Significance
Epigenetic regulation of cell fate determination is one of the hottest topics today. In this study, we isolated and characterized a zebrafish mutant in histone Lys-specific demethylase 1 (LSD1/KDM1A), and found that LSD1 plays a role in the initiation of hematopoietic differentiation in the hemangioblast, a bipotent cell that can give rise to hematopoietic or endothelial progenitors. In addition, we identified the major function of LSD1 in hemangioblasts to be the down-regulation of Ets variant 2 (Etv2), a critical regulator of hemangioblast development. Our results suggest that the LSD1-dependent shutdown of the Etv2 gene is the significant event required for hemangioblasts to initiate hematopoietic differentiation.
Keywords: cell differentiation, Gata1, gene regulation, histone demethylase, zebrafish
Abstract
The hemangioblast is a progenitor cell with the capacity to give rise to both hematopoietic and endothelial progenitors. Currently, the regulatory mechanisms underlying hemangioblast formation are being elucidated, whereas those controllers for the selection of hematopoietic or endothelial fates still remain a mystery. To answer these questions, we screened for zebrafish mutants that have defects in the hemangioblast expression of Gata1, which is never expressed in endothelial progenitors. One of the isolated mutants, it627, showed not only down-regulation of hematopoietic genes but also up-regulation of endothelial genes. We identified the gene responsible for the it627 mutant as the zebrafish homolog of Lys-specific demethylase 1 (LSD1/KDM1A). Surprisingly, the hematopoietic defects in lsd1it627 embryos were rescued by the gene knockdown of the Ets variant 2 gene (etv2), an essential regulator for vasculogenesis. Our results suggest that the LSD1-dependent shutdown of Etv2 gene expression may be a significant event required for hemangioblasts to initiate hematopoietic differentiation.
It has been proposed that hematopoietic and endothelial cells share a common progenitor, termed the “hemangioblast,” based largely on the intimate association of these lineages in the blood islands of the developing yolk sac (1–3). The sharing of markers between blood and blood vessel cells and the impairment of both tissues in mutants, such as the mouse Flk1 KO (4) and zebrafish cloche (5), are consistent with a common origin. Evidence for the presence of hemangioblasts has been provided by clonal studies using in vitro differentiating mouse and human ES cells (6–8) and in vivo analyses of both mouse and zebrafish embryos (9–11).
Precisely how blood cells are being formed from the hemangioblasts has been a topic of much interest. Although several transcription factors have been suggested to be required for hemangioblast formation, it is largely unknown how presumptive hematopoietic cells are selected from hemangioblasts to differentiate into the individual hematopoietic lineages. The transcription factor Scl/Tal1 has been demonstrated to play a critical role in the generation of the hemogenic endothelium, an endothelial intermediate between hemangioblasts and hematopoietic cells, but not in the development of hemangioblasts (12). Another transcription factor, Runx1/AML1, is dispensable for the generation of the hemogenic endothelium but is required for the production of definitive hematopoietic precursors from the endothelium (12, 13). In this way, the molecular mechanisms of hematopoietic development from the hemangioblast are beginning to be understood, but many pieces of this puzzle are still missing.
To identify these missing pieces, we focused our attention on the regulation of Gata1 gene expression. Gata1 encodes a transcription factor that transactivates a variety of erythropoietic genes. Gata1 gene KO mice and mutant zebrafish have been demonstrated to be lethally anemic because of drastic defects in erythropoiesis, and Gata1 has been considered to be a key factor required for erythropoiesis (14–16). Interestingly, the expression of Gata1 appears to define the hematopoietic commitment stage, because Gata1-positive cells display hematopoietic, but not endothelial, differentiation potential (17). We hypothesized that key factors involved in the commitment to the blood cell lineage are related to the factor(s) regulating Gata1 expression. The regulators of Gata1 expression have been studied for two decades, but it is still unknown how Gata1 expression is regulated in the early developmental stage, such as in hemangioblasts (18).
In this study, we tried to identify the factors that regulate the Gata1 gene in the early developmental stage through a genetic approach using zebrafish. We isolated a zebrafish mutant whose gata1 expression is reduced and identified the responsible gene as the zebrafish homolog of Lys-specific demethylase 1 (LSD1/KDM1A). LSD1 belongs to the FAD-dependent amine oxidases that demethylate mono- and dimethylated Lys4 (K4) and/or Lys9 (K9) within histone H3 through an oxidative process (19) and has been suggested to play key roles in multiple aspects of cell differentiation through epigenetic regulation (20).
The expression analyses of a variety of marker genes in lsd1 mutant embryos revealed that hematopoietic progenitors were reduced, whereas the number of endothelial progenitors was increased. Among a variety of hemangioblast markers examined, we found that the expression of Ets variant 2 gene (etv2), which is only expressed transiently in early progenitors in the mesoderm (21), was up-regulated in this mutant, suggesting that the hemangioblasts in the mutant embryos were generated but failed to initiate hematopoietic differentiation in the early step. It is noteworthy that the gene knockdown of etv2 could rescue the hematopoietic phenotypes of lsd1 mutants, suggesting that the up-regulation of etv2 was a principal cause of their hematopoietic defects. Based on these results, we hypothesized that the shutdown of Etv2 gene expression is a critical event required for hemangioblasts to initiate hematopoietic differentiation and that LSD1 regulates this event.
Results
LSD1 Is a Responsible Gene for the Hematopoietic Mutant it627.
A genetic screen was conducted to isolate zebrafish mutants that showed decreased gata1 expression in presumptive erythroid progenitors. The it627 mutation is one of the mutants isolated from this screen. The expression of the zebrafish Gata1 gene (gata1) is first observed in the posterior lateral plate mesoderm (LPM) at 11 h postfertilization (hpf) and is maintained after the formation of the intermediate cell mass (ICM) (22). The it627 mutant showed decreased expression of gata1 in both the LPM and ICM (Fig. 1A). The it627 mutation mapped by positional cloning to the zebrafish homolog gene for LSD1 (lsd1), which is a founding member of histone demethylase family (23). Zebrafish lsd1 encodes a protein that is 830 aa in length and shares 85% homology with human LSD1. LSD1 has been demonstrated to demethylate mono- and dimethylated K4 and/or K9 within histone H3 (19). Fig. S1A shows a phylogenetic tree, which indicates the high conservation of LSD1 proteins among vertebrates (24).
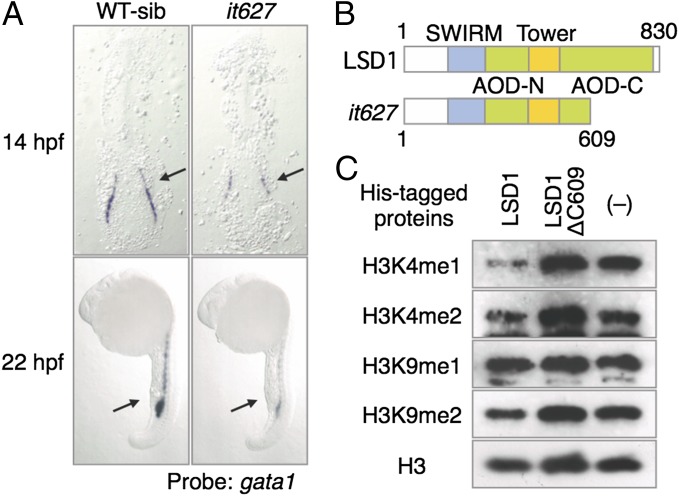
Fig. 1.
Isolation of LSD1 mutant. (A) Expression of gata1 in WT sibling (WT-sib) and it627 embryos. The arrows indicate hematopoietic tissues: posterior LPM (14 hpf) and ICM (22 hpf). (B) Structures of the LSD1 proteins. SWIRM, Swi3p, Rsc8p, and Moira domain; Tower, tower domain. (C) Results of the demethylation assay using bacterially expressed LSD1 proteins and bulk histones from calf thymus as substrates. Methylated proteins were detected by immunoblotting using specific antibodies.
