Significance
Polygynous marriage is commonly regarded as a harmful cultural practice, detrimental to women and children at the individual and group level. We present counterevidence that polygyny is often positively associated with food security and child health within communities and that, although polygyny and health are negatively associated at the group level, such differences are accounted for by alternative socioecological factors. These results support models of polygyny based on female choice and suggest that, in some contexts, prohibiting polygyny could be costly for women and children by restricting marital options. Our study highlights the dangers of naive analyses of aggregated population data and the importance of considering locally realizable alternatives and context dependency when considering the health implications of cultural practices.
Keywords: evolutionary anthropology, public health, family structure, child health, food security
Abstract
Polygyny is cross-culturally common and a topic of considerable academic and policy interest, often deemed a harmful cultural practice serving the interests of men contrary to those of women and children. Supporting this view, large-scale studies of national African demographic surveys consistently demonstrate that poor child health outcomes are concentrated in polygynous households. Negative population-level associations between polygyny and well-being have also been reported, consistent with the hypothesis that modern transitions to socially imposed monogamy are driven by cultural group selection. We challenge the consensus view that polygyny is harmful, drawing on multilevel data from 56 ethnically diverse Tanzanian villages. We first demonstrate the vulnerability of aggregated data to confounding between ecological and individual determinants of health; while across villages polygyny is associated with poor child health and low food security, such relationships are absent or reversed within villages, particularly when children and fathers are coresident. We then provide data indicating that the costs of sharing a husband are offset by greater wealth (land and livestock) of polygynous households. These results are consistent with models of polygyny based on female choice. Finally, we show that village-level negative associations between polygyny prevalence, food security, and child health are fully accounted for by underlying differences in ecological vulnerability (rainfall) and socioeconomic marginalization (access to education). We highlight the need for improved, culturally sensitive measurement tools and appropriate scales of analysis in studies of polygyny and other purportedly harmful practices and discuss the relevance of our results to theoretical accounts of marriage and contemporary population policy.
Recent years have witnessed growing recognition of the importance of gender in all aspects of international development (1). This shift includes domestic and international efforts to abolish so-called “harmful cultural practices,” a term used to describe practices of, typically nonwestern, cultures deemed detrimental to well-being, most often with regard to women and children (SI Text). Most attention has focused on female genital cutting and on child and forced marriage (2, 3). In many policy-orientated texts, this label is also given to polygynous marriage (hereafter polygyny). For example, the United Nations Convention on the Elimination of All Forms of Discrimination Against Women states that polygyny “contravene[s] a woman’s right to equality with men and can have such serious emotional and financial consequences for her and her dependents that such marriages ought to be discouraged and prohibited” (2). Such statements are frequently presented as stylized facts and made without discussion of supporting evidence. However, a recent spate of articles, mostly based on large-scale African Demographic and Health Surveys (DHS), conclude that polygyny is indeed harmful, reporting that children in polygynous households are consistently more likely to be of ill health or die in early childhood than children in monogamous households (4–8). Reviews of the literature have also informed policy in developed countries, including via the presentation of expert evidence in a recent retrial of the legal prohibition of polygyny in Canada (9).
Historically, more than 80% of preindustrial societies permitted polygyny (10). Today it is most prevalent in sub-Saharan Africa (11). If women and children do not benefit from polygyny then why is it so common? Evolutionary anthropologists have long puzzled the costs and benefits of polygyny (12). This literature, drawing on small-scale field studies of specific cultural contexts, reaches a consensus on the benefits of polygyny to men; polygynous men generally have higher reproductive success than their monogamous counterparts (13–16). The potential benefits of, and motivation for, polygyny for women are less clear. The “polygyny-threshold model” posits that polygyny occurs when the costs of sharing a husband are offset by equal or greater resource access than could otherwise be obtained via monogamy (17, 18). Supporting this model, polygynous men are typically wealthier than monogamous men (19, 20), and several studies show no apparent deficit in reproductive success or child health for polygynously married women (19, 21). However, in other cases, polygyny is associated with relatively poor child health (20, 22–24). Poor outcomes for women and/or children do not necessarily imply a rejection of the polygyny threshold model (12, 19). However, these findings have been interpreted as evidence of sexual conflict, with polygyny maximizing total reproductive success for men at the cost of suboptimal outcomes for individual wives and children (25). Drawing generalizable conclusions regarding the potential costs of polygyny from the anthropological literature alone is difficult (25, 26). Findings are mixed, study sites are rarely regionally or nationally representative, and small sample sizes raise issues of statistical power. Given these problems, the consistency of findings presented in recent large-scale, representatively sampled demographic studies of polygyny and child health is seductive (4–8). However, as we will argue, studies relying on highly aggregated data bring their own, often overlooked, methodological problems (27), problems that are acute when contrasting polygynous and monogamous households, in part because the former tend to be most common in remote and/or marginalized groups facing numerous socioecological barriers to health (SI Text).
Not only policy, but also grand theory, is built on the view that polygyny is harmful. It has been argued that cultural shifts to “socially imposed monogamy” in modern stratified societies can be accounted for by detrimental effects of polygyny at the group level, including costs to child health (28, 29). Most recently, Henrich et al. (28) assert that monogamy evolves by cultural group selection, with normative polygyny (i) incentivizing strategies of reduced paternal care, so that male effort is diverted into accumulating wives rather than raising offspring, and (ii) increasing the propensity for social unrest driven by a larger pool of unmarried men. To support the specific claim that polygyny has negative group-wide consequences for children, Henrich et al. (28) rely on data from large-scale demographic studies, as well as on selected population-specific contrasts where children in polygynous households experience relatively poor well-being. Consistent with the claim of greater social unrest in polygynous groups, the authors review evidence that the proportion of unmarried men positively predicts national rates of rape, murder, assault, theft, and fraud. However, such crude comparisons have limited inferential value in the face of many potential confounds. A recent review reveals no clear association between adult sex ratio, a likely correlate of the proportion of unmarried men, and violent crime (30).
Given the significance of the purported harmful effects of polygyny for both policy and our understanding of marriage systems, we conducted an innovative study addressing both individual and group-level relationships between polygyny, food security, and child health. We draw on multilevel data from 56 villages in northern Tanzania (Fig. S1). Tanzania experiences a high burden of food insecurity and malnutrition; 45% of children are stunted by World Health Organization (WHO) standards (31), a measure of developmental potential predictive of both later physical and cognitive functioning (32). One in four married women in rural Tanzania have at least one cowife (31), and female status is poor; internationally Tanzania scores 124/152 on the Gender Inequality Index (33). In many respects, our study combines the relative strengths of prior large-scale demographic and small-scale anthropological studies (SI Text). We sampled more households (n = 3,584) than the Tanzanian DHS for the same regions (34). However, unlike DHS studies, we incorporate data on ethnicity and livelihood-specific measures of household wealth (i.e., land cultivated and livestock owned), and, crucially, sufficient village-level data to enable a statistically robust consideration of within and between-village variation. Four main ethnic groups reside in the area, including the highly polygynous Maasai and Sukuma, the moderately polygynous Rangi and the predominantly monogamous Meru (34) (Tables S1 and S2). This setup provides a unique opportunity to consider relationships between polygyny and health in a context of varied and transitioning marital norms.
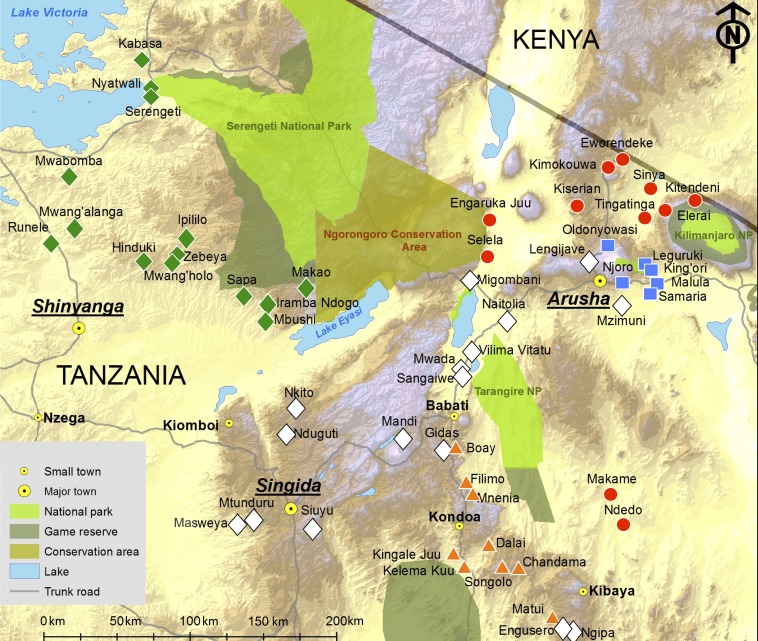
Fig. S1.
Location of the 56 study villages included in the Whole Village Project. Ethnicity is coded as the most common ethnicity in each village. Red circle, Maasai village; orange triangle, Rangi village; green diamond, Sukuma village; blue square, Meru village; white diamond, other ethnicity village. Reproduced from ref. 34.
Table S1.
Demographic characteristics by household type and ethnicity for working sample (n = 1764 households, 2833 children)
| Ethnic group | Male-headed monogamous | Male-headed polygynous | Female-headed polygynous | Female-headed monogamous |
| Sukuma | ||||
| No. of households (no. of children <5 y) | 289 (577) | 109 (223) | 23 (47) | 5 (8) |
| Mean head age in years (SD) | 44 (13) | 48 (13) | 40 (11) | 33 (8) |
| Mean household size (SD) | 8.2 (3.8) | 9.2 (4.3) | 8.7 (3.0) | 6.4 (1.1) |
| Mean no. <5 y (SD) | 2.0 (1.0) | 2.3 (1.3) | 2.0 (0.9) | 1.6 (0.9) |
| Mean no. 5-<15 y (SD) | 2.6 (1.8) | 2.7 (2.2) | 2.6 (1.4) | 1.8 (0.8) |
| Mean no. 15–64 y (SD) | 3.4 (2.0) | 4.0 (2.3) | 4.0 (1.9) | 3.0 (1.0) |
| Mean no. 65+ y (SD) | 0.2 (0.5) | 0.2 (0.5) | 0.1 (0.3) | 0.0 (0.0) |
| Maasai | ||||
| No. of households (no. of children <5 y) | 143 (207) | 82 (127) | 92 (145) | 42 (59) |
| Mean head age in years (SD) | 39 (13) | 47 (12) | 34 (11) | 30 (14) |
| Mean household size (SD) | 5.4 (1.9) | 6.1 (2.3) | 6.5 (3.0) | 5.5 (1.6) |
| Mean no. <5 y (SD) | 1.4 (0.7) | 1.6 (0.9) | 1.6 (0.8) | 1.4 (0.6) |
| Mean no. 5-<15 y (SD) | 1.5 (1.2) | 1.7 (1.3) | 2.3 (2.0) | 1.8 (1.2) |
| Mean no. 15–64 y (SD) | 2.4 (1.0) | 2.5 (1.0) | 2.5 (1.7) | 2.1 (0.9) |
| Mean no. 65+ y (SD) | 0.1 (0.3) | 0.2 (0.4) | 0.2 (0.6) | 0.2 (0.6) |
| Rangi | ||||
| No. of households (no. of children <5 y) | 149 (219) | 33 (51) | 4 (4) | 6 (9) |
| Mean head age in years (SD) | 40 (11) | 52 (14) | 32 (8) | 38 (13) |
| Mean household size (SD) | 6.2 (2.0) | 7.4 (2.3) | 4.0 (0.8) | 5.8 (1.0) |
| Mean no. <5 y (SD) | 1.4 (0.7) | 1.4 (0.8) | 1.0 (0.0) | 1.5 (0.8) |
| Mean no. 5-<15 y (SD) | 1.9 (1.2) | 2.8 (1.6) | 0.8 (1.0) | 2.0 (0.9) |
| Mean no. 15–64 y (SD) | 2.8 (1.1) | 2.9 (1.3) | 2.3 (1.3) | 2.3 (1.4) |
| Mean no. 65+ y (SD) | 0.1 (0.3) | 0.2 (0.5) | 0.0 (0.0) | 0.0 (0.0) |
| Meru | ||||
| No. of households (no. of children <5 y) | 135 (170) | 3 (4) | 0 (0) | 9 (13) |
| Mean head age in years (SD) | 39 (9.6) | 56 (13) | — | 38 (8) |
| Mean household size (SD) | 5.6 1.8) | 5.3 (1.5) | — | 5.9 (0.9) |
| Mean no. <5 y (SD) | 1.1 (0.5) | 1.0 (1.0) | — | 1.3 (0.7) |
| Mean no. 5-<15 y (SD) | 1.7 (1.3) | 0.7 (0.6) | — | 2.3 (0.7) |
| Mean no. 15–64 y (SD) | 2.6 (1.1) | 3.3 (1.5) | — | 2.2 (0.8) |
| Mean no. 65+ y (SD) | 0.1 (0.4) | 0.3 (0.6) | — | 0.0 (0.0) |
| Other ethnicity | ||||
| No. of households (no. of children <5 y) | 500 (746) | 79 (136) | 35 (47) | 26 (41) |
| Mean head age in years (SD) | 40 (12) | 49 (14) | 33 (7.4) | 37 (13) |
| Mean household size (SD) | 6.3 (2.3) | 7.1 (2.3) | 6.0 (1.8) | 6.3 (2.5) |
| Mean no. <5 y (SD) | 1.5 (0.7) | 1.8 (1.0) | 1.3 (0.5) | 1.4 (0.6) |
| Mean no. 5-<15 y (SD) | 1.9 (1.4) | 1.9 (1.3) | 2.0 (0.9) | 2.2 (1.3) |
| Mean no. 15–64 y (SD) | 2.7 (1.3) | 3.2 (1.5) | 2.5 (1.1) | 2.6 (1.1) |
| Mean no. 65+ y (SD) | 0.1 (0.4) | 0.3 (0.5) | 0.1 (0.3) | 0.1 (0.4) |
Table S2.
