Significance
Human biology is typically studied within the framework of sex (evolved, innate factors) rather than gender (sociocultural factors), despite some attention to nature/nurture interactions. Testosterone is an exemplar of biology studied as natural difference: men’s higher testosterone is typically seen as an innate “sex” difference. However, our experiment demonstrates that gender-related social factors also matter, even for biological measures. Gender socialization may affect testosterone by encouraging men but not women toward behaviors that increase testosterone. This shows that research on human sex biology needs to account for gender socialization and that nurture, as well as nature, is salient to hormone physiology. Our paper provides a demonstration of a novel gender→testosterone pathway, opening up new avenues for studying gender biology.
Keywords: testosterone, gender, socialization, competition, sex
Abstract
Testosterone is typically understood to contribute to maleness and masculinity, although it also responds to behaviors such as competition. Competition is crucial to evolution and may increase testosterone but also is selectively discouraged for women and encouraged for men via gender norms. We conducted an experiment to test how gender norms might modulate testosterone as mediated by two possible gender→testosterone pathways. Using a novel experimental design, participants (trained actors) performed a specific type of competition (wielding power) in stereotypically masculine vs. feminine ways. We hypothesized in H1 (stereotyped behavior) that wielding power increases testosterone regardless of how it is performed, vs. H2 (stereotyped performance), that wielding power performed in masculine but not feminine ways increases testosterone. We found that wielding power increased testosterone in women compared with a control, regardless of whether it was performed in gender-stereotyped masculine or feminine ways. Results supported H1 over H2: stereotyped behavior but not performance modulated testosterone. These results also supported theory that competition modulates testosterone over masculinity. Our findings thus support a gender→testosterone pathway mediated by competitive behavior. Accordingly, cultural pushes for men to wield power and women to avoid doing so may partially explain, in addition to heritable factors, why testosterone levels tend to be higher in men than in women: A lifetime of gender socialization could contribute to “sex differences” in testosterone. Our experiment opens up new questions of gender→testosterone pathways, highlighting the potential of examining nature/nurture interactions and effects of socialization on human biology.
Testosterone is a major influence on bodily and behavioral features seen as male and/or masculine. Not surprisingly, then, research on testosterone in humans mainly focuses on men, with some notable exceptions (e.g., refs. 1–3). Women, however, also have naturally occurring testosterone, and testosterone sometimes functions via conversion to estradiol (4). Although testosterone exists and functions similarly in women and men, men have markedly higher average testosterone than women. This difference in testosterone is widely presumed to be a sex difference, that is, one that reflects maleness and femaleness caused by innate and evolved influences (3). Together, this leads to characterizations of testosterone as the essence of maleness, fixed and unchanging, and determined by only innate factors (3, 5). This occurs despite growing understandings of biology that emphasize plasticity and social modulation (for example in the brain, immune system, and genetics).
The view of testosterone as fixed and innate is empirically dubious, given a surprisingly large and underexplored nongenetic influence (6). This nongenetic influence includes meaningful and predictable variation from factors such as seasonal or diurnal rhythms (6). In addition, there are profound social influences on testosterone that are sometimes more clear than the more widely studied effects of hormones on behavior (7–9).
In what we call the “reverse relationship” (7), social modulation of hormones strongly implicates gender in the study of testosterone (3). Gender-related sociocultural experiences related to femininity (sociocultural habits and norms tied to women and girls, usually promoting communality and nurturance) (10) and masculinity (sociocultural habits and norms tied to men and boys, usually promoting agency and competition) (10) may affect physiological parameters such as testosterone. This could occur in ways similar to how social experiences related to poverty or harsh parenting exert profound neurobiological effects (11, 12). If social experiences related to gender can modulate androgens, this may lead to surprising biological consequences: a gender→testosterone pathway.
A gender→testosterone pathway would involve multiple inputs including neurobiological, sociocultural, and evolutionary factors. Testosterone responds to social phenomena that are evolutionarily salient, but not all social phenomena have been evolutionarily selected to modulate testosterone or do so in the same ways. Theory predicts that, for testosterone, one evolutionarily salient social context is competition (3). Competition, and its behavioral examples such as wielding power, are relevant to gender, testosterone, and evolution, making them especially relevant for testing possible gender→testosterone pathways.
Competition drives evolution; its outcomes influence key indicators of evolutionary fitness such as survival and reproduction (13). Competition involves attempts to acquire or defend real or perceived resources such as status, territory, partners, and, especially relevant to this article, power (14). Research demonstrates in nonhuman animals that competition can experimentally increase testosterone, including most notably in males of various bird species (15) but also in others. For example, male California mice who win competitions show increased testosterone (16). Like humans, male cichlid fish who watch each other fight show increased testosterone (17). In men and women, some types of competition, potentially especially those that are formalized or involve clear win/loss outcomes, have also been experimentally shown to increase testosterone (7, 9, 18). In addition, there is some correlational evidence that people—women and men—in roles that are oriented toward power, competition, and/or masculinity have higher testosterone than others, although socioeconomic status can moderate this (19–21). Also, mixed evidence suggests that “power poses” can increase testosterone (22, cf. ref. 23). Thus, evidence suggests that power, like competition, may increase testosterone.
