Significance
To our knowledge, this study is the first to directly link rapid microbial consumption of ancient permafrost-derived dissolved organic carbon (DOC) to CO2 production using a novel bioreactor. Rapid mineralization of the freshly thawed DOC was attributed to microbial decomposition of low–molecular-weight organic acids, which were completely consumed during the experiments. Our results indicate that substantial biodegradation of permafrost DOC occurs immediately after thaw and before downstream transport occurs. We estimate that, by 2100, between 5 to 10 Tg of DOC will be released from Yedoma soils every year given the most recent estimates for projected thaw. This represents 19–26% of annual DOC loads exported by Arctic rivers, yet it is so far undetectable likely due to rapid mineralization in soils and/or headwater streams.
Keywords: permafrost, dissolved organic carbon, carbon dioxide, organic acids, Pleistocene
Abstract
Northern permafrost soils store a vast reservoir of carbon, nearly twice that of the present atmosphere. Current and projected climate warming threatens widespread thaw of these frozen, organic carbon (OC)-rich soils. Upon thaw, mobilized permafrost OC in dissolved and particulate forms can enter streams and rivers, which are important processors of OC and conduits for carbon dioxide (CO2) to the atmosphere. Here, we demonstrate that ancient dissolved organic carbon (DOC) leached from 35,800 y B.P. permafrost soils is rapidly mineralized to CO2. During 200-h experiments in a novel high–temporal-resolution bioreactor, DOC concentration decreased by an average of 53%, fueling a more than sevenfold increase in dissolved inorganic carbon (DIC) concentration. Eighty-seven percent of the DOC loss to microbial uptake was derived from the low–molecular-weight (LMW) organic acids acetate and butyrate. To our knowledge, our study is the first to directly quantify high CO2 production rates from permafrost-derived LMW DOC mineralization. The observed DOC loss rates are among the highest reported for permafrost carbon and demonstrate the potential importance of LMW DOC in driving the rapid metabolism of Pleistocene-age permafrost carbon upon thaw and the outgassing of CO2 to the atmosphere by soils and nearby inland waters.
Northern permafrost soils contain an estimated 1,700 Pg of organic carbon (OC) that has been isolated from the modern carbon cycle since the Pleistocene (1, 2). Yedoma permafrost soils, deposited as windblown silts in the late Pleistocene across Siberia and Alaska and frozen in place, account for a significant portion of the northern permafrost OC pool. It is estimated that these frozen, organic-rich silts contain anywhere from 210 (2, 3) to 456 Pg (2, 4) of OC. Rising global temperatures are expected to enhance thawing of this vast reservoir of carbon. Regions of discontinuous permafrost, including much of the Yedoma complex, are particularly vulnerable to thaw (5). Upon thaw, this stored carbon is subject to mineralization and emission to the atmosphere as carbon dioxide, CO2, and methane, CH4 (6, 7), and to export to aquatic systems (8). Historically, it has been assumed that aged OC is substantially degraded relative to modern OC and therefore relatively less biodegradable (9). In the case of aged permafrost OC, this assumption has been challenged by recent studies that demonstrate high biodegradability (10–13). If a significant portion of the OC released from these thawing soils is indeed labile, either to microbial decomposition or photooxidation, it represents a vast potential source of greenhouse gas emissions to the atmosphere. These emissions would contribute to further warming and thereby accelerate permafrost thaw, a process known as the “permafrost climate feedback” (6, 11).
Thawed permafrost OC can be respired in situ in soils, or dissolved and mobilized in soil pore water, meltwater, or precipitation. Once dissolved and mobilized, permafrost-derived dissolved OC (DOC) can undergo mineralization within soil pore water or at any given point during subsurface or surface water transport to inland waters (14, 15). Aquatic pathways may be particularly important for permafrost soil-derived OC turnover following the physical disconnection of OC and decomposer communities occurring in the soil matrix (16). Evidence supporting these aquatic pathways is nearly ubiquitous around the world, and it has been widely demonstrated that fluvial systems are important processors of terrestrial OC and significant sources of CO2 to the atmosphere (17–19). Once permafrost DOC is mineralized in a fluvial environment, the byproducts (aged CO2 and CH4) are emitted from surface waters to the atmosphere over large spatial areas, depending on the rate of gas emission (19, 20). The potential for, and pathways of, translocation of permafrost DOC from source (thawed soil) to fate (downstream transport or outgassing) pose challenges for quantifying and constraining the amount of thawed permafrost OC entering the atmosphere via mineralization in both terrestrial and aquatic environments (15).
Although recent investigations into the aquatic pathway in Siberia and Alaska demonstrate export of highly biodegradable DOC from thawing Yedoma soils to fluvial systems (11, 21), they have focused on stream and river DOC that has already undergone transport and potentially extensive mineralization in soils and in headwater stream networks. To complicate matters, the labile component of DOC is often a small and quickly cycling subset of the overall OC pool (22), which means that most river biodegradation measurements quantify the biolability of the remaining bulk fraction (23) and not of the original source DOC. To most accurately estimate the quantity of CO2 released from thawing permafrost soils, the biodegradability of all DOC components must be assessed immediately upon thaw, before significant mobilization and/or mineralization occurs. To that end, we investigate three important unknowns regarding Yedoma-derived DOC decomposition in Arctic ecosystems: (i) What is the biodegradability of this DOC immediately upon soil thaw? (ii) How does the chemical composition of the DOC relate to its susceptibility to microbial decomposition? (iii) What proportion of DOC leached from Yedoma soils will be converted to CO2 for eventual emission to the atmosphere? Answering these questions is key to understanding where on the landscape thawing Yedoma-derived DOC is likely to be decomposed, and to explaining observed and projected CO2 and DOC patterns in Arctic watersheds.
