Abstract
Background:
Glioblastoma (GBM) is commonly diagnosed in patients older than 60 years, but the treatment protocols are mostly based on trials in patients aged up to 70 years. These lead to little consensus and to an absence of protocols regarding the standard treatments. The objective of this study is to analyze the prognostic factors, treatment efficacy, and adverse events in a cohort of elderly patients.
Methods:
A retrospective observational study of all patients aged ≥65 with histologically confirmed GBM followed at Centro Hospitalar S. João between 2005 and 2013. Demographic, clinical, radiographic, treatment, and outcome data were evaluated. Univariate and multivariate analyses were performed.
Results:
A total of 126 patients were reviewed. Median progression-free survival was 5 months (95% confidence interval [CI], 4.138 to 5.862 months). Median overall survival (OS) was 8 months (95% CI, 5.950 to 10.050 months). Univariate analysis showed the statistically significant associations between the higher OS and age <70 (P = 0.046), Karnofsky performance status ≥70 (P = 0.001), single lesions (P = 0.007), lesions affecting one lobe (P = 0.007), total resection (P = 0.048), and Charlson age-comorbidity index ≤5. Multivariate analysis identified the completion of 60 Gy radiotherapy and completion of 6 or more cycles of temozolomide chemotherapy as independent prognostic factors positively correlated with increased survival.
Conclusions:
Maximal resection and radiochemotherapy treatment completion are associated with longer OS, and age alone should not preclude elderly patients from receiving surgery and adjuvant treatment. However, only a few patients were able to finish the proposed treatments. Poor performance and high comorbidity index status might compromise the benefit of treatment aggressiveness and must be considered in therapeutic decision.
Keywords: Chemotherapy, elderly, glioblastoma, oncology, radiation therapy, resection
INTRODUCTION
Glioblastoma (GBM) is the most frequent primary malignant brain tumor, representing 15.6% of all primary brain tumors and 45.2% of malignant tumors, with an incidence of 3.19/100, 000 persons/year.[25] It is primarily diagnosed at older ages, with higher rates between 75 and 84 years old. GBM is 1.57 times more frequent in males. The relative survival estimates for GBM are low. Despite the advances in surgery, radiation, and chemotherapy, the prognosis remains dismal, with the 5-year overall survival (OS) being <5%.[25] Current standard treatment consists of maximal safe resection followed by radiotherapy (RT) and concomitant and adjuvant chemotherapy with temozolomide (TMZ).[32] This treatment protocol was based on a randomized clinical trial, in which patients were aged 70 years or younger. In fact, the age has been recognized as a poor prognostic factor in patients with GBM.[4,31] Furthermore, the treatment of elderly patients appears to be associated with greater toxicity and reduced efficacy as compared to younger patients.[18] In general, the elderly patients receive less aggressive treatments and are under-represented in the clinical trials of new cancer treatments.[18,28,37] There is no consensus regarding the treatment of elderly patients with newly diagnosed GBM. The authors proposed to retrospectively review a cohort of patients diagnosed with GBM aged 65 or older and analyze the prognostic factors, treatment efficacy, and adverse events in this population subgroup, with particular emphasis on functional status and comorbidities data.
MATERIALS AND METHODS
Study design and study population
This is a retrospective observational study. All patients aged 65 or older with histologically confirmed GBM followed at Centro Hospitalar S. João between 2005 and 2013 were eligible. The follow-up took place until March 2015. A total of 126 patients comprised the study population.
The information was collected from each patient's clinical chart, using both electronic and paper records.
This study was approved by the local Institutional Ethics Committee.
Clinical data
For each patient, the clinical data included: (1) Demographics, such as gender and age at diagnosis; (2) comorbidities as assessed by Charlson age-comorbidity index (CACI);[8] (3) Karnofsky performance status (KPS); (4) tumor characteristics: Location, corpus callosum invasion, affected hemispheres, and the involvement of eloquent areas; radiographic pattern (single lesion vs. multifocal/multicentric lesions); (5) clinical manifestations at diagnosis, namely epilepsy, intracranial hypertension, headache, focal deficits, and brainstem/cerebellum changes; (6) extent of resection, number of surgeries, and postoperative complications; (7) treatment data, specifically radiation therapy dose and duration, type of chemotherapy, number of chemotherapy cycles, and adverse events; (8) date and type of progression if present; and (9) date of death or date of the last visit.
Tumor characteristics were obtained by the analysis of the preoperative T1- and T2- magnetic resonance imaging (MRI) weighted images. Multifocal tumors were defined as the result of growth or dissemination via established routes. Multicentric lesions were considered to be those that represent widely separated lesions and whose origin cannot be explained following common pathways.[26]
The following areas were regarded as eloquent: Primary motor and sensory cortex, left fronto-temporo-parietal opercular area, the primary visual cortex, basal ganglia, thalamus, and insula.[9]
Resection grade was determined by the analysis of the postoperative T1- and T2- MRI weighted images performed within 72h after the procedure and further classification according to the following criteria: (1) Total resection - absence of enhancement; (2) subtotal resection - linear enhancement along the resection margins or nodule <5 mm; and (3) partial resection - none of the above.
Concerning radiation therapy, the patients were divided into three groups: (1) Stupp - patients treated with RT plus concomitant and adjuvant TMZ, according to the Stupp protocol of chemoradiotherapy;[31] (2) standard RT - patients undergoing radiation therapy alone, aimed to achieve 60 Gy; and (3) hypofractionated RT - patients submitted to radiation treatment in which the total dose of radiation (60 Gy) was divided into larger and less frequent doses. The last two regimens were reserved for patients with the worse overall condition, namely worse KPS, and high comorbidities, according to multidisciplinary neuro-oncological team meeting decision.
Among the patients who received chemotherapy, the first-line agents were: (1) TMZ; (2) carmustine; and (3) TMZ plus bevacizumab. The second-line agents were: (1) Bevacizumab plus irinotecan; (2) procarbazine, lomustine, and vincristine (PCV); and (3) continuous TMZ. The third-line agents were: (1) PCV; and (2) continuous TMZ.