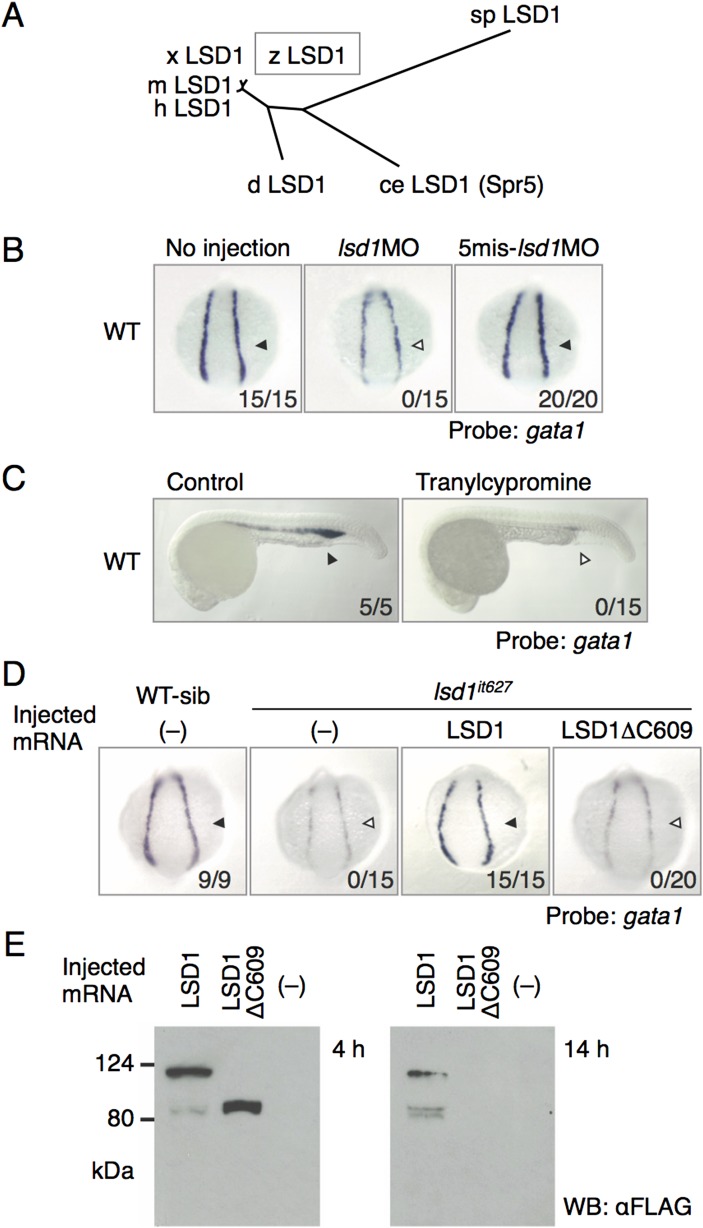
Fig. S1.
Identification of LSD1 as a responsive gene for the it627 mutant. (A) Phylogenetic tree constructed by the neighbor-joining method for the full-length amino acid sequences of LSD1 proteins. ce, Caenorhabditis elegans; d, Drosophila melanogaster; h, human; m, mouse; sp, Schizosaccharomyces pombe; x, Xenopus laevis; z, zebrafish. (B) Expression of gata1 in the LPM at 14 hpf in WT embryos injected or not injected with 1 pmol of the indicated morpholino oligonucleotides. Arrowheads denote gata1 expression. Numbers indicate the percentage of embryos exhibiting strong gata1 expression. 5mis, 5-mismatch. (C) Effects of tranylcypromine on gata1 expression. The embryos were treated with 300 μM tranylcypromine in WT embryos from 12 hpf or earlier to 24 hpf. (D) Expression of gata1 at 14 hpf in WT or lsd1it627 embryos injected with or without mRNA encoding the indicated LSD1 proteins. WT-sib indicates WT or heterozygous mutants, and lsd1it627 designates homozygous mutants. (E) Expression and stability of overexpressed WT LSD1 and LSD1ΔC609 proteins in 4-hpf and 14-hpf embryos were examined by immunoblotting using anti-FLAG antibody. WB, Western blot.
We found a C-to-T nonsense mutation in exon 17 only in the DNA isolated from the mutants. As shown in Fig. 1B, LSD1 contains several structural domains, including AOD-N and AOD-C (the N- and C-terminal parts of the amine oxidase domain, respectively). The mutation replaces Gln609 with a stop codon that leads to an ∼200-aa truncation of AOD-C. AOD-C contains the FAD- and substrate-binding subdomains, suggesting that the truncation eliminates its enzymatic activity. The demethylase activity of the it627-type LSD1ΔC609 protein was investigated to test this assumption (Fig. 1C). WT LSD1 protein demethylated mono- and dimethylated histone H3-K4 but not H3-K9, consistent with the human and Drosophila LSD1 proteins (25, 26). In contrast, the LSD1ΔC609 protein was unable to demethylate either methylated histone H3-K4 or H3-K9, suggesting that the LSD1 protein in lsd1it627 mutants has lost its enzymatic activity.
The effects of lsd1 knockdown in zebrafish embryos were analyzed using morpholino techniques (Fig. S1B). The injection of morpholino antisense oligonucleotides against lsd1 (1 pmol) resulted in a marked reduction of gata1 expression, but the effect was eliminated when five-base mismatches were introduced. Moreover, the gata1 expression was reduced in WT embryos treated with tranylcypromine (Fig. S1C), a well-known LSD inhibitor (27, 28). We further examined whether LSD1 overexpression could rescue gata1 expression in lsd1it627 mutants or not (Fig. S1D). Mutant embryos overexpressing WT LSD1 showed complete rescue of gata1 expression, whereas overexpression of LSD1ΔC609 failed to restore it. Because overexpressed LSD1ΔC609 protein was unstable compared with the WT LSD1 (Fig. S1E), this instability of the truncated protein may be another reason, in addition to its enzymatic inactivity, why it could not rescue the phenotype in lsd1it627 mutants.
LSD1 Is Essential for Zebrafish Development.
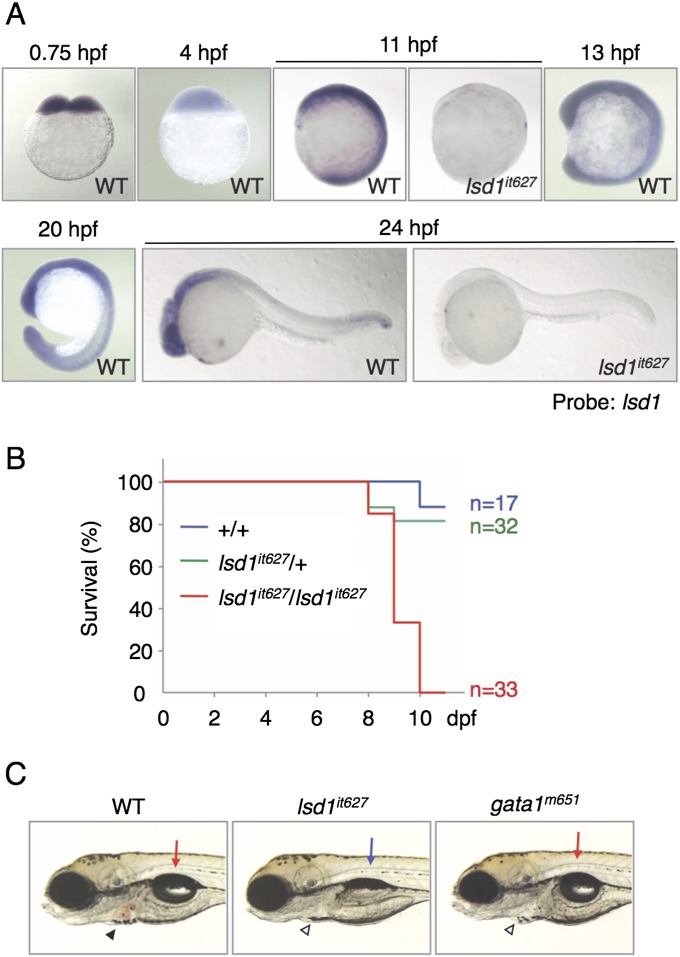
No obvious morphological defects, except for blood circulation, were observed in lsd1it627 embryos before hatching [2.5 d postfertilization (dpf)]. It was surprising to find that the obvious phenotype of lsd1it627 embryos was the only defect in hematopoiesis. This behavior is in contrast to LSD1 KO mice, which die around 6.5–7.5 d postcoitum, corresponding to the developmental stage of about 10 hpf in zebrafish (29–31). One possible explanation for this discrepancy between zebrafish and mice may be the differences in the expression profiles of LSD1 genes. To test this possibility, the expression of lsd1 was analyzed using WT and lsd1it627 mutant embryos (Fig. S2A). We found that lsd1 was expressed maternally in the early-stage embryos, which may be the reason for the phenotypic differences of LSD1-deficient animals between zebrafish and mice, because it was later ubiquitously expressed in every stage. Interestingly, the zygotic expression of lsd1 was greatly down-regulated in lsd1it627 mutants, suggesting that lsd1 positively regulates itself. Taking this observation into account, along with the low enzymatic activity and protein instability of the it627-type LSD1ΔC609 protein, lsd1it627, it was deduced to be a null mutation of lsd1 in zebrafish.
Fig. S2.
Nonhematopoietic defects in lsd1it627 embryos. (A) Expression of lsd1 at the indicated developmental stages was analyzed in WT or lsd1it627 embryos. Ubiquitous expression of lsd1 in zebrafish embryos was impaired in lsd1it627 mutants. (B) Survival rates of lsd1it627 mutants calculated using the Kaplan–Meier method. (C) Results of a comparison of the phenotypes between lsd1it627 and gata1m651 mutants at 4 dpf. The arrows and arrowheads indicate the swim bladder and erythrocytes in the heart, respectively. The presence of pale erythrocytes due to erythropoietic defects was detected in both lsd1it627 and gata1m651 mutants, whereas defects in the swim bladder were only observed in lsd1it627 mutants.
