Significance
Marfan syndrome (MFS) is a connective tissue disorder caused by mutations of fibrillin-1 (FBN1), the main component of extracellular matrix microfibrils. FBN1 mutations predispose to thoracic aortic aneurysm and rupture and are associated with increased TGFβ signaling. TGFβ is secreted from cells complexed with latent TGFβ binding protein (LTBP), a protein that targets TGFβ to the ECM through interaction with fibrillin-1. One hypothesis proposes that aortic disease in MFS is due to the release of LTBP/TGFβ complexes in the aortic wall. We suppressed the expression of Ltbp3 in an MFS mouse model and observed essentially no aortic aneurysm and rupture in these compound mice. Our data suggest a key role for LTBP-3 in MFS aortic disease and provide a potential therapeutic point for intervention.
Keywords: extracellular matrix, transforming growth factor beta, aneurysm, LTBP
Abstract
Marfan syndrome (MFS) is an autosomal dominant disorder of connective tissue, caused by mutations of the microfibrillar protein fibrillin-1, that predisposes affected individuals to aortic aneurysm and rupture and is associated with increased TGFβ signaling. TGFβ is secreted from cells as a latent complex consisting of TGFβ, the TGFβ propeptide, and a molecule of latent TGFβ binding protein (LTBP). Improper extracellular localization of the latent complex can alter active TGFβ levels, and has been hypothesized as an explanation for enhanced TGFβ signaling observed in MFS. We previously reported the absence of LTBP-3 in matrices lacking fibrillin-1, suggesting that perturbed TGFβ signaling in MFS might be due to defective interaction of latent TGFβ complexes containing LTBP-3 with mutant fibrillin-1 microfibrils. To test this hypothesis, we genetically suppressed Ltbp3 expression in a mouse model of progressively severe MFS. Here, we present evidence that MFS mice lacking LTBP-3 have improved survival, essentially no aneurysms, reduced disruption and fragmentation of medial elastic fibers, and decreased Smad2/3 and Erk1/2 activation in their aortas. These data suggest that, in MFS, improper localization of latent TGFβ complexes composed of LTBP-3 and TGFβ contributes to aortic disease progression.
Marfan syndrome (MFS) is an autosomal dominant connective tissue disorder caused by mutations in the gene encoding fibrillin-1 (FBN1), an extracellular matrix (ECM) glycoprotein that is the main component of microfibrils and that associates with elastin to form elastic fibers. In MFS, defects in microfibrils predispose individuals to thoracic aortic aneurysm (TAA), with ensuing vessel dissection and rupture (1, 2).
The vascular defects in MFS were initially considered a consequence of constitutive tissue weakness due to structurally abnormal fibrillin-1 microfibrils (3). However, mouse models of MFS revealed that abnormal fibrillin-1 resulted in an increase in signaling by transforming growth factor beta (TGFβ), a cytokine involved in cell proliferation, differentiation, and matrix synthesis. TGFβ signaling requires the cytokine to bind its type II cell surface receptor (TβRII), which recruits and phosphorylates the type I receptor (TβRI). TβRI phosphorylates SMAD2/3 (mothers against decapentaplegic homolog 2/3), which forms a heterodimeric complex with SMAD4 and enters the nucleus to activate the transcription of TGFβ-dependent genes. The TGFβ–TβRI–TβRII complex also can activate MAPK signaling pathways, including ERK1 and ERK2 (ERK1/2) (4). The levels of both active SMAD2/3 and ERK1/2 are heightened in the ascending aortas of MFS mouse models (5–7). Treatment of these animals with TGFβ neutralizing antibodies (TGFβ-Nab) prevents or impedes TAA progression in some studies (6, 7), while exacerbating arterial disease in others (5).
TGFβ is secreted from cells as part of a biologically inactive large latent complex (LLC), composed of LTBP-1, -3, or -4, the prodomain dimer of TGFβ, referred to as the latency associated peptide (LAP), and the mature TGFβ dimer. LAP associates noncovalently with mature TGFβ to form the small latent complex (SLC). Covalent binding of the SLC to an LTBP occurs in the secretory pathway through the formation of two disulfide bonds between LAP and the third 8-Cys domain of LTBP-1, -3, or -4. Of the four LTBPs, LTBP-1 and -3 bind efficiently to all three TGFβ (TGFβ1, -2, and -3) LAP isoforms whereas LTBP-4 binds very inefficiently and only to TGFβ1 LAP (8, 9). Moreover, LTBP-3 requires binding to TGFβ for secretion and is secreted only in the LLC form, suggesting an important role for LTBP-3 in the control of TGFβ availability (8, 9).
LTBPs regulate TGFβ activity by facilitating its secretion, by localizing the LLC to specific sites in the ECM, and by participating in latent TGFβ release from the ECM (9–12). For TGFβ to bind to its receptor, the interaction of LAP and TGFβ must be disrupted, a process known as latent TGFβ activation (13, 14). LTBP localization into the ECM is important for latent TGFβ activation. Abnormal localization is reported to alter TGFβ activity in both positive and negative ways: e.g., overexpression of a mutated form of LTBP-1 that binds TGFβ but does not interact with the ECM results in increased TGFβ activity (15) whereas mice in which the cysteines that link the propeptide of TGFβ1 to LTBP were mutated to serines, thereby blocking covalent interaction with LTBP and subsequent association to the ECM, have multiorgan inflammation resembling that observed in TGFβ1-null mice (16). In addition, cleavage of LTBP-1 by a bone morphogenetic protein 1 (BMP1)-like metalloproteinase liberates LLC from the ECM and leads to activation of TGFβ1 by MMP2 (17).
The mechanisms by which defective microfibrils perturb TGFβ signaling and cause aortic disease in MFS remain poorly understood. A current hypothesis proposes that abnormal fibrillin-1 fibers cause faulty LLC matrix incorporation, yielding increased TGFβ signaling with consequent aortic aneurysm and dissection (1, 2). However, there is no evidence demonstrating either the participation of the LLC in MFS aortic disease or which LTBP is involved. We previously reported the in vitro and in vivo absence of LTBP-3, but not LTBP-1, incorporation into matrices that lack fibrillin-1 microfibrils, implying that LTBP-3 is the functionally important LTBP affecting latent TGFβ in MFS (18). In the present study, we present data that identify LTBP-3 as an important contributor to TAA in MFS.
Results
Genetic Deletion of Ltbp3 Prevents Premature Death of Fbn1mgR/mgR Mice.
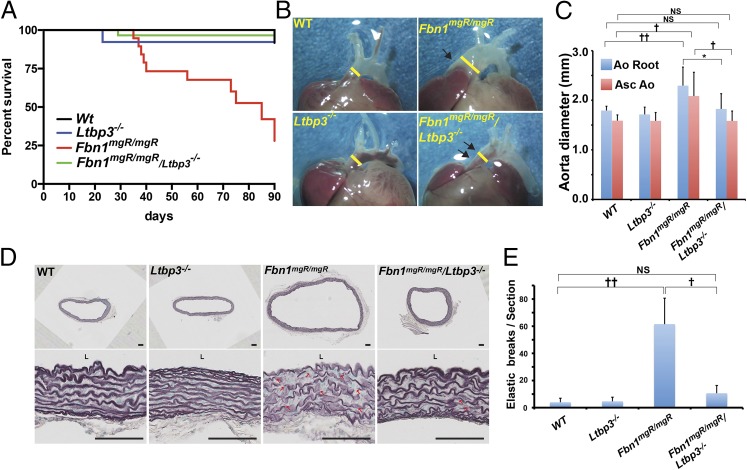
To identify a possible role of LTBP-3 in aortic disease in MFS, we generated Fbn1mgR/mgR:Ltbp3−/− mice. Fbn1mgR/mgR mice are a hypomorphic MFS mouse model and represent a progressively severe model of MFS because most of the affected animals die from dissecting TAA within 3 mo after birth (19, 20). The mutant allele encodes a WT protein, but the animals produce only 10–20% of the normal amount of fibrillin-1 (19). Survival studies were performed, and the mice were followed for 3 mo (Fig. 1A). More than 90% of Wt and Ltbp3−/− mice survived for this period whereas Fbn1mgR/mgR mice displayed 70% lethality by 90 d. Of the Fbn1mgR/mgR mice that died, 75% of deaths were due to ruptured ascending aneurysms, as determined by necropsy, in agreement with published data (19). Surprisingly, survival of Fbn1mgR/mgR:Ltbp3−/− mice was almost equivalent to Wt and Ltbp3−/− mice (Fig. 1A). There was no evidence of aortic rupture in any of the Wt, Ltbp3−/−, or Fbn1mgR/mgR:Ltbp3−/− mice. Together, these data suggest that the absence of LTBP-3 significantly attenuates aortic disease in Fbn1mgR/mgR mice and prolongs their survival.
Fig. 1.