Side effects, namely anemia, leukopenia, lymphopenia, neutropenia, thrombocytopenia, febrile neutropenia, infection, hypertension, weakness, anorexia, oral mucositis, nausea, vomiting, diarrhea, hyperglycemia, cerebral hemorrhage, epistaxis, and thromboembolic events were analyzed and graded according to the National Cancer Institute (US) Common Terminology Criteria for Adverse Events, version 4.0.[24]
Disease progression was evaluated according to Response Assessment in Neuro-Oncology criteria.[35] Progression was divided into three types: (1) Focal; (2) multifocal; and (3) infiltrative without gadolinium enhancement. In the remaining, the progression type considered was undetermined.
Statistical methods
Statistical analysis was performed with SPSS version 22 (IBM Corp., Armonk, NY, USA). The continuous variables were presented as median and range, and categorical variables as absolute and relative percentages.
OS was defined as the time interval from the date of surgery to death from any cause or last contact.
Progression-free survival (PFS) was defined as the time interval from the date of surgery to the date of progression.
The patients still alive were right-censored at last follow-up date. The Kaplan–Meier method was performed to calculate OS and PFS, and univariate and multivariate Cox proportional regression analyses were used to establish the independent prognostic factors of GBM in the elderly.
In multivariate analysis, variables with clinical relevance that showed significance at P < 0.20 in univariate analysis were included. A P < 0.05 was used as the criteria for statistical significance in the multivariate model. The results are expressed as hazard ratios (HRs) with 95% confidence interval (CI).
RESULTS
Patient demographics, presenting symptoms, and tumor characteristics
Data from 126 patients was available [Table 1]. There were 77 men and 49 women, with a median age of 71.0 years old (range 65–80). Comorbidities were common (72.2%), including hypertension in 57 patients (45.2%), diabetes mellitus in 27 patients (21.4%), and dyslipidemia in 26 patients (20.6). Evaluation by the CACI was performed as presented in Table 1.
Table 1.
Patients characteristics
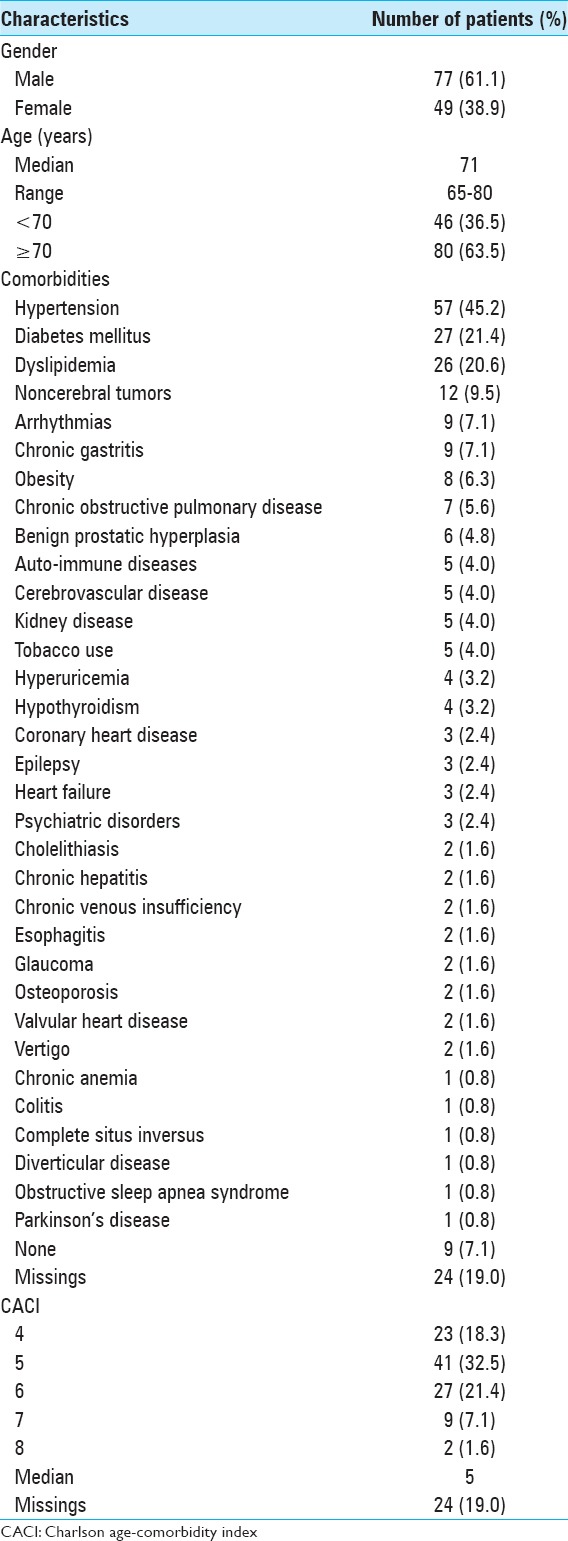
Tumor characteristics are summarized in Table 2. Lesions were located most frequently in the frontal lobe, in 40 patients (31.7%). Forty-three patients had affected two lobes (34.1%), and 21 patients had corpus callosum invasion (16.7%). The disease affected the left hemisphere in 61 patients (48.4%). Multifocal lesions were found in 16 patients (12.7%), and multicentric lesions were found in 12 patients (9.5%). Forty-six patients had tumors affecting eloquent areas (36.5%). Two patients (1.6%) had secondary GBM.
Table 2.