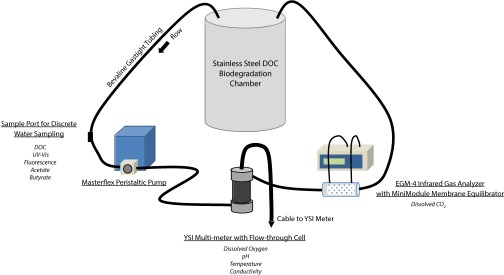
To address these questions, we collected Yedoma permafrost soils for DOC biodegradability experiments from a newly excavated extension of the Fox Permafrost Tunnel, operated by the Cold Regions Research and Engineering Laboratory in Fairbanks, Alaska. We thawed the soils at low temperature (2 °C) and immediately upon thaw leached them for DOC for incubation and chemical analysis. We measured DOC biodegradability using a novel incubation method with high-resolution subsampling and in situ instrumentation (Fig. S1), which allowed simultaneous characterization of changes in the DOC pool and tracking of the mineralization to dissolved inorganic C (DIC). Five replicate incubations were conducted at room temperature (20 °C), which is comparable to maximum summer temperatures in Arctic fluvial ecosystems (24, 25).
Fig. S1.
Schematic of DOC biodegradation chamber and components.
Results and Discussion
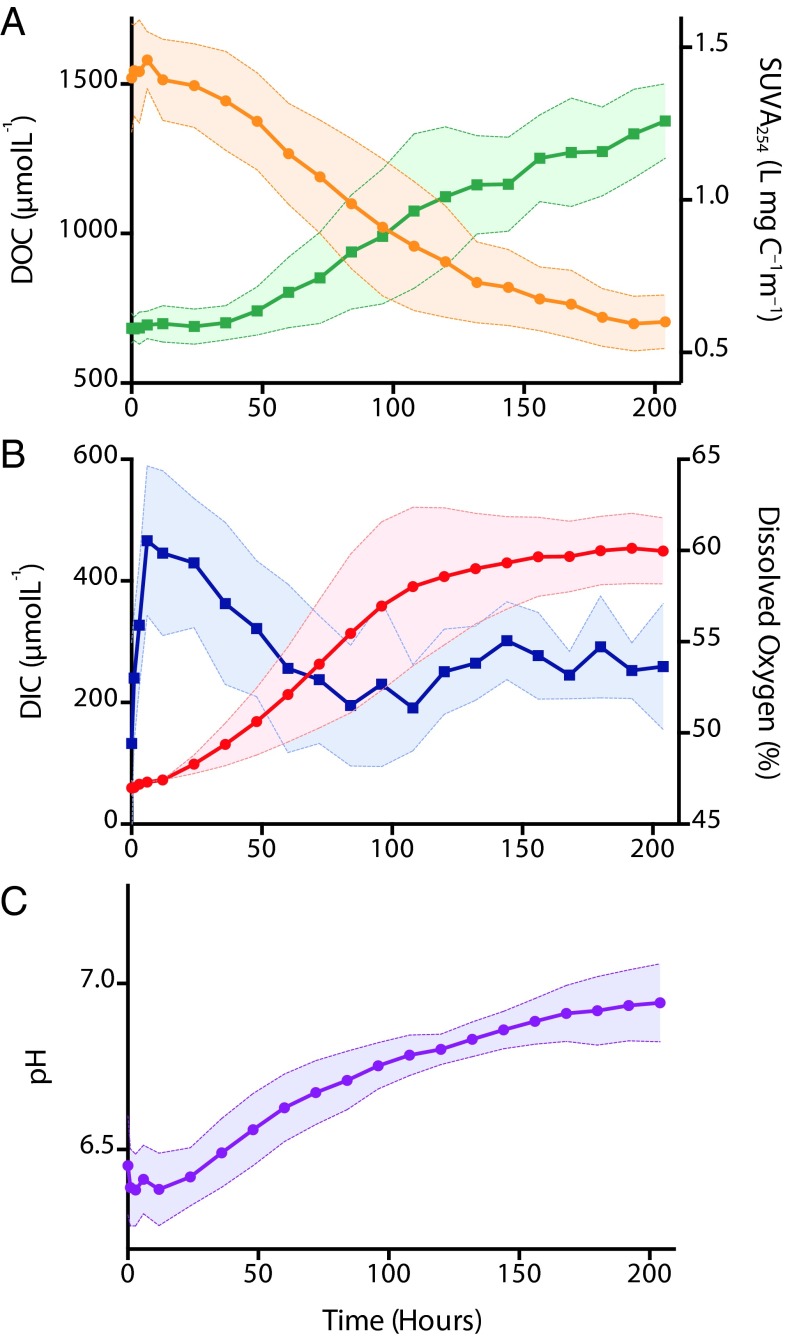
The permafrost soil had a radiocarbon age of 35,800 y B.P. (±200 y B.P.). The permafrost leachates yielded an average of 38.4 ± 3.69 μmol C as DOC per gram soil. Leached DOC had a radiocarbon age of 33,700 y B.P. (±270 y B.P.). The average C content of the permafrost soil was 1.36% by weight and thus the average DOC yield per gram soil C was 3.39%. Leachates were diluted for incubation with carbon-free Milli-Q water (>18 MΩ⋅cm), having an average initial diluted DOC concentration of 1,520 ± 181 μmol⋅L−1 (Table 1). Over the 200-h incubations, DOC concentrations decreased by an average of 53.5 ± 4.1%, with the majority of loss occurring in the first 100 h (Table 1 and Fig. 1A). We attribute DOC loss entirely to microbial uptake given the absence of other consumptive processes (i.e., photooxidation, sorption) in the closed incubation system. No DOC flocculation was observed. DOC mineralization resulted in a 7.4-fold average increase in DIC concentration (Fig. 1B; DIC = dissolved gaseous CO2 plus dissolved bicarbonate plus headspace gaseous CO2), which accounted for an average of 50% of the reduction in DOC concentration. Microbial biomass growth was estimated to account for the majority of the residual DOC loss (∼40%), although a small fraction of the loss may be due to DOC adsorption to biofilm produced over the course of the incubations (Materials and Methods). Assuming that the majority of the DOC loss was due to bacterial assimilation (BA) [equivalent to the sum of bacterial production (BP) and bacterial respiration (BR)], the bacterial growth efficiencies (BGE = BP/BA) were estimated to be 30–40%, which is relatively high for freshwater ecosystems (26). This range of BGE is approximate, because confounding factors such as secondary respiration or DOC adsorption would either increase or decrease growth efficiency, respectively. Dissolved oxygen concentrations decreased simultaneously with DIC production and DOC loss, starting at an average of 60.5% saturation and dropping to an average 53.6% by the end of the experiment (Fig. 1B). The biodegradable fraction of Yedoma permafrost DOC was respired to CO2 extremely rapidly (<100 h). The average DOC consumption rate was 114 ± 12.5 μmol C⋅L−1⋅d−1 and was relatively consistent across experiments.
