Significance
Within a given gene, there may be many possible mutations that are capable of producing a particular change in phenotype. However, if some sites have especially high rates of mutation to function-altering alleles, then such mutations may make disproportionate contributions to phenotypic evolution. We report the discovery that a point mutation at a highly mutable site in the β-globin gene of Andean house wrens has produced a physiologically important change in the oxygenation properties of hemoglobin (Hb). The mutant allele that confers an increased Hb–O2 affinity is present at an unusually high frequency at high altitude. These findings suggest that site-specific variation in mutation rate may exert a strong influence on the genetic basis of phenotypic evolution.
Keywords: biochemical adaptation, hemoglobin, high altitude, hypoxia, mutation bias
Abstract
A key question in evolutionary genetics is why certain mutations or certain types of mutation make disproportionate contributions to adaptive phenotypic evolution. In principle, the preferential fixation of particular mutations could stem directly from variation in the underlying rate of mutation to function-altering alleles. However, the influence of mutation bias on the genetic architecture of phenotypic evolution is difficult to evaluate because data on rates of mutation to function-altering alleles are seldom available. Here, we report the discovery that a single point mutation at a highly mutable site in the βA-globin gene has contributed to an evolutionary change in hemoglobin (Hb) function in high-altitude Andean house wrens (Troglodytes aedon). Results of experiments on native Hb variants and engineered, recombinant Hb mutants demonstrate that a nonsynonymous mutation at a CpG dinucleotide in the βA-globin gene is responsible for an evolved difference in Hb–O2 affinity between high- and low-altitude house wren populations. Moreover, patterns of genomic differentiation between high- and low-altitude populations suggest that altitudinal differentiation in allele frequencies at the causal amino acid polymorphism reflects a history of spatially varying selection. The experimental results highlight the influence of mutation rate on the genetic basis of phenotypic evolution by demonstrating that a large-effect allele at a highly mutable CpG site has promoted physiological differentiation in blood O2 transport capacity between house wren populations that are native to different elevations.
An important question in evolutionary genetics is whether certain mutations or certain types of mutation make disproportionate contributions to phenotypic evolution (1–6). Within a given gene, the mutations that contribute to evolutionary changes in phenotype may represent a biased, nonrandom subset of all possible mutations that are capable of producing the same functional effect. The preferential fixation of particular mutations (substitution bias) could have several causes. Most theoretical and empirical attention has focused on causes of fixation bias, i.e., mutations have different probabilities of being fixed once they arise, due to differences in dominance coefficients or the magnitude of deleterious pleiotropy (1, 2, 4, 7–9). In principle, substitution bias can also stem directly from mutation bias (some sites have higher rates of mutation to alleles that produce the change in phenotype) (4, 9–11). However, empirical evidence for the importance of mutation bias is scarce for an obvious reason: even in rare cases where it is possible to document the contributions of individual point mutations to evolutionary changes in phenotype, data on rates of mutation to function-altering alleles are typically lacking. Rare exceptions include cases where loss-of-function deletion mutations can be traced to hot spots of chromosomal instability or highly mutable changes in the copy number of repetitive elements (12). Documenting cases where genetic changes at highly mutable loci contribute to phenotypic divergence is therefore important for elucidating the evolutionary significance of mutation bias. This is especially true for cases where mutations cause fine-tuned modifications of protein activity rather than simple losses of function.
Here, we report the discovery that a single amino acid replacement at a mutational hot spot in the avian βA-globin gene has contributed to an evolutionary change in hemoglobin (Hb) function that has likely adaptive significance. By conducting experiments on native Hb variants and engineered recombinant Hb mutants, we demonstrate that a nonsynonymous mutation at a CpG dinucleotide in the βA-globin gene of Andean house wrens (Troglodytes aedon) has contributed to an evolved difference in Hb–O2 affinity between high- and low-altitude populations. In mammalian genomes, point mutations at CpG sites occur at a rate that is over an order of magnitude higher than the average for all other nucleotide sites (13, 14), and available data suggest a similar discrepancy in avian genomes (15, 16).
Andean house wrens are compelling subjects for studies of Hb function because this passerine bird species has an exceptionally broad and continuous elevational distribution, ranging from sea level to elevations >4,500 m (17). At 4,500-m elevation, the standard barometric pressure is ∼450 torr, so O2 partial pressure (PO2) is <60% that at sea level (∼96 torr compared to ∼160 torr). Under such conditions, enhancements of pulmonary O2 uptake and blood O2 transport capacity are required to sustain O2 flux to the tissue mitochondria in support of aerobic ATP synthesis (18). To complement changes in the cardiorespiratory system and microcirculation, changes in the O2-binding affinity and cooperativity of Hb can enhance the O2 capacitance of the blood (the total amount of O2 unloaded for a given arteriovenous difference in O2 tension). Because the optimal Hb–O2 affinity is expected to vary according to the ambient PO2, genetic variation in oxygenation properties of Hb may be subject to spatially varying selection between populations that inhabit different elevations. House wrens colonized South America in the late Pliocene or early Pleistocene via the newly formed Panamanian land bridge (19, 20), so the species may have been resident in the Andean highlands for up to ∼3 million years.
The Hb tetramer is composed of two semirigid α1β1 and α2β2 dimers that undergo a mutual rotation during the oxygenation-linked transition in quaternary structure between the deoxy (low-affinity “T”) conformation and the oxy (high-affinity “R”) conformation (21). This oxygenation-linked structural transition between the T and R states is the basis for cooperative O2 binding, and is central to the allosteric function of Hb as an O2 transport molecule. Our analysis of house wren Hb highlights the influence of mutation rate on the genetic basis of phenotypic divergence by demonstrating that mutation at a CpG dinucleotide produced a large-effect amino acid replacement at an α1β1 intradimer contact (β55Val→Ile)—a replacement that produced a significant increase in Hb–O2 affinity.
Results
We performed an integrative analysis of Hb polymorphism in Andean house wrens that combined a population genomic analysis of nucleotide variation with mechanistic studies of protein function. Our survey of Hb polymorphism in T. aedon was based on a total of 140 museum-vouchered specimens (Table S1) that we collected from a broad range of elevations in the Peruvian Andes (Fig. S1). Andean house wrens are characterized by a high degree of phylogeographic structure. In Peru alone, house wrens are divided into seven highly divergent mtDNA clades that have allopatric or parapatric distributions across the Andes (20). To minimize the confounding effects of population structure in our altitudinal survey of Hb polymorphism, we conducted a detailed population genetic analysis on a sample of 65 specimens from the western slope of the Andes in central Peru that are representatives of the same mtDNA clade (20). Comparisons between highland and lowland population samples were based on specimens collected from >3,000 and <1,000 m, respectively.
Table S1.
House wren samples used in this study with ARCTOS Museum of Southwestern Biology catalog web link, sampling details, and mtDNA clade from Galen and Witt (20)
| Catalog no. with embedded web link | NK | Day | Month | Elevation, m | Department | Latitude | Longitude | mtDNA clade |
| MSB:Bird:27052 | 159705 | 30 | Oct | 3,040 | Lima | −11.76 | −76.58 | 1 |
| MSB:Bird:27596 | 162008 | 3 | Jun | 366 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:27606 | 162022 | 3 | Jun | 366 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:27609 | 162025 | 3 | Jun | 366 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:31418 | 162982 | 8 | Jan | 372 | Lima | −12 | −76.92 | 1 |
| MSB:Bird:31425 | 162989 | 8 | Jan | 351 | Lima | −12 | −76.92 | 1 |
| MSB:Bird:31433 | 162997 | 8 | Jan | 372 | Lima | −12 | −76.92 | 1 |
| MSB:Bird:31450 | 163014 | 9 | Jan | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:31454 | 163018 | 9 | Jan | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:31456 | 163020 | 9 | Jan | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:31459 | 163023 | 9 | Jan | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:31469 | 163033 | 12 | Jan | 3,967 | Lima | −11.63 | −76.43 | 1 |
| MSB:Bird:31482 | 163046 | 12 | Jan | 3,959 | Lima | −11.63 | −76.43 | 1 |
| MSB:Bird:31489 | 163053 | 13 | Jan | 3,973 | Lima | −11.63 | −76.43 | 1 |
| MSB:Bird:31498 | 163062 | 13 | Jan | 3,981 | Lima | −11.63 | −76.43 | 1 |
