Significance
Adult stem cells are maintained in an undifferentiated state in response to factors provided by their surrounding niche microenvironment. The Drosophila limb development membrane protein-1 (LMBR1)/lipocalin-interacting membrane receptor (LIMR) type transmembrane protein Lilipod (Lili) is required in ovarian germ-line stem cells (GSC) for their self-renewal. In the fly ovary, niche-secreted bone morphogenetic protein (BMP) ligands activate signaling in the GSC to suppress transcription of the differentiation factor Bam. Lili is required for this suppression and functions as a modulator of BMP signal transduction in this and other contexts. LMBR1/LIMR type transmembrane proteins are conserved throughout the Metazoa, and this study provides the first example to our knowledge of physiological function in a model organism.
Keywords: stem cell, germline, Dpp, decapentaplegic, lipocalin receptor
Abstract
Limb development membrane protein-1 (LMBR1)/lipocalin-interacting membrane receptor (LIMR)-type proteins are putative nine-transmembrane receptors that are evolutionarily conserved across metazoans. However, their biological function is unknown. Here, we show that the fly family member Lilipod (Lili) is required for germ-line stem cell (GSC) self-renewal in the Drosophila ovary where it enhances bone morphogenetic protein (BMP) signaling. lili mutant GSCs are lost through differentiation, and display reduced levels of the Dpp transducer pMad and precocious activation of the master differentiation factor bam. Conversely, overexpressed Lili induces supernumerary pMad-positive bamP-GFP–negative GSCs. Interestingly, differentiation of lili mutant GSCs is bam-dependent; however, its effect on pMad is not. Thus, although it promotes stem cell self-renewal by repressing a bam-dependent process, Lilipod enhances transduction of the Dpp signal independently of its suppression of differentiation. In addition, because Lili is still required by a ligand-independent BMP receptor, its function likely occurs between receptor activation and pMad phosphorylation within the signaling cascade. This first, to our knowledge, in vivo characterization of a LMBR1/LIMR-type protein in a genetic model reveals an important role in modulating BMP signaling during the asymmetric division of an adult stem cell population and in other BMP signaling contexts.
The human lipocalin-interacting membrane receptor LIMR (also known as LMBR1L) and the closely related limb development membrane protein-1 (LMBR1) share a predicted multitransmembrane (TM) structure that is strikingly different from other well-characterized integral membrane receptors. LIMR was originally isolated by phage display through its interaction with lipocalin-1 (Lcn-1), a secreted lipid-binding carrier protein (1). Cell culture studies showed that LIMR could mediate the internalization of Lcn-1, suggesting a role in cell signaling (2). However, because the physiological roles of both Lcn-1 and LIMR are unknown, the significance of this observation is unclear. Less is known about the LMBR1 protein. Initially, the human locus was genetically linked to multiple congenital limb malformations. However, further studies of the human and mouse loci showed that the original association with limb defects was incidental because of the disruption of a long-range SHH enhancer located within an intron of LMBR1 (3). To date, no loss-of-function or gain-of-function analyses of LIMR or LMBR1 have been reported in any model system, and studies in vertebrates may be complicated by functional redundancy between the two family members.
Drosophila contains a single uncharacterized LIMR-like protein, CG5807, which is the shared ortholog of both LIMR and LMBR1. We have investigated the fly gene in vivo and show here that it functions in the germ-line stem cells (GSC) of the Drosophila ovary. In the fruit fly, oocytes are continually produced by GSCs that are housed within a structure called germarium (Fig. 1A); here, two to three GSCs adhere to cells of the somatic niche, the cap cells (CC) (4, 5). As the GSCs divide, one daughter cell remains in contact with the CCs and maintains stem cell identity, whereas the other forms away from the niche and turns into a differentiating cystoblast (CB), the progenitor of egg chambers and ultimately oocytes.
Fig. 1.
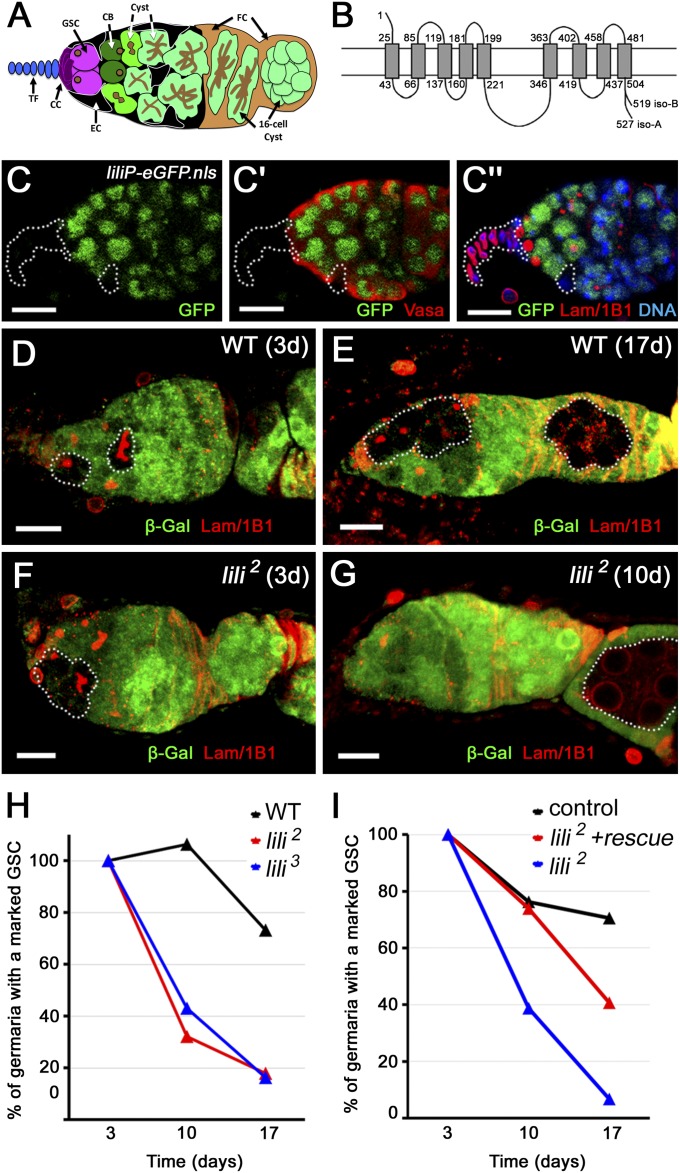
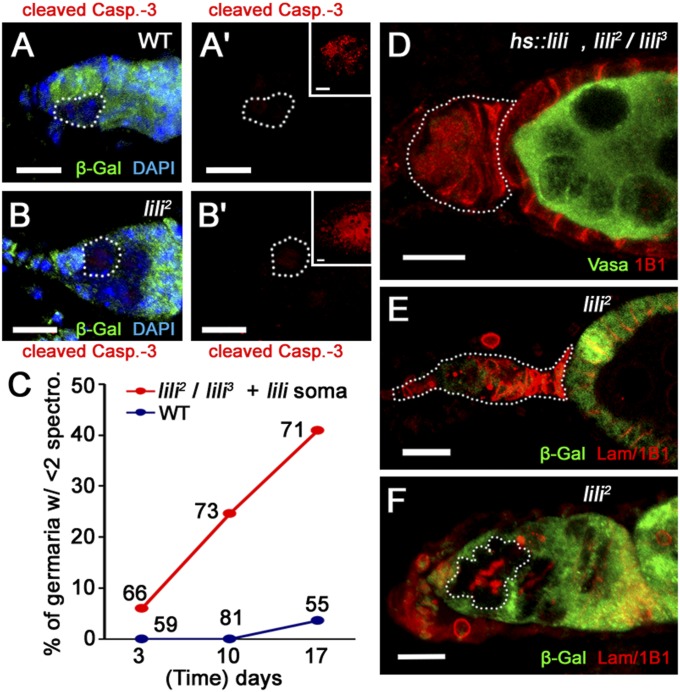