Table 1.
DOC, DIC, acetate, butyrate, and pH at 0 and 200 hours for all experiments
| DOC | DIC | Acetate | Butyrate | pH | ||||||
| Experiment | 0 h | 200 h | 0 h | 200 h | 0 h | 200 h | 0 h | 200 h | 0 h | 200 h |
| 1 | 1,630 | 753 (−53.7%) | 63.9 | 463 (+624%) | 417 | 1.67 (−99.6%) | 390 | <4.55 (greater than −98.8%) | 6.56 | 7.05 |
| 2 | 1,500 | 627 (−58.2%) | 77.6 | 481 (+520%) | 272 | 3.33 (−98.8%) | 425 | <4.55 (greater than −98.9%) | 6.63 | 7.04 |
| 3 | 1,330 | 600 (−54.7%) | 52.4 | 441 (+743%) | 291 | 9.17 (−96.8%) | 436 | <4.55 (greater than −99.0%) | 6.34 | 6.94 |
| 4 | 1,770 | 813 (−54.1%) | 71.1 | 550 (+674%) | 292 | 5.00 (−98.3%) | 432 | <4.55 (greater than −98.9%) | 6.46 | 6.92 |
| 5 | 1,380 | 733 (−46.9%) | 49.5 | 402 (+713%) | 253 | 3.33 (−98.7%) | 382 | <4.55 (greater than −98.8%) | 6.27 | 6.76 |
| Mean | 1,520 | 705 (−53.5%) | 62.9 | 468 (+655%) | 305 | 4.17 (−98.4%) | 413 | <4.55 (greater than −98.9%) | 6.45 | 6.94 |
Data other than pH are reported in micromoles per liter (percentage change). Acetate and butyrate data are reported in micromoles of carbon per liter. Average values reported as mean (mean percentage change between 0 and 200 hours) in micromoles per liter.
Fig. 1.
Dissolved organic carbon (DOC) (A, yellow circles), specific UV absorbance at 254 nm (SUVA254) (A, green squares), dissolved inorganic carbon (DIC) (B, red circles), dissolved oxygen (B, blue squares), and pH (C) over 200 h for five incubation replicates. Dark lines represent mean values at each time point, and shaded areas represent 1 SD from the mean.
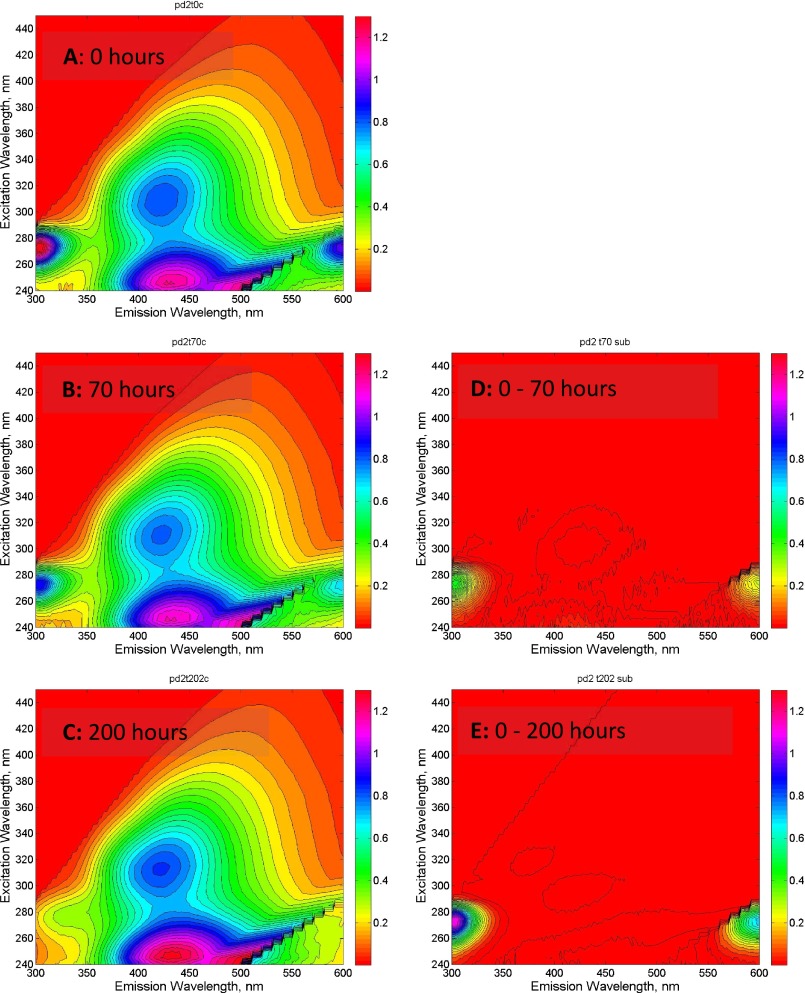
Yedoma leachate contained high initial average concentrations of the low–molecular-weight (LMW) organic acids acetate and butyrate (accounting for 20% and 27% of initial DOC on a molar basis, respectively). Over the course of the incubations, we measured near-complete losses of these LMW organic acids (Table 1). Consumption of both acetate and butyrate accounted for an average of 87% of the DOC consumed across experiments. Average DOC specific UV absorbance at 254 nm (SUVA254) was initially very low (0.58 L⋅mg C−1⋅m−1) and increased to 1.29 L⋅mg C−1⋅m−1 during the incubation (Fig. 1A). The time series of fluorescence excitation–emission matrices (EEMs) measured during the incubations indicate decreasing intensity of a LMW peak at emission of 310 nm/excitation of 275 nm (Fig. S2).
