Significance
Understanding how a protein sequence folds and orients in a lipid bilayer is central to establishing the molecular basis for membrane protein organization. How lipid environment affects membrane protein organization is understudied. We established that membrane protein orientation is dynamic during and after assembly, dependent on membrane lipid composition, and independent of other cellular factors. We developed a proteoliposome system in which lipid composition can be controlled before and after membrane protein reconstitution and used it to assess the kinetics of changes in transmembrane domain (TMD) orientation and phospholipid flipping within the lipid bilayer triggered by a change in lipid composition. We demonstrate that membrane proteins can undergo rapid postassembly TMD flipping in response to changes in the lipid environment.
Keywords: membrane protein, topology, lipid–protein interactions, phospholipids, real-time FRET
Abstract
A fundamental objective in membrane biology is to understand and predict how a protein sequence folds and orients in a lipid bilayer. Establishing the principles governing membrane protein folding is central to understanding the molecular basis for membrane proteins that display multiple topologies, the intrinsic dynamic organization of membrane proteins, and membrane protein conformational disorders resulting in disease. We previously established that lactose permease of Escherichia coli displays a mixture of topological conformations and undergoes postassembly bidirectional changes in orientation within the lipid bilayer triggered by a change in membrane phosphatidylethanolamine content, both in vivo and in vitro. However, the physiological implications and mechanism of dynamic structural reorganization of membrane proteins due to changes in lipid environment are limited by the lack of approaches addressing the kinetic parameters of transmembrane protein flipping. In this study, real-time fluorescence spectroscopy was used to determine the rates of protein flipping in the lipid bilayer in both directions and transbilayer flipping of lipids triggered by a change in proteoliposome lipid composition. Our results provide, for the first time to our knowledge, a dynamic picture of these events and demonstrate that membrane protein topological rearrangements in response to lipid modulations occur rapidly following a threshold change in proteoliposome lipid composition. Protein flipping was not accompanied by extensive lipid-dependent unfolding of transmembrane domains. Establishment of lipid bilayer asymmetry was not required but may accelerate the rate of protein flipping. Membrane protein flipping was found to accelerate the rate of transbilayer flipping of lipids.
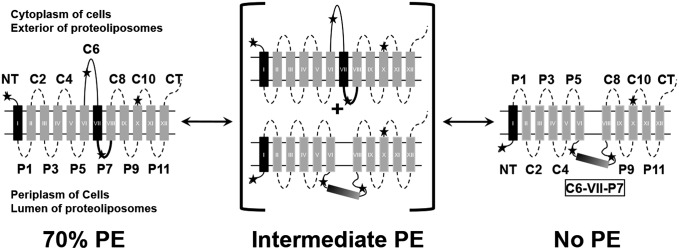
There is limited understanding of how lipid environment affects membrane protein folding during exit from the translocon and insertion into the lipid bilayer or how dynamic changes in lipid environment affect the structure and folding of membrane proteins. Membrane protein lipid environment can change rapidly through lateral movement within a membrane, during trafficking along the secretion pathway, and locally in response to stimuli. Lipid environment is a determinant of transmembrane domain (TMD) orientation for several secondary transporters of Escherichia coli at the time of initial protein folding, as well as after final folding in the cell membrane (1). We used lactose permease (LacY), the paradigm of secondary transporters throughout nature (2), from E. coli as a model membrane protein to study how lipid–protein interactions determine inherently dynamic protein organization and function. When assembled in cells lacking phosphatidylethanolamine (PE), the net neutral and major phospholipid of E. coli (3) (Fig. 1), LacY exhibits inversion of the N-terminal (NT) six-TMD α-helical bundle with respect to the plane of the membrane bilayer and the C-terminal (CT) five-TMD bundle (4); TMD VII becomes an extramembrane domain (EMD) exposed to the periplasm and acts as a molecular hinge allowing the two halves of LacY to respond independently to the lipid environment (4). By use of genetically modified strains of E. coli in which the levels of PE in the bilayer can be controlled and titrated, the reversibility in both directions of LacY topological arrangement after membrane insertion and initial folding was demonstrated (5, 6). Multiple topological isoforms of LacY stably coexist at steady state in the membrane governed by the level of PE in a dose-dependent manner (5). Therefore, structural and topological organization of LacY is highly dynamic in a lipid-dependent, reversible, and bidirectional manner.
Fig. 1.
Effect of changes in PE content on the orientation of domains C6, P7, or NT of LacY in lipid bilayers subjected to lipid exchange. TMD (I–XII) orientation is summarized for LacY in cell membranes or proteoliposomes containing 70% (Left), intermediate (Center), or 0% (Right) PE; the remaining lipid is PG plus CL. Stars indicate positions of single Cys or Trp replacements in EMDs used to determine topological orientation. The bold P7 EMD indicates proper folding of the P7 epitope only in the presence of PE.
The contribution of additional cellular factors, such as the membrane protein insertion machinery, molecular chaperones, or membrane potential, cannot be ruled out by in vivo experiments alone. To determine the necessary and sufficient determinants for initial and postassembly lipid-dependent membrane protein dynamic topological organization, purified LacY was reconstituted into liposomes containing only lipids and LacY (7, 8). The in vitro results mirrored the in vivo results in that the ratio of native to inverted topological LacY isoforms increased in proportion to the ratio of PE to the anionic lipids phosphatidylglycerol (PG) and cardiolipin (CL) in the proteoliposomes. Changes in the PE content after protein integration into proteoliposomes [fliposomes (7, 8)] resulted in postassembly TMD flipping (Fig. 1). Therefore, membrane protein folding and dynamic postinsertional rearrangements are thermodynamically driven processes dependent on intrinsic lipid–protein interactions that may not require other cellular factors.
We proposed the charge balance rule (1), which incorporates the influence of lipid environment into the positive inside rule (9–12) that governs the orientation of TMDs in the membrane. We found a synergistic relationship between the effective positive charge of EMDs as cytoplasmic retention signals and the negative charge density of the membrane surface determined by the ratio of net neutral [e.g., PE, phosphatidylcholine (PC)] to anionic (e.g., PG, CL) lipids. Therefore, postassembly topological rearrangements in response to alterations in the lipid environment and/or posttranslational modifications, such as phosphorylation, may dynamically alter organization of membrane proteins (1). The charge balance rule also provides a molecular basis for TMD orientation of proteins, such as the multi-drug transporter EmrE (13), and low-molecular-weight transporters (14) in E. coli, which coexist as stable dual topological conformers in the membrane. Although several recent reports (15–17) independently demonstrate that initial lipid composition of proteoliposomes determines steady-state TMD orientation, evidence for dynamic reorientation postassembly in vitro is limited (7).
The existence of reversible multiple topologies of a protein dependent on the lipid environment raises new questions and challenges current dogma concerning the mechanism of membrane protein topogenesis (1). However, due to the length of time between lipid exchange and biochemical assessment of the change in topological organization in proteoliposomes (7), no insight was possible into the kinetics, mechanism, or structural intermediates occurring during a change in lipid composition. How fast are the events occurring? Are they happening concomitantly or sequentially? Do the proteoliposomes exhibit some form of lipid asymmetry that assists the flipping process? Is a membrane protein extensively unfolded during reorientation? Fast topological switching would allow a protein to escape degradation due to protein quality control or to adopt a new structural organization, and possibly a new function, quickly. Herein, we have used time-resolved fluorescence spectroscopy to show that the process involves a sequence of events that starts with a change in the lipid composition of the outer leaflet of proteoliposomes, quickly followed by protein topological reorientation, and ending with a slower equilibration of lipid composition between the inner and outer bilayer leaflets.
Results
Use of Förster Resonance Energy Transfer for Topological Assessment.
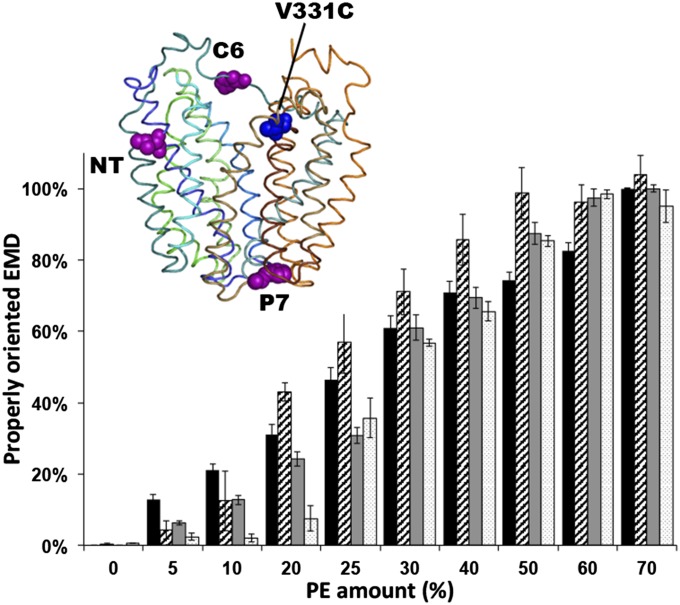
We used Förster resonance energy transfer (FRET) between Trp and 1,5-((((2-iodoacetyl)amino)ethyl)amino)naphthalene-1-sulfonic acid (IAEDANS) to follow topological changes in LacY in real time. A Cys replacement at position 331 (near the cytoplasmic end of TMD X) was labeled by IAEDANS as the acceptor of the FRET pair (Fig. 2). Because the second half of LacY (from TMD VIII to EMD CT) does not undergo any lipid-dependent topological switch (18), the labeled Cys331 will always remain on the outside of proteoliposomes. Starting with the single Cys at position 331, diagnostic Trp replacements were made in EMD NT, C6, or P7 at position 14, 205, or 250, respectively (Fig. 2). These replacements were performed in LacY containing all six native Trp residues, providing higher stability to LacY compared with single Trp mutants (19). We verified that these six Trp residues (all located in the NT seven TMDs) are silent to our FRET conditions (Fig. S1D). To validate our approach, LacY containing a Trp located in either NT or C6 was purified from PE-lacking cells, labeled with IAEDANS, reconstituted in proteoliposomes made of varying amounts of PE and examined by FRET. We have previously established (7) that LacY is properly oriented in proteoliposomes containing a lipid composition mimicking WT cells (70% PE and 30% PG plus CL) (Fig. 1). In the misoriented conformer in proteoliposomes lacking PE and containing only PG and CL, EMDs NT and C6 face the lumen (7). Additionally, we demonstrated a dose-dependent relationship between the amounts of PE in the proteoliposomes and the amounts of properly oriented EMDs C6 and NT, as detected by the substituted Cys accessibility method to determine TMD orientation (7).