lili is intrinsically required for GSC maintenance. (A) Illustration of Drosophila germarium highlighting germ line: GSC (magenta), CB (dark green), cysts (green and light green); and somatic cells of the niche: cap cells (CC; purple), terminal filament cells (TF; blue), and escort cells (EC; black). Follicle cells surrounding forming egg chambers are shown in light brown (FC). TFs and CCs show strong nuclear membrane staining with anti-Lamin C Ab and can be distinguished by their position and morphology at the anterior tip of the germarium. All germ-line cells express the cytoplasmic protein Vasa and can be distinguished by the presence of an intracellular structure (dark brown) detected by anti-Adducin Ab (1B1). This structure is called spectrosome and appears spherical in GSCs and CBs, whereas it is called fusome and appears branched across the interconnected cells of developing cysts. GSCs and CBs are distinguished by location: GSCs contact CCs and ECs, whereas CBs contact GSCs and ECs, but not CCs. (B) An illustration of predicted Lilipod topology. Isoforms A and B bear unique C termini (15 aa in A and 6 in B). (C–C′′) Anterior half of a liliP-eGFP.nls germarium stained for GFP (lili-reporter), Vasa (germ line), Lamin C (CC nuclear membrane), anti-1B1 (spectrosome/fusome), and DAPI (nuclei) as marked in images. Dashed outline mark TF and CCs, and one intercalating EC; none express lili-eGFP.nls. The continued presence of GFP in the differentiating germ line may reflect persistence of GFP protein. liliP-eGFP.nls must, however, be transcribed in GSCs. (D–G) Mosaic germaria containing clonal GSCs (β-gal negative) and progeny cysts (dashed outlines) at 3 (D) and 17 (E) d ACI stained with both anti-Lamin C and anti-1B1 (red) as well as anti–β-gal (green). (D and E) Mosaic germaria with control WT clones. (F and G) Mosaic germaria with lili2 homozygous mutant clonal GSCs and progeny at 3 d ACI (F), but only a clonal cyst and no lili2 mutant GSCs at 10 d ACI (G). (H) Progressive loss of lili mutant GSCs over time. Percentage of total germaria that contain at least one WT or mutant clonal GSC. (I) Expression of Lili (nos-Gal4vp16UASp-liliA) slows down lili mutant GSC loss. (Scale bars: 10 µm.)
The maintenance of ovarian stem cells is tightly regulated by multiple extrinsic and intrinsic factors. Some of these factors repress the differentiation program in the renewed GSC, whereas others relieve this repression in the CB. The major signaling system in this process is the BMP pathway (6). Specifically, the BMP2/4 ligand decapentaplegic (Dpp) is secreted by CCs and signals onto adjacent GSCs to prevent their differentiation. In the GSC, the signal is transmitted through the type I BMP receptor thickveins (Tkv) and the receptor-regulated R-SMAD Mothers against dpp (Mad) to the nucleus, thereby suppressing transcription of the differentiation-promoting factor Bag of marbles (Bam) (7, 8). Reduced BMP signaling favors differentiation resulting in stem cell loss; conversely, increased BMP signaling favors self-renewal resulting in GSC hyperplasia. In this context, modulators of signaling play a critical role in maintaining the optimal balance between self-renewal and differentiation. By enhancing BMP pathway activity in one daughter cell and antagonizing it in the other (9), they effect a dramatic switch from pathway on (in the renewed GSC) to pathway off (in the differentiating CB) during a single cell division.
We present here the first evidence to our knowledge of a physiological role for a LIMR/LMBR1-type protein in a model organism, showing that it contributes to the regulation of the BMP pathway in the female germ line. CG5807 is required in GSCs to promote BMP signaling and ensure the suppression of bam. Its loss leads to stem cell differentiation and sterility over time. Conversely, its overexpression increases the number of BMP-responsive cells, thereby expanding the GSC compartment. We also show that CG5807 affects the signaling cascade between the Tkv receptor and the Mad transducer, and that it operates in other BMP-signaling contexts in the soma. Based on these findings, we have named the CG5807 gene lilipod (lili) to reflect its sequence conservation and its function as a “LIMR-like promoter of ovarian dpp.”
Results
Lili Is Evolutionarily Conserved and Required in GSCs for Germ-Line Maintenance.
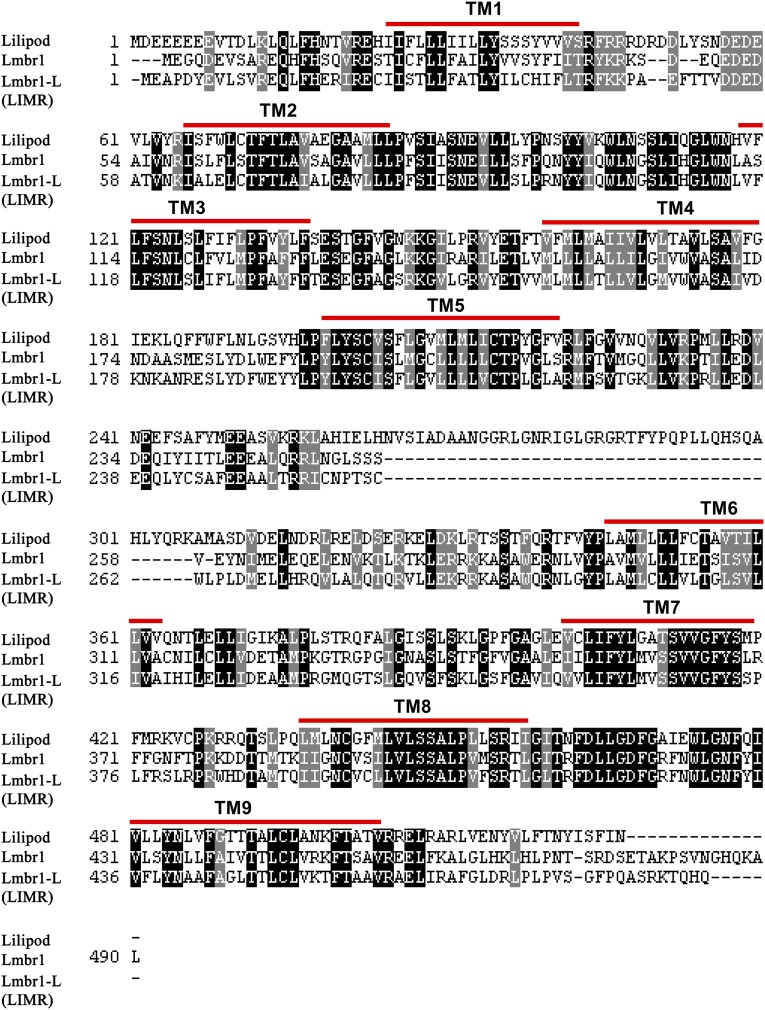
The Lilipod protein shares 44% (71%) amino acid identity (similarity) with human LIMR and 43% (72%) identity (similarity) with human LMBR1 (Fig. S1). TMpred and TMHMM (v2.0) predict a structure with nine TM spanning regions, extracellular N- and intracellular C-termini, and a large 125-aa central loop (Fig. 1B), similar to that originally proposed for human LIMR (2). Homozygous mutant animals (lili1, lili2, or lili3 allele) died as young larvae (during L1 and early in L2), but could be rescued to viability by a genomic p(liliWT) construct or by somatic expression of Lili isoform A (hs-Gal4 or actin5C-Gal4 with UASt-liliA).