Because the dispensability of LSD1 in zebrafish embryonic stages could be explained by its maternal expression, the viability of lsd1it627 mutants was next investigated in later larval stages (Fig. S2B). Homozygous mutants died around 10 dpf, indicating that lsd1 is an essential gene required for zebrafish development. Interestingly, the swim bladder of lsd1it627 larvae failed to inflate, whereas the swim bladder of a bloodless mutant, vlad tepes (gata1m651), was normal, as observed in WT larvae (Fig. S2C), thus suggesting that there are other nonhematopoietic functions of LSD1 in the larval stage.
Erythropoiesis and Ectopic Myelopoiesis Are Impaired in LSD1 Mutants.
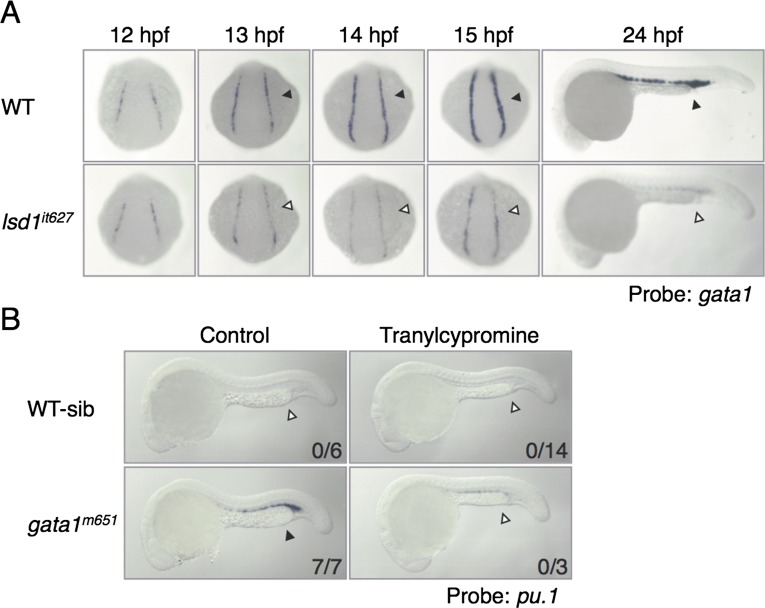
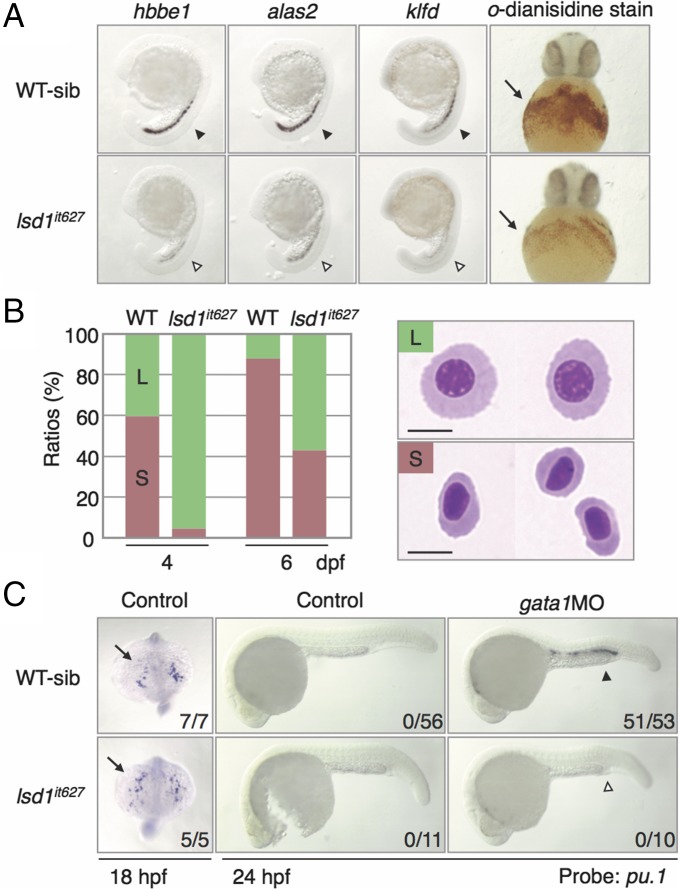
We next analyzed the detailed gata1 expression in lsd1it627 embryos (Fig. S3A). In WT embryos, the expression of gata1 in the posterior LPM was initiated around 11 hpf, and its level was up-regulated until 15 hpf. In lsd1it627 embryos, the gata1 expression at 12 hpf was similar to the gata1 expression in WT siblings, but its up-regulation after 13 hpf was impaired, suggesting that LSD1 is not required for the initiation of gata1 but is required for its up-regulation. To examine whether defects of gata1 up-regulation by the lsd1 mutation affect the expression of Gata1 downstream genes (16, 32, 33), the expression of hbbe1, alas2, and klfd was analyzed and demonstrated to be greatly reduced in lsd1it627 embryos (Fig. 2A). Cell staining with o-dianisidine, which reflects hemoglobin production, revealed that there were fewer o-dianisidine–positive cells in lsd1it627 embryos in comparison to the WT siblings (Fig. 2A).
Fig. S3.
Defects in the expression of hematopoietic markers by lsd1 down-regulation. (A) Defects in gata1 up-regulation in lsd1it627 embryos. The gata1 expression at the indicated developmental stages (arrowheads) was analyzed in WT or lsd1it627 embryos. (B) Effects of tranylcypromine on ectopic pu.1 expression in gata1m651 mutants. The embryos were treated with 300 μM tranylcypromine from 12 to 24 hpf. Arrowheads indicate ectopic pu.1 expression.
Fig. 2.
Hematopoietic defects in lsd1it627 embryos. (A) Expression of the Gata1 downstream genes at 20 hpf (arrowheads) and o-dianisidine staining in the blood islands at 36 hpf (arrows). The same expression profile was seen in both WT and heterozygous mutants (WT-sib), although expression in homozygous mutants (lsd1it627) was markedly diminished. hbbe1, hemoglobin beta embryonic 1; klfd, krüppel-like transcription factor d. (B) Wright–Giemsa staining of cytospin preparations of circulating blood cells. Green (L) and red (S) bars indicate large immature erythroblasts and small mature erythrocytes, respectively. (Scale bars, 5 μm.) (C) Expression of pu.1 at 18 hpf (anterior-dorsal views) or 24 hpf (lateral views) in WT or lsd1it627 embryos injected or not injected with 0.5 pmol of gata1MO. The arrows and arrowheads denote the anterior LPM and ICM, respectively. Numbers indicate the percentage of embryos exhibiting strong expression of indicated genes. The ectopic pu.1 expression in the ICM (closed arrowhead) induced by gata1MO injection was down-regulated in lsd1it627 embryos (open arrowhead).
In WT embryos, blood circulation initiates around 27 hpf, whereas in lsd1it627embryos, it began after 30 hpf. The number of circulating blood cells was dramatically lower in lsd1it627 embryos at 2 dpf in comparison to WT embryos (Movies S1 and S2). The difference in cell numbers became less apparent at 4 dpf, whereas the shapes of blood cells seem to be distinct (Movies S3 and S4). To examine the morphology of the blood cells, we isolated circulating blood cells (Fig. 2B). In larvae at 4 dpf, 40% of the WT cells were large immature erythroblasts and 60% were small mature erythrocytes, whereas almost all of them were large in the lsd1it627 larvae. Later at 6 dpf, 40% of the cells isolated from lsd1it627 larvae became mature erythrocytes, but the rest were still immature (Fig. 2B and Movies S5 and S6). All of these results indicate that the larvae with the LSD1 mutation have defects in the differentiation of erythroid cells.
In contrast to erythropoietic markers, no differences were observed between the WT and lsd1it627 embryos in the expression of myelopoietic markers, such as pu.1 (Fig. 2C, Left). In zebrafish, it has been demonstrated that the down-regulation of gata1 by gene mutations or gene knockdown reprograms the fate of the erythroid lineage to a myeloid lineage via the up-regulation of pu.1 (33, 34). Because gata1 was significantly down-regulated in lsd1it627 embryos, ectopic pu.1 expression in the ICM can be induced, but this event did not happen (Fig. 2C, Middle).
We thought that the level of gata1 down-regulation in lsd1it627 embryos might have been insufficient to induce ectopic pu.1 expression and injected a gata1-specific antisense morpholino oligonucleotide (gata1MO, 0.5 pmol) into the embryos to reduce gata1 expression further (Fig. 2C, Right). Unexpectedly, gata1MO-induced ectopic pu.1 expression in the ICM was only observed in WT siblings and not in lsd1it627 embryos, thus suggesting that ectopic myelopoiesis was impaired by the LSD1 mutation. To confirm this observation, we used gata1 mutant embryos (gata1m621) and an LSD1 inhibitor, tranylcypromine, instead of the gata1MO injection and lsd1it627 mutation, respectively, and compared the effects (Fig. S3B). Again, ectopic pu.1 expression was induced in gata1m621 embryos, whereas induction was abrogated when the embryos were treated with tranylcypromine. These results suggest that hematopoietic defects brought on by the lsd1it627 mutation could not be fully explained by the down-regulation of gata1.
Up-Regulation of etv2 and Endothelial Markers in LSD1 Mutants.