| MSB:Bird:31503 | 163067 | 14 | Jan | 3,967 | Lima | −11.63 | −76.43 | 1 |
| MSB:Bird:31739 | 163411 | 7 | May | 3,750 | Lima | −11.76 | −76.55 | 1 |
| MSB:Bird:31756 | 163428 | 21 | May | 2,400 | Lima | −11.74 | −76.61 | 1 |
| MSB:Bird:31766 | 163438 | 24 | May | 2,400 | Lima | −11.74 | −76.61 | 1 |
| MSB:Bird:31767 | 163439 | 24 | May | 2,400 | Lima | −11.74 | −76.61 | 1 |
| MSB:Bird:32902 | 168074 | 15 | Oct | 935 | Lima | −12.03 | −76.65 | 1 |
| MSB:Bird:32909 | 168081 | 15 | Oct | 935 | Lima | −12.03 | −76.65 | 1 |
| MSB:Bird:32910 | 168082 | 15 | Oct | 935 | Lima | −12.03 | −76.65 | 1 |
| MSB:Bird:32922 | 168094 | 16 | Oct | 935 | Lima | −12.03 | −76.65 | 1 |
| MSB:Bird:32923 | 168095 | 16 | Oct | 935 | Lima | −12.03 | −76.65 | 1 |
| MSB:Bird:32943 | 168115 | 18 | Oct | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:32950 | 168122 | 18 | Oct | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:32966 | 168138 | 18 | Oct | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:32967 | 168139 | 18 | Oct | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:32969 | 168141 | 18 | Oct | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:32982 | 168154 | 19 | Oct | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:32988 | 168160 | 19 | Oct | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:32991 | 168163 | 19 | Oct | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:33001 | 168173 | 19 | Oct | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:33006 | 168178 | 19 | Oct | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:33008 | 168180 | 19 | Oct | 352 | Lima | −12.01 | −76.92 | 1 |
| MSB:Bird:33310 | 168529 | 4 | Sep | 4,056 | Lima | −11.77 | −76.53 | 1 |
| MSB:Bird:33329 | 168548 | 6 | Sep | 3,910 | Lima | −11.76 | −76.54 | 1 |
| MSB:Bird:33351 | 168570 | 9 | Sep | 3,907 | Lima | −11.77 | −76.53 | 1 |
| MSB:Bird:33370 | 168589 | 12 | Sep | 3,905 | Lima | −11.77 | −76.53 | 1 |
| MSB:Bird:33416 | 168635 | 17 | Sep | 4,056 | Lima | −11.77 | −76.53 | 1 |
| MSB:Bird:34736 | 171462 | 3 | Jul | 309 | La Libertad | −8.39 | −78.65 | 1 |
| MSB:Bird:34739 | 171465 | 3 | Jul | 309 | La Libertad | −8.39 | −78.65 | 1 |
| MSB:Bird:34763 | 171489 | 4 | Jul | 309 | La Libertad | −8.39 | −78.65 | 1 |
| MSB:Bird:34830 | 171556 | 8 | Jul | 2,972 | Ancash | −8.75 | −78.05 | 1 |
| MSB:Bird:34832 | 171558 | 8 | Jul | 2,972 | Ancash | −8.75 | −78.05 | 1 |
| MSB:Bird:34892 | 171618 | 11 | Jul | 2,972 | Ancash | −8.75 | −78.05 | 1 |
| MSB:Bird:34903 | 171629 | 12 | Jul | 357 | La Libertad | −8.69 | −78.38 | 1 |
| MSB:Bird:34907 | 171633 | 13 | Jul | 357 | La Libertad | −8.69 | −78.38 | 1 |
| MSB:Bird:34916 | 171642 | 13 | Jul | 357 | La Libertad | −8.69 | −78.38 | 1 |
| MSB:Bird:34920 | 171646 | 14 | Jul | 357 | La Libertad | −8.69 | −78.38 | 1 |
| MSB:Bird:34921 | 171647 | 14 | Jul | 357 | La Libertad | −8.69 | −78.38 | 1 |
| MSB:Bird:34953 | 171679 | 16 | Jul | 3,439 | Ancash | −9.34 | −77.51 | 1 |
| MSB:Bird:34965 | 171691 | 17 | Jul | 3,439 | Ancash | −9.34 | −77.51 | 1 |
| MSB:Bird:34966 | 171692 | 17 | Jul | 3,439 | Ancash | −9.34 | −77.51 | 1 |
| MSB:Bird:34967 | 171693 | 17 | Jul | 3,439 | Ancash | −9.34 | −77.51 | 1 |
| MSB:Bird:35007 | 171733 | 21 | Jul | 3,714 | Ancash | −9.1 | −77.87 | 1 |
| MSB:Bird:35018 | 171744 | 21 | Jul | 3,714 | Ancash | −9.1 | −77.87 | 1 |
| MSB:Bird:35538 | 172264 | 8 | Aug | 3,200 | Arequipa | −15.81 | −72.67 | 1 |
| MSB:Bird:36014 | 173845 | 18 | May | 3,740 | Ancash | −8.74 | −78.04 | 1 |
| MSB:Bird:36049 | 173880 | 23 | May | 3,740 | Ancash | −9.02 | −77.54 | 1 |
| MSB:Bird:36081 | 173912 | 30 | May | 3,350 | Ancash | −8.84 | −77.93 | 1 |
| MSB:Bird:36568 | 175520 | 22 | Oct | 4,000 | Lima | −11.77 | −76.53 | 1 |
| MSB:Bird:36573 | 175525 | 24 | Oct | 4,116 | Lima | −11.77 | −76.53 | 1 |
| MSB:Bird:36574 | 175526 | 24 | Oct | 4,123 | Lima | −11.77 | −76.53 | 1 |
| MSB:Bird:32351 | 167523 | 15 | Jul | 2,052 | Amazonas | −6.1 | −78.34 | 2 |
| MSB:Bird:32619 | 167791 | 22 | Jul | 2,066 | Amazonas | −6.1 | −78.34 | 2 |
| MSB:Bird:32855 | 168027 | 28 | Jul | 2,073 | Amazonas | −6.1 | −78.34 | 2 |
| MSB:Bird:32862 | 168034 | 29 | Jul | 2,073 | Amazonas | −6.1 | −78.34 | 2 |
| MSB:Bird:33894 | 169120 | 26 | Dec | 143 | Lambayeque | −5.9 | −79.79 | 2 |
| MSB:Bird:34057 | 169283 | 22 | Dec | 2,215 | Piura | −5.84 | −79.51 | 2 |
| MSB:Bird:34076 | 169302 | 23 | Dec | 2,215 | Piura | −5.84 | −79.51 | 2 |
| MSB:Bird:34773 | 171499 | 4 | Jul | 309 | La Libertad | −8.39 | −78.65 | 2 |
| MSB:Bird:34902 | 171628 | 12 | Jul | 357 | La Libertad | −8.69 | −78.38 | 2 |
| MSB:Bird:27066 | 159722 | 26 | Nov | 3,120 | Cusco | −13.63 | −71.72 | 3 |
| MSB:Bird:27076 | 159732 | 27 | Nov | 3,120 | Cusco | −13.63 | −71.72 | 3 |
| MSB:Bird:27083 | 159740 | 28 | Nov | 3,120 | Cusco | −13.63 | −71.72 | 3 |
| MSB:Bird:27131 | 159789 | 4 | Dec | 4,300 | Cusco | −13.2 | −72.16 | 3 |
| MSB:Bird:27132 | 159790 | 4 | Dec | 4,300 | Cusco | −13.2 | −72.16 | 3 |
| MSB:Bird:27154 | 159814 | 8 | Dec | 3,380 | Cusco | −13.25 | −72.17 | 3 |
| MSB:Bird:27174 | 159834 | 9 | Dec | 3,380 | Cusco | −13.25 | −72.17 | 3 |
| MSB:Bird:27179 | 159840 | 9 | Dec | 3,380 | Cusco | −13.25 | −72.17 | 3 |
| MSB:Bird:27181 | 159842 | 10 | Dec | 3,380 | Cusco | −13.25 | −72.17 | 3 |
| MSB:Bird:27203 | 159868 | 12 | Dec | 3,380 | Cusco | −13.25 | −72.17 | 3 |
| MSB:Bird:27218 | 159887 | 13 | Dec | 3,380 | Cusco | −13.25 | −72.17 | 3 |
| MSB:Bird:31835 | 163507 | 18 | Jun | 3,710 | Junin | −11.98 | −74.93 | 3 |
| MSB:Bird:33119 | 168338 | 12 | Mar | 4,030 | Cusco | −13.19 | −72.23 | 3 |
| MSB:Bird:33650 | 168876 | 4 | Dec | 3,573 | Apurimac | −14.41 | −73.09 | 3 |
| MSB:Bird:33659 | 168885 | 5 | Dec | 3,548 | Apurimac | −14.41 | −73.09 | 3 |
| MSB:Bird:34084 | 169310 | 9 | Jan | 4,369 | Apurimac | −14.06 | −73.01 | 3 |
| MSB:Bird:34085 | 169311 | 9 | Jan | 4,454 | Apurimac | −14.06 | −73 | 3 |
| MSB:Bird:34089 | 169315 | 9 | Jan | 4,375 | Apurimac | −14.06 | −73.01 | 3 |
| MSB:Bird:34106 | 169332 | 9 | Jan | 4,384 | Apurimac | −14.06 | −73.01 | 3 |
| MSB:Bird:34109 | 169335 | 10 | Jan | 4,375 | Apurimac | −14.06 | −73.01 | 3 |
| MSB:Bird:34114 | 169340 | 10 | Jan | 4,401 | Apurimac | −14.06 | −73.01 | 3 |
| MSB:Bird:34202 | 169428 | 14 | Jan | 4,363 | Apurimac | −14.06 | −73 | 3 |
| MSB:Bird:34295 | 171021 | 30 | May | 1,500 | Cusco | −12.65 | −72.32 | 3 |
| MSB:Bird:34359 | 171085 | 3 | Jun | 1,500 | Cusco | −12.65 | −72.32 | 3 |
| MSB:Bird:35522 | 172248 | 8 | Aug | 3,200 | Arequipa | −15.81 | −72.67 | 3 |
| MSB:Bird:35700 | 172426 | 28 | Sep | 2,671 | Apurimac | −14.17 | −73.32 | 3 |
| MSB:Bird:35822 | 172637 | 5 | Aug | 3,201 | Cusco | −13.08 | −72.37 | 3 |
| MSB:Bird:35907 | 172722 | 21 | Sep | 2,672 | Apurimac | −14.17 | −73.32 | 3 |
| MSB:Bird:35908 | 172723 | 21 | Sep | 2,671 | Apurimac | −14.17 | −73.32 | 3 |
| MSB:Bird:28029 | 162535 | 20 | Jun | 322 | San Martín | −6.65 | −76.07 | 4 |
| MSB:Bird:33581 | 168807 | 19 | Nov | 2,500 | Cusco | −13.56 | −70.88 | 4 |
| MSB:Bird:36130 | 173961 | 13 | Jun | 1,673 | San Martin | −7.42 | −76.29 | 4 |
| MSB:Bird:36909 | 176089 | 23 | Jun | 292 | Madre de Dios | −11.71 | −69.21 | 4 |
| MSB:Bird:37014 | 176194 | 26 | Jun | 297 | Madre de Dios | −11.71 | −69.21 | 4 |
| MSB:Bird:37084 | 176264 | 28 | Jun | 297 | Madre de Dios | −11.71 | −69.21 | 4 |
| MSB:Bird:37149 | 176329 | 30 | Jun | 290 | Madre de Dios | −11.71 | −69.21 | 4 |
| MSB:Bird:37202 | 176382 | 1 | Jul | 297 | Madre de Dios | −11.71 | −69.21 | 4 |
| MSB:Bird:37318 | 176498 | 4 | Jul | 297 | Madre de Dios | −11.71 | −69.21 | 4 |
| MSB:Bird:37341 | 176521 | 5 | Jul | 297 | Madre de Dios | −11.71 | −69.21 | 4 |
| MSB:Bird:33720 | 168946 | 15 | Dec | 133 | Lambayeque | −5.9 | −79.79 | 5 |
| MSB:Bird:33725 | 168951 | 15 | Dec | 133 | Lambayeque | −5.9 | −79.79 | 5 |
| MSB:Bird:33758 | 168984 | 16 | Dec | 129 | Lambayeque | −5.9 | −79.78 | 5 |
| MSB:Bird:33778 | 169004 | 18 | Dec | 133 | Lambayeque | −5.9 | −79.79 | 5 |
| MSB:Bird:33779 | 169005 | 18 | Dec | 133 | Lambayeque | −5.9 | −79.79 | 5 |
| MSB:Bird:33844 | 169070 | 21 | Dec | 133 | Lambayeque | −5.9 | −79.79 | 5 |
| MSB:Bird:33889 | 169115 | 25 | Dec | 143 | Lambayeque | −5.9 | −79.79 | 5 |
| MSB:Bird:34698 | 171424 | 2 | Jul | 309 | La Libertad | −8.39 | −78.65 | 5 |
| MSB:Bird:34699 | 171425 | 2 | Jul | 309 | La Libertad | −8.39 | −78.65 | 5 |
| MSB:Bird:34893 | 171619 | 11 | Jul | 2,972 | Ancash | −8.75 | −78.05 | 5 |
| MSB:Bird:35250 | 171976 | 13 | Jul | 2,500 | Cajamarca | −7.4 | −78.78 | 5 |
| MSB:Bird:35330 | 172056 | 17 | Jul | 2,550 | Cajamarca | −7.4 | −78.78 | 5 |
| MSB:Bird:35393 | 172119 | 21 | Jul | 2,550 | Cajamarca | −7.4 | −78.78 | 5 |
| MSB:Bird:35402 | 172128 | 22 | Jul | 2,550 | Cajamarca | −7.4 | −78.78 | 5 |
| MSB:Bird:35035 | 171761 | 30 | Jul | 740 | Tacna | −17.56 | −70.67 | 6 |
| MSB:Bird:35043 | 171769 | 31 | Jul | 740 | Tacna | −17.56 | −70.67 | 6 |
| MSB:Bird:35046 | 171772 | 31 | Jul | 740 | Tacna | −17.56 | −70.67 | 6 |
| MSB:Bird:35047 | 171773 | 31 | Jul | 740 | Tacna | −17.56 | −70.67 | 6 |
| MSB:Bird:35057 | 171783 | 1 | Aug | 740 | Tacna | −17.56 | −70.67 | 6 |
| MSB:Bird:35442 | 172168 | 1 | Aug | 2,200 | Tacna | −17.39 | −70.35 | 6 |
| MSB:Bird:35476 | 172202 | 3 | Aug | 2,975 | Tacna | −17.32 | −70.25 | 6 |
| MSB:Bird:35491 | 172217 | 4 | Aug | 2,975 | Tacna | −17.32 | −70.25 | 6 |
| MSB:Bird:35493 | 172219 | 4 | Aug | 2,975 | Tacna | −17.32 | −70.25 | 6 |
| MSB:Bird:35507 | 172233 | 4 | Aug | 2,975 | Tacna | −17.32 | −70.25 | 6 |
| MSB:Bird:35512 | 172238 | 5 | Aug | 2,975 | Tacna | −17.32 | −70.25 | 6 |
| MSB:Bird:31626 | 163191 | 29 | Jan | 2,798 | Huánuco | −9.73 | −76.11 | 7 |
| MSB:Bird:35697 | 172423 | 27 | Nov | — | Ancash | — | — | 7 |
Fig. S1.