Fig. S1.
Sequence conservation of Lili and the human proteins LIMR/LMBR1-like and LMBR1.
lili expression is reported in several adult tissues with highest enrichment in the ovary and testis (flybase.org). To confirm expression in the ovary, we used a liliP-eGFP reporter containing 626 bp of 5′-flanking genomic DNA (Fig. S2) and detected GFP protein in the GSCs and their progeny but not in the somatic niche (cap, escort, or terminal filament cells) (Fig. 1 C–C′′); at a later stage, expression is also found in the somatic follicular epithelium around the forming egg chambers (Fig. S3). Thus, lili is transcribed at least in the GSCs and the follicle cells.
Fig. S2.
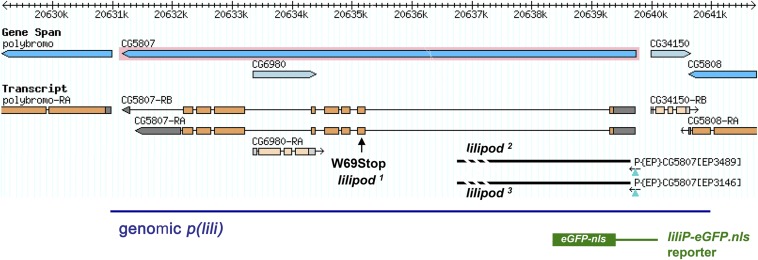
Illustration of the lili (CG5807) locus, lili mutations, and lili transgenes. A schematic representation of the lili genomic location as per Flybase. Orange boxes mark ORF and gray regions 5′ and 3′ UTRs, Orientation is with centromere to the left. The lili1 allele was obtained from the l(3)96Bb2 chromosome. Mobilization of P-elements P{EP}CG5807EP3489 and P{EP}CG5807EP3146 generated directed deletions (thick bar) of the first exon and part of the first intron, resulting in the lili2 and lili3 alleles. A 10,022-bp genomic rescue construct includes the lili transcription unit and flanking DNA (blue line). The liliP-eGFP.nls reporter contains the lili promoter region through the start codon (626 bp).
Fig. S3.
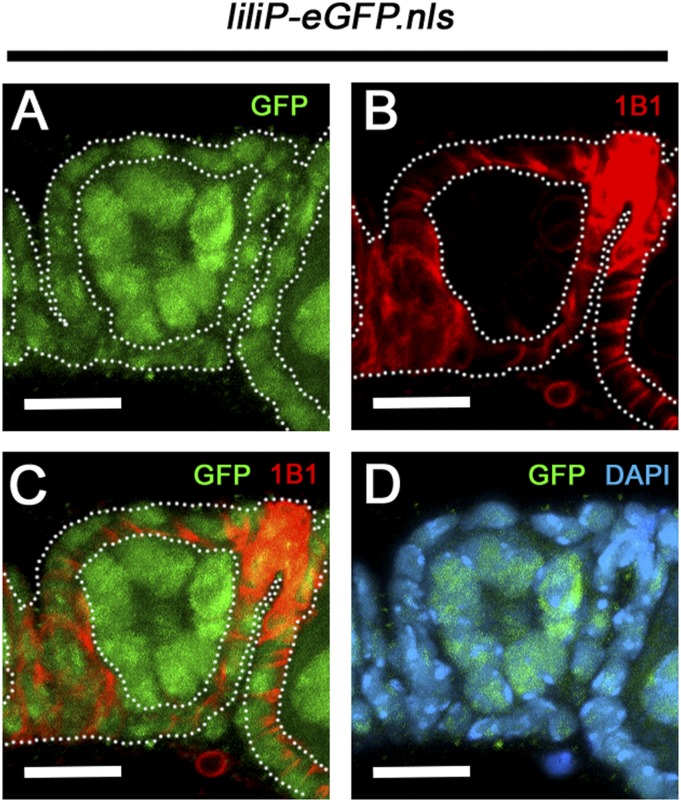
Expression of lili in the follicle cells around egg chambers. (A–C) The first separate egg chamber from a liliP-eGFP.nls female shows the continued presence of GFP (green) in the germ line, but also its expression in the surrounding follicle cells, which stain strongly with 1B1 (red). (D) DAPI is also shown. (Scale bars: 10 µm.)
To investigate lili’s function in the germ line, we analyzed homozygous mutant GSC clones induced in heterozygous females by the FLP/FRT method. Clonal GSCs and their progeny were identified by loss of the constitutive β-galactosidase marker arm-lacZ and GSC maintenance was assessed at days 3, 10, and 17 after clonal induction (ACI). Control GSCs (wt) were maintained over time, with 75–85% of germaria still containing a clonal GSC at 17 d ACI (level at 3 d ACI set as 100%) (Fig. 1 D, E, and H and Dataset S1). By contrast, at 10 and 17 d ACI, the frequency of lili mutant GSCs had dropped drastically (Fig. 1 F–H and Dataset S1). As expected, mutant stem cell loss was generally accompanied by replacement with a wt stem cell (Fig. 1G). To confirm that this phenotype was due to loss of Lili, we rescued the stem cell loss by driving expression of the wt protein in lili2 mutant GSCs (i.e., mutant clones in a nos-Gal4vp16 UASp-liliA background) (Fig. 1I and Dataset S1).
Altogether, these data establish that lili is expressed and required in the female germ line, where it promotes GSC maintenance.
lili Mutant GSCs Are Lost Through Differentiation.
Failure to self-renew is only one possible cause of stem cell loss, which can also result from cell death or, in mosaic germaria, from diminished fitness of mutant GSCs vis-à-vis WT ones (competition). Hence, we specifically investigated these possibilities in the case of lili.
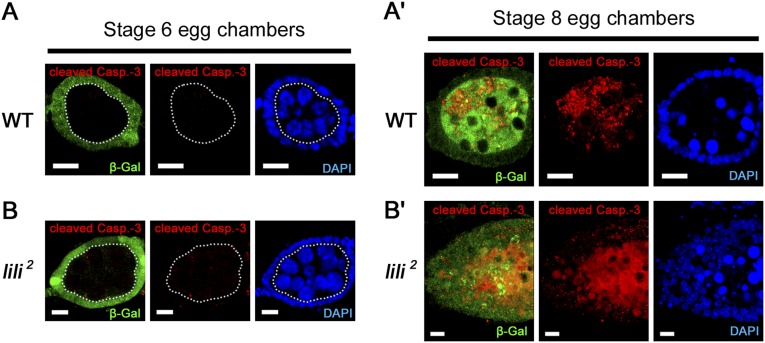
We assessed apoptotic death by staining mosaic ovaries for activated Caspase-3 at 8 d ACI. As for wild-type clones (Fig. 2A), we did not detect any activated Caspase-3 staining in lili 2 or lili3 mutant GSCs (n = 40 and n = 47, respectively) (Fig. 2B). Cell death was also not observed in WT or lili mutant egg chambers, but was clearly detected in stage 7–8 egg chambers when induced by poor nutrition (Fig. S4). In agreement with this finding, morphologically normal lili mutant cysts, egg chambers and eggs were regularly observed after clonal induction (Fig. 1 F and G). Altogether, these data indicate that lili is not required for GSC viability.
Fig. 2.