Tumor characteristics
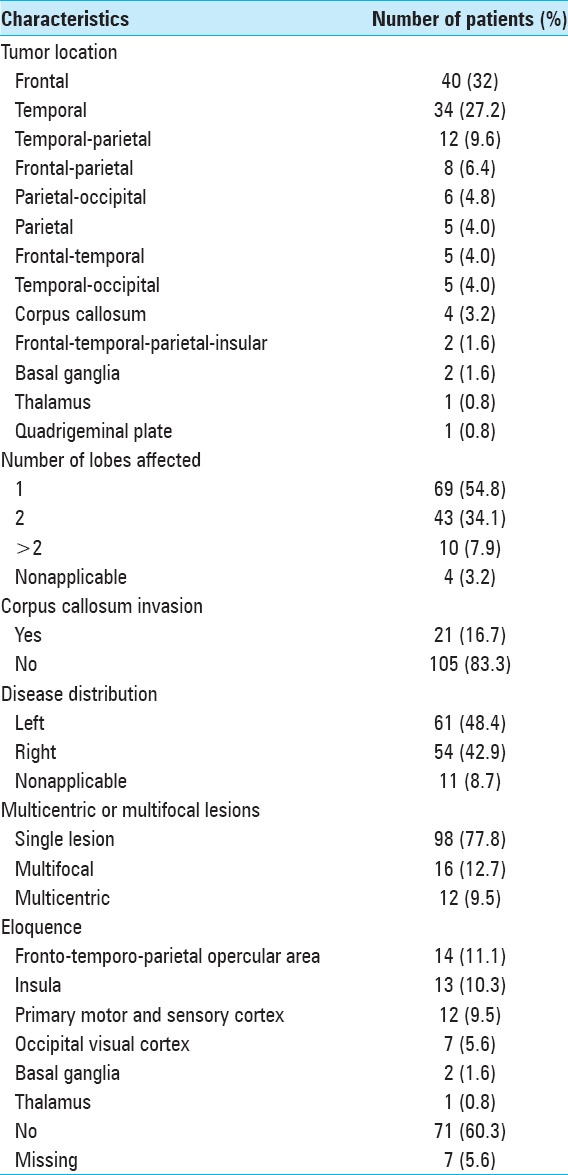
In terms of performance status [Table 3], the median KPS was 80 (range 20–100), with 85 (67.5%) patients having KPS equal or >70.
Table 3.
Clinical data
The most common clinical manifestation was motor deficit, in 61 patients (48.4%), followed by behavioral changes in 49 patients (38.9%), language deficits in 37 patients (29.4%), headache in 22 patients (17.5%), epilepsy in 18 patients (14.3%), and visual deficits in 10 patients (7.9%).
Surgical procedures and outcomes
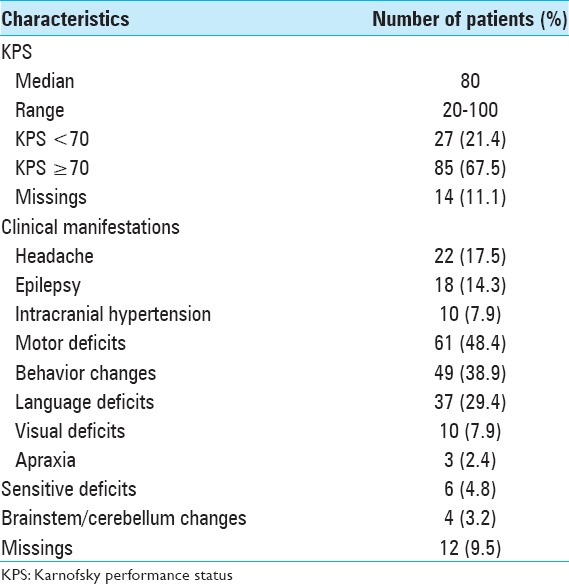
The initial surgical procedure [Table 4] was stereotaxic biopsy in 40 patients (31.7%) and tumor resection in 85 patients (67.5%). Nineteen patients (15.1%) underwent total resection, 28 patients (22.2%) underwent subtotal resection, and 34 patients (27%) underwent partial resection. Fourteen patients (11.1%) were submitted to a second surgery.
Table 4.
Information regarding the surgical procedure
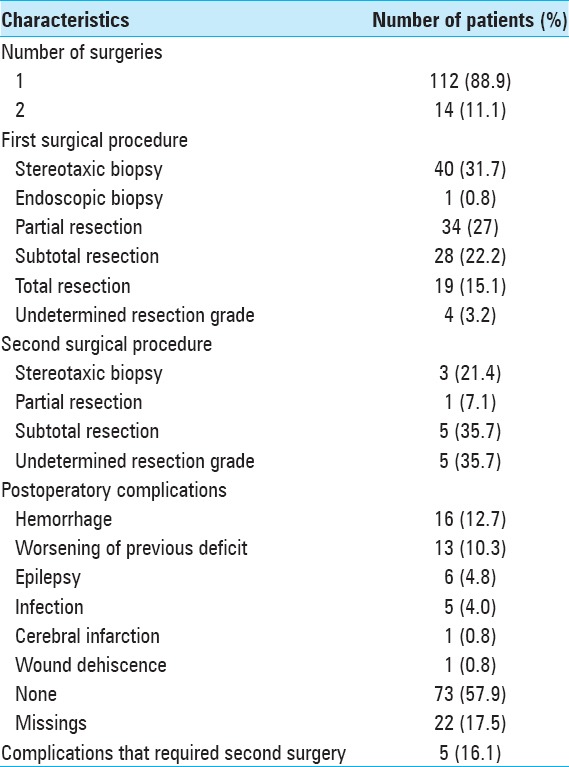
After the surgical procedure, 31 patients (24.6%) had complications. Sixteen patients (12.7%) had hemorrhage, and 13 patients (10.3%) suffered neurological worsening. Five patients (16.1%) had complications that required a second surgery.