In humans, competition can increase testosterone, but competition and wielding power are also subject to social forces: Western gender norms promote wielding power for men and discourage it for women (10, 24). Gender socialization can thus constrain women’s and men’s social behaviors, directly (through social enforcement of norms) or indirectly (via internalization of norms) (25). In addition, conceptualizations of masculinity tend to overlap with those of power and testosterone (3, 26). These gender considerations might influence how women and men engage in behaviors in ways that matter for testosterone. When men engage in more frequent competition than women do, or in ways that accord more with masculinity norms, this may have implications for testosterone. If men, more than women, are socialized to engage in competitive behaviors such as wielding power that increase testosterone, then this may partially explain why men have higher testosterone than women. Clearly, testosterone responds to social context, but huge gaps remain in understanding how neuroendocrine plasticity is shaped by social norms, especially those related to gender.
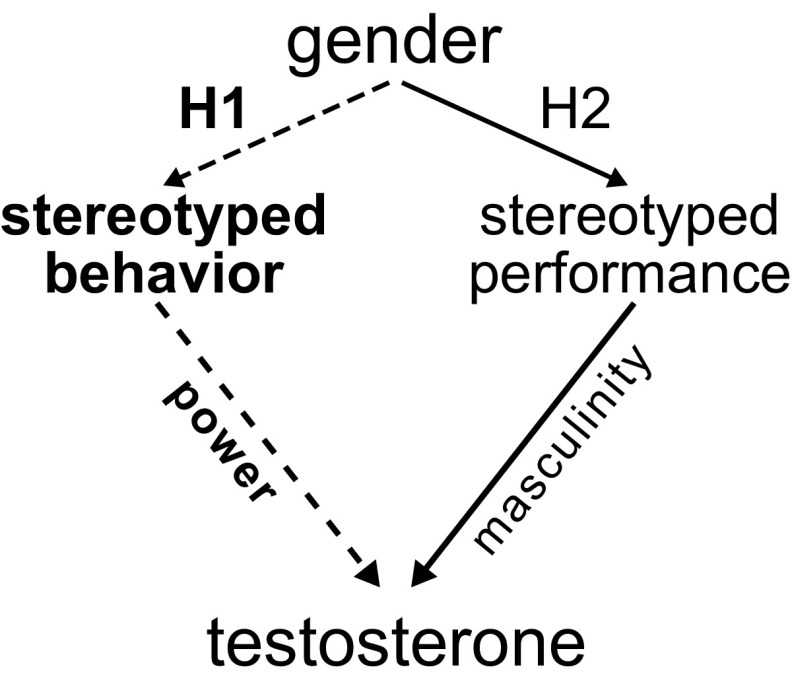
There may be more than one pathway from gender to testosterone because gender is multifaceted and includes behavior, stereotypes, roles, identity, and so on. In this experiment, we tested two potential pathways from gender to testosterone using power as a specific example of competition (Fig. 1): a stereotyped behavior pathway (i.e., what behaviors women and men do) vs. a stereotyped performance pathway (i.e., how men and women perform the same behaviors). In both pathways, we hypothesize that gender can influence testosterone.
Fig. 1.
Two hypothesized gender → testosterone pathways (our results supported the bolded pathway). In H1, the stereotyped behavior pathway, what behaviors women and men do influence testosterone: Wielding power, which social norms encourage for men but not women, increases testosterone. In H2, the stereotyped performance pathway, how men and women perform the same behaviors influence testosterone: Behavioral overlaps with masculine, but not feminine, gender stereotypes increase testosterone.
In the stereotyped behavior pathway (H1), we hypothesize that wielding power increases testosterone. Gender norms constrain these behaviors such that men wield power more frequently than women do. Accordingly, men could be engaging more frequently than women in behaviors that increase testosterone. In H1, we test whether wielding power would increase testosterone regardless of whether it is performed in gender-stereotyped masculine or feminine ways. In the stereotyped performance pathway (H2), we hypothesize that wielding power in gender-stereotyped ways modulates testosterone. Gender norms influence women to perform behaviors in stereotypically less masculine ways, and men to perform them in stereotypically more masculine ways. Accordingly, if masculine performance increases testosterone, men’s stereotypically more masculine performance of behavior may lead to more increases in testosterone. In H2, we test whether wielding power in stereotypically masculine, but not feminine, ways would increase testosterone. Recent theory predicts that competition and holding power, rather than masculinity per se, increases testosterone (3). Accordingly, we designed our experiment to disentangle power from masculinity where the two are typically conflated, so that we could assess two competing mediating pathways from gender to testosterone.
Would wielding power affect men and women equally? Testosterone has similar evolved functions in women’s and men’s bodies (27), but we have repeatedly found it easier to decrease testosterone in men and increase testosterone in women (3). This may stem from women having lower average testosterone than men: Lower baseline levels of testosterone are easier to increase (15). Evolutionary considerations thus might constrain the limits of social modulation of testosterone in this way, but also another: A higher baseline of competitive engagement actually predicts lower testosterone responses to any individual competition in some species (15), perhaps because the high frequency of competitions reduces an individual’s sensitivity to each subsequent one. Because gender norms encourage more competition for men and less for women (10, 24), men could actually show dampened testosterone responses to individual competitive events because of their higher rate of engagement in them. This may be especially the case for competitions enacted in social daily life because these are the ones with high frequencies of engagement (in contrast to infrequent formalized competitions with clear win/loss outcomes, where men’s testosterone can show an increase, e.g., refs. 7 and 28.