Phylogeographic population structure of house wrens sampled from throughout Peru. The tree (Left) shows relationships among well-defined mtDNA clades. In the map (Right), color coding of symbols shows the proportional representation of different mtDNA clades in samples of house wren specimens from each locality. Specimens comprising the “central Peru clade” served as the focus for the elevational survey of genomic polymorphism, as described in the main text.
Hb Isoform Composition of Red Blood Cells.
Most birds express two structurally distinct Hb isoforms during adult life: HbA and HbD (22). HbA is typically the major isoform, constituting ∼60–80% of adult Hb in passerine birds (22, 23). The major HbA isoform incorporates α-chain products of the αA-globin gene, and the minor HbD isoform incorporates products of the αD-globin gene; both isoforms incorporate β-chain products of the same βA-globin gene. To characterize the red cell Hb isoform composition of house wrens, we analyzed blood samples from individual specimens using a combination of isoelectric focusing (IEF) and tandem mass spectrometry (MS/MS). Consistent with data from other passerines, our wild-caught house wrens expressed two distinct isoforms, HbA (pI = 8.7) and HbD (pI = 7.1–7.2). There was no clear difference in relative isoform abundance in house wrens from different elevations: the relative percentages of HbD were 39% and 42% in high- and low-altitude specimens, respectively [n = 14 (7 from >3,900 m and 7 from <395 m)]. MS/MS analysis confirmed that subunits of the two adult Hb isoforms represent products of the αA-, αD-, and βA-globin genes; products of the embryonic α- and β-type globin genes were not detected.
Altitudinal Patterns of Amino Acid Polymorphism.
In birds, as in other amniotes, the subfamilies of α- and β-type globin genes are located on different chromosomes (23–26). All oscine passerines examined to date possess three tandemly linked α-type globin genes and four tandemly linked β-type globin genes (22–24, 27). Because the proteomic analyses confirmed that subunits of the two adult-expressed Hb isoforms are exclusively encoded by the αA-, αD-, and βA-globin genes, we surveyed nucleotide polymorphism at each of these three genes in the full panel of high- and low-altitude house wrens (n = 140) to identify amino acid changes that could potentially contribute to intraspecific variation in Hb function.
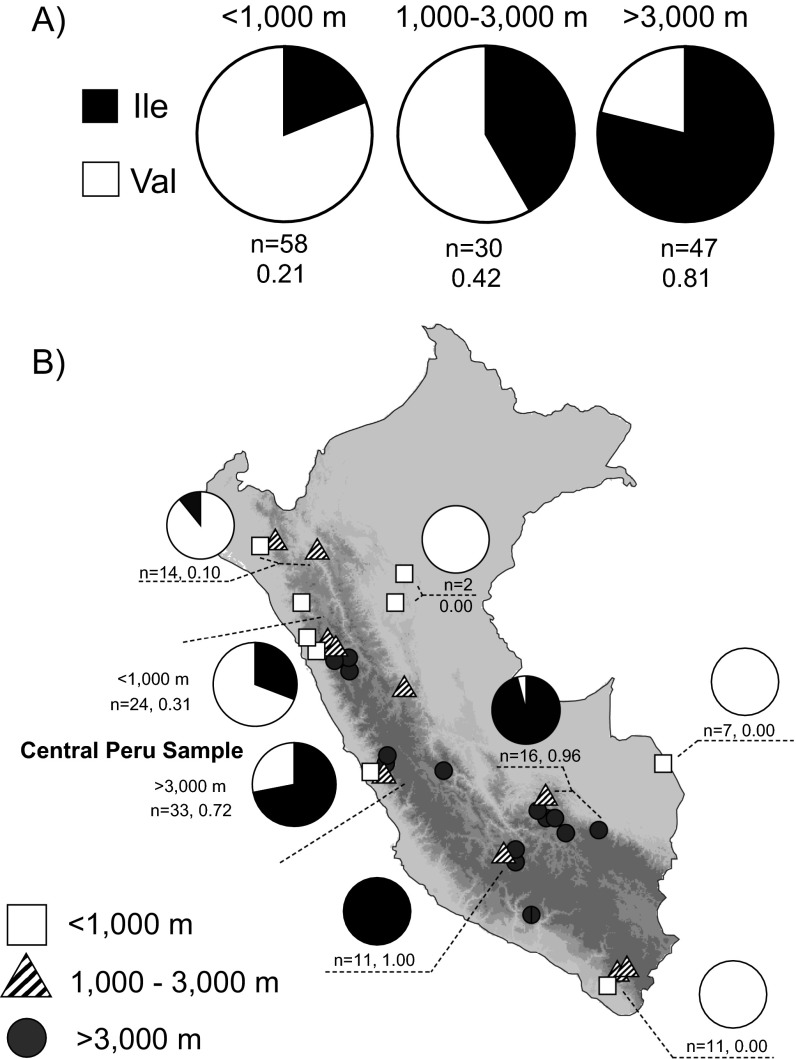
This survey revealed a number of low-frequency amino acid polymorphisms, but only one polymorphism, β55(Val/Ile), exhibited a significantly nonrandom pattern of allele frequency variation with respect to altitude (Fig. 1 and Fig. S2). In the central Peru sample, the frequency of the derived Ile variant was 0.72 at high altitude and 0.31 at low altitude. This allele frequency difference of 0.41 was roughly twofold higher than that of any other amino acid polymorphism in the α- or β-globin genes. The three adult-expressed globin genes exhibited silent-site diversities of π = 0.0016–0.0105 in the total sample of Andean house wrens (Table S2).
Fig. 1.
The β55(Val/Ile) polymorphism exhibits a striking altitudinal pattern of allele frequency variation among 14 natural populations of house wrens from throughout Peru. The derived β55Ile allele predominates at high altitude and the ancestral Val allele predominates at lower altitudes.
Fig. S2.
The β55(Val/Ile) polymorphism exhibits a striking altitudinal pattern of allele frequency variation in natural populations of Peruvian house wrens. (A) The β55Ile allele predominates at high altitude, and the Val allele predominates at lower altitudes. (B) Variation in frequency of the derived β55Ile allele across Peru. The central Peru sample comprises a phylogeographically defined set of specimens that served as the focus for the elevational survey of genomic polymorphism.
Table S2.
Summary of nucleotide polymorphism at adult-expressed globin genes of high- and low-altitude house wrens
| Gene | Sample | Na | Sb | h | Hd | π (Sil) | θW/bp (Sil) | Tajima’s D | 4Nc |
| αA-globin (671 bp) | High altitude | 44 | 7 | 5 | 0.385 | 0.0011 | 0.0019 | −0.9087 | 0.0000 |
| Low altitude | 66 | 12 | 9 | 0.724 | 0.0019 | 0.0047 | −1.5318 | 0.0033 | |
| Total | 110 | 13 | 10 | 0.626 | 0.0016 | 0.0048 | −1.6378 | 0.0004 | |
| αD-globin (345 bp) | High altitude | 48 | 11 | 16 | 0.850 | 0.0126 | 0.0162 | −0.6671 | 0.3953 |
| Low altitude | 64 | 8 | 13 | 0.694 | 0.0089 | 0.0101 | −0.3082 | 0.0241 | |
| Total | 112 | 13 | 22 | 0.769 | 0.0105 | 0.0147 | −0.7543 | 0.0823 | |
| βA-globin (1,297 bp) | High altitude | 46 | 35 | 35 | 0.989 | 0.0049 | 0.0078 | −1.2418 | 0.0818 |
| Low altitude | 58 | 41 | 44 | 0.984 | 0.0052 | 0.0089 | −1.3942 | 0.1258 | |
| Total | 104 | 58 | 72 | 0.991 | 0.0053 | 0.0111 | −1.6527 | 0.1096 |
Estimates of π, θW, and Tajima’s D are based on variation at silent sites. h, no. haplotypes; Hd, haplotype diversity; N, no. sampled chromosomes; S, no. segregating sites.
Recurrent Substitutions at a Mutational Hot Spot.