Absence of Ltbp3 prevents premature death, aneurysm of the ascending aorta, and elastic fiber breaks in Fbn1mgR/mgR mice. (A) Kaplan–Meier survival curves of WT (n = 34), Ltbp-3−/− (n = 26), Fbn1mgR/mgR (n = 21), and Fbn1mgR/mgR:Ltbp3−/− (n = 30), demonstrating a decreased rate of death of Fbn1mgR/mgR:Ltbp-3−/− mice compared with Fbn1mgR/mgR mice. Differences between Fbn1mgR/mgR and Wt or Fbn1mgR/mgR:Ltbp3−/− mice were statistically significant (P < 0.0001). There was no statistically significant difference between Wt and Fbn1mgR/mgR:Ltbp3−/− mice (P = 0.34). (B) Gross morphology of the ascending aorta in 2-mo-old Wt, Ltbp3−/−, Fbn1mgR/mgR, and Fbn1mgR/mgR:Ltbp3−/− mice. Fbn1mgR/mgR mice typically develop ascending aortic aneurysms (single arrows). Ablation of LTBP-3 in Fbn1mgR/mgR mice prevented aneurysm formation (double arrows). Yellow bars highlight aortic diameter. (C) Average aortic root (AoR) and ascending aorta (AscAo) dimensions measured by echocardiogram of 2-mo-old Wt (n = 8), Ltbp3−/− (n = 5), Fbn1mgR/mgR (n = 9), and Fbn1mgR/mgR:Ltbp3−/− (n = 5) mice. Fbn1mgR/mgR aortic diameter was significantly increased compared with Wt whereas there was no significant difference in aortic diameter between Fbn1mgR/mgR:Ltbp3−/− and Wt or Ltbp3−/− mice. Data are presented as the mean ± SD. *P < 0.05; †P < 0.02; ††P < 0.005. ANOVA P < 0.01. NS, not significant. (D) Staining of elastic fibers in cross-sections of ascending aortas from 2-mo-old Wt, Ltbp3−/−, Fbn1mgR/mgR, and Fbn1mgR/mgR/Ltbp3−/− mice. Arrows indicate elastic lamellae fragmentation. L, lumen. (Scale bars: all panels, 100 μm.) (E) Quantification of elastic breaks per aorta section for each genotype. Data are presented as the mean ± SD of at least three samples for each genotype. †P < 0.01; ††P < 0.001; ANOVA, P < 0.001. NS, not significant.
Ltbp3 Deletion Prevents Elastin Degradation and Aneurysm in Fbn1mgR/mgR Mice.
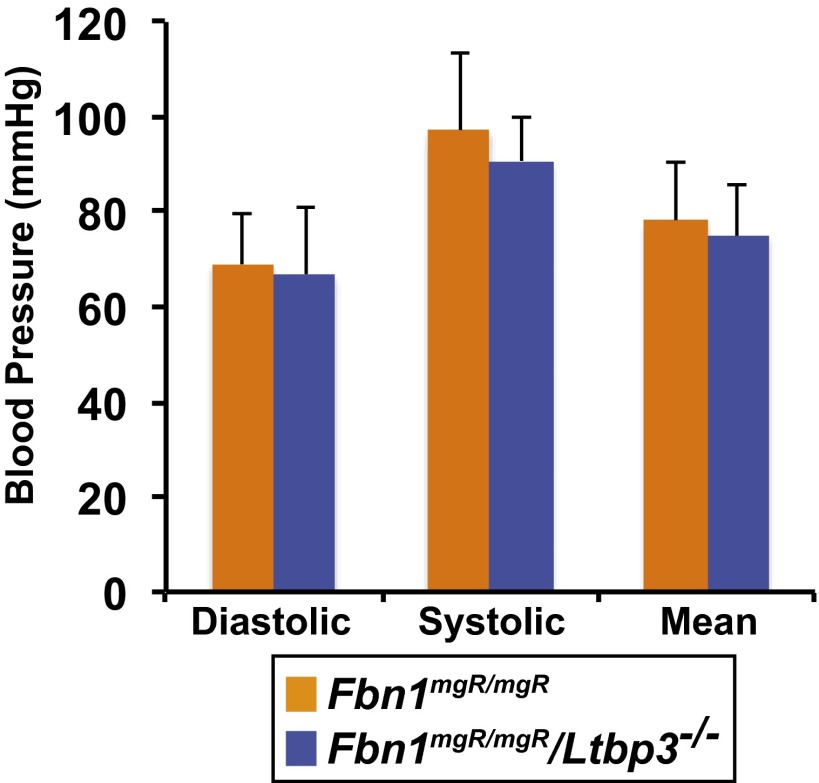
At 9 wk of age, obvious aneurysms of the ascending aortas on gross examination were observed in 63% (17 of 27) of killed Fbn1mgR/mgR mice compared with none observed in Wt, Ltbp3−/−, and Fbn1mgR/mgR:Ltbp3−/− mice (Fig. 1B). We evaluated ascending aortic diameters at 9 wk of age by transthoracic echocardiography. We observed a significant dilatation of the ascending aortas of Fbn1mgR/mgR mice (2.08 ± 0.48 mm) compared with both Wt (1.59 ± 0.11 mm) and Ltbp3−/− (1.59 ± 0.16 mm) mice. Absence of Ltbp3 in Fbn1mgR/mgR mice significantly attenuated the aortic enlargement (1.58 ± 0.19 mm), which was equivalent to the diameter of Wt or Ltbp3−/− aortas (Fig. 1C). Blood pressure measurements indicated no difference between Fbn1mgR/mgR and Fbn1mgR/mgR:Ltbp3−/− animals (Fig. S1).
Fig. S1.
Average systolic, diastolic, and mean blood pressures. Data are presented as the mean ± SD of four to five animals per genotype. No statistically significant differences were observed between the two groups (one-way ANOVA).
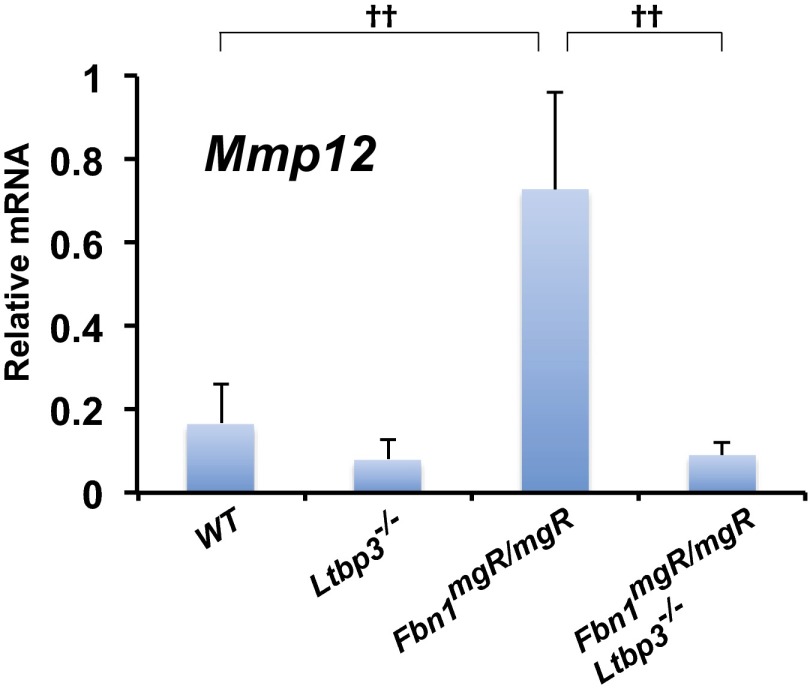
Previous studies of Fbn1mgR/mgR mice demonstrated progressive fragmentation of aortic elastic lamellae in the ascending aorta as the animals aged (19). Histological analysis of sections from 2-mo-old Fbn1mgR/mgR ascending aortas revealed disorganized media with extensive elastic fiber fragmentation (Fig. 1 D and E). On the other hand, the aortic wall architecture and the number of elastic breaks were normalized in Fbn1mgR/mgR mice lacking LTBP-3 (Fig. 1 D and E). In line with those observations, we observed a marked increased of the metalloelastase Mmp12 mRNA expression in Fbn1mgR/mgR compared with Wt (Fig. S2). Absence of LTBP-3 in Fbn1mgR/mgR significantly attenuated the expression of Mmp12 to a level of expression equivalent to Wt samples (Fig. S2). Together these data indicate that LTBP-3 absence in Fbn1mgR/mgR mice preserves aortic elastic fiber integrity and protects against TAA and rupture.
Fig. S2.
qPCR analysis of the expression of Mmp12 in ascending aortas from 2-mo-old Wt, Ltbp3−/−, Fbn1mgR/mgR, and Fbn1mgR/mgR:Ltbp3−/− mice. Total RNA was isolated, and mRNA expression levels were normalized to Gapdh. Data are presented as the mean ± SD of three to six aortas per genotype. ††P < 0.001; ANOVA, P < 0.001.