Adjuvant treatment and outcomes
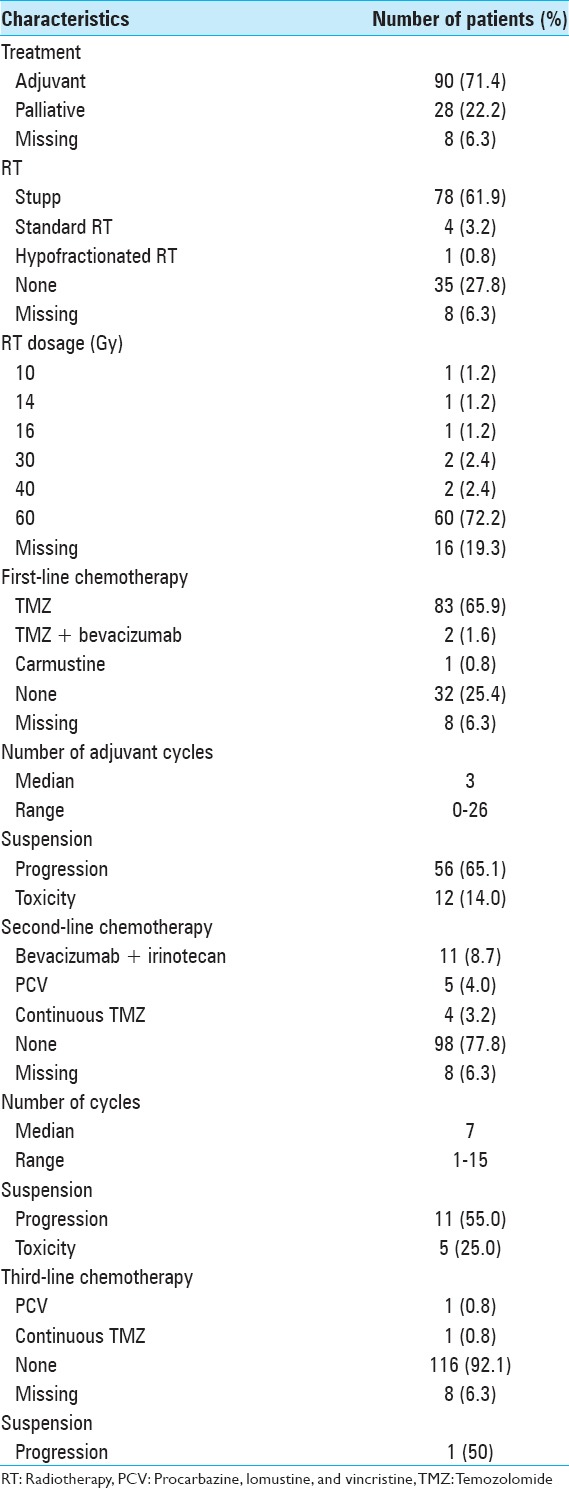
Ninety patients (71.4%) underwent the adjuvant therapy [Table 5]. Of these, 78 (61.9%) initiated Stupp protocol of radiochemotherapy. Most patients received 60 Gy radiations (72.2%). Twenty-five patients (32.1%) completed the Stupp protocol (6 or more cycles). Fifty-six patients (65.1%) suspended the first-line chemotherapy for progression, and 12 patients (14.0%) suspended it for toxicity. Twenty patients (15.9%) initiated the second-line chemotherapy, most of them bevacizumab plus irinotecan (8.7%).
Table 5.
Adjuvant treatment details
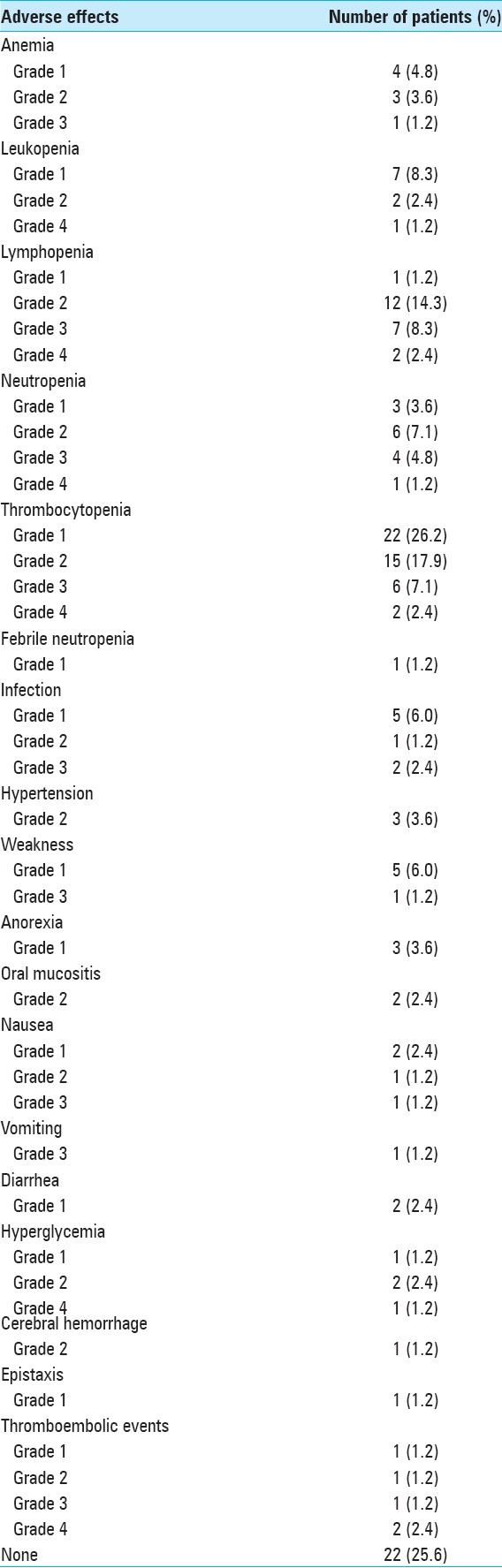
Adverse effects of chemotherapy [Table 6] were common (72.1%). Grade 3 or 4 complications occurred in 25 patients of those receiving chemotherapy (29.1%). The most common adverse effects found were Grade 1 (26.2%) and Grade 2 (17.9%) thrombocytopenia, Grade 2 lymphopenia (14.3%), and Grade 1 leukopenia (8.3%).
Table 6.
Chemotherapy adverse effects
Twenty-eight patients (22.2%) received palliative care only without any active tumor treatment.
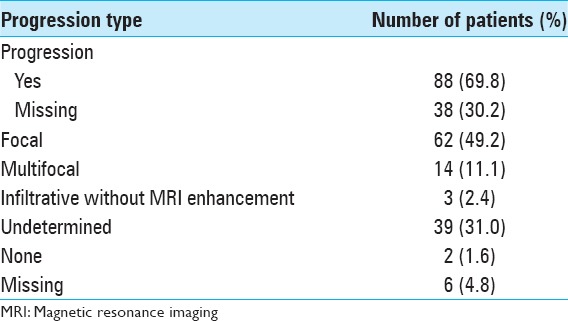
Disease progression [Table 7] was found in 118 patients (93.7%), mainly focal (49.2%). A diffuse infiltrative pattern without gadolinium enhancement was observed in 3 patients (2.4%), and in 39 patients (31.0%) progression type was not possible to assess, being classified as undetermined.
Table 7.
Disease progression
Progression-free and overall survival
At the time of analysis, 122 patients (96.8%) had died, 1 (0.8%) was lost to follow-up, and 3 (2.4%) were still alive at the end of the study.
