAHA.107.487280. [DOI] [PubMed] [Google Scholar]
- 133.Jeon SB, Choi HA, Badjatia N, Schmidt JM, Lantigua H, Claassen J, et al. Hyperoxia may be related to delayed cerebral ischemia and poor outcome after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2014;85:1301–1307. doi: 10.1136/jnnp-2013-307314. [DOI] [PubMed] [Google Scholar]
- 134.Rincon F, Kang J, Maltenfort M, Vibbert M, Urtecho J, Athar MK, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42:387–396. doi: 10.1097/CCM.0b013e3182a27732. [DOI] [PubMed] [Google Scholar]
- 135.Young P, Beasley R, Bailey M, Bellomo R, Eastwood GM, Nichol A, et al. The association between early arterial oxygenation and mortality in ventilated patients with acute ischaemic stroke. Crit Care Resusc. 2012;14:14–19. [PubMed] [Google Scholar]
- 136.Roffe C, Ali K, Warusevitane A, Sills S, Pountain S, Allen M, et al. The SOS pilot study: a RCT of routine oxygen supplementation early after acute stroke–effect on recovery of neurological function at one week. PLoS One. 2011;6:e19113. doi: 10.1371/journal.pone.0019113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ali K, Warusevitane A, Lally F, Sim J, Sills S, Pountain S, et al. The stroke oxygen pilot study: a randomized controlled trial of the effects of routine oxygen supplementation early after acute stroke–effect on key outcomes at 6 months. PLoS One. 2013;8:e59274. doi: 10.1371/journal.pone.0059274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 139.Kilgannon JH, Jones AE, Parrillo JE, Dellinger RP, Milcarek B, Hunter K, et al. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123:2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- 140.Bellomo R, Bailey M, Eastwood GM, Nichol A, Pilcher D, Hart GK, et al. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15:R90. doi: 10.1186/cc10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ihle JF, Bernard S, Bailey MJ, Pilcher DV, Smith K, Scheinkestel CD. Hyperoxia in the intensive care unit and outcome after out-of-hospital ventricular fibrillation cardiac arrest. Crit Care Resusc. 2013;15:186–190. [PubMed] [Google Scholar]
- 142.Nelskylä A, Parr MJ, Skrifvars MB. Prevalence and factors correlating with hyperoxia exposure following cardiac arrest–an observational single centre study. Scand J Trauma Resusc Emerg Med. 2013;21:35. doi: 10.1186/1757-7241-21-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Janz DR, Hollenbeck RD, Pollock JS, McPherson JA, Rice TW. Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Crit Care Med. 2012;40:3135–3139. doi: 10.1097/CCM.0b013e3182656976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee BK, Jeung KW, Lee HY, Lee SJ, Jung YH, Lee WK, et al. Association between mean arterial blood gas tension and outcome in cardiac arrest patients treated with therapeutic hypothermia. Am J Emerg Med. 2014;32:55–60. doi: 10.1016/j.ajem.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 145.Kuisma M, Boyd J, Voipio V, Alaspaa A, Roine RO, Rosenberg P. Comparison of 30 and the 100 % inspired oxygen concentrations during early post-resuscitation period: a randomised controlled pilot study. Resuscitation. 2006;69:199–206. doi: 10.1016/j.resuscitation.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 146.Schneider AG, Eastwood GM, Bellomo R, Bailey M, Lipcsey M, Pilcher D, et al. Arterial carbon dioxide tension and outcome in patients admitted to the intensive care unit after cardiac arrest. Resuscitation. 2013;84:927–934. doi: 10.1016/j.resuscitation.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 147.Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Trzeciak S. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation. 2013;127:2107–2113. doi: 10.1161/CIRCULATIONAHA.112.000168. [DOI] [PubMed] [Google Scholar]
- 148.Vaahersalo J, Bendel S, Reinikainen M, Kurola J, Tiainen M, Raj R, et al. Arterial blood gas tensions after resuscitation from out-of-hospital cardiac arrest: associations with long-term neurologic outcome. Crit Care Med. 2014;42:1463–1470. doi: 10.1097/CCM.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 149.Wang CH, Chang WT, Huang CH, Tsai MS, Yu PH, Wang AY, et al. The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta-analysis of observational studies. Resuscitation. 2014;85:1142–1148. doi: 10.1016/j.resuscitation.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 150.Elmer J, Scutella M, Pullalarevu R, Wang B, Vaghasia N, Trzeciak S, et al. The association between hyperoxia and patient outcomes after cardiac arrest: analysis of a high-resolution database. Intensive Care Med. 2015;41:49–57. doi: 10.1007/s00134-014-3555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Young P, Bailey M, Bellomo R, Bernard S, Dicker B, Freebairn R, et al. HyperOxic Therapy OR NormOxic Therapy after out-of-hospital cardiac arrest (HOT OR NOT): a randomised controlled feasibility trial. Resuscitation. 2014;85:1686–1691. doi: 10.1016/j.resuscitation.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 152.Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic. The effect of inspired oxygen on infection. Arch Surg. 1984;119:199–204. doi: 10.1001/archsurg.1984.01390140057010. [DOI] [PubMed] [Google Scholar]
