Photosynthetic organisms face a special challenge. They must absorb large numbers of solar photons to provide the energy to power their metabolism, yet too much energy can be extremely damaging and can lead to rapid cell death (1). Maintaining the balance between too little and too much energy, especially in the face of light intensity fluctuations that can change by orders of magnitude within milliseconds, requires a sophisticated system that has multiple levels of regulation and repair. The challenge is especially complex for oxygen-evolving photosynthetic organisms, which contain two coupled light-driven reaction centers that must coordinate electron flow despite significantly different kinetic and spectral properties. The job of collecting photons, efficiently distributing the energy to the place where it is needed, and protecting the organism against photodamage falls to the photosynthetic antenna system. These antenna systems are specialized for the absorption of photons and transfer of energy in the form of excited states or excitons to the photosynthetic reaction center, where electron transfer processes begin the long series of processes that lead to long-term energy storage in the form of biomass (2). In many cases, these antenna complexes can be purified away from the reaction centers and their light-absorbing and regulatory properties can be studied in molecular detail. In PNAS, Wang and Moerner (3) have carried out an in-depth analysis of a photosynthetic antenna complex, the allophycocyanin pigment protein found as part of the larger phycobilisome complex in cyanobacteria, revealing in remarkable detail the complexities of the energy transfer and photodegradation processes that take place.
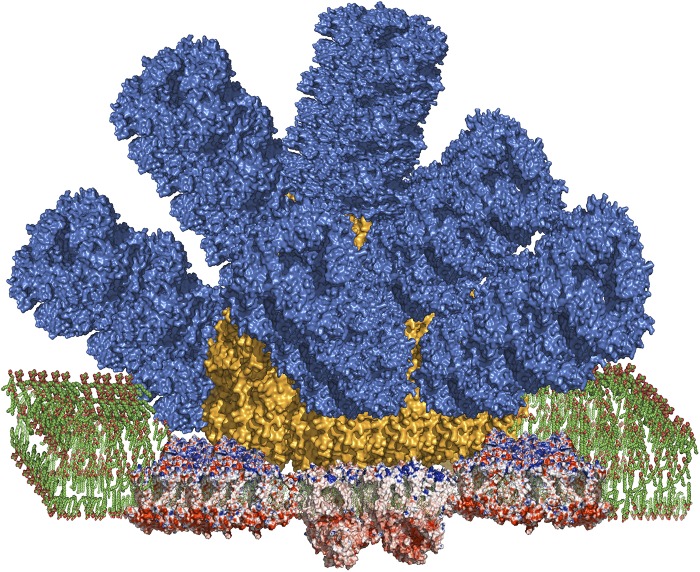
Phycobilisomes are large peripheral membrane pigment–protein complexes that are attached to the cytoplasmic side of the thylakoid membrane that contains the reaction centers (4–9). They are made up of several types of pigment-containing proteins, whose pigments are covalently attached open-chain tetrapyrrole pigments called bilins. Phycobilisomes also contain numerous colorless proteins collectively known as linkers, which help to organize the system and also fine-tune the spectral properties of the pigment-containing subunits to direct the energy flow to the reaction centers, which are integral membrane complexes that contain chlorophylls and other electron transfer cofactors. The entire phycobilisome complex can have a mass of several million daltons. Phycobilisomes have been known for many years to transfer energy mainly to photosystem II, the water-oxidizing photosystem, or, in some cases, to photosystem I (10). Recently, it has been shown that a “megacomplex” containing both photosystems and the phycobilisome is present (11). Fig. 1 shows a structural model that incorporates structural features of phycobiliproteins and reaction centers and their spatial relationships. The phycobiliproteins are organized into rods that feed energy into a central core structure, which then rapidly and efficiently transfers excitations to the reaction centers in the membrane.
Fig. 1.
Structural model of a phycobilisome antenna complex associated with both photosystem I and photosystem II in a megacomplex. The model is based on X-ray crystal structures of the phycocyanin and allophycocyanin proteins and the reaction centers. The relative positions and orientations of the phycobilisome and reaction centers are based on chemical cross-linking and MS studies (11). Color coding is as follows: phycocyanin, blue; allophycocyanin, orange; reaction centers are shown in red (negative charged residues), blue (positively charged residues), and tan (uncharged residues). Photosystem II is present as a dimeric complex directly under the core of the phycobilisome, with the downward-pointing lobes that make up the accessory subunits of the oxygen-evolving system. Photosystem I is present as two copies of a trimeric complex that flanks the central attachment site of the phycobilisome on photosystem II. The lipids of the thylakoid membrane bilayer are shown in green. Image courtesy of Haijun Liu (Washington University in St. Louis, St. Louis).
The pathway of energy transfer in phycobilisomes has been established to be directional along the rods, which are sometimes described as “light guides” (5). The allophycocyanin complex studied in detail by Wang and Moerner (3) is intermediate in the energy transfer system, receiving excitations from the phycocyanin complexes that make up the rods and transferring them to the terminal emitter complexes that, in turn, feed the reaction centers.
The basic architecture of the components of the phycobilisome is a trimeric building block. Each “monomer” subunit in the trimeric complex consists of a heterodimeric protein complex containing an alpha and beta protein chain. Each of the protein chains has one to three covalently attached bilin pigments, depending on the type of phycobiliprotein. In the allophycocyanin complex considered here, there are a total of six pigments in the trimeric assembly. These trimeric complexes then further assemble into hexameric discs, and several of the discs form the core structure of the phycobilisome, along with some additional subunits that facilitate the energy transfer to the reaction center. The phycocyanin subunits have a similar overall architecture but contain three bilin pigments per trimer. The hexameric discs of phycocyanin form the extended rods in the phycobilisome. In some species of cyanobacteria, a third type of phycobiliprotein called phycoerythrin is found at the tips of the rods.