Loss of lili mutant GSCs is not caused by cell death or cell competition. (A and B) Germaria containing either WT control (A) or lili mutant (B) clonal GSCs (β-gal negative; dashed lines) do not show any activated Caspase-3 staining (red) (A′ and B′). Positive cell death control (anti-activated Caspase-3 Ab; red) stage 7–8 egg chambers are shown in Insets (Fig. S4). Clone tissue is marked by absence of β-gal (green). (C) GSCs are lost over time in hs-Gal4/UASt-liliA; lili2/lili3 females. (D) Example of a “GSC-depleted” germarium (dashed outline) stained for the germ line (Vasa; green) and membranes/spectrosomes/fusomes (1B1; red) from a 10-d-old hs-Gal4/UASt-liliA; lili2/lili3 female. (E) A similarly depleted germarium (dashed outline) attached to a lili2 mutant clonal egg chamber (β-gal present in the soma but not the germ line) stained for the spectrosome, fusome, and cell membranes marker combination Lamin C/1B1 (red). (F) Example of a GSC-depleted germarium showing developing lili2 clonal cysts (dashed outline; β-gal absent) at the site normally occupied by GSCs, staining is with anti-LamC/anti-1B1 combination (red; branched fusome is clearly visible in clonal cyst). (Scale bars: 10 μm.)
Fig. S4.
Lack of cell death in lili mutant germ line. (A and A′) Cell death in WT clonal germ line is detected at the stage 7-8 checkpoint (A′) when induced by nutritional stress, but not at stage 6 (A). (B and B′) lili mutant clonal germ line behaves in an identical fashion. Dashed line in A and B separates the nonclonal soma (β-gal positive; green) from the clonal germ line (β-gal negative; green). Notice that egg chambers appear normal at stage 6 in both the WT (A) and lili mutant case (B) as shown by DAPI staining. (Scale bars: 10 µm.)
Loss of SCs in mosaic germaria can also be due to competition. This phenomenon occurs when metabolically less fit mutant cells compete for growth-promoting signals with neighboring WT cells; as a result, the mutant cells are eliminated and replaced by their WT competitors. In the germ line, this elimination occurs when less-fit mutant SCs are displaced from the niche by the healthier wt SCs, and are thereafter induced to differentiate (9, 10). A hallmark of this phenomenon is that in-niche retention of mutant SCs can be restored by absence of wt competitors (as shown for GSCs mutant for dm, the fly MYC homolog; ref. 10). To investigate this possibility, we turned to lili2/lili3 transheterozygote females rescued by hs-Gal4–driven expression of UASt-liliA, relying on the preferentially somatic expression from the UASt vector (11). In this background (somatically-rescued lili2/lili3 females), the all-mutant germ line still showed progressive GSC loss despite the lack of WT SC (no competition) (Fig. 2 C and D). Results consistent with this interpretation were also obtained from our clonal analyses (scoring the rare all-mutant samples presumably due to multiple FLP/FRT events in one germarium). In wt controls, germaria with all-clonal germ line were still present and normal at day 17 (Dataset S2). In contrast, when inducing lili mutant GSCs, no normal germaria with all-mutant germ line were observed at the later time point (although present at day 3 ACI). We detected instead “depleted” germaria, containing either no germ line at all or only mutant differentiating cysts (Fig. 2 E and F), and “arrested” germaria, with mutant spectrosome-containing cells but no cysts, right next to developing egg chambers (Dataset S2). Thus, loss of lili mutant GSCs in mosaic germaria does not result simply from competition between mutant and WT stem cells for critical resources.
Collectively, these findings suggest that Lili is required in GSCs for the promotion of self-renewal over differentiation, rather than GSC survival or cell fitness.
BMP Pathway Gain of Function Requires lilipod.
As mentioned in Introduction, BMP signaling plays a central role in balancing self-renewal versus differentiation, and the Dpp ligand signals to the GSC through the Tkv receptor to suppress expression of the potent differentiation factor Bam. In fact, either loss of BMP signaling or expression of exogenous Bam are sufficient to trigger GSC differentiation in the niche and, thus, SC loss (6, 12). Conversely, excessive BMP pathway activity or loss of bam delays differentiation and results in accumulation of stem cells outside the niche (GSCs hyperplasia) (6, 13). To investigate whether loss of lili mutant GSC might be due to a perturbation in BMP signaling, we investigated the relationship between lili and the pathway components bam and tkv.
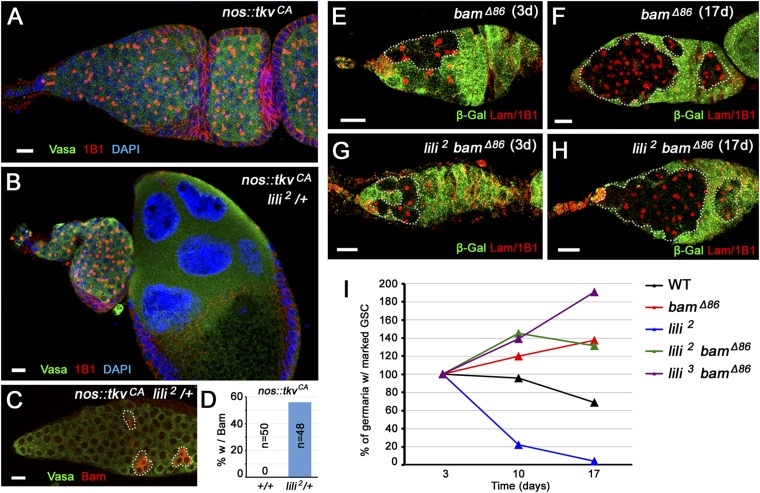
To assess whether pathway activity was sensitive to lili gene dosage, we relied on a genetic background that is hyperactive for BMP signaling but sensitized to the dosage of pathway components. Germ-line expression of a constitutively active ligand-independent form of the Tkv receptor (nos-Gal4vp16 UASp-tkvCA) induces a tumorous phenotype (all GSC-like cells no cysts) that can be partially suppressed by loss of one WT allele (−/+) of pathway components (14, 15). Interestingly, in this test, lili mutant alleles acted as dominant suppressors of the tumorous phenotype by restoring the presence of dividing cysts (Fig. 3 A and B). In this nos-Gal4vp16 UASp-tkvCA lili2/+ background, expression of the differentiation factor Bam was observed in 56% of the ovarioles (27 of 48 germaria), compared with never in nos-Gal4vp16 UASp-tkvCA ovarioles (0 of 48 germaria) (Fig. 3 C and D). Importantly, heterozygosity for lili does not by itself promote differentiation, i.e., it does not cause GSC loss (lili3/+ contained 5.40 ± 0.20 spectrosomes, n = 60 germaria; wild-type contained 5.14 ± 0.14 spectrosomes, n = 100). Hence, the sensitivity of TkvCA to lili gene dosage most likely reflects a limiting effect of lili on signaling by the BMP receptor.