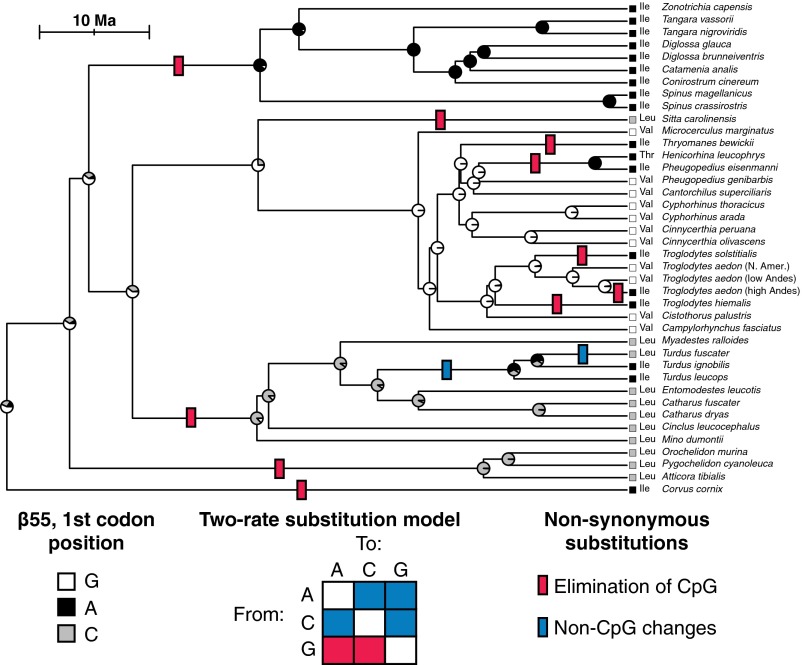
We sequenced the βA-globin gene in 38 songbird species, including 15 species in the wren family (Troglodytidae) and 23 species representing nine families of oscine passerines. Phylogenetic analysis revealed that repeated nonsynonymous substitutions at the first codon position of β55 were attributable to the recurrent elimination of an ancestral CpG dinucleotide. Specifically, recurrent G→A transition substitutions at the first codon position converted β55Val to Ile in Andean house wrens and seven other passerine lineages, G→C transversion substitutions converted β55Val to Leu in several lineages, and successive A↔C transversions (non-CpG changes) interconverted β55Ile and Leu in thrushes (Fig. 2). Depending on the methylation status of the cytosine, eliminations of CpG dinucleotides via point mutations at either site are expected to occur at a far higher rate than non-CpG mutations at the same sites (16, 17). Consistent with this expectation, the estimated per-path rate for observed substitutions that eliminate the CpG dinucleotide (CpG→CpA and CpG→CpC) was approximately fivefold higher than the rate for other possible substitutions at the same site (0.0196 vs. 0.0043, respectively), and a likelihood ratio test indicated that the two-rate model provided a significantly better fit to the data than a single-rate model (2ΔlnL = 4.75, P = 0.029).
Fig. 2.
Phylogeny of the wren family Troglodytidae and representative species from related oscine passerine families showing recurrent nonsynonymous substitutions at the first position of codon 55 in the βA-globin gene. Blue and red tick marks indicate the minimum number of changes that are consistent with maximum-likelihood estimates of ancestral states at each node (see pie diagrams). An ancestral CpG dinucleotide was eliminated multiple times independently by nonsynonymous G→A transition substitutions (which produced β55Val→Ile replacements in Andean house wrens and several other passerine lineages) and G→C transversion substitutions (which produced β55Val→Leu replacements in multiple lineages). The third position of the codon immediately preceding β55 was cystosine (C) in all examined species except for Cinclus leucocephalus, which had thymine (T) and is part of a clade in which the CpG dinucleotide had already been eliminated by a CpG→CpC substitution. Depending on the methylation status of the cytosine, the rate of elimination of the CpG dinucleotide by point mutations at either site is expected to be ∼10- to 15-fold higher than the mean mutation rate for non-CpG nucleotide sites. Maximum-likelihood analyses confirmed the expectation that the rate of substitutions that eliminated the CpG dinucleotide (CpG→CpA and CpG→CpC) was significantly higher than the rate of non-CpG substitutions at the same site.
Globin Gene Variation in Genome-Wide Context.
For the purpose of making comparisons with patterns of variation in the adult-expressed α- and β-type globin genes of Andean house wrens, we surveyed intronic sequence polymorphism in the ρ- and βH-globin genes, both of which are located immediately upstream of βA-globin, and we also surveyed intronic sequence of the unlinked myoglobin (Mb) gene. In comparisons between the high- and low-altitude population samples, the βA-globin gene exhibited a higher level of nucleotide differentiation than each of the other linked and unlinked globin genes (Table S3).
Table S3.
Nucleotide differentiation of globin genes between high- and low-altitude populations of Andean house wrens (<1,000 and >3,000 m above sea level, respectively)
| Gene | L, bp | N | S | FST |
| αD-globin | 345 | 112 | 13 | 0.006 |
| αA-globin | 671 | 110 | 13 | 0.023 |
| ρ-globin | 595 | 100 | 28 | 0.054 |
| βH-globin | 617 | 82 | 36 | 0.094 |
| βA-globin | 1,297 | 104 | 58 | 0.133 |
| myoglobin | 426 | 110 | 19 | 0.008 |
Estimates of FST are based on sets of specimens comprising the central Peru sample (see text for details). The α-type globin genes (αA and αD), the β-type globin genes (ρ, βH, and βA), and myoglobin are located on different chromosomes. L, length of sequenced fragment; n, number of sampled chromosomes; S, number of segregating sites.
To complement the multilocus survey of globin variation in the full panel of high- and low-altitude specimens, we used a subset of 28 specimens (14 highland, 14 lowland) in a genome-wide survey of single nucleotide polymorphisms (SNPs) in coding sequence. This allowed us to interpret altitudinal patterns of β55 polymorphism in a genome-wide context. We restricted the genomic analysis to 1,272 SNPs that mapped to putative protein-coding genes in a reference transcriptome (SI Methods). In the subset of specimens used in the genomic analysis, the site-specific FST value for β55 was 0.150, representing the upper 0.084 percentile of the empirical genome-wide distribution for coding SNPs. In the comparison between high- and low-altitude specimens comprising the central Peru sample (n = 108 alleles), the site-specific FST value for β55 was 0.269, representing the upper 0.013 percentile. The elevational differentiation in allele frequencies at the β55(Val/Ile) polymorphism thus provides suggestive evidence for a history of spatially varying selection.
Oxygenation Properties of Native HbA and HbD Variants.
We purified HbA and HbD variants from highland and lowland house wren specimens that had representative globin genotypes. Measured differences in functional properties between the native HbA and HbD variants of highland and lowland house wrens reflect the net effects of naturally occurring allelic variation at two β-chain sites: β55(Val/Ile) and β80(Gly/Ser). Allelic variation at β80 contributes to amino acid heterogeneity in the set of specimens used in our functional experiments, but—unlike the β55(Val/Ile) polymorphism—it represents a low-frequency polymorphism in the global population and it does not exhibit a consistent altitudinal pattern of allele frequency variation (Fig. S3).
Fig. S3.
In addition to the β55(Val/Ile) polymorphism, β80 is one of the only other amino acid sites that harbors segregating variation in natural populations of Peruvian house wrens. However, the ancestral Ser allele predominates in population samples from every elevational zone. (A) The derived β80Gly allele is present at moderate frequencies in population samples from the highlands of southern Peru. (B) Variation in frequency of the derived β80Gly allele across Peru. The central Peru sample comprises a phylogeographically defined set of specimens that served as the focus for the elevational survey of genomic polymorphism.
We measured O2 equilibria of purified native Hb solutions under a standardized set of experimental treatments that enabled us to test for physiological differences in O2 affinity between high- and low-altitude Hb variants while simultaneously providing insights into the molecular mechanism responsible for observed functional differences. We measured O2 equilibria (i) in the absence of allosteric effectors (“stripped”), (ii) in the presence of Cl− ions, added as 0.1 M KCl, (iii) in the presence of inositol hexaphosphate (IHP) (a chemical analog of the naturally occurring inositol pentaphosphate), at twofold molar excess over tetrameric Hb, and (iv) in the simultaneous presence of Cl− and IHP. This latter treatment is most relevant to in vivo conditions in avian red blood cells. For each treatment, we estimated P50, the PO2 at which Hb is 50% saturated, and the Hill coefficient, n50, a measure of cooperativity.
Because HbA and HbD share the same β-type subunit, functional effects of β-chain mutations should be manifest in comparisons between high- and low-altitude variants of both isoforms. Thus, data from both HbA and HbD provide replicate measurements of the mutations at β55 and β80 on two different α-chain backgrounds. The O2 equilibrium measurements revealed pronounced differences in O2 affinity between high- and low-altitude variants of both HbA and HbD (Table S4 and Fig. 3). P50(KCl+IHP) for the high-altitude HbA variant was 34% lower (O2 affinity was higher) than that of the low-altitude variant (17.07 vs. 25.88 torr). Similarly, P50(KCl+IHP) for the high-altitude HbD variant was 17% lower than that of the low-altitude variant (13.45 vs. 16.29 torr). In high- and low-altitude samples, O2 affinity differences between the two isoforms were consistent, as P50(KCl+IHP) was considerably higher for HbA relative to HbD (Table S4). Both isoforms exhibited cooperative O2 binding, as estimated Hill coefficients (n50 values) in the KCl-plus-IHP treatment were 1.36–2.11 for HbA, and 2.28–2.36 for HbD (Table S4).
Table S4.
O2 affinities (P50, torr; mean ± SEM) and cooperativity coefficients (n50) for native HbA and HbD isoforms of high- and low-altitude house wrens
| Property | Lowland HbA (β55Val) | Highland HbA (β55Ile) | Lowland HbD (β55Val) | Highland HbD (β55Ile) |
| P50, torr | ||||
| Stripped | 2.80 ± 0.25 | 2.47 ± 0.07 | 1.58 ± 0.03 | 1.59 ± 0.03 |
| +KCl | 4.57 ± 0.01 | 2.96 ± 0.20 | 2.67 ± 0.09 | 2.47 ± 0.04 |
| +IHP | 33.90 ± 1.61 | 21.39 ± 0.32 | 22.60 ± 0.74 | 17.54 ± 0.31 |
| KCl + IHP | 25.88 ± 1.22 | 17.07 ± 0.79 | 16.29 ± 0.19 | 13.45 ± 0.29 |
| n50 | ||||
| Stripped | 1.48 ± 0.15 | 1.53 ± 0.04 | 1.47 ± 0.07 | 1.36 ± 0.10 |
| +KCl | 1.91 ± 0.07 | 1.36 ± 0.09 | 1.92 ± 0.11 | 1.81 ± 0.02 |
| +IHP | 1.98 ± 0.25 | 1.37 ± 0.14 | 2.39 ± 0.06 | 2.22 ± 0.14 |
| KCl + IHP | 2.11 ± 0.13 | 1.36 ± 0.01 | 2.36 ± 0.12 | 2.28 ± 0.10 |
O2 equilibria of purified Hb solutions were measured in 0.1 M Hepes buffer at pH 7.40, 37 °C (heme, 0.3 mM). Measurements were conducted in the absence of anionic effectors (stripped), in the presence of 0.1 M KCl or IHP (IHP/Hb tetramer ratio = 2.0), and in the presence of both effectors, as indicated. [Heme], 0.3 mM. For each population sample, the predominant allelic state of site β55 is given in parentheses.
Fig. 3.
O2 affinities of HbA and HbD isoforms from high- and low-altitude populations of house wrens. (A) P50 values (mean ± SEM) for purified HbA variants of highland and lowland house wrens measured in 0.1 M Hepes buffer at pH 7.4 and 37 °C in the absence (stripped) and presence of allosteric effectors ([Cl−], 0.1 M; IHP/Hb tetramer ratio, 2.0; [heme], 0.3 mM). (B) P50 values for HbD variants of highland and lowland wrens (experimental conditions as in A).