Fig. S2.
Fluorescence excitation/emission matrices (EEMs) for a representative permafrost DOC incubation. EEMs measured at 0 h (A), 70 h (B), and 200 (C) are shown on the Left, whereas subtraction EEMs illustrating the change in fluorescence after 70 h (D) and change after 200 h (E) are on the Right. Note the loss of the peak at emission of 310/excitation of 275 over time.
The DOC biodegradability we measured, on a percent basis, is among the highest recorded for DOC derived from permafrost soils (10, 11, 21). A direct comparison of DOC biodegradability across studies using different incubation methods (e.g., static bottles, plug-flow bioreactors, circulating bioreactors) is difficult given the variety of physicochemical conditions they produce (27, 28). We designed our method to produce high-resolution data that allowed for tracking of the organic and inorganic C pools to address the key question of how much CO2 is generated from Yedoma permafrost DOC biodegradation. The biodegradation method in this study likely resulted in relatively conservative degradation rates compared with other methods, such as those that use bioreactors containing beads (27, 29), due to the relatively low surface area-to-volume ratio. Additionally, our method excluded soil particles and their associated microbes. Nevertheless, biodegradation rates were potentially accelerated at the incubation temperature we chose compared with average soil and surface water temperatures (30), although the incubation temperature is comparable to the reported maximum summer temperatures of Arctic fluvial ecosystems (24, 25). Although temperature can control the rate of respiration via bioenergetic constraints, it does not control which compounds are respired (i.e., the biodegradable fraction). Furthermore, decomposition of inherently biolabile DOC, such as LMW organic acids, is known to be less sensitive to temperature than more stable DOC (31). In other permafrost studies, bioenergetic constraints from lower temperature incubation did not alter the age or fraction of the used DOC (13). In light of these temperature dynamics and the fact that acetate and butyrate were exhausted over 200 h, percent loss is the preferred metric for both comparisons across incubation methods and for the determination of potential biodegradability in situ.
The measured 53% reduction in DOC concentration and corresponding 7.4-fold increase in DIC suggests that the freshly leached DOC collected immediately upon thaw is highly biodegradable relative to other studies and that we captured the maximum potential biodegradable fraction. Incubations of DOC in soil pore waters and surface waters likely miss a significant portion of this highly biodegradable pool (32) and should therefore be considered relatively conservative compared with the original source material (23). If we apply our average Yedoma permafrost soil DOC yield to the estimated thawed Yedoma soil OC pool by 2100 (2), we would expect between 260 and 480 Tg of rapidly biodegradable DOC released upon thaw by 2100 (Table 2), about one-half of which would be rapidly converted to DIC (130–240 Tg) for release as CO2 to the atmosphere.
Table 2.
Estimated DOC yields from Yedoma soils by 2100
The large DOC loss was fueled by rapid microbial consumption of LMW DOC, as evidenced by the complete loss of acetate and butyrate over the incubation, accounting for 87% of the total DOC loss. As observed in previous studies, the loss of these organic acids had a slight titration effect on water pH (33). Over 200 h, pH increased by an average of ∼0.5 units as a result of acetate and butyrate consumption (Fig. 1C). High acetate concentrations have been reported in other Yedoma permafrost soils (34), suggesting that the concentrations we measured are not unusual. Our study, however, is the first (to our knowledge) to directly link high concentrations of LMW organic acids to Yedoma permafrost soil-derived DOC biodegradability. In general, LMW organic acids such as acetate and butyrate are highly biodegradable (23, 35–37), explaining why they are largely absent from temperate soils (38).
Given the sedimentary history of Yedoma soils, we suggest that the LMW organic acids observed in this study were formed as the byproducts of fermentation by anaerobic bacteria (39). Little is known about the specific oxidation states of Yedoma sediments as they formed. One possibility is that rapid burial rates and saturated conditions led to anoxia and reducing conditions in these depositional environments. Under these conditions, fermenters would have been able to hydrolyze and metabolize complex carbohydrates into LMW compounds, which are known intermediates of methanogenesis (40). It is not clear why these LMW acids such as acetate would not undergo full conversion to methane, but it has been suggested that low pH can interrupt the progression from acetogenesis to acetoclastic methanogenesis (41). Regardless, the subsequent freezing of these soils would have preserved these highly biodegradable LMW organic compounds from microbial utilization. It is not known what percent of the standing Yedoma permafrost carbon stock is composed of LMW compounds, but if they exist in similar concentrations as observed in this study, a sizable (3.3–7.1 Pg) yet elusive pool of organic carbon is poised for turnover immediately upon thaw.