Fig. 3.
lili dominantly suppresses hyperactivated BMP pathway. (A and B) Germaria from either a nos-Gal4vp16 UASp-tkv CA female (A) or a nos-Gal4vp16 UASp-tkv CA female heterozygous for lili (lili 2/+) (B) stained with anti-Vasa and anti-1B1 (A and B). (C and D) In nos-Gal4vp16 UASp-tkv CA females heterozygous for lili (lili2/+), induction of Bam expression (anti-Bam Ab; red) is detected in the tumorous germ line (anti-Vasa Ab; green): detection in germarium (C) and quantification (D). Dashed outlines mark germ-line cells expressing Bam. (E–H) Germaria containing homozygous bamΔ86 (E and F) or lili2 bamΔ86 (G and H) clonal GSCs at 3 and 17 d ACI stained with anti–β-gal (green) and anti-Lamin C/anti-1B1 (red). Dashed outlines mark clonal germ-line cells. (I) Percentage of germaria containing a homozygous clonal lili bamΔ86 double mutant GSC, lili2, or bamΔ86 single mutant GSC, or control clonal GSC at 3, 10, and 17 d ACI. Increased accumulation of bamΔ86 GSC clones was reported by Jin et al. (19), due to increased competitiveness for the niche compared with wild-type GSCs; lili bamΔ86 double mutant GSCs show a similar effect. (Scale bars: 10 μm.)
Next, we reasoned that if Lili promotes GSC maintenance through dpp signaling, then it should contribute to the repression of bam, and the differentiation of lili mutant stem cells should be entirely bam-dependent (16–18). To test this prediction, we analyzed double-mutant lili bam GSCs. Maintenance of single mutant (lili2 or bamΔ86) and double mutant (lili2 bamΔ86 or lili3 bamΔ86) GSC clones was assessed on day 3, 10, and 17 ACI. As expected, lili2 mutant SCs were lost over time, whereas bamΔ86 mutant stem cells failed to properly differentiate, causing the accumulation of spectrosome-containing cells throughout the germarium (Fig. 3 E and F). In the double mutants, loss of bam blocked the differentiation of lili mutant GSCs, resulting in the accumulation of undifferentiated, spectrosome-containing lili bamΔ86 cells (Fig. 3 G–I and Dataset S3). No lili bamΔ86 double mutant germ-line cysts were observed at any time point, demonstrating that lili mutant GSCs completely depend on bam for differentiation. Interestingly, lili bam GSCs persisted in the niche longer than WT SCs, similarly to single mutant bam cells (19), pointing to a possibly enhanced association with the soma.
These findings suggest that lili functions to promote self-renewal over differentiation through the BMP pathway.
pMad Is Decreased in lilipod Mutant GSCs.
The genetic data described above were consistent with a model whereby Lili promoted signaling by Dpp. Hence, we decided to directly assess the activity of the dpp pathway in lili mutant GSCs.
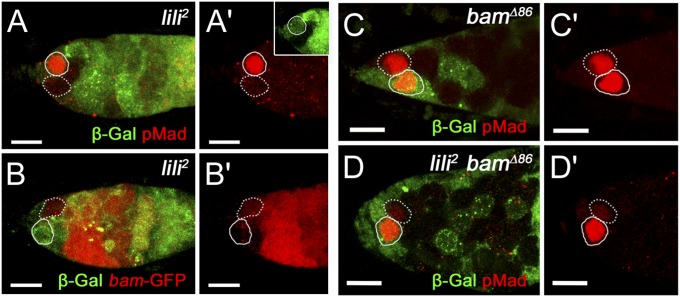
Pathway activity can be monitored at the level of activated Mad by using the anti-pMad Ab, and at the level of bam-silencing using the bamP-GFP reporter. We therefore compared pMad and bamP-GFP expression in germaria containing, still in the niche, an unmarked clonal GSC (either WT control or lili2 mutant clone) next to a β-galactosidase–marked WT GSC (at 8 d ACI). As expected, pMad expression was robust and bamP-GFP was undetectable in all WT GSCs, clonal and nonclonal. By contrast, a large fraction of lili2 mutant GSCs showed greatly reduced pMad levels compared with their neighboring WT stem cell (39%; 14 of 36 mutant-WT GSC pairs showed clearly lower pMad in the mutant SC; P < 0.005) (Fig. 4 A and A′). Conversely, a precocious, although still weak, up-regulation of bamP-GFP expression could be seen in many lili mutant GSCs (21%; 4 of 19 mutant-WT GSC pairs showed clearly higher GFP levels in the mutant SC; P < 0.005) (Fig. 4 B and B′). Considering the short experimental window between sufficient degradation of Lili protein after ACI and consequent stem cell loss to differentiation, these levels of pMad reduction and bam induction are significant.
Fig. 4.
lili is required for BMP signaling in the GSC. In all images, homozygous clonal GSCs are marked by a dashed outline, whereas neighboring nonclonal GSCs are marked by a solid outline. (A–B′) Germaria containing a lili2 mutant GSC (β-gal negative; green) next to a WT GSC (β-gal positive; green) stained for pMad (A and A′) or bamP-GFP (B and B′) expression (both shown in red). The lili mutant GSC have lower pMad and higher bamP-GFP compared with neighboring WT GSCs. (C and C′) Germarium containing a bamΔ86 mutant GSC (β-gal negative; green) next to a WT GSC (β-gal positive; green) stained for anti-pMad (red). Robust pMad was present in all clonal GSCs observed (n = 38). (D) Germarium containing a clonal lili2 bamΔ86 double mutant GSC (β-gal negative; not green) next to a WT GSC (β-gal positive; green) stained for pMad (red). Loss of pMad is observed even in the absence of differentiation. (Scale bars: 10 µm.)
To establish whether the decrease in pMad was a consequence of the precocious activation of bam, we then assessed pMad signaling in lili2 bamΔ86 double mutant GSCs. As reported (16, 20), pMad expression remained high in bamΔ86 mutant SCs in contact with cap cells (Fig. 4 C and C′). By contrast, pMad was reduced in nearly 60% of the lilipod2 bamΔ86 GSCs within the niche relative to their neighboring nonclonal GSC (reduced in 13 of 23 mutant-WT pairs; Fig. 4 D and D′). Hence, the disruption of BMP signaling in lili mutant GSCs is independent of bam activation, differentiation, or exit from the niche.
In conclusion, Lili is required to maintain normal levels of BMP signal transduction in the GSC and it does so independently of its ability to suppress differentiation.
Lilipod Overexpression Induces Supernumerary pMad+ bamP-GFP− GSCs.
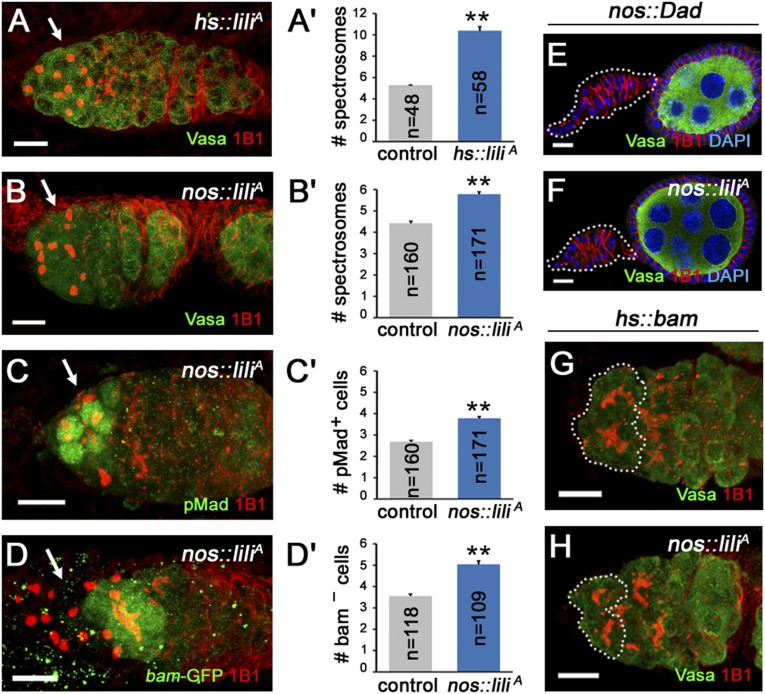
Lastly, to test whether Lili overexpression is not only required but also sufficient to promote self-renewal over differentiation, we expressed the protein ubiquitously (hs-Gal4) or in the germ line (nos-Gal4VP16) and then assessed the number of spectrosome-containing cells (GSCs + CBs) per germarium. A significant increase was observed in both genetic backgrounds (Fig. 5 A–B′). A single heat-shock pulse of homozygous hs-Gal4 UASp-liliA females was sufficient to induce a doubling of the average number of spectrosome-containing cells from 5.27 ± 0.03 (n = 48) in Gal4-only control to 10.38 ± 0.40 (n = 58) when UASp-liliA was also present (Fig. 5 A and A′). In the case of nos-Gal4vp16 UASp-liliA flies (single transgenes), the increase was more modest but still significant, from 5.06 ± 0.12 (n = 121) spectrosomes per germarium in nos-Gal4vp16 UASp-lacZ controls to 6.21 ± 0.15 (n = 107) in nos-Gal4vp16 UASp-liliA (P < 0.005) (Fig. 5 B and B′). Importantly, this was accompanied by corresponding increases in pMad-positive (Fig. 5 C and C′) and bam-P-GFP–negative spectrosome-containing cells (Fig. 5 C–D′). This effect is similar to the GSC expansion induced by nos-Gal4–driven expression of the WT Tkv receptor (21).