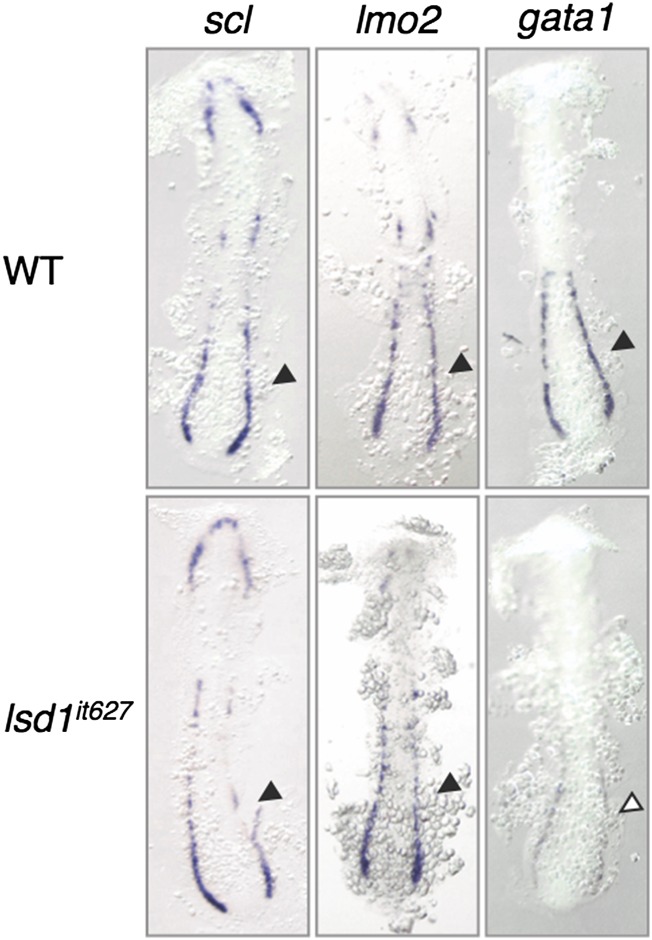
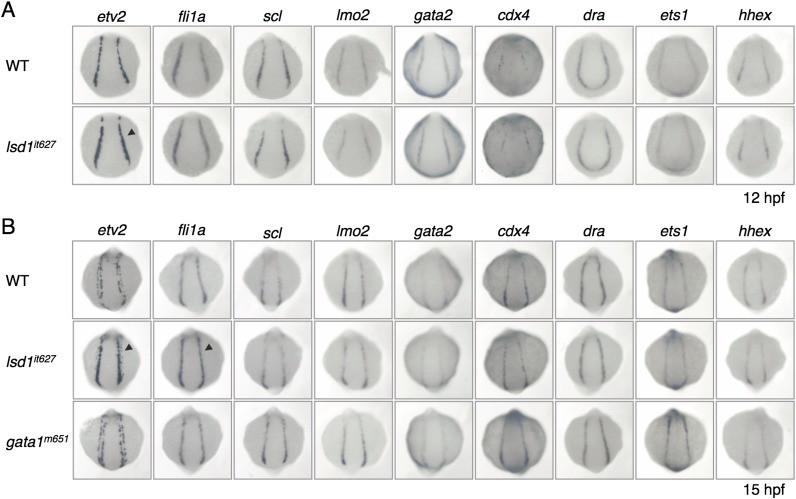
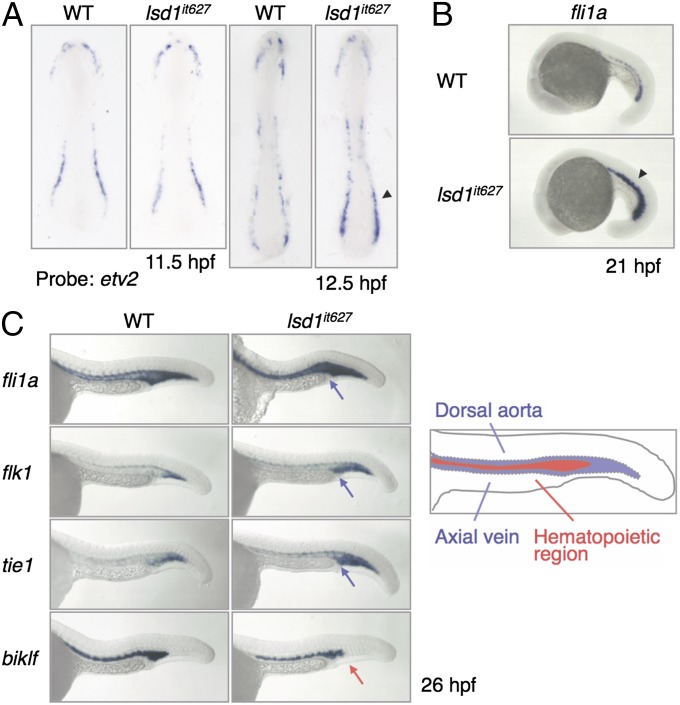
The presence of defects in both erythropoiesis and myelopoiesis suggests that the generation of common hematopoietic progenitors, such as hemangioblasts, may be impaired by the LSD1 mutation. We therefore analyzed the expression profiles of two hemangioblast markers, scl and lmo2, but no differences were observed between WT and lsd1it627 embryos (Fig. S4). This result implies that the LSD1 mutation has no effect on hemangioblast formation. To confirm this assumption, the expression of a variety of hemangioblast markers was analyzed using embryos at 12 hpf when hemangioblasts were forming (Fig. S5A). The expression of etv2 was slightly up-regulated in lsd1it627 embryos compared with WT embryos, although none of the other markers (fli1a, scl, lmo2, gata2, cdx4, draculin, ets1, and hhex) was affected. The up-regulation of etv2 was not observed in 11.5-hpf embryos, suggesting that LSD1 is not involved in the initiation of etv2 expression (Fig. 3A). Interestingly, up-regulation was only observed in the posterior (and not anterior) LPM (Fig. 3A).
Fig. S4.
Expression of scl and lmo2 in lsd1it627 embryos. The expression of scl, lmo2, and gata1 at 15 hpf in WT or lsd1it627 embryos is shown. Arrowheads indicate the posterior LPM.
Fig. S5.
Expression of hemangioblast markers in lsd1it627 embryos. The expression of the indicated hemangioblast markers at 12 hpf (A) and 15 hpf (B) in WT, lsd1it627, or gata1m651 embryos is shown. The arrowheads indicate up-regulation of etv2 and fli1a in the posterior LPM of lsd1it627 embryos. cdx4, caudal type homeobox 4; dra, draculin; ets1, E26 transformation-specific 1; hhex, hematopoietically expressed homeobox.
Fig. 3.
Up-regulation of endothelial markers in lsd1it627 embryos. (A) Expression of etv2 at the indicated developmental stages in WT or lsd1it627 embryos. The arrowhead indicates the up-regulation of etv2 in the posterior LPM. (B) Expression of fli1a at 21 hpf in WT or lsd1it627 embryos. The arrowhead indicates the up-regulation of fli1a in the ICM. (C) Expression of fli1a, flk1, tie1, and biklf at 26 hpf in the trunk region. The blue and red arrows indicate up-regulation of endothelial markers and down-regulation of a hematopoietic marker, respectively, in the hematopoietic region. (Right) Diagram of the hematopoietic and endothelial regions in WT embryos.
We also analyzed the expression of hemangioblast markers in 15-hpf embryos and observed enhanced expression of fli1a, in addition to etv2, in lsd1it627 embryos, although there were again no differences in the other markers (Fig. S5B). Because etv2 has been shown to regulate the expression of endothelial genes positively (35–37), the expression of fli1a and other endothelial markers (flk1 and tie1) was analyzed at later developmental stages (Fig. 3 B and C and Fig. S6). As expected, the expression levels of all three endothelial markers were up-regulated in lsd1it627 embryos. It was noted that the expression of these markers in the hematopoietic region at 24 hpf was decreased in WT embryos but not in lsd1it627 mutants (Fig. 3C, blue arrows). Instead, the hematopoietic marker biklf was down-regulated in this subregion (Fig. 3C, red arrow).
Fig. S6.
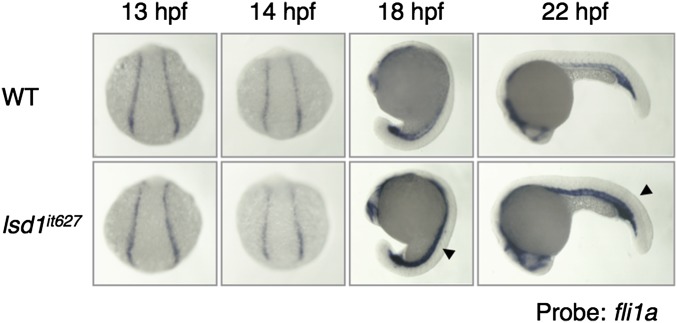
Expression of fli1a in lsd1it627 embryos. The expression of fli1a at the indicated developmental stages in WT or lsd1it627 embryos is shown. It is noted that no difference was observed between WT and lsd1it627 embryos before 15 hpf. The arrowheads indicate the up-regulation of fli1a in the ICM of lsd1it627 embryos.