In the case of the high- and low-altitude HbA variants, the slight difference in intrinsic O2 affinity [P50(stripped) = 2.47 vs. 2.80 torr, respectively] was greatly augmented in the presence of IHP and in the simultaneous presence of Cl− and IHP (Table S4 and Fig. 3A). In the case of the HbD isoforms, there was no discernible difference in intrinsic O2 affinity between the high- and low-altitude variants [P50(stripped) = 1.59 vs. 1.58 torr, respectively], but—as with the HbA variants—there was a highly significant affinity difference in the presence of Cl− and IHP (Table S4 and Fig. 3B). Results for high- and low-altitude variants of HbA and HbD indicate that allelic differences in Hb function stem from changes in both intrinsic O2 affinity and anion sensitivity. In both isoforms, these changes are clearly attributable to the independent or joint effect of shared amino acid mutations at β55 and β80.
Functional Effects of Individual Mutations.
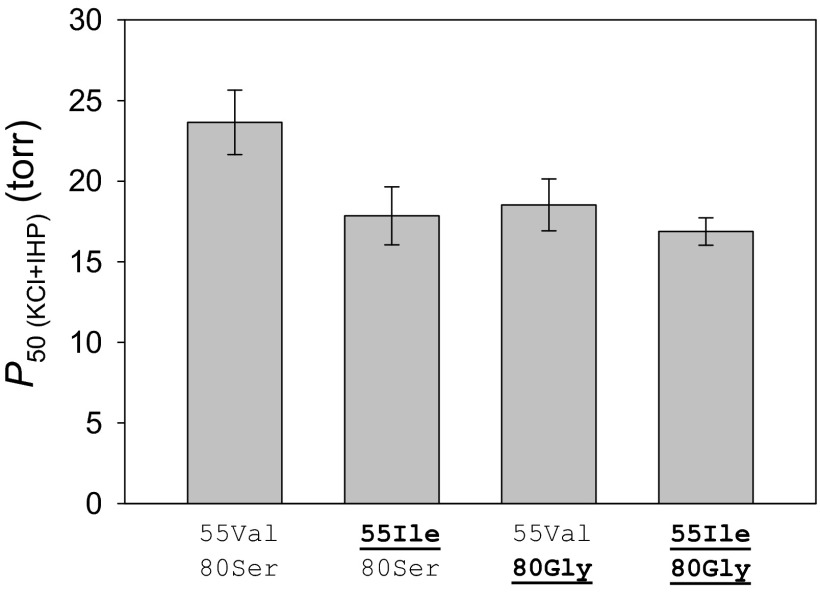
To measure the relative contributions of the mutations β55Val→Ile and β80Gly→Ser, we used site-directed mutagenesis to engineer four recombinant Hb (rHb) mutants representing each possible genotypic combination of allelic variation at the two sites. The measured O2 affinities of the ancestral genotype (55Val-80Ser) and the β55 single-mutant (55Ile-80Ser) recapitulated the measured difference between the native HbA variants from high- and low-altitude populations (Tables S4 and S5). The β55Val→Ile mutation produced a 25% reduction in P50(KCl+IHP) (an increase in O2 affinity) on the ancestral 55Val-80Ser background (difference in P50 = 5.79 torr, 95% confidence limits of ΔP50 = 11.06, 0.52), and a 9% reduction on the background with the derived Gly at β80 (Fig. 4 and Table S5) (difference in P50 = 1.65 torr, 95% confidence limits of ΔP50 = 5.22, −1.92). The β80Ser→Gly mutation also had a substantial affinity-enhancing effect on the ancestral background (Fig. 4 and Table S5).
Table S5.
O2 affinities (P50, torr; mean ± SEM) and cooperativity coefficients (n50) for purified house wren rHbs measured in 0.1 M Hepes buffer at pH 7.40, 37 °C
| Property | β55Val-β80Ser (LA) | β55Ile-β80Ser (HA) | β55Val-β80Gly | β55Ile-β80Gly |
| P50, torr | ||||
| Stripped | 3.21 ± 0.04 | 3.17 ± 0.07 | 2.74 ± 0.02 | 3.40 ± 0.08 |
| +KCl | 5.07 ± 0.06 | 4.03 ± 0.01 | 4.21 ± 0.09 | 4.17 ± 0.10 |
| +IHP | 30.82 ± 5.09 | 26.23 ± 4.93 | 27.87 ± 3.64 | 25.23 ± 1.05 |
| KCl + IHP | 23.65 ± 2.00 | 17.86 ± 1.80 | 18.53 ± 1.61 | 16.88 ± 0.85 |
| n50 | ||||
| Stripped | 1.61 ± 0.03 | 1.49 ± 0.05 | 1.52 ± 0.01 | 1.66 ± 0.06 |
| +KCl | 1.76 ± 0.04 | 1.77 ± 0.01 | 1.82 ± 0.06 | 1.46 ± 0.05 |
| +IHP | 1.00 ± 0.13 | 0.85 ± 0.11 | 1.17 ± 0.14 | 1.25 ± 0.05 |
| KCl + IHP | 1.30 ± 0.13 | 0.92 ± 0.09 | 1.30 ± 0.13 | 1.16 ± 0.06 |
Measurements were conducted in the absence of anionic effectors (stripped), in the presence of 0.1 M KCl or IHP (IHP/Hb tetramer ratio = 2.0), and in the presence of both effectors, as indicated. [Heme], 0.3 mM. “HA” and “LA” notations refer to two-site genotypes that are characteristic of high- and low-altitude house wrens, respectively.
Fig. 4.
O2 affinities [P50(KCl+IHP), torr; mean ± SEM] of purified house wren rHb mutants measured in the presence of physiological concentrations of Cl− ions (0.1 M KCl) and IHP (at twofold molar excess over tetrameric Hb). O2 equilibrium curves for each rHb mutant were measured in 0.1 M Hepes buffer at pH 7.40, 37 °C, and [heme], 0.3 mM. Numbers refer to residue positions in the β-chain subunit. “55Ile-80Ser” and “55Val-80Ser” are the two-site genotypes that predominate in high- and low-altitude house wrens, respectively. At each site, the derived (nonancestral) amino acid residues are underlined.
The Structural Mechanism Responsible for Changes in Hemoglobin–O2 Affinity.
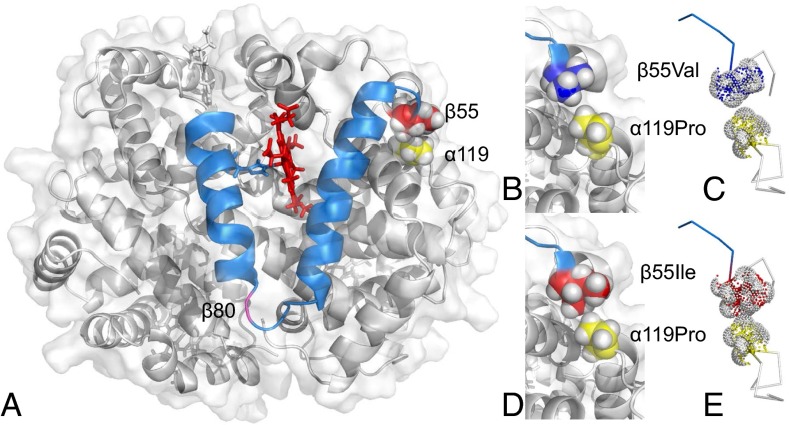
Results of homology modeling suggest that the β55Val→Ile mutation increases intrinsic Hb–O2 affinity through indirect effects on the β-chain distal heme pocket (the site of heme–ligand binding). The β55Val→Ile mutation results in the insertion of an additional carbonyl group in the α1β1 intradimer gap between β55 and α119Pro, thereby forming a van der Waals contact between the two residues (Fig. 5). In the deoxy state, this added atomic contact at the α1β1 interface induces strain on the adjacent D helix of the β-subunit, as indicated by a 1.3-fold increase in the single-residue frustration index for the derived β55Ile relative to Val on the ancestral background (Table S6). This effect propagates to the adjacent E helix, resulting in a subtle repositioning of key residues at the solvent interface that function as a gate for ligand entry/exit in the distal heme pocket and that directly or indirectly stabilize the heme–ligand complex (28).
Fig. 5.
Homology model of house wren HbA showing the location of amino acid replacements that distinguish high- and low-altitude variants. (A) The E and F helices of the β-chain subunit are shown in blue. The side chain of the proximal histidine, β92 (which covalently binds the fifth coordination site of the heme iron) is also shown in blue, and the residues forming the α1β1 intradimer contact between β55 and α119 are shown in red and yellow, respectively, with space-filling representation of van der Waals radii. The site of the β80Ser→Gly mutation in the EF interhelical loop is shown in pink. (B and C) There is no interchain atomic contact between β55Val and α119Pro at the α1β1 contact surface. (D and E) Because Ile has an additional carbon atom relative to Val, a van der Waals interaction is formed between β55Ile and α119Pro.
Table S6.
Single-residue frustration indices for two mutant sites in house wren HbA
| Single-residue frustration index, arbitrary units | ||
| Two-site genotype | β55 | β80 |
| β55Val-β80Ser | 1.5 | 0.9 |
| β55Ile-β80Ser | 2.0 | 1.0 |
| β55Val-β80Gly | 1.5 | 0.2 |
| β55Ile-β80Gly | 2.1 | 0.3 |
At β55 (the sixth residue position of the D helix), the replacement of Val with the more bulky Ile at the α1β1 intersubunit interface induces conformational strain on the β-chain D helix. This is reflected by the fact that β55Ile has a uniformly higher frustration index relative to Val at the same residue position. At β80, the hydroxyl side chain of Ser forms a helix-capping hydrogen bond with β83Asn, the penultimate C-terminal residue of the EF interhelical loop. The helix-capping hydrogen bond between β80Ser and β83Asn confers added rigidity to the E helix, and is reflected by the consistently higher frustration index for β80Ser relative to Gly at the same position.
SI Methods
Sample Collection.
All birds were live-trapped in mist nets and were sacrificed in accordance with protocols approved by the University of New Mexico Institutional Care and Use Committee (Protocol 08UNM033-TR-100117; Animal Welfare Assurance number A4023-01). All collections were authorized by permits issued by management authorities of Peru (004-2007-INRENA-IFFS-DCB, 135-2009-AG-DGFFS-DGEFFS, 0377-2010-AG-DGFFS-DGEFFS, 0199-2012-AG-DGFFS-DGEFFS, and 006-2013-MINAGRI-DGFFS/DGEFFS).