To test gender→testosterone pathways in men and women, we assessed participants’ testosterone before and after wielding power. We recruited trained actors to act out firing a subordinate, a context that demonstrates one’s own status and power and involves more regular social interactions and dynamics than more formalized competitions such as athletic events. Actor–participants received professional direction on acting out a workplace monologue, a format that controlled for complex workplace dynamics such as employee response. Participants performed the same monologue twice in counterbalanced order on different days controlling for time, once in a stereotypically masculine way (e.g., taking up space, dominance posturing, infrequent smiles) and once in a stereotypically feminine way (e.g., upending sentences, hesitancy, infrequent eye contact), and also engaged in a hormone-neutral control activity (watching a travel documentary; ref. 29). The involvement of trained actors provided a potentially crucial as well as innovative benefit: It maximized the likelihood that participants would be able to follow direction and act in gender-stereotypical ways that were, for one condition, counter to social gender norms. We measured testosterone before and after each condition to test the two competing hypotheses (Fig. 1: stereotyped behavior vs. stereotyped performance) of how gender might modulate testosterone.
Results
Participants Were Able to Perform in Gender-Stereotypical Ways.
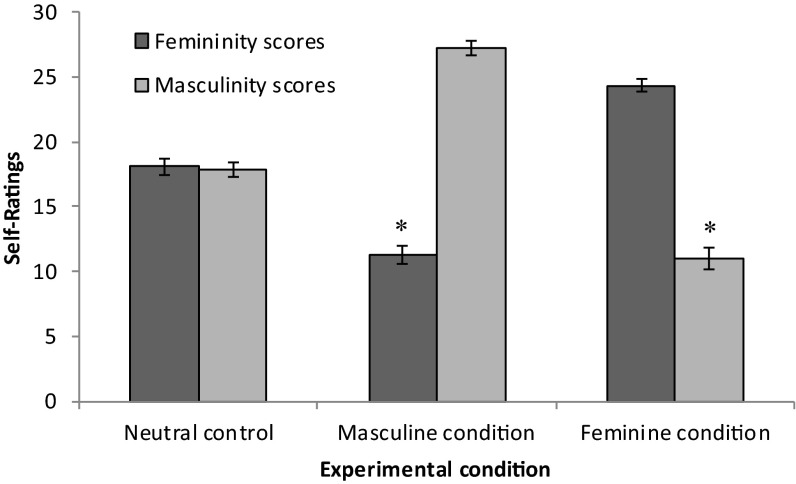
As a manipulation check, results confirmed that participants were able to act in gender-stereotypical ways. Raters scored participants in the masculine condition as significantly more masculine and less feminine and participants in the feminine condition as significantly more feminine and less masculine [femininity: multivariate F(1, 55) = 217.15, P < 0.001; masculinity: multivariate F(1, 55) = 282.59, P < 0.001]. Interrater reliability was moderate to substantial across conditions (Cohen’s K = 0.5745–0.6130). Participants also rated themselves similarly: both women and men had higher self-ratings on femininity after the feminine condition [multivariate F(2, 51) = 85.74, P < 0.001] and masculinity after the masculine condition [multivariate F(2, 51) = 102.27, P < 0.001] (Fig. 2).
Fig. 2.
Mean gender self-ratings on femininity and masculinity subscales (41, 42) by condition (neutral control, masculine, and feminine), with SE bars. Means are inclusive of men and women given no significant differences between them. Asterisks indicate that femininity and masculinity scores differ significantly in both experimental conditions at P < 0.05. N = 54.
Wielding Power Modulated Testosterone.
We tested two hypothesized gender→testosterone pathways: stereotyped behavior (H1), where wielding power increases testosterone regardless of its performance, vs. stereotyped performance (H2), where wielding power in masculine-stereotyped but not feminine-stereotyped ways increases testosterone. We tested these by conducting a repeated measures multivariate analysis of variance that assessed effects of condition (masculine performance, feminine performance, and control condition) on testosterone changes (via percent change scores, which are more sensitive to deviations in testosterone; ref. 6). Results with both women and men showed that wielding power significantly increased testosterone regardless of how it was done, supporting H1, the stereotyped behavior pathway [multivariate F(2, 38) = 5.70, P = 0.007, = 0.231]. However, this effect differed significantly for women and men [multivariate F(2, 38) = 3.38, P = 0.044, = 0.151], with only women showing a significant effect of condition on testosterone [multivariate F(2, 13) = 4.00, P = 0.044, = 0.381]. In women, the masculine condition significantly (P = 0.024, Cohen’s dz = 0.65) and the feminine condition marginally (P = 0.066, Cohen’s dz = 0.51) increased testosterone relative to the control condition. Wielding power in a feminine and especially in a masculine way thus increased testosterone in women, but this analysis did not reflect the contributions of potential confounds or (performance) stress, both of which may be relevant (6).