Fig. 5.
Overexpression of Lilipod results in ectopic GSC-like cells and increased BMP signaling activity. (A and B) Induction of Lili expression in hs-Gal4vp16 UASp-liliA females leads to an increase in GSC numbers. See example of germarium stained with 1B1 (red) and Vasa (green) to identify spectrosome-containing germ-line cells in A, quantification compared with hs-Gal4vp16 only control in A′. (B and C) Germaria from nos-Gal4vp16 UASp-liliA females also show a reproducible but more modest expansion of the GSCs+CBs compartment shown as spectrosome-containing cells in A (1B1 in red and Vasa in green). That this expansion reflects an increase in pMad-positive, bamP-GFP–negative GSCs is shown in C (1B1 in red and pMad in green) and D (1B1 in red and bamP-GFP in green). All quantifications compared with nos-Gal4vp16 controls are shown to the right (B′–D′). (E and F) Germaria from nos-Gal4vp16 UAS-Dad (E) and nos-Gal4vp16 UAS-Dad UASp-liliA (F) females both show complete loss of GSCs, dashed outlines mark depleted germaria, and notice attached egg chamber at right. (G and H) A HS-induced pulse of Bam expression either in nos-Gal4vp16 control (C) or nos-Gal4vp16 2XUASp-liliA (D) germaria results in forced differentiation of the germ line as shown by 1B1-stained fusomes in Vasa-positive cells in the GSCs position. Phenotypes shown in F and H were as penetrant as in controls (E and G). (Scale bars: 10 µm.)
Consistent with a model of GSC expansion through enhanced Dpp signaling, impairment of the Dpp pathway within Lili-overexpressing GSCs completely suppressed the supernumerary-GSCs phenotype. In fact, the coexpression of the inhibitory SMAD Dad with Lili (nos-Gal4vp16 UASp-liliA UASp-Dad) or the induction, by single heat shock, of Bam in Lili-overexpressing cells (hs-Bam with nos-Gal4vp16 UASp-liliA) resulted in GSCs loss through precocious differentiation (Fig. 5 E–H). Thus, the Lili-induced expansion of the GSC compartment appears to be Dpp-dependent.
Discussion
In this study, we show that the LIMR/LMBR1 type protein Lilipod functions to promote germ-line stem cell self-renewal in the Drosophila ovary. Lili is intrinsically required in GSCs to promote self-renewal over differentiation with loss or gain of Lili resulting in precocious or delayed differentiation, respectively. We show that Lili loss or gain affects the level of BMP pathway activity in the early germ-line lineage. Whereas precocious differentiation of lili mutant GSCs is entirely Bam-dependent, Lili’s modulation of Dpp signaling in the GSC is independent of the differentiation program, acting on a step in the cascade between the activated BMP receptor and the pMad transducer.
The activity of the BMP pathway is tightly regulated in the ovarian stem cell niche to maintain homeostatic balance between self-renewal and differentiation. This regulation of Dpp signaling involves multiple positive and negative modulators (21–23) in both GSC and CB daughter cells. In the GSC, germ-line factors positively (Lis-1) and negatively (the I-SMAD Dad) regulate the transduction of the signal intracellularly for an optimal balance of differentiation and self-renewal. They achieve this goal by modulating the activation and/or stability of the transducer Mad/pMad and the receptor Tkv (21, 22). In the forming CB, reduced signaling, as the cell moves away from the source of Dpp (the niche), leads to the stabilization of factors (Fu kinase and Smurf E3 ligase) that promote the degradation of Tkv and Mad/pMad (22). This step is then followed by a Bam-dependent shutdown of Mad synthesis through the translational repressor Brat (23). Ultimately, these mechanisms work to prevent the precocious differentiation of the SCs or their tumorous expansion.
The marked reduction in pMad levels within lili mutant GSCs suggests that Lili either enhances the activity or protects the stability of signaling components. The finding that removal of Bam from lili mutant GSCs suppresses their precocious differentiation without restoring normal levels of pMad indicates that Lili’s effect on the cascade occurs upstream of bam. Moreover, because the TkvCA receptor induces tumor formation even in the absence of functional Dpp ligand (24), the sensitivity of the TkvCA tumorous phenotype to lili gene dosage (Fig. 3B) places the activity of Lili downstream of the ligand–receptor interaction. We conclude, therefore, that lilipod modulates the intracellular transduction of the Dpp signal somewhere between the Tkv–Punt interaction (required even for TkvCA) and phosphorylated pMad levels. It remains to be seen whether Lilipod functions directly with receptor components and/or the transducer Mad (to enhance interactions, activity, or stability) or indirectly (through novel or already known regulators, such as Lis-1, Dad, or Fu/Smurf). However, loss- and gain-of-functions phenotypes for these factors are not readily compatible with the ones described here for lili; for instance, neither Lis-1 overexpression nor dad loss-of-function have been reported to induce GSC expansion and down-regulation of Fu or Smurf has a much stronger effect than Lili overexpression (6, 21, 22). Nonetheless, these possibilities remain to be explored through further studies of lili in vivo and in vitro.