Fig. 2.
Detection of topological rearrangements in LacY by Trp-IAEDANS FRET. The bar graph shows the percentage of normalized FRET (black and gray) or percentage of properly oriented LacY as determined by the Cys accessibility method (SCAM; diagonal and white) [data from Vitrac et al. (7)] as a function of PE content in proteoliposomes for Trp replacement in C6 (black) and NT (gray) and single Cys replacements in C6 (diagonal) and NT (white). The graph represents the average of three different experiments, with error bars indicating SD. Normalization of FRET was performed using proteoliposomes containing no PE or 70% PE as a minimal or maximal FRET value, respectively. Data represent the normalized FRET expressed as the ratio (F − F0%)/(F70% − F0%), as described in SI Methods. (Inset) Side view of LacY (Protein Data Bank ID code 2CFQ). TMD helices are rainbow-colored from green (TMD I) to orange (TMD XII). Diagnostic Trp replacements introduced individually in EMDs NT, C6, and P7 are shown with the indicated positions of Cα atoms of Trp (magenta spheres). The IAEDANS label is at Cys331 (blue sphere).
Fig. S1.
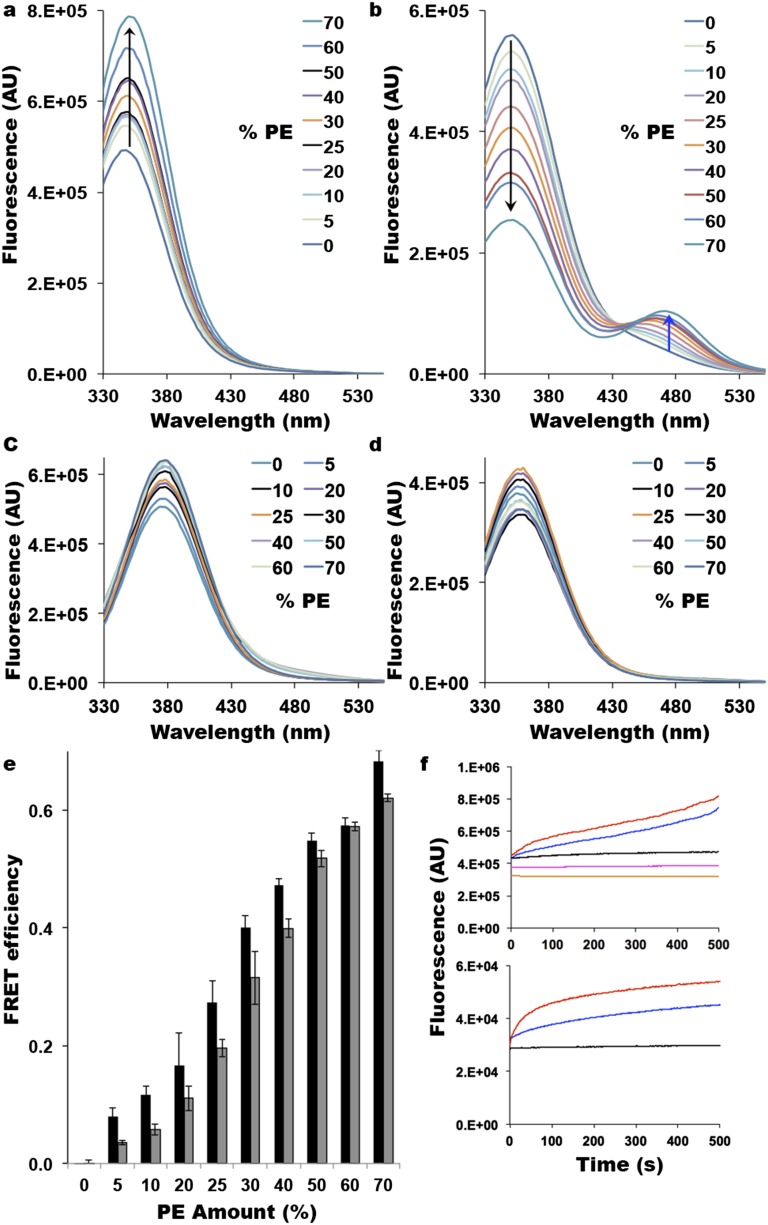
Use of Trp-IAEDANS FRET as a reporter of LacY topological rearrangement. (A) Trp fluorescence of LacY containing a diagnostic Trp replacement in EMD C6 observed with unlabeled H205W/V331C mutant. AU, arbitrary units. Spectra were recorded with excitation at 295 nm for 1 μM protein in 10 mM Tris⋅HCl (pH 7.5). An increase in Trp fluorescence as a function of PE content is indicated by the upward black arrow. (B) Quenching of Trp fluorescence (indicated by the black arrow) in the C6 EMD of LacY and appearance of Trp-IAEDANS FRET (indicated by the blue arrow) observed with IAEDANS-labeled H205W/V331C mutant. Spectra were recorded with excitation at 295 nm for 1 μM protein in 10 mM Tris⋅HCl (pH 7.5). (C) Fluorescence of LacY containing a diagnostic Trp replacement in EMD P7 observed with IAEDANS-labeled F250W/V331C mutant. Spectra were recorded with excitation at 295 nm for 1 μM protein in 10 mM Tris⋅HCl (pH 7.5). (D) Fluorescence of LacY containing only its six native Trp residues with IAEDANS-labeled V331C mutant. Spectra were recorded with excitation at 295 nm for 1 μM protein in 10 mM Tris⋅HCl (pH 7.5). (E) FRET efficiency as a function of the amount of PE present in LacY proteoliposomes. FRET efficiency (E) was calculated from steady-state fluorescence spectra as E = 1 − (FDA/FD), where FDA and FD are the donor fluorescence intensities at 340 nm in the presence and absence of the acceptor, respectively. Experiments were performed in triplicate, and error bars indicate SD (black, Trp in C6; gray, Trp in NT). (F) Real-time measurements of Trp fluorescence (Upper) and IAEDANS fluorescence (Lower) after excitation at 295 nm. (Upper) Red (C6) and blue (NT) traces indicate Trp fluorescence observed with unlabeled H205W/V331C and L14W/V331C mutants, respectively, upon addition of PE; the black trace indicates Trp fluorescence observed with unlabeled H205W/V331C in the absence of lipid exchange; and magenta and orange traces indicate Trp fluorescence observed with unlabeled V331C containing only endogenous Trp (no Trp replacement in EMDs) upon addition and dilution of PE, respectively. (Lower) Red (C6) and blue (NT) traces indicate FRET observed with IAEDANS-labeled H205W/V331C and L14W/V331C mutants, respectively, upon addition of PE, and the black trace indicates FRET observed with IAEDANS-labeled H205W/V331C in the absence of lipid exchange.
In properly oriented LacY (20), the Trp positioned in NT or C6 is located ∼17 Å or ∼19 Å, respectively, from the acceptor at position 331 (Fig. 2). However, when misoriented, the donor and acceptor will be on opposite sides of the membrane at a distance most likely ≥40 Å. Because the R0, the distance of half-maximal FRET intensity, of the FRET pair Trp-IAEDANS is 22 Å (21), we expected to observe FRET when the NT or C6 EMD is properly oriented, whereas there should be a significant FRET loss when these EMDs are misoriented. EMD P7 does not undergo transmembrane movement (4, 7) and should remain at a distance of ≥40 Å from position 331. The relationship between amounts of PE and measured FRET (Fig. 2) closely mimics the previously established relationship between amounts of PE in proteoliposomes and proper orientation of EMDs NT and C6 (5, 7). These results, complemented by the extended set of control studies using steady-state reconstitution of LacY in proteoliposomes and real-time fluorescence measurements presented in Fig. S1 (and in SI Results), establish the feasibility of the FRET measurements in estimating the topology of a single EMD.
Real-Time Measurement of Lipid Exchange in Proteoliposomes.