The allophycocyanin complex is also thought to be the site of action of the major regulatory process in phycobilisomes, energy quenching that is mediated by a water-soluble orange carotenoid protein (12). This protein has a photocycle in which, under excess light conditions, photons absorbed by the carotenoid cofactor convert the protein to a red form that docks on the phycobilisome and quenches excitations. The molecular details of how this quenching process works are not understood in detail, but MS cross-linking studies suggest that the orange carotenoid protein binds to the allophycocyanin near where pigment beta resides. The quenching is proposed to take place by Förster resonance energy transfer from the bilin pigment to the nearby carotenoid, which then safely dissipates the energy
Wang and Moerner have carried out an in-depth analysis of a photosynthetic antenna complex, the allophycocyanin pigment protein found as part of the larger phycobilisome complex in cyanobacteria.
as heat (13). The orange carotenoid protein undergoes a substantial conformational change upon the transition from the nonquenching orange form to the quenching red form, which is thought to position the carotenoid more favorably to quench excitations in the bound form (14–16).
The study of Wang and Moerner (3) uses the single molecule spectroscopy technique, which Moerner pioneered and for which he shared the 2014 Nobel Prize in Chemistry. They trapped both monomeric and trimeric complexes of allophycocyanin in an electrokinetic trap that cancels diffusion and then examined them in detail in terms of stability and spectral properties. Perhaps the most surprising aspect of the results reported by Wang and Moerner (3) is that the two pigments in each alpha/beta monomer subunit, although chemically identical, have significantly different properties, particularly their susceptibility to photodegradation. The trimers, which have six bilin pigments, show even more complex behavior with a number of distinct states that depend on the level of intactness of the complex during photobleaching. Their work provides an unprecedented level of molecular detail of the inner workings of the phycobilisome antenna complex and electronic properties of the pigments in their natural environment. It thus provides a solid foundation to understand the larger scale processes that take place in the complete phycobilisome, including its regulation.
Acknowledgments
This work was supported by Grant DE-FG02-07ER15902 from the Photosynthetic Systems Program of the US Department of Energy, Office of Science, Basic Energy Sciences.
Footnotes
The author declares no conflict of interest.
See companion article on page 13880.
References
- 1.Blankenship RE. Molecular Mechanisms of Photosynthesis. 2nd Ed Wiley–Blackwell; Oxford: 2014. [Google Scholar]
- 2.Croce R, van Amerongen H. Natural strategies for photosynthetic light harvesting. Nat Chem Biol. 2014;10(7):492–501. doi: 10.1038/nchembio.1555. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Moerner WE. Dissecting pigment architecture of individual photosynthetic antenna complexes in solution. Proc Natl Acad Sci USA. 2015;112:13880–13885. doi: 10.1073/pnas.1514027112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gantt E, Conti SF. Phycobiliprotein localization in algae. Brookhaven Symp Biol. 1966;19:393–405. [PubMed] [Google Scholar]
- 5.Glazer AN. Light harvesting by phycobilisomes. Annu Rev Biophys Biophys Chem. 1985;14:47–77. doi: 10.1146/annurev.bb.14.060185.000403. [DOI] [PubMed] [Google Scholar]
- 6.MacColl R. Cyanobacterial phycobilisomes. J Struct Biol. 1998;124(2-3):311–334. doi: 10.1006/jsbi.1998.4062. [DOI] [PubMed] [Google Scholar]
- 7.Adir N. Elucidation of the molecular structures of components of the phycobilisome: Reconstructing a giant. Photosynth Res. 2005;85(1):15–32. doi: 10.1007/s11120-004-2143-y. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe M, Ikeuchi M. Phycobilisome: Architecture of a light-harvesting supercomplex. Photosynth Res. 2013;116(2-3):265–276. doi: 10.1007/s11120-013-9905-3. [DOI] [PubMed] [Google Scholar]
- 9.Chang L, et al. Structural organization of an intact phycobilisome and its association with photosystem II. Cell Res. 2015;25(6):726–737. doi: 10.1038/cr.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullineaux CW. Phycobilisome-reaction centre interaction in cyanobacteria. Photosynth Res. 2008;95(2-3):175–182. doi: 10.1007/s11120-007-9249-y. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, et al. Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria. Science. 2013;342(6162):1104–1107. doi: 10.1126/science.1242321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirilovsky D. Modulating energy arriving at photochemical reaction centers: Orange carotenoid protein-related photoprotection and state transitions. Photosynth Res. 2015;126(1):3–17. doi: 10.1007/s11120-014-0031-7. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, et al. Molecular mechanism of photoactivation and structural location of the cyanobacterial orange carotenoid protein. Biochemistry. 2014;53(1):13–19. doi: 10.1021/bi401539w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leverenz RL, et al. PHOTOSYNTHESIS. A 12 Å carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection. Science. 2015;348(6242):1463–1466. doi: 10.1126/science.aaa7234. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, et al. Local and global structural drivers for the photoactivation of the orange carotenoid protein. Proc Natl Acad Sci USA. 2015;112(41):E5567–E5574. doi: 10.1073/pnas.1512240112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, et al. Mass spectrometry footprinting reveals the structural rearrangements of cyanobacterial orange carotenoid protein upon light activation. Biochim Biophys Acta. 2014;1837(12):1955–1963. doi: 10.1016/j.bbabio.2014.09.004. [DOI] [PubMed] [Google Scholar]