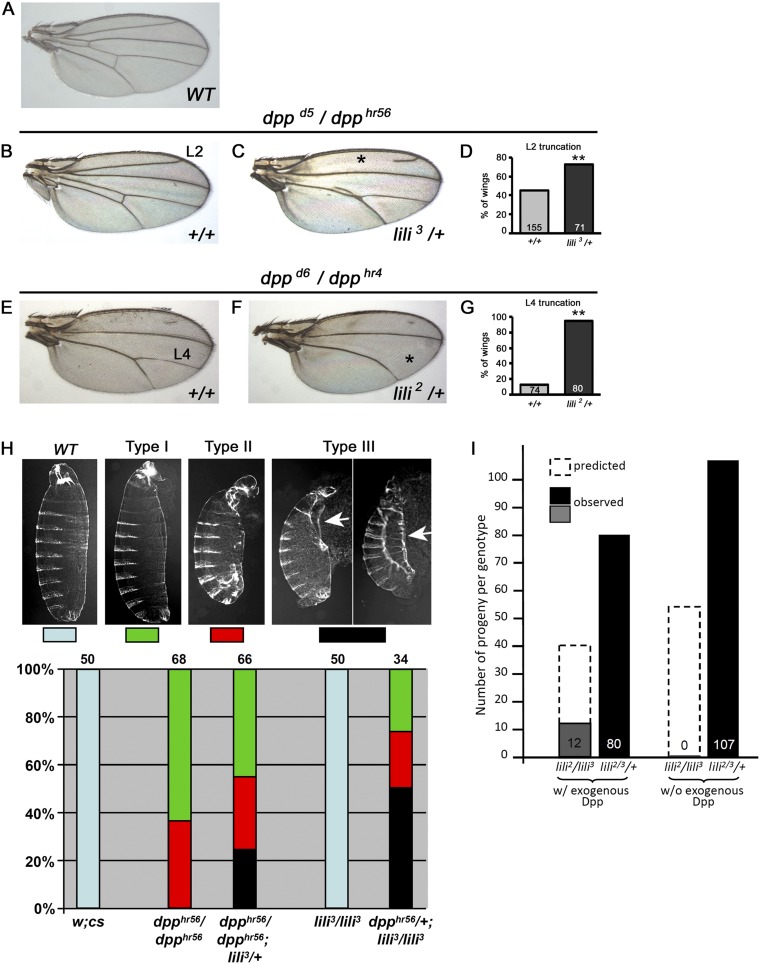
Does lili function as a universal modulator of BMP signaling or as a factor specific to the germ line? Whereas a thorough investigation of this issue is beyond the scope of this work, dpp–lili interactions in the soma point to roles in other BMP-signaling events. In fact, lili alleles dominantly enhance hypomorphic dpp mutant phenotypes observed in wings (Fig. S5 A–G) and embryonic cuticles (Fig. S5H), such that the range of mutant defects observed is more severe in dpp/dpp; lili/+ combinations than in dpp/dpp alone, and, conversely, introduction of one dpp mutant allele in a lili homozygous background has severe consequences for embryonic patterning. In addition, the lethality of lili alleles (delayed to the larval stage by a strong maternal contribution) can be rescued by ubiquitous low-level expression of exogenous Dpp (Fig. S5I). Although these findings do not exclude other non-Dpp-related functions for Lili, they confirm its significant role in regulating Dpp signaling in multiple developmental contexts.
Fig. S5.
lili functions in other BMP-signaling contexts. (A–H) Hypomorphic loss-of-function dpp alleles provide sensitized genetic backgrounds useful in querying the contribution of other factors to various BMP-signaling events. lili mutant alleles interact with Dpp signaling in the wing (A–G) and in the embryo (H). (A–G) Lili contributes to Dpp signaling during wing vein development. The reduction of Dpp signaling caused by the dppd5/dpphr56 and dppd6/dpphr4 genotypes results in wing vein patterning defects without affecting proliferation of the wing pouch. These phenotypes are sensitive to a reduction in the level of other BMP signaling components, such that heterozygosity increases the severity of vein defects (29, 30). (A) A WT adult wing. (B and C) In wings from dppd5/dpphr56 adults, the L2 vein is sometimes truncated (*) and the intervein region between L4 and L5 is sometimes reduced or lost. (C and D) Introducing one copy of lili3 in this background enhances the severity of both phenotypes. Quantification of L2 truncation is shown in D. (E) In the more severe dppd6/dpphr4 allelic combination, the L2 vein and L4–L5 intervein are mostly lost, but the L4 vein remains largely intact. (F) Introducing one copy of the lili2 mutant allele dramatically enhances the severity of the L4 loss (*); quantified in G. (H) A genetic interaction between dpp and lili mutant alleles is also observed in the embryo resulting in the enhancement of cuticle defects. All cuticles are shown with anterior on the top and ventral to the left. The embryonic lethal dpphr56/dpp hr56 genetic background produces a range of phenotypes, from cuticles with eight abdominal segments and variable deformities of the head and filtzkorper (Type I) to cuticles with seven or fewer abdominal segments, severe head defects, and internalization or complete loss of filtzkorper (Type II) (31). When one copy of WT lili is also removed (dpphr56/dpphr56; lili3/+), the range of mutant phenotypes becomes more severe, including embryos displaying the type III pattern (type II-like but with failure of dorsal closure; a process known to be dpp-dependent; ref. 32). Arrows point to failed dorsal closure; least and most severely affected cuticle are shown. Conversely, introduction of one dpphr56 allele in a lili3 homozygous background has severe consequences. Whereas all homozygous lili3/lili3 embryos and the large majority of heterozygous dpphr56/+ hatch with an essentially normal cuticle pattern (a small fraction, few %, of dpphr56/+ embryos die with anterior defects; ref. 31), lili3/lili3; dpphr56/+ embryos die with moderate to severe abnormalities including failure of dorsal closure. In these experiments, double heterozygous dpphr56/+; lili3/+ cuticles were indistinguishable from dpphr56/+; this result is not surprising because lili has a strong maternal component that likely masks its embryonic role in Dpp signaling unless the pathway is more severely compromised. These findings show that Lili contributes to BMP signaling during embryonic development as well. (I) All homozygous and transallelic lili-mutant combinations are 100% larval lethal. Leaky expression of Dpp in a hs-Gal4 UAS-dpp background (without heat shock) results in substantial rescue of the larval lethality. Numbers shown are total heterozygous adults (lili2 or 3/+ = lili2 or 3/TM6B) compared with total lili2/lili3 adults. In these crosses, the approximate expected ratio is 2:1 for lili2 or 3/TM6B to lili2/lili3, in the case of full rescue. Although early larval lethality has not been reported for any class of dpp alleles (33), the considerable rescue achieved by exogenous Dpp (∼1/3 of expected progeny) suggests that lili2/lili3 lethality is, at least in part, due to a dpp-dependent process.
Lastly, the high degree of sequence and structural similarity between LIMR and LMBR1 proteins from different species suggests conservation of function. The implication of Drosophila Lili in BMP signaling provides strong evidence for a signaling-related role for proteins of this kind. Given also the expression of a number of lipocalin-like factors in Drosophila, the fly offers an ideal system in which to dissect the function of Lili and investigate its possible regulation by a ligand.
Materials and Methods
Genetics.
Flies were grown at 25 °C unless otherwise stated. lili2; lili3 mutant alleles were generated by imprecise excision from P-element shown in Fig. S2 and delete the ORF in exon 1. The lili1 allele introduces a stop codon at position 69 and was isolated from chromosome l(3)96Bb2 (BL4526), which contains also a lethal hit in the Vps22 locus. The following chromosomes were generated by recombination: FRT lili1; FRT lili2; FRT lili3; FRT lili2 bamΔ86; FRT lili3 bamΔ86. Other stocks: hs-Gal4 (BL1799), Act5C-Gal4 (BL3954), nos-Gal4vp16 (BL4937), UAS-dpp (BL1486), bamΔ86 (BL5427), bamP-GFP (25), UASp-Dad (15), hs-bam (BL24636), UASp-tkvCA (16), dppd5 (BL2071), dppd6 (BL2062), and dpphr56 (BL36528); a dpphr56; lili3 recombinant stock was generated in the laboratory. GSC clones with marking were generated by FLP/FRT-mediated recombination by standard techniques (26) using FRT-82B arm-lacZ (BL7369). To induce lili mutant GSCs, 3- to 5-d-old adult females with a genotype of hs-FLP/+; FRT82B armadillo-lacZ/FRT82B lili (lili1, lili2, lili3, or FRT82B WT control) were heat-shocked at 37 °C for 1 h, twice per day, for 2 d. Bam expression was induced by a single 1-h heat shock, and ovarioles were scored 24 h later; Dad expression was induced through UAS-Dad and scored at day 3 after eclosion. All scoring was blind. Transgenic lines in w1118 were generated in-house: p(lili), 10,023-bp genomic DNA in pCaSpeR4; liliP-eGFP.nls, reporter containing upstream DNA through start codon; for the UASt-liliA-Myc, UASp-liliA, and UASp-eGFP-liliA expression constructs, cDNAs (isoform A) of identical sequence were obtained from BDGP (GH12663; GenBank AF132157) and by RT-PCR and cloned in UASt and UASp vectors.