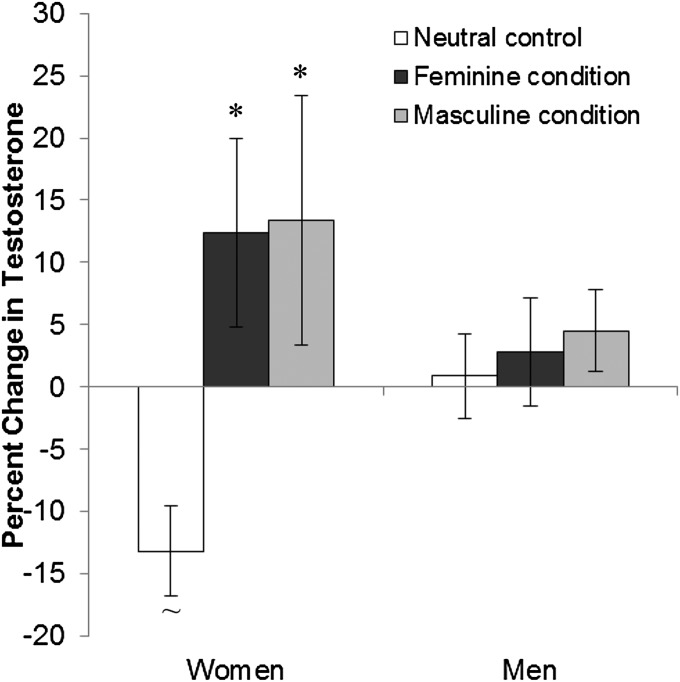
We next conducted a similar set of analyses, assessing the potential confounds listed in Materials and Methods. None affected our results except for relationship status, which is known to have a robust association with testosterone (30, 31). Controlling for relationship status showed a significant interaction between gender and condition [multivariate F(2, 34) = 5.84, P = 0.007, = 0.256], such that the effect was stronger in women and still showed no significant effect in men [multivariate F(2, 11) = 6.35, P = 0.015, = 0.536]. In women, both the masculine (P = 0.016, Cohen’s dz = 0.68) and the feminine (P = 0.020, Cohen’s dz = 0.69) conditions significantly and similarly increased testosterone over the control (Fig. 3). Wielding power increased testosterone in women regardless of the gender stereotyped way it was performed, supporting H1 (stereotyped behavior) over H2 (stereotyped performance).
Fig. 3.
Mean percent change in testosterone in women (n = 15) and men (n = 26) by condition, accounting for relationship status, with SE bars. Asterisk indicates a significant difference from ‟∼” at P < 0.05.
Including testosterone outliers in the above analyses generally gave the same pattern of results. Analyses with women were still significant, but analyses with women and men accounting for relationship status were marginally significant, and nonsignificant otherwise, perhaps reflecting the larger number of male (n = 6) vs. female (n = 2) outliers.
Cortisol is a confound of particular relevance given its potential links to both testosterone and status (32). Cortisol is also sensitive to stress activation (33) in ways that might matter for acting performance in general or stressful social interactions such as the one we used. However, incorporating cortisol into the analyses did not change the results, and wielding power did not significantly affect cortisol. The lack of stress axis activation means that changes in testosterone resulted from the hypothalamic–pituitary–gonadal axis and not from performance stress (androgens are also released from the adrenal gland, which releases cortisol). Thus, controlling for potential confounds including cortisol, and regardless of gender stereotypicality in performance, wielding power significantly increased testosterone in women.
Discussion
Our experiment provides evidence for a novel gender→testosterone pathway and points to possible mechanisms and mediation via gender-stereotyped behavior. We showed that wielding power increases testosterone in women regardless of whether it is done in stereotypically masculine or feminine ways, supporting the stereotyped behavior hypothesis (H1) over the stereotyped performance hypothesis (H2) for how gender might modulate testosterone. Our research design was able to disentangle wielding power from masculinity using trained actors, providing an innovative new paradigm. By involving actors, we were able to maximize the strengths and rigor of a within-subjects design, whereby participants were able to perform the same scenario with direction in two genders, including one that was not normative for participants. However, it remains to be seen whether effects generalize beyond actors role-playing to people engaging in everyday activities. Still, this paradigm opens up new avenues for research on gender and socially situated biology, by attending closely to the ways that gender norms constrain behaviors that themselves modulate physiology.
Testosterone is typically understood to underlie masculinity and maleness despite theory suggesting it is related to other phenomena such as competition (6). Our experiment supports this theory and falls in with empirical evidence, with results that support an association with competition rather than masculinity. Our results provided some suggestion, before controlling for confounds, that masculine stereotypes might increase testosterone more than feminine ones; our sample size was not large, which is a limitation, and it is possible that larger sample sizes might show multiple gender→testosterone pathways that include gender-stereotyped performances. However, the difference between feminine and masculine stereotypes disappeared when controlling for relationship status, which has been repeatedly shown to correlate with testosterone, supporting conclusions that gender stereotypes in this case were not modulating testosterone. Another limitation in our study lies in all participants performing the control condition before the experimental conditions (which were, themselves, counterbalanced); it remains possible that this could have influenced the pattern of findings, although it is unclear why this would happen in women but not in men.