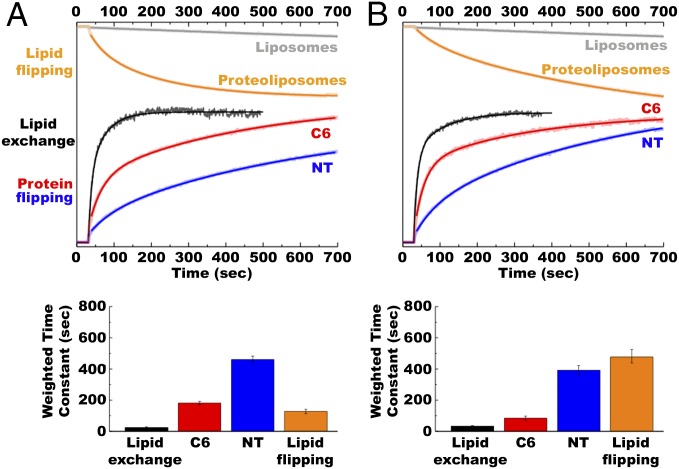
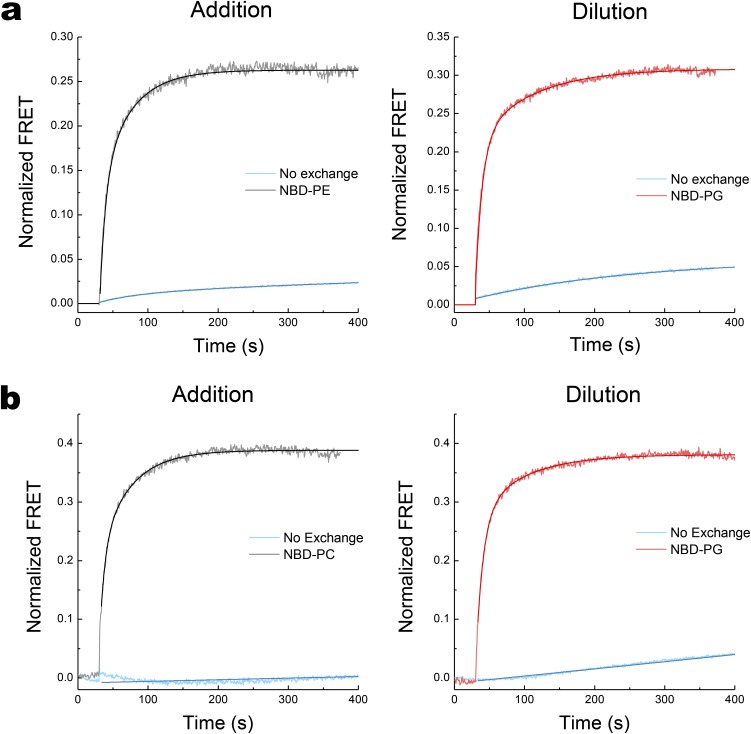
A methyl-β-cyclodextrin (MβCD)–induced lipid exchange protocol previously used (7) to demonstrate protein flipping in vitro was adapted to real-time measurements. Using E. coli naturally occurring lipids, we showed that lipid exchange between multilamellar vesicles (MLVs; donor lipid source) and proteoliposomes induced LacY topological switching, which was completed in less than 10 min at 37 °C (7). FRET experiments performed at this temperature led to kinetics that were too fast to monitor accurately. Therefore, all experiments were performed at 20 °C to obtain initial rates. MLVs were separately labeled with the FRET donor lipid probe 6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl (NBD) in the head group of PE or the fatty acid of PG and PC. Proteoliposomes were labeled with the acceptor lipid probe (head group labeled Rhodamine-PE). Upon transfer of phospholipid from the MβCD-loaded MLVs to proteoliposomes, a FRET signal (detected as Rhodamine emission at ∼585 nm resulting from NBD excitation at ∼470 nm) increased due to the proximity of the donor and acceptor probes in trace amounts. We previously determined steady-state end points (7) under similar conditions using quantitative lipid analysis. Proteoliposomes initially containing only PG plus CL contained about 55% PE after mixing with MLVs containing only PE. Proteoliposomes initially containing 70% PE and 30% PG plus CL contained about 40% PE after mixing with MLVs containing PG plus CL (7). The results obtained for addition and dilution of PE are displayed in Fig. 3 A and B (black traces), respectively (the full set of results is presented in Fig. S2). The respective time constants from these experiments are shown in Fig. 3 and Table S1.
Fig. 3.
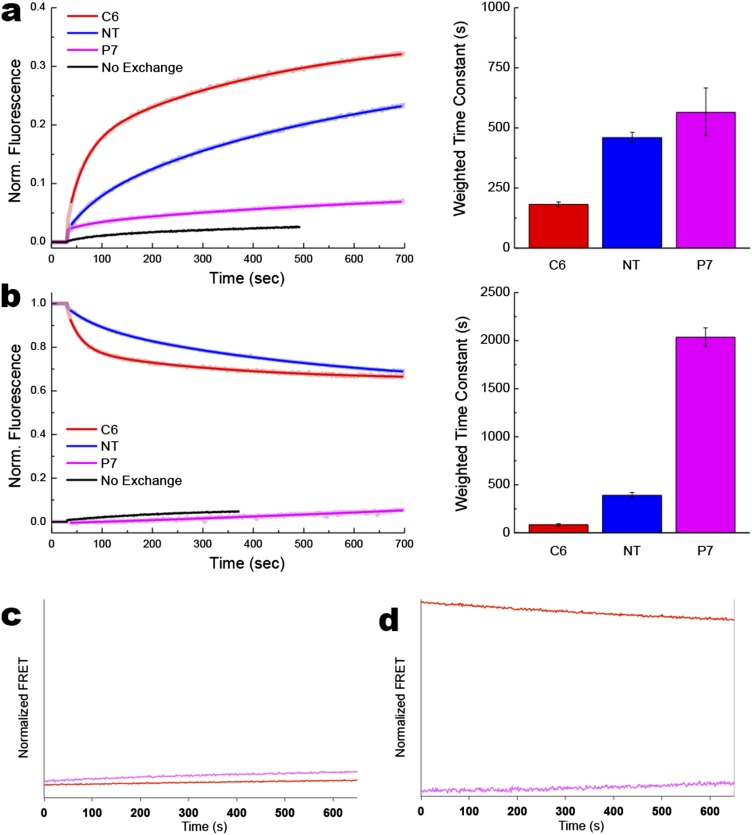
Rate of lipid exchange, protein flipping, and lipid flipping during addition (A) or dilution (B) of PE in proteoliposomes. Normalized FRET (y axis) represents values calculated in Figs. S2 and S3 and plotted on the same scale. (A) Addition of PE was performed with MLVs made of total lipid extracts from PE-containing E. coli (75% PE/15% PG/5% CL) and proteoliposomes or liposomes made of total lipid extracts from PE-lacking E. coli (50% PG/45% CL). (B) Lipid compositions for MLVs and proteoliposomes or liposomes were reversed to perform dilution of PE. Lipid exchange (black traces): Lipid exchange was triggered by addition of proteoliposomes to MβCD-loaded MLVs 30 s after stabilization of fluorescence, with FRET changes measured at 0.5-s intervals. Protein flipping: Lipid exchange was triggered by addition of MβCD-loaded MLVs to proteoliposomes 30 s after stabilization of fluorescence, with FRET changes measured at 0.5-s intervals. Red (C6) and blue (NT) traces indicate FRET observed with IAEDANS-labeled H205W/V331C and L14W/V331C mutants, respectively. Lipid flipping: NBD-PE quenching by dithionite for protein-free liposomes (gray traces) or proteoliposomes containing LacY (orange traces) under conditions that promote LacY flipping. Lipid exchange was triggered by addition of liposomes or proteoliposomes to MβCD-loaded MLVs 30 s after stabilization of fluorescence, with quenching kinetics measured at 1-s intervals. The experiments were repeated three to five times, and the data represent mean values ± lower and upper confidence limits from exponential fits of the data. The bar graph displays weighted average time constants from Table S1.
Fig. S2.
Characterization of the lipid exchange between MLVs and proteoliposomes. Real-time FRET measurements during addition or dilution of PE (A) or PC (B) are displayed. PE or PC was added to proteoliposomes containing total lipid extracts from PE-lacking E. coli (50% PG plus 45% CL) in the presence of MβCD-loaded MLVs containing total lipid extracts from PE-containing (75% PE/15% PG/5% CL) or PC-containing (70% PC/5% PG/26% CL) E. coli. Dilution of PE or PC was for proteoliposomes containing total lipid extracts from PE-containing or PC-containing E. coli in the presence of MβCD-loaded MLVs containing total lipid extracts from PE-lacking E. coli. FRET kinetics were measured every 0.5 s before and after starting the lipid exchange. Lipid exchange was triggered at 30 s after stabilization of the fluorescent signal by addition of proteoliposomes to MβCD-loaded MLVs. FRET was also measured in the absence of MβCD under conditions of no lipid exchange. Normalized FRET was determined using the ratio (F − Fmin)/(Fmax − Fmin) as described in SI Methods. The experiments were repeated four times, and the data represent mean values ± lower and upper confidence limits from exponential fits of the data.
Table S1.
Kinetic parameters for the real-time measurement of lipid exchange, protein flipping, and lipid flipping
| Experimental Condition | Fast tau (±LCL/UCL) | Slow tau (±LCL/UCL) | Area of fast tau (±LCL/UCL) | Weighted tau (±LCL/UCL) |
| Addition of PE | ||||
| Lipid exchange | 8.1 ± 0.9/0.4 | 42 ± 7/3 | 0.52 ± 0.03/0.04 | 25 ± 5/0.2 |
| Protein flipping | ||||
| C6 | 34 ± 0.8 | 404 ± 10 | 0.60 ± 0.02 | 181 ± 10 |
| NT | 59 ± 4 | 577 ± 24 | 0.22 ± 0.01 | 458 ± 22 |
| P7 | 47 ± 10 | 710 ± 97 | 0.22 ± 0.03 | 564 ± 100/97 |
| Lipid flipping | ||||
| LacY (flipping) | 36 ± 2 | 186 ± 3 | 0.39 ± 0.01 | 128 ± 14 |
| LacY (no flipping) | 61 ± 4 | 459 ± 20 | 0.29 ± 0.01 | 341 ± 23 |
| CscB (no flipping) | 53 ± 2 | 508 ± 14 | 0.31 ± 0.01 | 370 ± 40 |
| Dilution of PE | ||||
| Lipid exchange | 9.7 ± 1.2/0.4 | 79 ± 7.4/0.2 | 0.66 ± 0.2 | 33 ± 3/1 |
| Protein flipping | ||||
| C6 | 26 ± 0.9 | 296 ± 12 | 0.79 ± 0.04 | 83 ± 14/13 |
| NT | 57 ± 3 | 521 ± 16 | 0.28 ± 0.01 | 391 ± 30 |
| P7 | 2,035 ± 97 | 0 | 1 | 2,035 ± 97 |
| Lipid flipping | ||||
| LacY (flipping) | 61 ± 4 | 590 ± 26 | 0.21 ± 0.01 | 477 ± 19 |
| LacY (no flipping) | 54 ± 3 | 332 ± 9 | 0.37 ± 0.01 | 230 ± 26 |
| CscB (no flipping) | 54 ± 2 | 390 ± 7 | 0.34 ± 0.01 | 276 ± 24 |
| Addition of PC | ||||
| Lipid exchange | 7 ± 0.9 | 44 ± 2 | 0.97 ± 0.03/0.01 | 7.9 ± 1.4/2.4 |
| Protein flipping | ||||
| C6 | 28 ± 0.9 | 437 ± 7 | 0.59 ± 0.01 | 197 ± 18 |
| NT | 30 ± 0.7 | 602 ± 8 | 0.49 ± 0.01 | 320 ± 39 |
| P7 | 1,180 ± 161/140 | 0 | 1 | 1,180 ± 161/140 |
| Dilution of PC | ||||
| Lipid exchange | 10.1 ± 0.5 | 66 ± 3 | 0.97 ± 0.01 | 11.9 ± 0.8 |
| Protein flipping | ||||
| C6 | 29 ± 0.7 | 363 ± 9 | 0.66 ± 0.01 | 142 ± 12/14 |
| NT | 33.3 ± 0.7 | 403 ± 9 | 0.61 ± 0.01 | 178 ± 22 |
| P7 | 59 ± 13 | 801 ± 140 | 0.19 ± 0.01 | 661 ± 120 |
Weighted Tau = Percent Areafast * Taufast + (1 − Percent Areafast) * Tauslow. LCL, lower confidence limit from exponential fits of the data; UCL, upper confidence limit from exponential fits of the data.