Immunohistology.
Primary antibodies: mouse anti-Hts (1B1, 1:50; DSHB), mouse anti-Lamin C (LC28.26, 1:25; DSHB), rat anti-DE-cadherin (DCAD2, 1:50; DSHB), mouse anti-β-galactosidase (1:1,250; Promega), rabbit anti-β-galactosidase (1:2,000; Cappel), rabbit anti-GFP (1:10,000; Invitrogen), rabbit anti-Vasa (1:10,000; gift from P. Lasko, McGill University, Montreal), mouse anti-Bam (1:400; DSHB concentrated), rabbit anti-Cleaved caspase-3 (1:100; Cell Signaling), and rabbit anti-human p423/425 Smad3 (1:150; abcam52903) (27, 28). Secondary antibodies: Anti-mouse, anti-rat, and anti-rabbit Cy2-, Cy3- or Cy5-conjugated (1:200; Jackson Immuno Research Laboratories). Images were obtained with a Leica DM5500Q confocal system and processed with Adobe Photoshop.
Supplementary Material
Acknowledgments
We thank Drs. S. Zhu, A. Viczian, M. Zuber, and members of the F.P. laboratory for helpful discussions and comments on the manuscript; Dr. S. Ranade for imprecise-excision mutant alleles; and Bloomington Drosophila Stock Center and Developmental Studies Hybridoma Bank for fly stocks and Ab reagents. This work was supported by the NIH Grants R01GM110498 and R03HD082609 (to F.P.) and by Department of Ophthalmology of Upstate Medical University’s Research to Prevent Blindness Unrestricted Grant and Lions District 20-Y1 donations.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509856112/-/DCSupplemental.
References
- 1.Wojnar P, Lechner P, Merschak P, Redl B. Molecular cloning of a novel lipocalin-1 interacting human cell membrane receptor using phage display. J Biol Chem. 2001;276(23):20206–20212. doi: 10.1074/jbc.M101762200. [DOI] [PubMed] [Google Scholar]
- 2.Wojnar P, Lechner M, Redl B. Antisense down-regulation of lipocalin-interacting membrane receptor expression inhibits cellular internalization of lipocalin-1 in human NT2 cells. J Biol Chem. 2003;278(18):16209–16215. doi: 10.1074/jbc.M210922200. [DOI] [PubMed] [Google Scholar]
- 3.Lettice LA, et al. Disruption of a long-range cis-acting regulator for Shh causes preaxial polydactyly. Proc Natl Acad Sci USA. 2002;99(11):7548–7553. doi: 10.1073/pnas.112212199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290(5490):328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 5.Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296(5574):1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- 6.Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94(2):251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 7.Song X, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131(6):1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13(20):1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Harris RE, Ashe HL. Cease and desist: Modulating short-range Dpp signalling in the stem-cell niche. EMBO Rep. 2011;12(6):519–526. doi: 10.1038/embor.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhiner C, et al. Persistent competition among stem cells and their daughters in the Drosophila ovary germline niche. Development. 2009;136(6):995–1006. doi: 10.1242/dev.033340. [DOI] [PubMed] [Google Scholar]
- 11.Rørth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78(1-2):113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 12.Ohlstein B, McKearin D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development. 1997;124(18):3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- 13.McKearin D, Ohlstein B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development. 1995;121(9):2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- 14.Xi R, Doan C, Liu D, Xie T. Pelota controls self-renewal of germline stem cells by repressing a Bam-independent differentiation pathway. Development. 2005;132(24):5365–5374. doi: 10.1242/dev.02151. [DOI] [PubMed] [Google Scholar]
- 15.Jiang X, et al. Otefin, a nuclear membrane protein, determines the fate of germline stem cells in Drosophila via interaction with Smad complexes. Dev Cell. 2008;14(4):494–506. doi: 10.1016/j.devcel.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Casanueva MO, Ferguson EL. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development. 2004;131(9):1881–1890. doi: 10.1242/dev.01076. [DOI] [PubMed] [Google Scholar]
- 17.Chen D, McKearin D. Gene circuitry controlling a stem cell niche. Curr Biol. 2005;15(2):179–184. doi: 10.1016/j.cub.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Szakmary A, Cox DN, Wang Z, Lin H. Regulatory relationship among piwi, pumilio, and bag-of-marbles in Drosophila germline stem cell self-renewal and differentiation. Curr Biol. 2005;15(2):171–178. doi: 10.1016/j.cub.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Jin Z, et al. Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell. 2008;2(1):39–49. doi: 10.1016/j.stem.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci USA. 2003;100(8):4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, et al. Lissencephaly-1 controls germline stem cell self-renewal through modulating bone morphogenetic protein signaling and niche adhesion. Proc Natl Acad Sci USA. 2010;107(46):19939–19944. doi: 10.1073/pnas.1008606107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia L, et al. The Fused/Smurf complex controls the fate of Drosophila germline stem cells by generating a gradient BMP response. Cell. 2010;143(6):978–990. doi: 10.1016/j.cell.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Harris RE, Pargett M, Sutcliffe C, Umulis D, Ashe HL. Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev Cell. 2011;20(1):72–83. doi: 10.1016/j.devcel.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Z, Wang Z. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development. 2009;136(21):3627–3635. doi: 10.1242/dev.036939. [DOI] [PubMed] [Google Scholar]
- 25.Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003;130(6):1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- 26.Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121(11):3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- 27.Sun M, et al. Presynaptic contributions of chordin to hippocampal plasticity and spatial learning. J Neurosci. 2007;27(29):7740–7750. doi: 10.1523/JNEUROSCI.1604-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haerry TE. The interaction between two TGF-beta type I receptors plays important roles in ligand binding, SMAD activation, and gradient formation. Mech Dev. 2010;127(7–8):358–370. doi: 10.1016/j.mod.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Bangi E, Wharton K. Dpp and Gbb exhibit different effective ranges in the establishment of the BMP activity gradient critical for Drosophila wing patterning. Dev Biol. 2006;295(1):178–193. doi: 10.1016/j.ydbio.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Twombly V, et al. Functional analysis of saxophone, the drosophila gene encoding the BMP type I receptor ortholog of human ALK1/ACVRL1 and ACVR1/ALK2. Genetics. 2009;183(2):563–579, 1SI–8SI. doi: 10.1534/genetics.109.105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wharton KA, Ray RP, Gelbart WM. An activity gradient of decapentaplegic is necessary for the specification of dorsal pattern elements in the Drosophila embryo. Development. 1993;117(2):807–822. doi: 10.1242/dev.117.2.807. [DOI] [PubMed] [Google Scholar]
- 32.Fernández BG, Arias AM, Jacinto A. Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech Dev. 2007;124(11-12):884–897. doi: 10.1016/j.mod.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Irish VF, Gelbart WM. The decapentaplegic gene is required for dorsal-ventral patterning of the Drosophila embryo. Genes Dev. 1987;1(8):868–879. doi: 10.1101/gad.1.8.868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.