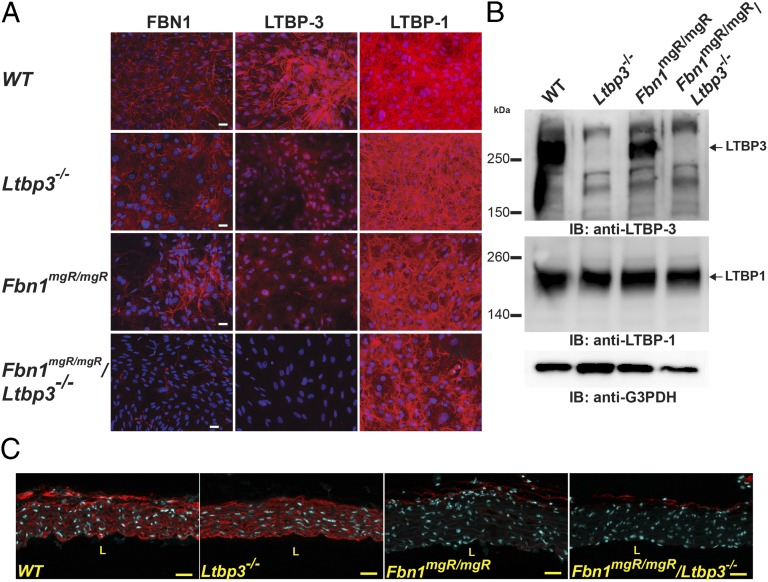
Decreased Incorporation of LTBP-3 in the ECM of Fbn1mgR/mgR Aortic Smooth Muscle Cells.
We determined whether hypomorphic expression of Fbn1 has an effect on matrix targeting of LTBP-3 in aortic smooth muscle cells (ASMCs) isolated from the ascending aorta. After 14 d in culture, immunoreactivity was detected in Wt cultures with antibodies against both LTBP-3 and fibrillin-1 (Fig. 2A). In Fbn1mgR/mgR and Fbn1mgR/mgR:Ltbp3−/− extracellular matrix, there were fewer detectable fibrillin-1 fibers than in Wt cultures. This absence correlated with decreased incorporation of LTBP-3 in Fbn1mgR/mgR extracellular matrix. As expected, staining for LTBP-3 was totally absent in the ECM of Ltbp3−/− and Fbn1mgR/mgR:Ltbp3−/− ASMCs (Fig. 2A). On the other hand, LTBP-1 seemed to be present in the ECM of all four genotypes in roughly equivalent amounts (Fig. 2A). We analyzed 24 h conditioned media from the ASMCs by Western blotting for LTBP-3. We observed the absence of LTBP-3 in the Ltbp3−/− and Fbn1mgR/mgR:Ltbp3−/− samples compared with those from Wt or Fbn1mgR/mgR cultures (Fig. 2B). There was a 60% decrease in the intensity of the LTBP-3 band in Fbn1mgR/mgR compared with Wt samples (Fig. 2B). Immunoblotting with an antibody specific for LTBP-1 revealed no differences in the amount of protein released by cells of the four genotypes (Fig. 2B). These experiments also revealed that, in nonreducing conditions, the molecular mass of the detected LTBP-3 band is consistent with that of the LTBP-3/LAP complex (Fig. 2B), implying that the changes in LTBP-3 levels would also affect latent TGFβ incorporation.
Fig. 2.
Decreased incorporation of LTBP-3 in Fbn1mgR/mgR ASMCs. (A) Analyses by immunofluorescence of the deposition of FBN1 and LTBP-1 and -3 into the ECM of Wt, Ltbp3−/−, Fbn1mgR/mgR, and Fbn1mgR/mgR:Ltbp3−/− ASMCs. Cells were cultured for 14 d on glass coverslips, fixed with ethanol, and stained with specific antibodies against LTBPs (red). Nuclei are stained with DAPI (blue). (Scale bars: all panels, 40 μm.) (B) Analyses of 24 h conditioned media from Wt and Fbn1mgR/mgR ASMCs. Conditioned media were collected and analyzed by Western blotting using antibodies specific for LTBP-3 and LTBP-1 isoforms. Housekeeping protein G3PDH is shown as a loading control. (C) Analysis by immunofluorescence of the distribution of FBN1 in Wt, Ltbp3−/−, Fbn1mgR/mgR, and Fbn1mgR/mgR:Ltbp3−/− ascending aortas. The fibrillin-1 staining intensity was equivalent in Wt and Ltbp3−/− samples whereas Fbn1mgR/mgR and Fbn1mgR/mgR:Ltbp3−/− samples displayed a decrease in staining intensity compared with Wt. (Scale bars: all panels, 40 μm.)
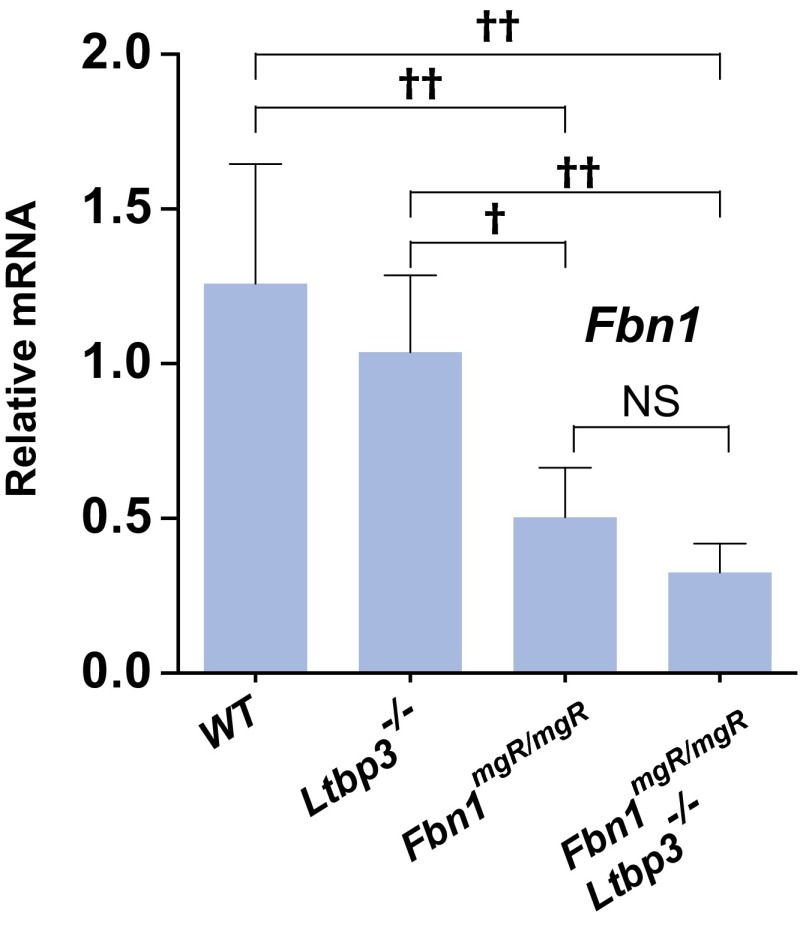
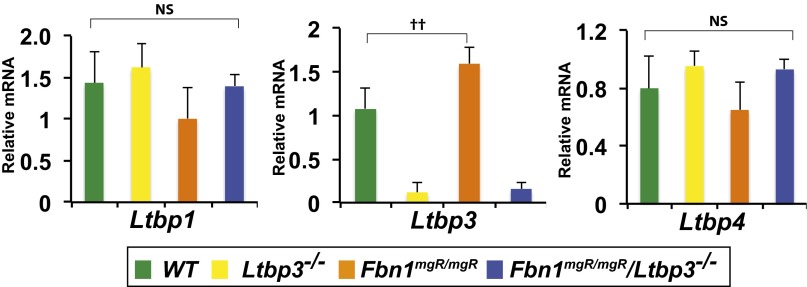
Next, we performed quantitative real-time PCR (qPCR) analyses to assess the relative levels of Fbn1 mRNA in the ascending aortas of the different genotypes. As expected, we observed a decrease of Fbn1 mRNA in Fbn1mgR/mgR and Fbn1mgR/mgR:Ltbp3−/− compared with Wt and Ltbp3−/− aortas (Fig. S3). There was no increase in Fbn1 expression in response to the absence of LTBP-3 in either Ltbp3−/− or Fbn1mgR/mgR:Ltbp3−/− tissues, indicating no compensation for fibrillin-1 expression in the Fbn1mgR/mgR:Ltbp3−/− ascending aortas. This result was confirmed by immunostaining against FBN1 on tissue sections of the ascending aorta. In Fbn1mgR/mgR and Fbn1mgR/mgR:Ltbp3−/− aortas, FBN1 staining in the aortic wall was greatly decreased compared with Wt or Ltbp3−/− (Fig. 2C). We observed equivalent levels of immunoreactivity against FBN1 in Fbn1mgR/mgR and Fbn1mgR/mgR:Ltbp3−/− samples, with no indication of an increase in FBN1 assembly in the absence of Ltbp3 (Fig. 2C). mRNA levels of LTBP-1, -3, and -4 in the ascending aortas of the four genotypes were analyzed by qPCR (Fig. S4). We observed similar levels of Ltbp1 and Ltbp4 transcripts in the four genotypes. Interestingly, there was a statistically significant increase in the mRNA level of Ltbp3 in Fbn1mgR/mgR compared with Wt (Fig. S4). The biological significance of this increase is unclear.
Fig. S3.