PFS for all patients is shown in Figure 1. The median PFS was 5 months (95% CI, 4.138 to 5.862 months).
Figure 1.
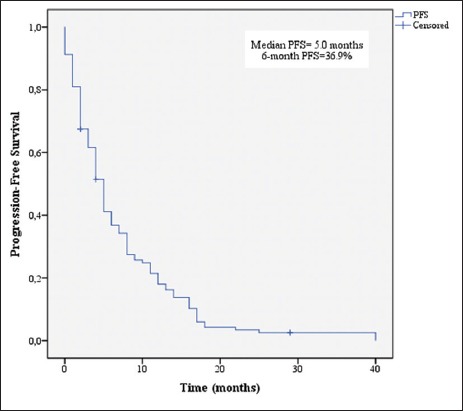
Kaplan–Meier estimates of progression-free survival
OS for all patients is shown in Figure 2. The median OS was 8 months (95% CI, 5.950 to 10.050 months). The 1-year and 2-year survival rates were 36.9% and 12.0%, respectively.
Figure 2.
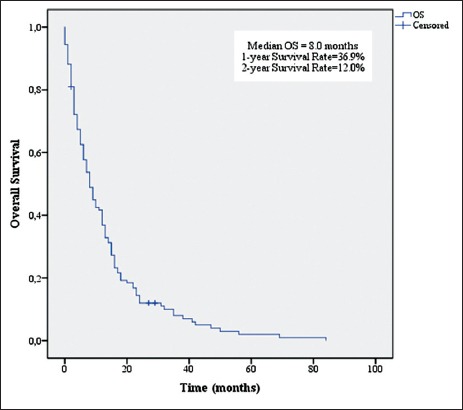
Kaplan–Meier estimates of overall survival
A univariate analysis was performed to assess the possible predictive factors of OS, namely patients’ age, KPS, CACI, frontal versus nonfrontal location, number of lobes affected, radiographic pattern of the tumor, eloquence, surgical procedure, surgery complications, secondary treatment, treatment completion, and adverse effects.
Age ≤70 years (P = 0.046), KPS ≤70 (P = 0.001), CACI (P = 0.004), total resection (P = 0.048), completion of 60 Gy of RT (P = 0.001), and completion of 6 or more cycles of TMZ (P = 0.001) were associated with increased survival [Figures 3-8].
Figure 3.
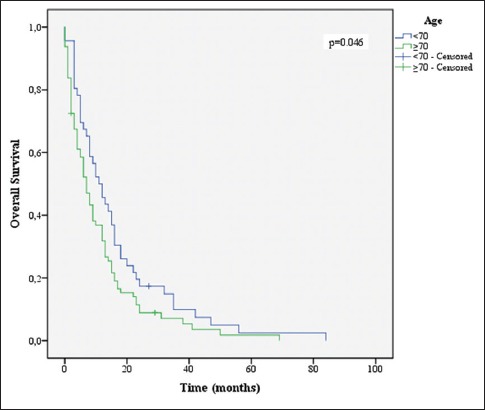
Overall survival stratified by age groups
Figure 8.
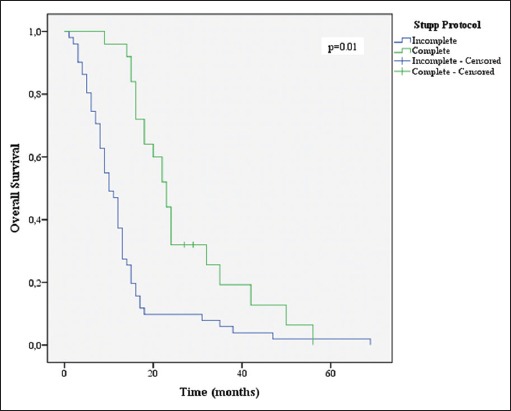
Overall survival stratified by Stupp protocol groups
Figure 4.
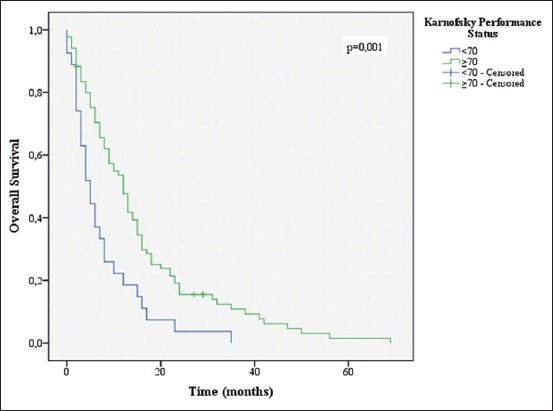
Overall survival stratified by Karnofsky performance status groups
Figure 5.
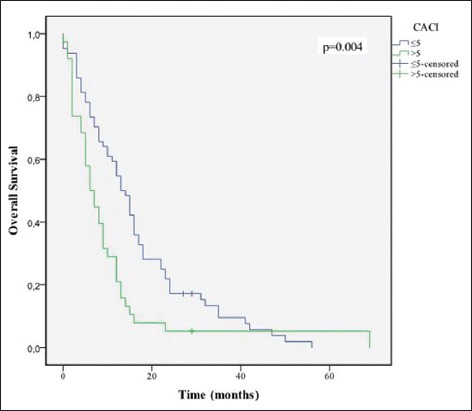
Overall survival stratified by Charlson age-comorbidity index groups
Figure 6.
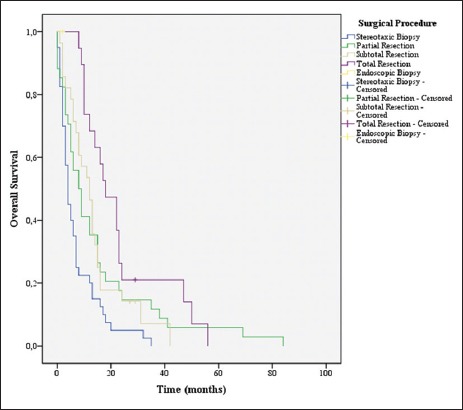
Overall survival stratified by surgical procedure groups
Figure 7.