To answer the question of whether excess endothelialization occurs in LSD1 mutants, we crossed the lsd1it627 mutant with the Tg(kdrl:EGFP)s843 line, which can visualize vasculogenesis (38), and analyzed the GFP expression in progeny lines. No obvious difference in GFP expression was observed between lsd1it627 mutants and WT embryos (Fig. S7), suggesting that the up-regulation of endothelial markers did not lead to excess endothelialization. Considering these results, together with the previous finding that the zebrafish Etv2 was a positive regulator of endothelial and anterior hematopoietic development (35, 39, 40), we speculated that the hematopoietic cells in the ICM region of lsd1it627 mutants were maintained in an undifferentiated state with endothelial characteristics.
Fig. S7.
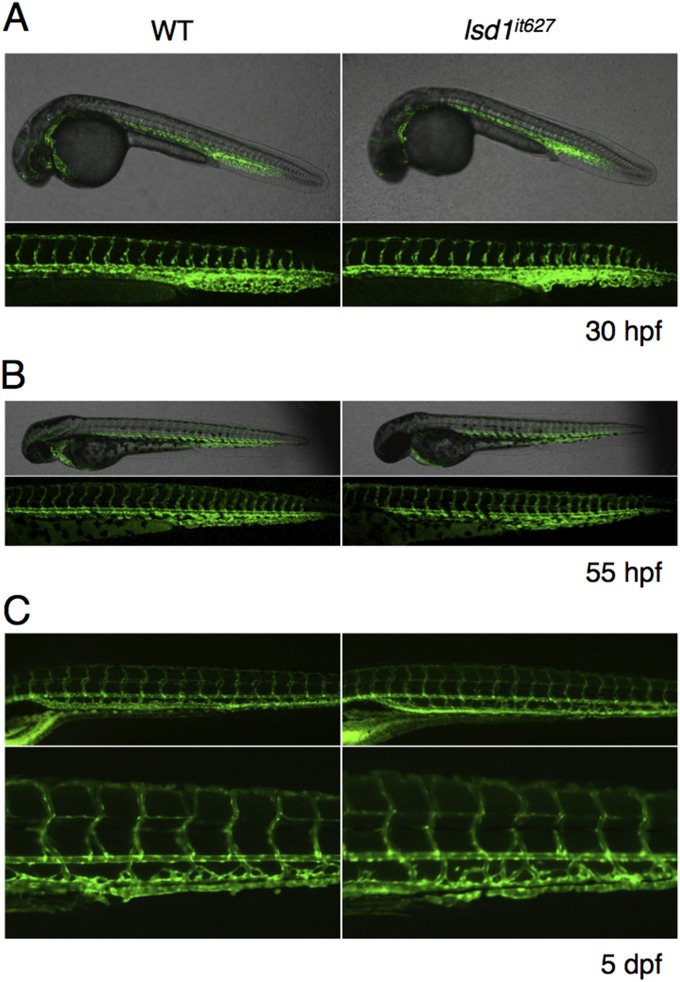
Effects of the lsd1 mutation on angiogenesis and vascular morphogenesis. Confocal images of Tg(kdrl:EGFP)s843 transgenic WT or lsd1it627 embryos at 30 hpf (A) and 55 hpf (B). (C) Fluorescent images of Tg(kdrl:EGFP)s843 transgenic WT or lsd1it627 embryos at 5 dpf.
Knockdown of the Etv2 Gene Can Rescue the Hematopoietic Phenotypes of LSD1 Mutants.
The earliest difference between WT and lsd1it627 mutants in the expression of hematopoietic/endothelial markers was etv2 at 12 hpf (Fig. S5A) and then gata1 at 13 hpf (Fig. S3A). The fli1a up-regulation in lsd1it627 mutants became obvious at 15 hpf, which was later compared with these genes (Figs. S5 and S6). To elucidate the relationship between these three genes, the expression of etv2 and fli1a was first analyzed in gata1m621 embryos (Fig. S5B). No effect by the gata1 mutation on the expression of either gene was observed, suggesting that the up-regulation of etv2 and fli1a in lsd1it627 embryos was not due to the gata1 reduction. Second, we examined the effects of the etv2 knockdown on the expression of gata1 and fli1a in lsd1it627 embryos. The reduced expression of gata1 in lsd1it627 embryos was rescued by injecting antisense morpholino oligonucleotide against etv2 (etv2MO, 0.5 pmol), whereas this effect was eliminated when five-base mismatches were introduced into the etv2MO (0.5 pmol). Importantly, the etv2 knockdown up-regulated only the reduced gata1 expression in lsd1it627 embryos and not its normal expression in WT embryos. In contrast, the expression of fli1a was not altered by the etv2 knockdown (Fig. S8A), suggesting that etv2 is not the reason for the fli1a up-regulation, consistent with a previous report showing etv2-independent regulation of fli1a in the posterior LPM (41). Because Fli1 was known to act at the top of the transcriptional network establishing the blood and endothelial programs (42), derepression of fli1a may contribute to some phenotypes in lsd1it627 mutants, either independently or in combination with etv2.
Fig. S8.
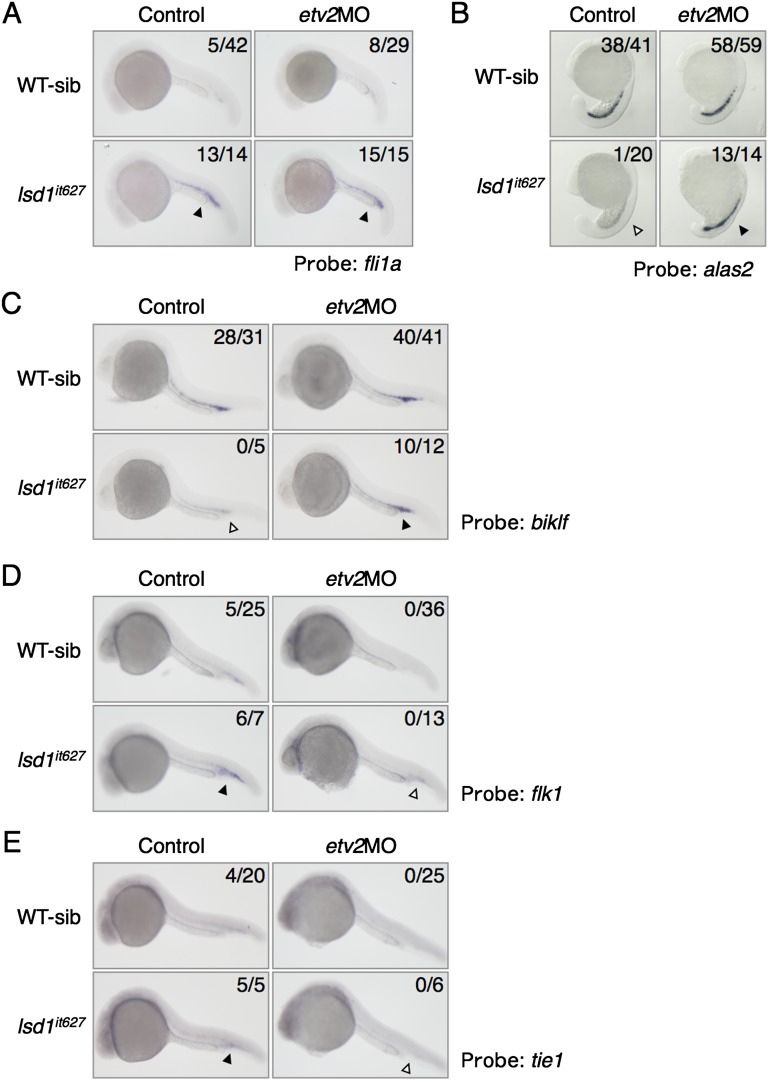
Expression of hematopoietic and endothelial cell markers in etv2 knockdown lsd1it627 embryos. The expression of fli1a (A), alas2 (B), biklf (C), flk1 (D), and tie1 (E) in WT or lsd1it627 embryos injected or not injected with 0.5 pmol of etv2MO is shown. The down-regulation of alas2 and biklf in lsd1it627 embryos (white arrowheads) was rescued by the etv2MO injection (black arrowheads). The up-regulation of flk1 and tie1, but not fli1a, in lsd1it627 embryos was rescued by the etv2MO injection (arrowheads).
To elucidate the effects of the etv2 knockdown on erythropoiesis, we analyzed the expression of alas2 and biklf and found that the reduced expression of these genes in lsd1it627 embryos was rescued by etv2MO injection (Fig. S8 B and C). To clarify the effects of the etv2 knockdown on myelopoiesis, the ectopic pu.1 expression in gata1-etv2 double-knockdown lsd1it627 embryos was analyzed (Fig. 4B). The defects of ectopic pu.1 expression in gata1MO-injected lsd1it627 embryos (Fig. 4B, open arrowhead) were rescued by coinjecting etv2MO (Fig. 4B, closed arrowhead). We also tested the effects of the etv2 knockdown on the endothelial markers flk1 and tie1 (Fig. S8 D and E) and showed that the up-regulated expression of these genes in lsd1it627 embryos was reduced by the etv2 knockdown. All of these results suggest that the up-regulation of etv2 is the principal cause of the hematopoietic defects and up-regulation of endothelial markers in lsd1it627 mutants.