Quantitative PCR analysis from Wt, Ltbp3−/−, Fbn1mgR/mgR, and Fbn1mgR/mgR:Ltbp3−/− ascending aortas. Total cellular RNA was isolated from 2-mo-old aortas, and the expression of Fbn1 was analyzed. mRNA expression levels were normalized to Gapdh. Data are presented as the mean ± SD of five to seven samples for each genotype. †P < 0.01; ††P < 0.001. ANOVA, P < 0.001. NS, not significant.
Fig. S4.
qPCR analysis of the expression of Ltbp-1, -3, and -4 in ascending aortas from 2-mo-old Wt, Ltbp3−/−, Fbn1mgR/mgR, and Fbn1mgR/mgR:Ltbp3−/− mice. Total RNA was isolated, and mRNA expression levels were normalized to Gapdh. Data are presented as the mean ± SD of three to five aortas per genotype. ††P < 0.01; ANOVA, P < 0.02. NS, not significant.
In summary, these data suggest that, in Fbn1mgR/mgR ASMCs, the LLC composed of LTBP-3, LAP, and TGFβ is poorly incorporated into the matrix and abrogation of Ltbp3 expression in Fbn1mgR/mgR mice prevents the formation of this complex and has no effect on FBN1 expression.
Absence of LTBP-3 Attenuates Activation of Smad2/3 and Erk1/2 in Fbn1mgR/mgR Aortas.
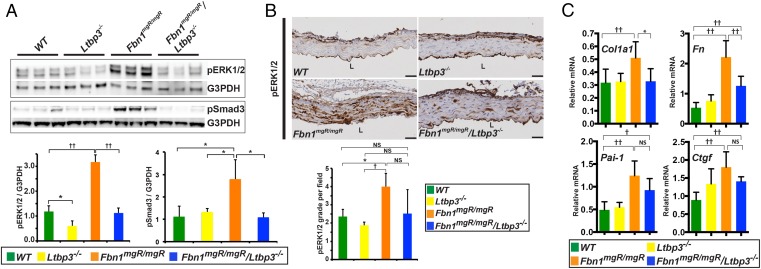
Increased ERK1/2 and Smad2/3 phosphorylation has been observed in the aortic wall in human tissues and in MFS mouse models (5, 7, 21). Moreover, treatment of Fbn1C1039G/- mice, a slowly progressing and mild form of MFS, with TGFβ-neutralizing antibodies, ERK1/2 inhibitor, or AT1r antagonists ameliorates the aortic phenotype in association with attenuated activation of Smad2/3 and ERK1/2 signaling (5, 7, 21). We therefore examined the effect of Ltbp3 deletion in Fbn1mgR/mgR mice on ERK1/2 and Smad3 phosphorylation in the ascending aorta. We performed Western blotting analysis on ascending aorta lysates from 12-wk-old mice. Strong up-regulation of phospho-ERK1/2 (p-ERK1/2) was observed in Fbn1mgR/mgR aortas compared with Wt or Ltbp3−/− samples (Fig. 3A). The absence of Ltbp3 in Fbn1mgR/mgR mice suppressed ERK1/2 activation to levels indistinguishable from Wt (Fig. 3A). Interestingly, we observed a small but significant decrease of ERK1/2 activation in Ltbp3−/− ascending aortas compared with Wt tissues (Fig. 3A). The significance of this change is unclear. Consistent with the Western blotting analysis, we observed, by immunostaining, increased p-ERK1/2 in the media of aortas from Fbn1mgR/mgR mice compared with Wt or Ltbp3−/− animals and no significant difference in Fbn1mgR/mgR:Ltbp3−/− compared with Wt or Ltbp3−/− samples (Fig. 3B).
Fig. 3.
Absence of LTBP-3 attenuates noncanonical (ERK1/2) and canonical (Smad2/3) TGFβ signaling in Fbn1mgR/mgR aortas. (A) Immunoblotting analysis of the ascending aorta of 12-wk-old mice. The results are expressed as densitometric levels of p-ERK1/2 and p-Smad3 intensity normalized to G3PDH. Fbn1mgR/mgR aortic tissue showed an increase in p-ERK1/2 compared with Wt or Ltbp3−/− tissues. Absence of LTBP-3 in Fbn1mgR/mgR mice substantially reduced p-ERK1/2. Fbn1mgR/mgR aortic tissues showed an increase in p-Smad3 compared with Wt, Ltbp3−/−, and Fbn1mgR/mgR:Ltbp3−/− tissues (P < 0.05). There was no significant difference in Smad3 activation between Fbn1mgR/mgR:Ltbp3−/− and Wt or Ltbp3−/− ascending aortas. Bars represent the means ± SD of three aortas per genotype. *P < 0.05; ††P < 0.01; ANOVA P < 0.01. NS, not significant. (B) Representative p-Erk1/2 immunostaining on cross-sections of ascending aorta from 2-mo-old mice. L, lumen. (Scale bars: all panels, 40 μm.) The graph represents p-ERK1/2 positive signal quantification scored according to grade indicated in SI Materials and Methods. Data are presented as the mean ± SD of at least three samples for each genotype. *P < 0.05; †P < 0.02; ANOVA, P < 0.05. NS, not significant. (C) qPCR analysis of TGFβ signaling target genes in ascending aortas from 2-mo-old Wt, Ltbp3−/−, Fbn1mgR/mgR, and Fbn1mgR/mgR:Ltbp3−/− mice. mRNA expression levels were normalized to Gapdh. Data are presented as the mean ± SD of five to seven aortas per genotype. *P < 0.05; †P < 0.02; ††P < 0.01; ANOVA, P < 0.02. NS, not significant.
We also assessed TGFβ pathway signaling by measuring the levels of phosphorylated Smad3 (p-Smad3) in the ascending aortas of 11-wk-old mice. Western blotting analysis showed increased p-Smad3 in Fbn1mgR/mgR compared with Wt or Ltbp3−/− samples (Fig. 3A). Absence of LTBP-3 in Fbn1mgR/mgR mice resulted in a statistically significant reduction of Smad3 activation levels (Fig. 3A). There was no significant difference in p-Smad3 levels between Fbn1mgR/mgR:Ltbp3−/− and Wt or Ltbp3−/− tissues (Fig. 3A).
To assess TGFβ signaling with a different approach, we examined the expression levels of collagen 1 alpha1 (Col1a1), fibronectin (Fn), plasminogen-activator inhibitor-1 (Pai1), and connective tissue growth factor (Ctgf), four genes whose expression is often induced by TGFβ (22). Col1a1, Fn, Pai1, and Ctgf expression were all significantly increased in the ascending aortas of Fbn1mgR/mgR mice compared with Wt (Fig. 3C). Expression of Col1a1 and Fn was significantly diminished in Fbn1mgR/mgR:Ltbp3−/− ascending aortas compared with Fbn1mgR/mgR samples (Fig. 3C). In conclusion, these data indicate that absence of LTBP-3 in Fbn1mgR/mgR mice reduces TGFβ signaling in the ascending aorta.
Absence of Ltbp-3 in Fbn1mgR/mgR Mice Normalizes Gene Expression Changes in the Aorta.
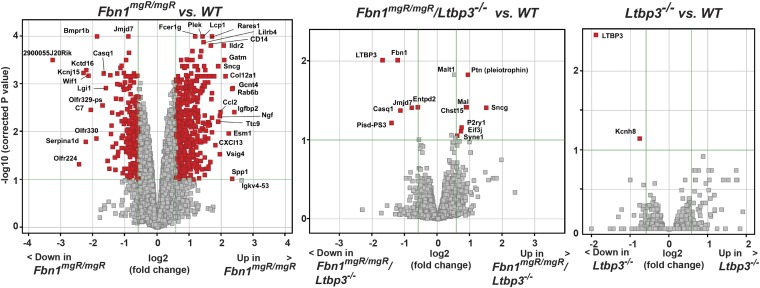
We performed gene expression analysis to identify genes whose expression is affected in the ascending aortas from Fbn1mgR/mgR and Fbn1mgR/mgR:Ltbp3−/− mice. Comparative gene array analysis of 2-mo-old Fbn1mgR/mgR versus Wt samples identified 786 differentially expressed genes using a threshold of 1.5 and a corrected P value of <0.10 (Fig. 4 and Dataset S1). However, in Fbn1mgR/mgR:Ltbp3−/− aortas, the expression of 773 genes out of 786 genes was reversed, and, as expected, two of the genes whose expression was not reversed were Fbn1 and Ltbp3 (Fig. 4 and Table S1). There were no major mRNA expression changes in Ltbp3−/− compared with Wt ascending aortas, except for Ltbp3 (>1.5-fold, corrected P value < 0.1) (Fig. 4 and Table S2). Overall, this result demonstrates that absence of LTBP-3 almost completely attenuates gene expression changes observed in MFS-like mice.
Fig. 4.
Gene expression changes in the ascending aorta of 2-mo-old Fbn1mgR/mgR mice are normalized by loss of LTBP-3. Volcano plot of findings from gene array data of 2-mo-old ascending aortas. Ltbp-3−/− (n = 3), Fbn1mgR/mgR (n = 4), and Fbn1mgR/mgR:Ltbp3−/− (n = 4) samples are compared with Wt (n = 4). Genes that met the inclusion criteria (P value of <0.1 and fold change of >1.5) were represented in the volcano plot. The –log10 of corrected P values (y axis) is plotted against the log2 of fold change between two groups. Within each of the three volcano plots, green lines delineate the cutoffs for genes significantly down-regulated (Left) or up-regulated (Right). Genes of highest statistical significance and fold change are sequestered into the upper left and upper right.