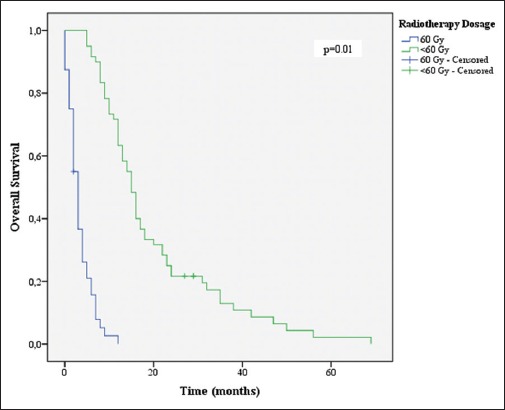
Overall survival stratified by radiotherapy dosage
Lesions affecting only one lobe and single lesions were also associated with increased survival (P = 0.007 for both).
On the opposite, frontal versus nonfrontal location, involvement of eloquent areas, presence of surgical complications, and Grade 3 or 4 chemotherapy adverse effects did not attain statistical significance (P = 0.535, P = 0.834, P = 0.426, and P = 0.624, respectively).
A comparison was made between the groups’ biopsy plus adjuvant treatment, biopsy plus palliative treatment, resection plus adjuvant treatment, and resection plus palliative treatment [Figure 9]. When compared to the groups resection plus palliative treatment, biopsy plus adjuvant treatment or biopsy plus palliative treatment, resection plus adjuvant treatment had a statistically significant benefit (P = 0.001, P = 0.005, and P < 0.001, respectively). The group biopsy plus adjuvant treatment had an advantage against the resection plus palliative treatment and biopsy plus palliative treatment (P = 0.028 and P < 0.001, respectively). No statistically significant difference was found between the groups resection plus palliative treatment and biopsy plus palliative treatment (P = 0.711); this stresses the importance of adjuvant treatment in the outcome.
Figure 9.
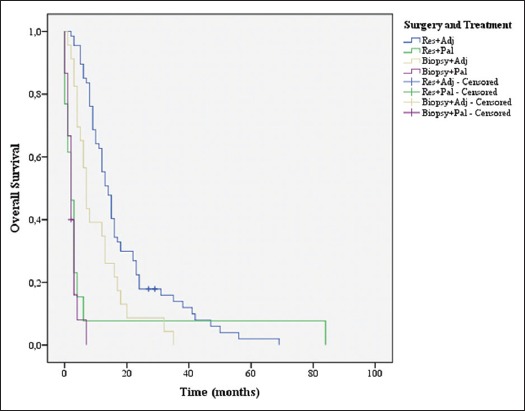
Overall survival stratified by surgery and treatment groups
When we correlate the comorbidities with treatment completion, a lower CACI (≤5), and the treatment completion were associated to a better outcome (χ2, P = 0.035).
Multivariate analysis was performed using Cox proportional hazard modeling, which included the variables with clinical relevance and P < 0.20, namely age, KPS, CACI, number of affected lobes, extent of resection, completion of 60 Gy RT, and completion of ≥6 cycles of TMZ. Both backward and forward stepwise modeling confirmed the prognostic significance of: Completion of 60 Gy RT (P = 0.04; HR, 4.052; 95% CI, 1.321 to 12.432) and completion of ≥ 6 cycles of TMZ (P = 0.03; HR, 2.572; 95% CI, 1.364 to 4.850). The KPS variable showed a trend toward a better survival, although it did not achieve the statistical significance (P = 0.06; HR, 2.222; 95% CI, 0.968 to 5.102).
DISCUSSION
Although the Stupp protocol is considered the standard of care for GBM, regarding to the elderly subpopulation, the benefits are not as clear. In fact, despite the increased incidence of GBM with advanced age, older patients remain under-represented in clinical trials, which lead to the greater difficulties in their management due to the lack of clear guidelines. According to a recent meta-analysis, there is little consensus regarding the treatment choices and although either a single-agent TMZ or hypofractionated RT alone may be rational options for patients who are not candidates to receive combined RT and chemotherapy, this should not be denied to patients submitted to surgical resection, with a KPS higher than 70.[37]
Therefore, the authors reviewed the impact of patient's clinical condition and current treatment modalities on OS, as well as the impact of treatment on the patient's condition, in 126 elderly patients with GBM.
Median OS was 8 months, and median PFS was 5 months. These results are consistent with previous studies in elderly GBM patients [Table 8] and reflect a worse prognosis in this subpopulation.[1,2,7,13,16,19,23,33,34]
Table 8.
Summary of the recent studies of elderly patients with newly diagnosed GBM
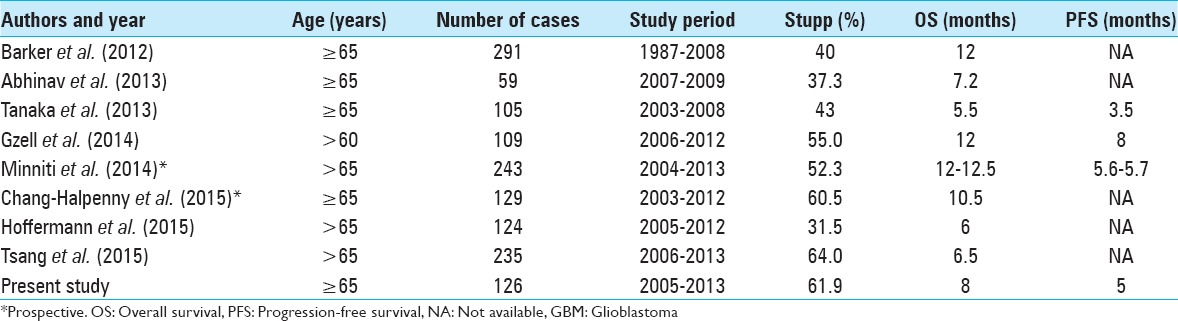
Several variables were correlated to higher OS in univariate analysis. As predicted, a KPS of 70 or more and age lower than 70 showed the association with increased survival (P = 0.01 and P = 0.046, respectively). Multiple studies have shown this in the past.[5,11,22,27]
The complete resection was a predictor of better prognosis (P = 0.048), which is in line with Hardesty and Sanai and other studies.[14,27]
As for factors regarding the tumor itself, the single lesions and tumors affecting only one lobe also showed the better outcomes (P = 0.007 for both), as expected.[30,33,36]
The development of postoperative complications, including a new postoperative motor or language deficits, is generally associated with decreased OS.[10,12,20] Nevertheless, the presence of surgical complications had no statistically significant impact on OS (P = 0.426). One possible explanation was the low rate of complications in the presented cohort, occurring in 31 patients (24.6%).