Fig. 4.
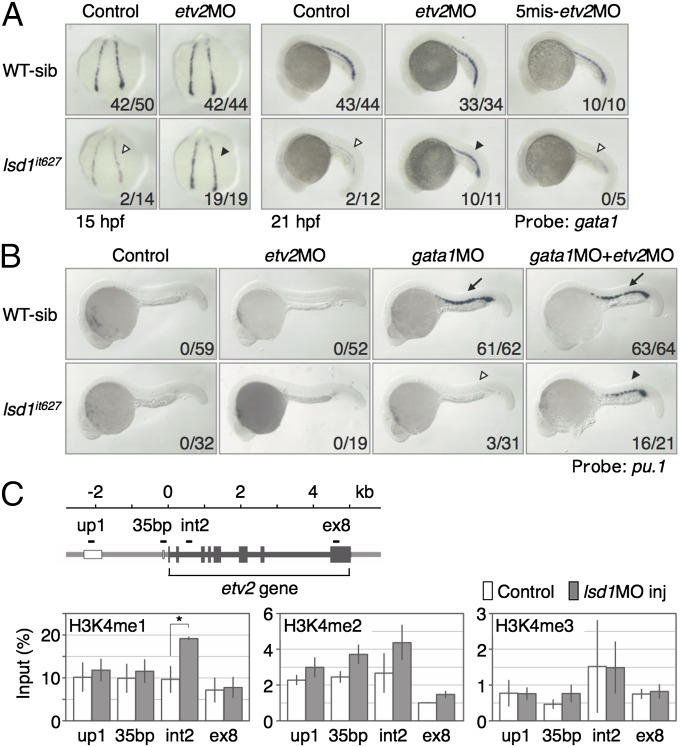
Rescue of the hematopoietic defects in lsd1it627 embryos by etv2 knockdown. (A) Expression of gata1 in WT or lsd1it627 embryos injected or not injected with 0.5 pmol of the indicated morpholino oligonucleotides. The down-regulation of gata1 in lsd1it627 embryos (open arrowheads) was rescued by the etv2MO injection (closed arrowheads). 5mis, 5-mismatch. (B) Expression of pu.1 at 24 hpf in WT or lsd1it627 embryos injected or not injected with 0.5 pmol of the indicated morpholino oligonucleotides. The gata1MO-induced ectopic pu.1 expression in the ICM (arrows) was lost in lsd1it627 embryos (open arrowhead) and recovered by the coinjection of etv2MO (closed arrowhead). (C) Methylation status of histone H3-K4 on the etv2 gene in 15-hpf embryos injected (gray bars) or not injected (white bars) with 1 pmol of lsd1MO (lsd1MO inj). Genomic regions (up1, int2, 35bp, ex8) examined by ChIP are shown as black bars. The results are means ± SEs of three independent experiments. *P < 0.05; Student’s t test.
To examine whether LSD1 modifies the in vivo methylation status of histone H3-K4 at the etv2 locus, ChIP analysis was carried out using LSD1 morphants (Fig. 4C). We analyzed histone methylation at three etv2 enhancers (up1, int2, and the 35-bp enhancer in the promoter), identified by Veldman and Lin (43), and at exon 8 (gene body). The results demonstrated that monomethylated histone H3-K4 at the int2 enhancer was significantly increased by LSD1 knockdown and dimethylated histone H3-K4 also showed a trend toward an increase at the 35-bp enhancer, suggesting that LSD1 directly down-regulates etv2 by histone modification.
Discussion
In this study, we isolated a zebrafish mutant that shows impaired hematopoiesis and identified that lsd1 was the responsible gene. Not only erythropoiesis but also ectopic myelopoiesis was diminished in the lsd1it627 mutants. The earliest hematopoietic/endothelial abnormality detected in the lsd1it627 embryos was the up-regulation of etv2 in hemangioblasts. The gene knockdown of etv2 rescued both the erythropoietic and myelopoietic defects in lsd1it627 embryos.
The importance of LSD1 in hematopoiesis has previously been shown using cultured cells (44–46), knockdown mice (47), and conditional KO mice (48). In addition, the contribution of LSD1 in leukemogenesis has been demonstrated, and the use of LSD1 inhibitors is being investigated as a new therapeutic strategy (49, 50). The roles of LSD1 in hematopoiesis are various, and include the differentiation switch between erythropoiesis and myelopoiesis (44), the terminal differentiation of some hematopoietic lineages (47, 48), maintenance of the undifferentiated state of plasma cells (46), globin switching (51–53), and the self-renewal of hematopoietic stem cells (48). Here, we unveiled new roles of LSD1, as a down-regulator of the endothelial characteristics and promoter of the hematopoietic commitment in hemangioblasts. Intriguingly, LSD1 seems to play many roles in hematopoiesis, probably binding to gene regulatory regions of key hematopoietic factors throughout the hematopoietic period and regulating them positively and/or negatively depending on the differentiation states. For its multifunctional roles, LSD1 may have a variety of hematopoietic partners (44–46, 52). Future studies will be needed to identify the LSD1 partners in hemangioblasts.
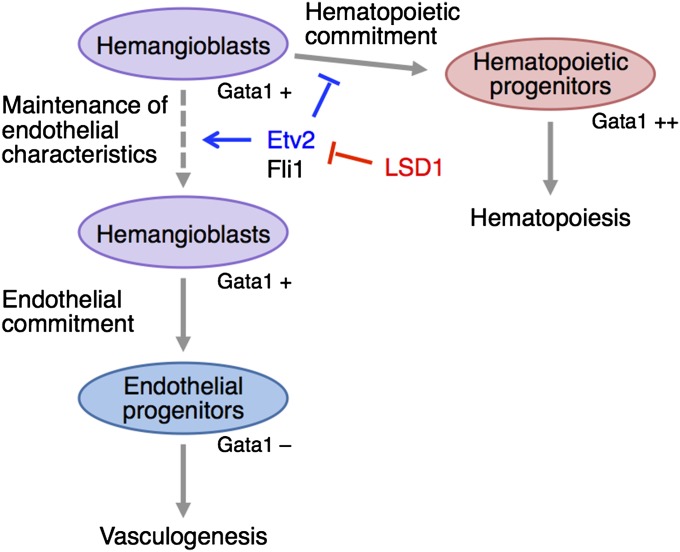
Another important finding of the present study is that the main role of LSD1 in hemangioblasts is the shutdown of Etv2 expression (Fig. S9). LSD1 may not directly transactivate the Gata1 gene but, rather, regulate the differentiation of weakly Gata1-positive hemangioblasts to generate strongly Gata1-positive hematopoietic progenitors. It is not easy to understand why the knockdown of etv2, which is essential for hematopoiesis rather than just endothelial development, can rescue the hematopoietic phenotypes of lsd1it627 embryos. Other important observations are that the LSD1 mutation reduced only posterior, and not anterior, hematopoiesis and that the up-regulation of etv2 was only detected in the posterior LPM (Fig. 3A). Previous reports demonstrated that etv2 plays no role in erythroid and posterior myeloid differentiation (39, 40), although it is strongly required for posterior endothelial development (36, 39). These findings, combined with our findings, indicate that the importance of the LSD1/Etv2 paradigm in hemangioblast differentiation may be restricted in the posterior hemangioblasts. We hypothesize that the Etv2 shutdown in hemangioblasts is an important process for hematopoiesis.
Fig. S9.
Model of the LSD1-Etv2 paradigm in hemangioblasts. Etv2 maintains the endothelial characteristics in hemangioblasts and inhibits the hematopoietic commitment. LSD1 negatively regulates the gene expression of Etv2 and promotes the initiation of hematopoietic differentiation.
It is interesting that the expression of mouse Etv2 was transiently detected in the beginning of hematopoiesis and was barely detectable in hematopoietic tissues at later stages, such as the aorta-gonad-mesonephros and fetal liver in embryos and in the bone marrow and spleen in adults (54–56). A dramatic down-regulation of Etv2 during hematopoietic differentiation is also observed in zebrafish (35) and Xenopus (57). The importance of the Etv2 shutdown in hematopoiesis has been experimentally demonstrated in mice (58). When Etv2 was persistently expressed in hematopoietic tissues using transgenic mouse techniques, abnormal hematopoiesis, especially erythropoiesis, occurred in both primitive and definitive hematopoiesis. Hayashi et al. (58) have proposed a model wherein persistent endothelialization due to forced Etv2 expression inhibited hematopoiesis. Their model fits well with our current results in zebrafish. It is well known that Etv2 overexpression also results in endothelialization in zebrafish (35, 37, 42, 59) and Xenopus (57). All of these observations suggest that Etv2 may play a conserved role in maintaining the endothelial characteristics of hemangioblasts in the early developmental periods.