Table S1.
Gene expression changes in Fbn1mgR/mgR;Ltbp3−/− compared with Wt samples
| Gene symbol | Gene description | Directional change | Fold change | P value corrected | P value | Transcripts cluster ID |
| Sncg | Synuclein, gamma | Up | 2.82 | 3.88E−02 | 1.03E−05 | 17305034 |
| Ptn | Pleiotrophin | Up | 1.91 | 1.50E−02 | 1.77E−06 | 17465856 |
| Chst15|Gm10584 | Carbohydrate (N-acetylgalactosamine 4-sulfate 6-O)sulfotransferase 15|predicted gene 10584 | Up | 1.87 | 3.83E−02 | 7.94E−06 | 17497076 |
| Mal | Myelin and lymphocyte protein,T cell differentiation protein | Up | 1.85 | 3.83E−02 | 7.53E−06 | 17391332 |
| P2ry1 | Purinergic receptor P2Y,G protein coupled 1 | Up | 1.68 | 6.87E−02 | 2.44E−05 | 17397957 |
| Gm9781|Eif3j | Predicted gene 9781|eukaryotic translation initiation factor 3, subunit J | Up | 1.64 | 7.79E−02 | 3.00E−05 | 17354128 |
| Syne1 | Synaptic nuclear envelope 1 | Up | 1.51 | 8.86E−02 | 3.93E−05 | 17238910 |
| Fbn1 | Fibrillin 1 | Down | 3.22 | 9.79E−03 | 5.07E−07 | 17390879 |
| Pisd-ps3 | Phosphatidylserine decarboxylase, pseudogene 3 | Down | 2.67 | 6.02E−02 | 1.96E−05 | 17532694 |
| Entpd2|Npdc1 | Ectonucleoside triphosphate diphosphohydrolase 2|neural proliferation, differentiation and control gene 1 | Down | 2.34 | 9.79E−03 | 5.80E−07 | 17367963 |
| Ltbp3 | Latent transforming growth factor beta binding protein 3 | Down | 2.19 | 4.27E−02 | 1.26E−05 | 17356526 |
| Casq1 | Calsequestrin 1 | Down | 1.74 | 3.88E−02 | 9.97E−06 | 17229851 |
| Jmjd7|Pla2g4b | Jumonji domain containing 7|phospholipase A2, group IVB (cytosolic) | Down | 1.53 | 3.83E−02 | 7.04E−06 | 17374972 |
Table S2.
Gene expression changes in Ltbp3−/− compared with Wt samples
| Gene symbol | Gene description | Directional change | Fold change | P value corrected | P value | Transcripts cluster ID |
| Ltbp3 | Latent transforming growth factor beta binding protein 3 | Down | 3.71 | 3.45E−03 | 1.02E−07 | 17356526 |
| Kcnh8 | Potassium voltage-gated channel, subfamily H (eag-related), member 8 | Down | 1.69 | 7.17E−02 | 4.25E−06 | 17338545 |
Upstream regulator analysis was applied to identify upstream molecular pathways that might explain the gene expression changes observed in Fbn1mgR/mgR aortas. The 786 genes affected in Fbn1mgR/mgR compared with Wt (>1.5-fold, P < 0.1) were analyzed with the Ingenuity Pathway Analysis (IPA) software package. This analysis confirmed the activation of the TGFβ pathway in Fbn1mgR/mgR mice (upstream analysis Z-score, 5.734; P value, 1.4 E−37) (Table S3). However, the TGFβ pathway was not activated in Fbn1mgR/mgR:Ltbp3−/− ascending aortas because the expression of none of those genes regulated by TGFβ was significantly affected in these double mutants. Only Fbn1, a TGFβ gene target, was affected, but this result was expected because those mice are homozygous for a hypomorphic mutation in Fbn1. We additionally identified the activation of molecular signatures associated with inflammation, fibrosis, and immune function (Table S3), which is consistent with reports that indicated the known participation of TGFβ in these processes (22, 23).
Table S3.
Molecular pathways activated or inhibited in Fbn1mgR/mgR compared with Wt
| Upstream regulator | Molecule type | Predicted activation state | Activation z-score |
| Lipopolysaccharide | Chemical drug | Activated | 8.570 |
| TNF | Cytokine | Activated | 8.188 |
| IFNG | Cytokine | Activated | 6.993 |
| IL1B | Cytokine | Activated | 6.164 |
| TGFB1 | Growth factor | Activated | 5.734 |
| Phorbol myristate acetate | Chemical drug | Activated | 5.430 |
| Tretinoin | Chemical—endogenous mammalian | Activated | 4.309 |
| IL13 | Cytokine | Activated | 4.307 |
| AKT1 | Kinase | Activated | 4.112 |
| IL27 | Cytokine | Activated | 4.054 |
| TGM2 | Enzyme | Activated | 4.045 |
| OSM | Cytokine | Activated | 3.957 |
| Akt | Group | Activated | 3.956 |
| NFKBIA | Transcription regulator | Activated | 3.880 |
| FOS | Transcription regulator | Activated | 3.710 |
| CEBPA | Transcription regulator | Activated | 3.509 |
| d-glucose | Chemical—endogenous mammalian | Activated | 3.497 |
| LEP | Growth factor | Activated | 3.407 |
| Palmitic acid | Chemical—endogenous mammalian | Activated | 3.398 |
| Cisplatin | Chemical drug | Activated | 3.348 |
| ERBB2 | Kinase | Activated | 3.337 |
| CCND1 | Transcription regulator | Activated | 3.308 |
| TNFRSF1A | Transmembrane receptor | Activated | 3.299 |
| LIF | Cytokine | Activated | 3.132 |
| NOS2 | Enzyme | Activated | 3.101 |
| E. coli serotype 0127B8 lipopolysaccharide | Chemical—endogenous nonmammalian | Activated | 2.945 |
| Mek | Group | Activated | 2.858 |
| CAMP | Other | Activated | 2.827 |
| SPI1 | Transcription regulator | Activated | 2.800 |
| RHOA | Enzyme | Activated | 2.792 |
| N-nitro-l-arginine methyl ester | Chemical drug | Activated | 2.775 |
| UTP | Chemical—endogenous mammalian | Activated | 2.772 |
| SPP1 | Cytokine | Activated | 2.764 |
| NRG1 | Growth factor | Activated | 2.755 |
| ITGB1 | Transmembrane receptor | Activated | 2.745 |
| Okadaic acid | Chemical toxicant | Activated | 2.742 |
| Deferoxamine | Chemical drug | Activated | 2.720 |
| EBI3 | Cytokine | Activated | 2.646 |
| Trovafloxacin | Chemical drug | Activated | 2.646 |
| Cholesterol | Chemical—endogenous mammalian | Activated | 2.627 |
| Methamphetamine | Chemical drug | Activated | 2.621 |
| PLAU | Peptidase | Activated | 2.618 |
| HRAS | Enzyme | Activated | 2.605 |
| RUNX2 | Transcription regulator | Activated | 2.585 |
| MAPKAPK2 | Kinase | Activated | 2.576 |
| C5 | Cytokine | Activated | 2.479 |
| SRC | Kinase | Activated | 2.477 |
| Monocrotaline | Chemical toxicant | Activated | 2.433 |
| Acetaminophen | Chemical drug | Activated | 2.433 |
| ESR1 | Ligand-dependent nuclear receptor | Activated | 2.379 |
| GnRH-A | Chemical reagent | Activated | 2.359 |
| Paclitaxel | Chemical drug | Activated | 2.358 |
| Prostaglandin E2 | Chemical—endogenous mammalian | Activated | 2.338 |
| IFNB1 | Cytokine | Activated | 2.315 |
| Hyaluronic acid | Chemical—endogenous mammalian | Activated | 2.254 |
| Doxorubicin | Chemical drug | Activated | 2.247 |
| CYP1B1 | Enzyme | Activated | 2.236 |
| HIPK2 | Kinase | Activated | 2.236 |
| HDAC6 | Transcription regulator | Activated | 2.207 |
| Streptozocin | Chemical drug | Activated | 2.203 |
| Captopril | Chemical drug | Activated | 2.191 |
| SP3 | Transcription regulator | Activated | 2.181 |
| LGALS3 | Other | Activated | 2.170 |
| Advanced glycation end products | Chemical—endogenous mammalian | Activated | 2.138 |
| KRT17 | Other | Activated | 2.121 |
| LEF1 | Transcription regulator | Activated | 2.077 |
| WNT3A | Cytokine | Activated | 2.054 |
| TGFB2 | Growth factor | Activated | 2.023 |
| Epigallocatechin-gallate | Chemical drug | Inhibited | −3.625 |
| SP600125 | Chemical—kinase inhibitor | Inhibited | −3.469 |
| SB-431542 | Chemical reagent | Inhibited | −3.464 |
| Curcumin | Chemical drug | Inhibited | −3.218 |
| Troglitazone | Chemical drug | Inhibited | −3.133 |
| Resveratrol | Chemical drug | Inhibited | −2.902 |
| Estrogen receptor | Group | Inhibited | −2.848 |
| KLF2 | Transcription regulator | Inhibited | −2.820 |
| POR | Enzyme | Inhibited | −2.538 |
| Pioglitazone | Chemical drug | Inhibited | −2.506 |
| Quercetin | Chemical drug | Inhibited | −2.418 |
| HLX | Transcription regulator | Inhibited | −2.416 |
| Atorvastatin | Chemical drug | Inhibited | −2.382 |
| Tacrolimus | Chemical drug | Inhibited | −2.343 |
| 15-deoxy-delta-12,14 -PGJ 2 | Chemical—endogenous nonmammalian | Inhibited | −2.257 |
| Metformin | Chemical drug | Inhibited | −2.253 |
| Ursolic acid | Chemical—endogenous nonmammalian | Inhibited | −2.236 |
| WISP2 | Growth factor | Inhibited | −2.219 |
| Triptolide | Chemical drug | Inhibited | −2.219 |
| PTEN | Phosphatase | Inhibited | −2.214 |
| TFAP2C | Transcription regulator | Inhibited | −2.213 |
| Ciglitazone | Chemical drug | Inhibited | −2.210 |
| FST | Other | Inhibited | −2.182 |
| AGTR2 | G protein coupled receptor | Inhibited | −2.178 |
| Dexamethasone | Chemical drug | Inhibited | −2.176 |
| Staurosporine | Chemical—kinase inhibitor | Inhibited | −2.149 |
| TCF3 | Transcription regulator | Inhibited | −2.125 |
| HDL | Complex | Inhibited | −2.121 |
| Mifepristone | Chemical drug | Inhibited | −2.114 |
| Hydrocortisone | Chemical—endogenous mammalian | Inhibited | −2.070 |
| CDH1 | Other | Inhibited | −2.035 |
| HMOX1 | Enzyme | Inhibited | −2.024 |
| Lovastatin | Chemical drug | Inhibited | −2.012 |
Discussion
The purpose of the present study was to investigate the role of TGFβ/LTBP/fibrillin-1 interactions in the pathogenesis of TAA in MFS. A current view is that, in MFS, abnormal ECM sequestration of LTBP/TGFβ complexes due to defective fibrilin-1 microfibrils leads to increased TGFβ signaling and results in TAA (24). However, the validity of this hypothesis and whether LTBP and latent TGFβ sequestration are involved in MFS have yet to be determined at a molecular level.