Most of the MβCD-induced lipid exchange occurs in less than 1 min and reaches a stable maximum after about 3 min. Fluorescence changes were reproducible and very similar for the type of exchange (addition or dilution of a particular phospholipid) or the type of lipid (PE, PC, or PG) exchanged (Fig. S2). Several NBD derivatives were used, having the NBD moiety grafted either onto the polar head of the phospholipid (NBD-PE) or onto one of the fatty acid tails (NBD-PG and NBD-PC). The lipid exchange kinetics observed when adding or diluting PE fit well to double, but not single, exponential functions (Fig. 3 A and B, black trace). The fast and weighted time constants for PE exchange were 8.1 s and 24.5 s, respectively, compared with 9.7 s and 32.9 s, respectively, for PG exchange (Fig. 3 A and B, Bottom and Table S1). Interestingly, the NBD-PC and NBD-PG lipid exchange kinetics were fit better to single exponential functions with similar fast time constants of 7 s and 10 s (Fig. S2 and Table S1). As a negative control, MLVs devoid of MβCD (no lipid exchange) were used, and FRET was monitored to verify that the variations observed in FRET are solely due to lipid exchange (Fig. S2). Slight changes in FRET observed upon mixing without lipid exchange can be attributed to spontaneous lipid exchange and/or local proximity of the two probes occurring when MLVs and proteoliposomes come into physical contact.
Real-Time Measurement of Lipid-Triggered TMD Flipping.
To trigger lipid exchange, MβCD-loaded MLVs containing PE/PG/CL or PG/CL were added to proteoliposomes containing PG/CL or PE/PG/CL, respectively, and the LacY derivatives containing Trp-IAEDANS pairs, after stabilization of the signal (first 30 s). The results obtained for supply and dilution of PE are summarized in Fig. 3 (red and blue traces for C6 and NT topological switches, respectively). For ease of comparison, real-time FRET measurements of protein flipping (NT and C6 EMDs) are shown as variations in FRET ratios in the case of dilution of PE (Fig. 3B). The actual increase (addition of PE) or decrease (dilution of PE) in FRET observed for NT and C6 EMDs is presented with the full set of results in Fig. S3. Data for addition or dilution of PC are presented in Fig. S4.
Fig. S3.
Protein topological rearrangements triggered by changes in PE content of the proteoliposomes. Real-time FRET measurements of proteoliposomes containing LacY and submitted to lipid exchange to trigger addition (A) or dilution (B) of PE. Changes in phospholipid composition were as described in Fig. S2. Lipid exchange was triggered by addition of MβCD-loaded MLVs to proteoliposomes 30 s after stabilization of fluorescence, with kinetics measured at 0.5-s intervals. Control lipid exchange kinetics [C and D (corresponding to A and B, respectively)] represent lipid exchange triggered between MβCD-loaded MLVs and proteoliposomes of the same lipid composition. Red (C6), blue (NT), and magenta (P7) traces indicate Trp-IAEDANS FRET observed with IAEDANS-labeled H205W/V331C, L14W/V331C, and F250W/V331C mutants, respectively. Black traces indicate measurements performed in the absence of lipid exchange. Data represent the normalized FRET expressed as the ratio (F(t) − F0)/(Fmax − F0), as described in SI Methods. The experiments were repeated three to five times, and the data represent mean values ± lower and upper confidence limits from exponential fits of the data. Bar graphs summarized time constants shown in Table S1.
Fig. S4.
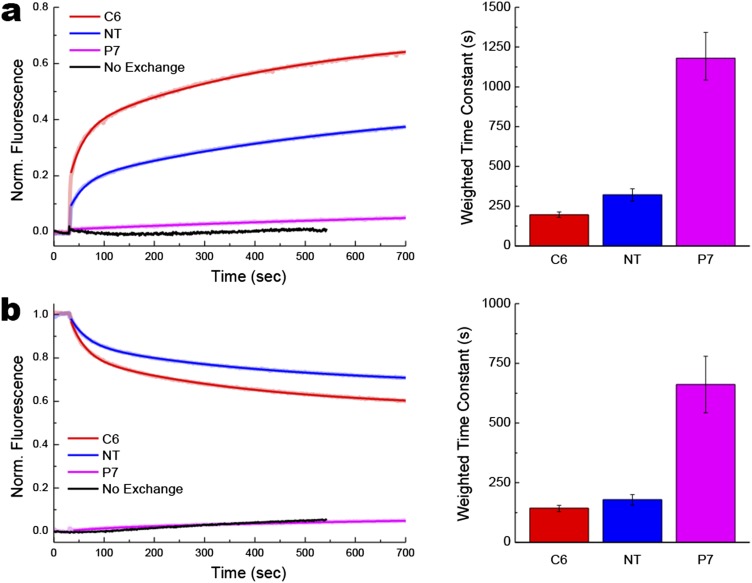
Protein topological rearrangements triggered by changes in PC content in the proteoliposomes. Real-time FRET measurements of proteoliposomes containing LacY and submitted to lipid exchange to trigger addition (A) or dilution (B) of PC. Changes in phospholipid composition were as described in Fig. S2. Lipid exchange was triggered by addition of MβCD-loaded MLVs to proteoliposomes 30 s after stabilization of fluorescence, with kinetics measured at 0.5-s intervals. Red (C6), blue (NT), and magenta (P7) traces indicate Trp-IAEDANS FRET observed with IAEDANS-labeled H205W/V331C, L14W/V331C, and F250W/V331C mutants, respectively. Black traces indicate measurements performed in the absence of lipid exchange as described in Fig. S3. Data represent the normalized FRET expressed as the ratio (F(t) − F0)/(Fmax − F0), as described in SI Methods. The experiments were repeated three to five times, and the data represent mean values ± lower and upper confidence limits from exponential fits of the data. Bar graphs summarized time constants shown in Table S1.
In both directions, protein flipping was rapid but lagged behind the rate of lipid exchange consistent with the previous topological studies (7) and steady-state FRET measurements (Fig. 2), indicating that proteoliposomes must contain ∼10% PE to exhibit detectable amounts of properly oriented LacY. The protein flipping kinetics observed when adding or diluting PE fit well to double exponential functions. As indicated by the fast and weighted time constants, the rates of C6 flipping (34 s and 181 s, respectively) were faster than the rates of NT flipping (59 s and 564 s, respectively) for addition of PE (Fig. 3A and Table S1). Dilution of PE also showed faster flipping of C6 (26 s and 83 s, respectively) than NT (57 s and 391 s, respectively) (Fig. 3B and Table S1). Flipping of C6 must occur concurrently with membrane exit or insertion of TMD VII, which acts as a molecular hinge allowing the two halves of LacY to respond independently to the lipid environment (6). This rearrangement appears to precede NT flipping in both directions. Protein flipping occurred on a second to minute scale, although none of these phenomena seem to reach a steady-state under our experimental conditions, further confirming that protein topological switching happens later and slower than lipid exchange. The fast time constant for flipping of C6 indicated a faster rate for PE dilution than PE addition, which was further accentuated in the weighted tau values, suggesting a greater contribution of the initial fast rate of flipping when PE was diluted. Interestingly, the fast and weighted time constants for PC dilution (29 s and 178 s, respectively) were essentially the same as for PC addition (28 s and 143 s, respectively) (Fig. S4 and Table S1). The possible significance of these differences will be discussed later.
The P7 domain does not cross the membrane during changes in lipid composition consistent with little change in FRET. However, small increases in FRET signal were observed when PE was added to proteoliposomes (Fig. S3). The native structure of the EMD P7 contains a continuous epitope that is recognized by the conformation-specific mAb 4B1 when LacY is in its native topological organization (22–24). We have shown that refolding of this domain appears to be more energetically favorable than “unfolding” of the preexisting properly folded P7 domain by dilution of the PE level (7). The lipid-dependent conformational changes in the P7 domain most likely affect the Trp250 environment, resulting in small variations in Trp emission fluorescence and/or shortening of the distance between Trp250 and Cys331. This phenomenon was not observed in the case of PC addition (Fig. S4), consistent with E. coli-made PC being less effective in supporting the native conformation of the 4B1 epitope in vitro (25).
As a negative control, MLVs devoid of MβCD (no lipid exchange) were used, and FRET was monitored to verify that the variations observed in FRET were due to lipid exchange (Figs. S2 A and B and S4). Another control was to trigger lipid exchange between proteoliposomes and MβCD-loaded MLVs having the same lipid composition. In this case, lipid exchange occurred without a change in phospholipid composition of the proteoliposomes or a change in fluorescence for LacY (Fig. S3 C and D).
Assessment of Lipid Asymmetry in Proteoliposomes.