The chromophoric dissolved organic matter data from the incubations are consistent with the measured LMW organic acid consumption. Average DOC SUVA254 was initially low relative to typical fluvial systems (42) and increased during the incubation, indicating a relative increase in the aromaticity of the DOC pool (43). Because the average UV254 absorbance did not change significantly over the incubation (P > 0.5), the increase in SUVA254 was driven solely by the loss of nonchromophoric, LMW DOC. The fluorescence time series shows the loss of a peak in what is typically denoted as the “protein-like” region (44). Compounds that fluoresce in this region can be simple amino acids and have been shown to be biodegradable (32, 44). Although changes in fluorescence cannot be used to ascertain quantitative changes in the DOC pool, the loss of the peak at emission of 310/excitation of 275 provides some insight as to the composition of the remaining 13% of the DOC loss not accounted for by acetate and butyrate. Overall, the increase in SUVA254 and the loss of fluorophores commonly associated with biodegradable compounds are consistent with LMW organic compounds as the dominant substrate for microbial respiration.
Current and future thawing of Pleistocene Yedoma permafrost soils will mobilize highly biodegradable LMW organic acids that fuel microbial metabolism and the production of CO2. Rapid decomposition rates in the presence of indigenous permafrost microbes suggests that the majority of these biodegradable LMW organic acids will be metabolized soon after thaw in soil pore waters, and promptly emitted as CO2 from soils or immediately upon export to adjacent surface waters before significant downstream translocation can occur. Furthermore, the rapid turnover of ancient DOC to CO2 observed in this study helps to explain the absence of a highly aged Yedoma DOC signature (12, 13, 45–47) at the mouths of large Arctic rivers, because any Yedoma permafrost-derived DOC was likely metabolized in soil pore waters and headwaters upstream of sample collection sites on major rivers. We estimate that thawing Yedoma soils may release 5.8–10.6 Tg of DOC⋅y−1 by 2100 across Siberia and Alaska (Table 2), which is equivalent to 19–26% of all DOC currently exported by Arctic rivers (48, 49). The majority of this Yedoma permafrost-derived DOC will not reach the main stem of major Arctic rivers and export to the Arctic Ocean, given the high biodegradability we measured here. This is consistent with research on streams and rivers that drain Yedoma soils in Siberia demonstrating the loss of an aged DOC permafrost signature downstream, with almost no trace present in the river’s main stem (12, 13).
An important consequence of this rapid mineralization is that it creates a blind spot with regard to assessing total DOC turnover to CO2. Recent work suggests that as much as 70–95% of DOC in Arctic inland waters may be processed via photochemical oxidation, with microbial processes playing a much smaller role (50). This likely misses the microbial removal of highly biodegradable DOC in soil pore waters that does not persist long enough to be measurable in receiving waters. The majority of the DOC leached from Yedoma permafrost soils is nonabsorbing within UV wavelengths and has high relative contributions of LMW organic acids (as evidenced by low SUVA254 values, Fig. 1A). Therefore, microbial processing of DOC in Arctic systems is indeed highly important, and large predominantly nonchromophoric DOC sources such as Yedoma permafrost soils (this study) and some fresh vegetation leachates (32) are primarily mineralized through microbial processes rather than photochemical processes. We assert that the continuum of DOC oxidation starts immediately upon leaching from source material, be it vegetation, litter, or soil, and that it is important to recognize the inherent influence of processing that occurs before export to surface waters.
Permafrost thaw increases flow pathlengths, water residence times, and microbial mineralization of DOC to DIC in the soil active layer (14). Incorporating data from this study, and related studies, we present an updated conceptual model for the mobilization of permafrost-derived DOC (Fig. 2) where highly biodegradable permafrost DOC is respired to the atmosphere directly from soils or headwater streams, and more stable DOC is exported downstream or oxidized by photochemical (50) or microbial (30) processes. The majority of mineralized permafrost DOC is emitted to the atmosphere as CO2 high in the watershed, after relatively short residence times. Generally, low-order streams exhibit higher concentrations of CO2 and emit CO2 more rapidly than larger rivers (19, 51).
Fig. 2.
Conceptual model for the mobilization and mineralization of permafrost-derived DOC.
Pore and headwater processing of permafrost-derived DOC poses a challenge for accurately quantifying and predicting the flux of permafrost C to the atmosphere upon thaw. Headwater ecosystems in the Arctic are generally inaccessible, are chemically and physically heterogeneous, and are distributed widely across the landscape. Furthermore, headwater ecosystems respond rapidly to hydrologic events and extremely detailed temporal studies are required to truly capture carbon fluxes from these systems (52, 53). New research into catchment-scale carbon cycling, including the integration of aquatic and terrestrial budgets, will help inform methods and target these important interface zones. Specifically, we suggest prioritizing headwater ecosystem sampling for detection of aged (14C-depleted) DOC and DIC. Additionally, more information is needed as to the ubiquity of highly biodegradable LMW organic acids in permafrost soils across the Arctic, as well as the conditions that produce and concentrate them. Such work will provide crucial information as to how different permafrost-dominated areas will respond to ongoing climate change in northern ecosystems.
Materials and Methods
Study Site.
Frozen permafrost soils were collected from a newly excavated extension of the Fox Permafrost Tunnel, which is operated by the Cold Regions Research and Engineering Laboratory in Fairbanks, Alaska. In total, 250 kg of frozen soil was collected, homogenized, and split into five different samples. Homogenization consisted of loosely mixing roughly 12 kg of frozen permafrost from different shipping containers by hand, removing any large woody debris that would impact the DOC yield ratios, and ensuring general uniformity before thaw.
Leachate Preparation.
For each of the five analytical replicates, 600 g of frozen Yedoma permafrost soil was leached into 2.8 L of deionized water (>18 MΩ⋅cm) for 24 h at 2 °C, with intermittent agitation. After 24 h, a 40-mL sample of leachate was collected and filtered with a 1.6-μm prerinsed Whatman GF/A 13-mm syringe filter for use as the inoculant. The remainder of the leachate was decanted through a sieve and centrifuged at 8,000 rpm Sorvall Superspeed RC2-B Automatic Refrigerated Centrifuge, National Research Project, US Geological Survey, Boulder, CO for 25 min at low temperature (∼10 °C) to remove fine particulates and suspended clays. Two liters of the centrifuged leachate were then filtered through a 0.45-μm prerinsed GeoTech capsule filter.