Household and village characteristics for working sample by ethnicity
| Household characteristics (n = 1,764 households) | Ethnicity of household head | |||||
| Sukuma | Maasai | Rangi | Meru | Other | ||
| Number of households | 426 | 359 | 192 | 147 | 640 | |
| Main livelihood of household head | Farming | 92% | 25% | 94% | 71% | 83% |
| Livestock | 0% | 67% | 1% | 3% | 4% | |
| Business | 4% | 3% | 4% | 12% | 7% | |
| Other/none | 4% | 5% | 2% | 14% | 6% | |
| Wealth index | Mean log(x + 1) (SD) | 1.4 (0.4) | 1.0 (0.5) | 1.4 (0.4) | 1.8 (0.4) | 1.4 (0.4) |
| Cultivates land? | % yes | 99% | 66% | 97% | 99% | 95% |
| Acres of land cultivated (for cultivators only) | Mean log(x + 1) (SD) | 1.9 (0.7) | 1.4 (0.7) | 1.7 (0.6) | 1.2 (0.5) | 1.5 (0.7) |
| Owns livestock? | % yes | 70% | 94% | 52% | 88% | 68% |
| Tropical livestock units (for livestock owners only) | Mean log(x + 1) (SD) | 1.8 (1.1) | 1.6 (0.9) | 1.4 (0.9) | 0.9 (0.4) | 1.4 (0.9) |
| Majority ethnicity of village | ||||||
| Village characteristics (n = 56 villages) | Sukuma | Maasai | Rangi | Meru | Other | |
| Number of villages where ethnicity is in the majority | 12 | 11 | 7 | 6 | 20 | |
| Polygyny prevalence (% of household heads polygynously married) (SD) | 25% (8) | 38% (9) | 14% (4) | 7% (4) | 16% (10) | |
| Mean annual rainfall in mm3 (SD) | 847 (69) | 626 (87) | 683 (27) | 973 (126) | 762 (166) | |
| Mean distance to District capital in km (SD) | 33 (19) | 35 (20) | 35 (16) | 20 (19) | 33 (16) | |
| Percent household heads with nonzero education (SD) | 70% (8) | 32% (11) | 68% (5) | 78% (7) | 75% (14) | |
Results
Contrasting Monogamous and Polygynous Households.
We first estimate relationships between polygyny, food security and the heights and weights of children under 5 y using linear regression aggregating data across all villages (Table S3). This method is analytically equivalent to existing studies of large-scale demographic surveys, which routinely ignore both ethnic variation and village-level spatial clustering of health (SI Text). Consistent with such studies, polygynous households have lower food security than monogamous households (β = −1.56, 95% confidence intervals (95%CI) = −2.31;-0.81, P < 0.001) and, using WHO standardized z-scores, lower child height-for-age (HAZ, β = −0.21, 95%CI = −0.34;−0.08, P < 0.01). Child weight-for-height (WHZ) did not significantly differ between polygynous and monogamous households (β = −0.06, 95% CI = −0.16; 0.05, P > 0.1).
Table S3.
Linear and multilevel regressions predicting household food security, HAZ, and WHZ
| Model set A: Monogamous vs. polygynous binary coding | Food security (n = 1,745) [β (95% CIs)] | Height-for-age Z-score (n = 2,704) [β (95% CIs)] | Weight-for-height Z-score (n = 2,711) [β (95% CIs)] | ||||
| Linear | Multilevel | Linear | Multilevel | Linear | Multilevel | ||
| Household type (reference: monogamous) | Polygynous | −1.56*** (−2.31; −0.81) | 0.26 (−0.47; 0.98) | −0.21** (−0.34; −0.08) | −0.07 (−0.20; 0.06) | −0.06 (−0.16; 0.05) | 0.00 (−0.12; 0.11) |
| Child age (mo) (centered at 30 mo) | — | — | −0.09*** (−0.10; −0.08) | −0.09*** (−0.10; −0.07) | −0.03*** (−0.04; −0.01) | −0.02*** (−0.04; −0.01) | |
| Child age squared (mo2) (centered at 30 mo2) | — | — | 0.001*** (0.001; 0.002) | 0.001*** (0.001; 0.002) | 0.000 (0.000; 0.000) | 0.000 (0.000; 0.000) | |
| Child sex (reference: boy) | Girl | — | — | 0.12* (0.01; 0.24) | 0.13* (0.02; 0.24) | 0.06 (−0.04; 0.15) | 0.05 (−0.05; 0.14) |
| Age of household head (y) (centered at 43 y) | 0.00 ns (−0.02; 0.03) | −0.02† (−0.05; 0.00) | 0.01** (0.00; 0.01) | 0.00 (0.00; 0.01) | 0.01** (0.00; 0.01) | 0.00† (0.00; 0.01) | |
| Season (reference: not hunger) | Hunger | — | −2.66** (−4.32; −0.99) | — | −0.37** (−0.62; −0.12) | — | −0.05 (−0.23; 0.14) |
| Intercept | 17.33*** (16.94; 17.71) | 20.62*** (18.07; 23.17) | −2.02*** (−2.13; −1.91) | −1.54*** (−1.93; −1.15) | 0.11* (0.02; 0.21) | 0.17 (−0.13; 0.46) | |
| Random effects variance | Cons | — | 8.65 | — | 0.17 | — | 0.09 |
| Residual | — | 39.58 | — | 2.09 | — | 1.56 | |
| Model set B: Monogamous vs. polygynous four-category coding | |||||||
| Household type (reference: monogamous male-headed) | Polygynous male-headed | −0.38 (−1.28; 0.51) | 0.86* (0.01; 1.70) | −0.16* (−0.31; −0.01) | −0.09 (−0.24; 0.06) | −0.00 (−0.13; 0.13) | 0.01 (−0.12; 0.14) |
| Polygynous female-headed | −4.20*** (−5.38; −3.02) | −1.16† (−2.34; 0.01) | −0.37*** (−0.58; −0.16) | −0.07 (−0.30; 0.15) | −0.17† (−0.36; 0.01) | 0.01 (−0.18; 0.20) | |
| Monogamous female-headed | −2.72*** (−4.25; −1.19) | −0.34 (−1.78; 1.10) | −0.32* (−0.59; −0.04) | −0.10 (−0.38; 0.17) | 0.04 (−0.19; 0.27) | 0.18 (−0.05; 0.41) | |
| Child age (mo) (centered at 30 mo) | — | — | −0.09*** (−0.10; −0.08) | −0.09*** (−0.10; −0.07) | −0.03*** (−0.04; −0.01) | −0.02*** (−0.04; −0.01) | |
| Child age squared (mo2) (centered at 30 mo2) | — | — | 0.001*** (0.001; 0.002) | 0.001*** (0.001; 0.002) | 0.000 (0.000; 0.000) | 0.000 (0.000; 0.000) | |
| Child sex (reference: boy) | Girl | — | — | 0.12* (0.01; 0.24) | 0.13* (0.02; 0.24) | 0.06 (−0.04; 0.15) | 0.05 (−0.05; 0.14) |
| Age of household head (y) (centered at 43 y) | −0.02 (−0.05; 0.01) | −0.03* (−0.06; −0.01) | 0.01* (0.00; 0.01) | 0.00 (0.00; 0.01) | 0.01** (0.00; 0.01) | 0.00* (0.00; 0.01) | |
| Season (reference: not hunger) | Hunger | — | −2.59** (−4.22; −0.97) | — | −0.37** (−0.61; −0.12) | — | −0.05 (−0.24; 0.14) |
| Intercept | 17.45*** (17.05; 17.84) | 20.54*** (18.05; 23.03) | −2.00*** (−2.11; −1.89) | −1.54*** (−1.92; −1.15) | 0.11* (0.02; 0.20) | 0.16 (−0.13; 0.45) | |
| Random effects variance | Cons | — | 8.18 | — | 0.17 | — | 0.09 |
| Residual | — | 39.43 | — | 2.09 | — | 1.56 | |
P < 0.1, *P < 0.05, **P < 0.01, and **P < 0.001. Statistically significant estimates at P < 0.1 are in bold.
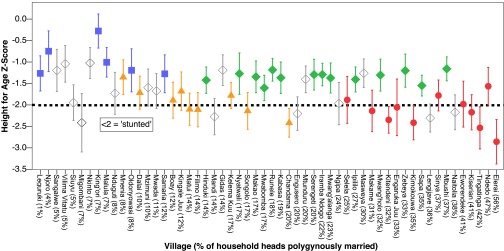
However, there is a clear tendency for relatively polygynous villages and ethnic groups (particularly the Maasai) to have poor food security and child health (Fig. 1) (see ref. 34 for a comphensive analysis of ethnic differences in food security and child health). Accounting for this variance by including a random effect for village demonstrates that neither food security nor child health are significantly associated with polygyny when contrasted within villages (food security: β = 0.26, 95% CI = −0.47; 0.98, P > 0.1; HAZ: β = −0.07, 95% CI = −0.20; 0.06, P > 0.1; WHZ: β = 0.00, 95% CI = −0.12; 0.11, P > 0.1; Table S3). As such, multilevel analysis reveals a Simpson’s paradox (27), i.e., village-level differences obscure underlying relationships between polygyny, food security, and child health within villages.
Fig. 1.