Hematopoietic cells in lsd1it627 mutants may change their fate to become endothelial cells. However, this possibility is considered to be unlikely, because no ectopic or thickened blood vessels were detected in this mutant (Fig. S7). Instead, we believe that the differentiation of hemangioblasts to hematopoietic cells may be suspended, and, as a result, immature hemangioblasts that possess endothelial characteristics are accumulated. LSD1 has been proposed to be involved in shutting down programs of ES cells through enhancer decommission and to promote cell differentiation (60). A similar situation may occur in hemangioblasts. As observed for Sox2 or Fbox15 in the case of ES cells, LSD1 may also shut down Etv2 in hemangioblasts.
Materials and Methods
Fish and Chemicals.
AB and Tupfel long fin (TL) strains were used as WT zebrafish. The Tg(kdrl:EGFP)s843 transgenic line was described previously (38). For genotyping lsd1it627, genomic DNA at the mutation sites was amplified by PCR using the primers 5′-ctggcagaggagggacaaactgcacggccggcggcagct and 5′-gcagtcatctgactgtgcg, and was digested with PvuII. Genotyping of gata1m651 was carried out as described previously (16). Tranylcypromine was purchased from Enzo Life Sciences.
N-Ethyl-N-Nitrosourea Mutagenesis.
N-ethyl-N-nitrosourea mutagenesis was carried out as described previously (61). Seven hundred sixteen F2 families were screened, and four mutants with reduced gata1 expression were isolated. The gene locus of the it627 mutant was roughly mapped by a bulked-segregant analysis using 333 polymorphic Z-markers (62). Fine mapping with additional polymorphic markers was carried out using 1,656 homozygous mutant embryos in the AB/TL hybrid F1 generation. Polymorphic markers were designed using the database of the zebrafish genome project (www.ensembl.org/Danio_rerio/Info/Index).
cDNA Cloning.
Full-length cDNA of lsd1 was synthesized by RT-PCR assay and subcloned into the pBluescript II KS, pET15b, and pCS2FL (63) vectors. The constructs pET15lsd1ΔC609 and pCS2FLlsd1ΔC609 were made by introducing a Gln-to-Stop point mutation at amino acid 609. The nucleotide sequence data of zebrafish lsd1 have been deposited in the DNA Data Base in Japan/European Molecular Biology Laboratory/GenBank databases (accession no. AB494456).
Staining and Imaging Analysis.
Whole-mount in situ hybridization was performed using digoxigenin-labeled RNA probes as described previously (64). The o-dianisidine staining was performed as described previously (22). Blood cells for Wright–Giemsa staining were collected from embryos after cardiac puncture, followed by cytospin concentration at 700 rpm for 3 min using a Cytospin 4 (Thermo Scientific). Fluorescent images were taken with a Leica SP5 confocal or Leica AF6000 epifluorescence microscope. Genotyping of the stained embryos was carried out after taking photographs.
Demethylation Assay.
A histone demethylation assay was carried out basically as described previously (23). His-tagged LSD1 proteins were expressed in Escherichia coli strain BL21(DE3) and purified using a nickel-nitrilotriacetic acid affinity column (Qiagen). Purified proteins were incubated in a reaction buffer [100 mM Tris (pH 8.5), 40 mM KCl, 20 mM MgCl2, 20 mM mercaptoethanol, 5% (vol/vol) glycerol] with bulk histones from calf thymus (Sigma–Aldrich) at 37 °C for 12 h, and they were then assessed by an immunoblot analysis using specific antibodies: anti-monoMeK4H3 (ab8895; Abcam), anti-diMeK4H3 (UP07-030; Upstate), anti-monoMeK9H3 (UP07-450; Upstate), anti-diMeK9H3 (UP07-441; Upstate), and anti-histone H3 (ab1791; Abcam).
Microinjection.
Morpholino oligonucleotides or capped mRNAs were injected into one-cell-stage embryos using an IM300 microinjector (Narishige) as described previously (65). The nucleotide sequences of the morpholino oligonucleotides (Gene Tools) were 5′-gttattcacaccttgttgagatttc [lsd1 morpholino oligonucleotide (lsd1MO)], 5′-gttattgacacgttcttcagatatc (5mis-lsd1MO), 5′-ctgcaagtgtagtattgaagatgtc (gata1MO) (33), 5′-ttggtacatttccatatcttaaagt (etv2MO) (35), and 5′-ttgctagatttcgatatgttaaact (5mis-etv2MO). The lsd1 mRNA was transcribed from linearized pCS2 plasmids using the mMESSAGE mMACHINE kit (Ambion). Overexpressed proteins were analyzed by immunoblotting using anti-FLAG antibody (Sigma–Aldrich) as described previously (64).
ChIP.
ChIP analysis was basically carried out as described previously (66, 67) using specific antibodies: anti-monoMeK4H3 (ab8895; Abcam), anti-diMeK4H3 (UP07-030; Upstate), and anti-triMeK4H3 (ab8580; Abcam). Two hundred fifty embryos were used for each assay, and cells were sonicated by a Branson Sonifier 250. Quantitative PCR analysis was performed using a 7500 Fast Real-Time PCR System (Life Technologies), FastStart Universal SYBR Green Master (Roche), and the specific primers listed in Table S1.
Table S1.
Oligonucleotide primers for ChIP analysis
| Target regions | Names | Sequences |
| up1 enhancer | etv2.up1.f1 | 5′-TCGCACAGTTTGGCAACTTAC |
| etv2.up1.r1 | 5′-AGTGCTTTGGACAGATAAGACC | |
| 35-bp enhancer | etv2.35bp.f1 | 5′-AATCTGTGGTCGTGACTCCTG |
| etv2.35bp.r1 | 5′-GTCTGTGCTGGACTGAGGAC | |
| int2 enhancer | etv2.int2.f2 | 5′-AACATTTCCGCTGTCCTTGAG |
| etv2.int2.r2 | 5′-GACATCCCATTCCTAAATCTCAG | |
| exon 8 | etv2.exon8.f1 | 5′-ACGTCTACCGCTTTGTCTGTG |
| etv2.exon8.r1 | 5′-GCTTGTGTTTGCTACAGACTG |
Supplementary Material
Acknowledgments
We thank Y. Kametani, S. Takada, and D. Y. Stainier for the kind gift of the Tg(kdrl:EGFP)s843 line; the Sakura Motion Picture Co. Ltd for their assistance with the movies; H. Kato at Leica Microsystems for the confocal microscopy; and M. Eguchi, M. Komeda, Y. Nakayama, and Y. Wada for help with the mutant screening and fish maintenance. We also thank R. Patient for valuable suggestions; H. Daitoku, A. Fukamizu, A. Fukuda, M. Hibi, H. Hirata, K. Hisatake, A. Kawakami, Y. Kishimoto, S. Koshida, M. Nagano, Y. Nakajima, R. Nakamura, M. Okuwaki, R. Shimizu, H. Takeda, K. Yamagata, and M. Yonezawa for technical advice; A. Hirano, L. Li, M. Miyamoto, K. Mukaigasa, Y. Nakajima-Takagi, K. Nishikawa, and T. Tsujita for experimental help and discussions; and K. Igarashi, A. Kawahara, and K. Ohneda for critical reading of the manuscript. This work was supported by grants-in-aid from the Japan Society for the Promotion of Science (to Miki Takeuchi) and the Japan Science and Technology Corporation (Exploratory Research for Advanced Technology) (to M.Y.), and by Grants 20052007, 24116504, 25118705, and 26116705 from the Ministry of Education, Science, Sports and Culture of Japan (to M.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The nucleotide sequence data of zebrafish lsd1 have been deposited in the DNA Data Base in Japan/European Molecular Biology Laboratory/GenBank databases (accession no. AB494456).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517326112/-/DCSupplemental.