Experimental Setup.
The bioreactor consisted of a gas-tight, 12-L stainless-steel container with inlet and outlet ports (Fig. S1). A Masterflex peristaltic pump drew water from the bioreactor and pumped it through a gas-tight exterior flow-through system at a rate of 1.1 L⋅min−1 to ensure continuous mixing (complete turnover time, 10 min). The flow-through system was equipped with a YSI Professional Plus Multimeter, and a Liqui-Cel MiniModule membrane gas equilibrator connected to a PP Systems EGM-4 CO2 Analyzer for continuous in-line measurements of dissolved oxygen, pH, temperature, conductivity, and dissolved CO2. Discrete water samples were collected from an in-line sample port with a three-way valve. A system blank was performed, and no DOC was found to have leached from the incubation system. Five replicate incubations were carried out under identical conditions. In each experiment, 2 L of filtered leachate were added to the bioreactor and were diluted to a total volume of 11 L with carbon-free MilliQ water (>18 MΩ⋅cm). The leachate was inoculated with the 40 mL of 1.6-μm-filtered water, and mixed by shaking for 1 min. Incubations were run at 20 °C. Initial water samples (t0) were collected, and the bioreactor was sealed and connected to the flow-through system. Fifty-milliliter water samples were collected at 1, 3, 6, and 12 h followed by every 12 h for a total of 21 samples over 204 h. Each time a water sample was collected, 50 mL of CO2-free air was added to the headspace to maintain constant pressure. Data from the CO2 Analyzer and the YSI were collected continuously for the duration of the experiment. Discrete water samples were immediately filtered with a 0.45-μm syringe filter and analyzed immediately for UV absorbance and fluorescence (t0, t70, and t200). Sample splits were preserved with 30 μL of H3PO4 for DOC measurements, stored refrigerated in the dark until analysis, and frozen for LMW organic acids analysis (t0 and t200).
Analyses.
Soil radiocarbon ages were measured on homogenized, oven-dried (50 °C) samples, and DOC radiocarbon ages were measured on initial filtered soil leachates. 14C-DOC samples were freeze-dried and subsequently acidified under HCl vapors to remove inorganic carbon before combustion. All radiocarbon samples were analyzed at the Accelerator Mass Spectrometry Facility at the Woods Hole Oceanographic Institute on triplicate samples. Soil organic carbon content was measured using standard techniques on an elemental analyzer (Costech ECS 4010) (54). All incubation experiments were carried out at the US Geological Survey (USGS) National Research Program laboratories in Boulder, Colorado. UV and visible light absorbance were measured using a Hewlett-Packard model 8453 photodiode array spectrophotometer (wavelength, 200–800 nm) and a 1-cm pathlength quartz cell. Change of UV254 absorbance over time was tested using a paired t test of significance. DOC samples were analyzed using an O.I. 700 TOC Analyzer via the platinum-catalyzed persulfate wet oxidation method (55). Three-dimensional fluorescence EEMs were generated for t0, t70, and t200 time points using unacidified water extracted via the discrete sampling port and analyzed on a Jobin-Yvon Horiba Fluoromax 3 fluorometer. Acetate, butyrate, and formate concentrations were analyzed on a Dioxnex DX-600 Ion Chromatograph using established protocols (e.g., ref. 34). Formate was not detected in any samples.
Carbon Balance Calculations.
We tracked the speciation of carbon among the organic and inorganic pools using our time series measurements of DOC concentration, dissolved CO2 concentration, pH, temperature, and system volumes. Concentrations of dissolved gaseous CO2, bicarbonate (HCO3−), and headspace CO2 were derived from the CO2 concentration measured in the aqueous phase, pH, and the volumes of both the water and the headspace. Total DIC in the system is the sum of the dissolved and gaseous inorganic C constituents. Because the water and headspace were assumed to be in equilibrium over the course of the incubation, any CO2 exchanged to the headspace originated as dissolved gaseous CO2 or bicarbonate. For this reason, calculated headspace CO2 concentrations were included in the reported total DIC. Particulate organic C (POC) (i.e., microbial biomass) concentrations were assumed to be negligible at the beginning of each experiment because the leachates were filtered at 0.45 μm. At the end of the experiments, final POC concentrations were estimated by scraping and suspending microbial biomass formed as a biofilm on the bioreactor walls, vigorously shaking the bioreactor, and filtering the water through preweighed 0.45-μm precombusted glass fiber filters. The filters were air-dried and reweighed, and the difference was attributed to microbial biomass. We assumed that 50% of the microbial biomass was C (56). DOC adsorption to biofilms was assumed to play a minor role in overall DOC loss (or POC production) given the lack of observed flocculation, the absence of a substrate (i.e., soil particles) for adsorption, the simultaneous increase in DIC, and that the DOC lost was composed largely of biolabile and monovalent LMW organic acids, which are known to weakly adsorb to a soil’s solid phase (57). However, any small fraction of DOC lost to adsorption to biofilms would mean that our estimated BGEs are somewhat elevated, because we assumed all DOC loss was due to bacterial utilization.
Acknowledgments
We thank Kevin Bjella (US Army Engineer Research and Development Center, Cold Regions Research and Engineering Laboratory) and Mikhail Kanevskiy (University of Alaska–Fairbanks) for allowing and assisting with sample collection from the permafrost tunnel; Mark Dornblaser [US Geological Survey (USGS)] for technical assistance and project design feedback; Brett Uhle, Blaine McCleskey, and Kenna Butler (USGS) for analytical assistance; and Brett Poulin (University of Colorado–Boulder; USGS) for assistance with data analysis and review of an early draft of the manuscript. This work was funded by the National Research Program, USGS, and National Science Foundation Grants PLR-1500169/ANT-1203885 (to R.G.M.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511705112/-/DCSupplemental.