For each of the 140 house wren specimens, we collected 20–60 μL of whole blood from the brachial or ulnar vein using heparinized microcapillary tubes. Red blood cells were separated from the plasma fraction by centrifugation, and the packed red cells were flash-frozen in liquid nitrogen. We collected liver and pectoral muscle from each specimen as sources of genomic DNA and globin mRNA, respectively. Muscle samples were flash-frozen or preserved using RNAlater. All tissue and blood samples were subsequently stored at −80 °C.
Tandem Mass Spectrometry.
Database searches of MS/MS spectra were performed using Mascot (Matrix Science; version 1.9.0). Specifically, peptide mass fingerprints derived from the MS/MS analysis were used to query a custom database of avian α- and β-type globin sequences. These amino acid sequences were derived from conceptual translations of the adult-expressed αA-, αD-, and βA-globin genes of T. aedon, in addition to the full complement of embryonic and adult α- and β-type globin genes that have been annotated in the genome assemblies of other birds (22–24, 27, 56). We identified all significant protein hits that matched more than one peptide with P < 0.05. After separating the HbA and HbD isoforms by native gel IEF and identifying each band on the gel by MS/MS, the relative abundance of the different isoforms was quantified densitometrically.
PCR, Cloning, and Sanger Sequencing.
We extracted genomic DNA from frozen tissues of each of the 140 house wren specimens using the DNeasy Blood and Tissue Kit (Qiagen). We used the PCR to amplify six autosomal loci, including full-length coding sequences of the adult-expressed α- and β-type globin genes (αA-, αD-, and βA-globin), intron 2 sequences of ρ-globin and βH-globin (β-type globin genes that are located just upstream of βA-globin), and intron 2 of the unlinked myoglobin gene. Negative controls were included in each PCR to control for contamination. All PCR amplicons were purified using ExoSap-IT (USB) and were sequenced in both directions using dye terminator cycle-sequencing (BigDye; ABI) on an ABI 3130 automated sequencer (Applied Biosystems).
For the 14 specimens used in the experimental analyses of Hb function, we extracted RNA from pectoral muscle tissue using the RNeasy kit (Qiagen) and we amplified full-length cDNAs of the αA, αD-, and βA-globin genes using a OneStep RT-PCR kit (Qiagen). We designed paralog-specific primers using 5′- and 3′-UTR sequences from passerine species, as described previously (23). We cloned RT-PCR products using the TOPO TA Cloning Kit (Life Technologies), and we sequenced at least five clones per gene to recover both alleles of each globin gene. This enabled us to determine full diploid genotypes for each of the three adult-expressed globin genes in each specimen.
Population Genetic Analysis.
We computed summary statistics of nucleotide polymorphism for each of the adult-expressed globin genes (αA-, αD-, and βA-globin). As a measure of nucleotide variation, we calculated nucleotide diversity, π, and Watterson’s θW, an estimator of the population mutation rate (=4Nμ, where N is the effective population size and μ is the mutation rate per nucleotide). We calculated Tajima’s D to characterize the distribution of allele frequencies at silent sites and we calculated Hudson’s (57) estimator of the population recombination rate, 4Nc, where c is the rate of crossing over between adjacent nucleotides. To test whether measured values of Tajima’s D deviated from neutral-equilibrium expectations, we obtained critical values for each statistic by conducting 10,000 coalescent simulations (no recombination) that were conditioned on the observed number of segregating sites.
Genome-Wide Survey of Nucleotide Differentiation Using a Genotyping-by-Sequencing Approach.
To more broadly survey patterns of genomic differentiation between high- and low-elevation populations, we produced multiplexed, reduced-representation Illumina libraries following Parchman et al. (58). Briefly, we digested genomic DNA samples for a total of 28 individuals (14 from high elevation and 14 from low elevation; Table S1) with two restriction endonucleases (EcoRI and Mse1). We then ligated double-stranded adaptor oligonucleotides that contained Illumina sequencing binding sites and a unique 8- to 10-bp barcode for individual identification, and PCR amplified these adaptor-ligated fragments. Details on the adaptor sequences as well as the digestion and PCR conditions can be found in the study by Parchman et al. (58). We pooled the barcoded amplicons from each individual in equimolar concentrations, and electrophoresed them on 2.5% agarose gel for size selection. Fragments that were between 350 and 500 bp in length were excised from the gel and purified using a QIAquick Gel Extraction Kit (Qiagen). The pooled library was sequenced in a single lane on the Illumina HiSEq 1000 platform as 100-nt single-end reads at the Keck Center for Comparative and Functional Genomics at the University of Illinois, Urbana–Champaign.
We parsed the resulting reads by individual barcodes and trimmed adaptor sequences and low-quality bases using custom Perl scripts, resulting in a final mean read length of 87 nt. To limit our analysis to putative protein-coding genes, we mapped individual T. aedon reads to the published transcriptome of a closely related passerine, Zontrichia leucophrys (59), using the sensitive-local settings in Bowtie2 (60). Transcript-aligned reads were then processed using the program STACKS (61) to identify single-nucleotide polymorphisms (SNPs) in reads that mapped to known transcripts using the following input parameters for pstacks: - m3, –model_type snp, –alpha 0.05. All 28 individuals were included when compiling the SNP catalog in cstacks. Downstream population genetic analysis were restricted to loci that were genotyped in at least 10 individuals per population with a minimum sequencing depth of 5 reads per locus per individual and a minor allele frequency of 0.05, resulting in a final dataset of 1,272 unique loci. We calculated locus-specific FST values using the program POPULATIONS implemented in STACKS (61).
Protein Purification and in Vitro Analysis of Hb Function.
We purified HbA and HbD variants from pooled hemolysates from seven highland specimens and seven lowland specimens. There was a nearly fixed difference between the two samples at β55, with Ile and Val alleles predominating in the highland and lowland specimens, respectively, but there was also a low level of amino acid heterogeneity at site β80: a derived Gly allele was present at frequencies of 0.36 and 0.07 in the samples of highland and lowland specimens, respectively (n = 14 alleles in each sample).
Using purified Hb solutions (0.3 mM heme) that were stripped of organic phosphates and other allosteric effectors, we measured O2 equilibrium curves at 37 °C, 0.1 M Hepes, pH 7.4, in the absence (“stripped”) and presence of 0.1 M KCl, IHP (at twofold molar excess over tetrameric Hb), and in the simultaneous presence of KCl and IHP. We measured O2 equilibria of 3-μL thin-film samples in a modified diffusion chamber where absorption at 436 nm was monitored during stepwise changes in equilibration gas mixtures generated by precision Wösthoff gas-mixing pumps. We estimated values of P50 and n50 (Hill’s cooperativity coefficient at half-saturation) by fitting the Hill equation Y = PO2n/(P50n + PO2n) to the experimental O2 saturation data by means of nonlinear regression (Y = fractional O2 saturation; n, cooperativity coefficient) (22, 62–64). The model fitting was based on five to eight equilibration steps between 30% and 70% oxygenation. Free Cl− concentrations were measured with a model 926S Mark II chloride analyzer (Sherwood Scientific Ltd.).
Expression and Purification of Recombinant Hbs.
Recombinant Hb expression was carried out in the JM109 (DE3) E. coli strain. Bacterial cells were selected in LB agar with dual antibiotics (ampicillin and kanamycin) to ensure that transformants received both pGM and pCO-MAP plasmids for expression. The expression of each rHb mutant was carried out in 1.5 L of TB medium. Bacterial cells were grown in 37 °C in an orbital shaker at 200 rpm until absorbance values reached 0.6–0.8 at 600 nm. The bacterial cultures were induced by 0.2 mM isopropyl β-d-1-thiogalactopyranoside and were then supplemented with hemin (50 μg/mL) and glucose (20 g/L). The bacterial culture conditions and the protocol for preparing cell lysates are described in the study by Natarajan et al. (53).
We purified each rHb sample by means of two step ion-exchange chromatography as described previously (32, 37, 38, 40, 53). Samples were passed through a cation-exchange column (HiTrap SP-Sepharose; GE Healthcare; 17-1152-01) followed by equilibration with 20 mM Tris buffer (0.5 mM EDTA, 0.5 mM DTT, pH 6.0) and elution using a linear gradient of 0–0.5 M NaCl. The eluted fractions were passed through an anion-exchange column (HiTrap Q-Sepharose; GE Healthcare; 17-1153-01), followed by equilibration with 20 mM Tris buffer (0.5 mM EDTA, 0.5 mM DTT, pH 8.5), and elution using a linear gradient of 0–0.5 M NaCl. The samples were desalted by dialysis against 10 mM Hepes buffer (pH 7.6) at 4 °C. The eluted fractions of each rHb sample were concentrated by using centrifugal filtrate. The purified rHb samples were analyzed by SDS-polyacrylamide gel electrophoresis and isoelectrofocusing (IEF). After preparing rHb samples as oxyHb, deoxyHb, and carbonmonoxy derivatives, we measured absorbance at 450–600 nm to confirm that the absorbance maxima match those of the native HbA samples.
Discussion
Possible Adaptive Significance of Altitudinal Differences in Hb–O2 Affinity.
The evolved difference in Hb–O2 affinity between the highland and lowland house wrens is consistent with theoretical and empirical results demonstrating that the optimal blood–O2 affinity varies according to the ambient PO2, reflecting an unavoidable trade-off between the need to preserve arterial O2 saturation under hypoxia while simultaneously ensuring adequate O2 unloading in the peripheral circulation (29–31).
Patterns of convergence in Hb function among different high-altitude vertebrates also provide insights into the possible adaptive significance of changes in Hb–O2 affinity. Comparative studies of Andean hummingbirds revealed that species with extraordinarily high elevational range limits consistently have higher Hb–O2 affinities than closely related lowland species (32). Studies of birds and mammals have documented altitude-related differences in Hb–O2 affinity in some cases (32–38), but not in others (39–42). Andean house wrens provide the first example (to our knowledge) of a continuously distributed bird species in which high-altitude natives have evolved a derived increase in Hb–O2 affinity relative to lowland conspecifics. In contrast to recently documented cases where changes in Hb function between populations or closely related species evolved via multiple mutational changes that had individually minor effects (37, 38, 41), the increased Hb–O2 affinity in high-altitude house wrens is clearly attributable to a single, large-effect mutation.
Insights into Structural Mechanism.
Results of the protein-engineering experiments clearly demonstrate the affinity-enhancing effect of the β55Val→Ile mutation. A different amino acid substitution at this same site (β55Leu→Ser) has been implicated in the evolution of an increased Hb–O2 affinity in the Andean goose (33, 34), although phylogenetic surveys of βA-globin sequence variation have revealed that the β55Ser character state is not uniquely derived in the Andean goose—it is actually a shared, ancestral character state in South American sheldgeese, and most species in this group are lowland natives (43). The β55Leu→Ser substitution eliminates a van der Waals interaction between β55Leu and α119Pro at the α1β1 intradimer interface, thereby destabilizing the T-state conformation and shifting the allosteric equilibrium in favor of the high-affinity R state, resulting in an increase in overall O2 affinity. Engineering the same β55Leu→Ser substitution into recombinant human Hb produced the predicted increase in O2 affinity, corroborating the hypothesized structural mechanism (33, 34).