As an initial approach to analyze the interactions of LTBPs and fibrillin-1 in MFS, we previously examined the matrix assembly of LTBPs in aortic tissue from Fbn1-null mice (18) and found diminished ECM incorporation of LTBP-3 and, by inference, its bound TGFβ. In the present study, we extended this observation to ASMCs isolated from Wt and Fbn1mgR/mgR mice (18, 20). Fbn1mgR/mgR mice represent a murine model that replicates the clinically severe and progressive form of human MFS, with death from aortic dissection and rupture during the first year of life accompanied by enhanced TGFβ signaling in the media of the thoracic aorta (19, 25). As with Fbn1−/− ASMCs, Fbn1mgR/mgR cells exhibited impaired LTBP-3 incorporation into the matrix with no significant change in LTBP-1 incorporation.
To test the role of LTBP-3 in the aortic disease observed in MFS, we generated mice containing mutations in both Fbn1 (Fbn1mgR/mgR) and Ltbp3 genes. We reasoned that, if enhanced activation of latent TGFβ complexes containing LTBP-3 were causative for the vascular disease in MFS, the absence of LTBP-3 should prevent the formation of the LLC that contributes to pathological TGFβ signaling and thus attenuate aortic disease progression in Fbn1mgR/mgR. Ltbp3 deletion dramatically reduced aortic disease in Fbn1mgR/mgR mice and increased their survival to a level similar to that of Wt mice. Fbn1mgR/mgR;Ltbp3−/− mice had aortas with diameters equivalent to those of normal mice, the number of discontinuities in the elastic lamellae was decreased by 80%, signaling through both Smad3 and ERK1/2 pathways was normalized, and expression of two genes regulated by TGFβ was reverted to levels approximating that observed in WT mice.
To our knowledge, this work is the first study in which ablation of a gene involved in the regulation of TGFβ action improves the aortic disease phenotype in MFS mice. Genetic ablation of Mmp2 results in prolonged survival of Fbn1mgR/mgR mice, but it is uncertain whether this finding reflects a diminution of TGFβ signaling due to the absence of proteolytic activation of latent TGFβ by MMP2 or is a consequence of decreased aortic media degeneration caused by MMP2 enzymatic activity (25). Conversely, mutations in several genes that encode components of the TGFβ pathway either alone or in combination with an MFS mouse model (e.g., loss-of-function mutations in Tgfbr1 or Tgfbr2 genes, Smad3-null mutation, and Smad4 or Tgfb2 haploinsufficiency in Fbn1C1039G/+ mice) either induce or worsen TAA (7, 26–30). Curiously, these mutations are expected to attenuate TGFβ signaling but yield paradoxical activation of TGFβ signaling in the aortas. However, deletion of Ltbp3 in Fbn1mgR/mgR mice did not result in paradoxical increase in TGFβ signaling and prevented aortic disease in Fbn1mgR/mgR mice although LTBP-3 deletion is expected to decrease TGFβ levels and accordingly to worsen aortic disease (31).
A possible explanation for these discrepancies is that, whereas Tgfbr1 and -2 mutations, Smad3 deletion, and Smad4 haploinsufficiency affect signaling of all three TGFβ isoforms, Ltbp3 deletion disturbs only the subset of latent TGFβ molecules bound to this specific carrier protein. It should be noted that our results also indicate that LTBP-1 present in the aorta cannot compensate for the loss of LTBP-3 even though both molecules bind all three isoforms of TGFβ. This observation implies functional specificity for these two LTBPs. It is still unclear which TGFβ isoform is the mediator of the aortic disease found in MFS because LTBP-3 interacts with TGFβ1, -β2, and -β3 isoforms with similar efficiency (9). However, it is unlikely that ablation of Ltbp3 in Fbn1mgR/mgR mice diminished TGFβ2 signaling because mice with Tgfb2 haploinsufficiency, when bred with Fbn1C1039G/+ mice, display increased aortic dilatation and morbidity, indicating a protective role for TGFβ2 (28). Accordingly, we propose that specifically lowering either TGFβ1 or -3 in MFS mice should mitigate TAA and rupture.
To date, only pharmacological inhibition of TGFβ with either TGFβ-neutralizing antibodies (Nabs), the MEK1/2 inhibitor RDEA119, the angiotensin-II type 1 receptor-blocker losartan, or doxycycline ameliorates the aortic phenotype in MFS mice (6, 7, 25, 32). However, it is unclear whether the efficacy of losartan or doxycycline in attenuating aortic disease in MFS mice is due to direct impairment of TGFβ signaling or whether TGFβ-independent mechanisms are involved (5, 25). Pharmacological inhibition of TGFβ with neutralizing antibodies has also underscored the complex, controversial, and context-dependent roles of TGFβ in aneurysm. Whereas earlier studies of systemic TGFβ neutralization with the Fbn1C1039G/+ mouse model prevented TAA formation, recent results with the Fbn1mgR/mgR mouse model have indicated that TGFβ can exert opposite effects on TAA pathology that broadly correlate with the early and late stages of TAA progression (5). Thus, early treatment (postnatal day 16; P16) with TGFβ-neutralizing antibodies enhances aneurysm formation whereas later treatment (P45) diminishes aneurysm formation. This context-dependent role of TGFβ was also underscored by a recent study in which induction of aortic dissection was dependent on the age at which Tgfbr2 was ablated in smooth muscle cells, with enhanced incidence of the phenotype with young mice and almost no in cidence after 9 wk (33). Differential contributions of TGFβ signaling to aortic physiology early and later after birth may explain the greater incidence of aortic dissection in young mice upon targeted ablation of Tgfbr2 in smooth muscle cells (33). Accordingly, we propose that the protective effect of LTBP-3 loss might represent the specific diminution of the pathological effects of LTBP-3/TGFβ complexes during aortic disease with no perturbation of basal physiological TGFβ signaling involved in aorta development or aortic tissue homeostasis and repair pathways (33, 34). In support of this postulate, we have not observed any evidence of vascular phenotype in this study or other studies with Ltbp3−/− mice (31, 35).
Finally, we cannot exclude the possibility that LTBP-3 has TGFβ-independent functions that participate in aortic pathology. TGFβ-independent activities have been identified for LTBP-4 in stabilizing microfibril bundles and regulating elastic fiber assembly and for LTBP-2 in the development of ciliary zonule microfibrils (36–38). Thus, LTBP-3 might directly contribute to the integrity of the matrix or the mechanical compliance of the aortic wall (39).