References
- 1.Tarnocai C, et al. Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochem Cycles. 2009;23(2):GB2023. [Google Scholar]
- 2.Schuur EAG, et al. Climate change and the permafrost carbon feedback. Nature. 2015;520(7546):171–179. doi: 10.1038/nature14338. [DOI] [PubMed] [Google Scholar]
- 3.Strauss J, et al. The deep permafrost carbon pool of the Yedoma region in Siberia and Alaska. Geophys Res Lett. 2013;40(23):6165–6170. doi: 10.1002/2013GL058088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimov SA, Schuur EAG, Chapin FS., 3rd Climate change. Permafrost and the global carbon budget. Science. 2006;312(5780):1612–1613. doi: 10.1126/science.1128908. [DOI] [PubMed] [Google Scholar]
- 5.Schuur EAG, et al. Vulnerability of permafrost carbon to climate change: Implications for the global carbon cycle. Bioscience. 2008;58(8):701–714. [Google Scholar]
- 6.Schuur EAG, et al. The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature. 2009;459(7246):556–559. doi: 10.1038/nature08031. [DOI] [PubMed] [Google Scholar]
- 7.Osterkamp TE, Romanovsky VE. Evidence for warming and thawing of discontinuous permafrost in Alaska. Permafrost Periglac Process. 1999;10:17–37. [Google Scholar]
- 8.Guo L, Ping C, Macdonald RW. Mobilization pathways of organic carbon from permafrost to Arctic rivers in a changing climate. Geophys Res Lett. 2007;34(13):L13603. [Google Scholar]
- 9.Marín-Spiotta E, et al. Paradigm shifts in soil organic matter research affect interpretations of aquatic carbon cycling: Transcending disciplinary and ecosystem boundaries. Biogeochemistry. 2014;117(2-3):279–297. [Google Scholar]
- 10.Dutta K, et al. Potential carbon release from permafrost soils of Northeastern Siberia. Glob Change Biol. 2006;12:2336–2351. [Google Scholar]
- 11.Vonk JE, et al. High biolability of ancient permafrost carbon upon thaw. Geophys Res Lett. 2013;40:2689–2693. [Google Scholar]
- 12.Spencer RGM, et al. Detecting the signature of permafrost thaw in Arctic rivers. Geophys Res Lett. 2015;42(8):2830–2835. [Google Scholar]
- 13.Mann PJ, et al. Utilization of ancient permafrost carbon in headwaters of Arctic fluvial networks. Nat Commun. 2015;6:7856. doi: 10.1038/ncomms8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Striegl RG, et al. A decrease in discharge-normalized DOC export by the Yukon River during summer through autumn. Geophys Res Lett. 2005;32(21):L21413. [Google Scholar]
- 15.Vonk JE, Gustafsson O. Permafrost-carbon complexities. Nat Geosci. 2013;6:675–676. [Google Scholar]
- 16.Schmidt MW, et al. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478(7367):49–56. doi: 10.1038/nature10386. [DOI] [PubMed] [Google Scholar]
- 17.Battin TJ, et al. The boundless carbon cycle. Nat Geosci. 2009;2:598–600. [Google Scholar]
- 18.Aufdenkampe AK, et al. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front Ecol Environ. 2011;9:53–60. [Google Scholar]
- 19.Raymond PA, et al. Global carbon dioxide emissions from inland waters. Nature. 2013;503(7476):355–359. doi: 10.1038/nature12760. [DOI] [PubMed] [Google Scholar]
- 20.Striegl RG, Dornblaser MM, McDonald CP, Rover JR, Stets EG. Carbon dioxide and methane emissions from the Yukon River system. Global Biogeochem Cycles. 2012;26(4):GB0E05. [Google Scholar]
- 21.Abbott BW, et al. Elevated dissolved organic carbon biodegradability from thawing and collapsing permafrost. J Geophys Res Biogeosci. 2014;119:2049–2063. [Google Scholar]
- 22.Sondergaard M, et al. Dynamics of biodegradable DOC produced by freshwater plankton communities. Aquat Microb Ecol. 2000;23:73–83. [Google Scholar]
- 23.Roehm CL, et al. Bioavailability of terrestrial organic carbon to lake bacteria: The case of a degrading subarctic permafrost mire complex. J Geophys Res Biogeosci. 2009;114(G3):G03006. [Google Scholar]
- 24.Irons JG, Oswood MW. Seasonal temperature patterns in an Arctic and two subarctic Alaskan (USA) headwater streams. Hydrobiologia. 1992;237(3):147–157. [Google Scholar]
- 25.Whitefield J, Winsor P, McClelland J, Menemenlis D. A new river discharge and river temperature climatology data set for the pan-Arctic region. Ocean Model. 2015;88:1–15. [Google Scholar]
- 26.Del Giorgio PA, Cole J. Bacterial growth efficiency in natural aquatic systems. Annu Rev Ecol Syst. 1998;29:503–541. [Google Scholar]
- 27.Kaplan LA, Newbold JD. Measurement of streamwater biodegradable dissolved organic carbon with a plug-flow bioreactor. Water Res. 1995;29(12):2696–2706. [Google Scholar]
- 28.Frias J, Ribas F, Lucena F. Comparison of methods for the measurement of biodegradable organic carbon and assimilable organic carbon in water. Water Res. 1995;29(12):2785–2788. [Google Scholar]
- 29.Choi Y, Johnson K, Hayes DF, Sung N, Xu H. Dissolved organic matter and nitrogen removal by advanced aerated submerged bio-film reactor. Desalination. 2010;250(1):368–372. [Google Scholar]
- 30.Wickland KP, et al. Biodegradability of dissolved organic carbon in the Yukon River and its tributaries: Seasonality and importance of inorganic nitrogen. Global Biogeochem Cycles. 2012;26(4):GB0E03. [Google Scholar]