Child height-for-age by village sorted by polygyny prevalence. There is strong ethnic and village-level variation in child health. Relatively monogamous Meru villages tend to have relatively good child health, whereas relatively polygynous Maasai villages tend to have relatively poor child health. The dashed line represents the WHO cutoff for chronic malnutrition. Ethnicity is coded as the majority ethnic group residing in each village. Error bars represent 95% CIs. Red circle, Maasai; green diamond, Sukuma; orange triangle, Rangi; blue square, Meru; white diamond, other ethnicity.
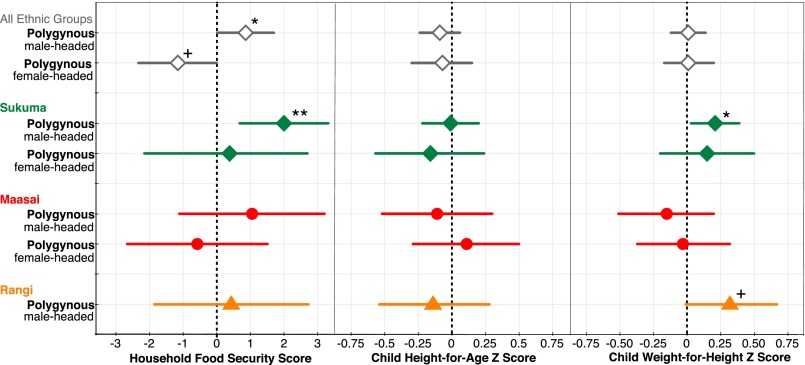
Polygynous men generally resided with their first wife (SI Text), and in only 10% of male-headed polygynous households did multiple wives coreside (most commonly among the Sukuma, where 17% of polygynously married male household heads lived with multiple wives). Second or later cowives and their children typically lived in separate, but often adjacent, dwellings to their husbands. Distinguishing between these household types reveals that male-headed polygynous households have significantly higher food security than monogamous households within villages (β = 0.86, 95% CI = 0.01; 1.70, P < 0.05). Stratified analysis confirms that a trend toward higher food security for male-headed polygynous households is present in all three ethnic groups with a substantial prevalence of polygyny (Fig. 2), although this is only statistically significant in the Sukuma (β = 2.00, 95% CI = 0.68; 3.32, P < 0.01). Furthermore, in both the Sukuma and Rangi, children in male-headed polygynous households also had higher WHZ (Sukuma: β = 0.21, 95% CI = 0.03; 0.39, P < 0.05, Rangi: β = 0.33, 95% CI = −0.01; 0.67, P = 0.06). Overall, female-headed polygynous households had lower food security than monogamous households within the same village (β = −1.16, 95% CI = −2.34; 0.01, P = 0.05), although this pattern did not approach statistical significance in stratified analyses (Fig. 2 and Tables S3 and S4).
Fig. 2.
Food security and child health by household type. Within villages polygyny is associated with relatively high food security when households are headed by a male and relatively low food security when headed by a female (typically later wife households). Stratified analysis confirms higher food security in the Sukuma and relatively improved child weight-for-height in both the Sukuma and Rangi, for male-headed polygynous households. The reference category (dashed line) is male-headed monogamous households (Table S4 for full model output). +P < 0.1, *P < 0.05, **P < 0.01, and ***P < 0.001.
Table S4.
Multilevel regressions predicting household food insecurity, HAZ, and WHZ stratified by ethnic group
| Independent variable | Food security [β (95% CIs)] | Height-for-age Z-score [β (95% CIs)] | Weight-for-height Z-score [β (95% CIs)] | |||||||
| Sukuma (n = 426) | Maasai (n = 358) | Rangi (n = 191) | Sukuma (n = 829) | Maasai (n = 500) | Rangi (n = 272) | Sukuma (n = 832) | Maasai (n = 506) | Rangi (n = 269) | ||
| Household type (reference: monogamous male-headed) | Polygynous male-headed | 2.00** (0.68; 3.32) | 1.05 (−1.12; 3.21) | 0.43 (−1.86; 2.73) | −0.01 (−0.22; 0.20) | −0.11 (−0.53; 0.30) | −0.13 (−0.54; 0.28) | 0.21* (0.03; 0.39) | −0.15 (−0.51; 0.20) | 0.33† (−0.01; 0.67) |
| Polygynous female-headed | 0.38 (−2.16; 2.92) | −0.58 (−2.67; 1.51) | 2.07 (−3.56; 7.71) | −0.16 (−0.57; 0.24) | 0.11 (−0.29; 0.50) | −0.67 (−2.15; 0.81) | 0.15 (−0.20; 0.50) | −0.03 (−0.38; 0.32) | 0.04 (−1.15; 1.23) | |
| Monogamous female-headed | −2.37 (−7.64; 2.90) | −0.39 (−3.17; 2.39) | 0.07 (−4.53; 4.67) | −0.51 (−1.45; 0.43) | 0.32 (−0.22; 0.86) | −0.40 (−1.26; 0.45) | −0.09 (−0.90; 0.72) | 0.26 (−0.20; 0.73) | 0.10 (−0.59; 0.79) | |
| Child age (mo) (centered at 30 mo) | — | — | — | −0.08*** (−0.10; −0.06) | −0.06** (−0.09; −0.02) | −0.11*** (−0.15; −0.08) | −0.01 (−0.03; 0.01) | −0.07*** (−0.10; −0.03) | −0.01 (−0.04; 0.01) | |
| Child age squared (mo2) (centered at 30 mo2) | — | — | — | 0.001*** (0.001; 0.002) | 0.001* (0.000; 0.001) | 0.002*** (0.001; 0.002) | 0.000 (0.000; 0.000) | 0.001* (0.000; 0.001) | 0.000 (−0.001; 0.000) | |
| Child sex (reference: boy) | — | — | — | — | 0.09 (−0.09; 0.27) | −0.03 (−0.34; 0.27) | 0.21 (0.10; 0.51) | −0.01 (−0.17; 0.15) | 0.17 (−0.09; 0.43) | −0.17 (−0.42; 0.08) |
| Age of household head (y) (centered at 43 y) | 0.00 (−0.05; 0.04) | −0.05 (−0.12; 0.02) | 0.02 (−0.05; 0.09) | 0.00 (−0.01; 0.00) | 0.02** (0.01; 0.03) | 0.00 (−0.02; 0.01) | 0.00* (0.00; 0.01) | 0.01† (0.00; 0.02) | −0.01† (−0.02; 0.00) | |
| Season (reference: not hunger) | Hunger | −1.96** (−3.40; −0.51) | −1.37 (−4.47; 1.74) | −1.16 (−3.26; 0.95) | 0.00 (−0.23; 0.22) | −0.44* (−0.85; −0.04) | −0.20 (−0.60; 0.19) | 0.01 (−0.15; 0.17) | −0.08 (−0.55; 0.39) | −0.09 (−0.51; 0.32) |
| Intercept | 20.42*** (18.17; 22.66) | 14.45*** (9.02; 19.88) | 17.95*** (15.21; 20.70) | −1.73*** (−2.10; −1.35) | −1.58*** (−2.32; −0.84) | −2.13*** (−2.68; −1.58) | 0.29* (0.02; 0.57) | −0.15 (−0.99; 0.69) | 0.18 (−0.42; 0.77) | |
| Random effects variance | Cons | 0.74 | 6.11 | 0.26 | 0.01 | 0.05 | 0.00 | 0.00 | 0.13 | 0.05 |
| Residual | 34.66 | 57.38 | 31.56 | 1.76 | 2.99 | 1.61 | 1.33 | 2.24 | 1.02 | |
P < 0.1, *P < 0.05, **P < 0.01, and ***P < 0.001. Statistically significant estimates at P < 0.1 are in bold.
Polygyny and Wealth.
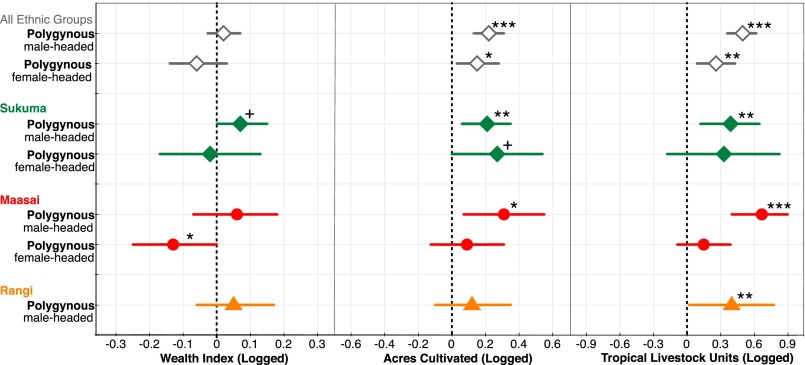
Wealth was measured by an asset-based household wealth index (SI Text), a generic measure favored by large-scale surveys and used across rural and urban contexts (35). This measure indicates minimal differences in wealth between monogamous and polygynous households. However, livelihood-specific measures of wealth reveal that polygynous households, particularly when male-headed, both cultivate more land (β = 0.22, 95% CI = 0.14; 0.31, P < 0.001) and own more livestock (β = 0.49, 95% CI = 0.36; 0.62, P < 0.001) than monogamous households (Fig. 3). These differences are apparent in all major ethnic groups in stratified analyses and are robust to statistical adjustment for the number of adults and young dependents in the household (Tables S5 and S6). Thus, consistent with the polygyny threshold model, higher wealth presents a strong candidate mechanism for superior food security and child nutrition in male-headed polygynous households.
Fig. 3.
Wealth index, land cultivated, and livestock owned by household type. Within villages polygynous households, particularly when headed by males, cultivate more land and own more livestock than monogamous households. The reference category (dashed line) is male-headed monogamous households (Table S6 for full model output). +P < 0.1, *P < 0.05, **P < 0.01, and ***P < 0.001.
Table S5.
Multilevel regressions predicting household wealth index, land cultivated, and livestock owned
| Model set A: Without adjustment for number of adults and dependents in household | Wealth index logged (n = 1,721) [β (95% CIs)] | Acres cultivated logged (n = 1,546) [β (95% CIs)] | Tropical livestock units logged (n = 1,292) [β (95% CIs)] | |
| Household type (reference: monogamous male-headed) | Polygynous male-headed | 0.02 (−0.03; 0.07) | 0.22*** (0.14; 0.31) | 0.49*** (0.36; 0.62) |
| Polygynous female-headed | −0.06 (−0.13; 0.03) | 0.15* (0.03; 0.28) | 0.26** (0.09; 0.43) | |
| Monogamous female-headed | −0.05 (−0.14; 0.03) | 0.00 (−0.15; 0.16) | −0.01 (−0.23; 0.21) | |
| Age of household head (y) (centered at 43 y) | 0.002** (0.001; 0.004) | 0.01*** (0.01; 0.01) | 0.01*** (0.01; 0.01) | |
| Season (reference: not hunger) | Hunger | −0.12† (−0.25; 0.01) | −0.15 (−0.34; 0.05) | 0.08 (−0.12; 0.29) |
| Intercept | 1.54*** (1.33; 1.74) | 1.73*** (1.43; 2.03) | 1.25*** (0.94; 1.57) | |
| Random effects variance | Cons | 0.06 | 0.12 | 0.12 |
| Residual | 0.14 | 0.34 | 0.70 | |
| Model Set B: With adjustment for number adults and dependents in household | Wealth index logged (n = 1,657) [β (95% CIs)] | Acres cultivated logged (n = 1,494) [β (95% CIs)] | Tropical livestock units logged (n = 1,239) [β (95% CIs)] | |
| Household type (reference: monogamous male-headed) | Polygynous male-headed | 0.01 (−0.04; 0.06) | 0.21*** (0.13; 0.29) | 0.47*** (0.34; 0.59) |
| Polygynous female-headed | −0.07* (−0.14; 0.00) | 0.10 (−0.03; 0.22) | 0.21* (0.04; 0.37) | |
| Monogamous female-headed | −0.07† (−0.16; 0.01) | −0.02 (−0.16; 0.13) | 0.00 (−0.22; 0.22) | |
| Age of household head (y) (centered at 43 y) | −0.002 (−0.002; 0.001) | 0.002 (−0.001; 0.004) | 0.005* (0.001; 0.009) | |
| Number of adults in household (15+ y) (centered on three persons) | 0.06*** (0.04; 0.07) | 0.11*** (0.09; 0.13) | 0.08*** (0.05; 0.12) | |
| Number of dependents in household (<15 y) (centered on four persons) | 0.01 (0.00; 0.02) | 0.05*** (0.03; 0.07) | 0.06*** (0.03; 0.09) | |
| Season (reference: not hunger) | Hunger | −0.11 (−0.24; 0.02) | −0.13 (−0.31; 0.05) | 0.12 (−0.09; 0.32) |
| Intercept | 1.53*** (1.33; 1.73) | 1.73*** (1.46; 2.00) | 1.22*** (0.90; 1.53) | |
| Random effects variance | Cons | 0.06 | 0.10 | 0.12 |
| Residual | 0.13 | 0.30 | 0.66 | |
P < 0.1, *P < 0.05, **P < 0.01, and ***P < 0.001. Statistically significant estimates at P < 0.1 are in bold.