The original lipid exchange protocol was developed to create liposomes (lacking an incorporated membrane protein) with mammalian-like properties, including lipid asymmetry in the bilayer (26). The formation and stability of lipid asymmetry using E. coli phospholipids have not been explored. Therefore, we designed a set of experiments to assess the transbilayer flipping of exchanged lipids in proteoliposomes. An established method for monitoring bilayer asymmetry is accessibility of NBD-labeled lipids to reduction with the water-soluble reducing agent dithionite (27). Under our conditions, the fluorescently labeled lipid was first located in the MLVs, followed by transfer to the outer leaflet of the small unilamellar vesicles (SUVs) during MβCD-induced lipid exchange. We used either protein-free liposomes or proteoliposomes made of nonfluorescent lipids in which 10 mM dithionite was encapsulated in the lumen. If asymmetry is maintained, the NBD-labeled phospholipid (originally contained in MLVs) will remain on the outer leaflet of the SUVs, and its fluorescence will remain constant during lipid exchange. However, if transbilayer movement of lipid occurs, the lipid would be exposed to the lumen, where reduction of NBD would occur with accompanied quenching of NBD fluorescence. As expected, maximum NBD fluorescence was determined when only MLVs were present and minimal fluorescence was measured after detergent disruption of the vesicles in the presence of dithionite, leading to full quenching of NBD. The real-time measurement of NBD fluorescence quenching associated with lipid flipping is summarized in Fig. 3 (orange and gray traces). The full set of results for lipid flipping is presented in Fig. 4.
Fig. 4.
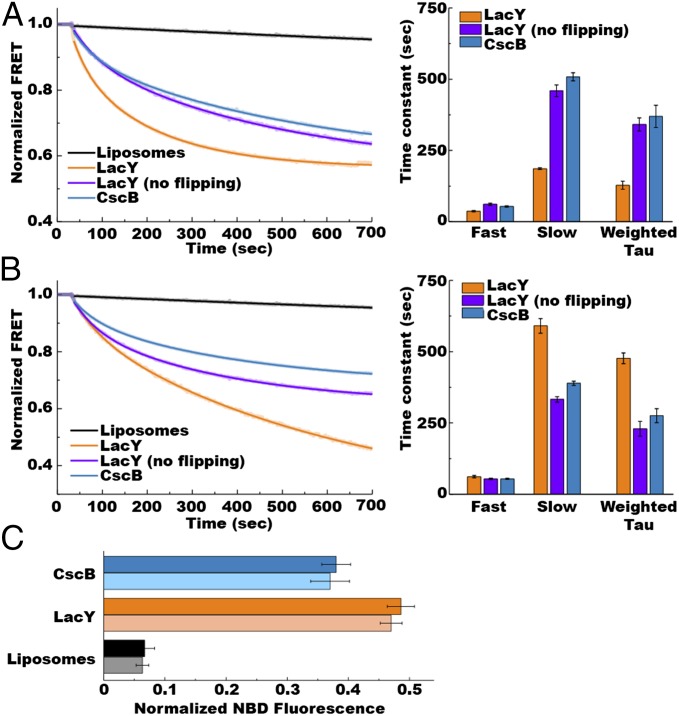
Lipid flipping across the lipid bilayers upon changes in phospholipid composition. Real-time NBD-PE quenching by dithionite measurements of protein-free liposomes (black traces) or proteoliposomes containing either LacY (orange and purple traces) or CscB (blue traces) and submitted to lipid exchange to trigger addition (A) or dilution (B) of PE is displayed. (A) Addition of PE was performed with MLVs made of total lipid extracts from PE-containing E. coli (75% PE/15% PG/5% CL) and proteoliposomes or from liposomes made of total lipid extracts from PE-lacking E. coli (50% PG/45% CL). (B) Lipid compositions for MLVs and proteoliposomes or liposomes were reversed to perform dilution of PE. Lipid exchange was triggered by addition of liposomes or proteoliposomes to MβCD-loaded MLVs 30 s after stabilization of fluorescence, with quenching kinetics measured at 1-s intervals. (C) Histogram shows percentage of normalized NBD fluorescence measured after 30 min of lipid exchange in liposomes and proteoliposomes containing LacY or CscB when performing resupply (dark-colored bars) or dilution (light-colored bars) of PE. Data represent the mean ± SD of six independent experiments. Data represent the normalized NBD-PE fluorescence expressed as the ratio (F(t) − F0)/(Fmax − F0), as described in SI Methods. The experiments were repeated three to five times, and the data represent mean values ± lower and upper confidence limits from exponential fits of the data. The bar graph displays time constants from Table S1.
When protein-free liposomes were used, no significant quenching of NBD was observed over the course of 10 min, no matter the type of lipid exchange (Fig. 3 A and B, gray traces and Fig. 4 black traces), confirming that spontaneous lipid flipping is very slow. Presence of a membrane protein under conditions where no protein flipping occurred accelerated the rate of lipid flipping to the same extent with the same fast and weighted time constants (Fig. 4 and Table S1). Lipid flipping was measured using either LacY (Fig. 4, purple traces) when performing lipid exchange while maintaining the same lipid composition (donor MLVs and recipient proteoliposomes have the same lipid composition) or using nonflipping sucrose permease (CscB) (18) when performing an alteration of the lipid composition (Fig. 4, light blue traces). Finally, LacY-containing proteoliposomes were used under conditions where LacY (Figs. 3 and 4, orange traces in both) undergoes TMD flipping (lipid exchange-induced modulations of PE amounts). In this case, we observed a further acceleration of transbilayer lipid flipping with both the fast and weighted time constants for PE addition (36 s and 128 s, respectively) (Figs. 3A and 4A, orange traces in both), indicating faster lipid flipping than for PE dilution (61 s and 477 s, respectively) (Figs. 3B and 4B, orange traces in both and Table S1). Because the processes monitored here did not reach equilibrium and could proceed long after lipid exchange is completed, we decided to examine the extent of NBD quenching after 30 min of incubation. At this time point, we know that both lipid exchange and protein flipping have reached equilibrium. The results are presented in Fig. 4C. In the case of protein-free liposomes, less than 10% NBD quenching was observed, confirming the generation of asymmetrical vesicles. When using proteoliposomes containing LacY or CscB, about 50% or 40% of NBD quenching was observed, respectively. These results point out that lipid transbilayer movements are facilitated by the presence of an α-helical membrane protein inserted into the bilayer, confirming previous observations (28, 29). The differences in lipid flipping rates observed when LacY is also flipping may be due to a specific association of LacY with PE, as will be discussed later.
SI Results
Use of Trp-IAEDANS FRET as a Reporter of LacY Topological Rearrangement.
To investigate in real time the kinetics of LacY flipping upon changes in membrane phospholipid composition in proteoliposomes, we designed a set of LacY mutants containing (i) a single Cys replacement at position V331 (where labeling by the acceptor fluorescent molecule IAEDANS can be performed) and (ii) diagnostic Trp replacements in EMD NT, C6, or P7. The choice of the Trp-IAEDANS fluorophore pair stems from the need to maintain LacY EMD charge content unaltered. These diagnostic Trp replacements were made in LacY containing all six native Trps, providing higher stability to LacY compared with Trp-less LacY (19).
First, we tested the feasibility of the approach by monitoring the fluorescence emission in the range of 330–550 nm after excitation at 295 nm, both in unlabeled (Fig. S1A) and IAEDANS-labeled (Fig. S1B) LacY. Spectra obtained for LacY with a Trp replacement in EMD C6 and V331C either unlabeled (Fig. S1A) or labeled with IAEDANS (Fig. S1B) reconstituted in proteoliposomes made of increasing amounts of PE are shown. The fluorescence spectra display either one maximum (no IAEDANS labeling; Fig. S1A) or two maxima (Fig. S1B): the emission of the donor visualized around 340 nm and the emission of the acceptor visualized around 475 nm. Fig. S1A depicts the effect of PE amounts in proteoliposomes (i.e., the amount of correctly oriented LacY) on the fluorescence of the Trp located in EMD C6. The emission of the donor at 340 nm increases with the amount of PE. In Fig. S1B, the emission of the donor decreases as PE content in proteoliposomes increases, whereas fluorescence emission of the acceptor increases.
When performing the same type of experiment using the Trp replacement in EMD P7 (which stays on the opposite side of the membrane from the donor, independent of PE amounts), we observed that there is a low increase in the fluorescence of the donor as a function of PE amounts, whereas the fluorescence of the acceptor shows only a slight increase at high PE amounts (Fig. S1C). We also verified that the native six Trp residues (all located in TMDs) are silent to our FRET conditions by assessing the fluorescence of both the donor (Trp) and the acceptor (IAEDANS) when LacY carrying a single Cys at position V331 was labeled with IAEDANS and reconstituted in proteoliposomes containing increasing amounts of PE (Fig S1D). No fluorescence of the acceptor is observed, whereas small variations in the fluorescence of the donor are noticeable. These variations appear to be PE-independent. These various results clearly established the feasibility of our approach, allowing us to monitor lipid-dependent protein topological switching in real time.
The relationship between FRET efficiency (E) and amounts of PE is depicted in Fig. S1E. The FRET efficiency E is calculated using the following equation:
where FDA and FD are the donor fluorescence intensities at 340 nm in the presence and absence of the acceptor, respectively. Although the fluorescence intensity of Trp increases in the absence of acceptor, it is quenched when the acceptor is present. It can be concluded that both normalized FRET (at 475 nm; Fig. 2) and FRET efficiency are representative of LacY topological rearrangement [i.e., although FD for Trp increases as its lipid environment changes (which could be interpreted as an initial change not directly associated with flipping), FDA also decreases in parallel (which we interpret as a result of Trp coming closer to the acceptor)].