In summary, our work addresses the significance of the interaction between LTBP and fibrillin-1 microfibrils in the control of TGFβ action by using a genetic approach. Our results demonstrate the potential importance of the interaction between the LTBP-3/TGFβ complex and fibrillin-1 microfibrils in the control of detrimental TGFβ signaling involved in TAA pathogenesis in MFS. Blocking activation of latent TGFβ associated with LTBP-3 might represent a potentially novel and specific therapeutic approach to preventing aortic disease in MFS.
Materials and Methods
Mice.
Fbn1mgR/mgR and Ltbp3−/− mice have been previously described (19, 31). Colonies of Wt and mutant mice were maintained on a mixed C57BL/6;Sv129;SW genetic background. Only age-matched males were used in all experiments. For tissue analysis, animals were euthanized by CO2 asphyxiation. Survival of the different genotypes was calculated from weaning through death or collection at the intended time points. All procedures were performed according to the regulations of the New York University Langone Medical Center Institutional Animal Care and Use Committee.
Antibodies and Reagents.
Antibodies used for Western blotting are described in SI Materials and Methods.
Isolation of Vascular Smooth Muscle Cells.
Vascular smooth muscle cells (VSMCs) were isolated from 12-wk-old male ascending aortas of the different genotypes as described in SI Materials and Methods.
RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR Analysis.
Total RNA from ascending aortas was extracted using the RNeasy Fibrous Tissue Mini Kit (Qiagen) with DNase treatment included. See SI Materials and Methods for details. Primers used for qPCR are listed in SI Materials and Methods.
Immunocytochemistry.
ASMCs were plated on glass coverslips and stained as described by Zilberberg et al. (18). Adjustments of brightness and contrast were applied to the whole image. Images from different genotypes underwent the same adjustments.
Conditioned Media Analysis.
Cell cultures were washed with serum-free DMEM and incubated with fresh serum-free DMEM for 24 h. The conditioned media (CM) was collected, and protease inhibitors were added. CM was clarified by centrifugation and stored at –80 °C.
Echocardiography.
Transthoracic echocardiography was performed in standard fashion as described in SI Materials and Methods.
Histology and Immunohistochemistry.
Ascending aortas were harvested and fixed in 10% (vol/vol) buffered formalin, processed, and embedded in paraffin. Five-micrometer sections were used in all studies. Elastin was stained using the orcinol–new fuchsin technique (40). The number of elastic fiber breaks for the entire aortic ring was counted manually for each genotype. Immunohistochemistry was performed using rabbit anti-mouse p-ERK1/2 (clone D13.14.4E; Cell Signaling) as described in SI Materials and Methods.
Gene Expression Analysis.
Total RNA (100 ng) from the 8-wk-old ascending aortas was analyzed as described in SI Materials and Methods.
Statistics.
Data are presented as the mean ± SD in bar graphs. The unpaired two-tailed Student’s t test was used to determine the significance between two groups, assuming significance at P < 0.05. Analyses between multiple groups used one-way ANOVA with P < 0.05 considered as statistically significant. Kaplan–Meier survival curves were constructed and analyzed using the log-rank (Mantel–Cox) test (GraphPad Prism software).
SI Materials and Methods
Isolation of Vascular Smooth Muscle Cells.
Vascular smooth muscle cells (VSMCs) were isolated from 12-wk-old male ascending aortas of the different genotypes. The ascending aortas were digested with collagenase Type II (Worthington) to remove the adventitia, and the endothelium was scraped off by gently rubbing the intimal surface with a scalpel blade. The aortas were transferred to 35-mm culture dishes containing DMEM supplemented with 10% FBS, 2 mM l-glutamine, and 100 units/mL penicillin/streptomycin and left overnight in a cell culture incubator (37 °C, 5% CO2). Tissues were subsequently digested with Elastase (Sigma) and Collagenase type II (Worthington) for 20 min. Enzyme digestion was stopped by adding DMEM supplemented with 20% FBS. Aorta fragments were plated on 35-mm Primaria tissue culture dishes and allowed to adhere to the plate. VSMCs that grow out of the pieces of tissue were expanded in SmGM-2 media (CC-3182; Lonza) until passage 3 and frozen for subsequent experiments. Cells between passages 3 and 6 were used in the experiments.
Antibodies and Reagents.
Rabbit polyclonal anti-LTBP-1 (Ab39) was a gift from Carl-Henrik Heldin (Ludwig Institute for Cancer, Uppsala) and Kohei Miyazono (Tokyo University, Tokyo). Rabbit polyclonal anti-FBN1 (pAb 9543) was a gift from Lynn Sakai (Shriners Hospital for Children, Portland, OR), and rabbit polyclonal anti-LTBP-3 (pAb952) was previously described (8, 18). Mouse anti-G3PDH (clone 6C5) was purchased from Santa Cruz (sc-32233), rabbit monoclonal anti-pERK1/2 from Cell Signaling (4370), and rabbit polyclonal anti-pSmad3 from Abcam (Ab51451).
Blood Pressure Measurements.
Blood pressure measurements were acquired in 2-mo-old conscious animals using the noninvasive volume-pressure recording tail cuff method (CODA-2; Kent Scientific). Mice were placed in the holder 10 min before obtaining pressure measurements. The average systolic and diastolic pressures, measured in 5–10 recordings, were used for each animal.
RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR Analysis.
Total RNA from cells of ascending aortas was extracted using the RNeasy Fibrous tissue Mini Kit (Qiagen) with DNase treatment included. cDNA was generated from 50 ng of RNA with SuperScript III Reverse transcriptase following the manufacturer's instructions (Invitrogen) and used for quantitative real-time PCR (qPCR) analysis. qPCRs were performed using specific primers and a QuantiFast SYBR Green PCR Kit (Qiagen) on an iCycler Thermal Cycler (Bio-Rad). The amount of each gene was calculated using the Ct value and corresponding standard curve. Each target transcript expression was quantified relative to the G3PDH gene. Primers used were as follows: Gapdh sense, ACGGCCGCATCTTCTTGTGC, antisense, TGGTGACCAGGCGCCCAATAC; Ltbp-1 sense, AGCACCATCACCTCTGCTCT, antisense, CAGACACTGCTGTCCTCCAA; Ltbp-3 sense, ACGGCCTCAGTTGCATAGAC, antisense, AAAGAGCCTGGTGTGTTCGT; Ltbp-4 sense, TGACCTCCGATACAACACCA, antisense, AGGCAGAAAGCCTGTAGGTG; Fbn1 sense, GATCAACGGCTACCCAAAAC, antisense, GTTGGCTTCCATCTCAGACC; Col1a1 sense, CACCTACAGCACCCTTGTGG, antisense, GGGAGGTCTTGGTGGTTTTG; Fn sense, AAGGTTCGGGAAGAGGTTGT, antisense, CCGTGTAAGGGTCAAAGCAT; Ctgf sense, CAAGGAGTGGGTGTGTGACGA, antisense, TCTTCCAGTCGGTAGGCAGCT; Pai1 sense, CGGGGGTGGTGAACTCAGTGT, antisense, ACGCCTGGTGCTGGTGAATG. The Annealing temperature was 60 °C.
Immunoblotting.
For aortic tissue analysis, the ascending aorta (root to right brachiocephalic trunk) was removed, flash frozen in liquid nitrogen, and stored at −80 °C. Protein concentrations were determined using a BCA protein assay kit (Thermo Scientific). Equivalent amounts of protein were separated by SDS/PAGE using 4–20% gradient polyacrylamide mini gels (Thermo Scientific) in reducing or nonreducing condition and transferred to nitrocellulose membranes (Whatman) by electroblotting. Membranes were blocked in PBS/0.1% Tween 20 containing 2.5% BSA for 1 h at room temperature and incubated with primary antibodies overnight at 4 °C. The membranes were washed three times in PBS/0.1% Tween 20 and incubated with horseradish peroxidase-linked anti-rabbit or anti-mouse secondary antibody (GE Healthcare) for 1 h at room temperature. Immunoreactive bands were revealed using Amersham ECL Prime Western Blotting Detection Reagent, and images were acquired with an ImageQuant LAS 4000 digital imaging system (GE Healthcare Life Sciences). The intensity of the bands was evaluated using ImageJ software and normalized with G3PDH.
Echocardiography.
Transthoracic echocardiography was performed in standard fashion as described previously (41, 42), using a Vevo 770 ultrasound system with a 40-MHz center frequency transducer (Visual Sonics). Mice were anesthetized with 1% isoflurane mixed with room air via nose cone, with strict thermoregulation (core temperature 37 ± 1 °C) to optimize physiological conditions and reduce hemodynamic variability. Analyses included aortic root and ascending aortic diameters (obtained at the largest diameter, in systole). Basic cardiac rhythm was monitored by electrocardiographic limb leads on the warming pad on which the mouse lay and was displayed with echocardiographic images simultaneously. All measurements were performed in triplicate, averaged, and performed without knowledge of genotype.
Histology and Immunohistochemistry.
Immunohistochemistry was performed using rabbit anti-mouse pERK1/2 (clone D13.14.4E; Cell Signaling). Antibody incubation and detection were performed on a Discovery XT platform using Ventana’s reagent buffer and detection kits. Images were digitally captured using a whole slide digital scanner (Leica SCN 400) and Leica SlidePath software. Four pictures per aorta section were taken randomly, and the pERK1/2-positive signal was evaluated by two blinded observers. Staining was scored based on the following scale: 0, no staining; 1, minimal; 2, mild; 3, moderate; 4, maximal. The pERK1/2 staining score for each sample was calculated by averaging the results of the two blinded observers.