Table S6.
Multilevel regressions predicting household wealth index, land cultivated, and livestock owned stratified by ethnic group
| Model Set A: Without adjustment for number of adults and dependents in household | Wealth index logged [β (95% CIs)] | Acres cultivated logged [β (95% CIs)] | Tropical livestock units logged [β (95% CIs)] | |||||||
| Sukuma (n = 422) | Maasai (n = 340) | Rangi (n = 190) | Sukuma (n = 420) | Maasai (n = 216) | Rangi (n = 185) | Sukuma (n = 296) | Maasai (n = 338) | Rangi (n = 99) | ||
| Household type (reference: monogamous male-headed) | Polygynous male-headed | 0.07† (0.00; 0.15) | 0.06 (−0.07; 0.18) | 0.05 (−0.07; 0.17) | 0.21** (0.06; 0.35) | 0.31* (0.07; 0.55) | 0.12 (−0.10; 0.35) | 0.39** (0.12; 0.65) | 0.65*** (0.40; 0.90) | 0.40* (0.02; 0.78) |
| Polygynous female-headed | −0.02 (−0.17; 0.13) | −0.13* (−0.25; 0.00) | −0.01 (−0.30; 0.29) | 0.27† (0.00; 0.54) | 0.09 (−0.13; 0.31) | 0.03 (−0.51; 0.56) | 0.33 (−0.18; 0.83) | 0.15 (−0.09; 0.39) | 0.17 (−0.57; 0.92) | |
| Monogamous female-headed | −0.13 (−0.44; 0.17) | −0.16† (−0.32; 0.00) | 0.04 (−0.20; 0.29) | −0.27 (−0.84; 0.30) | −0.15 (−0.46; 0.16) | 0.03 (−0.42; 0.47) | −0.53 (−1.95; 0.89) | −0.11 (−0.43; 0.21) | −0.39 (−1.42; 0.64) | |
| Age of household head (y) (centered at 43 y) | 0.00 (0.00; 0.00) | 0.03 (−0.00; 0.01) | 0.005** (0.001; 0.009) | 0.01*** (0.01; 0.02) | 0.00 (0.00; 0.01) | 0.01*** (0.01; 0.02) | 0.02*** (0.01; 0.03) | 0.00 (−0.01; 0.00) | 0.02** (0.01; 0.03) | |
| Season (reference: not hunger) | Hunger | 0.07 (−0.04; 0.18) | −0.08 (−0.28; 0.12) | −0.03 (−0.14; 0.08) | 0.03 (−0.28; 0.33) | −0.31† (−0.67; 0.05) | −0.12 (−0.48; 0.24) | 0.24 (−0.08; 0.56) | 0.06 (−0.29; 0.41) | −0.24 (−0.85; 0.37) |
| Intercept | 1.23*** (1.06; 1.40) | 1.23*** (0.88; 1.57) | 1.46*** (1.32; 1.60) | 1.73*** (1.25; 2.20) | 1.91*** (1.27; 2.54) | 1.87*** (1.37; 2.38) | 1.24*** (0.75; 1.72) | 1.28*** (0.67; 1.89) | 1.49*** (0.68; 2.30) | |
| Random effects variance | Cons | 0.01 | 0.03 | 0.00 | 0.08 | 0.08 | 0.06 | 0.04 | 0.07 | 0.12 |
| Residual | 0.11 | 0.18 | 0.09 | 0.39 | 0.38 | 0.28 | 1.01 | 0.73 | 0.50 | |
| Model set B: With adjustment for number adults and dependents in household | Sukuma (n = 417) | Maasai (n = 315) | Rangi (n = 186) | Sukuma (n = 415) | Maasai (n = 202) | Rangi (n = 181) | Sukuma (n = 292) | Maasai (n = 313) | Rangi (n = 98) | |
| Household type (reference: monogamous male-headed) | Polygynous male-headed | 0.05 (−0.02; 0.13) | 0.04 (−0.09; 0.17) | 0.06 (−0.06; 0.17) | 0.17** (0.04; 0.29) | 0.28* (0.04; 0.52) | 0.07 (−0.15; 0.28) | 0.35** (0.10; 0.61) | 0.58*** (0.33; 0.83) | 0.33† (0.05; 0.72) |
| Polygynous female-headed | −0.07 (−0.21; 0.07) | −0.13* (−0.27; 0.00) | −0.05 (−0.24; 0.33) | 0.13 (0.10; 0.37) | 0.02 (−0.22; 0.25) | 0.22 (−0.29; 0.72) | 0.19 (−0.28; 0.67) | 0.14 (−0.12; 0.40) | 0.46 (−0.31; 1.23) | |
| Monogamous female-headed | −0.16 (−0.44; 0.13) | −0.19* (−0.36; 0.03) | 0.07 (−0.16; 0.30) | −0.31 (−0.80; 0.18) | −0.18 (−0.49; 0.12) | 0.05 (−0.36; 0.46) | −0.53 (−1.86; 0.80) | −0.12 (−0.45; 0.21) | −0.39 (−1.41; 0.64) | |
| Age of household head (y) (centered at 43 y) | 0.00 (0.00; 0.00) | 0.00 (0.00; 0.00) | 0.00 (0.00; 0.01) | 0.00 (0.00; 0.00) | 0.00 (−0.01; 0.01) | 0.01* (0.00; 0.01) | 0.01* (0.00; 0.02) | −0.01 (−0.01; 0.00) | 0.02** (0.01; 0.03) | |
| Number of adults in household (15+ y) (centered on three persons) | 0.06*** (0.04; 0.08) | 0.06** (0.02; 0.11) | 0.08*** (0.05; 0.12) | 0.16*** (0.13; 0.19) | 0.08* (0.01; 0.15) | 0.11*** (0.05; 0.16) | 0.14*** (0.08; 0.20) | 0.03 (−0.06; 0.12) | 0.05 (−0.07; 0.17) | |
| Number of dependents in household (<15 y) (centered on four persons) | 0.00 (−0.01; 0.02) | 0.02 (−0.01; 0.05) | 0.02 (−0.01; 0.05) | 0.04 ** (0.01; 0.07) | 0.06* (0.00; 0.11) | 0.10*** (0.05; 0.16) | 0.06* (0.01; 0.11) | 0.04 (−0.02; 0.10) | 0.11* (0.00; 0.22) | |
| Season (reference: not hunger) | Hunger | 0.09 (−0.03; 0.21) | −0.05 (−0.22; 0.13) | −0.01 (−0.11; 0.10) | 0.05 (−0.20; 0.29) | −0.29 (−0.65; 0.07) | −0.12 (−0.50; 0.25) | 0.22 (−0.11; 0.56) | 0.07 (−0.29; 0.43) | −0.14 (−0.74; 0.45) |
| Intercept | 1.19*** (1.01; 1.37) | 1.20*** (0.91; 1.50) | 1.46*** (1.33; 1.60) | 1.62*** (1.24; 2.00) | 1.92*** (1.29; 2.54) | 1.94*** (1.40; 2.47) | 1.10*** (0.60; 1.61) | 1.29*** (0.68; 1.90) | 1.40*** (0.62; 2.18) | |
| Random effects variance | Cons | 0.01 | 0.02 | 0.00 | 0.05 | 0.08 | 0.08 | 0.05 | 0.08 | 0.10 |
| Residual | 0.10 | 0.18 | 0.08 | 0.29 | 0.35 | 0.24 | 0.88 | 0.70 | 0.49 | |
P < 0.1, *P < 0.05, **P < 0.01, and ***P < 0.001. Statistically significant estimates at P < 0.1 are in bold.
Contrasting Monogamous and Polygynous Villages.
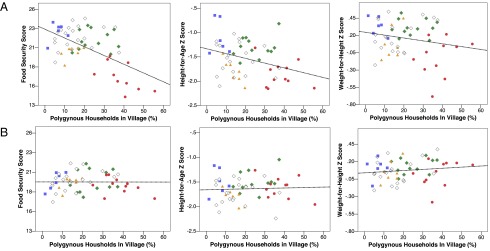
We next consider how village characteristics predict individual measures of food security and child health using multilevel regression including village-level random and fixed effects (SI Text and Table S7). Independently of individual marital status, each 10% increase in the proportion of polygynous households sampled per village is associated with an estimated −1.52 unit decrease in food security (β = −1.52, 95% CI = −2.09; −0.95, P < 0.001), a −0.15 reduction in child HAZ (β = −0.15, 95% CI = −0.25; −0.05, P < 0.001), and a −0.07 reduction in child WHZ (β = −0.07, 95% CI = −0.15; 0.01, P < 0.1). However, once we adjust analyses for village-level proxies for ecological vulnerability (annual rainfall) and socioeconomic marginalization (distance to district capital and the proportion of household heads with nonzero education), these associations dramatically attenuate and become statistically nonsignificant in the case of food security and child HAZ, whereas the proportion of polygynous households in a village becomes positively associated with child WHZ (β = 0.08, 95% CI = −0.01; 0.18, P = 0.09; Fig. 4). As such, our analyses do not support the idea that polygyny has negative group-level consequences on well-being.
Table S7.