- 31.Davidson EA, Janssens IA. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440(7081):165–173. doi: 10.1038/nature04514. [DOI] [PubMed] [Google Scholar]
- 32.Wickland KP, Neff JC, Aiken GR. Dissolved organic carbon in Alaskan boreal forest: Sources, chemical characteristics, and biodegradability. Ecosystems (N Y) 2007;10:1323–1340. [Google Scholar]
- 33.Wang ZA, et al. Inorganic carbon speciation and fluxes in the Congo River. Geophys Res Lett. 2013;40:1–6. [Google Scholar]
- 34.Strauss J, et al. Organic-matter quality of deep permafrost carbon—a study from Arctic Siberia. Biogeosciences. 2014;12:2227–2245. [Google Scholar]
- 35.Smith MR, Mah RA. Acetate as sole carbon and energy source for growth of Methanosarcina strain 227. Appl Environ Microbiol. 1980;39(5):993–999. doi: 10.1128/aem.39.5.993-999.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieth A, et al. Water extraction of coals—potential for estimating low molecular weight organic acids as carbon feedstock for the deep terrestrial biosphere. Org Geochem. 2008;39(8):985–991. [Google Scholar]
- 37.Berggren M, Laudon H, Haei M, Ström L, Jansson M. Efficient aquatic bacterial metabolism of dissolved low-molecular-weight compounds from terrestrial sources. ISME J. 2010;4(3):408–416. doi: 10.1038/ismej.2009.120. [DOI] [PubMed] [Google Scholar]
- 38.van Hees PAW, et al. The carbon we do not see—the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: A review. Soil Biol Biochem. 2005;37:1–13. [Google Scholar]
- 39.Hädrich A, Heuer VB, Herrmann M, Hinrichs KU, Küsel K. Origin and fate of acetate in an acidic fen. FEMS Microbiol Ecol. 2012;81(2):339–354. doi: 10.1111/j.1574-6941.2012.01352.x. [DOI] [PubMed] [Google Scholar]
- 40.Bridgham SD, Cadillo-Quiroz H, Keller JK, Zhuang Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob Change Biol. 2013;19(5):1325–1346. doi: 10.1111/gcb.12131. [DOI] [PubMed] [Google Scholar]
- 41.Duddleston KN, et al. Anaerobic microbial biogeochemistry in a northern bog: Acetate as a dominant metabolic end product. Global Biogeochem Cycles. 2002;16:11-1–11-9. [Google Scholar]
- 42.Spencer RGM, et al. Dissolved organic carbon and chromophoric dissolved organic matter properties of rivers in the USA. J Geophys Res. 2012;117(G3):G03001. [Google Scholar]
- 43.Weishaar JL, et al. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol. 2003;37(20):4702–4708. doi: 10.1021/es030360x. [DOI] [PubMed] [Google Scholar]
- 44.Fellman JB, Hood E, Spencer RGM. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol Oceanogr. 2010;55(6):2452–2462. [Google Scholar]
- 45.Raymond PA, et al. Flux and age of dissolved organic carbon exported to the Arctic Ocean: A carbon isotopic study of the five largest Arctic rivers. Global Biogeochem Cycles. 2007;21(4):GB4011. [Google Scholar]
- 46.Striegl RG, Dornblaser MM, Aiken GR, Wickland KP, Raymond PA. Carbon export and cycling by the Yukon, Tanana, and Porcupine rivers, Alaska, 2001–2005. Water Resour Res. 2007;43(2):W02411. [Google Scholar]
- 47.Aiken GR, Spencer RGM, Striegl RG, Schuster PF, Raymond PA. Influences of glacier melt and permafrost thaw on the age of dissolved organic carbon in the Yukon River basin. Global Biogeochem Cycles. 2014;28(5):525–537. [Google Scholar]
- 48.Spencer RGM, et al. Utilizing chromophoric dissolved organic matter measurements to derive export and reactivity of dissolved organic carbon exported to the Arctic Ocean: A case study of the Yukon River, Alaska. Geophys Res Lett. 2009;36(6) [Google Scholar]
- 49.Holmes RM, et al. Seasonal and annual fluxes of nutrients and organic matter from large rivers to the Arctic Ocean and surrounding seas. Estuaries Coasts. 2012;35(2):369–382. [Google Scholar]
- 50.Cory RM, Ward CP, Crump BC, Kling GW. Carbon cycle. Sunlight controls water column processing of carbon in Arctic fresh waters. Science. 2014;345(6199):925–928. doi: 10.1126/science.1253119. [DOI] [PubMed] [Google Scholar]
- 51.Butman D, Raymond PA. Significant efflux of carbon dioxide from streams and rivers in the United States. Nat Geosci. 2011;4:839–842. [Google Scholar]
- 52.Andrews DM, et al. Hot spots and hot moments of dissolved organic carbon export and soil organic carbon storage in the Shale Hills catchment. Vadose Zone J. 2011;10:943–954. [Google Scholar]
- 53.Dornblaser MM, Striegl RG. Switching predominance of organic versus inorganic carbon exports from an intermediate-size subarctic watershed. Geophys Res Lett. 2015;42:386–394. [Google Scholar]
- 54.Spencer RGM, et al. An initial investigation into the organic matter biogeochemistry of the Congo River. Geochim Cosmochim Acta. 2012;84:614–627. [Google Scholar]
- 55.Aiken GR, et al. Isolation of hydrophilic organic acids from water using nonionic macroporous resins. Org Geochem. 1992;18:567–573. [Google Scholar]
- 56.Bratbak G, Dundas I. Bacterial dry matter content and biomass estimations. Appl Environ Microbiol. 1984;48(4):755–757. doi: 10.1128/aem.48.4.755-757.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones DL, Dennis PG, Owen AG, van Hees PAW. Organic acid behavior in soils—misconceptions and knowledge gaps. Plant Soil. 2003;248:31–41. [Google Scholar]