Our findings add to growing evidence for the reverse relationship and extend it to gender: that gendered behavior modulates testosterone. Our results would support a pathway from gender to testosterone that is mediated by men engaging more frequently than women in behaviors such as wielding power that increase testosterone. This suggests that, when gender norms constrain behaviors that affect testosterone, gender norms can mediate effects of gender on testosterone, for example by encouraging wielding power for men and discouraging it for women (24).
Why do men have higher testosterone than women? Clearly, heritability—nature—plays a large role in this difference (34, 35). Our research points to an additional reason for differences in testosterone: the understudied role of nurture—social context. Social context akin to gender norms may have biological consequences when gender norms overlap with evolutionarily salient phenomena such as wielding power. Because wielding power is subject to gender-specific socialization, gender socialization can constrain how frequently women and men engage in behaviors that affect testosterone. Testosterone thus reflects some combination of both heritable and social influences.
We found that wielding power increased testosterone for women but not for men, consistent with some of our other studies where experimental manipulations were more successful at increasing testosterone in women (29, 36, 37). Although evidence is mixed, some forms of competition do increase testosterone in men, although these are mainly formalized ones with clear win/loss outcomes as with athletic engagements (7, 18). Our study used a more social interactive engagement, with the opportunity for rich social communication consistently marking power rather than merely outcome. This may map more closely onto daily interactions; even though firing someone is not a daily occurrence for most people, social interactions that involve leveraging power can be. However, because men are encouraged to engage in more competitions and to wield power more frequently, this might paradoxically lead to dampened testosterone responses in men to individual competitions, as has been shown in other species (15). Lived experiences related to gender might therefore alter testosterone directly via behavior, and/or indirectly by influencing the saliency of social interactions in ways that have implications for testosterone responsivity.
A major implication of our experiment is that gender socialization can contribute to variation in human testosterone levels. Our findings show that discrete events of gender-related socialization may account for some portion of the observed “sex” difference in adult testosterone levels. This adds to growing evidence that gender and sex are more permeable categories than is typically accounted for in bioscientific research (38) and opens up new questions about physiological pathways that link gender socialization to human biology.
Materials and Methods
Participants.
The initial sample consisted of 108 participants: 65 men (mean age = 28.35 y, SD = 12.3) and 43 women (mean age = 25.67 y, SD = 9.8) recruited from the community and the University of Michigan’s Center for Research on Learning and Teaching (CRLT) theater group. Recruitment materials specified that participants must be at least 18 y old and be experienced actors. Participants reported an average of 10.2 y of acting experience (SD = 10.1). Most participants (n = 100) had at least some college education, and a little over half (n = 63) were currently students. Participants identified their race/ethnicity, which we categorized as follows: African American/Black (n = 11), Asian/Asian American (n = 9), Caucasian/White (n = 78), European (n = 2), Hispanic/Latino/a (n = 2), Indian (n = 1), and Bi/Multiracial (n = 5).
In our statistical analyses, we included only those participants who completed all sessions, were not testosterone outliers (3 SDs from the mean, of which there were two women and six men; we assess outliers using the same process for all studies from our laboratory), and were not using medications affecting testosterone, including hormonal contraceptives. Our analyses for testosterone were thus based on a smaller sample of 41 participants who met these criteria, which was still robust for repeated-measures analyses: 26 men (mean age = 28.88 y, SD = 12.1) and 15 women (mean age = 29.67 y, SD = 12.8). This subset of participants reported an average of 11.2 y of acting experience (SD = 10.6) and was overall similar to the initial sample in terms of demographic characteristics. Among participants included in analyses, all but one had at least some college education, and many (n = 27) were currently students. Participants identified their race/ethnicity, which we categorized as follows: African American/Black (n = 5), Asian/Asian American (n = 5), Caucasian/White (n = 27), European (n = 1), Hispanic/Latino/a (n = 1), Indian (n = 1), and Bi/Multiracial (n = 1).
Materials.
Health and demographics questionnaire.
This questionnaire included items about demographic characteristics and potential hormone confounds (e.g., height and weight to calculate body mass index, sleep/wake habits, nicotine and alcohol use, and relationship status). Participants chose which of several options best described their relationship status based on definitions we provided (39), and we categorized responses as single (no sexual or romantic contacts), casually partnered (e.g., dating), or in a committed relationship.
Acting experiences questionnaire.
At their baseline laboratory session, participants responded to 10 items about their typical acting style (emotional vs. cognitive) and indicated their number of years of experience with acting. Participants also completed a “state” version of this questionnaire after both performances, referencing their emotional/cognitive acting experience during the performance.
Positive and negative affect schedule.
The positive and negative affect schedule (PANAS) (40) is widely used to measure positive mood (10 items) and negative mood (10 items). Participants completed the PANAS three times at each experimental session: before the manipulation (neutral control, masculine condition, or feminine condition), immediately postmanipulation, and 15 min postmanipulation. Participants indicated the extent to which each item described their feelings on a scale from 1 = “Very slightly or not at all” to 5 = “Extremely.” At premanipulation and 15 min postmanipulation time points, participants rated their current feelings, and at immediately postmanipulation participants rated their feelings during the performance or control condition.