Multilevel regressions predicting household food security, HAZ, and WHZ
| Independent variable | Food security (n = 1,745) [β (95% CIs)] | Height-for-age Z-score (n = 2,704) [β (95% CIs)] | Weight-for-height Z-score (n = 2,711) [β (95% CIs)] | ||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | ||
| Household type (reference: monogamous male-headed) | Polygynous male-headed | 1.05* (0.20; 1.90) | 1.04* (0.19; 1.89) | −0.06 (−0.21; 0.10) | −0.05 (−0.20; 0.10) | 0.03 (−0.10; 0.16) | 0.03 (−0.10; 0.16) |
| Polygynous female-headed | −0.85 (−2.03; 0.33) | −0.74 (−1.92; 0.44) | −0.02 (−0.25; 0.20) | −0.01 (−0.24; 0.21) | 0.05 (−0.15; 0.24) | 0.06 (−0.14; 0.25) | |
| Monogamous female-headed | −0.16 (−1.60; 1.29) | −0.10 (−1.54; 1.34) | −0.07 (−0.35; 0.20) | −0.07 (−0.34; 0.21) | 0.20† (−0.03; 0.43) | 0.22+ (−0.02; 0.45) | |
| Child age (mo) (centered at 30 mo) | — | — | −0.09*** (−0.10; −0.07) | −0.09*** (−0.10; −0.07) | −0.02*** (−0.03; −0.01) | −0.02*** (−0.03; −0.01) | |
| Child age squared (mo2) (centered at 30 mo2) | — | — | 0.001*** (0.001; 0.002) | 0.001*** (0.001; 0.002) | 0.000 (0.000; 0.000) | 0.000 (0.000; 0.000) | |
| Child sex (reference: boy) | Girl | — | — | 0.13* (0.02; 0.24) | 0.13* (0.02; 0.24) | 0.05 (−0.05; 0.14) | 0.04 (−0.05; 0.14) |
| Age of household head (y) (centered at 43 y) | −0.03* (−0.06; −0.01) | −0.03* (−0.06; −0.01) | 0.00 (0.00; 0.01) | 0.00 (0.00; 0.01) | 0.00* (0.00; 0.01) | 0.00† (0.00; 0.01) | |
| Season (reference: not hunger) | Hunger | −1.39† (−2.80; 0.02) | −1.75** (−2.81; −0.69) | −0.25* (−0.50; −0.01) | −0.32** (−0.53; −0.11) | 0.00 (−0.19; 0.19) | −0.03 (−0.18; 0.13) |
| Polygyny prevalence (per 10%) (centered at 22%) | −1.52*** (−2.09; −0.95) | −0.02 (−0.68; 0.64) | −0.15** (−0.25; −0.05) | 0.04 (−0.09; 0.17) | −0.07† (−0.15; 0.01) | 0.08† (−0.01; 0.18) | |
| Annual rainfall (per 100 mm3) (centered at 780 mm3) | — | 0.58** (0.24; 0.91) | — | 0.07* (0.01; 0.14) | — | 0.09*** (0.04; 0.14) | |
| Percent nonzero education (per 10%) (centered at 64%) | — | 1.08*** (0.66; 1.49) | — | 0.13** (0.05; 0.21) | — | 0.11*** (0.05; 0.17) | |
| Distance to capital (per 10 km) (centered at 33 km) | — | 0.03 (−0.26; 0.32) | — | −0.06† (−0.11; 0.00) | — | 0.04† (0.00; 0.08) | |
| Intercept | 18.57*** (16.39; 20.75) | 19.26*** (17.61; 20.91) | −1.73*** (−2.11; −1.34) | −1.61*** (−1.94; −1.28) | 0.07 (−0.23; 0.38) | 0.13 (−0.12; −0.38) | |
| Random effects variance | Cons | 5.12 | 2.23 | 0.15 | 0.09 | 0.09 | 0.04 |
| Residual | 39.40 | 39.40 | 2.08 | 2.08 | 1.56 | 1.56 | |
P < 0.1, *P < 0.05, **P < 0.01, and ***P < 0.001. Statistically significant estimates at P < 0.1 are in bold.
Fig. 4.
Village differences in food security and child health by polygyny prevalence. Predicted village intercepts before (A) and after (B) adjustment for village-level differences in ecological vulnerability (annual rainfall) and socioeconomic marginalization (distance to district capital and proportion of household heads with nonzero education). After adjustment, polygyny prevalence is unrelated to food security and HAZ, and positively predicts WHZ. Intercepts are mean/mode centered for household characteristics. See text for estimated coefficients and Table S7 for corresponding model output. Red circle, Maasai village; green diamond, Sukuma village; orange triangle, Rangi village; blue square, Meru village; white diamond, other ethnicity village.
Discussion
We challenge the widespread notion that polygyny is harmful to children. Consistent with prior studies (4–8), polygyny is predictive of relatively low food security and poor child health in aggregated data. However, such associations are driven entirely by the tendency of polygyny to be more common in marginalized and ecologically vulnerable villages and ethnic groups. Within villages, polygynous households, at least those headed by males, often had higher food security and better child outcomes than monogamous households. Polygynous households were also wealthier in terms of livelihood-specific forms of wealth (land and livestock), although not in asset ownership, which is the foundation of wealth indices favored by national demographic surveys (35). These findings are consistent with classic evolutionary and economic models suggesting that sharing a husband can be in a woman’s strategic interest, at least in contexts where women depend on men for resources, by enabling access to equal or greater wealth than could be achieved by opting for monogamy (17, 18). Our results also highlight the inherent weaknesses of highly aggregated samples such as the DHS, the primary data source for population scientists studying family structure and health in sub-Saharan Africa (36).
That polygyny is associated with better outcomes for specifically male-headed households indicates that cowives resident with their husband are most likely to benefit from polygyny. Female-headed polygynous households in contrast may often lose cowife conflicts over shared resources. We found that female-headed polygynous households had lower food security than monogamous households when considering the sample as a whole, although child health did not differ (Fig. 2). In this context, first wives are most often coresident with their husband. Advantages to first wives have been reported elsewhere (13, 24). In rural Ethiopia, Gibson and Mace (13) found first wives were in better physical health and had more surviving offspring than monogamously married women and that relatively poor child health was only associated with polygyny for second or later cowives. This result may reflect selection effects, i.e., women of good health/social standing are less likely to enter polygynous marriages as later wives, such that differences in child outcomes, or indeed food security, cannot be seen as consequences of polygyny itself (13, 16). Alternatively, first wives may benefit from exclusivity before sharing their husband and subsequent seniority over later wives. Thus, to the extent that deficits in child health or food security are unequally portioned among wives, we note that polygyny may, in some instances, be considered harmful.
We demonstrate ethnic variation in the relationship between polygyny and health. Findings from prior small-scale studies suggest such variation, but comparing results across studies is hampered by methodological differences (25). Specifically, we detect an advantage of being raised in male-headed polygynous households for the Sukuma (the largest ethnic group in Tanzania) and the Rangi, but not for the Maasai. Although our stratified analyses here have relatively low statistical power, at least two factors may account for these differences: low status of Maasai women and the relative poverty of this ethnic group. Previous studies emphasize low female status in the Maasai (37), restricting women’s control over their marital arrangements (including divorce and the addition of cowives) and/or preventing women from effectively allocating household resources to children (38). The Maasai also suffered the greatest burden of food insecurity and poor health in our study (34). Borgerhoff Mulder (39, 40) found that polygyny was negatively associated with child survival only in the poorest households in Kenyan Kipsigis. Strassmann (22) observed negative associations between polygyny and child health in the Dogon of Mali in all but one “exceptionally large and wealthy village” (p. 10,897). Thus, it might be that polygyny fails to provide better circumstances in conditions of relative resource scarcity where children are most vulnerable to biased intrahousehold resource allocation, accounting for the differences between the Maasai and neighboring ethnic groups.
Our analyses do not support the assertion that polygyny has group-wide costs on child health (28). Instead, it seems parsimonious that highly polygynous, predominantly Maasai, villages do poorly not because of polygyny, but because of vulnerability to drought, low service provision, and broader sociopolitical disadvantages. Highly monogamous, predominantly Meru, villages on the other hand occupy the relatively high rainfall, fertile slopes of Mount Meru close to Arusha city, benefiting from improved health care and education infrastructure (34). It is possible that polygyny has negative group-level consequences on unmeasured aspects of well-being. However, we are skeptical of the theoretical foundation of such arguments. Recent reformulations of sexual selection theory emphasize facultative responses to partner availability, predicting that the more common sex will cater to the preferences of the rarer sex to acquire and retain mates. As such when polygyny leads unmarried women to be in relatively short supply we might expect higher not lower levels of paternal investment (30, 41). Consistent with this perspective, our adjusted analyses found that child WHZ was marginally higher in the most polygynous villages (Fig. 4).
If polygyny does not bestow group-level costs on women and children, as suggested by Henrich et al. (28), how can we account for observed transitions to socially imposed monogamy with economic development? In Tanzania, the spread of both Islam and Christianity have clearly influenced marital norms. Missionary influence may be partially responsible for the ubiquity of monogamy among the Meru (42). However, explanations based solely on religion are unsatisfactory because religious prescriptions and marriage patterns most likely coevolve, constrained to some extent by systems of production (43). Fortunato and Archetti (44) propose that monogamy evolves via the maximization of individual, not group benefits, and is best understood as an inheritance strategy favored when intergenerational resource transfers are critical to descendant success. Monogamy may thus be beneficial to both men and women when returns to parental investment favor offspring quality over quantity. In line with this account, the Meru were early adopters of relatively intensified agriculture (42), where productivity is limited by land inheritance, as opposed to low intensity agriculture and pastoralism, which may be relatively labor limited. The Meru also have the highest educational attainment (34), which is associated with transitions to low fertility. Once individuals opt for smaller family sizes, a pattern best understood as motivated by economic rather than reproductive success (45), the reproductive advantages of polygyny are likely outweighed by novel opportunities to invest more per child, e.g., via formal education.
Although we make important methodological advancements, our study shares several limitations with prior studies of polygyny. Our use of the standard demographic household definition (SI Text) often cleaves polygynous families into distinct survey units, preventing direct contrasts of children of first and later wives sharing the same husband. Cross-sectional data also limit our ability to infer causality, preventing explicit consideration of the impact of additional wives on previously monogamous women and their children. A recent retrospective study in Bolivia reports that, although women in polygynous marriages had lower fertility than women in monogamous marriages overall, the addition of a second wife did not impact on the fertility of the first wife in intraindividual analyses (16). Self-selection may thus be responsible for reported effects of polygyny in some cross-sectional studies (13). We also caution that the relatively small number of female-headed polygynous households (at least for the Sukuma and Rangi; Table S1) in our study may have resulted from disagreement between the village register sampling frame and household definition used by enumerators on the ground. If unsampled and sampled households systematically differ this may bias our estimates. The common use of rigid household definitions is coming under increasing criticism for obscuring the measurement of complex demographic phenomena, and we support recent calls (46) for experimentation with alternative survey methodologies that more accurately cater to the reality of African family structure.
Our study concerns food security and child health and cannot tell us about the wider potential of polygyny to cause harm. Other aspects of physical and mental well-being may be influenced by polygyny (47). Recent studies counter simple intuition. Polygyny is associated with lower HIV prevalence at both national and regional levels across Africa. Reniers and colleagues (48) suggest polygyny increases individual exposure, but selective recruitment of HIV-positive women into polygynous marriages where coital frequency is lower isolates transmission risks from the wider population. A recent study in Tanzania also found no evidence for an association between polygyny and maternal anxiety and depression (49). Whatever the outcome, we do not anticipate universal relationships between polygyny and well-being. We have demonstrated variation in the estimated consequences of polygyny both between women (by coresidence with husband) and between ethnic groups. Moreover, the vital insight of both economic and anthropological theory is that cultural diversity in marriage practices stems in large part from context-dependency in the pay-offs to alternative behavioral strategies (50). As anthropologists have long emphasized, polygyny itself is also a diverse institution with considerable cultural variation in associated norms of spousal recruitment and residence (51).
We particularly advocate that policy makers distinguish low female autonomy from polygyny rather than treat the latter as a definitive indicator of the former. Where women have control over marital placements, we do not anticipate costs to polygyny. Indeed, if there are large differences in male wealth, prohibiting polygyny may be disadvantageous to women by restricting marital options. Levirate marriage or widow inheritance, whereby a women marries the close male relative of her deceased spouse as a polygynous bride, is also likely to offer women and their children substantially better prospects than living as a single widow in many contexts (52). On the other hand, if female autonomy is low, and/or when polygyny is not associated with differences in male wealth, marital placements may logically be prone to negative impacts of male coercion. We also recommend future research prioritizes data analysis at the level of social groups (i.e., villages, neighborhoods). Institutions for marriage and child-raising are rapidly changing across the globe, and their gendered impacts are increasingly taking center stage in discussions of international development (1). Policy analysts concerned with these transformations need to consider appropriate comparison groups, selection effects, and broader community confounds. Only by making meaningful contrasts, which capture alternatives readily available to individuals, and by taking into account the distribution of specific traditions across different communities and ecologies, can we expect to achieve a true understanding of the health implications of cultural practices.