- 153.Togioka B, Galvagno S, Sumida S, Murphy J, Ouanes JP, Wu C. The role of perioperative high inspired oxygen therapy in reducing surgical site infection: a meta-analysis. Anesth Analg. 2012;114:334–342. doi: 10.1213/ANE.0b013e31823fada8. [DOI] [PubMed] [Google Scholar]
- 154.Hovaguimian F, Lysakowski C, Elia N, Tramer MR. Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2013;119:303–316. doi: 10.1097/ALN.0b013e31829aaff4. [DOI] [PubMed] [Google Scholar]
- 155.Schietroma M, Cecilia EM, Sista F, Carlei F, Pessia B, Amicucci G. High-concentration supplemental perioperative oxygen and surgical site infection following elective colorectal surgery for rectal cancer: a prospective, randomized, double-blind, controlled, single-site trial. Am J Surg. 2014;208:719–726. doi: 10.1016/j.amjsurg.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 156.Kotani N, Hashimoto H, Sessler DI, Muraoka M, Hashiba E, Kubota T, et al. Supplemental intraoperative oxygen augments antimicrobial and proinflammatory responses of alveolar macrophages. Anesthesiology. 2000;93:15–25. doi: 10.1097/00000542-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 157.Qadan M, Battista C, Gardner SA, Anderson G, Akca O, Polk HC., Jr Oxygen and surgical site infection: a study of underlying immunologic mechanisms. Anesthesiology. 2010;113:369–377. doi: 10.1097/ALN.0b013e3181e19d1d. [DOI] [PubMed] [Google Scholar]
- 158.Meyhoff CS, Jorgensen LN, Wetterslev J, Christensen KB, Rasmussen LS. Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow-up of a randomized clinical trial. Anesth Analg. 2012;115:849–854. doi: 10.1213/ANE.0b013e3182652a51. [DOI] [PubMed] [Google Scholar]
- 159.Meyhoff CS, Jorgensen LN, Wetterslev J, Siersma VD, Rasmussen LS. Risk of new or recurrent cancer after a high perioperative inspiratory oxygen fraction during abdominal surgery. Br J Anaesth. 2014;113(Suppl 1):i74–i81. doi: 10.1093/bja/aeu110. [DOI] [PubMed] [Google Scholar]
- 160.Meyhoff CS, Wetterslev J, Jorgensen LN, Hennebegr SW, Hogdall C, Lundvall L, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery. JAMA. 2009;302:1543–1550. doi: 10.1001/jama.2009.1452. [DOI] [PubMed] [Google Scholar]
- 161.Kurz A, Fleischmann E, Sessler DI, Buggy DJ, Apfel C, Akça O, et al. Effects of supplemental oxygen and dexamethasone on surgical site infection: a factorial randomized trial. Br J Anaesth. 2015;115:434–443. doi: 10.1093/bja/aev062. [DOI] [PubMed] [Google Scholar]
- 162.Wetterslev J, Meyhoff CS, Jørgensen LN, Gluud C, Lindschou J, Rasmussen LS. The effect of high perioperative inspiratory oxygen fraction for adult surgical patients (review). Cochrane Database Syst Rev. 2015;6:CD008884. [DOI] [PMC free article] [PubMed]
- 163.de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PH, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12:R156. doi: 10.1186/cc7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, Abu-Hanna A, de Keizer NF, de Jonge E. Associations of arterial carbon dioxide and arterial oxygen concentrations with hospital mortality after resuscitation from cardiac arrest. Crit Care. 2015;19:348. doi: 10.1186/s13054-015-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eastwood G, Bellomo R, Bailey M, Taori G, Pilcher D, Young P, et al. Arterial oxygen tension and mortality in mechanically ventilated patients. Intensive Care Med. 2012;38:91–98. doi: 10.1007/s00134-011-2419-6. [DOI] [PubMed] [Google Scholar]
- 166.Sutton AD, Bailey M, Bellomo R, Eastwood GM, Pilcher DV. The association between early arterial oxygenation in the ICU and mortality following cardiac surgery. Anaesth Intensive Care. 2014;42:730–735. doi: 10.1177/0310057X1404200608. [DOI] [PubMed] [Google Scholar]
- 167.Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18:711. doi: 10.1186/s13054-014-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, de Jonge E. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, metaanalysis, and meta-regression of cohort studies. Crit Care Med. 2015;43:1508–1519. doi: 10.1097/CCM.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 169.Helmerhorst HJ, Schultz MJ, van der Voort PH, Bosman RJ, Juffermans NP, de Jonge E, et al. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care. 2014;4:23. doi: 10.1186/s13613-014-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rixen D, Siegel JH. Bench-to-bedside review: oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care. 2005;9:441–453. doi: 10.1186/cc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Barbee RW, Reynolds PS, Ward KR. Assessing shock resuscitation strategies by oxygen debt repayment. Shock. 2010;33:113–122. doi: 10.1097/SHK.0b013e3181b8569d. [DOI] [PubMed] [Google Scholar]
- 172.Suzuki S, Eastwood GM, Glassford NJ, Peck L, Young H, Garcia-Alvarez M, et al. Conservative oxygen therapy in mechanically ventilated patients: a pilot before-and-after trial. Crit Care Med. 2014;42:1414–1422. doi: 10.1097/CCM.0000000000000219. [DOI] [PubMed] [Google Scholar]