Gene Expression Analysis.
Total RNA (100 ng) from the 8-wk-old ascending aortas was converted to double-stranded (ds) cDNA using the Ambion WT Expression Kit (Life Technologies). The dscDNA was fragmented and labeled with the Affymetrix biotin marker using the Affymetrix Genechip WT Terminal Labeling and Hybridization kit. Ten micrograms of fragmented, labeled single-stranded cDNA was added to the hybridization mixture according to the protocol outlined in the Affymetrix Genechip WT Terminal Labeling and Hybridization kit. The hybridization mixture was injected into Genechip Mouse Gene 2.0 ST array chips and allowed to hybridize for 16 h at 45 °C and 60 RPM. The array chips were stained in the Affymetrix Genechip Fluidics Station 450. After staining, the arrays were scanned in the Affymetrix Genechip Scanner 3000 7G. Affymetrix GeneChip Mouse Gene 2.0 ST arrays were used. The CEL files were processed by GeneSpring 12.6.1 (Agilent Technologies, Inc.) software. The values were robust multiarray analysis (RMA)-summarized and quantile-normalized. An unpaired t test with Benjamini–Hochberg multiple testing correction was used to identify significant differentially expressed genes between groups.
Supplementary Material
Acknowledgments
We thank M. Vassallo for excellent technical assistance. We acknowledge the use of the New York University Langone Medical Center (NYUMC) Histopathology and Transgenic Mouse Cores supported by NYUMC Cancer Center Grant P30CA016087 and the Genome Technology Center supported in part by Grant UL1 TR00038 from the National Center for Advancing Translational Sciences, National Institutes of Health. This work was supported by NIH Grants R01 CA034282 and P01 AR49698 (to D.B.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507652112/-/DCSupplemental.
References
- 1.Dietz HC, Loeys B, Carta L, Ramirez F. Recent progress towards a molecular understanding of Marfan syndrome. Am J Med Genet C Semin Med Genet. 2005;139C(1):4–9. doi: 10.1002/ajmg.c.30068. [DOI] [PubMed] [Google Scholar]
- 2.Ramirez F, Sakai LY, Dietz HC, Rifkin DB. Fibrillin microfibrils: Multipurpose extracellular networks in organismal physiology. Physiol Genomics. 2004;19(2):151–154. doi: 10.1152/physiolgenomics.00092.2004. [DOI] [PubMed] [Google Scholar]
- 3.Doyle JJ, Gerber EE, Dietz HC. Matrix-dependent perturbation of TGFβ signaling and disease. FEBS Lett. 2012;586(14):2003–2015. doi: 10.1016/j.febslet.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook JR, et al. Dimorphic effects of transforming growth factor-β signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler Thromb Vasc Biol. 2015;35(4):911–917. doi: 10.1161/ATVBAHA.114.305150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habashi JP, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312(5770):117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holm TM, et al. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332(6027):358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Dabovic B, Annes JP, Rifkin DB. Latent TGF-beta binding protein-3 (LTBP-3) requires binding to TGF-beta for secretion. FEBS Lett. 2002;517(1-3):277–280. doi: 10.1016/s0014-5793(02)02648-0. [DOI] [PubMed] [Google Scholar]
- 9.Saharinen J, Keski-Oja J. Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol Biol Cell. 2000;11(8):2691–2704. doi: 10.1091/mbc.11.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallas SL, et al. Role of the latent transforming growth factor beta binding protein 1 in fibrillin-containing microfibrils in bone cells in vitro and in vivo. J Bone Miner Res. 2000;15(1):68–81. doi: 10.1359/jbmr.2000.15.1.68. [DOI] [PubMed] [Google Scholar]
- 11.Flaumenhaft R, et al. Role of the latent TGF-beta binding protein in the activation of latent TGF-beta by co-cultures of endothelial and smooth muscle cells. J Cell Biol. 1993;120(4):995–1002. doi: 10.1083/jcb.120.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol. 1994;124(1-2):171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyytiäinen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: Extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41(3):233–264. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- 14.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: Orchestrators of TGF-beta availability. J Biol Chem. 2005;280(9):7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 15.Mazzieri R, et al. Expression of truncated latent TGF-beta-binding protein modulates TGF-beta signaling. J Cell Sci. 2005;118(Pt 10):2177–2187. doi: 10.1242/jcs.02352. [DOI] [PubMed] [Google Scholar]
- 16.Yoshinaga K, et al. Perturbation of transforming growth factor (TGF)-beta1 association with latent TGF-beta binding protein yields inflammation and tumors. Proc Natl Acad Sci USA. 2008;105(48):18758–18763. doi: 10.1073/pnas.0805411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge G, Greenspan DS. BMP1 controls TGFbeta1 activation via cleavage of latent TGFbeta-binding protein. J Cell Biol. 2006;175(1):111–120. doi: 10.1083/jcb.200606058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zilberberg L, et al. Specificity of latent TGF-β binding protein (LTBP) incorporation into matrix: Role of fibrillins and fibronectin. J Cell Physiol. 2012;227(12):3828–3836. doi: 10.1002/jcp.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira L, et al. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci USA. 1999;96(7):3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwill S, et al. The fibrillin-1 hypomorphic mgR/mgR murine model of Marfan syndrome shows severe elastolysis in all segments of the aorta. J Vasc Surg. 2013;57(6):1628–1636, 1636.e1–1636.e3. doi: 10.1016/j.jvs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Habashi JP, et al. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332(6027):361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74(2):196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Travis MA, Sheppard D. TGF-β activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaartinen V, Warburton D. Fibrillin controls TGF-beta activation. Nat Genet. 2003;33(3):331–332. doi: 10.1038/ng0303-331. [DOI] [PubMed] [Google Scholar]
- 25.Xiong W, Meisinger T, Knispel R, Worth JM, Baxter BT. MMP-2 regulates Erk1/2 phosphorylation and aortic dilatation in Marfan syndrome. Circ Res. 2012;110(12):e92–e101. doi: 10.1161/CIRCRESAHA.112.268268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boileau C, et al. National Heart, Lung, and Blood Institute (NHLBI) Go Exome Sequencing Project TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet. 2012;44(8):916–921. doi: 10.1038/ng.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallo EM, et al. Angiotensin II-dependent TGF-β signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest. 2014;124(1):448–460. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsay ME, et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet. 2012;44(8):922–927. doi: 10.1038/ng.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeys BL, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37(3):275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 30.Ye P, et al. GM-CSF contributes to aortic aneurysms resulting from SMAD3 deficiency. J Clin Invest. 2013;123(5):2317–2331. doi: 10.1172/JCI67356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dabovic B, et al. Bone abnormalities in latent TGF-[beta] binding protein (Ltbp)-3-null mice indicate a role for Ltbp-3 in modulating TGF-[beta] bioavailability. J Cell Biol. 2002;156(2):227–232. doi: 10.1083/jcb.200111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G, Espinosa E, Oemar BS, Lüscher TF. Bimodal effects of angiotensin II on migration of human and rat smooth muscle cells: Direct stimulation and indirect inhibition via transforming growth factor-beta 1. Arterioscler Thromb Vasc Biol. 1997;17(7):1251–1257. doi: 10.1161/01.atv.17.7.1251. [DOI] [PubMed] [Google Scholar]
- 33.Li W, et al. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest. 2014;124(2):755–767. doi: 10.1172/JCI69942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choudhary B, et al. Absence of TGFbeta signaling in embryonic vascular smooth muscle leads to reduced lysyl oxidase expression, impaired elastogenesis, and aneurysm. Genesis. 2009;47(2):115–121. doi: 10.1002/dvg.20466. [DOI] [PubMed] [Google Scholar]
- 35.Colarossi C, et al. Lung alveolar septation defects in Ltbp-3-null mice. Am J Pathol. 2005;167(2):419–428. doi: 10.1016/S0002-9440(10)62986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dabovic B, et al. Function of latent TGFβ binding protein 4 and fibulin 5 in elastogenesis and lung development. J Cell Physiol. 2015;230(1):226–236. doi: 10.1002/jcp.24704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue T, et al. Latent TGF-β binding protein-2 is essential for the development of ciliary zonule microfibrils. Hum Mol Genet. 2014;23(21):5672–5682. doi: 10.1093/hmg/ddu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noda K, et al. Latent TGF-β binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5. Proc Natl Acad Sci USA. 2013;110(8):2852–2857. doi: 10.1073/pnas.1215779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheehan DC, Hrapchak BB. 1980. Theory and Practice of Histotechnology (Mosby, St. Louis), 2nd Ed.
- 41.Collis LP, et al. Expression of a sorcin missense mutation in the heart modulates excitation-contraction coupling. FASEB J. 2007;21(2):475–487. doi: 10.1096/fj.06-6292com. [DOI] [PubMed] [Google Scholar]
- 42.Danielson LS, et al. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. FASEB J. 2013;27(4):1460–1467. doi: 10.1096/fj.12-221994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.