Materials and Methods
Data (Dataset S1) were collected between 2009 and 2011 as part of the Whole Village Project (WVP), coordinated by Savannas Forever Tanzania, the University of Minnesota (UM), and the Tanzanian National Institute of Medical Research (NIMR). The WVP received ethical approval from the UM Institutional Review Board (code 0905S65241) and NIMR. Between 60–75 households were randomly selected from 56 villages (Fig. S1), leading to a sample of 3,584 households, 2,268 of which contained children under 5 y of age. Nearly half (45%) provided anthropometric data on more than one child (two children, 35%; three or more children, 10%). Four ethnicities, the Maasai, Sukuma, Rangi, and Meru, make up 65% of households. Maasai are traditionally seminomadic pastoralists but have recently diversified into cultivation. Sukuma, Rangi, and Meru are all characterized as agro-pastoralists. Rangi and Meru primarily identify as Muslims and Protestants, respectively. Sukuma and Maasai identify with either Christian or indigenous religions (34). Our analysis is limited to households with a married head of at least 16 y, bringing our working sample to 1,764 households, containing 2,833 children (averaging 32 households and 51 children per village). The Household Food Insecurity Access Scale assesses food insecurity during the last month on a 27-point scale. We reversed this measure so that a higher score indicates greater food security (mean: 16.9; SD: 7.0). Anthropometrics were WHO standardized. HAZ assesses chronic malnourishment (mean: −1.6; SD: 1.6) and WHZ assesses acute malnourishment (mean: 0.2; SD:1.3). Z-scores less than −2.0 indicate stunting and wasting, respectively. Relatedness data are available for villages 15–56 only: 80% of children were biological children of the head, 14% were grandchildren, 6% were other relatives. A wealth index was calculated by principal component analysis applied to the ownership of 37 assets. Acres cultivated and livestock units were recorded separately. Wealth measures were transformed (log x + 1) to approximate normal distributions. Village mapping was used to compute distance to district capital and estimated annual rainfall. SI Text provides further information on child, household, and village data. Regressions were fit using maximum likelihood estimation and include controls for child age and sex, age of household head, and hunger season (for details, see SI Text and Tables S3–S7).
SI Text
Harmful Cultural Practices.
The terms “harmful cultural practice” and “harmful traditional practice” are used interchangeably by the international development and human rights community to refer to nonwestern cultural practices deemed detrimental to individual well-being, most often with regard to women and children. The concept was initially developed by the United Nations (UN) to name and combat ostensibly blatant forms of male domination of women, culminating in a 1995 UN Fact sheet devoted to the issue (53). We avoid the more commonly used term harmful traditional practice in our text following concern that “traditional” falsely implies that modern/western practices are exempt from potential to cause harm and that cultural subordination of women is limited to traditional populations (53). There is no single universally agreed list of harmful cultural practices, but the concept is most frequently used in reference to female genital cutting, gendered violence, child, early, and forced marriage, and polygynous marriage. Attention on harmful cultural practices has grown in recent years, in line with an increased focus on gender in all spheres of international development (1). For further discussion of current and historical negative characterizations of polygynous marriage from both human rights and theological perspectives, see refs. 2, 54, and 55.
Methodological Limitations of Prior Research on Polygyny and Child Health.
Our study overcomes important limitations of prior research, combining methodological strengths of prior small-scale anthropological and large-scale demographic studies. The main limitations of small-scale anthropological studies, particularly from the perspective of public health, is that sample sizes are generally extremely small (often n < 100). Furthermore, we can’t directly contrast results across small-scale studies to compare specific cultural and ecological contexts because of idiosyncratic variation in statistical methodology and study design (e.g., differences in sampling, definition, and use of independent and dependent variables, inclusion of controls for potential confounders). Our study provides a large sample more characteristic of large-scale demographic studies. Indeed, our initial sample (n = 3,584 households) surpasses the Tanzania DHS for the same regions (31, 34). Using parallel sampling and analysis methods across multiple ethnic groups, our study also enables effective estimation of context dependency in relationships between polygyny, food security, and child health. However, we caution that, although our study site encompasses a large area of northern Tanzania, our results cannot be taken as nationally representative. Rather, our findings should only be treated as representative of our specific study villages (Fig. S1). Most notably we sampled a high proportion of Maasai households. Our study site contains 22% Maasai households compared with the 1996 Tanzanian DHS, which included only 2% Maasai households (34). The Maasai are exceptional for primarily relying on pastoralism as opposed to agriculture, high levels of polygyny relative to other Tanzanian ethnic groups, and for experiencing high levels of socioeconomic marginalization (34).
In contrast to small-scale anthropological studies, the primary concern with large-scale demographic studies is their inherent vulnerability to confounding between ecological and individual determinants of health (i.e., vulnerability to the “ecological fallacy”). Previous studies of DHS data have attempted to deal with this problem in various ways we believe are largely unsatisfactory. First, several studies have included random effects to adjust for hierarchical clustering at the national level only (e.g., ref. 4). Second, other studies have incorporated random effects at the subnational regional level (e.g., ref. 6). National or subnational clusters are likely to only crudely map spatial covariance in marriage and health outcomes. Subnational regions are an improvement but still aggregate data across much structured diversity in both health and cultural practices. For example, in our case, Maasai and Meru villages are often directly adjacent but offer the most extreme comparisons in terms of both polygyny prevalence and health outcomes (Fig. S1) (34). Finally, Wagner and Rieger (5) incorporate random effects at the level of primary sampling units (PSUs). Although PSUs offer higher resolution, their value is questionable. We question the value of including random effects for PSU for two reasons. The first reason is that very few households are surveyed per PSU by most DHS [e.g., only 16–22 households in Tanzania (ref. 31, p. 10)], and among those sampled, sample size per PSU cluster is further reduced by data restrictions (most obviously in this case many households will not contain children < 5 y old). Small cluster size can lead to estimation problems, particularly when analysis rests on the estimation of many parameters that may vary to differing degrees within each cluster. The second reason is that PSUs are usually based on census enumeration areas, which do not necessarily correspond with specific villages or cohesive communities (including, for example, adjacent urban zones within towns and cities), which means they are not ideal for contextual analysis. For this reason previous studies have avoided the incorporation of PSUs as a random effect (ref. 6, p. 347). Our study has the advantage of using clearly defined village units as random effects with relatively high-density sampling per cluster (averaging 32 households and 51 children per village included in our final analyses).
Interestingly, Wagner and Rieger (5), who adjust for PSUs as a random effect in a mixed urban and rural sample of 26 African DHS surveys, estimate that, whereas in the majority of countries there was a negative relationship between polygyny and child anthropometrics, a positive and nonsignificant relationship was estimated for Tanzania (ref. 5, p. 17). As we have noted, our study site is not representative of Tanzania as a whole and therefore direct comparisons of effect estimates should be avoided. However, this finding could be seen as consistent with our conclusion that, once low-level spatial clusters are adjusted for, polygyny is no longer predictive of child health for Tanzanian families. Unfortunately, Wagner and Reiger (5) do not report country-specific estimates both with and without adjustment for PSUs, so we cannot infer from this study whether or not adjusting for PSU specifically modifies their effect estimates compared with more aggregated analyses. We are skeptical of the use of PSUs as random effects for the reasons outlined above, but advocate future researchers explore alternative methods for dealing with spatial clustering with DHS data. Ultimately the adequacy of using PSUs as clusters will depend on the specific number of cases per cluster, the nature of the sample (e.g., rural vs. urban), and the specific analysis methodology implemented.
The final major advantage of our study is the utilization of additional household-level data generally not incorporated into large-scale demographic studies of polygyny and child health. Previous studies of the DHS have used a standardized household wealth index to measure wealth. We use an equivalent measure based on the distribution of asset-based wealth in our population, but also livelihood specific forms of wealth in the form of the acres of land cultivated and amount of livestock owned (see below). As our results demonstrate, these measures reveal wealth differences between households obscured when relying on generic wealth indices alone. We also incorporate data on ethnicity. With the exception of Gyimah (8), no DHS study of polygyny and child health has adjusted or stratified estimates by ethnicity. However, ethnicity covaries strongly with both culturally shared marital norms and broader social and ecological determinants of child health. As such there is considerable margin for error in the interpretation of large-scale DHS analyses neglecting ethnicity. This issue is particularly salient to Tanzania, where ethnicity data have not been made available for DHS data for almost two decades. Five DHSs have thus far been conducted in Tanzania (1991/2, 1996, 1999, 2004/5, and 2010), and to our knowledge, only the 1991/2 and 1996 DHS provide ethnicity data.
Sampling and Ethnicity.
Overall, 56 villages were sampled by the WVP, between mid-2009 and mid-2011, across the northern and central Tanzanian regions of Arusha (19 villages), Manyara (11 villages), Dodoma (7 villages), Singida (5 villages), Shinyanga (8 villages), Mwanza (3 villages), and Mara (3 villages). The sampling of villages was based in part on the priorities of development agency partners and the permission of government leaders, although effort was made to randomize village sampling where possible and to ensure a wide geographic spread. Fig. S1 shows the location of each village, color coded by the majority ethnic group (recorded at the household level), in relation to major settlements, main roads, national parks, and game reserves. Lawson et al. (34) provide information on the exact number of households and children sampled by village, district, and region. Maasai, Sukuma, Meru, and Rangi were the most common ethnic groups sampled, collectively accounting for 60.4% of households sampled. Other ethnic groups were also sampled at relatively low frequency. These groups include the Arusha (6.4%), the Wanda (4.7%), the Iraqw (4.3%), the Turu (3.0%), the Mbugwe (2.9%), the Gogo (2.0%), and a large number of ethnic groups each accounting for <2% of the sampled households.
Within each village, between 60 and 75 households were randomly selected for participation from a list provided by village administrators, leading to a total of 3,584 surveyed households. Household head marital status is used to contrast monogamous and polygynous family settings. Heads were identified as the person responsible for household upkeep and households defined as “a group of persons who live together in the same house or compound, share the same house-keeping arrangments, and eat together as one unit.” Informed oral consent was obtained from participants, and all individual data were anonymized before analysis. Anthropometric measurements were taken for all resident children under 5 y of age. Of 3,584 sampled households, 2,268 (63%) contributed child anthropometric data, and just under half of those households provided data on more than one child (two children: 35%; three or more children: 10%), leading to a total of 3,586 surveyed children. The sample for our current analysis is limited to households with a verified currently married head of at least 16 y, bringing our working sample to 1,764 households, containing 2,833 children.
Child, Household, and Village Data.
Child anthropometrics.
The mean age of sampled children was 28.9 mo, with roughly even sampling across the age range of zero to 60 mo and evenly split by sex (34). Child weight was measured to the nearest 100 g using a Salter-type spring hanging scale for infants and electronic scales for children able to stand. Child height was measured to the nearest millimeter using a measuring board for young children and using a stadiometer for children of 2 y or older. All measurements were made once and immediately entered into a database. Children were measured by different field staff depending on the village sampled, but training of enumerators by United Nations Children’s Fund staff and oversight of anthropometric sessions by the Tanzanian National Institute of Medical Research personnel ensured high levels of interrater reliability before data collection.
Three anthropometric indicators were derived using WHO age- and sex-specific growth standards (56). HAZ serves as an indicator of long-term effects of malnutrition. A child with a HAZ of <−2 SDs from the WHO reference is considered stunted, i.e., chronically malnourished, which reflects failure to receive adequate nutrition over a long period and is influenced by recurrent and chronic illness. Weight-for-height Z-scores (WHZ) measure body mass in relation to body height/length and describes current nutritional status. A child with a WHZ <−2 SDs is considered acutely malnourished (i.e., wasted), which represents the failure to achieve adequate nutrition in the period immediately preceding measurement and may result from inadequate food intake or illness. HAZ and WHZ scores were derived in IBM SPSS v.20 using WHO-supplied syntax, which automatically removes extreme cases, including those likely to have resulted from measurement error, i.e., incorrect recorded child age, height, or weight. HAZ scores of <−6 or >6 are removed, and WHZ scores of <−5 or >5 are removed. Applying these criteria reduces our sample of child anthropometric data from 2,833 to 2,704 and 2,711 valid HAZ and WHZ scores, respectively. The mean HAZ score is −1.61 with an SD of 1.56, with 40.5% categorized as stunted. The mean WHZ score is 0.17 with a SD of 1.33, with 4.1% categorized as wasted.