Although the frontal tumors are frequently associated with higher OS, we did not find this location to have a statistically significant impact on OS (P = 0.535).[17,29]
Multivariate analysis identified the two prognostic independent factors: Completion of 60 Gy RT and completion of 6 or more cycles of TMZ. Radiation therapy is known to be an independent prognostic factor in all ages, and we have found that benefit specifically in elderly patients.[21,23,27] The addition of chemotherapy, specifically TMZ, to regimens including radiation and surgery has already been proven as beneficial in the elderly population. Interestingly, although the 6 cycle regimen is the current standard treatment, our data showed that the number of TMZ cycles was a strong predictor for survival, regardless of the KPS.[5,6]
Grade 3 or 4 toxicity in our series occurred in 25 patients (19.8%), which is in line with previously reported series in elderly patients.[3,13,16] The completion of Stupp protocol improved the OS and PFS (23 months and 14 months, respectively), with greater impact when compared to Stupp et al. (OS of 14.6 months and PFS of 6.9 months).[32] However, only 32.1% of the patients who initiated the protocol were able to finish it, in contrast with Stupp et al. in which 47% of the patients completed the treatment.[32] We could theorize that this low completion rate derived from the incidence of Grade 3 or 4 adverse effects (30.8%). Nonetheless, the interruption of the chemotherapy was also due to the fact that a significant number of patients under TMZ had progression (65.1% vs. 39% previous studies) before the completion of the protocol, which implicated escalation of chemotherapy to the second-line modalities, or in worst cases, definitive the suspension of adjuvant treatment.
To refine the comorbidities assessment and correlate it with the outcome, we analyzed the CACI. With this we tried to explore the weight of physiological age over the chronological age. Interestingly, age per se did not play a major role in the outcome and therefore, in determining the therapeutic modality. In fact, the comorbidities’ burden appears to have a more clinical and therapeutic impact.
This raises a pressing question: Are elderly patients being over-treated? How relevant his physiological age in the outcome of GBM patients? To better treat this subgroup, it is of great importance to find the possible predictors of response to TMZ. In fact, one of the limitations of this study is the absence of the determination of the methylation status of the methyl-guanine methyl transferase gene (MGMT), which identifies the patients most likely to benefit from TMZ.[5,15,31,32,37] MGMT testing was not routinely carried out during the study period.
One of the most interesting results was the importance of the association of surgical resection and the ability to complete adjuvant treatment [Figure 9]. This comes to show that resection is the best option when it is foreseeable that the patient has adequate clinical conditions to be further submitted to the adjuvant treatment. This evidence is in accordance with former studies.[7,13,16,34]
Another interesting finding relates to the association between the comorbidities and the ability to complete the treatment, showing that physiological parameters might define the groups of patients who benefit the most of aggressive therapy.
The main strengths of this study were the large study population, a good functional status and morbidities characterization, and the fact that little data was missing.
In contrast, given the fact that our cohort is retrospective and included all patients in the considered period, the patients were not randomized into the treatment groups, which mean selection bias cannot be excluded.
CONCLUSION
This study demonstrates that maximal safe resection followed by adjuvant chemoradiotherapy is associated with significantly longer survival for elderly patients with newly diagnosed GBM. On the other hand, the evidence strongly suggests that KPS and comorbidities index (e.g., CACI) are important factors with influence in the treatment response and outcome. In fact, in spite of the well-established value of resection on OS, the patient's clinical condition must be considered first as a possible predictor of initiation and response to adjuvant treatment.
Elderly patients benefit from active treatment, particularly those with high KPS and low morbidity index who complete at least 6 cycles of TMZ. However, in our series only a third of patients were able to complete the treatment; actually, this therapeutic benefit does not extend across the entire elderly population. Therefore, the selection of patients and treatment modalities must not be left to chance, making the elaboration of treatment protocols in this age group of utter importance. It is essential to consider other potential prognostic factors prior to surgery, to maximize the therapeutic effectiveness, and OS without compromising the patient's quality of life, in an attempt to avoid unnecessary therapeutic aggression.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicst of interest.
Footnotes
Contributor Information
André F. Pereira, Email: andrepereira5@gmail.com.
Bruno F. Carvalho, Email: bmfcarvalho@gmail.com.
Rui M. Vaz, Email: ruimcvaz@gmail.com.
Paulo J. Linhares, Email: paulojlinhares@yahoo.com.