References
- 1.Orkin SH, Zon LI. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132(4):631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lancrin C, et al. Blood cell generation from the hemangioblast. J Mol Med (Berl) 2010;88(2):167–172. doi: 10.1007/s00109-009-0554-0. [DOI] [PubMed] [Google Scholar]
- 3.Ciau-Uitz A, Monteiro R, Kirmizitas A, Patient R. Developmental hematopoiesis: Ontogeny, genetic programming and conservation. Exp Hematol. 2014;42(8):669–683. doi: 10.1016/j.exphem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Shalaby F, et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89(6):981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 5.Stainier DY, Weinstein BM, Detrich HW, 3rd, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121(10):3141–3150. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- 6.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125(4):725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125(9):1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 8.Zambidis ET, et al. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112(9):3601–3614. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432(7017):625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 10.Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443(7109):337–339. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z, et al. An adult uterine hemangioblast: Evidence for extramedullary self-renewal and clonal bilineage potential. Blood. 2010;116(16):2932–2941. doi: 10.1182/blood-2010-01-266882. [DOI] [PubMed] [Google Scholar]
- 12.Lancrin C, et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457(7231):892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457(7231):887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci USA. 1996;93(22):12355–12358. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi S, et al. Arrest in primitive erythroid cell development caused by promoter-specific disruption of the GATA-1 gene. J Biol Chem. 1997;272(19):12611–12615. doi: 10.1074/jbc.272.19.12611. [DOI] [PubMed] [Google Scholar]
- 16.Lyons SE, et al. A nonsense mutation in zebrafish gata1 causes the bloodless phenotype in vlad tepes. Proc Natl Acad Sci USA. 2002;99(8):5454–5459. doi: 10.1073/pnas.082695299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimoto T, et al. Step-wise divergence of primitive and definitive haematopoietic and endothelial cell lineages during embryonic stem cell differentiation. Genes Cells. 2001;6(12):1113–1127. doi: 10.1046/j.1365-2443.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Yamamoto M. Regulation of GATA1 gene expression. J Biochem. 2007;142(1):1–10. doi: 10.1093/jb/mvm122. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol Cell. 2007;25(1):1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Nottke A, Colaiácovo MP, Shi Y. Developmental roles of the histone lysine demethylases. Development. 2009;136(6):879–889. doi: 10.1242/dev.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lammerts van Bueren K, Black BL. Regulation of endothelial and hematopoietic development by the ETS transcription factor Etv2. Curr Opin Hematol. 2012;19(3):199–205. doi: 10.1097/MOH.0b013e3283523e07. [DOI] [PubMed] [Google Scholar]
- 22.Detrich HW, 3rd, et al. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci USA. 1995;92(23):10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, Ma H. Evolutionary history of histone demethylase families: Distinct evolutionary patterns suggest functional divergence. BMC Evol Biol. 2008;8:294. doi: 10.1186/1471-2148-8-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi YJ, et al. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19(6):857–864. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Rudolph T, et al. Heterochromatin formation in Drosophila is initiated through active removal of H3K4 methylation by the LSD1 homolog SU(VAR)3-3. Mol Cell. 2007;26(1):103–115. doi: 10.1016/j.molcel.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 27.Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem Biol. 2006;13(6):563–567. doi: 10.1016/j.chembiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Forneris F, Binda C, Battaglioli E, Mattevi A. LSD1: Oxidative chemistry for multifaceted functions in chromatin regulation. Trends Biochem Sci. 2008;33(4):181–189. doi: 10.1016/j.tibs.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, et al. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature. 2007;446(7138):882–887. doi: 10.1038/nature05671. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, et al. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat Genet. 2009;41(1):125–129. doi: 10.1038/ng.268. [DOI] [PubMed] [Google Scholar]
- 31.Foster CT, et al. Lysine-specific demethylase 1 regulates the embryonic transcriptome and CoREST stability. Mol Cell Biol. 2010;30(20):4851–4863. doi: 10.1128/MCB.00521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishikawa K, et al. Self-association of Gata1 enhances transcriptional activity in vivo in zebra fish embryos. Mol Cell Biol. 2003;23(22):8295–8305. doi: 10.1128/MCB.23.22.8295-8305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galloway JL, Wingert RA, Thisse C, Thisse B, Zon LI. Loss of gata1 but not gata2 converts erythropoiesis to myelopoiesis in zebrafish embryos. Dev Cell. 2005;8(1):109–116. doi: 10.1016/j.devcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Rhodes J, et al. Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev Cell. 2005;8(1):97–108. doi: 10.1016/j.devcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4(1):e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pham VN, et al. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol. 2007;303(2):772–783. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong KS, Proulx K, Rost MS, Sumanas S. Identification of vasculature-specific genes by microarray analysis of Etsrp/Etv2 overexpressing zebrafish embryos. Dev Dyn. 2009;238(7):1836–1850. doi: 10.1002/dvdy.21990. [DOI] [PubMed] [Google Scholar]
- 38.Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132(23):5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- 39.Liu F, Patient R. Genome-wide analysis of the zebrafish ETS family identifies three genes required for hemangioblast differentiation or angiogenesis. Circ Res. 2008;103(10):1147–1154. doi: 10.1161/CIRCRESAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- 40.Glenn NO, et al. Distinct regulation of the anterior and posterior myeloperoxidase expression by Etv2 and Gata1 during primitive Granulopoiesis in zebrafish. Dev Biol. 2014;393(1):149–159. doi: 10.1016/j.ydbio.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu F, et al. Enhanced hemangioblast generation and improved vascular repair and regeneration from embryonic stem cells by defined transcription factors. Stem Cell Rep. 2013;1(2):166–182. doi: 10.1016/j.stemcr.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol. 2008;18(16):1234–1240. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 43.Veldman MB, Lin S. Etsrp/Etv2 is directly regulated by Foxc1a/b in the zebrafish angioblast. Circ Res. 2012;110(2):220–229. doi: 10.1161/CIRCRESAHA.111.251298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saleque S, Kim J, Rooke HM, Orkin SH. Epigenetic regulation of hematopoietic differentiation by Gfi-1 and Gfi-1b is mediated by the cofactors CoREST and LSD1. Mol Cell. 2007;27(4):562–572. doi: 10.1016/j.molcel.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 45.Hu X, et al. LSD1-mediated epigenetic modification is required for TAL1 function and hematopoiesis. Proc Natl Acad Sci USA. 2009;106(25):10141–10146. doi: 10.1073/pnas.0900437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su ST, et al. Involvement of histone demethylase LSD1 in Blimp-1-mediated gene repression during plasma cell differentiation. Mol Cell Biol. 2009;29(6):1421–1431. doi: 10.1128/MCB.01158-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sprüssel A, et al. Lysine-specific demethylase 1 restricts hematopoietic progenitor proliferation and is essential for terminal differentiation. Leukemia. 2012;26(9):2039–2051. doi: 10.1038/leu.2012.157. [DOI] [PubMed] [Google Scholar]
- 48.Kerenyi MA, et al. Histone demethylase Lsd1 represses hematopoietic stem and progenitor cell signatures during blood cell maturation. eLife. 2013;2:e00633. doi: 10.7554/eLife.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris WJ, et al. The histone demethylase KDM1A sustains the oncogenic potential of MLL-AF9 leukemia stem cells. Cancer Cell. 2012;21(4):473–487. doi: 10.1016/j.ccr.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Schenk T, et al. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat Med. 2012;18(4):605–611. doi: 10.1038/nm.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi L, Cui S, Engel JD, Tanabe O. Lysine-specific demethylase 1 is a therapeutic target for fetal hemoglobin induction. Nat Med. 2013;19(3):291–294. doi: 10.1038/nm.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu J, et al. Corepressor-dependent silencing of fetal hemoglobin expression by BCL11A. Proc Natl Acad Sci USA. 2013;110(16):6518–6523. doi: 10.1073/pnas.1303976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui S, et al. The LSD1 inhibitor RN-1 induces fetal hemoglobin synthesis and reduces disease pathology in sickle cell mice. Blood. 2015;126(3):386–396. doi: 10.1182/blood-2015-02-626259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee D, et al. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2(5):497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kataoka H, et al. Etv2/ER71 induces vascular mesoderm from Flk1+PDGFRα+ primitive mesoderm. Blood. 2011;118(26):6975–6986. doi: 10.1182/blood-2011-05-352658. [DOI] [PubMed] [Google Scholar]
- 56.Wareing S, Eliades A, Lacaud G, Kouskoff V. ETV2 expression marks blood and endothelium precursors, including hemogenic endothelium, at the onset of blood development. Dev Dyn. 2012;241(9):1454–1464. doi: 10.1002/dvdy.23825. [DOI] [PubMed] [Google Scholar]
- 57.Salanga MC, Meadows SM, Myers CT, Krieg PA. ETS family protein ETV2 is required for initiation of the endothelial lineage but not the hematopoietic lineage in the Xenopus embryo. Dev Dyn. 2010;239(4):1178–1187. doi: 10.1002/dvdy.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayashi M, et al. Endothelialization and altered hematopoiesis by persistent Etv2 expression in mice. Exp Hematol. 2012;40(9):738–750.e11. doi: 10.1016/j.exphem.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Veldman MB, et al. Transdifferentiation of fast skeletal muscle into functional endothelium in vivo by transcription factor Etv2. PLoS Biol. 2013;11(6):e1001590. doi: 10.1371/journal.pbio.1001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whyte WA, et al. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature. 2012;482(7384):221–225. doi: 10.1038/nature10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi M, et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29(2):493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimoda N, et al. Zebrafish genetic map with 2000 microsatellite markers. Genomics. 1999;58(3):219–232. doi: 10.1006/geno.1999.5824. [DOI] [PubMed] [Google Scholar]
- 63.Li L, et al. Molecular evolution of Keap1. Two Keap1 molecules with distinctive intervening region structures are conserved among fish. J Biol Chem. 2008;283(6):3248–3255. doi: 10.1074/jbc.M708702200. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi M, Nishikawa K, Suzuki T, Yamamoto M. The homeobox protein Six3 interacts with the Groucho corepressor and acts as a transcriptional repressor in eye and forebrain formation. Dev Biol. 2001;232(2):315–326. doi: 10.1006/dbio.2001.0185. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi M, et al. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7(8):807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 66.Lindeman LC, Vogt-Kielland LT, Aleström P, Collas P. Fish’n ChIPs: Chromatin immunoprecipitation in the zebrafish embryo. Methods Mol Biol. 2009;567:75–86. doi: 10.1007/978-1-60327-414-2_5. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura R, et al. Large hypomethylated domains serve as strong repressive machinery for key developmental genes in vertebrates. Development. 2014;141(13):2568–2580. doi: 10.1242/dev.108548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.