The use of WHO standardized growth scores is ubiquitous in both the demographic and anthropological literature on polygyny and child health, and we have chosen to use these measurement standards to enhance comparability with prior research. We caution that the application of WHO reference standards to an ethnically and socio-ecologically diverse sample cannot take into account differing genetic capacity for growth and the potential for environmental variation to modify the local health significance of growth indicators. However, we have previously (34) confirmed that differences in child anthropometric indicators between ethnic groups closely map onto differences in subjective ratings of child health, recorded illnesses, food insecurity, and recent food consumption at this site. In all cases, Maasai households, particularly when primarily reliant on pastoralism, appear substantially disadvantaged. This result suggests that differences in child anthropometry between ethnic groups make appropriate proxies for health. The alternative of making Z-scores specific to each ethnic group is not feasible because (i) the “Other” ethnic group category contains many ethnic groups with no clear point of internal reference, and (ii) our sample is composed of children from 0 to 60 mo with relatively few cases for each age and sex combination to make robust age- and sex-specific estimates.
Household food security.
The Household Food Insecurity Access Scale (HFIAS) was used to measure food security (57). It is a brief survey instrument developed by the US Agency for International Development (USAID) funded Food and Nutrition Technical Assistance (FANTA) Project to improve the measurement of food security and ultimately better target interventions to the most vulnerable households. The scale is based on a household’s reported experience of problems regarding three domains of food insecurity argued to be universal across cultures: (i) feelings of uncertainty or anxiety about household food supplies; (ii) perceptions that household food is of insufficient quality (including variety and food type preference); and (iii) insufficient food intake and its physical consequences. The HFIAS is composed of nine questions recording the occurrence and frequency of specific problems along these domains. Responses were scored so that “never” received a score of 0, “rarely” scored 1, “sometimes” scored 2 and “often” scored 3, so that when summed, the lowest possible score was 0 and the highest 27. This measure was then reversed for our study so that a higher value represents higher food security rather than food insecurity. For our sample of 1,764 households, complete responses were available for all but 19 households. For the 1,745 households with complete data, the mean food security score was 16.91 with an SD of 7.04. A categorical measure can also be computed on the basis of the HFIAS questions. By this measure, 46% of all sampled households can be categorized as severely food insecure (34), meaning they cut back on meal size or number of meals often and/or experience any of the three most severe conditions (running out of food, going to bed hungry, or going a whole day and night without eating) at least once a month (57).
Household type.
We categorized households by the sex and marital status of the household head. Out of our working sample of 1,764 households, 69% of households were headed by a monogamously married male, 17% were headed by a polygynously married male, 9% by a polygynously married female, and 5% by a monogamously married female. Table S1 provides household descriptive data by household type and ethnicity for the working sample. Formal data on wife rank were not collected, but field observations confirmed that male-headed polygynous households typically consisted of a husband, his first wife, and their shared children, whereas female-headed polygynous households typically consisted of later cowives and her children living separately. In only a small proportion of cases was more than one wife coresident in a male-headed polygynous household (17% in the Sukuma, 7% in Maasai, 6% in the Rangi, 0% in the Meru, and 8% in the other ethnicity group.) We did not collect data linking households containing cowives for the same husband. This limitation prevents us from making contrasts between children of different mothers sharing the same father.
Household wealth.
Table S2 provides supporting data on household soioeconomic characteristics. A Household Wealth Index was calculated on the basis of a principal components analysis (PCA). The PCA was applied to a total of 37 dichotomous variables representing ownership of assets and characteristics of assets at the household level. Owning a particular asset or a better asset increases the value of the index by different amounts determined by the household’s score for the first principal component. The index is scaled so that its minimum is zero, i.e., by construction the poorest household has a score of zero. The following assets were included: drinking water source, household flooring type, household roofing type, type of toilet, owns land, house, cart, hoe, motorcycle, bicycle, plow, sewing machine, lantern, wheelbarrow, computer, radio, water tank, video, chair, sofa, bed, cupboard, chest, dining set, car, cell phone, solar panel, watch or clock, and drum. Note the index does include land ownership (yes/no) and items relating to farming such as a plow or hoe but does not include livestock ownership, despite cattle having a clear economic value. Therefore, it should be interpreted as a non-livestock wealth index. Based on household surveys, we were able to complete a wealth index for 3,480/3,584 (97.1%) of surveyed households. Livestock ownership was measured separately in tropical livestock units with the following conversion rates: cattle (0.7 units); goats and sheep (0.1 units); pigs (0.2 units); and donkey/horses/mules (0.5 units).
Village-level data.
Table S2 shows supporting data on the village-level indicators by the majority ethnic group. Polygyny prevalence and the percentage of heads with nonzero education are measured as the percentage of sampled households within each village (using the complete sample of 3,584 households). Annual rainfall data for each village were derived from the WorldClim climatic data resource as the mean annual total precipitation over the period covering 1950–2000 at a resolution of 1 km2 mapped to a central point of each village (34). Distance to the district capital was calculated for each village using the straight-line distance between the mean coordinates of all sampled households within each village and the central point of the district capital in kilometers. Villages were sampled across an extensive geographic area that straddles two climatic zones experiencing either bimodal or unimodal rainy seasons, influencing the timing of so-called hunger or lean seasons. Although annual rainfall patterns are erratic, the hunger season generally occurs from October to December in the bimodal zone; whereas in the unimodal zone, an overlapping but longer hunger season generally falls from November to February. Based on this monthly categorization, we coded whether or not each village was sampled during in the hunger season, with all villages from the regions of Arusha, Mara, and Mwanza considered to be in the bimodal zone and the regions of Shinyanga, Dodoma, Manyara, and Singida in the unimodal zone. A binary coding of not hunger season (31 villages) vs. hunger season (25 villages) was included in all models.
Analytical Strategy and Full Model Output.
Model estimation.
All models were fit using maximum likelihood estimation in Stata version 13 using the “regress” command for standard linear regression and the “xtmixed’’ command for multilevel linear regression. Models predicting household-level variables (food security, wealth index, land cultivated, and total livestock units) adjust for the age of the household head in years (centered at 43 y). Models predicting child-level outcomes (i.e., HAZ and WHZ) adjust for age of the household head in years (centered at 43 y), child age, and child age squared in months (centered at 30 mo). Standard linear regression models aggregate all data across villages, effectively estimating relationships across the full study area without consideration for the hierarchical spatial clustering of data. Multilevel linear regression models include a random intercept for village. Note that, although child-level outcomes are also clustered within households (because some households contribute data on more than one child), we do not include a random effect for household when predicting child anthropometrics. This strategy is followed because when clusters (i.e., households) are unbalanced and sparsely populated (i.e., ≤2 cases per level), both fixed and random effects may be overestimated (58). All multilevel regression models for both household- and child-level outcome variables also include a village-level fixed effect for whether or not the village was sampled during a hunger season.
Contrasting monogamous and polygynous households.
Table S3 shows the results of a set of standard linear vs. multilevel regression models predicting household food security, child HAZ, and child WHZ, using a dichotomous coding of polygynous vs. monogamous households. Table S3 also shows the results of a parallel set of models using a four-category variable for household type (i.e., male-headed monogamous household, female-headed monogamous household, male-headed polygynous household, and female-headed polygynous household). Comparing the results from standard linear and multilevel regression analyses in these tables demonstrates that adjustment for village-level differences in food security and child health substantially modifies the statistical significance and magnitude of effect estimates. Estimates from Table S3 further demonstrate that the estimated effect of polygyny differs depending on whether households are male or female headed. Table S4 reports the results of a stratified analysis with models run for the three main ethnic groups with a substantial proportion of polygynous households (the Sukuma, the Maasai, and the Rangi). Contrasts in this analysis have relatively low statistical power due to reduced sample size. However, they strongly imply context dependency by ethnic group in the estimated effects of polygyny on food security and child health. Effect estimates from this stratified analysis are graphically represented in Fig. 2. Note that very few female-headed polygynous households were sampled in the Rangi (only four households). Therefore, estimates for the difference between male-headed monogamous households and female-headed polygynous households for the Rangi are deemed unreliable and not graphically represented in Fig. 2.
Polygyny and wealth.
Table S5 reports the results of multilevel regression analyses predicting household wealth index, the number of acres cultivated, and tropical livestock units. Note for both acres cultivated and tropical livestock units, analyses are restricted to cases that own at least some land and some cattle, respectively. Table S6 reports the results of stratified analyses for the same outcomes by ethnic group. Effect estimates from this stratified analysis are graphically represented in Fig. 3. Tables S5 and S6 also show estimates adjusted for the number of adults (i.e., age 15 y and over) and number of dependents (i.e., aged <15 y) in the household. Data are incomplete for these variables on a small fraction of cases, leading to a slight change in sample size between models.
Contrasting monogamous and polygynous villages.
Finally, Table S7 reports the results of multilevel linear regressions predicting individual household food insecurity, child HAZ, and child WHZ that further incorporate village-level fixed effects. These analyses enable us to determine what influence the proportion of polygynous households in a village has over and above the marital status of an individual household. Model 1 for each outcome in Table S7 is identical to the multilevel regression models shown in Table S3 with the four-category coding of household type, except that they also include a village-level fixed effect for polygyny prevalence, with each unit increase representing an additional 10% of sampled households being polygynous rather than monogamous (centered at 22% prevalence). Model 2 for each outcome in Table S7 also includes additional village-level fixed effects for annual rainfall in 100s of cubic millimeters (centered at 780 mm3), the proportion of sampled household heads with nonzero educational attainment (centered at 64%), and distance to the district capital in 10s of kilometers (centered at 33 km). Comparing the results of model 1 and model 2 for each outcome demonstrates that the effects of polygyny prevalence are substantially modified by adjusting estimates for independent determinants of village differences in food security and child health. Fig. 4 graphically represents this comparison by plotting polygyny prevalence against predicted village intercepts for each outcome before and after adjustment for annual rainfall, proportion of household heads with nonzero education, and village distance from the district capital.
Other Supporting Information Files
Supplementary Material
Acknowledgments
We thank the village residents, C. Packer, D. Levison, K. Hartwig, M. Kaziya, F. Rabison Msangi, J. Felix, and E. Sandet for contributions to the Whole Village Project (WVP) and L. Fortunato, M. Grote, C. Moya, R. Sear, G. Stulp, C. Uggla, and the Human Behavioral Ecology and Cultural Evolution Group at University of California, Davis, for comments. The WVP was funded by the US Agency for International Development, Partners for Development, the University of Minnesota, and the Canadian Foodgrains Bank. The Wellcome Trust supported E.N., B.N., and S.G.M.M. during project implementation. D.W.L. is funded by the UK Medical Research Council and Department for International Development (Grant MR/K021672/1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.H.J. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507151112/-/DCSupplemental.