Based on these results, kinetic measurements were undertaken. Fig. S1F depicts the raw values of Trp fluorescence (Upper) and IAEDANS fluorescence (FRET; Lower) measured during kinetics of lipid exchange. Because the various LacY mutants have been designed in a LacY template containing all six native Trp residues (located in TMD I, II, V, VI, and VII), we verified that the Trp fluorescence signal from these native Trp residues did not interfere with our topological assessment. To do so, we performed real-time measurement of Trp fluorescence during MβCD-induced lipid exchange (Fig. S1F, Upper; the magenta trace shows the addition of PE, and the orange trace shows the dilution of PE). The data observed confirmed the absence of contribution of the six native Trp residues to the topological assessment of LacY.
Discussion
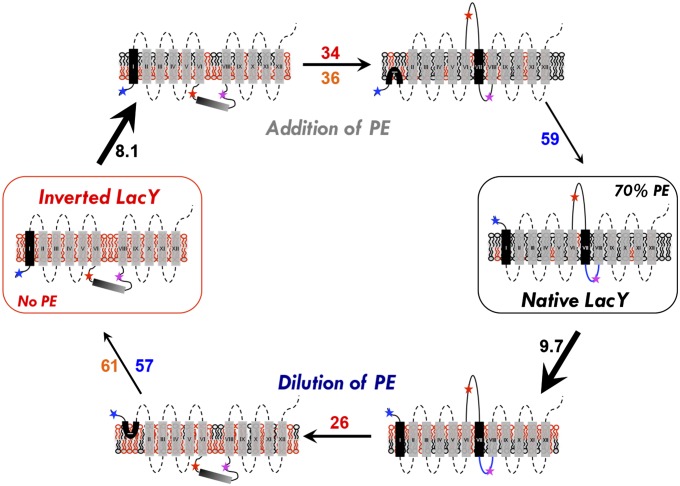
This is the first report to our knowledge of a real-time assessment of lipid-induced membrane protein topological flipping. The analysis of fluorescence kinetics led to determination of the sequence of events occurring during the resupply or dilution of PE (or PC), as well as a better understanding of the complexity of the events involved in the postassembly lipid-induced topological rearrangement of LacY. For each measurement, the individual time constants reflect the complexity of the events, whereas the weighted average time constants reflect the global order of the events. A summary scheme of these various events is presented in Fig. 5.
Fig. 5.
Schematic model of the events occurring during lipid-induced topological switching of LacY in proteoliposomes. LacY topology is shown when assembled in proteoliposomes lacking PE (inverted LacY, Left) and in proteoliposomes containing WT amounts of PE (Native LacY, Right), before and during lipid exchange. Stars indicate positions of diagnostic Trp replacements used to determine topological orientation of EMDs (blue, red, and magenta correspond to NT, C6, and P7, respectively). The blue P7 loop indicates proper folding of the P7 epitope. Membrane phospholipids are depicted as follows: black, PE; red, anionic lipids (PG and CL). The time constant value (s−1) of each event is indicated as follows: black, lipid exchange; red, C6 flipping; blue, NT flipping; orange, lipid flipping. In both addition and dilution of PE (or PC), lipid exchange occurs first and is followed by protein topological rearrangement and transbilayer movement of lipid. During lipid exchange, lipids from the outer leaflet of proteoliposomes are exchanged and become enriched with PE (addition of PE) or PG/CL (dilution of PE), leading to transient generation of lipid asymmetry. Protein flipping occurs subsequent to lipid exchange, with C6 EMD flipping being more sensitive to PE (or PC) content than the NT EMD. P7 EMD folding/unfolding is PE-dependent and linked to the topological switch. The rate of C6 EMD flipping is faster during PE dilution, which may be due to specific interaction of LacY with PE. Asymmetrical distribution of lipids across the lipid bilayer may accelerate the rate of protein flipping.
Because PE exchange kinetics fit double exponential functions and PC exchange kinetics fit a single exponential function, PE exchange appears to be more complex than PC exchange. Although PE and PC share the ability to dilute the high negative charge density of the membrane surface due to PG and CL, they mainly differ in their ability to form a lipid bilayer. The nonbilayer-prone properties of PE could lead to variations in the membrane curvature of proteoliposomes as the PE content is varied, which would not occur when varying PC content. This difference in properties could account for the divergent kinetics of exchange.
After triggering lipid exchange, protein flipping and lipid transbilayer flipping showed clear double exponential time courses. Because the lipid modulation acts to trigger topological rearrangement, the double exponential nature of this lipid stimulus will necessarily reverberate onward to any subsequent processes. In the case of PC, despite the single exponential nature of the stimulus, each subsequent event displayed a double exponential time course as well, which reflects the complex nature of the events leading to membrane protein topological rearrangement.
Besides the surprisingly rapid rate of lipid-induced protein flipping, a differential effect of LacY protein flipping on transbilayer movement of NBD-PE was observed depending on whether PE was added or diluted. The time constants for NBD-PE transbilayer flipping were similar for CscB and LacY under conditions where no protein flipping occurred. However, under conditions where LacY flipping occurred, there was a significant acceleration in the fast rate for NBD-PE transbilayer flipping during addition (36 s) vs. dilution (61 s) of PE. Flipping of C6 occurred about as fast as lipid translocation during addition of PE (34 s vs. 36 s), whereas the rate of C6 flipping was accelerated during the dilution of PE (26 s) over the rate of lipid translocation (61 s). The latter differences are even greater for the weighted average time constants. These results suggest that LacY flipping accelerates the rate of PE translocation during addition of PE even though the rate of LacY flipping is slower than when PE is diluted. How can these differences be rationalized? Suárez-Germà et al. (30, 31) demonstrated by FRET between pyrene-labeled lipids and LacY containing a single Trp residue that PE preferentially associates with LacY due to the exclusion of PG and CL. When adding PE to PG/CL proteoliposomes, newly introduced PE could preferentially interact with LacY and rapidly displace PG/CL from the annular region, resulting in LacY flipping accelerating PE flipping. A lower degree of asymmetry would be generated at early time points because LacY flipping would equilibrate PE more rapidly across the bilayer. During dilution of PE, the newly introduced lipids (including NBD-PE) may not displace PE molecules already tightly associated with LacY as rapidly. LacY topological rearrangement may initially only drag along associated PE, resulting in a decreased rate of NDB-PE flipping and generating bilayer lipid asymmetry at early time points that might accelerate LacY flipping. These results suggest that bilayer lipid asymmetry (higher during PE dilution) accelerates the rate of protein TMD flipping. There was little difference in the rate of C6 EMD flipping in experiments where PC was added or diluted with PG. This may be due to the reduced ability of PC vs. PE in supporting native structure and function of LacY in vitro (25). Future experiments with proteoliposomes exhibiting asymmetrical lipid distribution need to be carried out and would be of particular interest with respect to lipid asymmetry in biological membranes.
The results presented here demonstrate that (i) lipid exchange occurs first on a second-scale at 20 °C and is followed by protein topological rearrangement and transbilayer movement of lipid, (ii) protein flipping occurs rapidly on a second to minute scale at 20 °C, (iii) the hinge region (C6-TMD VII-P7) constitutes the lipid-sensor region undergoing the earliest topological rearrangement in both directions, (iv) transbilayer lipid flipping occurs faster in the presence of an α-helical membrane protein and is further enhanced by a protein that undergoes TMD flipping, (v) rates of flipping of individual lipid classes may depend on their type of interactions with membrane proteins, and (vi) asymmetrical distribution of lipids across the lipid bilayer may accelerate the rate of protein flipping. Our results further document the dynamic properties of membrane proteins and demonstrate that TMD orientation within even complex 12-TMD proteins is not fixed at the time of initial membrane insertion. These results may explain how topological heterogeneity can arise cotranslationally through perturbations of intermediate protein folding equilibriums sensitive to the effective charge of EMDs as modulated by the negative surface charge density of the membrane (charge balance rule).
Lack of FRET between the endogenous Trp resides and the acceptor (Fig. S1D) indicates we are measuring only events related to the added Trp residues. Although numerous structural changes are occurring during protein flipping, the small changes in endogenous Trp residue fluorescence indicate there is surprisingly little unfolding of the NT six-TMD helical bundle. Therefore, it is unlikely that the lipid-dependent fluorescence changes and subsequent FRET associated with Trp residues in NT and C6 are mainly due to local unfolding events rather than protein flipping. In addition, we know that the P7 domain undergoes sufficient structural changes to affect its recognition by a mAb (22–24). However, lipid-dependent changes in P7 fluorescence and resulting FRET were very minor (Fig. S1C).
Preliminary measurements of protein flipping at 37 °C in our system were too fast to measure with any precision. This phenomenon would therefore occur fast enough in vivo to be of physiological significance with no mandatory requirement for other cellular factors. Assuming a Q10 (the fold change in a rate for a change of 10 °C in temperature) of 2 for these events suggests that protein topology switching and lipid flipping might occur ∼3.5-fold faster at physiological temperatures. The rapid rate at which such topological changes occur in the absence of other cellular factors supports the possibility of such interconversions in any cell membrane at any time. Thus, demonstration of rapid topological switching may explain lipid-dependent postassembly functional changes and responses to stimuli in other membrane proteins. Because changes in the net charge of EMDs also affect orientation of neighboring TMDs (1), phosphorylation of membrane proteins may also result in rapid topological changes.
Methods
More detailed methods and results can be found in SI Methods.