Gender self-ratings.
We used the personal attributes questionnaire (PAQ) (41, 42) as a measure of gendered characteristics along three dimensions: masculinity (stereotypically more characteristic of men; e.g., “very competitive”), femininity (stereotypically more characteristic of women; e.g., “very emotional”), and masculinity–femininity (characteristics where socialization pressures for women and men to differ are especially strong; e.g., “very submissive vs. very dominant”). For each of 24 items, participants were asked to choose where they fall on a 5-point scale between two extreme responses. Participants responded to this scale immediately postmanipulation (indicating how they felt during the performance or control condition) and again 15 min postmanipulation (indicating their current feelings). The PAQ measures femininity and masculinity as personality trait-like attributes; we adapted it to use as a state measure by asking participants to report “how you felt as your character during the scene.” Others have used it this way as well (e.g., ref. 43). It is important to note that femininity and masculinity are not necessarily opposites (44): Individuals can be high on both, but our experiment was designed to increase one or the other.
Monologue.
The monologue script was written by coauthor J.S., a professional theater director, with input from coauthors. The text and form were in part developed to allow for differently gendered performances. For example, the monologue incorporates several interruptions that could be differentially dealt with according to gender norms.
Saliva samples and assays.
Saliva samples are widely used as a less-invasive alternative to blood sampling in behavioral research, and salivary assays for testosterone and cortisol are well-validated (6). Although salivary assays may underestimate the actual strength of testosterone–behavior links in women (45), our within-subjects design addresses this problem (6). Participants provided saliva samples for hormones by spitting into 17-mL polystyrene tubes. Samples were frozen until assay at the Core Assay Facility at the University of Michigan. Women’s testosterone was measured using enzyme immunoassay (EIA) kits from Salimetrics. The interassay coefficients of variation (CVs) were 7.08 and 13.81% for high and low testosterone, respectively, and the intraassay CV was 6.36%. Cortisol and men’s testosterone were measured using RIA kits from Siemens. For men’s testosterone, interassay CVs were 7.33 and 15.25% at high and low testosterone, respectively, and intraassay CV was 16.10%. For cortisol, interassay CVs were 6.12 and 14.91% at high and low cortisol, respectively, and intraassay CV was 7.25%.
Procedure.
Participants attended four laboratory sessions: a baseline session, a “direction session,” and two experimental sessions (the order of the feminine and masculine performances was counterbalanced across participants). Participants were reimbursed for laboratory sessions and up to 2 h of preparation time; the rate was $17/h for participants recruited from the population, or $18/h for participants who were recruited from CRLT (which has uniform pay rates). All procedures were approved by the University of Michigan Institutional Review Board.
Baseline session.
Participants provided informed consent and completed the health and demographics and acting experiences questionnaires. They then provided a saliva sample and completed the PANAS before watching a 5-min emotionally neutral travel documentary. Immediately after the video, participants completed a second PANAS and the gender self-ratings and waited 15 min before providing the postmanipulation saliva sample, because effects of social stimuli on hormones occur at a delay (6). They then completed a final PANAS and second set of gender self-ratings while providing the second sample.
Direction session.
During the direction session the director worked with participants on their performance, providing detailed instructions on style and motivation. These were derived from the broad literature on gender-stereotypical and normative communication styles (e.g., refs. 46 and 47).
For the masculine condition, participants were instructed that their gestures, movements, and behaviors should involve: taking up space, dominance posturing, infrequent smiles, leading positionality rather than echoing it, interrupting, and eye contact. Participants were instructed that their motivations should involve desire to show annoyance to employee, confidence in decision to fire employee, comfort with position of power, superiority, and wanting to be respected. All of these were explained in detail to participants.
For the feminine condition, participants were instructed that their gestures, movements, and behaviors should involve upending sentences, higher voice register, taking up little space, frequent smiles, hesitancy, and infrequent eye contact. Participants were instructed that their motivations should involve wanting to be liked, concern about others’ judgments, discomfort with firing people, trying to be nice, and being unsure whether they were doing the right thing. All of these were explained in detail to participants.
Experimental sessions.
Participants completed the PANAS and provided a saliva sample. Then, they performed the feminine or masculine version of the monologue for an audience of two experimenters, one of whom was blind to the gendered version of the performance. The experimenters served to heighten the saliency of the performance and also rated the performance on masculinity and femininity on scales from 1 = “Not at all” to 7 = “Extremely.” Immediately following the performance, participants completed the PANAS, gender self-ratings, and state acting experiences questionnaire. Finally, 15 min after the performance, participants provided a second saliva sample and completed the PANAS and gender self-ratings again.
Statistical Analyses.