In house wren Hb, an amino acid replacement at the same site also produces a dramatic change in O2 affinity, but the structural mechanism is completely different. The affinity-enhancing β55Leu→Ser replacement in Andean goose Hb eliminates an atomic contact between α119 and β55, thereby destabilizing the low-affinity T-state conformation. By contrast, the affinity-enhancing β55Val→Ile replacement in house wren Hb adds an intradimer atomic contact in the same α1β1 interface (Fig. 5), which indirectly affects deoxy β-heme reactivity by reorienting the E helix. This illustrates how substitutions involving different pairs of amino acid residues at the same site can alter protein function via different structural and functional mechanisms.
Effects of Mutation Bias on Propensities of Molecular Adaptation.
Results of our molecular evolution analysis demonstrated a quantitative asymmetry in rates of CpG and non-CpG substitution in the first codon position of β55, and results of our protein-engineering experiments demonstrated that the mutationally favored Val→Ile replacement at this site produces a significant increase in Hb–O2 affinity on the ancestral genetic background (Fig. 4 and Table S5). The direction of character state change is consistent with the expectation that an increased Hb–O2 affinity is adaptive at high altitude. This inference is bolstered by results of the population genomic analysis, which suggest that the altitudinal shift in frequency of the derived β55-Ile variant is attributable to a history of spatially varying selection. Our results therefore demonstrate how a mutationally favored amino acid change produced a large phenotypic effect that has likely adaptive significance. The important question is whether the increased rate of mutation to the function-altering allele made the observed evolutionary outcome especially likely to occur. This is relevant to the more general question of whether propensities of mutational change cause propensities in pathways of adaptive molecular evolution (10, 11, 44, 45).
Studies of naturally occurring mutations in human Hb and engineered mutations in recombinant Hbs, as well as comparative studies of Hbs from different animal species, demonstrate that there are numerous possible amino acid changes that are capable of producing fine-tuned increases in Hb–O2 affinity (46–49). As an adaptive solution to the respiratory challenges of O2 transport at high altitude, there is no reason to think that the β55Val→Ile replacement was a forced option; any number of amino acid mutations in the same protein presumably could have produced a quantitatively similar phenotypic effect. Assuming that an increased Hb–O2 affinity confers a fitness benefit in birds living at high altitude, there seems little reason to suppose that the observed β55Val→Ile mutation would have had a higher fixation probability than any number of other possible affinity-enhancing mutations. However, if the rate of β55Val→Ile mutation is 10-fold higher than the rate of mutation to any other affinity-enhancing amino acid at any other site in the protein, then—in the absence of contributions from standing variation—this would bias evolutionary outcomes in the same way as a 10-fold higher probability of fixation. When adaptive evolution is mutation-limited, an increase in the rate of mutation to a particular allele and a commensurate increase in the mutant allele’s probability of fixation have the same effect on the odds that the allele will be the next to fix (4, 10, 11, 50). The extent to which adaptation in natural populations approximates the mutation-limited scenario envisioned by origin-fixation models remains an open question in evolutionary genetics (50). Our findings suggest that variation in the mutation rate to function-altering alleles may be an important factor influencing the preferential fixation of mutations during phenotypic evolution.
Methods
Sample Collection.
We collected 140 house wren specimens from a range of elevations (120–4,454 m above sea level) in the Peruvian Andes and adjacent lowlands. All specimens were preserved as vouchers in the ornithological collection of the Museum of Southwestern Biology of the University of New Mexico and the Centro de Ornitología y Biodiversidad (CORBIDI) (Lima, Peru). Birds were handled in accordance with protocols approved by the University of New Mexico Institutional Care and Use Committee (Protocol 08UNM033-TR-100117; Animal Welfare Assurance number A4023-01). Complete specimen data are available via the ARCTOS online database (Table S1). Details regarding specimen collection and permits are provided in SI Methods.
Characterization of Hb Isoform Composition.
We characterized Hb isoform composition in the mature erythrocytes of 14 house wren specimens (7 highland and 7 lowland). Native Hb components were separated by means of IEF, gel bands were excised and digested with trypsin, and MS/MS was used to identify the resultant peptides, as described in SI Methods.
Molecular Cloning and Sequencing.
Details regarding cloning and sequencing protocols are provided in SI Methods. All sequences were deposited in GenBank under accession numbers KT759682–KT760400.
Phylogenetic Survey of βA-Globin Sequence Variation in Oscine Passerines.
We sequenced the βA-globin gene in representative wren species and species from related oscine passerine families. We used a time-scaled supertree (51) and the “ace” function of the R package ape (52) to test alternative maximum-likelihood models of character state change and to estimate ancestral character states for the first codon position of β55. Our model was based on a 3 × 3 rate matrix representing all possible interconversions among observed character states at the focal site: A, C, and G (T was not an observed character state). The null model used a single rate parameter for all six substitution types. The alternative model included a second rate parameter for substitutions that eliminated the CpG dinucleotide (CpG→CpA and CpG→CpC). We used a likelihood ratio test to compare the one-rate and two-rate models.
Population Genetic Analysis.
For each of the adult-expressed globin genes (αA-, αD-, and βA-globin), we computed summary statistics of nucleotide polymorphism, as described in SI Methods.
Survey of Genomic Differentiation.
We used a genotyping-by-sequencing approach to survey genome-wide patterns of nucleotide differentiation in coding sequence. Briefly, we used genomic DNA samples from 28 house wren specimens to produce multiplexed, reduced-representation Illumina libraries. Details of library preparation, library sequencing, and quality control filtering are provided in SI Methods. Parsed Illumina reads have been deposited in the National Center for Biotechnology Information Sequence Read Archive (SRA) (PRJNA295865).
Protein Purification and in Vitro Analysis of Hb Function.
The experimental analysis of native HbA and HbD variants was based on pooled hemolysates from seven highland specimens and seven lowland specimens that had representative genotypes. For each of the pooled hemolysates, we isolated and purified the HbA and HbD isoforms by means of anion-exchange fast-protein liquid chromatography, using a HiTrap QHP column (GE Healthcare). Details regarding sample preparation and the measurement of O2 equilibrium curves are provided in SI Methods.
Vector Construction and Site-Directed Mutagenesis.
The αA- and βA-globin sequences were synthesized by Eurofins MWG Operon after optimizing the nucleotide sequences in accordance with Escherichia coli codon preferences. The synthesized αA-βA globin gene cassette was cloned into a custom pGM vector system along with the methionine aminopeptidase (MAP) gene, as described previously (37, 53). We engineered each of the β-chain codon substitutions using the QuikChange II XL Site-Directed Mutagenesis kit from Stratagene. Each engineered codon change was verified by DNA sequencing.
Expression and Purification of Recombinant Hbs.
Recombinant Hb expression was carried out in the E. coli JM109 (DE3) strain as described previously (53). Additional details are provided in SI Methods.
Structural Modeling.
Homology-based structural modeling was performed on the SWISS-MODEL server (54), using human deoxyHb (Protein Data Bank ID 2hhb) as template. To predict mutational effects on conformational stress, we computed an index of energetic frustration using the Frustratometer program (55). Graphics were produced by the PyMol (Schrödinger).
Acknowledgments
We thank the Peruvian government agencies Instituto Nacional de Recursos Naturales and Servicio Nacional Forestal y de Fauna Silvestre for permits. We thank T. Valqui, E. Bautista, and CORBIDI for assistance in the field; J. Projecto-Garcia and E. Ellebæk Petersen for assistance in the laboratory; and two anonymous reviewers for constructive criticism. This work was funded by the Frank M. Chapman Fund of the American Museum of Natural History (S.C.G.); the Department of Animal Biology, University of Illinois (Z.A.C.); the National Institutes of Health/National Heart, Lung, and Blood Institute [Grant HL087216 (to J.F.S.)]; the National Science Foundation [Grant IOS-0949931 (to J.F.S.), Grant IOS-1354390 (to J.F.S.), Grant MCB-1517636 (to J.F.S.), Grant IOS-1354934 (to Z.A.C.), Grant DEB-1146491 (to C.C.W.), and Grant MCB-1516660 (to C.C.W.)]; the Danish Council for Independent Research, Natural Sciences [Grant 10-084-565 (to A.F.)]; and the Faculty of Science and Technology, Aarhus University (R.E.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 13753.
Data deposition: The newly generated sequences reported in this paper have been deposited in the GenBank database (accession nos. KT759682–KT760400). The parsed Illumina reads reported in this paper have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA), www.ncbi.nlm.nih.gov/sra (PRJNA295865).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507300112/-/DCSupplemental.