Strain AL95 (null for PE and LacY synthesis) with (PE-containing) or without (PE-lacking) plasmid pDD72GM (encodes for PE synthesis) and strain AL95 with plasmid pAC-PCSlp-Sp-Gm (encodes for PC synthesis in place of PE) were grown (6, 32) and used to prepare lipid extracts for preparation of proteoliposomes, protein-free liposomes, and MLVs (7) as previously described. Strain AL95 with or without plasmid pDD72GM (6) was used as the host for expression of plasmid pT7-5/C-less LacY (ampR, ColE1 replicon) encoding LacY in which Cys residues are replaced by Ser, plasmid pT7-5/C-less LacY/V331C (ampR, ColE1 replicon) encoding a single Cys replacement at Val331 in otherwise Cys-less LacY, or plasmid pSP72/CscB-WT (ampR, ColE1 replicon) encoding WT CscB; plasmids were provided by H. R. Kaback (University of California, Los Angeles). Trp replacements were made in EMDs NT, C6, and P7 at positions 14, 205, and 250, respectively (Figs. 1 and 2). All LacY derivatives and CscB were purified from cells grown as previously described. Labeling of LacY with IAEDANS was carried out at 100 μM protein in 10 mM Tris⋅HCl/0.05% β-d-dodecylmaltoside (pH 7.5). Lipid exchange was carried out using a modified protocol (7) adapted from Cheng et al. (26). Real-time lipid exchange was monitored at an excitation wavelength of 470 nm (for NBD) and at an emission wavelength of 580 nm (for Rhodamine), both with a 0.5-nm band pass. Real-time protein flipping was monitored at an excitation wavelength of 295 nm (for Trp) and at an emission wavelength of 475 nm (for IAEDANS), both with a 1-nm band pass. The transbilayer flipping of head group-labeled NBD-PE was measured using the dithionite method (27).
SI Methods
Reagents.
Fluorescent head group-labeled 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl (NBD-PE), fatty acid-labeled 1-oleoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-[phospho-rac-(1-glycerol)] (NBD-PG), fatty acid-labeled 1-oleoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphocholine (NBD-PC), and headgroup-labeled 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine Rhodamine B sulfonyl) (Rhodamine-PE) were purchased from Avanti Polar Lipids. IAEDANS was purchased from Molecular Probes. HiTrap columns, the PD10 desalting column, and Vivaspin concentrators (50,000 molecular weight cutoff) were purchased from GE Healthcare. The enhanced chemiluminescence (ECL) kit, Imperial protein stain solution, horse radish peroxidase (HRP)-labeled secondary antibody, micro-bicinchoninic acid assay (BCA) protein reagent assay, Slide-A-Lyzer G2 Dialysis Cassettes, and avidin-HRP were purchased from Thermo Pierce. Octyl glucoside and β-d-dodecylmaltoside (DDM) were purchased from Anatrace. The QuikChange Lightning site-directed mutagenesis kit was purchased from Agilent Technologies. Complete protease inhibitor was purchased from Roche Molecular Biochemicals. SpinOUT columns were purchased from G Biosciences. DNase and MβCD and all other reagents were purchased from Sigma.
Bacterial Strains and Plasmids.
Strain AL95 (null for PE and LacY synthesis) with (WT lipid composition) or without (lacking PE and containing mostly PG and CL) plasmid pDD72GM (encodes PE synthesis) and strain AL95 with plasmid pAC-PCSlp-Sp-Gm (encodes for PC synthesized under anhydrotetracycline control in place of PE) were grown as previously described and used to prepare lipid extracts for preparation of proteoliposomes and protein-free liposomes and MLVs (6, 32). Strain AL95 with or without plasmid pDD72GM (6) was used as the host for expression of plasmid pT7-5/C-less LacY encoding LacY in which Cys residues are replaced by Ser, plasmid pT7-5/C-less LacY/V331C encoding a single Cys replacement at Val331 in otherwise Cys-less LacY, or plasmid pSP72/CscB-WT encoding WT CscB; plasmids were provided by H. R. Kaback (University of California, Los Angeles).
Construction of LacY Derivatives.
LacY Trp derivatives were constructed by site-specific mutagenesis using the QuikChange Lightning site-directed mutagenesis kit and plasmid pT7-5/C-less LacY/V331C as a template. Trp replacements were made in EMDs NT, C6, and P7 at positions 14, 205, and 250, respectively (Figs. 1 and 2A).
Growth Conditions and Protein Purification.
All LacY derivatives and CscB were engineered with a His6-tag at the C terminus to facilitate purification and were expressed under control of LacY operator-promoter (OPlac) by growth of cells in the presence of 1 mM isopropyl β-thiogalactoside (IPTG). Cells were grown in rich LB containing ampicillin (100 μg/mL) to an OD600 of 0.6, induced by addition of IPTG, and grown until cell arrest occurred. Purification of LacY derivatives and CscB was carried out at 4 °C or on ice as previously described (7). Protein content during purification and the concentration of protein in proteoliposomes were determined by the micro-BCA protein assay according to the manufacturer’s instructions.
Preparation of Total Lipid Extracts.
Total lipid extracts from PE-containing (AL95/pDD72GM), PE-lacking (AL95), and PC-containing (AL95/pAC-PCSlp-Sp-Gm) cells were prepared from cells grown to an OD600 of 0.7–0.8 in LB with appropriate additives. Cells were harvested by centrifugation, and phospholipids were quantitatively extracted from cell pellets by vigorously vortexing in chloroform/methanol (2:1 vol/vol). After centrifugation, the pellet was resuspended for a second extraction in methanol containing 0.1 N of acetic acid. Supernatants from the two extractions were pooled and concentrated under vacuum using a SpeedVac concentrator (Savant) and stored at −20 °C in chloroform/methanol (9:1 vol/vol) until further use.
IAEDANS Labeling.
Trp replacement derivatives of LacY/V331C were purified from PE-containing cells because previous results demonstrated that proteoliposome lipid composition, and not the cell source lipid composition, determines LacY organization (7). The protein used in the labeling reaction was freshly purified and diluted (if necessary) to a final concentration of 1 mg/mL. Labeling of LacY with IAEDANS was carried out at 100 μM protein in 10 mM Tris⋅HCl/0.05% (wt/vol) DDM (pH 7.5). A 10-fold molar excess of IAEDANS was added drop-wise to initiate the labeling reaction. The reaction was left in the dark for 2 h at room temperature, and an additional batch of 10-fold molar excess IAEDANS was then added for an additional 2 h at room temperature. The reaction was stopped by addition of a 10-fold excess of DTT, and unreacted dye was removed by buffer exchange against 10 mM Tris⋅HCl/0.05% DDM (pH 7.5) using SpinOUT columns. The labeling ratio was quantified by comparing the absorption spectrum of labeled protein with unlabeled protein using extinction coefficients of ε334 = 5,700 M−1⋅cm−1 for IAEDANS. Typical labeling efficiency was above 85%.
Preparation of Proteoliposomes and Lipid-Exchanged Proteoliposomes.
All natural and synthetic lipid compositions are in mol %. Proteoliposomes were formed by reconstitution of protein into SUVs of various lipid compositions with or without 2% Rhodamine-PE as previously described (7), by incorporation of non-IAEDANS–labeled LacY, CscB, or IAEDANS-labeled LacY into preformed liposomes. MLV populations composed of various lipid compositions with or without either 0.5% head group-labeled NBD-PE or 0.5% fatty acid-labeled NBD-PG or NBD-PC were prepared as previously described (7). MLVs composed of various lipid compositions with 1% head group-labeled NBD-PE were prepared as previously described (7) and used to monitor lipid flipping across the bilayer. A total of 500 μL of MLVs at 25 mM total lipid concentration was mixed with 95 μL of 390 mM MβCD and incubated at 37 °C for 2 h to generate MβCD-loaded MLVs. Lipid exchange between MLVs (at 625 μM) and proteoliposomes (SUV at 500 μM) dependent on addition of MβCD was carried out using a modified protocol (7) adapted from Cheng et al. (26). Depending on the type of fluorescent measurement performed, different mixing orders of the components were performed (described below). Liposomes and MLVs were made from total lipid extracts from PE-containing (75% PE/15% PG/5% CL), PE-lacking (50% PG/45% CL), or PC-containing (70% PC/3% PG/26% CL) E. coli.
Fluorescence Measurements.
The various fluorescence measurements were performed using a QuantaMaster model QM3-SS (Photon Technology International), a cuvette-based fluorescence spectrometer. Using a Peltier TE temperature controller, the sample was held at a constant 20 °C. Data were collected and analyzed using Felix 32 software.
Real-time lipid exchange of head group-labeled NBD-PE, fatty acid-labeled NBD-PG, or NBD-PC from MLVs to proteoliposomes containing Rhodamine-PE was monitored at an excitation wavelength of 470 nm (for NBD) and at an emission wavelength of 580 nm (for Rhodamine), both with a 0.5-nm band pass. A MLV-MβCD mixture was prepared as previously described (7), with the addition of 0.5% NBD-labeled phospholipid. The MLV-MβCD mixture (50 μL of the MLV-MβCD mixture at 25 mM phospholipids in a 2-mL final reaction volume) was first equilibrated at 20 °C. Lipid exchange was triggered by addition of proteoliposomes containing 1 μM LacY (Cys-less) at a lipid-to-protein ratio (wt/wt) of 500 containing 2% N-Rhodamine-PE under constant stirring at 20 °C. To normalize the data, liposomes and proteoliposomes of various phospholipid compositions containing 2% Rhodamine-PE or liposomes and proteoliposomes of various phospholipid compositions containing both 0.5% NBD-labeled phospholipid and 2% Rhodamine-PE were used to determine the minimum (Fmin) or maximum (Fmax) of fluorescence, respectively. The measured FRET values were the normalized using Eq. S1:
| [S1] |
Real-time protein flipping was monitored at an excitation wavelength of 295 nm (for Trp) and at an emission wavelength of 475 nm (for IAEDANS), both with a 1-nm band pass. The proteoliposomes [containing 1 μM IAEDANS-labeled LacY at a lipid-to-protein ratio (wt/wt) of 500] were first equilibrated at 20 °C. Lipid exchange was triggered by addition of the MLV-MβCD mixture (50 μL of the MLV-MβCD mixture at 25 mM phospholipids in a 2-mL final reaction volume) under constant stirring at 20 °C. The fluorescence spectra recorded between 330 and 550 nm, after excitation of Trp at 295 nm, displayed two maxima: the emission of the donor visualized around 340 nm and the emission of the acceptor visualized around 475 nm. We used the FRET values observed at 0% (F0%) and 70% (F70%) PE to normalize the FRET values (F) and to plot the relationship between amounts of PE and FRET (Fig. 2) using Eq. S2:
| [S2] |
In the case of the Trp replacement in EMD P7 (where no maximal FRET will be observed because this EMD stays across the membrane from the acceptor), normalization was performed using maximal FRET values obtained for Trp replacement in EMD C6. In all cases, before starting kinetics measurements, care was taken to measure Trp fluorescence before lipid exchange to verify that the fluorescence signal from the donor was in the range of what was to be expected.