As per methodological guidelines and work on social modulation of human testosterone (6), we assessed the following confounds in our analyses because all have been shown to be important sources of variation in testosterone: age, time of testing, date of testing, body mass index, waking time, time to sleep, nicotine use, alcohol use, exercise, and relationship status. We also assessed the following variables as potential sources of error variation related specifically to our experimental manipulation: years spent acting, emotional vs. cognitive acting styles, mood via the PANAS, and gender self-ratings. Testosterone was measured via different assays for women (EIA) and men (RIA) because of assay constraints, so we confirmed that analyses incorporating both in one repeated measures analysis showed the same pattern of results as analyses conducted separately. EIA and RIA results have been shown to be highly correlated (45).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.S.H. is a guest editor invited by the Editorial Board.
References
- 1.Cain KE, Ketterson ED. Competitive females are successful females; phenotype, mechanism and selection in a common songbird. Behav Ecol Sociobiol. 2012;66(2):241–252. doi: 10.1007/s00265-011-1272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa SM, Horan CM, Johnson PA, Adkins-Regan E. Copulatory behaviors and body condition predict post-mating female hormone concentrations, fertilization success, and primary sex ratios in Japanese quail. Horm Behav. 2011;59(4):556–564. doi: 10.1016/j.yhbeh.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 3.van Anders SM. Beyond masculinity: testosterone, gender/sex, and human social behavior in a comparative context. Front Neuroendocrinol. 2013;34(3):198–210. doi: 10.1016/j.yfrne.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc Biol Sci. 2002;269(1493):823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fine C, Jordan-Young R, Kaiser A, Rippon G. Plasticity, plasticity, plasticity…and the rigid problem of sex. Trends Cogn Sci. 2013;17(11):550–551. doi: 10.1016/j.tics.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 6.van Anders SM, Goldey KL, Bell SN. Measurement of testosterone in human sexuality research: Methodological considerations. Arch Sex Behav. 2014;43(2):231–250. doi: 10.1007/s10508-013-0123-z. [DOI] [PubMed] [Google Scholar]
- 7.van Anders SM, Watson NV. Social neuroendocrinology : Effects of social contexts and behaviors on sex steroids in humans. Hum Nat. 2006;17(2):212–237. doi: 10.1007/s12110-006-1018-7. [DOI] [PubMed] [Google Scholar]
- 8.Goldey KL, van Anders SM. Sexual modulation of testosterone: Insights for humans from across species. Adapt Human Behav Physiol. 2015;1(2):93–123. [Google Scholar]
- 9.Hamilton LD, Carre JM, Mehta PH, Olmstead N, Whitaker JD. Social neuroendocrinology of status: A review and future directions. Adapt Human Behav Physiol. 2015;1(2):202–230. [Google Scholar]
- 10.Eagly AH, Steffen VJ. Gender stereotypes stem from the distribution of women and men into social roles. J Pers Soc Psychol. 1984;46(4):735–754. [Google Scholar]
- 11.Teicher MH, et al. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1-2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 12.Lipina SJ, Posner MI. The impact of poverty on the development of brain networks. Front Hum Neurosci. 2012;6:238. doi: 10.3389/fnhum.2012.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West-Eberhard MJ. Sexual selection, social competition, and evolution. Proc Am Philos Soc. 1979;123(4):222–234. [Google Scholar]
- 14.van Anders SM, Goldey KL, Kuo PX. The steroid/peptide theory of social bonds: Integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology. 2011;36(9):1265–1275. doi: 10.1016/j.psyneuen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. The “challenge hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat. 1990;136(6):829–846. [Google Scholar]
- 16.Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA. Testosterone release and social context: When it occurs and why. Front Neuroendocrinol. 2009;30(4):460–469. doi: 10.1016/j.yfrne.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira RF, Lopes M, Carneiro LA, Canário AV. Watching fights raises fish hormone levels. Nature. 2001;409(6819):475. doi: 10.1038/35054128. [DOI] [PubMed] [Google Scholar]
- 18.Archer J. Testosterone and human aggression: An evaluation of the challenge hypothesis. Neurosci Biobehav Rev. 2006;30(3):319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Pearcey SM, Docherty KJ, Dabbs JM., Jr Testosterone and sex role identification in lesbian couples. Physiol Behav. 1996;60(3):1033–1035. doi: 10.1016/0031-9384(96)00132-1. [DOI] [PubMed] [Google Scholar]
- 20.Dabbs JM, Jr, de La Rue D, Williams PM. Testosterone and occupational choice: Actors, ministers, and other men. J Pers Soc Psychol. 1990;59(6):1261–1265. doi: 10.1037/0022-3514.59.6.1261. [DOI] [PubMed] [Google Scholar]