References
- 1.Coles A, Gray L, Momsen J. The Routledge Handbook of Gender and Development. New York: Routledge; 2015. [Google Scholar]
- 2.Gaffney-Rhys R. A comparison of child marriage and polygamy from a human rights perspective: Are the arguments equally cogent? J Soc Welf Fam. 2012;34(1):49–61. [Google Scholar]
- 3.Wadesango N, Rembe S, Chabaya O. Violation of women’s rights by harmful traditional practices. Anthropologist. 2011;13(2):121–129. [Google Scholar]
- 4.Omariba D, Boyle M. Family structure and child mortality in sub‐Saharan Africa: Cross‐national effects of polygyny. J Marriage Fam. 2007;69(2):528–543. [Google Scholar]
- 5.Wagner N, Rieger M. Polygyny and child growth: Evidence from twenty-six African countries. Fem Econ. 2014;21(2):1–26. [Google Scholar]
- 6.Smith-Greenaway E, Trinitapoli J. Polygynous contexts, family structure, and infant mortality in sub-saharan Africa. Demography. 2014;51(2):341–366. doi: 10.1007/s13524-013-0262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amey FK. Polygyny and child survival in West Africa. Soc Biol. 2002;49(1-2):74–89. doi: 10.1080/19485565.2002.9989050. [DOI] [PubMed] [Google Scholar]
- 8.Gyimah SO. Polygynous marital structure and child survivorship in sub-Saharan Africa: some empirical evidence from Ghana. Soc Sci Med. 2009;68(2):334–342. doi: 10.1016/j.socscimed.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 9.Henrich J. 2010 Polygyny in Cross-Cultural Perspective: Theory and Implications. Affidavit submitted to the Supreme Court of British Columbia in the matter of the constitutionality of s. 293 of the Criminal Code of Canada, R.S.C. 1985, c. C-46, July 15, 2010. Available at www.alliancealert.org/2010/2010071902.pdf. Accessed October 8, 2015.
- 10.Murdock GP, White DW. Standard cross-cultural sample. Ethnology. 1969;8(4):329–369. [Google Scholar]
- 11.Westoff C. 2003. Trends in marriage and early childbearing in developing countries. DHS Comparative Reports No. 5 (ORC Macro, Calverton, MD)
- 12.Fortunato L. 2015. Marriage systems, evolution of. International Encylopedia of Social and Behavioural Sciences, 2nd Ed, ed Wright J (Elsevier, Oxford, UK), pp 611–619.
- 13.Gibson MA, Mace R. Polygyny, reproductive success and child health in rural Ethiopia: why marry a married man? J Biosoc Sci. 2007;39(2):287–300. doi: 10.1017/S0021932006001441. [DOI] [PubMed] [Google Scholar]
- 14.Cronk L. Wealth, status, and reproductive success among the Mukogodo of Kenya. Am Anthropol. 1991;93(2):345–360. [Google Scholar]
- 15.Mulder B. On cultural and reproductive success : Kipsigis evidence. Am Anthropol. 1987;89(3):617–634. [Google Scholar]
- 16.Winking J, Stieglitz J, Kurten J, Kaplan H, Gurven M. 2013. Polygyny among the Tsimane of Bolivia: An improved method for testing the polygyny-fertility hypothesis. Proc Roy Soc B Biol Sci 280(1756):20123078.
- 17.Orians GH. On the evolution of mating systems in birds and mammals. Am Nat. 1969;103(934):589–603. [Google Scholar]
- 18.Becker GS. A Treatise on the Family. Harvard Univ Press; Cambridge, UK: 1981. [Google Scholar]
- 19.Borgerhoff Mulder M. Kipsigis women’s preferences for wealthy men: Evidence for female choice in mammals? Behav Ecol Sociobiol. 1990;27(4):255–264. doi: 10.1007/BF00164897. [DOI] [PubMed] [Google Scholar]
- 20.Strassmann BI. Polygyny as a risk factor for child mortality among the Dogon. Curr Anthropol. 1997;28(4):688–695. [Google Scholar]
- 21.Sear R, Steele F, McGregor IA, Mace R. The effects of kin on child mortality in rural Gambia. Demography. 2002;39(1):43–63. doi: 10.1353/dem.2002.0010. [DOI] [PubMed] [Google Scholar]
- 22.Strassmann BI. 2011. Cooperation and competition in a cliff-dwelling people. Proc Natl Acad Sci USA 108(Suppl 2):10894–10901.
- 23.Hadley C. Is polygyny a risk factor for poor growth performance among Tanzanian agropastoralists? Am J Phys Anthropol. 2005;126(4):471–480. doi: 10.1002/ajpa.20068. [DOI] [PubMed] [Google Scholar]
- 24.Sellen DW. Polygyny and child growth in a traditional pastoral society: The case of the Datoga of Tanzania. Hum Nat. 1999;10(4):329–371. doi: 10.1007/s12110-999-1007-8. [DOI] [PubMed] [Google Scholar]
- 25.Lawson D, Uggla C. 2014. Family Structure and Health in the Developing World : What Can Evolutionary Anthropology Contribute to Population Health Science? Applied Evolutionary Anthropology: Darwinian Approaches to Contemporary World Issues, eds Gibson MA, Lawson DW (Springer, New York), pp 85–118.
- 26.Borgerhoff Mulder M. Women’s strategies in polygynous marriage : Kipsigis, Datoga, and other East African cases. Hum Nat. 1992;3(1):45–70. doi: 10.1007/BF02692266. [DOI] [PubMed] [Google Scholar]
- 27.Pollet TV, Tybur JM, Frankenhuis WE, Rickard IJ. What can cross-cultural correlations teach us about human nature? Hum Nat. 2014;25(3):410–429. doi: 10.1007/s12110-014-9206-3. [DOI] [PubMed] [Google Scholar]
- 28.Henrich J, Boyd R, Richerson PJ. The puzzle of monogamous marriage. Philos Trans R Soc Lond B Biol Sci. 2012;367(1589):657–669. doi: 10.1098/rstb.2011.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander RD, Hoogland JL, Howard RD, Noonan KM, Sherman PW. Sexual dimorphisms and breeding systems in pinnipeds, ungulates, primates and humans. In: Chagnon NA, Irons W, editors. Evolutionary Biology and Human Social Behaviour: An Anthropological Perspective. North Scituate, MA: Duxbiry Press; 1979. pp. 402–435. [Google Scholar]
- 30.Schacht R, Rauch KL, Borgerhoff Mulder M. Too many men: The violence problem? Trends Ecol Evol. 2014;29(4):214–222. doi: 10.1016/j.tree.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 31.National Bureau of Statistics Tanzania and ICF Macro . 2011. Tanzania Demographic and Health Survey 2010. (NBS and ICF Macro, Dar es Salaam, Tanzania) [Google Scholar]
- 32.Grantham-McGregor S, et al. International Child Development Steering Group Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.UNDP 2014. Human Development Report 2014. Sustaining Human Progress: Reducing Vulnerabilities and Building Resilience (United Nations, New York)
- 34.Lawson DW, et al. Ethnicity and child health in northern Tanzania: Maasai pastoralists are disadvantaged compared to neighbouring ethnic groups. PLoS One. 2014;9(10):e110447. doi: 10.1371/journal.pone.0110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutstein SO. 2008. The DHS Wealth Index : Approaches for Rural and Urban Areas (Macro International, Calverton, MD)
- 36.David P, Haberlen S. 10 best resources for...measuring population health. Health Policy Plan. 2005;20(4):260–263. doi: 10.1093/heapol/czi030. [DOI] [PubMed] [Google Scholar]
- 37.Hodgson DL. Pastoralism, patriarchy and history: Changing gender relations among Maasai in Tanganyika, 1890-1940. J Afr Hist. 1999;40(1):41–65. doi: 10.1017/s0021853798007397. [DOI] [PubMed] [Google Scholar]
- 38.Carlson GJ, Kordas K, Murray-Kolb LE. Associations between women’s autonomy and child nutritional status: A review of the literature. Matern Child Nutr. 2014;11(4):452–482. doi: 10.1111/mcn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borgerhoff Mulder M. Hamilton’s rule and kin competition: The Kipsigis case. Evol Hum Behav. 2007;28(5):299–312. [Google Scholar]
- 40.Borgerhoff Mulder M. Marrying a Married Man: A Postscript. In: Betzig L, editor. Human Nature: A Critical Reader. Oxford University Press; New York: 1997. pp. 115–117. [Google Scholar]
- 41.Kokko H, Jennions MD. Parental investment, sexual selection and sex ratios. J Evol Biol. 2008;21(4):919–948. doi: 10.1111/j.1420-9101.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 42.Spear T. Mountain Farmers: Moral Economies of Land and Agricultural Development in Arusha and Meru. Oakland, CA: Univ of California Press; 1997. [Google Scholar]
- 43.Goody J. Polygyny, economy, and the role of women. In: Goody J, editor. The Character of Kinship. Cambridge Univ Press; London: 1973. pp. 175–190. [Google Scholar]
- 44.Fortunato L, Archetti M. Evolution of monogamous marriage by maximization of inclusive fitness. J Evol Biol. 2010;23(1):149–156. doi: 10.1111/j.1420-9101.2009.01884.x. [DOI] [PubMed] [Google Scholar]
- 45.Goodman A, Koupil I, Lawson DW. 2012. Low fertility increases descendant socioeconomic position but reduces long-term fitness in a modern post-industrial society. Proc Roy Soc B Biol Sci 279(1746):4342–4351. [DOI] [PMC free article] [PubMed]
- 46.Randall S, Coast E. Poverty in African households: The limits of survey and census representations. J Dev Stud. 2014;51(2):162–177. [Google Scholar]
- 47.Bove R, Valeggia C. Polygyny and women’s health in sub-Saharan Africa. Soc Sci Med. 2009;68(1):21–29. doi: 10.1016/j.socscimed.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 48.Reniers G, Tfaily R. Polygyny, partnership concurrency, and HIV transmission in Sub-Saharan Africa. Demography. 2012;49(3):1075–1101. doi: 10.1007/s13524-012-0114-z. [DOI] [PubMed] [Google Scholar]
- 49.Patil C, Hadley C. Symptoms of anxiety and depression and mother’s marital status: An exploratory analysis of polygyny and psychosocial stress. Am J Hum Biol. 2008;20(4):475–477. doi: 10.1002/ajhb.20736. [DOI] [PubMed] [Google Scholar]
- 50.Gibson MA, Lawson DW. Applying evolutionary anthropology. Evol Anthropol Issues. Rev. 2015;24(1):3–14. doi: 10.1002/evan.21432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White D. Rethinking polygyny: Co-wives, codes and cultural systems. Curr Anthropol. 1988;19(4):529–572. [Google Scholar]
- 52.Palmore E. Cross-cultural perspectives on widowhood. J Cross Cult Gerontol. 1987;2(1):93–105. doi: 10.1007/BF00117178. [DOI] [PubMed] [Google Scholar]
- 53.Winter B, Thompson D, Jeffreys S. The UN approach to harmful traditional practices. Int Fem J Polit. 2002;4(1):72–94. [Google Scholar]
- 54.Jonas O. The practice of polygamy under the scheme of the Protocol to the African Charter on Human and Peoples’ Rights on the Rights of Women in Africa: A critical appraisal. J Afr Stud Dev. 2012;4(5):142–149. [Google Scholar]
- 55.Witte J., Jr . The Western Case for Monogamy Over Polygamy. Cambridge Univ Press, Cambridge, UK; 2015. [Google Scholar]
- 56.de Onis M, et al. WHO Multicentre Growth Reference Study Group Worldwide implementation of the WHO Child Growth Standards. Public Health Nutr. 2012;15(9):1603–1610. doi: 10.1017/S136898001200105X. [DOI] [PubMed] [Google Scholar]
- 57.Coates J, Swindale A, Bilinsky P. 2007. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide (v.3) (Food and Nutrition Technical Assistance Project, Academy for Educational Development, Washington DC)
- 58.Clarke P. When can group level clustering be ignored? Multilevel models versus single-level models with sparse data. J Epidemiol Community Health. 2008;62(8):752–758. doi: 10.1136/jech.2007.060798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.