The transbilayer flipping of head group-labeled NBD-PE was measured using the dithionite method (27). Liposomes or proteoliposomes containing either LacY or CscB were prepared in the presence of 50 mM potassium phosphate (KPi; pH 7.5), 2 mM β-mercaptoethanol, and 10 mM sodium dithionite as previously described (27). Unincorporated dithionite was removed by size exclusion chromatography on a PD10 column equilibrated with 50 mM KPi (pH 7.5), 2 mM β-mercaptoethanol, and 10 mM NaCl. MβCD-equilibrated MLVs (50 μL of the MLV-MβCD mixture at 25 mM phospholipids in a 2-mL final reaction volume) containing head group-labeled NBD-PE at 1% lipid were prepared and equilibrated at 20 °C. Transbilayer movement of phospholipids was monitored at an excitation wavelength of 470 nm and at an emission wavelength of 540 nm (for NBD), both with a 0.5-nm band pass. Initial NBD fluorescence emission (Fmax) was measured. Lipid exchange was then triggered by addition of liposomes or proteoliposomes (SUVs at 500 μM) containing encapsulated sodium dithionite under constant stirring at 20 °C. The fluorescence emission intensity (F(t)) was recorded vs. time. The maximum fluorescence quenching (F0) was obtained after vesicles were solubilized using Triton X-100 in the presence of additional 10 mM dithionite. The fraction of NBD-PE protected from internal dithionite due to location in the outer leaflet was normalized [F(t)norm] using Eq. S3:
| [S3] |
Data Analysis and Fitting.
For data analysis, the time course of F(t)norm was averaged across experiments and fitted to a single or double exponential function beginning shortly after triggering lipid exchange (∼35–38 second time point) using Origin 8.6 (OriginLab Corp.). In all figures, solid lines indicate these fits and error bars mark the upper and lower confidence limits (95%) for the fitted parameters. In all graphs, we used weighted tau as a representation of the relative timing of each event. Weighted tau was calculated using Eq. S4:
| [S4] |
Acknowledgments
We thank Dr. H. R. Kaback (University of California, Los Angeles) for providing plasmids encoding LacY and CscB. This work was supported by NIH Grants R37 GM 20478 (to W.D.) and R01 GM 113212 and R01 GM 094246-04 (to V.J.) and by the John Dunn Research Foundation (W.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512994112/-/DCSupplemental.
References
- 1.Bogdanov M, Dowhan W, Vitrac H. Lipids and topological rules governing membrane protein assembly. Biochim Biophys Acta. 2014;1843(8):1475–1488. doi: 10.1016/j.bbamcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaback HR. A chemiosmotic mechanism of symport. Proc Natl Acad Sci USA. 2015;112(5):1259–1264. doi: 10.1073/pnas.1419325112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowhan W. A retrospective: Use of Escherichia coli as a vehicle to study phospholipid synthesis and function. Biochim Biophys Acta. 2013;1831(3):471–494. doi: 10.1016/j.bbalip.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdanov M, Xie J, Heacock P, Dowhan W. To flip or not to flip: Lipid-protein charge interactions are a determinant of final membrane protein topology. J Cell Biol. 2008;182(5):925–935. doi: 10.1083/jcb.200803097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogdanov M, Dowhan W. Lipid-dependent generation of dual topology for a membrane protein. J Biol Chem. 2012;287(45):37939–37948. doi: 10.1074/jbc.M112.404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogdanov M, Heacock PN, Dowhan W. A polytopic membrane protein displays a reversible topology dependent on membrane lipid composition. EMBO J. 2002;21(9):2107–2116. doi: 10.1093/emboj/21.9.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitrac H, Bogdanov M, Dowhan W. In vitro reconstitution of lipid-dependent dual topology and postassembly topological switching of a membrane protein. Proc Natl Acad Sci USA. 2013;110(23):9338–9343. doi: 10.1073/pnas.1304375110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Bogdanov M, Dowhan W. Topology of polytopic membrane protein subdomains is dictated by membrane phospholipid composition. EMBO J. 2002;21(21):5673–5681. doi: 10.1093/emboj/cdf571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 1986;5(11):3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989;341(6241):456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]
- 11.von Heijne G. Membrane-protein topology. Nat Rev Mol Cell Biol. 2006;7(12):909–918. doi: 10.1038/nrm2063. [DOI] [PubMed] [Google Scholar]
- 12.von Heijne G, Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]
- 13.Seppälä S, Slusky JS, Lloris-Garcerá P, Rapp M, von Heijne G. Control of membrane protein topology by a single C-terminal residue. Science. 2010;328(5986):1698–1700. doi: 10.1126/science.1188950. [DOI] [PubMed] [Google Scholar]
- 14.Fontaine F, Fuchs RT, Storz G. Membrane localization of small proteins in Escherichia coli. J Biol Chem. 2011;286(37):32464–32474. doi: 10.1074/jbc.M111.245696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIlwain BC, Vandenberg RJ, Ryan RM. Transport rates of a glutamate transporter homologue are influenced by the lipid bilayer. J Biol Chem. 2015;290(15):9780–9788. doi: 10.1074/jbc.M114.630590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serdiuk T, Sugihara J, Mari SA, Kaback HR, Müller DJ. Observing a lipid-dependent alteration in single lactose permeases. Structure. 2015;23(4):754–761. doi: 10.1016/j.str.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tunuguntla R, et al. Lipid bilayer composition can influence the orientation of proteorhodopsin in artificial membranes. Biophys J. 2013;105(6):1388–1396. doi: 10.1016/j.bpj.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vitrac H, Bogdanov M, Heacock P, Dowhan W. Lipids and topological rules of membrane protein assembly: Balance between long and short range lipid-protein interactions. J Biol Chem. 2011;286(17):15182–15194. doi: 10.1074/jbc.M110.214387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang X, et al. Evidence for an intermediate conformational state of LacY. Proc Natl Acad Sci USA. 2012;109(12):E698–E704. doi: 10.1073/pnas.1201107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar H, et al. Structure of sugar-bound LacY. Proc Natl Acad Sci USA. 2014;111(5):1784–1788. doi: 10.1073/pnas.1324141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu P, Brand L. Conformational flexibility in a staphylococcal nuclease mutant K45C from time-resolved resonance energy transfer measurements. Biochemistry. 1994;33(34):10457–10462. doi: 10.1021/bi00200a029. [DOI] [PubMed] [Google Scholar]
- 22.Bogdanov M, Sun J, Kaback HR, Dowhan W. A phospholipid acts as a chaperone in assembly of a membrane transport protein. J Biol Chem. 1996;271(20):11615–11618. doi: 10.1074/jbc.271.20.11615. [DOI] [PubMed] [Google Scholar]
- 23.Frillingos S, Kaback HR. Monoclonal antibody 4B1 alters the pKa of a carboxylic acid at position 325 (helix X) of the lactose permease of Escherichia coli. Biochemistry. 1996;35(31):10166–10171. doi: 10.1021/bi960995r. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Wu J, Carrasco N, Kaback HR. Identification of the epitope for monoclonal antibody 4B1 which uncouples lactose and proton translocation in the lactose permease of Escherichia coli. Biochemistry. 1996;35(3):990–998. doi: 10.1021/bi952166w. [DOI] [PubMed] [Google Scholar]
- 25.Vitrac H, Bogdanov M, Dowhan W. Proper fatty acid composition rather than an ionizable lipid amine is required for full transport function of lactose permease from Escherichia coli. J Biol Chem. 2013;288(8):5873–5885. doi: 10.1074/jbc.M112.442988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng HT, Megha, London E. Preparation and properties of asymmetric vesicles that mimic cell membranes: Effect upon lipid raft formation and transmembrane helix orientation. J Biol Chem. 2009;284(10):6079–6092. doi: 10.1074/jbc.M806077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIntyre JC, Sleight RG. Fluorescence assay for phospholipid membrane asymmetry. Biochemistry. 1991;30(51):11819–11827. doi: 10.1021/bi00115a012. [DOI] [PubMed] [Google Scholar]
- 28.Kol MA, van Dalen A, de Kroon AI, de Kruijff B. Translocation of phospholipids is facilitated by a subset of membrane-spanning proteins of the bacterial cytoplasmic membrane. J Biol Chem. 2003;278(27):24586–24593. doi: 10.1074/jbc.M301875200. [DOI] [PubMed] [Google Scholar]
- 29.Van Voorst F, De Kruijff B. Role of lipids in the translocation of proteins across membranes. Biochem J. 2000;347(Pt 3):601–612. doi: 10.1042/0264-6021:3470601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suárez-Germà C, Hernández-Borrell J, Prieto M, Loura LM. Modeling FRET to investigate the selectivity of lactose permease of Escherichia coli for lipids. Mol Membr Biol. 2014;31(4):120–130. doi: 10.3109/09687688.2014.915351. [DOI] [PubMed] [Google Scholar]
- 31.Suárez-Germà C, et al. Phospholipid-lactose permease interaction as reported by a head-labeled pyrene phosphatidylethanolamine: A FRET study. J Phys Chem B. 2013;117(22):6741–6748. doi: 10.1021/jp402152n. [DOI] [PubMed] [Google Scholar]
- 32.Bogdanov M, Heacock P, Guan Z, Dowhan W. Plasticity of lipid-protein interactions in the function and topogenesis of the membrane protein lactose permease from Escherichia coli. Proc Natl Acad Sci USA. 2010;107(34):15057–15062. doi: 10.1073/pnas.1006286107. [DOI] [PMC free article] [PubMed] [Google Scholar]