- 21.White RE, Thornhill S, Hampson E. Entrepreneurs and evolutionary biology: The relationship between testosterone and new venture creation. Organ Behav Hum Dec. 2006;100(1):21–34. [Google Scholar]
- 22.Carney DR, Cuddy AJC, Yap AJ. Power posing: Brief nonverbal displays affect neuroendocrine levels and risk tolerance. Psychol Sci. 2010;21(10):1363–1368. doi: 10.1177/0956797610383437. [DOI] [PubMed] [Google Scholar]
- 23.Ranehill E, et al. Assessing the robustness of power posing: No effect on hormones and risk tolerance in a large sample of men and women. Psychol Sci. 2015;26(5):653–656. doi: 10.1177/0956797614553946. [DOI] [PubMed] [Google Scholar]
- 24.Eagly AH, Wood W. In: Handbook of Theories of Social Psychology. Van Lange PAM, Kruglanski AW, Higgins ET, editors. Sage; Thousand Oaks, CA: 2012. pp. 458–476. [Google Scholar]
- 25.Wood W, Christensen PN, Hebl MR, Rothgerber H. Conformity to sex-typed norms, affect, and the self-concept. J Pers Soc Psychol. 1997;73(3):523–535. doi: 10.1037//0022-3514.73.3.523. [DOI] [PubMed] [Google Scholar]
- 26.Vescio TK, Schlenker KA, Lenes JG. In: The Social Psychology of Power. Guinote A, Vescio TK, editors. Guilford; New York: 2010. pp. 363–380. [Google Scholar]
- 27.Adkins-Regan E. Neuroendocrinology of social behavior. ILAR J. 2009;50(1):5–14. doi: 10.1093/ilar.50.1.5. [DOI] [PubMed] [Google Scholar]
- 28.Carré JM, McCormick CM, Hariri AR. The social neuroendocrinology of human aggression. Psychoneuroendocrinology. 2011;36(7):935–944. doi: 10.1016/j.psyneuen.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Goldey KL, van Anders SM. Sexy thoughts: Effects of sexual cognitions on testosterone, cortisol, and arousal in women. Horm Behav. 2011;59(5):754–764. doi: 10.1016/j.yhbeh.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Gray PB, et al. Human male pair bonding and testosterone. Hum Nat. 2004;15(2):119–131. doi: 10.1007/s12110-004-1016-6. [DOI] [PubMed] [Google Scholar]
- 31.van Anders SM, Gray PB. Hormones and human partnering. Annu Rev Sex Res. 2007;18(1):60–93. [Google Scholar]
- 32.Mehta PH, Josephs RA. Testosterone and cortisol jointly regulate dominance: Evidence for a dual-hormone hypothesis. Horm Behav. 2010;58(5):898–906. doi: 10.1016/j.yhbeh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Seeman TE, McEwen BS. Impact of social environment characteristics on neuroendocrine regulation. Psychosom Med. 1996;58(5):459–471. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Kuijper EA, et al. Heritability of reproductive hormones in adult male twins. Hum Reprod. 2007;22(8):2153–2159. doi: 10.1093/humrep/dem145. [DOI] [PubMed] [Google Scholar]
- 35.Harris JA, Vernon PA, Boomsma DI. The heritability of testosterone: A study of Dutch adolescent twins and their parents. Behav Genet. 1998;28(3):165–171. doi: 10.1023/a:1021466929053. [DOI] [PubMed] [Google Scholar]
- 36.Ritchie LL, van Anders SM. There’s jealousy… and then there’s jealousy: Differential effects of jealousy on testosterone. Adapt Human Behav Physiol. 2015;1(2):231–246. [Google Scholar]
- 37.Goldey KL, van Anders SM. Sexual thoughts: Links to testosterone and cortisol in men. Arch Sex Behav. 2012;41(6):1461–1470. doi: 10.1007/s10508-011-9858-6. [DOI] [PubMed] [Google Scholar]
- 38.Fausto-Sterling A. The bare bones of sex: Part 1-sex and gender. Signs (Chic Ill) 2005;30(2):1491–1527. [Google Scholar]
- 39.van Anders SM, Goldey KL. Testosterone and partnering are linked via relationship status for women and ‘relationship orientation’ for men. Horm Behav. 2010;58(5):820–826. doi: 10.1016/j.yhbeh.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 41.Spence JT, Helmreich RL. Masculinity and Femininity: Their Psychological Dimensions, Correlates, and Antecedents. Univ of Texas Press; Austin, TX: 1978. [Google Scholar]
- 42.Helmreich RL, Spence JT, Wilhelm JA. A psychometric analysis of the personal attributes questionnaire. Sex Roles. 1981;7(11):1097–1108. [Google Scholar]
- 43.Cota AA, Dion KL. Social ties: subgroup differences in costs and benefits. J Pers Soc Psychol. 1986;50(4):770–776. doi: 10.1037//0022-3514.51.4.770. [DOI] [PubMed] [Google Scholar]
- 44.Bem SL. The measurement of psychological androgyny. J Consult Clin Psychol. 1974;42(2):155–162. [PubMed] [Google Scholar]
- 45.Shirtcliff EA, Granger DA, Likos A. Gender differences in the validity of testosterone measured in saliva by immunoassay. Horm Behav. 2002;42(1):62–69. doi: 10.1006/hbeh.2002.1798. [DOI] [PubMed] [Google Scholar]
- 46.Hall JA, Carter JD, Horgan TG. In: Gender and Emotion: Social Psychological Perspectives. Fischer AH, editor. Cambridge Univ Press; Cambridge, UK: 2000. pp. 97–117. [Google Scholar]
- 47.Vrugt A, Luyerink M. The contribution of bodily posture to gender stereotypical impressions. Soc Behav Pers. 2000;28(1):91–103. [Google Scholar]