References
- 1.Stern DL, Orgogozo V. The loci of evolution: How predictable is genetic evolution? Evolution. 2008;62(9):2155–2177. doi: 10.1111/j.1558-5646.2008.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern DL, Orgogozo V. Is genetic evolution predictable? Science. 2009;323(5915):746–751. doi: 10.1126/science.1158997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gompel N, Prud’homme B. The causes of repeated genetic evolution. Dev Biol. 2009;332(1):36–47. doi: 10.1016/j.ydbio.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 4.Streisfeld MA, Rausher MD. Population genetics, pleiotropy, and the preferential fixation of mutations during adaptive evolution. Evolution. 2011;65(3):629–642. doi: 10.1111/j.1558-5646.2010.01165.x. [DOI] [PubMed] [Google Scholar]
- 5.Stern DL. The genetic causes of convergent evolution. Nat Rev Genet. 2013;14(11):751–764. doi: 10.1038/nrg3483. [DOI] [PubMed] [Google Scholar]
- 6.Nei M. Mutation-Driven Evolution. Oxford Univ Press; Oxford: 2013. [Google Scholar]
- 7.Otto SP. Two steps forward, one step back: The pleiotropic effects of favoured alleles. Proc Biol Sci. 2004;271(1540):705–714. doi: 10.1098/rspb.2003.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll SB. Evo-devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell. 2008;134(1):25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Chevin L-M, Martin G, Lenormand T. Fisher’s model and the genomics of adaptation: Restricted pleiotropy, heterogenous mutation, and parallel evolution. Evolution. 2010;64(11):3213–3231. doi: 10.1111/j.1558-5646.2010.01058.x. [DOI] [PubMed] [Google Scholar]
- 10.Yampolsky LY, Stoltzfus A. Bias in the introduction of variation as an orienting factor in evolution. Evol Dev. 2001;3(2):73–83. doi: 10.1046/j.1525-142x.2001.003002073.x. [DOI] [PubMed] [Google Scholar]
- 11.Stoltzfus A. Mutation-biased adaptation in a protein NK model. Mol Biol Evol. 2006;23(10):1852–1862. doi: 10.1093/molbev/msl064. [DOI] [PubMed] [Google Scholar]
- 12.Chan YF, et al. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science. 2010;327(5963):302–305. doi: 10.1126/science.1182213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachman MW, Crowell SL. Estimate of the mutation rate per nucleotide in humans. Genetics. 2000;156(1):297–304. doi: 10.1093/genetics/156.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondrashov AS. Direct estimates of human per nucleotide mutation rates at 20 loci causing Mendelian diseases. Hum Mutat. 2003;21(1):12–27. doi: 10.1002/humu.10147. [DOI] [PubMed] [Google Scholar]
- 15.Webster MT, Axelsson E, Ellegren H. Strong regional biases in nucleotide substitution in the chicken genome. Mol Biol Evol. 2006;23(6):1203–1216. doi: 10.1093/molbev/msk008. [DOI] [PubMed] [Google Scholar]
- 16.Ellegren H. The evolutionary genomics of birds. Annu Rev Ecol Evol Syst. 2013;44:239–259. [Google Scholar]
- 17.Fjeldsa J, Kessler M. Birds of the High Andes. University of Copenhagen and Apollo Books; Svendborg, Denmark: 1990. [Google Scholar]
- 18.Storz JF, Scott GR, Cheviron ZA. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J Exp Biol. 2010;213(Pt 24):4125–4136. doi: 10.1242/jeb.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith BT, Klicka J. The profound influence of the Late Pliocene Panamanian uplift on the exchange, diversification, and distribution of New World birds. Ecography. 2010;33(2):333–342. [Google Scholar]
- 20.Galen SC, Witt CC. Diverse avian malaria and other haemosporidian parasites in Andean house wrens: Evidence for regional co-diversification by host-switching. J Avian Biol. 2014;45(4):374–386. [Google Scholar]
- 21.Perutz MF. Mechanisms of cooperativity and allosteric regulation in proteins. Q Rev Biophys. 1989;22(2):139–237. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- 22.Grispo MT, et al. Gene duplication and the evolution of hemoglobin isoform differentiation in birds. J Biol Chem. 2012;287(45):37647–37658. doi: 10.1074/jbc.M112.375600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opazo JC, et al. Gene turnover in the avian globin gene families and evolutionary changes in hemoglobin isoform expression. Mol Biol Evol. 2015;32(4):871–887. doi: 10.1093/molbev/msu341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann FG, Storz JF, Gorr TA, Opazo JC. Lineage-specific patterns of functional diversification in the α- and β-globin gene families of tetrapod vertebrates. Mol Biol Evol. 2010;27(5):1126–1138. doi: 10.1093/molbev/msp325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann FG, Opazo JC, Storz JF. Whole-genome duplications spurred the functional diversification of the globin gene superfamily in vertebrates. Mol Biol Evol. 2012;29(1):303–312. doi: 10.1093/molbev/msr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storz JF, Opazo JC, Hoffmann FG. Gene duplication, genome duplication, and the functional diversification of vertebrate globins. Mol Phylogenet Evol. 2013;66(2):469–478. doi: 10.1016/j.ympev.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang G, et al. Avian Genome Consortium Comparative genomics reveals insights into avian genome evolution and adaptation. Science. 2014;346(6215):1311–1320. doi: 10.1126/science.1251385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birukou I, Soman J, Olson JS. Blocking the gate to ligand entry in human hemoglobin. J Biol Chem. 2011;286(12):10515–10529. doi: 10.1074/jbc.M110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turek Z, Kreuzer F, Turek-Maischeider M, Ringnalda BEM. Blood O2 content, cardiac output, and flow to organs at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflugers Arch. 1978;376(3):201–207. doi: 10.1007/BF00584951. [DOI] [PubMed] [Google Scholar]
- 30.Bencowitz HZ, Wagner PD, West JB. Effect of change in P50 on exercise tolerance at high altitude: A theoretical study. J Appl Physiol. 1982;53(6):1487–1495. doi: 10.1152/jappl.1982.53.6.1487. [DOI] [PubMed] [Google Scholar]
- 31.Willford DC, Hill EP, Moores WY. Theoretical analysis of optimal P50. J Appl Physiol. 1982;52(4):1043–1048. doi: 10.1152/jappl.1982.52.4.1043. [DOI] [PubMed] [Google Scholar]
- 32.Projecto-Garcia J, et al. Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proc Natl Acad Sci USA. 2013;110(51):20669–20674. doi: 10.1073/pnas.1315456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jessen TH, Weber RE, Fermi G, Tame J, Braunitzer G. Adaptation of bird hemoglobins to high altitudes: Demonstration of molecular mechanism by protein engineering. Proc Natl Acad Sci USA. 1991;88(15):6519–6522. doi: 10.1073/pnas.88.15.6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber RE, Jessen TH, Malte H, Tame J. Mutant hemoglobins (α 119-Ala and β 55-Ser): Functions related to high-altitude respiration in geese. J Appl Physiol (1985) 1993;75(6):2646–2655. doi: 10.1152/jappl.1993.75.6.2646. [DOI] [PubMed] [Google Scholar]
- 35.Storz JF, et al. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc Natl Acad Sci USA. 2009;106(34):14450–14455. doi: 10.1073/pnas.0905224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storz JF, Runck AM, Moriyama H, Weber RE, Fago A. Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol. 2010;213(Pt 15):2565–2574. doi: 10.1242/jeb.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Natarajan C, et al. Epistasis among adaptive mutations in deer mouse hemoglobin. Science. 2013;340(6138):1324–1327. doi: 10.1126/science.1236862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tufts DM, et al. Epistasis constrains mutational pathways of hemoglobin adaptation in high-altitude pikas. Mol Biol Evol. 2015;32(2):287–298. doi: 10.1093/molbev/msu311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Revsbech IG, et al. Hemoglobin function and allosteric regulation in semi-fossorial rodents (family Sciuridae) with different altitudinal ranges. J Exp Biol. 2013;216(Pt 22):4264–4271. doi: 10.1242/jeb.091397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheviron ZA, et al. Integrating evolutionary and functional tests of adaptive hypotheses: A case study of altitudinal differentiation in hemoglobin function in an Andean sparrow, Zonotrichia capensis. Mol Biol Evol. 2014;31(11):2948–2962. doi: 10.1093/molbev/msu234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natarajan C, et al. Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol Biol Evol. 2015;32(4):978–997. doi: 10.1093/molbev/msu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janecka JE, et al. Genetically based low oxygen affinities of felid hemoglobins: Lack of biochemical adaptation to high-altitude hypoxia in the snow leopard. J Exp Biol. 2015;218(Pt 15):2402–2409. doi: 10.1242/jeb.125369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCracken KG, Barger CP, Sorenson MD. Phylogenetic and structural analysis of the HbA (alphaA/betaA) and HbD (alphaD/betaA) hemoglobin genes in two high-altitude waterfowl from the Himalayas and the Andes: Bar-headed goose (Anser indicus) and Andean goose (Chloephaga melanoptera) Mol Phylogenet Evol. 2010;56(2):649–658. doi: 10.1016/j.ympev.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 44.Stoltzfus A. Mutationism and the dual causation of evolutionary change. Evol Dev. 2006;8(3):304–317. doi: 10.1111/j.1525-142X.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 45.Stoltzfus A, Yampolsky LY. Climbing mount probable: Mutation as a cause of nonrandomness in evolution. J Hered. 2009;100(5):637–647. doi: 10.1093/jhered/esp048. [DOI] [PubMed] [Google Scholar]
- 46.Bellelli A, Brunori M, Miele AE, Panetta G, Vallone B. The allosteric properties of hemoglobin: Insights from natural and site directed mutants. Curr Protein Pept Sci. 2006;7(1):17–45. doi: 10.2174/138920306775474121. [DOI] [PubMed] [Google Scholar]
- 47.Weber RE. High-altitude adaptations in vertebrate hemoglobins. Respir Physiol Neurobiol. 2007;158(2-3):132–142. doi: 10.1016/j.resp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Storz JF, Moriyama H. Mechanisms of hemoglobin adaptation to high altitude hypoxia. High Alt Med Biol. 2008;9(2):148–157. doi: 10.1089/ham.2007.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varnado CL, et al. Development of recombinant hemoglobin-based oxygen carriers. Antioxid Redox Signal. 2013;18(17):2314–2328. doi: 10.1089/ars.2012.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCandlish DM, Stoltzfus A. Modeling evolution using the probability of fixation: History and implications. Q Rev Biol. 2014;89(3):225–252. doi: 10.1086/677571. [DOI] [PubMed] [Google Scholar]
- 51.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. The global diversity of birds in space and time. Nature. 2012;491(7424):444–448. doi: 10.1038/nature11631. [DOI] [PubMed] [Google Scholar]
- 52.Paradis E, Claude J, Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20(2):289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 53.Natarajan C, et al. Expression and purification of recombinant hemoglobin in Escherichia coli. PLoS One. 2011;6(5):e20176. doi: 10.1371/journal.pone.0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biasini M, et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42(Web Server issue):W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jenik M, et al. Protein frustratometer: A tool to localize energetic frustration in protein molecules. Nucleic Acids Res. 2012;40(Web Server issue):W348–W351. doi: 10.1093/nar/gks447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann FG, Storz JF. The alphaD-globin gene originated via duplication of an embryonic α-like globin gene in the ancestor of tetrapod vertebrates. Mol Biol Evol. 2007;24(9):1982–1990. doi: 10.1093/molbev/msm127. [DOI] [PubMed] [Google Scholar]
- 57.Hudson RR. Estimating the recombination parameter of a finite population model without selection. Genet Res. 1987;50(3):245–250. doi: 10.1017/s0016672300023776. [DOI] [PubMed] [Google Scholar]
- 58.Parchman TL, et al. Genome-wide association genetics of an adaptive trait in lodgepole pine. Mol Ecol. 2012;21(12):2991–3005. doi: 10.1111/j.1365-294X.2012.05513.x. [DOI] [PubMed] [Google Scholar]
- 59.Balakrishnan CN, et al. Brain transcriptome sequencing and assembly of three songbird model systems for the study of social behavior. PeerJ. 2014;2:e396. doi: 10.7717/peerj.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA. Stacks: An analysis tool set for population genomics. Mol Ecol. 2013;22(11):3124–3140. doi: 10.1111/mec.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber RE. Use of ionic and zwitterionic (Tris/BisTris and HEPES) buffers in studies on hemoglobin function. J Appl Physiol (1985) 1992;72(4):1611–1615. doi: 10.1152/jappl.1992.72.4.1611. [DOI] [PubMed] [Google Scholar]
- 63.Storz JF, Weber RE, Fago A. Oxygenation properties and oxidation rates of mouse hemoglobins that differ in reactive cysteine content. Comp Biochem Physiol A Mol Integr Physiol. 2012;161(2):265–270. doi: 10.1016/j.cbpa.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weber RE, Fago A, Malte H, Storz JF, Gorr TA. Lack of conventional oxygen-linked proton and anion binding sites does not impair allosteric regulation of oxygen binding in dwarf caiman hemoglobin. Am J Physiol Regul Integr Comp Physiol. 2013;305(3):R300–R312. doi: 10.1152/ajpregu.00014.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]