REFERENCES
- 1.Abhinav K, Aquilina K, Gbejuade H, La M, Hopkins K, Iyer V. A pilot study of glioblastoma multiforme in elderly patients: Treatments, O-6-methylguanine-DNA methyltransferase (MGMT) methylation status and survival. Clin Neurol Neurosurg. 2013;115:1375–8. doi: 10.1016/j.clineuro.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 2.Barker CA, Chang M, Chou JF, Zhang Z, Beal K, Gutin PH, et al. Radiotherapy and concomitant temozolomide may improve survival of elderly patients with glioblastoma. J Neurooncol. 2012;109:391–7. doi: 10.1007/s11060-012-0906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behm T, Horowski A, Schneider S, Bock HC, Mielke D, Rohde V, et al. Concomitant and adjuvant temozolomide of newly diagnosed glioblastoma in elderly patients. Clin Neurol Neurosurg. 2013;115:2142–6. doi: 10.1016/j.clineuro.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Brandes AA, Bartolotti M. Neuro-oncology: Treatment decisions in elderly patients with glioblastoma. Nat Rev Neurol. 2012;8:664–5. doi: 10.1038/nrneurol.2012.220. [DOI] [PubMed] [Google Scholar]
- 5.Brandes AA, Franceschi E, Tosoni A, Benevento F, Scopece L, Mazzocchi V, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: Correlation with MGMT promoter methylation status. Cancer. 2009;115:3512–8. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
- 6.Brandes AA, Vastola F, Basso U, Berti F, Pinna G, Rotilio A, et al. A prospective study on glioblastoma in the elderly. Cancer. 2003;97:657–62. doi: 10.1002/cncr.11097. [DOI] [PubMed] [Google Scholar]
- 7.Chang-Halpenny CN, Yeh J, Lien WW. Elderly patients with glioblastoma multiforme treated with concurrent temozolomide and standard- versus abbreviated-course radiotherapy. Perm J. 2015;19:15–20. doi: 10.7812/TPP/14-083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 9.Della Puppa A, De Pellegrin S, d’Avella E, Gioffrè G, Rossetto M, Gerardi A, et al. 5-aminolevulinic acid (5-ALA) fluorescence guided surgery of high-grade gliomas in eloquent areas assisted by functional mapping. Our experience and review of the literature. Acta Neurochir (Wien) 2013;155:965–72. doi: 10.1007/s00701-013-1660-x. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson H, Carico C, Nuño M, Mukherjee D, Ortega A, Black KL, et al. Unplanned readmissions and survival following brain tumor surgery. J Neurosurg. 2015;122:61–8. doi: 10.3171/2014.8.JNS1498. [DOI] [PubMed] [Google Scholar]
- 11.Fiorica F, Berretta M, Colosimo C, Stefanelli A, Ursino S, Zanet E, et al. Glioblastoma in elderly patients: Safety and efficacy of adjuvant radiotherapy with concomitant temozolomide. Arch Gerontol Geriatr. 2010;51:31–5. doi: 10.1016/j.archger.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Gulati S, Jakola AS, Nerland US, Weber C, Solheim O. The risk of getting worse: Surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011;76:572–9. doi: 10.1016/j.wneu.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Gzell C, Wheeler H, Guo L, Kastelan M, Back M. Elderly patients aged 65–75 years with glioblastoma multiforme may benefit from long course radiation therapy with temozolomide. J Neurooncol. 2014;119:187–96. doi: 10.1007/s11060-014-1472-8. [DOI] [PubMed] [Google Scholar]
- 14.Hardesty DA, Sanai N. The value of glioma extent of resection in the modern neurosurgical era. Front Neurol. 2012;3:140. doi: 10.3389/fneur.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 16.Hoffermann M, Bruckmann L, Kariem Mahdy A, Asslaber M, Payer F, von Campe G. Treatment results and outcome in elderly patients with glioblastoma multiforme – A retrospective single institution analysis. Clin Neurol Neurosurg. 2015;128:60–9. doi: 10.1016/j.clineuro.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis. Neuro Oncol. 2004;6:227–35. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Jung TY, Jung S, Kim IY, Jang WY, Moon KS, et al. Performance status during and after radiotherapy plus concomitant and adjuvant temozolomide in elderly patients with glioblastoma multiforme. J Clin Neurosci. 2013;20:503–8. doi: 10.1016/j.jocn.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 19.Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–26. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 20.McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–9. doi: 10.1227/01.NEU.0000349763.42238.E9. [DOI] [PubMed] [Google Scholar]
- 21.Mineo JF, Bordron A, Baroncini M, Ramirez C, Maurage CA, Blond S, et al. Prognosis factors of survival time in patients with glioblastoma multiforme: A multivariate analysis of 340 patients. Acta Neurochir (Wien) 2007;149:245–52. doi: 10.1007/s00701-006-1092-y. [DOI] [PubMed] [Google Scholar]
- 22.Minniti G, De Sanctis V, Muni R, Filippone F, Bozzao A, Valeriani M, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88:97–103. doi: 10.1007/s11060-008-9538-0. [DOI] [PubMed] [Google Scholar]
- 23.Minniti G, Scaringi C, Lanzetta G, Terrenato I, Esposito V, Arcella A, et al. Standard (60 Gy) or short-course (40 Gy) irradiation plus concomitant and adjuvant temozolomide for elderly patients with glioblastoma: A propensity-matched analysis. Int J Radiat Oncol Biol Phys. 2015;91:109–15. doi: 10.1016/j.ijrobp.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009. National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events (CTCAE). Rev. Edition. [Google Scholar]
- 25.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1–56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patil CG, Eboli P, Hu J. Management of multifocal and multicentric gliomas. Neurosurg Clin N Am. 2012;23:343–50. doi: 10.1016/j.nec.2012.01.012. x. [DOI] [PubMed] [Google Scholar]
- 27.Scott JG, Suh JH, Elson P, Barnett GH, Vogelbaum MA, Peereboom DM, et al. Aggressive treatment is appropriate for glioblastoma multiforme patients 70 years old or older: A retrospective review of 206 cases. Neuro Oncol. 2011;13:428–36. doi: 10.1093/neuonc/nor005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma A, Sharma DN, Julka PK, Rath GK. Treatment options in elderly patients with glioblastoma. Lancet Oncol. 2012;13:e460–1. doi: 10.1016/S1470-2045(12)70459-X. [DOI] [PubMed] [Google Scholar]
- 29.Simpson JR, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: Results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26:239–44. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 30.Stark AM, van de Bergh J, Hedderich J, Mehdorn HM, Nabavi A. Glioblastoma: Clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg. 2012;114:840–5. doi: 10.1016/j.clineuro.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 32.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka S, Meyer FB, Buckner JC, Uhm JH, Yan ES, Parney IF. Presentation, management, and outcome of newly diagnosed glioblastoma in elderly patients. J Neurosurg. 2013;118:786–98. doi: 10.3171/2012.10.JNS112268. [DOI] [PubMed] [Google Scholar]
- 34.Tsang DS, Khan L, Perry JR, Soliman H, Sahgal A, Keith JL, et al. Survival outcomes in elderly patients with glioblastoma. Clin Oncol (R Coll Radiol) 2015;27:176–83. doi: 10.1016/j.clon.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 35.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 36.Yabroff KR, Harlan L, Zeruto C, Abrams J, Mann B. Patterns of care and survival for patients with glioblastoma multiforme diagnosed during 2006. Neuro Oncol. 2012;14:351–9. doi: 10.1093/neuonc/nor218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zarnett OJ, Sahgal A, Gosio J, Perry J, Berger MS, Chang S, et al. Treatment of elderly patients with glioblastoma: A systematic evidence-based analysis. JAMA Neurol. 2015;72:589–96. doi: 10.1001/jamaneurol.2014.3739. [DOI] [PubMed] [Google Scholar]


